Abstract
Freshwater fishes are faced with increasing threats due to intensification of agriculture. This study evaluated the haematological and genotoxic effects of exposure of the African Catfish, Clarias gariepinus to sublethal concentrations of commonly used pesticides in agricultural settings. The evaluated pesticides were abamectin, carbofuran, chlorpyrifos, cypermethrin, deltamethrin, dichlorvos, dimethoate, fipronil, lambda-cyhalothrin and paraquat. The fishes were initially exposed singly to the pesticides for 96 h periods to determine their LC50, followed by exposure to sublethal concentrations (1/100th 96 h LC50) over a 21 d period. In all cases, a control experiment with catfishes kept in dechlorinated municipal water was monitored simultaneously. The 96 h LC50 values was found to vary widely from 2.043 μgL-1 (Lambda-cyhalothrin) to 10284.288 μgL-1(Paraquat). Significant differences (P < 0.05) were observed between mean haematological parameters-WBC, RBC, HGB, HCT, MCH and MCHC in the exposed and control catfishes. More so, micronucleus and nuclear abnormalities occurred at significantly higher proportions in pesticide exposed catfishes. Holistic cradle to grave approach as well as fate analysis is required to mitigate the potential harmful effects of pesticides to fresh water fishes.
Keywords: Agricultural science, Agrochemicals, Aquatic pollution, Mutagenesis, Oxidative stress
Agricultural science; Agrochemicals; Aquatic pollution; Mutagenesis; Oxidative stress.
1. Introduction
The United Nations Environment Programme (UNEP, 2005) defines pesticides as any substance or mixture of substances intended for preventing, destroying, repelling or mitigating any pest. Their use in agriculture has led to a significant improvement in crop yield per hectare (Aktar et al., 2009). However, the indiscriminate and intensive use of pesticides in agriculture, animal husbandry and post-harvest technology is a threat to the natural water system, public health and welfare of mankind (Tilak et al., 2007). Cessna and Allan (2009), noted that not all pesticides reach their target. In fact, over 98% of sprayed insecticides and 95% of herbicides have been reported to reach a destination other than their target species, because they are sprayed or spread across entire agricultural fields (George, 2004). Tang et al. (2012) reported that runoff can carry pesticides into aquatic ecosystems while Tashkent (1998) noted that wind can carry them to other fields, grazing areas or human settlements, potentially affecting other species. Pesticide residues have also been found in rain and ground water (Kellogg et al., 2000) with far reaching threats to humans and other biota. Pesticides are often applied in excess resulting in degradation of soil, their biomass, microorganisms, fungi and earthworms (Joko et al., 2017).
Environmental threats to freshwater ecosystems are increasing at faster rates as industrialization, urbanization and agricultural activities intensify. Billions of kilograms of industrical chemicals find their way into fresh water bodies around the world annually including 140 billion kilograms of pesticides (Mensah et al., 2014). Toxicologists consider pesticides to have become a necessary evil and like many discoveries or developments, people have been quick to reap their benefits, but extra slow to comprehend and deal with their negative consequences. These effects may be sometimes irreversible, and harmful to humans and the environment. Given that their properties differs, toxicity to fishes can vary with each pesticide group, with insecticides typically the most toxic (Sabra and Mehana, 2015).
In Nigeria, agrochemicals that contain pesticides especially chlorinated hydrocarbons and the organophosphates, are routinely employed as part of the integrated farming practice to protect crops and animals from insects, weeds and diseases (Fafioye et al., 2001; Ezike, 2017). The increasing use of various pesticides for pest control has caught much attention for extensive investigations on the mode of toxic actions of pesticides especially on aquatic fauna (Ayoola and Ajani, 2008). Among these pesticides are abamectin, carbofuran, chlorpyrifos, cypermethrin, deltamethrin, dichlorvos, dimethoate, fipronil, lambda-cyhalothrin and paraquat which are commonly deployed in Lagos, Nigeria (Sourced from market survey in an unpublished research work).
Blood is the most essential and abundant body fluid and is a vehicle for quickly mobilizing defence against trauma and ill health (Adewumi et al., 2018). In assessing the toxic effects of chemicals in aquatic organisms, the use of haematological techniques has become more relevant in recent times, because of the relevance of blood in maintaining homeostasis and life functions of fishes (Musa and Omoregie, 1999; Gabriel et al., 2006). Studies have revealed that when the aquatic quality is affected by contaminants, any physiological variations will be revealed in values of one or more haematological parameters of aquatic animals (Akinrotimi et al., 2007; Gabriel et al., 2007; Adewumi et al., 2018). Samprath et al. (1993) observed that the relevance of haematological studies in fish, lies in the possibility that the blood will reveal anomalies within the body of the fish long before there is any outward manifestation of symptoms of disease or effects of unfavourable environmental factors. To this end, many laboratory studies have also elucidated effects of toxicants on the haematology of Clarias gariepinus including exposure to chlorpyrifos and DDforce (Adewumi et al., 2018), cypermethrin (Akinrotimi et al., 2012; Gabriel and Ugbomeh, 2016), as well as dichlorvos (Ezike, 2017), thereby supporting earlier assertions.
Ololade and Oginni (2010) opined that the most common haematological variables measured during stress included Red and White blood cells count, haemoglobin content, and haematocrit value and red blood cells indices (Suganthi et al., 2015). In their independent research works, Abdulkareem and Owolabi (2014) and Nwani et al. (2015) have reported significant changes in the white blood cell (WBC), red blood cell (RBC) and other haematological parameters of Clarias gariepinus exposed to various pesticides.
Fishes exposed to toxicants may also exhibit stress responses, defined as a state of re-established homeostasis, a complex suite of mal-adaptive responses (Chrousos, 1998). Under such conditions, physiological and biochemical responses may be compromised, resulting in detrimental effects on fish's health and well-being (Ayanda et al., 2017). Such stresses have been associated with DNA damage in living cells and initiation of a range of alterations at the cellular, individual, community and population level (Lee et al., 2013). Genotoxicity is a property possessed by some substances that makes them harmful to the genetic information contained in an organism (Shahi and Singh, 2014; Sabzar et al., 2016). The micronucleus assay, developed by Schmidt (1975), is an in vivo and in vitro short-time screening method which is sensitive and therefore extensively used as a tool for detecting mutagenic and genotoxic effects of chemicals in the environment. The micronucleus (MN) test which basically involves assessment of nuclear abnormalities (NA) (da Silva and Fontanetti, 2006; Rivero-Wendt et al., 2013), due to its simplicity, is one of the most applicable techniques to identify genomic alterations in animals (Bolognesi and Hayashi, 2011). The incidences of micronuclei in fish peripheral erythrocytes have been used as an important tool for monitoring genotoxicity in aquatic environments as well as laboratory treatments in vivo (Ayllon and Garcia-Vazquez, 2000). The formation of nuclear abnormalities, such as blebbed, lobed and notched nuclei as described by Carrasco et al. (1990), have been reported in fish erythrocytes as a consequence of exposure to environmental and chemical contaminants (Muranli and Güner, 2011).
Fishes in their early life stages are known to be extremely vulnerable and have been used as sensitive species for toxicant studies (Byrne, 2012) Some pesticides can bioaccumulate, or build up to toxic levels in the bodies of organisms that consume them overtime, affecting species high on the food chain. Repeated exposure to sub-lethal concentrations of pesticides have been associated with physiological and behavioural changes that reduce fish populations, decreased immunity to diseases and decreased predator avoidance (Araújo and Blasco, 2019).
In an effort to bring to the fore the threats faced by tropical fishes due to pesticides exposure, this study investigated the toxicological responses in the African Catfish, Clarias gariepinus, a commonly consumed tropical freshwater fish. This fish is one of the most cultured both inside and outside its natural range of tropical and subtropical environment (Adewolu et al., 2008). They are also one of the most cultured fishes in the world (FAO, 2014) and are ubiquitous in Nigeria, where they are noted as the most cultivated fish in the country (FAO, 2017). Thus, given their ubiquity in natural and culture settings, they are inadvertently exposed to run offs from farmlands, hence their importance as sentinels in this study.
This investigation was therefore aimed at elucidating the acute toxicity of the most commonly used pesticides on fish early life stages and the long term hamenatological and genotoxic responses associated with chronic exposures which are common in agricultural settings where farmlands are in close proximity to rivers.
