Abstract
The phenomenon of enhanced invasion and metastasis of residual tumor cells has been observed in an increasing number of patients receiving chemoradiotherapy recently, and tumor metastasis will undoubtedly limit patient prognosis. However, the key mechanism by which chemoradiotherapy affects the invasion and metastasis of tumor cells remains unclear. Studies have shown that chemoradiotherapy may directly act on tumor cells and alter the tumor microenvironment, or induce cell apoptosis and autophagy to promote tumor cell survival and metastasis. In this review, we summarize the potential mechanisms by which chemoradiotherapy may affect the biological behavior of tumor cells and open up new avenues for reducing tumor recurrence and metastasis after treatment. These insights will improve the efficacy of chemoradiotherapy.
Keywords: Biological behavior, Cancer, Chemoradiotherapy, Mechanism, Invasion and metastasis
Introduction
Chemoradiotherapy is the combination of radiotherapy and chemotherapy, which utilizes chemotherapy as a radiosensitizer that renders tumor cells more sensitive to radiotherapy to achieve better local control effect.1,2 In several cases of liver cancer, however, the therapeutic effect of chemoradiotherapy was observed as unexpected, reflected in opposite effect of chemotherapy. Tumor volume rapidly decreased in a short period after patients received a high dose of chemoradiotherapy, but metastasis would occur after discontinuation of chemoradiotherapy.3 A similar phenomenon has been observed in non-small cell lung cancer (NSCLC), where cell proliferation is accelerated after the cessation of chemotherapy and progression becomes faster than in untreated tumors.4 Similarly, in oropharyngeal cancer, the potential doubling time of tumors after induction of chemotherapy was shorter after treatment than before treatment. This was indicative of an increased proliferation of oropharyngeal tumor cells after poor response to chemotherapy.5 In extrahepatic cholangiocarcinoma, chemoradiotherapy combined with surgery is potentially beneficial to local control by improving tumor resectability and survival rate, but systemic metastases threaten patient prognosis and chemoradiotherapy needs to be considered more critically.6 Chemoradiotherapy also induces the metastasis of ovarian cancer cells to the bone marrow and other organs.7 The mechanism underlying rapid tumor metastasis after discontinuation of chemoradiotherapy is still unclear.
In a study, local treatment with 4–10 Gy radiation for oral mammary carcinoma resulted in increased lung metastasis in tumor-bearing mice compared with that in the sham irradiation control group.8 A similar pattern is noted in breast cancer, where even low-dose preoperative exposure can lead to increased lung metastasis.9 Although the Radiation Therapy Oncology Group (RTOG) noted that,in NSCLC the tolerated radiation dose can be as high as 83.8 Gy using three-dimensional conformal techniques.10 However, RTOG0617 data present different perspectives. This clinical experiment compared the prognosis of lung cancer patients receiving 74Gy and 60Gy.11 The results showed that although no increased toxic side effects were observed in 74Gy radiation-treated patients, the risk of death and local failure of these patients was higher than those in 60Gy radiation-treated patients, and most of them died of metastatic recurrence. Therefore, it is speculated that high-dose radiation therapy may promote tumor invasion and metastasis.
Although preoperative chemoradiotherapy can reduce tumor burden and achieve tumor downstaging, it may increase the risk of distant metastasis and reduce disease-free survival. Tumor microenvironment of metastasis (TMEM) score and TMEM-related isoform protein expression were increased in residual breast cancer cells in patients treated with doxorubicin plus cyclophosphamide with neoadjuvant paclitaxel. This was indicative of a decrease in tumor size, but the risk of metastatic transmission also increased.12 Analysis of the 10-year data from the German CAO/ARO/AIO-94 phase 3 trial on rectal cancer showed that the prognosis of patients after chemoradiotherapy was generally poor and the migration of tumors increased after downstaging.13 An association between radiotherapy and metastases of the oropharynx and hypopharynx tumors was discovered in 1978.14 Subsequent studies have also shown that radiotherapy increases the risk of distant metastasis by increasing the number of circulating tumor cells in NSCLC and bladder cancer.15 Although the reduction of tumor burden by chemoradiotherapy is beneficial, we need to consider the impact of the associated increase in the risk of distant metastasis on patient prognosis.
While most studies in the field of tumor chemoradiotherapy focus on how to reduce tumor burden, we attempt to clarify how the key mechanisms of chemoradiotherapy affect the biological behavior of tumor cells, and explore their reasonable interventions. This may provide insights for reducing the chemoradiotherapy-related tumor recurrence and metastasis, and improve the efficacy of chemoradiotherapy.
Mechanisms of chemoradiotherapy affecting tumor cell invasion and metastasis
Chemoradiotherapy directly affects tumor cells, influencing invasion and metastasis
DNA damage affects tumor metastasis
DNA damage is an important mechanism for the understanding of the development of cancer. A consensus revealed that DNA damage induced by chemoradiotherapy is associated with cancer development.16 When DNA damage occurs, the cell is confronted with various processes, including regulation of cell cycle and programmed cell death. The micellar formulations of talazoparib and buparlisib reinforce DNA damage in breast cancer radiotherapy, causing cell apoptosis and inhibiting tumor metastasis.17 It has also been reported that radiation-induced lung DNA damage promotes breast cancer lung-metastasis.18 Some studies also show that DNA repair is related to tumor metastasis. In melanoma, DNA repair genes are upregulated in metastatic tumors compared to primary tumors.19,20 DNA damage repair-related proteins can enhance chemoradioresistance and alleviate DNA damage caused by chemoradiotherapy. A study showed that silencing MBD1, a DNA damage repair-related protein, reduced chemoradiotherapy resistance in pancreatic cancer.21 This may explain why DNA damage repair affects the recurrence and metastasis of tumors after radiotherapy and chemotherapy. DNA damage caused by chemoradiotherapy, therefore, can stimulate tumor cell metastasis, and controlling it may be beneficial to tumor therapy.
