Abstract
Background
In many patients, the risk of cardiovascular (CV) events persists despite statin treatment and attaining target LDL–c levels. This residual risk is in part attributed to atherogenic dyslipidemia (AD). We studied the clinical effectiveness of the CNIC-polypill in improving the lipid profile, and lipid ratios and indices indicative of AD that are more accurate in predicting lipid-related CV risk.
Methods
Post-hoc analysis of a multicenter, observational, non-comparative, prospective registry in 533 patients in Mexico. We evaluated blood lipids at baseline (usual care) and after 12 months of treatment with the CNIC-polypill (Sincronium®), including total cholesterol (TC), triglycerides (TG), cholesterol low-density lipoproteins (LDL–c), cholesterol high-density lipoproteins (HDL–c), and cholesterol non-high-density lipoproteins (non-HDL–c). We also calculated and compared AD-related lipid ratios and indices, including remnant cholesterol (RC), Castelli's risk index-I (CRI–I), atherogenic index (AI), atherogenic coefficient (AC), a surrogate of insulin resistance (IRS), atherogenic index of plasma (AIP), and lipoprotein combined index (LCI).
Results
At 1 year of treatment, there was a significant reduction in the levels of TC (−22.6%), TG (−29.2%), LDL–c (−13.8%), and non-HDL–c (−29.2%) (all p < 0.001). The likelihood that patients attained their corresponding target LDL–c and TG levels was almost three-fold and seven-fold higher, respectively (p < 0.001). The values of the AD-related ratios RC, CRI–I, AI, AC, AIP, and LCI were all significantly lower (p < 0.001) after one year of treatment.
Conclusions
In patients with or at high risk of CVD, one-year treatment with the CNIC-polypill significantly lowered lipid ratios indicative of AD compared to baseline.
Keywords: Polypill, Cardiovascular risk, Lipid ratios, Secondary prevention, High-risk primary prevention, Atherogenic dyslipidemia
1. Introduction
The major traditional risk factors for cardiovascular disease (CVD)2 include both non-modifiable factors (e.g., age, male sex, or familial predisposition) and modifiable factors such as smoking, physical inactivity, obesity, hypertension, dyslipidemia, and type 2 diabetes mellitus (T2DM) [1]. Typically, these risk factors cluster in individuals and have a synergistic effect on the risk of CVD, also resulting in increased CV mortality [2]. At the disease management level, this implies that most patients on primary prevention, and all of those on secondary prevention, need a multi-targeted treatment approach based on combination therapy with two or more drugs. This polypharmacy frequently leads to complex medication regimens that result in poor therapeutic adherence and persistence, which in turn is associated with increased risk of recurrent or new CV events and poorer clinical outcomes [3]. A strategy nowadays endorsed by several clinical guidelines to circumvent the above-mentioned drawbacks is the use of the CV polypill [1], [4], [5]. It consists of a single oral dosage form composed of active ingredients with well-supported evidence for the prevention of CV events, in general a minimum of a statin, an antiplatelet drug, and an antihypertensive agent [6]. Several clinical trials for secondary prevention of CVD have shown that, compared to the standard of care, the CV polypill improves systolic blood pressure (BP) and low-density lipoprotein cholesterol (LDL–c), might reduce the risk of all-cause mortality compared to usual care, and it is cost-effective [6], [7], [8].
In Europe and Latin America (LATAM), the CNIC-polypill (i.e. aspirin, ramipril, and atorvastatin/simvastatin) was the first CV polypill approved and marketed for the secondary prevention of CVD [6], [9]. A recent real-world, prospective, observational study conducted in Mexico (SORS study) in patients with or at high risk of CVD reported that, after one year of treatment with the CNIC-polypill, there were significant reductions in systolic and diastolic BP, total cholesterol (TC), LDL–c, triglycerides (TG), and an increase in high-density lipoprotein cholesterol (HDL–c) [10]. In clinical practice, and beyond lifestyle recommendations, LDL–c reduction is the primary target in the management of dyslipidemia and reduction of cardiovascular risk (CVR), with statins as the first line lipid-lowering therapy [1]. Nonetheless, the risk for CV events commonly persists in spite of statin treatment and having achieved the recommended or even below-target LDL–c levels, and this residual risk is attributed to other lipid factors with potential atherogenic action [11]. The largest contributor to this residual risk of lipid origin is atherogenic dyslipidemia (AD), defined as the combination of elevated LDL–c and TG levels, low HDL–c levels, and a preponderance of small-dense LDL–c particles [12]. As a consequence, patients may remain untreated or inadequately treated if their LDL–c levels are not substantially elevated [13], highlighting the need to carefully address the components of the lipid profile beyond LDL–c. This is especially true for Latin-American countries, where the prevalence of AD is higher than in other geographical regions [14]. In the particular case of Mexico, some 30% of obese or overweight subjects have hypertriglyceridemia and 18% of them mixed dyslipidemias [15].
