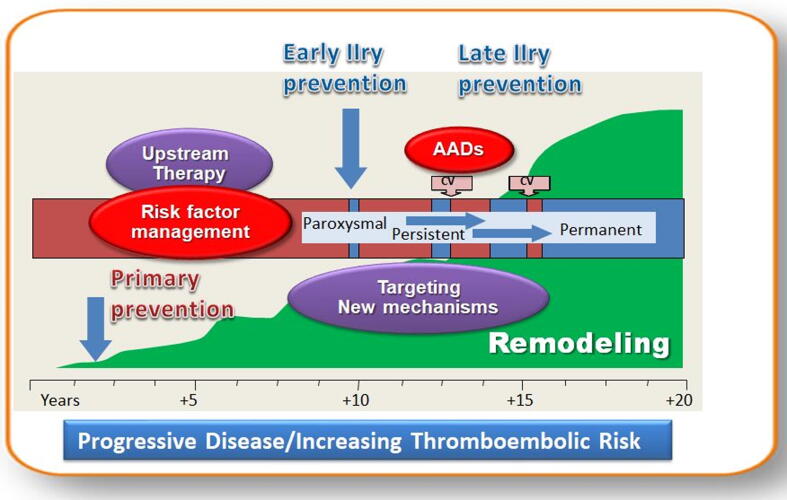
The traditional atrial fibrillation (AF) paradigm created by Wiffels in 1995 [1] “AF begets AF” was recently further developed by introducing the new holistic concept of “Atrial Cardiomyopathy” (ACMP) [2]. Corresponding to this pathophysiological concept, AF is the result (marker) of any type atrial electrical and structural remodeling induced by risk factors and at the same time AF itself induces atrial remodeling acting as a risk factor for ACMP. The new concept is important to understand the “two hits” hypothesis for AF prevention: prevention and treatment of risk factors and prevention and lowering of AF burden (Fig. 1).
Fig. 1.
Prevention of AF and AF burden. CV: cardioversion; AADs: traditional anti-arrhythmic drugs Modified after Cosio [3].
Agents that target the remodelling process could prevent new-onset AF by behaving as non-traditional AAD (upstream therapy). Activated renin-angiotensin-aldosterone system (RAAS) is up-regulated in AF [4], [5] and angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) proved encouraging in preventing AF in preclinical studies [6], [7] and appear, as shown by retrospective analyses and reports from the studies in which AF was a pre-specified secondary endpoint, to prevent new-onset AF in patients with LV dysfunction, LV hypertrophy and in hypertensive patients [8], [9]. Statins are attractive candidates for upstream therapy particularly because the causative role of the inflammatory mechanism in AF was recently established [10], [11]. However in an adequately designed RCT [12] statins failed to show a beneficial preventive effect. Evidences from RCTs have shown that mineralocorticoid receptor antagonists (MRA) reduce new-onset atrial arrhythmias in patients with heart failure with reduced ejection fraction in parallel with improvement of other cardiovascular outcome [13]. The positive impact of MRA was also shown in patients with heart failure with preserved ejection fraction [14]. Recently the RACE 3 study [15] offered new insights for upstream therapy. In this study of patients with mild and moderate heart failure upstream rhythm control including ACEI and/or ARB, MRA, statins, cardiac rehabilitation therapy, and intensive counselling on dietary restrictions, exercise maintenance, and drug adherence confirmed the importance of assessing underlying conditions in targeting upstream therapy and that targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent AF.
Conversely, classic antiarrhythmic drugs (AAD) are only moderately effective likely because they target directly the electrophysiological properties and do not modulate the complex signaling mechanisms involved in AF-promoting atrial remodeling and its perpetuation. Moreover there are important warnings concerning the safety of AAD as shown by repeated meta-analyses and systematic reviews proving the moderate to low efficacy of current AAD in controlling sinus rhythm at the expense of frequent side effects including severe proarrhythmia and a high withdrawal rate [16]. New drugs are continuously developed in parallel with the evolution of knowledge; however, a huge gap exists between currently available AAD and contemporary practical expectations [17]. Newer drugs should target both the extrinsic and intrinsic drivers and mechanisms of ACMP and AF including genetic background, atrial proteomics and metabolomics, fibrosis, inflammation and neurobiology factors [18]. Recent studies have diversified the cellular and molecular targets for AF therapy. Not only cardiomyocyte but also fibroblasts, macrophages or adipocytes could represent potential new targets. The molecular targets responsible for the AF-related electrophysiologic abnormalities and also those involved in AF progression as calcium-dependent intracellular mechanisms or inflammatory drivers are promising targets for a new framework for AF drug developement [10], [11], [19].