2. Materials and methods
2.1. Collection and acclimatization of test animals for the bioassays
The African Catfish (Clarias gariepinus) fry, with mean weight 0.2 ± 0.1 g and mean length 2 ± 0.7 cm were obtained from the Department of Marine Biology and Fisheries, University of Lagos fish culture ponds for the acute toxicity tests. Those for the sub-lethal tests were juveniles of the same species (mean weight 29.68 ± 3.96 g and mean length 15.5 ± 0.2 cm) to ensure ease of blood sample collection after bioassays. They were transported to the Ecotoxicology Laboratory, Department of Zoology Laboratory Annex, within five minutes in plastic bags with sufficient air early in the day (7.00 am–9.00 am). In each case, the fishes were gently transferred into aerated acclimatization tanks (40 × 30 × 30 cm) filled with dechlorinated water and allowed to acclimatize together. They were acclimatized under laboratory conditions (ambient temperature 28 °C ± 0.5; relative humidity 70 ± 5%; Light: Darkness, 12: 12 h) for seven days in rectangular glass tanks, three-quarters filled with dechlorinated water (after an initial 48 h stabilization period as required by OECD Test 203 Guidelines) and covered with a mosquito net to prevent the escape of the fish prior to any experiment and entry of insects into the tanks. During this period, the fishes were fed with Coppens® feed twice daily ad libitum and the stock water was changed once every other day to prevent the accumulation of decaying food particles and waste metabolites. The acclimatization tank was continuously aerated with 220 V air pumps and airstones throughout the period.
Permission was sought and obtained from the University of Lagos Ethical Committee at the College of Medicine in order to use animals for our studies on pesticides (CMUL/HREC/12/19/708). All test fishes were handled humanely in accordance with the ethical guidelines for use of animals in scientific research in accordiance with the EU Directive 2010/63/EU for animal experiments.
2.2. Selection of test chemicals
Ten pesticides (abamectin- 1.8% EC carbofuran-100%, chlorpyrifos-20% EC, cypermethrin-10% EC, deltamethrin-12.5 gL-1, dichlorvos-1000 gL−1, dimethoate-40% EC, fipronil-2.5% EC, lambda-cyhalothrin-2.5% EC and paraquat-200 gL−1) found to be commonly used by farmers in the city of Lagos, Nigeria were obtained from a major pesticide market for this study.
2.3. Acute toxicity bioassay
This was conducted using 4 L transparent glass tanks which were thoroughly washed and carefully dried before use. For the liquid pesticides, 1 ml of each liquid pesticide was injected in 1 L of water to obtain the stock solution (1 mlL-1) while for the solid form (Carbofuran), 1 g was weighed and dissolved in 1 L of water to obtain the stock solution (1 gL-1). Various concentrations were measured into bioassay tanks for range finding and definitive tests. Each experiment was conducted in duplicates of 10 test fishes each including five pairs of test chambers and one pair of control chamber (with dechlorinated water). The fishes were randomly introduced into the experimental tanks with continuous changes in the order of introduction to minimize bias. The definitive test concentrations were as follows:
| Abamectin (C95H142O28): 6.4, 9.6, 12.8, 16.0, 19.2 and 0.0 μgL-1 |
| Carbofuran (C12H15NO3): 20.0, 40.0, 60.0, 80.0, 100.0 and 0.0 μgL-1 |
| Chlorpyrifos (C9H11Cl3NO3PS): 32.0, 40.0, 48.0, 56.0, 64.0 and 0.0 μgL-1 |
| Cypermethrin (C22H19Cl2NO3): 8.0, 10.0, 12.0, 14.0, 16.0 and 0.0 μgL-1 |
| Deltamethrin (C22H19Br2NO3): 17.5, 20.0, 22.5, 25.0, 30.0 and 0.0 μgL-1 |
| Dichlorvos (C4H7Cl2O4P): 800,1200, 1600, 2000, 2400, and 0.0 μgL-1 |
| Dimethoate (C5H12NO3PS2): 1600, 2400, 3200, 4000, 4800 and 0.0 μgL-1 |
| Fipronil (C12H4Cl2F6N4OS): 6.5, 7.5, 8.5, 9.5, 10.5 and 0.0 μgL-1 |
| Lambda-cyhalothrin (C23H19ClF3NO3): 1.5, 2.0, 2.5, 3.0, 3.5 and 0.0 μgL-1 |
| Paraquat (C12H14Cl2N2): 4000, 12000, 20000, 28000, 36000 and 0.0 μgL-1 |
The definitive experiment lasted for 96 h, with mortality recorded every 24 h. Dead fishes were carefully removed from the bioassay containers to prevent contamination using forceps. More so, both exposed and control fishes were not fed during the 96 h study. All experiments were initiated early in the day (7.00 am–10.00 am) to minimize variations in physiological status at the start of the experiments and also ensure consistency of observation times.
2.4. Sub-lethal exposure bioassay
The containers used for the sub-lethal toxicity test on Clarias gariepinus juveniles were rectangular 10 L transparent glass tanks which were thoroughly washed with brine and carefully dried before using them for the experiment.
A dilution factor of one-hundredth (1/100th) of the respective 96 h LC50 value for the ten selected pesticides was used for the sub-lethal tests. Six active juveniles of similar-sized catfishes were randomly picked from the acclimatization tank using a plastic sieve and carefully transferred to tanks containing the sub-lethal concentrations of the respective pesticides for the bioassay. Both definitive and control experiments were conducted in duplicates (3 fishes per tank per pesticide). As with the acute toxicity test, the control fishes were maintained in similar glass tanks but in dechlorinated municipal water only. The test was performed using a semi-static (renewal) bioassay system in which the test media was changed into a fresh solution of the same concentration of test chemicals and untreated control every 72 h to maintain toxicant strength and level of dissolved oxygen as well as reduce the levels ammonia from excretion during the bioassay. The fishes were fed throughout the 21-day sub-lethal exposure period ad libitum, twice daily (7.00 am–9.00 am) and (5.00 pm–7.00 pm). Feeding was done slowly in relation to the fish appetite to minimize accumulation of waste.
2.5. Haematological effect assessment
At the end of the 21-day experiment, at least 1 ml of blood samples were drawn from the caudal vein of three randomly selected juvenile catfishes per pesticide and the control using 2 ml syringes and decanted into EDTA anti-coagulant bottles. The haematological examination was carried out using a computerized Automated Haematological Analyser (SysmexR KX-21) using standard methods (Alexander et al., 1993). The haematological parameters analysed were White Blood Cell Count (WBC), Red Blood Cell Count (RBC), Haemoglobin (HGB), Hematocrit (HCT), Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Mean Corpuscular Haemoglobin Concentration (MCHC) and Platelet (PLT). Samples were not homogenized, rather they were analysed separately and the mean values of their readings were calculated using values for three sample analysis each.
2.6. Genotoxic effects assessment
Immediately after collecting blood from caudal vein of the exposed fishes, well-labelled slides were prepared by smearing one drop of blood to make thin films by spreading with another glass slide at 45°. Three slides were prepared per test chemical and the control. They were air-dried for 30 min, fixed in ethanol for a few seconds and then allowed to air-dry at room temperature for 10 min to minimize red blood cell (RBC) damaged. The slides were then stained with May-Grunwald and allowed to air-dry for 10 min before rinsing gently with water and allowed to air-dry for about 1 h. The slides were subsequently counter stainined using Giemsa, rinsed and then air-dried. During the microscopy, a drop of oil (immersion) was smeared on a stained glass slide to enhance the refactive index. The slides were placed under a binocular Olympus® microscope and nuclear abnormalities (micronucleus, binucleated as well as bud, notch, blebbed, lobed and 8-shaped nucleus) were assessed, observed and counted using a manual hand-held counter based on the keys presented in Anifowoshe et al. (2020). One thousand RBC were examined per slide, while the results were expressed as percentages of the total number of cells observed.
2.7. Statistical analysis
All analyses were carried out using the Statistical Package for the Social Sciences (SPSS) Version 20 software (IBM). The dose-response from the acute toxicity tests involving 20 fry per concentration of five gradients of the ten pesticides were each subjected to probit (regression) analysis to determine the median lethal concentrations (LC50). The toxicity of the pesticides to the fishes were ranked using toxicity factor as follows:
Significant differences (at P < 0.05) in the measurements of haematological parameters and red blood cell nuclear abnormalities in the exposed juvenile catfishes were analyzed using one-way analysis of variance (ANOVA), while means were separated using Duncan Multiple Range Test (DMRT) and Least Significant Difference (LSD).
3. Results
3.1. Acute toxicity concentration-response of the catfishes to pesticides exposure
The results of the acute toxicity evaluation involving the ten pesticides evaluated indicated a consistent pattern of concentration-response relationship (Tables 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10). The results of the acute toxicity evaluation indicated a significant difference (P < 0.05) in the range of sensitivity of the catfishes to the ten pesticides evaluated (Table 11).
