Abstract
Background
Cisplatin (CP) is a common antineoplastic agent widely used to treat a broad spectrum of cancers. However, its usage for cancer treatment was restricted due to various side effects such as neurotoxicity, nephrotoxicity, hepatotoxicity and ototoxicity. Neurotoxicity in patients who have undergone a complete course of chemotherapy is clinically evident. CP administration caused problems in rats with memory and learning.
Methods
The effect of combination of CP with either thymoquinone (TQ) or geraniol (Ger) on cell viability of human breast cancer cells (MCF-7) was detected by MTT assay. Forty male Wistar albino rats, healthy and adult, were divided into four groups: normal control, CP-treated group, CP + TQ-treated group and CP + Ger-treated group.
Results
Our results demonstrated that prophylactic treatment with either TQ or Ger plus CP enhanced the anticancer effect of CP in MCF-7 cell line. In vivo study showed that CP-treated rats had higher depressives like behavior in open field and Morris water maze test while prophylactic treatment with either TQ or Ger and CP significantly enhanced the performance of depressive-like behavior. Also, histopathological evaluation of brain tissues proved the neurotoxic effect of CP and the possible protective activity of either TQ or Ger.
Conclusion
The findings of the present work revealed that TQ or Ger along with CP may enhance the antitumor effect of CP. Also, spontaneous administration of CP with either TQ or Ger as natural antioxidants may prevent CP-induced neurotoxicity in rats through diminishing the memory and learning impairment.
Keywords: Cisplatin, Thymoquinone, Geraniol, Neurotoxicity, Behavior test, Rats, Behavioral neuroscience, Oxidative stress, Antioxidant, Chemotherapy
Cisplatin; Thymoquinone; Geraniol; Neurotoxicity; Behavior test; Rats; Behavioral neuroscience; Behavioral test; Oxidative stress; Antioxidant; Chemotherapy.
1. Introduction
Cisplatin (CP), cis-diaminedichloroplatinum (II), is one of the most powerful antineoplastic agents used to treat a wide range of human malignancies such as testicular, ovarian, bladder, head and neck, cervical, esophageal and small cell lung cancer (Desoize and Madoulet, 2002; Chen et al., 2009; Amptoulach and Tsavaris, 2011). CP treatment is hampered by significant side effects such as neurotoxicity, nephrotoxicity, ototoxicity and vomiting (Yang et al., 2011; Hinduja et al., 2015; Liu et al., 2014; Wood et al., 2014). Thirty percent of CP-treated patients suffered from neurotoxicity because CP can cross the blood-brain barrier and accumulate in repeated doses (Namikawa et al., 2000; Cavaletti, 2008; Windebank and Grisold, 2008).
Cancer patients undergoing chemotherapy treatment have been shown symptoms of depression and anxiety with an incidence of 12–24% (Linden et al., 2012). Furthermore, following exposure to CP treatment direct neurotoxicity is a common and most often dose-limiting complication in the form of changes in acute consciousness, cerebral infarctions, neuropathy, seizures, paralysis and ototoxicity (Beinert et al., 2000; Cavaliere and Schiff, 2006). It was reported that CP damages the mitochondria and causing neurotoxicity (Waseem and Parvez, 2013).
Many studies have revealed that antioxidant supplementation prevents neurotoxicity associated with platinum-based chemotherapy that works together to increase anticancer activity and reduce adverse effects (Pace et al., 2010; Mendonça et al., 2013; Turan et al., 2014).
Thymoquinone (TQ) is the primary bioactive constituent derived from Nigella sativa (NS). In the Middle and the Far East, the NS seeds were used as a traditional medicine for treating a broad spectrum of diseases (Salem, 2005). TQ is a member of the bioflavonoids that has several properties such as antioxidant, anti-inflammatory, neuroprotective, antiallergenic, antiviral, anti-diabetic and anti-carcinogenic activities (Budancamanak et al., 2006; Tekeoglu et al., 2007; Ashraf et al., 2011; Badr et al., 2011; Kanter, 2011). It has been noted that TQ neutralizes oxygen-free radicals i.e acts as a scavenger and increases antioxidant enzyme activities (Nader et al., 2010). The second natural antioxidant drug used in this study is geraniol (Ger). Ger (3,7 dimethyl-2,6 octadien-1-ol) is monoterpene alcohol which naturally found in small amounts in geranium, lemon and other essential oils from medicinal plants and is the aromatic component of many cosmetic products (Vieira et al., 2011). Ger has been reported to have many therapeutic characteristics including its antioxidant and anticancer potential (Tiwari and Kakkar, 2009; Ahmad et al., 2011).
Based on this data, the main purpose of this study was to evaluate the protective effect of prophylactic treatment with either TQ or Ger against neurotoxicity induced by CP through evaluating behavioral changes in rats. Our findings support issues faced by TQ's or Ger's neuroprotective effects against CP-induced neurotoxicity in rats.