In the present issue of the Journal [20] Berlin et al. present the design of a phase II randomized study with a new non-traditional antiarrhythmic molecule, OMT-28, aimed to demonstrate the ability of sinus rhythm stabilization after electrical cardioversion of AF. OMT-28 is a synthetic analog of 17,18-epoxyeicosatetetraenoic acid, an active lipid mediator derived from the omega-3 fatty acid eicosapentaenoic acid. Omega-3 epoxyeicosanoids were demonstrated to prevent cardiomyocyte calcium overload, to protect from hypoxia or reoxygenation injury, to improve mitochondrial function and to inhibit pro-inflammatory signals through inhibition of NF-kβ [21]. The proposed study will test 3 different dosages of active drug (4 mg, 12 mg and 24 mg) versus placebo in a 1:1:1:1 design. One important attribute of the study, fulfilling the modern requirement for AF screening, is the calculation of AF burden with continuous rhythm monitoring using insertable loop monitoring devices. In this way the study is aimed to demonstrate, besides the safety, pharmacokinetics and pharmacodynamics of the new molecule, the impact of a potential new antifibrillatory drug on the real AF burden (including silent AF) and also the rate of recurrences and time to recurrences.
There are several important conclusions to be drawn from this study. First, it emphasizes the imminent need for a new paradigm [22] in the prevention and treatment of AF but also in the enlarging the concept of AADs beyond the traditional concept of the pure electrophysiological actions as described in the historical Vaughan-Williams-Singh classification of AADs [17]. Second, this study could help to gain further insights into the complexity of AF beyond a simple electrical phenotype. Not surprisingly there is growing evidence in favor of an intimate relationship between inflammation and AF initiation and perpetuation [10], [11]. Not only inflammatory biomarkers such as interleukin-1β are correlated with prevalence and prognosis of AF. but the arrhythmia mechanism its involves multiple inflammatory pathways, which offer attractive targets for the development of new therapeutic options based on anti-inflammatory, non-traditional AAD [23].
Large and unexpected doors are now opening to new AAD development confirming the prediction of Hamlet: “There are more things in heaven and earth, …. Than are dreamt of in your philosophy”.
References
- 1.Wijffels M.C.E.F., Kirchhof C.J.H.J., Dorland R., Allessie M.A. Atrial fibrillation begets atrial fibrillation: A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 2.Goette A., Kalman J.M., Aguinaga L., Akar J., Cabrera J.A., Chen S.A., Chugh S.S., Corradi D., D'Avila A., Dobrev D., Fenelon G., Gonzalez M., Hatem S.N., Helm R., Hindricks G., Ho S.Y., Hoit B., Jalife J., Kim Y.H., Lip G.Y., Ma C.S., Marcus G.M., Murray K., Nogami A., Sanders P., Uribe W., Van Wagoner D.R., Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosio F.G., Aliot E., Botto G.L., Heidbüchel H., Geller C.J., Kirchhof P., De H.-C., Frank R., Villacastin J.P., Vijgen J., Crijns H. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10:21–27. doi: 10.1093/europace/eum276. [DOI] [PubMed] [Google Scholar]
- 4.Goette A., Staack T., Röcken C., Arndt M., Geller J.C., Huth C., Ansorge S., Klein H.U., Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J. Am. Coll. Cardiol. 2000;35:1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 5.Thanigaimani S., Lau D.H., Agbaedeng T., Elliott A.D., Mahajan R., Sanders P. Molecular mechanisms of atrial fibrosis: implications for the clinic. Expert Rev. Cardiovasc. Ther. 2017;15:247–256. doi: 10.1080/14779072.2017.1299005. [DOI] [PubMed] [Google Scholar]