The clinical effectiveness of the CNIC-polypill in terms of the improvement in lipid ratios indicative of AD and thus associated with lipid-related CV risk has not previously been studied. Therefore, the current study aims to assess its clinical effect on the comprehensive lipid profile of Mexican patients at high risk of or with pre-existing CVD participating in the SORS study through the inclusion of a panel of atherogenic parameters known to be predictors or surrogate markers of atherosclerosis and CVD.
2. Materials and methods
2.1. Study design and population
This was a post hoc analysis of the SORS study, which was a multicenter, prospective, non-comparative observational study conducted in Mexico, details of which have been published previously [10]. Briefly, the study analyzed 1193 patients who initiated treatment for CV prevention with the CNIC-polypill (acetylsalicylic acid 100 mg, ramipril 5 or 10 mg, and simvastatin 40 mg). For the current analysis, we selected only those patients with full data on all lipid parameters both at study entry (on standard of care) and at the end of the follow-up (after 12 months of treatment).
The study was approved by the corresponding Health Authorities, and all patients signed a written informed consent form prior to participation. The study was conducted in accordance with the Declaration of Helsinki (1975).
2.2. Study measures
As well as patient demographics, baseline medication, history of CV events, BMI, and presence of CV risk factors, the study collected fasting blood samples both at baseline and at the end of the study to determine plasma glucose and blood lipid levels, which included TC, LDL–c (estimated according to the Friedewald formula) [16], HDL–c, and TG. In addition, other non-conventional lipid parameters and markers of AD included the following biochemical ratios and indices: 1) Non-HDL–c, 2) remnant cholesterol (RC; TC minus HDL–c minus LDL–c) [17], 3) Castelli's risk index-I (CRI–I; TC/HDL–c) [18], 4) Castelli's risk index-II or atherogenic index (AI; LDL–c/HDL–c) [18], 5) atherogenic coefficient (AC; non-HDL–c/HDL–c) [19], 6) surrogate marker of insulin resistance (IRS; TG/HDL–c) [20], 7) the atherogenic index of plasma (AIP; log[TG/HDL–c]) [21], and 8) the lipoprotein combined index (LCI; TC × TG × LDL/HDL–c).
2.3. Statistical analysis
The LDL–c, non-HDL–c, RC, CRI–I, AI, AC, IRS, AIP, and LCI ratios were calculated. Categorical variables are presented as absolute and relative frequency, and continuous variables are expressed as mean ± standard deviation (SD). The difference in the mean value of the different lipid parameters and indices from baseline to end of follow-up is expressed as % change with 95% confidence intervals (CI), and the statistical significance of the change was assessed using paired sample Student's t-test for repeated measures. A further analysis was conducted in patients with on-target LDL–c levels (i.e. < 70 mg/dL for those with a previous event and < 100 mg/dL for all others at moderate risk) and in patients with normal or borderline high TG levels (<150 and < 200 mg/dL, respectively). The target levels for LDL–c and recommended levels for TG were those in the 2016 European Society of Cardiology (ESC) guidelines on lipid management, [22] since this observational study was performed before the publication of the latest 2019 ESC guidelines [23]. To determine the likelihood of achieving on-target values after one year of treatment we used McNemar’s test for dichotomous repeated measures, and results were expressed as Odds Ratio (OR) and 95% CI. All the statistical analyses were performed using the statistical package SPSS 22.0 (SPSS Inc., Chicago, Illinois). All P values were two-tailed, and p < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of the study population
The original SORS study included 1193 patients [10] whose baseline treatment was acetyl salicylic acid in 85.5% of patients, statins in 94%, 94.2% received either ACEi (angiotensin-converting-enzyme inhibitors) or ARBs (angiotensin II receptor blockers), and 97.3% of patients were treated with all three drug classes. Out of the whole population, 533 had a full set of data on traditional lipid parameters at both baseline and after one year of treatment with the CNIC-polypill. Sociodemographic, clinical, and CVR factors are shown in Table 1. The mean age of the studied population was 57.3 years (SD = 14.3), there was a higher proportion of males (53.4%), and the mean BMI was in the range of overweight (29.4; SD = 4.5). With regard to CVR factors, the vast majority of participants had arterial hypertension or hypercholesterolemia (85.1% and 86.3%, respectively), more than half of them had hypertriglyceridemia (63%), about a third had T2DM (29.7%), and most (90.6%) had more than two of the above risk factors (including obesity). Finally, 57.6% of patients had pre-existing CVD, with stable angina being the most common condition (45.1%), followed by myocardial infarction (39.8%) and unstable angina (12.8%).