2. Materials and methods
2.1. Chemicals
Cisplatin was purchased from Mylan S.A.S. (Saint-Priest, France). TQ and Ger were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Corn oil has been purchased from the local market. Both glasses and plastic were washed and rinsed with distilled water. Dimethyl sulfoxide (DMSO), trypan and 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) blue dye were obtained from Sigma (St. Louis, Mo., USA). Fetal Bovine serum, L-glutamine, RPMI-1640, gentamycin, HEPES buffer solution and 0.25% Trypsin-EDTA were purchased from Lonza (Belgium).
2.2. Mammalian breast cancer cell lines
MCF-7 cells (the cell line of human breast cancer) were collected from American Type Tissue Culture. The cells were propagated in Dulbecco's modified Eagle medium (DMEM) with 10% heat-inactivated bovine fetal serum, 50 μg/ml gentamycin and 1% L-glutamine, HEPES buffer; these cells were kept at 37°C in a humidified atmosphere with 5% CO2 and subcultured twice a week.
2.3. Assay of cell viability
First Experiment: CP, Ger and TQ: each drug was used as a treatment alone against the MCF-7 cells for 48 h.
Second Experiment: Ger and TQ were tested at the IC50 concentration in combination with different concentrations (2, 5, 10, 20, 40 and 80 μg/ml) of CP compared with CP, TQ and Ger alone against the MCF-7 cells for 48 h.
2.4. Cytotoxicity evaluation using MTT viability assay
The cells were seeded in 96-well plates at a cell concentration of -1×104 cells per well in a growth medium of 100 μl for cytotoxicity assay. After 24 h of seeding the fresh medium containing different test sample concentrations was applied. Using a multichannel pipette, the required concentrations from the tested compound were applied to convergent cell monolayers dispensed into 96-well, flat-bottomed microtiter plates (Falcon, NJ, USA). The microtiter plates were incubated at 37°C in a humidified incubator with 5% CO2 for a period of 48 h. Three wells were used for each concentration of the test sample. Control cells were incubated without test samples and with or without DMSO. The little percentage of DMSO present in the wells (maximal 0.1%) was found not to affect the experiment. After incubation of the cells at 37°C for 48 h, the viable cells yield was determined by a colorimetric MTT method.
In summary, the medium was withdrawn from the 96 well plates and substituted for each well with 100 μl fresh culture medium RPMI 1640 without phenol red then 10 μl of 12 mM MTT stock solution (5 mg MTT in 1 mL PBS) including untreated controls. Next, the 96 well plates were incubated for 4 h at 37°C and 5% CO2. The wells were extracted from an 85 μl media aliquot and added 50 μl of DMSO to each well thoroughly mixed with the pipette and incubated for 10 min at 37°C. The optical density was then determined at 590 nm using the microplate reader (Sunrise, TECAN, Inc, USA) to estimate the number of viable cells and the viability percentage was calculated at [ODt/ODc] x 100% where ODt is the mean optical density of wells treated with the sample and ODc is the mean optical density of untreated cells. The correlation between the surviving cells and the concentration of drugs was drawn after treatment with the specified compound to achieve the survival curve of tumor cell line (Mosmann, 1983; Gomha et al., 2015).
2.5. Animals
In the current study, forty healthy adult male Wistar albino rats weighing 150–200 g; 3 months old were obtained from El Nahda University's Animal House (Beni-Suef, Egypt). They were placed in stainless steel cages at a well-ventilated animal house. They were held under normal laboratory conditions (12 h light/dark cycle, 24 ± 1°C) for at least one week for adaptation before the experiment and all of these conditions were maintained until the end of the study with sufficient food and water. The current study protocol was carried out in line with the protocol accepted by the Experimental Animal Ethics Committee of the University of Beni-Suef (BSU/FS/2018/13).
2.6. Experimental design
The rats were divided into four groups each containing ten rats and treated as follows:
Group I (normal control group): rats received 2 ml/kg/day corn oil orally for 33 consecutive days (Geng et al., 2012) and injected with saline 2 ml/kg intraperitoneally (i.p.) twice a week (a total of nine injections).
Group II: rats received CP 2 mg/kg body weight i.p. twice a week starting on the 5th day for 33 days (a total of nine injections) (Al Moundhri et al., 2013).
Group III: rats received TQ 20 mg/kg body weight orally in corn oil three times a week for 33 days (Wilson et al., 2015) as well as CP as mentioned before in group II.
Group IV: rats were received Ger 100 mg/kg body weight orally in corn oil for 33 consecutive days (Wang et al., 2016) as well as CP as mentioned before in group II.