The process of DNA damage and repair is complex. The ataxia-telangiectasia mutated (ATM) protein and p53 are greatly important in DNA damage response, and once activated, they regulate many substrates for DNA damage-induced responses.22 An investigation of cell apoptosis, using different doses of chemoradiotherapy, confirmed that the ATM/p53 pathway is directly involved in DNA damage induced by chemoradiotherapy in breast cancer cell lines, and rhodamine can be used to inhibit the survival of tumor cells via the ATM/p53 pathway.23 Radiotherapy for breast cancer induces DNA damage and p53 activation, accompanied by lung injury and stimulation of lung metastasis.18 DNA repair in tumor cells of patients with brain metastases, after radiotherapy, promotes cell proliferation, survival, metastasis, and drugs that block G2/M checkpoint under irradiation disrupt DNA damage repair and inhibit brain metastases.24 Hence, DNA damage is one of the possible mechanisms, as shown in Fig. 1, that leads to tumor metastasis caused by chemoradiotherapy. Therefore, understanding DNA damage mechanisms is beneficial to the treatment of cancer, and the prevention of side effects of chemoradiotherapy.
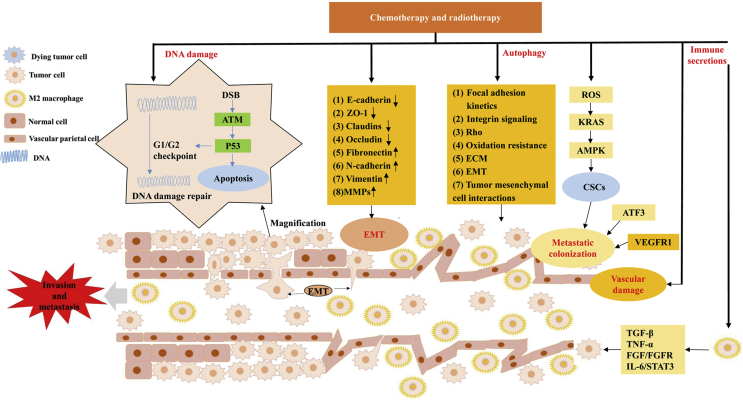
Figure 1.
A highly simplified diagram shows the mechanism by which chemoradiotherapy may induce invasion and metastasis of tumor cells. DNA damage (DNA double-strand breaks, DSB) may trigger apoptosis, and necessitate the escape of tumor cells for survival. At the site of EMT, the matrix is locally degraded, intercellular connections are lost, cytoplasmic processes are protruded, and the contraction of the cytoskeleton causes the cell body to separate from its neighbors to complete ectopia. Under adverse microenvironmental conditions such as chemoradiotherapy, functional autophagy response supports the survival of tumor cells and promotes tumor invasion and metastasis. Vascular injury can directly accelerate tumor cell overflow, and the resulting dysmorphogenesis of blood vessels and macrophages is conducive for creating the TMEM and promoting tumor invasion and metastasis. Chemoradiotherapy also promotes the stem-like properties of CSCs and activates metastatic colonization. Following chemoradiotherapy, M2 macrophages release inflammatory cytokines, which trigger tumor cell metastasis. The process of promoting tumor cell invasion and metastasis by chemoradiotherapy involves many and complex molecules, but only a few representative star molecules were listed in the above diagram.
Epithelial-mesenchymal transition (EMT) affects tumor invasion and metastasis
Tumor cells acquire the migration phenotype through the loss of polarity of epithelial cells and acquisition of mesenchymal characteristics, a process known as EMT.25,26 The mechanism of this process is complex, as cancer cell migration from original locations results from the synergy of multiple molecules that cause the decreased epithelial marker and increased mesenchymal marker.27 EMT can also reduce the sensitivity of tumor cells to chemoradiotherapy.28,29 On the other hand, chemoradiotherapy can induce EMT, and enhance the invasion and metastasis of tumor cells. The possible mechanisms involved in the induction of EMT by chemoradiotherapy are shown in Fig. 1. A study evaluated the relationship between EMT markers, E-cadherin, vascular endothelial growth factor and epidermal growth factor receptor, and overall survival of patients after chemoradiotherapy and intracavitary brachytherapy. The results showed that EMT markers were associated with tumor proliferation and angiogenesis after chemoradiotherapy, and overall survival of patients with cervical cancer.30 Colorectal cancer cells treated with radiotherapy showed typical EMT changes, such as polarity loss, spindle cell traits, intercellular separation, pseudopod formation, enhancement of cell migration, and invasion observed at the molecular level.31 The EMT changes caused by chemoradiotherapy were directly observed through changes in cell morphology, hence, it may be used as a marker to determine the risk of clinical tumor metastasis. After 30 days of radiotherapy for liver cancer in nude mice, although the expression of vascular endothelial growth factor decreased to the control level, overexpression of TMPRSS4 induced E-cadherin transcriptional repressor results in the loss of E-cadherin and promotes the EMT related spread and metastasis of residual tumors in mice.32 Similarly, molecular changes consistent with EMT have been observed in oxaliplatin-treated liver cancer cells, accompanied by significantly enhanced migration and invasion.33 Studies have found that inhibition of EMT by tyroserleutide decreases the invasive and metastatic potential of radiation-induced hepatocellular carcinoma.34 The inhibition or promotion of EMT corresponds to a proportional inhibition or promotion of tumor metastasis. This emphasizes EMT as an important contributor to distant tumor metastasis induced after radiotherapy and chemotherapy.