Table 1.
Baseline demographic and clinical characteristics of the patients.
| Variable | Subjects treated with the CNIC-Polypill N = 533 |
|---|---|
| Age (years), mean (SD) | 57.3 (14.3) |
| Gender (male), n (%) | 285 (53.4) |
| BMI (kg/m2), mean (SD) | 29.4 (4.5) |
| Arterial hypertension, n (%) | 453 (85.1) |
| Hypercholesterolemia, n (%) | 459 (86.3) |
| T2DM, n (%) | 158 (29.7) |
| Hypertriglyceridemia, n (%) | 336 (63.0) |
| Presence of > 2 CV risk factors,* n (%) | 483 (90.6) |
| Previous CV event, n (%) | 307 (57.6) |
| Stable angina, n (%) | 139 (45.1) |
| Myocardial infarction, n (%) | 122 (39.8) |
| Unstable angina, n (%) | 39 (12.8) |
| Others, n (%) | 7 (2.2) |
BMI, body mass index; CV, cardiovascular; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Defined as more than two of the following: BMI > 30 kg/m2, arterial hypertension, hypercholesterolemia, diabetes mellitus, or hypertriglyceridemia.
3.2. Traditional lipid profile after one year of treatment
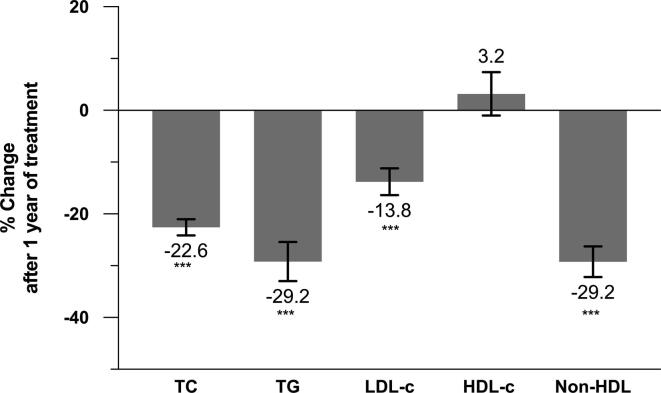
One year of treatment with the CNIC-polypill, compared with baseline, significantly lowered the levels of TC (from 241 mg/dL [SD = 62] to 187 mg/dL [SD = 36]), TG (from 227 mg/dL [SD = 101] to 161 mg/dL [SD = 49]), LDL–c (from 130 mg/dL [SD = 36] to 112 mg/dL [SD = 30]), and overall non-HDL–c (from 192 mg/dL [SD = 64] to 134 mg/dL [SD = 39]), while mean plasma HDL–c levels experienced a slight but non-significant increase (from 49 mg/dL [SD = 24] to 50 mg/dL [SD = 14]). The mean percentage difference before the CNIC-polypill and after one year of treatment was statistically significant for TC, TG, LDL–c, and non-HDL–c (all p < 0.001), with the greatest reductions observed in TG and non-HDL–c levels (−29.2%), followed by TC (−22.6%) and LDL–c levels (−13.8%) (Fig. 1).
Fig. 1.