2.7. Behavioral tests
2.7.1. Open field test
The experiment was carried out in a wooden square box (80 × 80 × 40 cm) divided into 16 equal squares (20 × 20 cm) at the bottom. Each rat was located in the open field center individually and permitted for 3 min of free exploration of the area. After each test, the floor and walls were cleaned to avoid potential bias due to the odors of previous rats. For later off-line evaluation, a video camera was set at the top of the box to monitor rat behavior. The number of squares passed (ambulation) and the number of times the animal standing on the hind limbs (rearing), as well as the actual time during monitoring due to lack of movement (immobility), were recorded (Kafkafi, 2003; Onaolapo et al., 2015).
2.7.2. Morris water maze test
Morris water-maze (MWM) test is the most widely used behavioral model to test rodent spatial memory and learning (Morris et al., 1982). In the last week of the experiment, MWM was carried out as previously explained elsewhere (Vorhees and Williams, 2006). Rats were thus put in a circular pool and allowed in the training session to swim freely for 60 s. The animals were given five daily trials from each 3 starting points daily. Rats were educated in each trial to learn the location of the hidden platform based on visual signs outside the maze. The animals that are unable to find the hidden platform within 60 s were directed to the platform and permitted to stay 15 s on the platform (acquisition trials).
At the end of each training trial, the latency to find the escape platform (sec) was recorded. The test was done on the sixth day; the escape platform was excluded and placed rats in the pool for a total duration of 60 s. The time to reach the hidden platform and the time spent in the target quadrant (sec) were calculated in these probe trials. The digital camera was fixed above the maze to monitor the animals during the different stages of the test.
2.8. Histological examination
Brain samples from three animals of each group were fixed into 10% neutral formalin and were embedded in paraffin. The sections were cut into 4–6 μm thickness, mounted on slides and stained with hematoxylin and eosin (H&E). Histopathological changes in the brains were evaluated by assessing the morphological changes in the sections using a standard technique (Bancroft and Gamble, 2008).
2.9. Statistical analysis
All measurements were performed using SPSS 16.0 (SPSS, Chicago, Ill, USA) computer program. The differences between test groups were analyzed using the ANOVA followed by the Tukey post-hoc test where p < 0.05 was considered significant. The data as clearly indicated is reported as mean ± standard error (SE) values. All graphs were plotted using Graph pad Prism software (San Diego, CA. USA).
3. Results
3.1. Cell viability
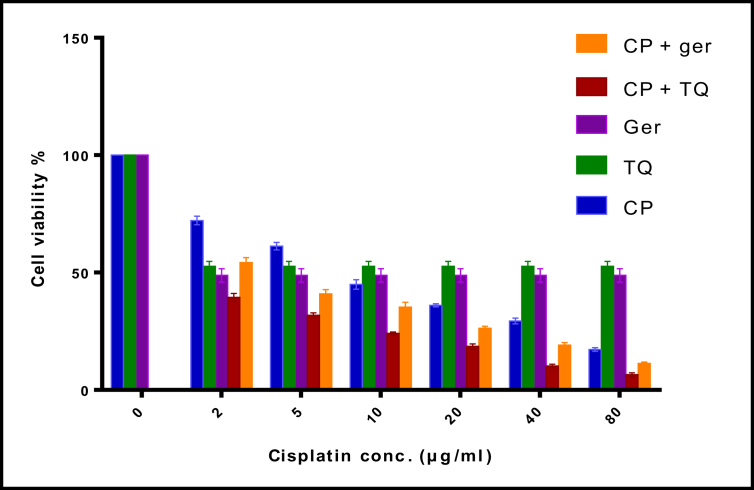
In this study, MCF-7 cells were treated with various concentrations of CP (2, 5, 10, 20, 40 or 80 μg/ml) and their viability was measured by MTT assay for 48 h (Figure 1). We aimed to determine whether prophylactic treatment with TQ or Ger can affect the antitumor activity of CP or not in MCF-7 cells in vitro. First, the combination of TQ or Ger plus CP was tested in cell viability assay compared to CP, TQ and Ger alone. The drug combination induced synergistic inhibitory effects on cell viability. The anticancer effect of CP did not decrease with either TQ or Ger treatment. The maximum concentration of TQ that could be safely used in cell line is 15 μg/ml and 420 μg/ml for Ger and the maximum concentration of CP is 1 μg/ml. Our results showed that CP had a dramatic effect on the viability of MCF-7 cells with an IC50 of 1.06 μg/ml. These results suggested that cell viability could be significantly decreased with increasing CP, TQ and Ger concentrations.
Figure 1.
MTT assay results of cell viability for MCF-7 cell line after treatment with CP, TQ and Ger for 48 h. MCF-7 cells were incubated with (2, 5, 10, 20, 40 or 80 μg/ml) CP, 15 μg/ml TQ and 420 μg/ml Ger for 48 h. The values are presented as the mean ± SE. CP: cisplatin; TQ: thymoquinone; Ger: geraniol.