Autophagy affects tumor invasion and metastasis
Macroautophagy (here called autophagy) is an intracellular degradation pathway that mediates the isolation of intracellular entities within the double-membrane sac, known as autophagosomes, and delivers them to lysosomes for degradation and recovery.35 For cancer, autophagy plays a dual role in tumor cell fate: it can inhibit the occurrence of tumors via the elimination of damaged organelles in transformed cells, protect cells from oxidative stress, and prevent malignant transformation. Autophagy may also act as a tumor-supporter by triggering cancer cell survival and inhibiting apoptosis. This can promote chemoresistance and EMT mediated metastasis.36 Over the past several years, emerging evidence has revealed a direct role of autophagy in inducing invasion and metastasis in cancer cells. For instance, highly expressed ATG10 in colorectal cancer is related to lymphovascular invasion and lymph node metastasis, and ATG10 may act as a potential prognostic marker in colorectal cancer.37 Furthermore, advanced human tumors typically exhibit increased autophagy fluxes, which are associated with invasion/metastasis phenotypes, high nuclear grade, and poor disease outcomes.38,39 Autophagy regulates several mechanisms that facilitate metastasis and these include (1) focal adhesion kinetics, (2) integrin signaling and transport, (3) Rho GTPase-regulated cytoskeletal remodeling, (4) oxidation resistance, (5) ECM composition, (6) epithelial-mesenchymal transformation (EMT) signaling, and (7) tumor mesenchymal cell interactions.40
There have also been increasing evidence in support of the activation of autophagy in tumor cells by radiation, chemotherapy, and targeted therapy.41 Multiple studies have revealed that 5-FU therapy triggers autophagy of cancer cells in vivo, and suppressing autophagy potentiated the anticancer effects of 5-FU.42,43 In colorectal cancer, chemotherapy induces genotoxic stress followed by the elevation of p53, and activated p53 regulates autophagy via the activation of AMPK and inhibition of mTOR.44 A previous study showed that p53 promotes cell survival and chemotherapeutic resistance of liver cancer by regulating autophagy activation in the absence of nutrients.45 Linifanib has been shown to suppress PDGFR-β and the downstream Akt/mTOR and Mek/Erk signaling, which increases autophagy in hepatocarcinoma cells, leading to their survival in vitro and in vivo.46 Cisplatin promotes the activation of GFRA1, which induces autophagy via SRC-AMPK signaling in osteosarcoma cells.47 Ionizing radiation triggers ROS-mediated macromolecular (mainly DNA) damage and ER stress response, resulting in autophagy.48 Overall, autophagy triggers invasion and metastasis in cancer, and chemotherapy activates autophagy. Therefore, chemoradiotherapy may contribute to the invasion and metastasis via activating autophagy (Fig. 1). Further research of autophagy in the tumor microenvironment may help develop new inhibitors and clinical trial strategies.
Chemoradiotherapy induces metastatic colonization of cancer cells
The formation of premetastatic niche and colonization of cancer cells are important programs for metastasis. Emerging evidence has shown that chemoradiotherapy may facilitate premetastatic niche formation and cancer cell colonization at distant sites. Paclitaxel demonstrates the pro-metastatic effect of chemotherapy by facilitating tumor diffusion and metastasis formation via the generation of a pro-metastatic niche and enhancement of metastatic colonization.49 Chang et al revealed that paclitaxel and cyclophosphamide can trigger tumor cell dissemination and metastatic colonization via the recruitment of myeloid progenitors to the primary, as well as secondary, sites in a pattern that relies on stress-inducible gene Atf3.50 Paclitaxel and cisplatin promote the retention of tumor cells in pulmonary vessels of mice, leading to metastasis and colonization. A possible mechanism is that chemotherapy induces the activity of vascular endothelial growth factor receptor 1 (VEGFR1), which enhances the adhesion of endothelial cell and tumor cell, as well as the paracrine interactions.51 Radiation following 10 days of subcutaneous implantation in mice can stimulate tumor cell colonization via capillaries. This phenomenon was confirmed by the increased infiltration of CD31 positive cells, following local irradiation in mice with Lewis lung cancer.52 Overall, chemoradiotherapy may facilitate cancer cell seeding and colonization, resulting in tumor metastasis.