Plot of the mean percentage of change in lipid parameters compared to baseline after one year of treatment with the CNIC-polypill. ***P < 0.001. HDL–c, high-density lipoprotein cholesterol; LDL–c, low-density lipoprotein cholesterol; Non-HDL–c, non-high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
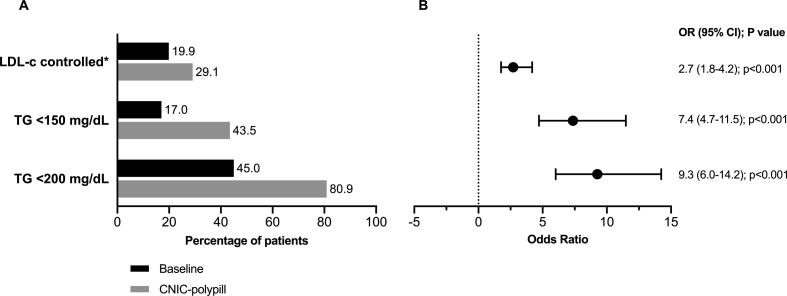
There was a significant improvement after one year of treatment with the CNIC-polypill in the proportion of patients attaining target levels (p < 0.001) (Fig. 2A). Ten percent more patients achieved recommended LDL–c levels (namely < 70 mg/dL for those with a previous event and < 100 mg/dL for all others at moderate risk), 27% more attained TG levels of < 150 mg/dL, and 36% more < 200 mg/dL, in the latter case with as many as 81% of the population at such levels. Furthermore, the likelihood that patients achieved their corresponding target LDL–c levels was almost 2.7 times higher after one year of treatment with the CNIC-polypill (p < 0.001; Fig. 2B). This improvement vs. pre-treatment was more evident in relation to the attainment of recommended TG levels, with a 7.4 times greater likelihood of being below 150 mg/dL, and 9.3 times greater odds of being below 200 mg/dL (p < 0.001; Fig. 2B).
Fig. 2.
Proportion of patients with lipid parameters at target or recommended levels before and after one year of treatment with the CNIC-polypill (A) and odds of attaining the target (B). *<70 mg/dL for those with a previous event and < 100 mg/dL for high-risk patients.[22]. CI, confidence interval; LDL–c, low-density lipoprotein cholesterol; OR, odds ratio; TG, triglycerides.
3.3. Parameters of atherogenic dyslipidemia after one year of treatment
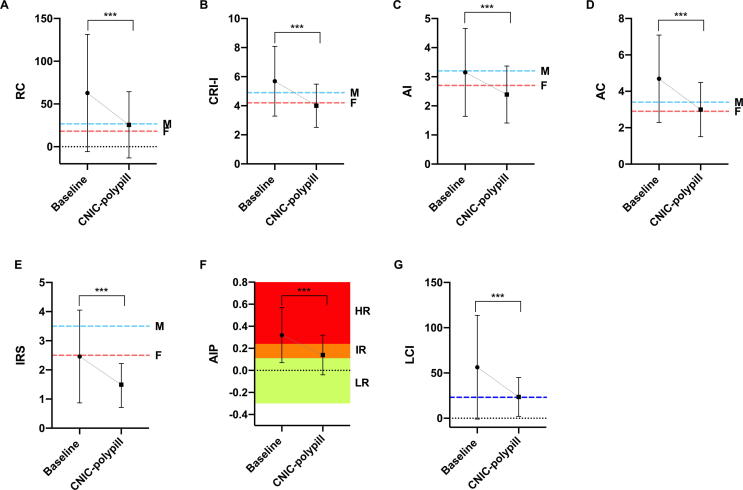
When subjects were treated as per standard of care, all atherogenic dyslipidemia indices (except the surrogate marker of insulin resistance) were above normal mean values (Fig. 3A-E and G), including a mean AIP value in the range of high risk for CVD (above 0.24) (Fig. 3F). After one year of treatment with the CNIC-polypill, all ratios decreased to normal or almost normal reference values and, remarkably, AIP decreased to a ratio considered to be associated with low-intermediate CV risk (0.14). When the mean difference between standard of care and polypill treatment was quantified as the percentage of change, the observed reductions for all studied AD markers were statistically significant (p < 0.001). These reductions were>55% in the case of RC (−59%), AIP (−56%), and LCI (−58%), while for the other lipid ratios the decrease was less pronounced but still significant at − 39% for IRS, −30% for CRI–I, and − 24% for AI.