Table 1.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Chloropyrifos.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 32 | 1.50 | 2 | 13.3 | 6 | 40 | 7 | 46.7 | 7 | 46.7 |
| 40 | 1.60 | 2 | 13.3 | 7 | 46.7 | 7 | 46.7 | 9 | 60 |
| 48 | 1.68 | 10 | 66.7 | 10 | 66.7 | 12 | 80 | 13 | 86.7 |
| 56 | 1.75 | 13 | 86.7 | 13 | 86.7 | 15 | 100 | 15 | 100 |
Table 2.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Fipronil.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 6.5 | 0.81 | 1 | 6.67 | 4 | 26.7 | 9 | 60 | 9 | 60 |
| 7.5 | 0.88 | 0 | 0 | 5 | 33.3 | 11 | 73.3 | 13 | 86.7 |
| 8.5 | 0.93 | 7 | 46.7 | 7 | 46.7 | 8 | 53.3 | 13 | 86.7 |
| 9.5 | 0.98 | 7 | 46.7 | 11 | 73.3 | 14 | 93.3 | 14 | 93.3 |
| 10.5 | 1.021 | 14 | 93.3 | 14 | 93.3 | 14 | 93.3 | 15 | 100 |
Table 3.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Dimethoate.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 1600 | 3.20 | 3 | 20 | 4 | 26.7 | 4 | 26.7 | 4 | 26.7 |
| 2400 | 3.38 | 5 | 33.3 | 7 | 46.7 | 8 | 53.3 | 9 | 60 |
| 3200 | 3.50 | 8 | 53.3 | 9 | 60 | 11 | 73.3 | 12 | 80 |
| 4000 | 3.60 | 12 | 80 | 13 | 86.7 | 15 | 100 | 15 | 100 |
| 4800 | 3.68 | 14 | 95 | 15 | 100 | 15 | 100 | 15 | 100 |
Table 4.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Cypermethrin.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 8 | 0.90 | 1 | 6.67 | 2 | 13.3 | 4 | 26.7 | 6 | 40 |
| 10 | 1 | 3 | 20 | 4 | 26.7 | 6 | 40 | 8 | 53.3 |
| 12 | 1.079 | 7 | 46.7 | 8 | 53.3 | 9 | 60 | 11 | 73.3 |
| 14 | 1.15 | 12 | 80 | 12 | 80 | 13 | 86.7 | 14 | 93.3 |
| 16 | 1.20 | 15 | 100 | 15 | 100 | 15 | 100 | 15 | 100 |
Table 5.
Concentration-response relationship of African Catfish, clarias gariepinus exposed to Lambda-cyalothrin.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 0.18 | 0 | 0 | 1 | 6.67 | 2 | 13.3 | 3 | 20 |
| 2.0 | 0.30 | 0 | 0 | 5 | 33.3 | 6 | 40 | 7 | 46.7 |
| 2.5 | 0.40 | 7 | 46.7 | 10 | 66.7 | 10 | 66.7 | 10 | 66.7 |
| 3.0 | 0.48 | 7 | 46.7 | 8 | 53.3 | 11 | 73.3 | 13 | 86.7 |
| 3.5 | 0.54 | 9 | 60 | 11 | 73.3 | 11 | 73.3 | 15 | 100 |
Table 6.
Concentration-response relationship of African Catfish, clarias gariepinus exposed to Carbofuran.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 1.30 | 0 | 0 | 3 | 20 | 4 | 26.7 | 5 | 33.3 |
| 40 | 1.60 | 1 | 6.67 | 3 | 20 | 6 | 40 | 7 | 46.7 |
| 60 | 1.78 | 1 | 6.67 | 4 | 26.7 | 7 | 46.7 | 7 | 46.7 |
| 80 | 1.90 | 5 | 33.3 | 7 | 46.7 | 8 | 53.5 | 11 | 73.3 |
| 100 | 2 | 9 | 93.3 | 10 | 66.7 | 11 | 73.3 | 12 | 80 |
Table 7.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Paraquat (n = 15).
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 4000 | 3.60 | 2 | 13.30 | 3 | 20.00 | 3 | 20.00 | 3 | 20.00 |
| 12000 | 3.08 | 0 | 0.00 | 0 | 0.00 | 5 | 33.30 | 7 | 46.70 |
| 20000 | 4.30 | 1 | 6.67 | 4 | 26.7 | 6 | 40.00 | 10 | 66.70 |
| 28000 | 4.45 | 5 | 33.30 | 9 | 60.00 | 11 | 73.30 | 15 | 100.00 |
| 36000 | 4.56 | 9 | 60.00 | 12 | 80.00 | 15 | 100.00 | 15 | 100.00 |
Table 8.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Abamectin.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 6.4 | 0.81 | 0 | 0.00 | 2 | 13.30 | 4 | 26.70 | 4 | 26.70 |
| 9.6 | 0.98 | 2 | 13.30 | 3 | 20.00 | 5 | 33.30 | 7 | 46.70 |
| 12.8 | 1.18 | 6 | 40.00 | 7 | 46.70 | 8 | 53.30 | 12 | 80.00 |
| 16 | 1.20 | 8 | 53.3 | 8 | 53.30 | 9 | 60.00 | 14 | 93.30 |
| 19.2 | 1.28 | 12 | 80.00 | 13 | 86.70 | 15 | 100.00 | 15 | 100.00 |
Table 9.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Dichlorvos.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 800 | 2.90 | 3 | 20.00 | 4 | 27.00 | 6 | 40.00 | 6 | 40.00 |
| 1200 | 3.08 | 8 | 53.30 | 9 | 60.00 | 9 | 60.0 | 10 | 66.70 |
| 1600 | 3.20 | 11 | 73.30 | 11 | 73.30 | 11 | 73.30 | 12 | 80.00 |
| 2000 | 3.30 | 6 | 40.00 | 9 | 60.00 | 11 | 73.30 | 13 | 86.70 |
| 2400 | 3.38 | 10 | 66.70 | 12 | 80.00 | 12 | 80.00 | 15 | 100.00 |
Table 10.
Concentration-response relationship of African Catfish, Clarias gariepinus exposed to Deltamethrin.
| Concentration μg/l |
Log of conc. | 24 h | % | 48 h | % | 72 h | % | 96 h | % |
|---|---|---|---|---|---|---|---|---|---|
| 17.5 | 1.24 | 0 | 0.00 | 2 | 13.30 | 2 | 93.30 | 2 | 13.30 |
| 20 | 1.30 | 1 | 6.67 | 2 | 13.30 | 3 | 46.70 | 4 | 26.70 |
| 22.5 | 1.35 | 0 | 0.00 | 3 | 20.00 | 7 | 46.70 | 11 | 73.30 |
| 25 | 1.40 | 3 | 20.00 | 4 | 26.70 | 7 | 20.00 | 12 | 80.00 |
| 30 | 1.48 | 12 | 80.00 | 14 | 93.30 | 14 | 13.30 | 15 | 100.00 |
Table 11.
Relative 96 h acute toxicity of the Pesticides on fry of the African Catfish, Clarias gariepinus.
| Pesticides | LC5 (μgL−1) | LC50 (μgL−1) | LC95 (μgL−1) | DF | S.E | Probit equation | TF | Relative toxicity ranking | Sub-lethal Conc (1/100th LC50) (μgL−1) |
|---|---|---|---|---|---|---|---|---|---|
| Abamectin | 4.623 (2.570–6.020) | 8.939 (7.303–10.303) | 17.286 (14.269–25.641) | 3 | 1.194 | -5.8 + 6x | 4.375 | 3 | 0.089 |
| Carbofuran | 4.876 (0.044–12.94) | 37.675 (16.218–58.155) | 291.123 (132.167–11692.4) | 2 | 0.632 | -3.2856 + 2.14x | 18.44 | 7 | 0.377 |
| Chlorpyriphos | 14.438 (0.013–24.13) | 34.554 (8.693–41.316) | 82.697 (58.797–7267.14) | 2 | 1.922 | -8.6 + 5.6x | 16.91 | 6 | 0.346 |
| Cypermethrin | 5.225 (2.384–6.823) | 9.461 (7.602–10.637) | 17.133 (14.315–28.082) | 3 | 1.637 | - 5.7 + 6x | 4.60 | 4 | 0.095 |
| Deltamethrin | 16.492 (13.64–18.02) | 21.244 (19.971–22.492) | 27.366 (25.185–32.573) | 3 | 3.044 | -16.5 + 12.5x | 10.4 | 5 | 0.212 |
| Dimethoate | 1122.415 (557.8–1423) | 2112.339 (1696.712–2444.) | 3975.337 (3247.0–6477.6) | 2 | 1.429 | -17 + 5x | 1033.9 | 9 | 21.123 |
| Dichlorfos | 338.603 (0–735.188) | 884.330 (0–1235.81) | 2309.601 (1774.2–199642300.) | 2 | 1.947 | -7.733 + 2.67x | 432.9 | 8 | 8.843 |
| Fipronil | 3.977 (1.119–5.262) | 6.148 (3.802–7.026) | 9.503 (8.384–14.448) | 2 | 2.888 | -5.2 + 7x | 3.00 | 2 | 0.061 |
| Lambda-cyalothrin | 1.207 (0.772–1.476) | 2.043 (1.768–2.278) | 3.460 (2.957–4.816) | 3 | 1.495 | -1.6 + 6.25x | 1.00 | 1 | 0.020 |
| Paraquat | 2382.084 (513–4314.2) | 10284.288 (42.719–14262.4) | 44400.865 (27254–148764.54) | 2 | 0.621 | -7.75 + 1.875x | 5033.9 | 10 | 102.843 |
The lowest (32 μgL-1) and highest (56 μgL-1) concentrations of exposure for Chlopyrifos resulted in 46.7% and 100% mortality of catfishes respectively after 96 h (Table 1). Fipronil exposure resulted in range of 60%–100% for the lowest (6.5 μgL-1) and highest (10.5 μgL-1) exposure concentrations within the same period (Table 2) compared to 26.7% (1600 μgL-1) and 100% (4800 μgL-1) recorded for Dimethoate exposure (Table 3). The catfish mortality range recorded for Cypermethrin exposure were 40% (8 μgL-1) and 100% (16 μgL-1) (Table 4) compared to 20% (1.5 μgL-1) and 100% (3.5 μgL-1) for Lambda-cyalothrin exposure (Table 5). Carbofuran exposure resulted in a range of 33.3% (20 μgL-1) to 80% (100 μgL-1) (Table 6) mortality while for Paraquat it was 20% (4000 μgL-1) and 100% (36000 μgL-1) respectively (Table 7). Abamectin exposure resulted in a range of 26.7% (6.4 μgL-1) to 100% (19.2 μgL-1) mortality (Table 8) while for Dichlorvos it was 40% (800 μgL-1) to100% (2400 μgL-1) (Table 9). Deltamethrin exposure caused mortality within the range of 13.3% and 100% for the lowest (17.5 μgL-1) and highest (30 μgL-1) concentrations respectively after 96 h (Table 10).