Chemoradiotherapy enhances the stem-like properties of cancer stem cells (CSCs)
Active metastatic colonization is contingent upon the dissemination of CSCs that can re-initiate tumor growth. CSCs are a small subset of tumors that can self-renew and proliferate, and play crucial roles in tumor progression and recurrence. Although chemotherapy and radiotherapy can kill most tumor cells, they do not affect CSCs, leading to their enrichment and development into more refractory tumors.53 A previous study showed that typical CSCs account for about 1–10% of the total number of cells in head and neck cancer, whereas this proportion may elevate with the increase of therapeutic radiation dose.54 Eradication of CSCs is essential for effective therapy of cancer, as CSCs can promote regrowth of the cancer cells and lead to tumor recurrence. Nevertheless, it is difficult to eliminate CSCs as they are usually resistant to therapy. The stem-like characteristics of CSCs are potentially necessary for successful metastatic colonization, and increasing evidence support the claim that chemoradiotherapy enhances the stem-like characteristics of CSCs.51 A previous study showed that chemotherapy stress reinforced the sphere-forming ability of CSCs in breast cancer, and induced the change of cell morphology and EMT-related genes at the mRNA level. Meanwhile, the migration capacity of CSCs was not affected.55 Cancer cells that develop EMT have been claimed to obtain stemness and experience metabolic alterations, although these ideas are controversial. Irradiation is known to stimulate the characteristics of CSCs, including dedifferentiation and self-renewal, and enhance oncogenic metabolism via activating the EMT-inducing signaling pathways.56 It has been shown that Gemcitabine may induce the stem-like cell properties in pancreatic cancer via the involvement of ROS/KRAS/AMPK signaling pathway.57 These findings suggest that chemoradiotherapy enhances the stem-like properties of CSCs and the resultant active metastatic colonization.
Chemoradiotherapy affects invasion and metastasis through the TMEM
Tumor metastasis depends on the TMEM, and it is not independent of the direct contact of three cell types: tumor cell, endothelial cell and macrophage.58 The effects of radiotherapy and chemotherapy on tumor metastasis, from the tumor microenvironment, associated with endothelial and immune cells.
Chemoradiotherapy affects tumor invasion and metastasis by facilitating vascular damage
Chemoradiotherapy induced modifications of the host, including immunosuppression and vascular damage, can facilitate invasion and metastasis.59,60 For instance, a single fraction dose of 5–10 Gy can cause minor vascular damage, and more than 10 Gy can induce more severe vascular damage.61 Recent studies have shown that chemoradiotherapy may produce acute and late, local and systemic side effects including vascular damage.62,63 In chemotherapy, bleomycin administration can result in damage to the endothelial cells lining the common pulmonary arteries and veins.64,65 This bleomycin-induced endothelial cell retraction, with increased lung endothelial permeability, is related to increased retention of I.V. injected tumor cells and enhancement of lung nodule formation.66 After X-irradiation, the enhancement of experimental metastasis has been attributed to the vascular damage associated with this treatment.67,68 Radiotherapy, therefore, causes damage to the DNA of NSCLC cells and increases the number of circulating tumor cells in the blood, which may result from damage to the vessels by radiation. Thus, tumor cells can easily break the barrier and enter the blood circulation to complete distant migration.69 Compared with untreated mice with cancer cells that were stationary in blood vessels, mice treated with the cyclophosphamide showed proliferation, extravasation, and extravascular colony formation of cancer cells in the vessels.70 These suggest that normal vascular injury caused by chemoradiotherapy possibly provides an outlet for tumor metastasis and promotes angiogenesis, and this may be the precondition for tumor metastasis. Additionally, several markers associated with vascular damage have been identified.71 Therefore, after chemoradiotherapy, preventing or reversing vascular damage may reduce the recurrence of tumor metastasis.
Notably, the structure and function of vessels in solid tumors are abnormal, and anti-angiogenesis therapy promotes tumor metastasis. In a study on sorafenib-treated mice, tumor growth was accelerated, mouse survival was decreased, lung metastasis was increased, and sorafenib downregulated the expression of HIV-1 Tat interactive protein 2 (HTATIP2) through the JAK-STAT3 signaling pathway. This, in turn, promoted the invasive and metastatic potential of orthotopic tumors of hepatocellular carcinoma cells in the mice.72 In another study, aspirin minimized the pro-metastasis effect of sorafenib by mediating the inhibition of COX2 to upregulate tumor suppressor HTATIP2.73 VEGFR-2 tyrosine kinase inhibitor AZD2171 was also found to inhibit tumor growth, both in vivo and in vitro, and promote tumor sensitivity to radiation, limiting metastasis.74 The upregulation of fibroblast growth factor-2, a pleiotropic angiogenesis inducer, is thought to be the mechanism by which tumors evade anti-VEGF therapy.69
Immune secretions affect tumor cell invasion and metastasis
Immune cells, including macrophages and platelets, promote tumor metastasis after stimulation by chemoradiotherapy, and the possible mechanisms are shown in Fig. 1. Condeelis et al believe that macrophages are located at the center of the TMEM and have six characteristics for promoting malignant tumors: tumor cell invasion, inflammation, matrix remodeling, angiogenesis, seeding at distant sites and intravasation.75 Chemoradiotherapy promotes the release of inflammatory cytokines by macrophages, such as tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta 1 (TGF-β), which aggravates tumor injury.76 Tumor-associated macrophages are the main cells for the expression of immunoreactive C-X-C chemokine receptor 4 in mouse tumor cells, whereas M2-related macrophages, especially those in direct contact with vessels, may stimulate tumor recurrence and metastasis after chemotherapy induction.77 FGF and their receptor (FGFR) regulate a variety of cellular processes, including proliferation, survival and motility, and in a study, FGF2 was highly expressed in recurrent cancer tissues, after chemoradiation in esophageal cancer patients. The 2-year and 5-year local recurrence-free survival rates of patients with high expression of FGF-2 were 15.4% and 0% respectively, while those of patients low expression of FGF-2 were 45.8% and 33.3% respectively.78 FGF2 and its related signaling pathway can promote tumor metastasis in various sites including breast cancer, lung cancer, colorectal cancer, melanoma, etc.70,71,79,80
Chemotherapy also induces tumor cell senescence, which irreversibly prevents cell proliferation and suppresses tumors. It also induces normal cell senescence, which leads to local and systemic inflammation and increases tumor growth and metastasis.81 In a study, IL-6/STAT3 signaling loop and platelet-derived growth factor-BB/PDGF receptor pathway were upregulated in a conditioned medium (CM) from senescent cells; besides, CM can promote angiogenesis in the chicken chorioallantoic membrane by endothelial cell invasion.82 The effect of macrophages on tumor cell metastasis is systematic and comprehensive. Thus, numerous immune cell secretions affect the invasion and metastasis of cells via TMEM alteration after induction of chemoradiotherapy.