Fig. 3.
Mean change in parameters of atherogenic dyslipidemia between baseline and after one year of treatment with the CNIC-polypill: A) remnant cholesterol (RC), B) Castelli's risk index–I (CRI–I), C) atherogenic index (AI); D) atherogenic coefficient (AC), E) TG/HDL–c ratio, used as a surrogate marker of insulin resistance (IRS), F) atherogenic index of plasma (AIP), and G) lipoprotein combined index (LCI). F, females; M, males. Dotted lines indicate the mean reference values (RC, CRI, AI, and LCI); the cut-off value for the detection of metabolic syndrome (AC); or the cut-off value for increased CV risk (IRS and AIP) [17], [18], [34], [39], [40], [41]. RC = TC − HDL–c − LDL–c; CRI–I = TC/HDL–c; AI = LDL–c/HDL–c; AC = non-HDL–c/HDL–c; IRS = TG/HDL–c; AIP = log(TG/HDL–c); LCI = TC × TG × LDL/HDL–c.
4. Discussion
The decrease in TC and LDL–c parameters observed after one year of treatment with the CNIC-polypill was within the same range as previous randomized controlled trials (RCT) that assessed patients with CVD and patients without previous event [7], [8]. Expressed as a percentage of change, we observed a reduction of 23% in TC levels and 29% in TG levels, which is in line with those of a recent study also conducted in Mexico that included patients in secondary prevention [24]. However, the percentage LDL–c reduction in our study was 14%, while in the Mendez-Garcia et al. study it was 40% [24]. The smaller decrease in LDL–c levels in our study could be attributable to higher mean pre-treatment LDL–c values in their case (135.8 mg/dL vs. 129.6 mg/dL in ours).
The better performance of the polypill strategy has been attributed to a better adherence, estimated to be improved by about 40% compared with usual care (i.e. the 3 drugs given separately) [6], [7]. This is relevant because low adherence is an issue that exists worldwide and a striking problem in low- or medium-income countries, such as many of the countries in LATAM [25]. Moreover, the previously observed pharmacodynamic synergy between monocomponents when administered together could have impacted in these results [26]. In the particular case of the CNIC-polypill, which contains atorvastatin 40 mg, ramipril 10 mg, and acetylsalicylic acid 100 mg, it has recently been reported that it exerts a significantly (7%) greater reduction in LDL–c than atorvastatin alone [26]. However, it should be taken into account that the lipid-lowering component used in our study was simvastatin and these results should therefore be assessed with caution.
Of note, the proportion of subjects attaining their target LDL–c levels in our study and the probability of their doing so was about 10% higher than before the intervention (30% of patients in total at the end of the study). This figure is in line with a meta-analysis of RCTs comparing the polypill strategy with usual care in patients at high risk of or with CVD [27]. Our results are remarkable if we consider that the proportion of patients taking lipid-lowering treatment in addition to the polypill was low, with only 3.9% and 0.4% of patients being treated with fibrates and ezetimibe, respectively [10]. Certainly, it is necessary to bear in mind that, although the polypill may be used as baseline treatment, it can be up-titrated with other lipid-lowering drugs when LDL–c levels remain off-target and/or TG higher than recommended.
Regarding the achievement of TG levels, the difference compared to baseline was much more pronounced, with 81% of subjects attaining levels < 200 mg/dL and almost half of them at a target of < 150 mg/dL at the end of the study, representing improvements of 18% and 36%, respectively. This is highly relevant to clinical practice because cholesterol and TG are the two main lipids in plasma and, although LDL–c is considered the primary atherogenic cholesterol-rich particle, elevated TG are associated with increased CV risk and are independently associated with CV events even in patients treated with statins and with target LDL–c levels [28], [29], [30]. It is worth noting that non-HDL–c levels in our study experienced an additional 29% reduction compared with pre-treatment.