3.2. Effect of prophylactic treatment with either TQ or Ger on rats behavior in the open field
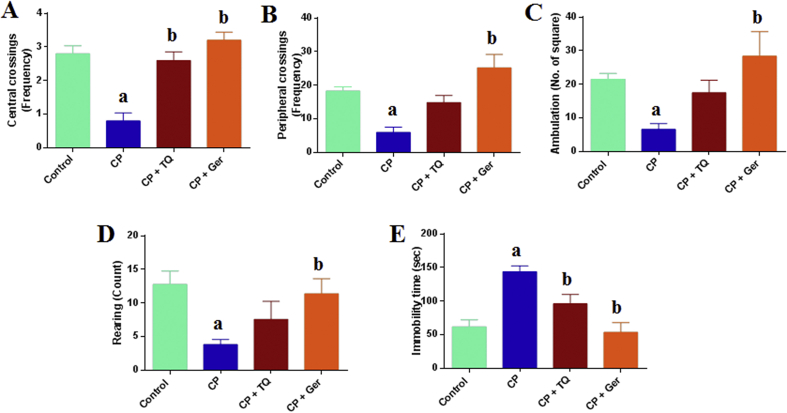
Cisplatin-treated rats showed an elevation in depression-like activities. Our results demonstrated that CP treatment caused a significant reduction in central crossings (F = 17.53, p < 0.05), peripheral crossing (F = 10.54, p < 0.05), ambulation (F = 13.30, p < 0.05) and rearing frequencies (F = 6.61, p < 0.05) along with a significant increase in immobility time compared to the normal control group (F = 22.42, p < 0.05). Prophylactic treatment with TQ or Ger reduced the aforementioned behavioral changes in the CP-treated rats. TQ treatment caused a significant increase in the central crossings and insignificant elevation of peripheral crossings, ambulation and rearing while the administration of Ger significantly increased the central crossings, peripheral crossing, ambulation and rearing, whereas both drugs significantly decreased the immobility time in the open field test as compared to the CP-treated group (Figure 2).
Figure 2.
Effect of prophylactic treatments of TQ or Ger against CP-induced changes in rats' behavior in the open field test. (A) Central crossings, (B) Peripheral crossings, (C) Ambulation, (D) Rearing and (E) Immobility time. The data are presented as the mean ± SE of 10 rats. ap < 0.05 versus control group. bp < 0.05 versus CP group. CP: cisplatin; TQ: thymoquinone; Ger: geraniol.
3.3. Effect of prophylactic treatment with either TQ or Ger on rats behavior in the Morris water maze test
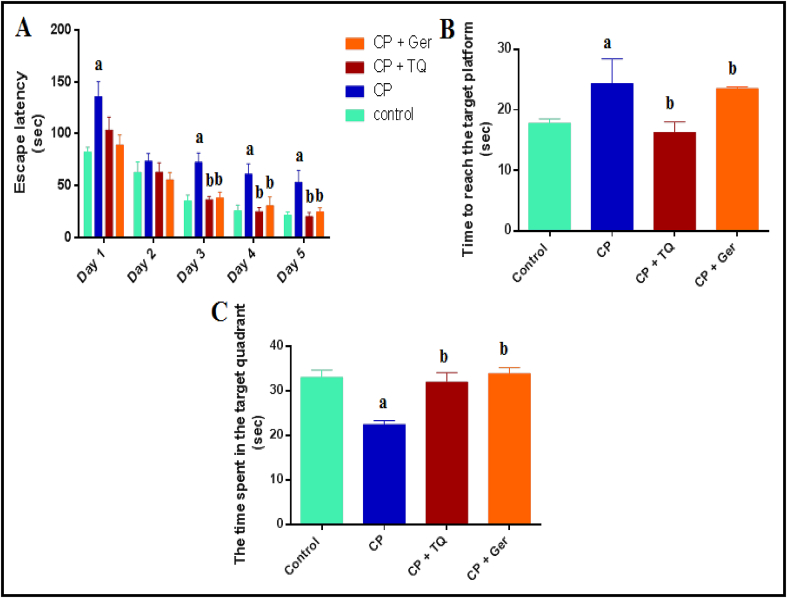
On the first training trial, the mean escape latency did not distinguish among the groups and the CP-treated rats demonstrated insignificant longer escape latencies when compared to the normal control, TQ- and Ger-treated groups in successive training trials (F = 6.97, F = 2.24, p < 0.05). Additionally, TQ and Ger supplementation in the CP-treated animals significantly decreased mean latency in the last three days of the training session (F = 25.01, F = 14.31, F = 18.99, p < 0.05). In the probe trial, after elimination of the platform, the time to find the target platform in the CP-treated group was significantly longer than those treated with either TQ or Ger (F = 7.89, p < 0.05). The time spent in the target quadrant was significantly reduced in the CP-treated animals compared to the normal control group while prophylactic treatment with TQ or Ger plus CP significantly (F = 18.70, p < 0.05) increased the time spent in the target quadrant compared to that of the CP-treated group (Figure 3).