Accumulating evidence shows that under certain conditions, radiation can be used in combination with immune checkpoint inhibitors to enhance the efficacy.83 Anti-CTLA4 alone fails to suppress tumor growth or increase survival, while radiotherapy alone could delay the growth of primary lesions. The combination of Anti-CTLA4 therapy and radiotherapy, however, can significantly increase the overall survival and reduce lung metastasis.84 PD-1 blockers also increase the anti-tumor response. Combination radiotherapy/αPD-L1 mAb therapy generated efficacious CD8 (+) T-cell responses that increased long-term survival and prevented tumor recurrence in a study.85 Immune secretions affect tumor cell invasion and metastasis, and the combination of radiation and immunotherapy that targets them presents a potential novel therapeutic approach.
Summary and outlook
The beneficial effects of chemoradiotherapy on cancer treatment has been emphasized, however, cancer recurrence and metastasis of tumor cells after chemoradiotherapy should not be overlooked. Tumor metastasis is influenced by several factors, and the effect of chemoradiotherapy on tumor cells is not simply a trade-off effect. We conclude that chemoradiotherapy may directly affect tumor cells and promote tumor cell survival and metastasis by altering the TMEM or cell apoptosis and autophagy. Understanding the mechanism of action linking tumor metastasis and chemoradiotherapy will help us develop more effective therapeutic drugs and increase treatment options. This can reduce patient burden and improve prognosis.
For a follow-up study, the following recommendations should provide direction for improving cancer chemoradiotherapy. First, an in-depth study of the molecular mechanism of chemoradiotherapy in promoting tumor cell invasion and metastasis should be undertaken. This is required to provide a theoretical basis for clinical control of tumor metastasis. Second, appropriate drugs should be developed to be used in combination with chemoradiotherapy to inhibit the invasion and metastasis of tumor cells and improve the efficiency of chemoradiotherapy. Finally, the minimum dosage of chemoradiotherapy associated with the lowest rate of residual tumor metastasis and strong tumor-suppressive ability needs to be determined. This has practical significance for improving the survival of patients. The realization of the above recommendations will promote the effective application of chemoradiotherapy, and improve patient survival. This study highlighted the side effects, and summarized the possible mechanisms, of chemoradiotherapy in promoting invasion and metastasis of tumor cells. It also highlighted the side effects of chemoradiotherapy and deepened the understanding of the relationship between chemoradiotherapy and cancer. Consequently, new insights for clinically improving the effectiveness of chemoradiotherapy, and controlling cancer metastasis and recurrence, were demonstrated.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Top Yong Talents of Ten Thousand Talent Program of Yunnan Province.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Wen-Hui Li, Email: wenhuili64@yeah.net.
Zhao-You Tang, Email: zytang88@163.com.
References
- 1.Eyck B.M., Van der Wilk B., Lagarde S. Neoadjuvant chemoradiotherapy for resectable oesophageal cancer. Best Pract Res Clin Gastroenterol. 2018;36:37–44. doi: 10.1016/j.bpg.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Seiwert T.Y., Salama J.K., Vokes E.E. The concurrent chemoradiation paradigm—general principles. Nat Clin Pract Oncol. 2007;4(2):86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 3.Chung Y.L., Jian J.J., Cheng S.H. Sublethal irradiation induces vascular endothelial growth factor and promotes growth of hepatoma cells: implications for radiotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12(9):2706–2715. doi: 10.1158/1078-0432.CCR-05-2721. [DOI] [PubMed] [Google Scholar]
- 4.El Sharouni S.Y., Kal H.B., Battermann J.J. Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br J Cancer. 2003;89(12):2184–2189. doi: 10.1038/sj.bjc.6601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourhis J., Wilson G., Wibault P. Rapid tumor cell proliferation after induction chemotherapy in oropharyngeal cancer. Laryngoscope. 1994;104(4):468–472. doi: 10.1288/00005537-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Nelson J.W., Ghafoori A.P., Willett C.G. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73(1):148–153. doi: 10.1016/j.ijrobp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunjal P.M., Schneider G., Ismail A.A., Kakar S.S., Kucia M., Ratajczak M.Z. Evidence for induction of a tumor metastasis-receptive microenvironment for ovarian cancer cells in bone marrow and other organs as an unwanted and underestimated side effect of chemotherapy/radiotherapy. J Ovarian Res. 2015;8(1):20. doi: 10.1186/s13048-015-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan H.S., Murphy E.D. The effect of local roentgen irradiation on the biological behavior of a transplantable mouse carcinoma; increased frequency of pulmonary metastasis. J Natl Cancer Inst. 1949;9(5–6):407–413. [PubMed] [Google Scholar]