The rationale behind the calculation and use of lipid ratios is that they reflect the balance of both atherogenic and protective lipoproteins [31]. In the present study, we observed significant reductions of between 59% and 24% in the baseline scores of all studied AD markers. Of note, the mean scores for RC, CRI–I, AI, AC, AIP, and LCI showed that patients were at risk of CV events when treated based on the standard of care, but those values then reverted to normal or near-normal after one year of treatment with the CNIC-polypill, indicating average or low-risk CV risk scores. The fact that different lipid ratios were substantially improved is probably due to various reasons. Firstly, statins inhibit intracellular cholesterol biosynthesis, thus reducing LDL–c, but they also reduce the synthesis and entrance in the circulation of small size lipoproteins (apolipoprotein B), and are able to lower serum concentrations of TG and remnant lipoproteins [32]. Secondly, the different components of AD often present as a cluster because they share metabolic and physiopathological mechanisms, and therefore the polypill strategy might have improved multiple components simultaneously [33].
The clinical relevance of the clear improvement in AD markers observed in our study could potentially be in relation to a decreased risk of atherosclerosis. For instance, the mean AIP value in our study fell from 0.32 at baseline to 0.14 after one year of treatment with the CNIC-polypill, which correspond to values associated with high and low-to-intermediate CV risk, respectively [34].
The results obtained with the present study are relevant in particular in LATAM, where CVD is the leading cause of death and disability, with 70% of cases due to acute myocardial infarction, this being mainly due to the high prevalence of CVR factors in these regions [14]. However, in LATAM, up to 31% of patients with CVD do not receive any pharmacological treatment, and <4% receive the 3 or 4 proven therapies (i.e. antiplatelet medications, beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, and statins) [35]. Additionally, the prevalence of AD is higher in LATAM than in other geographical regions, with low HDL–c levels present in 34–53% and high TG levels in 26–31% of the population compared to 30% and 24% in the US, or 22% and 21% in Europe, respectively [14], [36]. These data are even more worrying in the case of Mexico, with an estimated 10.3% of its population having diabetes mellitus,[37] 44% metabolic syndrome, and 55% considered to be at high risk of CVD [38]. Moreover, the prevalence of low HDL–c and mixed hyperlipidemia is amongst the highest in the world, with about 30% of obese or overweight subjects having hypertriglyceridemia and 18% of them mixed dyslipidemias [15].
5. Study limitations
The present study has strengths and limitations that must be acknowledged. The major strength is the observational design, which captures data in real-world clinical practice. However, there are inherent weaknesses associated with this design, such as the lack of a control group, the presence of multiple confounders and biases introduced by the heterogeneity of the selected population. Moreover, the study’s sample size was relatively small and, although representative of the Mexican population at high risk of CVD, may not reflect the response to treatment in other countries with a different prevalence of AD components or profile of CV risk factors.
6. Conclusions
The present real-world study showed that, additional to standard of care, patients with or at high risk of CVD treated for one year with the CNIC-polypill experienced a significant reduction in lipid markers indicative of AD. These results not only confirm that the CV polypill strategy can control lipid parameters better than traditional combination therapy, but also that it can reduce the residual CV risk of lipid origin, thus potentially exerting more intense protective effects on the overall CVD risk.
Declaration of Competing Interest
E. Ruiz is an employee in the Medical Department in Ferrer. C. Ponte-Negretti and E. Góme-Álvarez have received speaker honoraria from Ferrer outside the content of this manuscript. The other authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
The authors would like to thank Jose Luis Lorenzo, employee of Ferrer, who helped editing the manuscript. Medical writing and editorial support were provided by Mònica Gratacòs funded by Ferrer.
Footnotes
Abbreviations: AC, atherogenic coefficient; AD, atherogenic dyslipidemia; AIP, atherogenic index of plasma; BMI, body mass index; BP, blood pressure; CRI-I, Castelli's risk index-I; CV, cardiovascular; CVD, cardiovascular disease; CVR, cardiovascular risk; HDL-c, high-density lipoproteins; IRS, insulin resistance surrogate; LATAM, Latin America; LCI, lipoprotein combined index; LDL-c, low-density lipoproteins; RC, remnant cholesterol; TC, total cholesterol; TD2M, type 2 diabetes mellitus; TG, triglycerides; TRL, triglyceride-rich lipoproteins
Contributor Information
Emilio Ruíz, Email: jruizo@ferrer.com.
Marco Martínez Ríos, Email: dirgral2@cardiologia.org.mx.