Overall, the assessed 96 h LC50 values for the ten pesticides on C. gariepinus indicated that lambda-cyhalothrin was the most toxic with a value of 2.043 μgL-1 while Paraquat was the least toxic with a value of 10284.288 μgL-1. The high range in toxicity differences implies that the most toxic pesticide, lambda-cyhalothrin was at least 5033 more toxic than the least toxic one, indicating a considerable difference in chemical composition and mechanisms of action.
The relative toxicity after 96 h exposure can be summarized as follows: Lambda-cyhalothrin > Fipronil > Abamectin > cypermethrin > Deltamethrin > chlorpyrifos > carbofuran > dichlorvoss > dimethoate > paraquat (Table 11).
3.2. Haematological effects of exposure to the sub-lethal concentrations of the pesticides
The results from the haematological analysis of the exposed juvenile C. gariepinus are presented in Table 12. Statistical analysis revealed significant differences (P < 0.05) between the mean values of WBC, RBC, HGB, HCT, MCH and MCHC in the exposed and control fishes.
Table 12.
Changes in haematological parameters of Clarias gariepinus Juveniles exposed to sub-lethal concentrations of ten pesticides.
| Pesticide | Haematological Parameters |
|||||||
|---|---|---|---|---|---|---|---|---|
| WBC (109 L−1) | RBC (1012 L−1) | HGB (gdL−1) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (gdL−1) | PLT (109 L−1) | |
| Control | 49.60 ± 11.61ab | 1.28 ± 0.18a | 5.66 ± 0.80a | 13.90 ± 2.36a | 107.73 ± 3.25a | 44.13 ± 0.68b | 41.10 ± 1.62b | 72.33 ± 31.54a |
| Abamectin | 58.53 ± 27.37abcd | 4.32 ± 2.56b | 9.90 ± 2.07ab | 27.53 ± 6.53ab | 94.43 ± 25.97a | 34.73 ± 10.05ab | 36.33 ± 0.91ab | 52.66 ± 22.83a |
| Carbofuran | 54.73 ± 17.79abc | 1.34 ± 0.30a | 6.03 ± 1.12a | 15.63 ± 3.27a | 118.07 ± 4.30a | 45.96 ± 2.52b | 39.03 ± 1.72b | 70.0 ± 6.11a |
| Chlorpyrifos | 20.43 ± 16.54a | 1.85 ± 1.20ab | 5.90 ± 3.37a | 16.90 ± 9.59a | 100.87 ± 10.76a | 28.03 ± 8.86a | 27.86 ± 7.69a | 153.67 ± 117.23a |
| Cypermethrin | 101.60 ± 5.95bcde | 2.17 ± 0.01ab | 9.13 ± 0.17ab | 23.90 ± 1.10ab | 110.37 ± 4.56a | 42.03 ± 0.59b | 38.26 ± 1.20b | 45.0 ± 15.94a |
| Deltamethrin | 79.16 ± 8.40cdef | 1.78 ± 0.11ab | 7.60 ± 0.60a | 20.46 ± 0.86ab | 115.33 ± 2.65a | 42.46 ± 0.78b | 36.96 ± 1.49b | 90.0 ± 26.0a |
| Dichlorvos | 71.96 ± 15.91bcd | 1.63 ± 0.21ab | 6.83 ± 0.83a | 17.70 ± 1.75a | 109.67 ± 3.71a | 41.90 ± 0.49b | 38.40 ± 0.85b | 23.0 ± 9.64a |
| Dimethoate | 59.90 ± 9.30abcd | 1.39 ± 0.14ab | 6.40 ± 0.75a | 15.30 ± 2.08a | 109.83 ± 4.0a | 45.90 ± 0.63b | 42.03 ± 1.58b | 87.33 ± 17.63a |
| Fipronil | 121.27 ± 12.34ef | 2.43 ± 0.26ab | 10.13 ± 1.33ab | 28.96 ± 5.83a | 116.97 ± 10.21a | 41.43 ± 1.12b | 35.83 ± 2.50ab | 48.33 ± 6.66a |
| Lambda-cyhalothrin | 139.50 ± 9.71f | 2.96 ± 0.18ab | 12.66 ± 0.93ab | 33.66 ± 2.96ab | 113.77 ± 5.38a | 42.70 ± 0.49b | 37.70 ± 1.67b | 158.33 ± 37.40a |
| Paraquat | 105.10 ± 17.96def | 2.31 ± 0.35ab | 9.76 ± 1.44ab | 26.50 ± 4.20ab | 114.57 ± 1.29a | 42.30 ± 1.02b | 37.06 ± 1.31b | 85.33 ± 58.40a |
WBC: white blood cell, RBC: red blood cell, HGB: haemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular haemoglobin MCHC: mean corpuscular haemoglobin concentration, PLT: platelets. Values are means of three replicates ± Standard Error Mean. Column values with different superscripts are significantly different (P < 0.05).
Specifically, there was a significant increase (P < 0.05) in WBC count in the C. gariepinus exposed to the selected pesticides compared to the control, except for Chlorpyrifos (20.43 ± 16.54 × 109 L−1) which showed a significant decrease (P < 0.05) in WBC compared to the control (49.60 ± 11.61 × 109 L−1) after the 21 d period.
A significant increase (P < 0.05) in RBC counts was observed in catfishes exposed to nine pesticides compared to the control, except for those exposed to carbofuran (1.34 ± 0.30 × 1012 L−1) for which there was no significant difference (P > 0.05) compared to the control (1.28 ± 0.18 × 1012 L−1). RBC was significantly higher (P < 0.05) in those exposed to Abamectin compared to the other pesticides and the control. HGB also significantly increased (P < 0.05) in Abamectin, Cypermethrin, Fipronil, Lamda-cyhalothrin exposed catfishes compared to the control.
The HCT levels in catfishes exposed to Abamectin, Cypermethrin, Deltamethrin, Lambda-cyhalothrin and Paraquat were significantly higher (P < 0.05) compared to the control. There was a slight increase in the level of MCV in all of the pesticides exposure except in Abamectin which showed a slight decrease. There was no significant difference (P > 0.05) in the MCV levels in those exposed to Abamectin compared to the control. Only MCH levels in Abamectin (34.73 ± 10.05 pg) and Chlorpyrifos (28.03 ± 8.86 pg) exposed catfishes showed significant decreases (P < 0.05) compared to the control. The MCHC in catfish exposed to Chlorpyrifos, Abamectin and Fipronil showed significant decrease in comparison to the control. There was no significant difference (P > 0.05) between the PLT level in the experimental groups and the control.
3.3. Genotoxic effects of exposure to sub-lethal concentrations of the pesticides
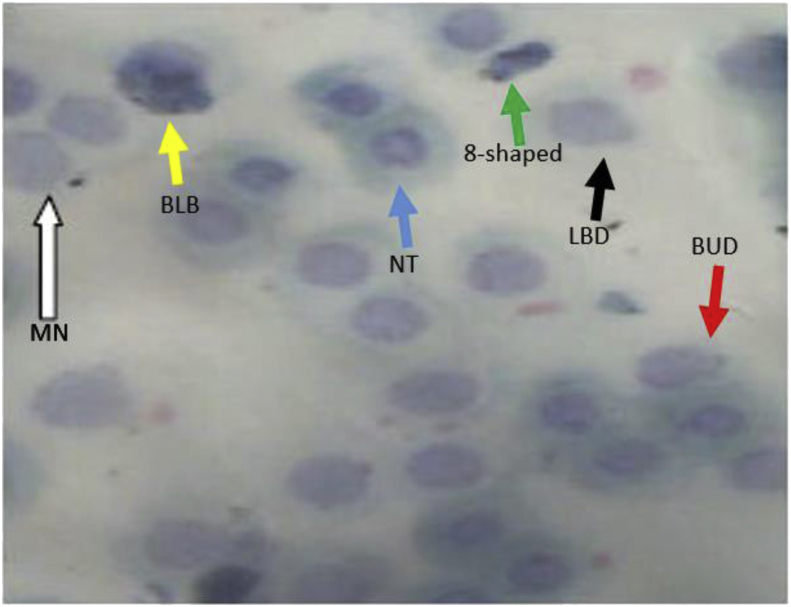
The red blood cell nucleus abnormalities observed in the exposed catfishes were micronuclei (MN), binuclei (BN), bud shaped nuclei, lobed shaped nuclei, 8-shaped nuclei, notched nuclei and blebbed shape (Figure 1). The frequencies of occurrence of these abnormalities in the C. gariepinus juveniles exposed to sub-lethal concentrations of ten selected pesticides are presented in Table 13.