- 9.Sheldon P., Fowler J. The effect of low-dose pre-operative X-irradiation of implanted mouse mammary carcinomas on local recurrence and metastasis. Br J Cancer. 1976;34(4):401–407. doi: 10.1038/bjc.1976.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley J., Graham M.V., Winter K. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61(2):318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 11.Wakelee H.A. Commentary: highlights in NSCLC from the 15th world conference on lung cancer. Clin Adv Hematol Oncol. 2014;12(1 Suppl 1):17–21. [PubMed] [Google Scholar]
- 12.Karagiannis G.S., Pastoriza J.M., Wang Y. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med. 2017;9(397):eaan0026. doi: 10.1126/scitranslmed.aan0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fokas E., Liersch T., Fietkau R. Downstage migration after neoadjuvant chemoradiotherapy for rectal cancer: the reverse of the Will Rogers phenomenon? Cancer. 2015;121(11):1724–1727. doi: 10.1002/cncr.29260. [DOI] [PubMed] [Google Scholar]
- 14.Strong M.S., Vaughan C.W., Kayne H.L. A randomized trial of preoperative radiotherapy in cancer of the oropharynx and hypopharynx. Am J Surg. 1978;136(4):494–500. doi: 10.1016/0002-9610(78)90268-4. [DOI] [PubMed] [Google Scholar]
- 15.Dorsey J.F., Kao G.D., MacArthur K.M. Tracking viable circulating tumor cells (CTC s) in the peripheral blood of non–small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer. 2015;121(1):139–149. doi: 10.1002/cncr.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastan M.B. DNA damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture. Mol Cancer Res. 2008;6(4):517–524. doi: 10.1158/1541-7786.MCR-08-0020. [DOI] [PubMed] [Google Scholar]
- 17.DuRoss A.N., Neufeld M.J., Landry M.R. Micellar formulation of talazoparib and buparlisib for enhanced DNA damage in breast cancer chemoradiotherapy. ACS Appl Mater Interfaces. 2019;11(13):12342–12356. doi: 10.1021/acsami.9b02408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feys L., Descamps B., Vanhove C. Radiation-induced lung damage promotes breast cancer lung-metastasis through CXCR4 signaling. Oncotarget. 2015;6(29):26615. doi: 10.18632/oncotarget.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winnepenninckx V., Lazar V., Michiels S. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98(7):472–482. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- 20.Kauffmann A., Rosselli F., Lazar V. High expression of DNA repair pathways is associated with metastasis in melanoma patients. Oncogene. 2008;27(5):565–573. doi: 10.1038/sj.onc.1210700. [DOI] [PubMed] [Google Scholar]
- 21.Xu J., Zhu W., Xu W. Silencing of MBD1 reverses pancreatic cancer therapy resistance through inhibition of DNA damage repair. Int J Oncol. 2013;42(6):2046–2052. doi: 10.3892/ijo.2013.1901. [DOI] [PubMed] [Google Scholar]
- 22.Banin S., Moyal L., Shieh S. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281(5383):1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z.-F., Sun W.-Y., Yu D.-H. Rotundic acid enhances the impact of radiological toxicity on MCF-7 cells through the ATM/p53 pathway. Int J Oncol. 2018;53(5):2269–2277. doi: 10.3892/ijo.2018.4544. [DOI] [PubMed] [Google Scholar]
- 24.Li X., Wang Y., Wang J. Enhanced efficacy of AZD3759 and radiation on brain metastasis from EGFR mutant non-small cell lung cancer. Int J Canc. 2018;143(1):212–224. doi: 10.1002/ijc.31303. [DOI] [PubMed] [Google Scholar]
- 25.Guarino M. Epithelial–mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39(12):2153–2160. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 28.Ni J., Cozzi P., Hao J. Epithelial cell adhesion molecule (EpCAM) is associated with prostate cancer metastasis and chemo/radioresistance via the PI3K/Akt/mTOR signaling pathway. Int J Biochem Cell Biol. 2013;45(12):2736–2748. doi: 10.1016/j.biocel.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Ni J., Cozzi P.J., Hao J.L. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate. 2014;74(6):602–617. doi: 10.1002/pros.22775. [DOI] [PubMed] [Google Scholar]
- 30.Rojas-Puentes L., Cardona A.F., Carranza H. Epithelial–mesenchymal transition, proliferation, and angiogenesis in locally advanced cervical cancer treated with chemoradiotherapy. Cancer Med. 2016;5(8):1989–1999. doi: 10.1002/cam4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamoto A., Yokoe T., Tanaka K. Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol Rep. 2012;27(1):51–57. doi: 10.3892/or.2011.1485. [DOI] [PubMed] [Google Scholar]
- 32.Li T., Zeng Z., Wang L. Radiation enhances long-term metastasis potential of residual hepatocellular carcinoma in nude mice through TMPRSS4-induced epithelial–mesenchymal transition. Cancer Gene Ther. 2011;18(9):617–626. doi: 10.1038/cgt.2011.29. [DOI] [PubMed] [Google Scholar]
- 33.Xiong W., Ren Z.-G., Qiu S.-J. Residual hepatocellular carcinoma after oxaliplatin treatment has increased metastatic potential in a nude mouse model and is attenuated by Songyou Yin. BMC Cancer. 2010;10(1):219. doi: 10.1186/1471-2407-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia J.-B., Wang W.-Q., Sun H.-C. A novel tripeptide, tyroserleutide, inhibits irradiation-induced invasiveness and metastasis of hepatocellular carcinoma in nude mice. Invest N Drugs. 2011;29(5):861–872. doi: 10.1007/s10637-010-9435-1. [DOI] [PubMed] [Google Scholar]