References
- 1.Piepoli M.F., Hoes A.W., Agewall S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancia G. Total cardiovascular risk: a new treatment concept. J. Hypertens. Suppl. 2006;24(2):S17–24. doi: 10.1097/01.hjh.0000220099.12154.c1. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury R., Khan H., Heydon E. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur. Heart J. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B., James S., Agewall S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.Williams B., Mancia G., Spiering W. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 6.Webster R., Castellano J.M., Onuma O.K. Putting polypills into practice: challenges and lessons learned. Lancet. 2017;389(10073):1066–1074. doi: 10.1016/S0140-6736(17)30558-5. [DOI] [PubMed] [Google Scholar]
- 7.Bahiru E., de Cates A.N., Farr M.R. Fixed-dose combination therapy for the prevention of atherosclerotic cardiovascular diseases. Cochrane Database Syst. Rev. 2017;CD009868 doi: 10.1002/14651858.CD009868.pub3. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster R., Patel A., Selak V. Effectiveness of fixed dose combination medication ('polypills') compared with usual care in patients with cardiovascular disease or at high risk: A prospective, individual patient data meta-analysis of 3140 patients in six countries. Int. J. Cardiol. 2016;205:147–156. doi: 10.1016/j.ijcard.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Castellano J.M., Fuster V. Polypill, el policomprimido cardiovascular: del concepto a la realidad en España. Rev. Espanola de Cardiol. Supl. 2015;15:19–24. doi: 10.1016/s1131-3587(16)30005-x. [DOI] [Google Scholar]
- 10.Castellano J.M., Verdejo J., Ocampo S. Clinical Effectiveness of the Cardiovascular Polypill in a Real-Life Setting in Patients with Cardiovascular Risk: The SORS Study. Arch. Med. Res. 2019;50(1):31–40. doi: 10.1016/j.arcmed.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Chapman M.J., Ginsberg H.N., Amarenco P. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 2011;32(11):1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy S.M. Small LDL, atherogenic dyslipidemia, and the metabolic syndrome. Circulation. 1997;95(1):1–4. doi: 10.1161/01.cir.95.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson T.A., Case C.C., Roberts S. Characteristics of US adults with the metabolic syndrome and therapeutic implications. Diabetes Obes. Metab. 2004;6(5):353–362. doi: 10.1111/j.1462-8902.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 14.Ponte-Negretti C.I., Isea-Perez J.E., Lorenzatti A.J. Atherogenic Dyslipidemia in Latin America: Prevalence, causes and treatment: Expert's position paper made by The Latin American Academy for the Study of Lipids (ALALIP) Endorsed by the Inter-American Society of Cardiology (IASC), the South American Society of Cardiology (SSC), the Pan-American College of Endothelium (PACE), and the International Atherosclerosis Society (IAS) Int. J. Cardiol. 2017;243:516–522. doi: 10.1016/j.ijcard.2017.05.059. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar-Salinas C.A., Olaiz G., Valles V. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nationwide survey. J. Lipid Res. 2001;42(8):1298–1307. [PubMed] [Google Scholar]
- 16.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 17.McNamara J.R., Shah P.K., Nakajima K. Remnant lipoprotein cholesterol and triglyceride reference ranges from the Framingham Heart Study. Clin. Chem. 1998;44(6 Pt 1):1224–1232. [PubMed] [Google Scholar]
- 18.Castelli W.P., Abbott R.D., McNamara P.M. Summary estimates of cholesterol used to predict coronary heart disease. Circulation. 1983;67(4):730–734. doi: 10.1161/01.cir.67.4.730. [DOI] [PubMed] [Google Scholar]
- 19.Brehm A., Pfeiler G., Pacini G., Vierhapper H., Roden M. Relationship between serum lipoprotein ratios and insulin resistance in obesity. Clin. Chem. 2004;50(12):2316–2322. doi: 10.1373/clinchem.2004.037556. [DOI] [PubMed] [Google Scholar]
- 20.Ray S., Bairagi A.K., Guha S. A simple way to identify insulin resistance in non-diabetic acute coronary syndrome patients with impaired fasting glucose. Indian J. Endocrinol. Metab. 2012;16(Suppl 2):S460–464. doi: 10.4103/2230-8210.104132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobiasova M., Frohlich J., Sedova M., Cheung M.C., Brown B.G. Cholesterol esterification and atherogenic index of plasma correlate with lipoprotein size and findings on coronary angiography. J. Lipid Res. 2011;52(3):566–571. doi: 10.1194/jlr.P011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catapano A.L., Graham I., De Backer G. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016;37(39):2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 23.The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS), 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 290 (2019) 140-205. https://doi.org/10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed]