Figure 1.
A typical blood smear showing abnormalities observed in the erythrocyte of some of the juvenile African Catfish, Clarias gariepinus exposed to the pesticides. Arrows (white = micronucleus-MN, blue = notched-NT, red = bud-BUD, green = eight shaped, yellow = blebbed-BLB, black = lobed-LBD).
Table 13.
Frequencies of nuclear abnormalities in Clarias gariepinus Juveniles exposed to sub-lethal concentrations of the ten selected pesticides (n = 1000 erythrocytes per pesticide).
| Pesticide | MN (%) | BN (%) | BUD (%) | NOTCH (%) | 8-SHAPED (%) | BLEBBED (%) | LOBED (%) |
|---|---|---|---|---|---|---|---|
| Control | 1 (0.1) | 2 (0.2) | |||||
| Abamectin | 7 (0.7) | 3 (0.3) | 41 (4.1) | 7 (0.7) | 18 (1.8) | ||
| Carbofuran | 2 (0.2) | 15 (1.5) | 3 (0.3) | 1 (0.1) | |||
| Chlorpyrifos | 4 (0.4) | 37 (3.7) | 13 (1.3) | ||||
| Cypermethrin | 1 (0.1) | 18 (1.8) | 2 (0.2) | 3 (0.3) | |||
| Deltamethrin | 22 (2.2) | 2 (0.2) | |||||
| Dichlorvos | 9 (0.9) | 32 (3.2) | 8 (0.8) | ||||
| Dimethoate | 42 (4.2) | 8 (0.8) | |||||
| Fipronil | 30 (3.0) | 1 (0.1) | 1 (0.1) | 4 (0.4) | |||
| Lambda-cyhalothrin | 3 (0.3) | 35 (3.5) | 7 (0.7) | ||||
| Paraquat | 25 (2.5) | 1 (0.1) | 1 (0.1) |
MN: Micronucleus, BN: Binucleated.
All the cells of the exposed catfishes displayed at least two nuclear abnormalities with the bud shaped nucleus being observed in all exposure groups. Among the fishes exposed to the various pesticides, overall differences in blood cell nuclear abnormalities were not significant (P > 0.05). The most commonly observed nuclear abnormality was bud-shaped nuclei with an occurrence of 4.2% in the blood cells of those exposed to Dimethoate while 8-shaped nuclei had the least occurrence of 0.1% (in those exposed to Fipronil).
Micronuclei was observed in three exposure group (Abamectin, Chlorpyrifos and Lambda-cyhalothrin) while binuclei occurred in four of the pesticides groups (Abamectin, Carbofuran, Cypermethrin and Dichlorvos). Notched and lobed nuclei had the second highest occurrence, appearing in four exposure groups (Table 13).
4. Discussion
Pesticides are economic poisons whose use are inevitable in the present world but their effects on non-target organisms perhaps outweigh their effects on the target pests. This is an overwhelming challenge which resonates with the findings of this study. All the tested pesticides exhibited acute toxicity to the exposed catfishes with median lethal toxicity ranging from 2.043 to 10284.28 μgL-1. The non-consideration of sex as a selection criterion is a shortcoming for the present study. Moreover, early life stages were employed, during which sex differentiation is difficult to perform effectively. Thus, selection was entirely random and fishes were from the same fertilization stock to minimize this shortcoming. More so, there are limited literature citing the influence of sex on the respons of fishes to pesticide exposure. Barata et al., 2002 considered sex related variation in Marine Copepod, Acartia tonsa exposed to cypermethrin but found significant concentration-mortality response only in the first 24 h.
Among the pesticides examined, Lambda-cyhalothrin, a chlorinated hydrocarbon, and Fipronil (phenylpyrazole chemical family) were the most toxic. The least toxic were Dimethoate, an organophosphate compound, and Paraquat, an herbicide. This might likely be linked to the differences in their respective chemical composition and to varying degrees, peharps their specific mechanism of action. For example, Fipronil is a neurotoxin, a known blocker of GABA receptors as have been reported in experiments using fathead minnow (Bencic et al., 2013). Dimethoate on the other hand is a neurotoxin acts by inhibiting of actetylcholinesterase (USEPA, 2008). This implies that a more intrinscic investication of relative mode of action of these pesticides is require to further elucidate the reasons for the observed actute toxicity responses. Moreover, insecticides have been reported to be more toxic than herbicides in a study by Mesnage et al. (2014) who compared toxicity of active ingredients of three classes of pesticides. Thus corroborates the finding in this study for which the herbicide, Paraquat was by far the least toxic pesticide.
There is no universal toxicity to organisms by pesticides rather responses are species-specific as well as pesticide-specific, depending on organismal susceptibility even within same species, age, environmental conditions as well as the particular chemical characteristics of the pesticides. For instance, the 96-h LC50 value of 8.939 μgL-1 recorded for Abamectin in the present study is higher than 3.2 μg/L reported for rainbow trout but much lower than that of carp (42 μgL-1) (Jenčič et al., 2006) for the same pesticide. The species specificity is emphasized when the 96-h LC50 (37.7 μgL-1) of Carbofuran in Clarias gariepinus for this study is compared with that of Clarias batrachus (13 μgL-1) reported by Singh et al., 2003. The differences in toxicity to the same African Catfish, C. gariepinus exposed to cypermethrin in studies conducted within the same institution indicated an almost 7-fold increase in toxicity (96-hour LC50 values) with 9.461 μg/L reported in the present study compared to 63 μgL-1 reported by Ayoola and Ajani (2008). Deltamethrin affects the nervous system and has high toxicity for benthic arthropods and fishes, but is less toxic for mammals and birds (Lahr et al., 2000). The 96-h LC50 for exposure to Deltamethrin in the present study (21.24 μgL-1) was considerably higher than those recorded by Mestres and Mestres (1992), who reported values for rainbow trout (Salmo gairdneri) as 0.39 μgL-1; common carp (Cyprinus carpio) 1.84 μgL-1 and Mozambique tilapia (Sarotherodon mossambica) as 3.50 μgL-1. These differences emphasize the difficulty in making one-fits-all decisions with respect to pesticide use and also emphasize the need for locally thought out decisions in specific farming areas. A universal recommendation in this regards will be centred around increasing distance of farmlands to natural water bodies as well as minimizing pesticide use as much as practicable.
The haematological results showed that the exposure of C. gariepinus to sub-lethal concentrations of the ten selected pesticides caused a significant increase in WBC count of the fish except for chlorpyrifos which showed a significant decrease in WBC count. The most significant increase was observed in fish exposed to lambda cyhalothrin, and this is an indication of the high toxicity of the pesticide on C. gariepinus. An increase in WBC count can be correlated with an increase in antibody production, which helps in survival and recovery of fish exposed to sub-lethal concentration of pesticides (Joshi et al., 2003). This response was also observed in the same fish species exposed to chlorpyrifos and DDforce (Adewumi et al., 2018), and in Cyprinus carpio after acute exposure to phenithrotion and dichlorvos (Svobodová, 1991, 1996). This may be due to the release of WBC from spleen in the blood stream to combat the toxicant. As suggested by Muralidharan (2012), several chemical compounds including insecticides generate antibodies owing to their interference with the immune system.
The significant increase observed due to pesticide exposures in the levels of RBC (Abamectin, Chlorpyrifos, Cypermethrin, Deltamethrin, Dichlorvos, Dimethoate, Fipronil, Lambda-cyhalothrin, Paraquat), HGB (Abamectin, Cypermethrin, Fipronil, Lambda-cyhalothrin, Paraquat) and HCT (Abamectin, Cypermethrin, Deltamethrin, Lambda-cyhalothrin, Paraquat) recorded in this study differs with findings of some previous investigations using similar pesticides, indicating some level of inconsistencies in haematological responses. For instance, a significant decrease in RBC and HGB level after exposure to pesticides and other toxicants were reported by several investigators (Adewoye, 2010; Gafaar et al., 2010; Jaya and Singh, 2010; Akinrotimi et al., 2013). These changes observed in this study can be attributed to direct responses of structural damage to RBC membrane resulting in haemolysis and also the subsequent need to quickly produce replacement blood cells to minimize risk of anaemia. According to Singh and Srivastava (2010), the RBC elevation may be due to blood cell reserve combined with cell shrinkage as a result of osmotic alterations of blood by the actions of the pesticides. In agreement with results of the present study, Riaz-ul-Haq et al. (2018) have previously observed an increase in HCT (or PCV) of freshwater fish Channa punctatus exposed to endosulphan pesticide. The significant decrease in the MCH and MCHC level of fishes exposed to sub-lethal concentrations of Abamectin and Fipronil in this study is a good indicator of RBC swelling in the fish which has been related to hypoxia. In a study carried out by Pakanit and Kinchareon (2011), flowerhorn fishes showed significant increase in RBC, WBC, HGB and HCT after exposure to conditions of hypoxia. Given that RBC is important in oxygen transport to cells, increased RBC counts would ensure that cells continue to receive enough oxygen for metabolism. Previous studies involving catfishes have reported that under extreme hypoxia, reduced activity is reflection of the differences in blood respiratoty functions of different species (Wells et al., 2005).