- 35.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokarram P., Albokashy M., Zarghooni M. New frontiers in the treatment of colorectal cancer: autophagy and the unfolded protein response as promising targets. Autophagy. 2017;13(5):781–819. doi: 10.1080/15548627.2017.1290751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo Y.K., Kim S.C., Park I.J. Increased expression of ATG10 in colorectal cancer is associated with lymphovascular invasion and lymph node metastasis. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazova R., Camp R.L., Klump V., Siddiqui S.F., Amaravadi R.K., Pawelek J.M. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18(2):370–379. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikhaylova O., Stratton Y., Hall D. VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer cell. 2012;21(4):532–546. doi: 10.1016/j.ccr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dower C.M., Wills C.A., Frisch S.M., Wang H.-G. Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy. 2018;14(7):1110–1128. doi: 10.1080/15548627.2018.1450020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorburn A., Thamm D.H., Gustafson D.L. Autophagy and cancer therapy. Mol Pharmacol. 2014;85(6):830–838. doi: 10.1124/mol.114.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J., Hou N., Faried A., Tsutsumi S., Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer. 2010;46(10):1900–1909. doi: 10.1016/j.ejca.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Guo X-l, Li D., Hu F. Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett. 2012;320(2):171–179. doi: 10.1016/j.canlet.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Tang J.-C., Feng Y.-L., Liang X., Cai X.-J. Autophagy in 5-fluorouracil therapy in gastrointestinal cancer: trends and challenges. Chin Med J. 2016;129(4):456. doi: 10.4103/0366-6999.176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo X-l, Hu F., Zhang S-s. Inhibition of p53 increases chemosensitivity to 5-FU in nutrient-deprived hepatocarcinoma cells by suppressing autophagy. Cancer Lett. 2014;346(2):278–284. doi: 10.1016/j.canlet.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Pan H., Wang Z., Jiang L. Autophagy inhibition sensitizes hepatocellular carcinoma to the multikinase inhibitor linifanib. Sci Rep. 2014;4:6683. doi: 10.1038/srep06683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M., Jung J.-Y., Choi S. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy. 2017;13(1):149–168. doi: 10.1080/15548627.2016.1239676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaurasia M., Bhatt A.N., Das A., Dwarakanath B.S., Sharma K. Radiation-induced autophagy: mechanisms and consequences. Free Radic Res. 2016;50(3):273–290. doi: 10.3109/10715762.2015.1129534. [DOI] [PubMed] [Google Scholar]
- 49.D'Alterio C., Scala S., Sozzi G., Roz L., Bertolini G. Paper Presented at: Seminars in Cancer Biology. 2019. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. [DOI] [PubMed] [Google Scholar]
- 50.Chang Y.S., Jalgaonkar S.P., Middleton J.D., Hai T. Stress-inducible gene Atf3 in the noncancer host cells contributes to chemotherapy-exacerbated breast cancer metastasis. Proc Natl Acad Sci Unit States Am. 2017;114(34):E7159–E7168. doi: 10.1073/pnas.1700455114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karagiannis G.S., Condeelis J.S., Oktay M.H. Chemotherapy-induced metastasis: mechanisms and translational opportunities. Clin Exp Metastasis. 2018;35(4):269–284. doi: 10.1007/s10585-017-9870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundahl N., Duprez F., Ost P., De Neve W., Mareel M. Effects of radiation on the metastatic process. Mol Med. 2018;24(1):16. doi: 10.1186/s10020-018-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vengoji R., Ponnusamy M.P., Rachagani S. Novel therapies hijack the blood–brain barrier to eradicate glioblastoma cancer stem cells. Carcinogenesis. 2019;40(1):2–14. doi: 10.1093/carcin/bgy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid P.A., Wilson P., Li Y., Marcu L.G., Bezak E. Current understanding of cancer stem cells: review of their radiobiology and role in head and neck cancers. Head Neck. 2017;39(9):1920–1932. doi: 10.1002/hed.24848. [DOI] [PubMed] [Google Scholar]
- 55.Li X., Strietz J., Bleilevens A., Stickeler E., Maurer J. Chemotherapeutic stress influences epithelial–mesenchymal transition and stemness in cancer stem cells of triple-negative breast cancer. Int J Mol Sci. 2020;21(2):404. doi: 10.3390/ijms21020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S.Y., Jeong E.K., Ju M.K. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Canc. 2017;16(1):10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao H., Wu S., Li H. ROS/KRAS/AMPK signaling contributes to gemcitabine-induced stem-like cell properties in pancreatic cancer. Mol Ther Oncol. 2019;14:299–312. doi: 10.1016/j.omto.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harney A.S., Arwert E.N., Entenberg D. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage–derived VEGFA. Cancer Discov. 2015;5(9):932–943. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baldo B.A., Pham N.H.J.C., Reviews M. Adverse reactions to targeted and non-targeted chemotherapeutic drugs with emphasis on hypersensitivity responses and the invasive metastatic switch. Cancer Metastasis Rev. 2013;32(3–4):723–761. doi: 10.1007/s10555-013-9447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMillan T., Hart I.J.C., Reviews M. Can cancer chemotherapy enhance the malignant behaviour of tumours? Cancer Metastasis Rev. 1987;6(4):503–520. doi: 10.1007/BF00047465. [DOI] [PubMed] [Google Scholar]