- 24.Mendez-Garcia L.A., Gonzalez-Chavez A., Trejo-Millan F. Six Month Polypill Therapy Improves Lipid Profile in Patients with Previous Acute Myocardial Infarction: The Heart-Mex Study. Arch. Med. Res. 2019;50(4):197–206. doi: 10.1016/j.arcmed.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Khatib R., McKee M., Shannon H. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet. 2016;387(10013):61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 26.González-Juanatey J.R., Tamargo J., Torres F., Weisser B., Oudovenko N. Pharmacodynamic study of the cardiovascular polypill. Is there any interaction among the monocomponents? Revista Española de Cardiología (English Edition) 2020 doi: 10.1016/j.rec.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Selak V., Bullen C., Stepien S. Do polypills lead to neglect of lifestyle risk factors? Findings from an individual participant data meta-analysis among 3140 patients at high risk of cardiovascular disease. Eur. J. Prev. Cardiol. 2016;23(13):1393–1400. doi: 10.1177/2047487316638216. [DOI] [PubMed] [Google Scholar]
- 28.Hokanson J.E., Austin M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk. 1996;3(2):213–219. [PubMed] [Google Scholar]
- 29.Nordestgaard B.G., Benn M., Schnohr P., Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 30.Langsted A., Freiberg J.J., Tybjaerg-Hansen A., Schnohr P., Jensen G.B., Nordestgaard B.G. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J. Intern. Med. 2011;270(1):65–75. doi: 10.1111/j.1365-2796.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- 31.Langlois M.R., Chapman M.J., Cobbaert C. Quantifying Atherogenic Lipoproteins: Current and Future Challenges in the Era of Personalized Medicine and Very Low Concentrations of LDL Cholesterol. A Consensus Statement from EAS and EFLM. Clin. Chem. 2018;64(7):1006–1033. doi: 10.1373/clinchem.2018.287037. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer E.J., McNamara J.R., Tayler T. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. Am. J. Cardiol. 2004;93(1):31–39. doi: 10.1016/j.amjcard.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Xiao C., Dash S., Morgantini C., Hegele R.A., Lewis G.F. Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: The Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol. Diabetes. 2016;65(7):1767–1778. doi: 10.2337/db16-0046. [DOI] [PubMed] [Google Scholar]
- 34.Dobiasova M. AIP–atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr. Lek. 2006;52(1):64–71. [PubMed] [Google Scholar]
- 35.Avezum A., Oliveira G.B.F., Lanas F. Secondary CV Prevention in South America in a Community Setting: The PURE Study. Glob. Heart. 2017;12(4):305–313. doi: 10.1016/j.gheart.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Halcox J.P., Banegas J.R., Roy C. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc. Disord. 2017;17(1):160. doi: 10.1186/s12872-017-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.INEGI, Instituto Nacional de Salud Pública. Secretaría de Salud. Estados Unidos Mexicanos. Encuesta Nacional de Salud y Nutrición 2018. Presentación de resultados. Available from: https://ensanut.insp.mx/encuestas/ensanut2018/doctos/informes/ensanut_2018_presentacion_resultados.pdf. (Accessed Feb 2020).
- 38.Waters D.D., Brotons C., Chiang C.W. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120(1):28–34. doi: 10.1161/CIRCULATIONAHA.108.838466. [DOI] [PubMed] [Google Scholar]
- 39.Kim S.W., Jee J.H., Kim H.J. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int. J. Cardiol. 2013;168(3):2678–2683. doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Salazar M.R., Carbajal H.A., Espeche W.G. Relation among the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio-metabolic risk factors in men and women. Am. J. Cardiol. 2012;109(12):1749–1753. doi: 10.1016/j.amjcard.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Cai G., Shi G., Xue S., Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine (Baltimore) 2017;96(37):e8058. doi: 10.1097/MD.0000000000008058. [DOI] [PMC free article] [PubMed] [Google Scholar]