The findings of this study indicated a relationship between pesticide exposure and induction of micronucleus (MN) in the red blood cell of catfishes. Micronucleus have been reported in different fish organs (Srivastava et al., 2016) and their induction in fishes have been associated with environmental factors as well as rate of cell proliferation (Arkhipchuk and Garanko, 2005). Sub-lethal concentrations of all ten pesticides assessed in this study induced varying degrees of nuclear abnormalities (NA) as well. Nuclear abnormalities are induced in response to genotoxic agents and this have been affirmed by previous reports as being associated with exposure to heavy metals (Ergene et al., 2007) and pesticides (Malla and Ganesh, 2009) in fishes. Notably, Ansari et al. (2009) repotred that deltamethrin exposure ranging from 0.4 and 1.2 μgL-1 induced MN and significant increase (P < 0.001) in NA, such as binucleus, blebbed and lobed nucelus in erythrocytes of the freshwater fish Channa punctate within only 72 h. Similarly, Grisolia (2002) reported increased MN frequency in erythrocytes of Tilapia rendalli exposed to commercial deltamethrin. Also, lambda-cyhalothrin has been found to be a genotoxic agent in the erythrocytes of Cheirodon interruptus interruptus (Campana et al., 1999) and Garra rufa (Cavas and Ergene-Gözükara, 2003). Further in support of the genotoxic markers observed in the present study for C. gariepinus exposed to environmental concentrations of various pesticides (0.02–102.834), Muranli and Güner (2011) reported that lambda-cyhalothrin (1 × 10−4 gL-1, 2 × 10−4 gL-1, 4 × 10−4 gL-1) induced MN and NA (notched, lobed, blebbed nuclei) in the mosquitofish (Gambusia affinis) after 48 h period. The slower rate of DNA repair in fishes compared to mammals make them important sentinel species in assessing environmental genotoxicity (Espina and Weis, 1995) but the general similarity of toxic agents in vertebrates, assessing genotoxicity in fishes presents an opportunity of establishing risk potentials in higher vertebrates. The findings from the present study and available literature (Ullah et al., 2016; Ambreen and Javed, 2018; D'Costa et al., 2018) point to the oxidative stress potentials of the pesticides, given that oxidation is a major precursor for DNA damage. It also raises concerns about the mutagenicity of pesticides not just on the fishes but to humans as well for which several investigators have determine the effects of acute and chronic pesticide exposures (Benedetti et al., 2014). The public health concerns of pesticide runoffs in fresh water bodies are associated with comsuption of fishes which have bioaccumulated these pesticides (Yu et al., 2012; Eqani et al., 2013) and their use as a source of domestic water supply (Sankhla et al., 2018). Sankhla et al. (2018) reported that pesticide exposures to humans are associated with reproductive and developmental defects as well as immunological abnormalities and hematopoietic cancers. Basil et al. (2007) in a review of literature on the association of pesticides with cancers, noted that high and long term exposures to pesticides showed association with cancers in humans especially for brain and prostate cancers where the most correlation were recorded. These effects in humans can be due to their ability to impair anti-oxidative defenses by altering redox equilibria (Benedetti et al., 2014) These findings are consistent with the chromosomal abberations expressed as NA observed in the erythrocytes of the fishes even at sub-lethal levels and resounds the impacts of pesticides at sub-cellular levels. The results of the present study however, show that herbicides (Paraquat) offer less mutagenic threats compared to the insecticides investigated. This may be attributed to the fact that plants are their primary target, compared to insectides which are designed to penetrate animal cell membranes and more acute damage inflict damage (Sabra and Mehana, 2015).
5. Conclusion
The acute toxicity effects, alteration of haematological parameters and induction genotoxic effects in C. gariepinus observed for all pesticides assessed, including herbicides implies that commonly used agrochemicals which find their way into fresh water remain a serious health concern to aquatic organisms. In view of the toxicity of pesticides to fishes, it is important to consider distance to natural water bodies when situating farmlands in order to minimize the risk of intoxication of fishes by pesticides through surface surface runoffs and atmospheric depositions. More than ever, as global populations and food demands rise, increased attention needs to be focused on formulating environmentally friendly, more target-specific and fast degrading pesticides in order to mitigate the potential negative consequences on fishes.
Declarations
Author contribution statement
Nnamdi Amaeze: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Abdulbashir Salako, Benjamin Komolafe: Analyzed and interpreted the data; Wrote the paper.
Kingsley Akagha, Micheal Femi, Opeyemi Olatinwo, Tam-Miete Dawari Briggs: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Department of Zoology, University of Lagos for provision of reagents and laboratory space for this study.
References
- Abdulkareem S.I., Owolabi O.D. Toxicity of sub-lethal concentrations of Monocrotophos (MCP) on the haematological, biochemical and growth responses of hybrid catfish, Heteroclarias and contaminated-Heteroclarias fed rats. Int. J. Curr. Microbiol. Appl. Sci. 2014;3(6):917–931. [Google Scholar]
- Adewolu M.A., Ogunsanmi A.O., Yunusa A. Studies on growth performance and feed utilization of two Clariid catfish and their hybrid reared under different culture systems. Eur. J. Sci. Res. 2008;23(2):252–260. [Google Scholar]
- Adewoye S.O. Haematological and biochemical changes in Clarias gariepinus exposed to Trephosia vogelii extract. Adv. Appl. Sci. Res. 2010;1(1):74–79. [Google Scholar]
- Adewumi B., Germaine Akinola Ogunwole G.A., Akingunsola E., Falope O.C., Eniade A. Effects of sub-lethal toxicity of chlorpyrifos and DDforce pesticides on haematological parameters of Clarias gariepinus. Int. J. Environ. Res. Publ. Health. 2018;5(5):62–71. [Google Scholar]
- Akinrotimi O.A., Ansa E.J., Owhonda K.N., Onunkwo D.N., Edun O.M. Effects of transportation stress on haematological parameters of black Chin Tilapia, Sarotherodon melanotheron. J. Animal Vet. Adv. 2007;6:841–845. [Google Scholar]
- Akinrotimi O.A., Gabriel U.U., Ariweriokuma S.V. Haematotoxicity of cypermethrin to African catfish Clarias gariepinus under laboratory conditions. J. Environ. Eng. Technol. 2012;1(2):20–25. [Google Scholar]
- Akinrotimi O.A., Gabriel U.U., Orokotan O.O. Changes in enzymes activities of Clarias gariepinus brood fish exposed to anaesthetics metomidate. Appl. Ecol. Environ. Sci. 2013;1(3):37–40. [Google Scholar]
- Aktar W., Sengupta D., Chowdhury A. Impact of pesticides use in agriculture; their benefits and hazards. Interdiscipl. Toxicol. 2009;2(1):1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S., Katz F., Reeves B.R., Kearney L., Chessells J. Leukaemia arising in donor cells following allogeneic bone marrow transplantation for β thalassaemia demonstrated by immunological, DNA and molecular cytogenetic analysis. Br. J. Haematol. 1993;85(2):326–331. doi: 10.1111/j.1365-2141.1993.tb03174.x. [DOI] [PubMed] [Google Scholar]
- Ambreen F., Javed M. Pesticide mixture induced DNA damage in peripheral blood erythrocytes of freshwater fish, Oreochromis niloticus. Pakistan J. Zool. 2018;50(1):339–346. [Google Scholar]
- Anifowoshe A.T., Oyebanji J.A., Oladipo O.S., Oyeyemi F.B., Abdulrahim M.Y., Abdulkareem S.I., Mustapha M.K. Histological changes, micronuclei induction and nuclear abnormalities in the peripheral erythrocytes of Clarias gariepinus (Burchell 1822) exposed to water sample from Apodu Reservoir. J. Life Bio-Sci. Res. 2020;1(1):1–7. [Google Scholar]