- 6.Kumagai K., Nakashima H., Urata H., Gondo N., Arakawa K., Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J. Am. Coll. Cardiol. 2003;41:2197–2204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 7.Schneider M.P., Hua T.A., Böhm M., Wachtell K., Kjeldsen S.E., Schmieder R.E. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J. Am. Coll. Cardiol. 2010;55:2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Healey J.S., Baranchuk A., Crystal E., Morillo C.A., Garfinkle M., Yusuf S., Connolly S.J. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J. Am. Coll. Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 9.Anand K., Mooss A.N., Hee T.T., Mohiuddin S.M. Meta-analysis: inhibition of renin-angiotensin system prevents new-onset atrial fibrillation. Am. Heart J. 2006;152:217–222. doi: 10.1016/j.ahj.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Yao C., Veleva T., Scott L., Cao S., Li L., Chen G., Jeyabal P., Pan X., Alsina K.M., Abu-Taha I., Ghezelbash S., Reynolds C.L., Shen Y.H., Lemaire S.A., Schmitz W., Müller F.U., El-Armouche A., Tony Eissa N., Beeton C., Nattel S., Wehrens X.H.T., Dobrev D., Li N. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heijman J., Muna A.P., Veleva T., Molina C.E., Sutanto H., Tekook M.A., Wang Q., Abu-Taha I., Gorka M., Künzel S., El-Armouche A., Reichenspurner H., Kamler M., Nikolaev V.O., Ravens U., Li N.a., Nattel S., Wehrens X.HT., Dobrev D. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for post-operative atrial fibrillation. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.316710. (epub on July 30, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Z., Jayaram R., Jiang L., Emberson J., Zhao Y., Li Q., Du J., Guarguagli S., Hill M., Chen Z., Collins R., Casadei B. Perioperative rosuvastatin in cardiac surgery. N. Engl. J. Med. 2016;374:1744–1753. doi: 10.1056/NEJMoa1507750. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K., Zannad F., McMurray J.J.V., Krum H., van Veldhuisen D.J., Shi H., Vincent J., Pitt B., EMPHASIS-HF Study Investigators Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J. Am. Coll. Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 14.Cikes M., Claggett B., Shah A.M., Desai A.S., Lewis E.F., Shah S.J., Anand I.S., O’Meara E., Rouleau J.L., Sweitzer N.K., Fang J.C., Saksena S., Pitt B., Pfeffer M.A., Solomon S.D. Atrial fibrillation in heart failure with preserved ejection fraction: The TOPCAT trial. JACC Heart Fail. 2018;6:689–697. doi: 10.1016/j.jchf.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Rienstra M., Hobbelt A.H., Alings M., Tijssen J.G.P., Smit M.D., Brügemann J., Geelhoed B., Tieleman R.G., Hillege H.L., Tukkie R., Van V.DJ., Crijns H.J.G.M., Van G.IC. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: Results of the RACE 3 trial. Eur. Heart J. 2018;39:2987–2996. doi: 10.1093/eurheartj/ehx739. [DOI] [PubMed] [Google Scholar]
- 16.Lafuente-Lafuente C., Valembois L., Bergmann J.-F., Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2015;3:CD005049. doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 17.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int. J. Cardiol. Heart Vasc. 2018;21:11–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ang Y.S., Rajamani S., Haldar S.M., Hüser J. A new therapeutic framework for atrial fibrillation drug development. Circ. Res. 2020;127:184–201. doi: 10.1161/CIRCRESAHA.120.316576. [DOI] [PubMed] [Google Scholar]
- 19.Nattel S., Heijman J., Zhou L., Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ. Res. 2020;127:51–72. doi: 10.1161/CIRCRESAHA.120.316363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berlin S., Goette A., Summo L., Lossie J., Gebauer A., Al-Saady N., Calo L., Naccarelli G., Schunck W.H., Fischer R., Camm A.J., Dobrev D. Assessment of OMT-28, a synthetic analog of omega-3 epoxyeicosanoids, in patients with persistent atrial fibrillation: Rationale and design of the PROMISE-AF phase II study. Int. J. Cardiol. Heart Vasc. 2020;29:100573. doi: 10.1016/j.ijcha.2020.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darwesh A.M., Jamieson K.L., Wang C., Samokhvalov V., Seubert J.M. Cardioprotective effects of CYP-derived epoxy metabolites of docosahexaenoic acid involve limiting NLRP3 inflammasome activation. Can. J. Physiol. Pharmacol. 2019;97:544–556. doi: 10.1139/cjpp-2018-0480. [DOI] [PubMed] [Google Scholar]
- 22.Dan G.A. Changing the paradigm to understand and manage atrial fibrillation. In: Dan G.A., Bayés de Luna A., Camm J., editors. Atrial Fibrillation Therapy. Springer; London: 2014. pp. 127–164. [Google Scholar]
- 23.Scott L., Li N., Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int. J. Cardiol. 2019;287:195–200. doi: 10.1016/j.ijcard.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]