Figure 3.
Effect of prophylactic treatments of TQ or Ger against CP-induced changes in rats' behavior in the Morris water maze test. (A) Escape latency in the first training trials (sec), (B) Time to reach the target platform in the probe trial (sec) and (C) The time spent in the target quadrant. The data are presented as the mean ± SE of 10 rats. ap < 0.05 versus control group. bp < 0.05 versus CP group. CP: cisplatin; TQ: thymoquinone; Ger: geraniol.
3.4. Brain histopathology
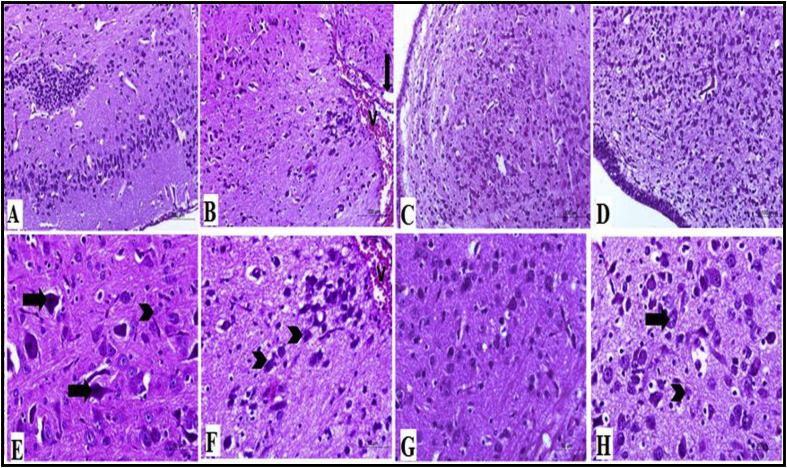
Histopathological examination of the rat cerebral tissues showed that CP treatment caused a significant damage to the brain tissues with congested cerebral blood vessels and degeneration of nerve cells (Figure 4B & F) when compared to normal control group which had normal cerebral tissue with normal neurons and neuroglia (Figure 4A & E), had a cerebral tissue. TQ treatment had normal and degenerated nerve cells and neuroglia (Figure 4C & G) and Ger-treated group also showed normal nerve cells and neuroglia cells and degenerated neurons (Figure 4D & H).
Figure 4.
Histopathological examination of the brain tissues showing cerebral tissues of different studied groups. (A) Brain tissues of the normal control group showing nerve cells and neuroglia cells appeared with normal structure, H&E stain X100, (E) higher magnification of (A), showing normal nerve cells (arrow) and normal neuroglia (arrowhead), H&E stain X400. (B) Brain tissues of CP-treated group showing congestion (V) and edema (arrow) of the cerebral blood vessels, H&E stain X100, (F) higher magnification of (B), showing congestion of blood vessels (V), sever degeneration of nerve cells (arrowhead), H&E stain X400. (C) Brain tissues of CP + TQ-treated group showing numerous nerve cells are normal with few degenerated nerve cells, H&E stain X100, (G) higher magnification of (C), showing normal nerve cells and neuroglia cells, H&E stain X400. (D) CP + Ger-treated group, brain tissues showing normal and degenerated nerve cells and neuroglia, H&E stain X100, (H) higher magnification of (D), showing normal nerve cells and neuroglia cells (arrow) and degenerated neurons (arrowhead), H&E stain X400. CP: cisplatin; TQ: thymoquinone; Ger: geraniol.
4. Discussion
Cisplatin is considered one of the most important and effective antineoplastic drug used for the treatment of numerous types of malignancies. Although its effectiveness it caused serious side effects as neurotoxicity, a main adverse effect resulted in changes in behavior and memory functions in patients who completed CP therapy. CP interacts with DNA forming DNA-Pt adducts resulted in DNA damage, crosslinking and mitochondrial dysfunction leading to apoptosis. Cognitive impairment is a neurological complication of CP chemotherapy, but the underlying mechanisms are not fully explained. It has been demonstrated that CP can pass the BBB under changed physiological conditions such as short-term hypoxia (Minami et al., 1996). The degeneration of the Purkinje cell line in the cerebellar cortex after CP treatment also suggests that CP may penetrate the BBB to some degree (Abou-Elghait et al., 2010).
It is well established that the energy levels and oxidative stress in the cell explain the progressive cell division or apoptotic cell death. The cell undergoes necrosis when the stress level within the cell exceeds a certain limit with a higher energy deficit while apoptosis occurs when the stress is moderate with a moderate energy level (Lieberthal et al., 1996; Hassan et al., 2010, 2012). Motor and cognitive disorders and abnormal behaviors have also been reported in rats as a result of changes in hippocampal and cerebellar functions (Shabani et al., 2012).