- Ansari R.A., Kaur M.F., Ahmad S., Rahman H., Rashid F., Islam S., Raisuddin S. Genotoxic and oxidative stress-inducing effects of deltamethrin in the erythrocytes of a freshwater biomarker fish species Channa punctatus. Environ. Toxicol. 2009;24:429–436. doi: 10.1002/tox.20445. [DOI] [PubMed] [Google Scholar]
- Araújo C.V.M., Blasco J. Spatial avoidance as a response to contamination by aquatic organisms in non-forced, multi-compartmented exposure systems: a complementary approach to the behavioral response. Environ. Toxicol. Chem. 2019;38:312–320. doi: 10.1002/etc.4310. [DOI] [PubMed] [Google Scholar]
- Arkhipchuk V.V., Garanko N.N. Using the molecular biomarker and micronuclei test on in vivo fish fin cells. Ecotoxicol. Environ. Saf. 2005;62:42–52. doi: 10.1016/j.ecoenv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Ayanda O.I., Oniye S.J., Auta J.A. Behavioural and some physiological assessment of glyphosate and paraquat toxicity to juveniles of African catfish, Clarias gariepinus. Pakistan J. Zool. 2017;49(1):183–190. [Google Scholar]
- Ayllon F., Garcia-Vazquez E. Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna, an assessment of the fish micronucleus test. Mutat. Res. 2000;467:177–186. doi: 10.1016/s1383-5718(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Ayoola S.O., Ajani E.K. Histopathological effect of cypermethrin on juvenile African catfish (Clarias gariepinus) World J. Biol. Res. 2008;1(2):1–14. [Google Scholar]
- Barata C., Medina M., Telfer T., Baird D., J. Determining demographic effects of cypermethrin in the marine copepod Acartia tonsa: stage-specific short tests versus life-table tests. Arch. Environ. Contam. Toxicol. 2002;43(3):0373–0378. doi: 10.1007/s00244-002-1268-2. [DOI] [PubMed] [Google Scholar]
- Bassil K.L., Vakil C., Sanborn M., Cole D.C., Kaur J.S., Kerr K.J. Cancer health effects of pesticides: systematic review. Can. Fam. Physician. 2007;53(10):1704–1711. [PMC free article] [PubMed] [Google Scholar]
- Benedetti D., Silva F.R.D., Kvitko K., Fernandes S.P., Silva J.D. InTech; Munich, Germany: 2014. Genotoxicity Induced by Ocupational Exposure to Pesticides; pp. 29–51. [Google Scholar]
- Bencic D.C., Villeneuve D.L., Biales A.D., Blake L., Durhan E.J., Jensen K.M., Kahl M.D., Makynen E.A., Martinović-Weigelt D., Ankley G.T. Effects of the insecticide fipronil on reproductive endocrinology in the fathead minnow. Environ. Toxicol. Chem. 2013;32(8):1828–1834. doi: 10.1002/etc.2254. [DOI] [PubMed] [Google Scholar]
- Bolognesi C., Hayashi M. Micronucleus assay in aquatic animals, Review. Mutagenesis. 2011;26(1):205–213. doi: 10.1093/mutage/geq073. [DOI] [PubMed] [Google Scholar]
- Byrne M. Global change ecotoxicology: identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 2012;76:3–15. doi: 10.1016/j.marenvres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Campana M.A., Panzeri A.M., Morenof V.J., Dulout F.N. Genotoxic evaluation of the pyrethroid lambda-cyhalothrin using the micronucleus test in erythrocytes of the fish Cheirodon interruptus interruptus. Mutat. Res. 1999;438:155–161. doi: 10.1016/s1383-5718(98)00167-3. [DOI] [PubMed] [Google Scholar]
- Carrasco K.R., Tilbury K.L., Myers M.S. Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Can. J. Fish. Aquat. Sci. (Ottawa) 1990;47:2123–2136. [Google Scholar]
- Cavas T., Ergene-Gözükara S. Micronuclei, nuclear lesions and interphase silver-stained nucleolar organizer regions (AgNORs) as cyto-genotoxicity indicators in Oreochromis niloticus exposed to textile mill effluent. Mutat. Res. 2003;538(1-2):81–91. doi: 10.1016/s1383-5718(03)00091-3. [DOI] [PubMed] [Google Scholar]
- Cessna A.J., Allan J. Research Centre; Lethbridge, AB: 2009. Pesticides in the Environment: Real or Imagined. Agriculture and Agri-Food Canada. [Google Scholar]
- Chrousos G.P. Stressors, stress and neuroendocrine integration of the adaptative response. Ann. N. Y. Acad. Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- da Silva S.T., Fontanetti C.S. Micronucleus test and observation of nuclear alterations in erythrocytes of Nile tilapia exposed to waters affected by refinery effluent. Mutat. Res. 2006;605:87–93. doi: 10.1016/j.mrgentox.2006.02.010. [DOI] [PubMed] [Google Scholar]
- D’Costa A.H., Shyama S.K., Kumar M.P., Fernandes T.M. Induction of DNA damage in the peripheral blood of zebrafish (Danio rerio) by an agricultural organophosphate pesticide, monocrotophos. Int. Aquat. Res. 2018;10(3):243–251. [Google Scholar]
- Ergene S., Çavaş T., Çelik A., Köleli N., Aymak C. Evaluation of river water genotoxicity using the piscine micronucleus test. Environ. Mol. Mutagen. 2007;48(6):421–429. doi: 10.1002/em.20291. [DOI] [PubMed] [Google Scholar]
- Espina N.G., Weis P. DNA repair in fish from polluted estuaries. Mar. Environ. Res. 1995;39(1-4):309–312. [Google Scholar]
- Eqani S.A.M.A.S., Malik R.N., Cincinelli A., Zhang G., Mohammad A., Qadir A. Uptake of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) by river water fish: the case of River Chenab. Sci. Total Environ. 2013;450:83–91. doi: 10.1016/j.scitotenv.2013.01.052. [DOI] [PubMed] [Google Scholar]
- Ezike C.O. Acute toxicity and haematology of Clarias gariepinus (Burchell, 1822) exposed to 2, 2-dichlorovinyl dimethyl phosphate (dichlorvos) Int. J. Fish. Aquat. Stud. 2017;5(5):100–105. [Google Scholar]
- Fafioye O.O., Adeogun O.A., Olayinka E.A., Ayoade A.A. Effect of Sub-lethal concentrations of lead on growth of Clarias gariepinus. Niger. Exp. Biol. 2001;5(1):61–68. [Google Scholar]
- FAO . Food and Agriculture Organization of the United Nations; Rome: 2014. FAO Year Book, Fishery and Aquaculture Statistics. [Google Scholar]
- FAO . FAO Fisheries and Aquaculture Department, Rome. 2017. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950-2015 (FishstatJ) [Google Scholar]
- Gabriel U.U., Anyanwu P.E., Akinrotimi O.A. Comparative effects of different acclimation media on haematological characteristics of Brackish water tilapia, Sarotherodon melanotheron (Rupell, 1852) J. Fish. Int. 2007;2:195–199. [Google Scholar]
- Gabriel U.U., Ezeri G.N.O., Ikeme P.M., Ikwut E.F. Haematoxicity of proxone to juvenile Clarias gariepinus (Burchell, 1822) under laboratory conditions. Pollut. Res. 2006;25(2):413. [Google Scholar]
- Gabriel U.U., Ugbomeh A.P. Effects of cypermethrin on haematological parameters of African freshwater catfish, Clarias gariepinus. J. Aquat. Sci. 2016;31(2B):433–442. [Google Scholar]
- Gafaar A.Y., El-manakhly E.M., Soliman M.K., Hassan S.M., Soufy H. Some pathological, biochemical and haematological investigation on Nile Tilapia (Oreochromis niloticus) following exposure to edifenphos pesticide. J. Am. Sci. 2010;6(10):542–551. [Google Scholar]
- George T.M. Thomson/Brooks/Cole; 2004. Sustaining the Earth, an Intergrated Approach; pp. 211–216. [Google Scholar]
- Grisolia C.K. A comparison between mouse and fish micronucleus test using cyclophosphamide, mitomycin C and various pesticides. Mutat. Res. 2002;518:145–150. doi: 10.1016/s1383-5718(02)00086-4. [DOI] [PubMed] [Google Scholar]
- Jaya S., Singh A. A comparative study on the piscicidal activity of synthetic pesticides and plant origin pesticides, to fish Channa punctatus. World J. Zool. 2010;5(1):20–24. [Google Scholar]
- Jenčič V., Cerne M., Erzen N.K., Kobal S., Cerkvenik-Flajs V. Abamectin effects on rainbow trout (Oncorhynchus mykiss) Ecotoxicology. 2006;15(3):249–257. doi: 10.1007/s10646-006-0056-6. [DOI] [PubMed] [Google Scholar]
- Joko T., Anggoro S., Sunoko H.R., Rachmawati S. Pesticides usage in the soil quality degradation potential in wanasari subdistrict, Brebes, Indonesia. Appl. Environ. Soil Sci. 2017 [Google Scholar]
- Joshi S.C., Mathur R., Gajraj A., Sharma T. Influence of methyl parathion on reproductive parameters in male rats. Environ. Toxicol. Pharmacol. 2003;14(3):91–98. doi: 10.1016/S1382-6689(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Kellogg R., Nehring R., Grube A., Goss D.W., Plotkin S. USDA Economic Research Service Conference-Agricultural Productivity, Data, Methods, and Measures. 2000. Environmental indicators of nitrogen and pesticide leaching and run-off from farm fields; pp. 9–10. [Google Scholar]