- 61.Park H.J., Griffin R.J., Hui S., Levitt S.H., Song CWJRr. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177(3):311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 62.Dörr WJBcr . Vol. 4. 2009. pp. 169–190. (Pathogenesis of normal tissue side effects). [Google Scholar]
- 63.Bentzen S.M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 64.Adamson I.Y., Bowden D.H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77(2):185. [PMC free article] [PubMed] [Google Scholar]
- 65.Catravas J.D., Lazo J.S., Dobuler K.J., Mills L.R., Gillis C.N. Pulmonary endothelial dysfunction in the presence or absence of interstitial injury induced by intratracheally injected bleomycin in rabbits. Am Rev Resp Dis. 1983;128(4):740–746. doi: 10.1164/arrd.1983.128.4.740. [DOI] [PubMed] [Google Scholar]
- 66.Orr F., Adamson I., Young L.J.C.R. Promotion of pulmonary metastasis in mice by bleomycin-induced endothelial injury. Cancer Res. 1986;46(2):891–897. [PubMed] [Google Scholar]
- 67.Withers H.R., Milas L.J.C.R. Influence of preirradiation of lung on development of artificial pulmonary metastases of fibrosarcoma in mice. Cancer Res. 1973;33(8):1931–1936. [PubMed] [Google Scholar]
- 68.Mount D., Bruce WJRr. Local plasma volume and vascular permeability of rabbit skin after irradiation. Radiat Res. 1964;23(3):430–445. [PubMed] [Google Scholar]
- 69.Martin O.A., Anderson R.L., Russell P.A. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88:395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 70.Kensuke Y., Meng Y., Katsuhiro H. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 2008;68:516. doi: 10.1158/0008-5472.CAN-07-3063. [DOI] [PubMed] [Google Scholar]
- 71.Venkatesulu B.P., Sanders K.L., Hsieh C.E., Kim B.K., Krishnan S. Biomarkers of radiation-induced vascular injury. Cancer Rep. 2018;2:e1152. doi: 10.1002/cnr2.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W., Sun H.C., Wang W.Q. Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology. 2012;143(6):1641–1649. doi: 10.1053/j.gastro.2012.08.032. e1645. [DOI] [PubMed] [Google Scholar]
- 73.Lu L., Sun H.-C., Zhang W. Aspirin minimized the pro-metastasis effect of sorafenib and improved survival by up-regulating HTATIP2 in hepatocellular carcinoma. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0065023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Albert J., Cao C., Lu B. 2473: VEGFR-2 tyrosine kinase inhibitor AZD2171 and radiotherapy in mouse models of lung cancer. Int J Radiat Oncol Biol Phys. 2006;66(3):S473. doi: 10.1158/0008-5472.CAN-06-2414. [DOI] [PubMed] [Google Scholar]
- 75.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Kim S.-H., Lim D.-J., Chung Y.-G. Expression of TNF-alpha and TGF-beta 1 in the rat brain after a single high-dose irradiation. J Kor Med Sci. 2002;17(2):242. doi: 10.3346/jkms.2002.17.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hughes R., Qian B.-Z., Rowan C. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75(17):3479–3491. doi: 10.1158/0008-5472.CAN-14-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y., Li X., Yang H. Expression of basic fibroblast growth factor, CD31, and α-smooth muscle actin and esophageal cancer recurrence after definitive chemoradiation. Tumor Biol. 2014;35(7):7275–7282. doi: 10.1007/s13277-014-1987-9. [DOI] [PubMed] [Google Scholar]
- 79.Tsunoda S., Sakurai H., Saito Y., Ueno Y., Koizumi K., Saiki I. Massive T-lymphocyte infiltration into the host stroma is essential for fibroblast growth factor-2-promoted growth and metastasis of mammary tumors via neovascular stability. Am J Pathol. 2009;174(2):671–683. doi: 10.2353/ajpath.2009.080471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andreucci E., Bianchini F., Biagioni A. Roles of different IRES-dependent FGF2 isoforms in the acquisition of the major aggressive features of human metastatic melanoma. J Mol Med. 2017;95(1):97–108. doi: 10.1007/s00109-016-1463-7. [DOI] [PubMed] [Google Scholar]
- 81.Demaria M., O'Leary M.N., Chang J. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Canc Discov. 2017;7(2):165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu Y.-C., Yang P.-M., Chuah Q.-Y. Radiation-induced senescence in securin-deficient cancer cells promotes cell invasion involving the IL-6/STAT3 and PDGF-BB/PDGFR pathways. Sci Rep. 2013;3:1675. doi: 10.1038/srep01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manukian G., Bar Ad V., Lu B., Argiris A., Johnson J.M. Combining radiation and immune checkpoint blockade in the treatment of head and neck squamous cell carcinoma. Front Oncol. 2019;9:122. doi: 10.3389/fonc.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demaria S., Kawashima N., Yang A.M. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2):728–734. [PubMed] [Google Scholar]
- 85.Dovedi S.J., Adlard A.L., Lipowska-Bhalla G. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]