In the present study, MTT assay confirmed that the cell viability was markedly decreased following CP treatment. Meanwhile, the prophylactic treatment with either TQ or Ger plus CP reduced the cell viability in MCF-7 cells and did not alter the antitumor activity of CP.
In this work, we illustrated the mechanisms of CP-induced neurotoxicity by using a rat model to evaluate the effects on neuronal integrity and cognitive function of CP regimens. Our findings revealed that chronic treatment with CP affected the functions of learning and memory in rats. The open field and the MWM tests were used in the present work to measure the functions of learning and memory in rats. We have demonstrated some behavioral tests in this study to evaluate the effect of CP on rat memory and learning activities and the role of natural antioxidant drugs in amelioration of the memory and learning problems.
These results were in an agreement with those of previous studies of (Oz et al., 2015; Abdelkader et al., 2017) which demonstrated that CP treatment-induced learning and memory problems in rats in the open field and the MWM tests, respectively.
TQ the natural main bioactive component of NS has been found to exhibit antioxidant and anti-inflammatory activities as it acts as a free radical scavenger augmented by many factors, such as its quinone structure's redox properties and its ability to cross biological barriers (Badary et al., 2003; Ragheb et al., 2009). Moreover, the beneficial effect of NS on cognition, attention and memory was documented by other researches (Sayeed et al., 2013). Geraniol is an acyclic monoterpenoid and is the major ingredient of rose oil and palmarosa oil and the main component of ginger, lemon, lime, lavender, nutmeg, orange and rose essential oil. In several previous studies, Ger has antioxidant, antitumor and anti-inflammatory properties (Manoharan and Vasantha Selvan, 2012; Chaudhary et al., 2013; Khan et al., 2013; de Carvalho et al., 2014; Soubh et al., 2015).
Recently, our study (Kandeil et al., 2019) proved that the neuroprotective effects of TQ or Ger against CP-induced neurotoxicity in rats were through downregulating of gene expression of some apoptotic markers including p38 mitogen-activated protein kinase (MAPK), STAT-1, p53, p21, MMP9 and FMO3. In addition, the treatment with either TQ or Ger as natural antioxidants protected against cisplatin-induced oxidative stress in brain tissues via increasing the activity of antioxidant enzyme superoxide dismutase (SOD), reducing myeloperoxidase activity, inhibition of lipid peroxidation by decreasing 8-isoprostane levels besides the brain function biomarkers such as glutamate levels and lactate dehydrogenase (LDH) activity which were reduced when compared to CP-treated rats.
According to the present results, the prophylactic treatment with either TQ or Ger alleviated CP-induced neurotoxicity and cognitive impairments in rats via improving behavioral changes, learning and memory deficits. These effects may be due to their potent antioxidant activities.
According to the previous findings (Abdel-Zaher et al., 2017) that reported the neuroprotective efficacy of TQ against pentylenetetrazole (PTZ) which caused learning and cognitive impairment of mice through its antioxidant activity and improved memory deficits in MWM test. TQ administration plus PTZ resulted in the attenuation of nitric oxide (NO) overproduction induced by inducible NO synthase and alleviated oxidative stress through the reduction of the malondialdehyde (MDA) and nitrite besides increasing the glutathione (GSH) level and glutathione peroxidase activity in the brain.
Also, Khan et al. (2012) indicated the neuroprotective properties of TQ against β-amyloid-induced neurotoxicity in rats through the restoration of elevated AChE activity, decreasing NO production, reducing lipid peroxidation and increasing the GSH levels, glutathione peroxidase and glutathione reductase activities in brain tissues which lead to prevention of disorders of central nervous system and improving learning and memory deficits.
As mentioned before, the protective effect of TQ and Ger may be due to their antioxidant effect as reported by our group (Kandeil et al., 2019) that attributed the neuroprotective effects of both natural compounds against CP-induced neurotoxicity in rats to their antioxidant activities through reducing the glutamate levels and LDH activity, the main brain function marker, and enhancing the activity of SOD in brain tissues.
The neuroprotective effect of Ger discussed in previous studies (Rekha et al., 2013; Prasad, 2014) revealed that Ger administration was found to markedly decrease the elevated MDA, increase GSH levels and protect dopaminergic neurons, through increasing the dopamine formation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson's disease in mice and acrylamide-induced behavioral deficits in rats respectively resulted in improving the behavioral problems.
In this study, the histolopathological examination of the brain tissues also confirmed the brain damage after CP administration and the protective effect of TQ or Ger against CP-induced neurotoxicity in rats.
Considering the possible neuroprotective and antioxidant activities of both TQ or Ger as discussed above, the present study has been established to research the potential protective effects of these agents against CP-induced neurotoxicity in rats.