- Lahr J., Diallo A.O., Gadji B., Diouf P.S., Bedaux J.J.M., Badji A. Ecological effects of experimental insecticide applications on invertebrates in sahelian temporary ponds. Environ. Toxicol. Chem. 2000;19(5):1278–1289. [Google Scholar]
- Lee S., Liu X., Takeda S., Choi K. Genotoxic potentials and related mechanisms of bisphenol A and other bisphenol compounds, a comparison study employing chicken DT40 cells. Chemosphere. 2013;93(2):434–440. doi: 10.1016/j.chemosphere.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Malla T.M., Ganesh N. Cytogenetic and tissue toxicity by synthetic sindoor in fresh water catfish Heteropneustes fossils. Biomed. Pharmacol. J. 2009;2(1):85–89. [Google Scholar]
- Mensah P.K., Palmer C.G., Muller W.J. Pesticides: Toxic Aspects. InTech Publica-tions; Rijeka, Croatia: 2014. Lethal and Sublethal Effects of Pesti-cides on Aquatic Organisms: the Case of a Freshwater Shrimp Exposure to Roundup®; pp. 163–185. [Google Scholar]
- Mesnage R., Defarge N., Spiroux de Vendômois J., Séralini G.E. Major pesticides are more toxic to human cells than their declared active principles. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres R., Mestres G. Deltamethrin, uses and environmental safety. Rev. Environ. Contam. Toxicol. 1992;24:1–18. doi: 10.1007/978-1-4612-2864-6_1. [DOI] [PubMed] [Google Scholar]
- Muralidharan L. Haemato-biochemical alterations induced by chronic exposure to fenthion in cyprinus carpio. Trends. Fish. Res. 2012;1:19–25. [Google Scholar]
- Muranli F.D.G., Güner U. Induction of micronuclei and nuclear abnormalities in erythrocytes of mosquito fish (Gambusia affinis) following exposure to the pyrethroid insecticide lambda-cyhalothrin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011;726(2):104–108. doi: 10.1016/j.mrgentox.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Musa S.O., Omoregie E. HAematological Changes in mudfish, Clarias gariepinus (Burchell) exposed to malachite green. J. Aquat. Sci. 1999;14:37–42. [Google Scholar]
- Nwani D.C., Ekwueme H.I., Ejere V.C., Peace S.O. Physiological effects of paraquat in juvenile African catfish Clarias gariepinus (Burchel 1822) J. Coastal Life Med. 2015;3(10):35–43. [Google Scholar]
- Ololade I.A., Oginni O. Toxic stress and hematological effects of nickel on African catfish, Clarias gariepinus, fingerlings. J. Environ. Chem. Ecotoxicol. 2010;2(2):14–19. [Google Scholar]
- Pakanit K., Kinchareon W. Hematological and biochemical responses of the Flowerhorn fish to hypoxia. J. Anim. Vet. Adv. 2011;10(20):2631–2638. [Google Scholar]
- Riaz-ul-Haq M., Javeed R., Iram S., Rasheed M.A., Amjad M., Iqbal F. Effect of Diafenthiuron exposure under short and long term experimental conditions on hematology, serum biochemical profile and elemental composition of a non-target organism, Labeo rohita. Environ. Toxicol. Pharmacol. 2018;62:40–45. doi: 10.1016/j.etap.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Rivero-Wendt C.L.G., Miranda-Vilela A.L., Ferreira M.F.N., Borges A.M., Grisolia C.K. Cytogenetic toxicity and gonadal effects of 17 α-methyltestosterone in Astyanax bimaculatus (Characidae) and Oreochromis niloticus (Cichlidae) Genet. Mol. Res. 2013;12(3):3862–3870. doi: 10.4238/2013.September.23.4. [DOI] [PubMed] [Google Scholar]
- Sabra F.S., Mehana E.S.E.D. Pesticides toxicity in fish with particular reference to insecticides. Asian J. Agricult. Food Sci. 2015;3(1):40–60. [Google Scholar]
- Sabzar A., Yousuf R.A., Balkhi M.H. An introduction about genotoxicity methods as tools for monitoring aquatic ecosystem, present status and future perspectives. Fish. Aquacult. J. 2016;7(158):2. [Google Scholar]
- Samprath K., Velamnial S., Kennedy I.J., James R. Haematological changes and their recovery in Oreochromis massambicus as a function of exposure period and sub-lethal levels of Ekalus. Acta Hydrobiol. Sin. 1993;35:73–83. [Google Scholar]
- Sankhla M.S., Kumari M., Sharma K., Kushwah R.S., Kumar R. Water contamination through pesticide & their toxic effect on human health. Int. J. Res. Appl. Sci. Eng. Technol. (IJRASET) 2018;6(1):967–970. [Google Scholar]
- Schmidt W. The micronucleus test. Mutat. Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- Shahi J., Singh A. Genotoxic and haematological effect of commonly used fungicide on fish Clarias batracus. J. Biol. Earth Sci. 2014;4(2):137–143. [Google Scholar]
- Singh N.N., Srivastava A.K. Haematological parameters as bioindicators of insecticide exposure in teleosts. Ecotoxicology. 2010;19(5):838–854. doi: 10.1007/s10646-010-0465-4. [DOI] [PubMed] [Google Scholar]
- Singh R.K., Singh R., L., Sharma B. Acute toxicity of carbofuran to a freshwater teleost, Clarias batrachus. Bull Environ Contam Toxicol. 2003;70(6):1259–1263. doi: 10.1007/s00128-003-0118-x. [DOI] [PubMed] [Google Scholar]
- Srivastava P., Singh A., Pandey A.K. Pesticides toxicity in fishes: biochemical, physiological and genotoxic aspects. Biochem. Cell. Arch. 2016;16(2):199–218. [Google Scholar]
- Suganthi P., Murali M., Sadiq Bukhari A., Syed Mohamed H.E., Basu H., Singhal R.K. Haematological studies on freshwater Tilapia treated with ZnO nanoparticles. J. Adv. Appl. Sci. Res. 2015;1:41–67. [Google Scholar]
- Svobodová Z., Pravda D., Paláčková J. Manuals of Research Institute of Fish Culture and Hydrobiology, Vodňany, Czech Republic, University of South Bohemia, 22. 1991. Unified methods of haematological examination of fish. [Google Scholar]
- Svobodová Z., Beklova M., Machala M., Drabek P., Dvorakova D., Kolarova J., Modra H. Evaluation of the effect of chemical substances, preparation, wastes and waste waters to organisms in the aquatic environment. Bull VURH Vodnany. 1996;32:76–96. [Google Scholar]
- Tang X., Zhu B., Katou H. A review of rapid transport of pesticides from sloping farmland to surface waters: processes and mitigation strategies. J. Environ. Sci. 2012;24(3):351–361. doi: 10.1016/s1001-0742(11)60753-5. [DOI] [PubMed] [Google Scholar]
- Tashkent I. Biodiversity Conservation National Strategy and Action Plan of Republic of Uzbekistan. 1998. Part 1, conditions and provisions for developing a national strategy for Biodiversity conservation. [Google Scholar]
- Tilak K.S., Veeraiah K., Butchiram M.S. Effect of phenol on haematological components of India major carps Catla, Labeo rohita and Cirrhinus mrigala. J. Environ. Biol. 2007;28:177–179. [PubMed] [Google Scholar]
- Ullah S., Begum M., Dhama K., Ahmad S., Hassan S., Alam I. Malathion induced DNA damage in freshwater fish, Labeo rohita (Hamilton, 1822) using alkaline single cell gel electrophoresis. Asian J. Anim. Vet. Adv. 2016;11(2):98–105. [Google Scholar]
- UNEP . United Nations Environment Programme, Chemin des Anemone; Geneva, Switzerland: 2005. Ridding the World of Persistent Organic Pollutants, A Guide to the Stockholm Convention on Persistent Organic Pollutants. [Google Scholar]
- USEPA . Pesticide Effects Determination, Environmental Fate and Effects Division Office of Pesticide Programs Washington, D.C. 20460. 2008. Risks of dimethoate use to the federally-listed California red legged frog (Rana aurora draytonii) [Google Scholar]
- Wells R.M., Baldwin J., Seymour R.S., Christian K., Brittain T. Red blood cell function and haematology in two tropical freshwater fishes from Australia. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2005;141(1):87–93. doi: 10.1016/j.cbpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Yu Y., Li C., Zhang X., Zhang X., Pang Y., Zhang S., Fu J. Route-specific daily uptake of organochlorine pesticides in food, dust, and air by Shanghai residents, China. Environ. Int. 2012;50:31–37. doi: 10.1016/j.envint.2012.09.007. [DOI] [PubMed] [Google Scholar]