5. Conclusion
In conclusion, the current study proved that TQ or Ger did not alter the anticancer effect of CP in human breast cancer cells besides the protective action of TQ or Ger against CP-induced neural damage through amelioration of the learning and memory impairments. Thus prophylactic treatment with TQ or Ger plus CP offered a good adjuvant therapy for the treatment of cognitive impairments induced by CP.
Declarations
Author contribution statement
Mohamed A. Kandeil: Conceived and designed the experiments.
Mohamed O. Mahmoud: Analyzed and interpreted the data; Wrote the paper.
Safaa B. Gomaa: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors would like to thank Dr. Abdel-Razik H. Abdel-Razik, Department of Histology, Faculty of Veterinary Medicine, Beni-Suef University for his assessment in the histopathological study.
References
- Abdel-Zaher A.O., Farghaly H.S., Farrag M.M., Abdel-Rahman M.S., Abdel-Wahab B.A. A potential mechanism for the ameliorative effect of thymoquinone on pentylenetetrazole-induced kindling and cognitive impairments in mice. Biomed. Pharmacother. 2017;88:553–561. doi: 10.1016/j.biopha.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Abdelkader N.F., Saad M.A., Abdelsalam R.M. Neuroprotective effect of nebivolol against cisplatin-associated depressive-like behavior in rats. J. Neurochem. 2017;141(3):449–460. doi: 10.1111/jnc.13978. [DOI] [PubMed] [Google Scholar]
- Abou-Elghait A., El-Gamal D.A., Abdel-Sameea A.R., Mohamed A.A. Effect of cisplatin on the cerebellar cortex and spinal cord of adult male albino rat and the possible role of vitamin E: light and electron microscopic study. Jew Hist. 2010;33(2):202–212. [Google Scholar]
- Ahmad S.T., Arjumand W., Seth A., Nafees S., Rashid S., Ali N., Sultana S. Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways. Toxicology. 2011;290(1):69–81. doi: 10.1016/j.tox.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Al Moundhri M.S., Al-Salam S., Al Mahrouqee A., Beegam S., Ali B.H. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: some behavioral, biochemical, and histopathological studies. J. Med. Toxicol. 2013;9(1):25–33. doi: 10.1007/s13181-012-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amptoulach S., Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemo. Res. Practice. 2011;2011:843019. doi: 10.1155/2011/843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S.S., Rao M.V., Kaneez F.S., Qadri S., Al-Marzouqi A.H., Chandranath I.S., Adem A. Nigella sativa extract as a potent antioxidant for petrochemical-induced oxidative stress. J. Chromatogr. Sci. 2011;49(4):321–326. doi: 10.1093/chrsci/49.4.321. [DOI] [PubMed] [Google Scholar]
- Badary O.A., Taha R.A., Gamal El-Din A.M., Abdel-Wahab M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2003;26(2):87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- Badr G., Alwasel S., Ebaid H., Mohany M., Alhazza I. Perinatal supplementation with thymoquinone improves diabetic complications and T cell immune responses in rat offspring. Cell. Immunol. 2011;267(2):133–140. doi: 10.1016/j.cellimm.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bancroft J., Gamble A. sixth ed. Churchill-Livingstone; Edinburgh, London, Melbourne, New York: 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- Beinert T., Masuhr F., Mwela E., Schweigert M., Flath B., Harder H., Binder D., Oehm C., Behse F., Possinger K. Neuropathy under chemotherapy. Eur. J. Med. Res. 2000;5(10):415–423. [PubMed] [Google Scholar]
- Budancamanak M., Kanter M., Demirel A., Ocakci A., Uysal H., Karakaya C. Protective effects of thymoquinone and methotrexate on the renal injury in collagen-induced arthritis. Arch. Toxicol. 2006;80(11):768–776. doi: 10.1007/s00204-006-0094-0. [DOI] [PubMed] [Google Scholar]
- Cavaletti G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat. Rev. Canc. 2008;8(1):72. doi: 10.1038/nrc2167-c1. 72. [DOI] [PubMed] [Google Scholar]
- Cavaliere R., Schiff D. Neurologic toxicities of cancer therapies. Curr. Neurol. Neurosci. Rep. 2006;6(3):218–226. doi: 10.1007/s11910-006-0009-8. [DOI] [PubMed] [Google Scholar]
- Chaudhary S.C., Siddiqui M.S., Athar M., Alam M.S. Geraniol inhibits murine skin tumorigenesis by modulating COX-2 expression, Ras-ERK1/2 signaling pathway and apoptosis. J. Appl. Toxicol. 2013;33(8):828–837. doi: 10.1002/jat.2739. [DOI] [PubMed] [Google Scholar]
- Chen D., Milacic V., Frezza M., Dou Q.P. Metal complexes, their cellular targets and potential for cancer therapy. Curr. Pharmaceut. Des. 2009;15(7):777–791. doi: 10.2174/138161209787582183. [DOI] [PubMed] [Google Scholar]
- de Carvalho K.I.M., Bonamin F., dos Santos R.C., Périco L.L., Beserra F.P., de Sousa D.P., Barbosa Filho J.M., da Rocha L.R.M., Hiruma-Lima C.A. Geraniol—a flavoring agent with multifunctional effects in protecting the gastric and duodenal mucosa. N. Schmied. Arch. Pharmacol. 2014;387(4):355–365. doi: 10.1007/s00210-013-0947-z. [DOI] [PubMed] [Google Scholar]
- Desoize B., Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol.-Hematol. 2002;42(3):317–325. doi: 10.1016/s1040-8428(01)00219-0. [DOI] [PubMed] [Google Scholar]
- Geng X.C., Li B., Zhang L., Song Y., Lin Z., Zhang Y.Q., Wang J.Z. Corn oil as a vehicle in drug development exerts a dose-dependent effect on gene expression profiles in rat thymus. J. Appl. Toxicol. 2012;32(10):850–857. doi: 10.1002/jat.2773. [DOI] [PubMed] [Google Scholar]
- Gomha S.M., Riyadh S.M., Mahmmoud E.A., Elaasser M.M. Synthesis and anticancer activities of thiazoles, 1, 3-thiazines, and thiazolidine using chitosan-grafted-poly (vinylpyridine) as basic catalyst. Heterocycles. 2015;91(6):1227–1243. [Google Scholar]
- Hassan I., Chibber S., Khan A.A., Naseem I. Riboflavin ameliorates cisplatin induced toxicities under photoillumination. PloS One. 2012;7(5) doi: 10.1371/journal.pone.0036273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I., Chibber S., Naseem I. Ameliorative effect of riboflavin on the cisplatin induced nephrotoxicity and hepatotoxicity under photoillumination. Food Chem. Toxicol. 2010;48(8-9):2052–2058. doi: 10.1016/j.fct.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Hinduja S., Kraus K.S., Manohar S., Salvi R.J. d-Methionine protects against cisplatin-induced neurotoxicity in the hippocampus of the adult rat. Neurotox. Res. 2015;27(3):199–204. doi: 10.1007/s12640-014-9503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkafi N. Extending SEE for large-scale phenotyping of mouse open-field behavior. Behav. Res. Methods Instrum. Comput. 2003;35(2):294–301. doi: 10.3758/bf03202555. [DOI] [PubMed] [Google Scholar]
- Kandeil M.A., Mahmoud M.O., Abdel-Razik A.-R.H., Gomaa S.B. Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci. 2019;228:145–151. doi: 10.1016/j.lfs.2019.04.065. [DOI] [PubMed] [Google Scholar]
- Kanter M. Protective effects of thymoquinone on the neuronal injury in frontal cortex after chronic toluene exposure. J. Mol. Histol. 2011;42(1):39–46. doi: 10.1007/s10735-010-9305-3. [DOI] [PubMed] [Google Scholar]
- Khan A., Vaibhav K., Javed H., Khan M.M., Tabassum R., Ahmed M.E., Srivastava P., Khuwaja G., Islam F., Siddiqui M.S. Attenuation of Aβ-induced neurotoxicity by thymoquinone via inhibition of mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2012;369(1-2):55–65. doi: 10.1007/s11010-012-1368-x. [DOI] [PubMed] [Google Scholar]
- Khan A.Q., Khan R., Qamar W., Lateef A., Rehman M.U., Tahir M., Ali F., Hamiza O.O., Hasan S.K., Sultana S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: possible role of p38 MAP Kinase and NF-κB. Exp. Mol. Pathol. 2013;94(3):419–429. doi: 10.1016/j.yexmp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Lieberthal W., Triaca V., Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am. J. Physiol. Ren. Physiol. 1996;270(4):F700–F708. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- Linden W., Vodermaier A., MacKenzie R., Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012;141(2-3):343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang H., Zhang Y., Li N., Wen Y., Cao F., Ai H., Xue X. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol. Cell. 2014;37(12):865–872. doi: 10.14348/molcells.2014.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan S., Vasantha Selvan M. Chemopreventive potential of geraniol in 7, 12-dimethylbenz (a) anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. J. Environ. Biol. 2012;33(2):255–260. [PubMed] [Google Scholar]
- Mendonça L.M., da Silva Machado C., Teixeira C.C.C., de Freitas L.A.P., Bianchi M.d.L.P., Antunes L.M.G. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 2013;34:205–211. doi: 10.1016/j.neuro.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Minami T., Ichii M., Okazaki Y. Detection of platinum in the brain of mice treated with cisplatin and subjected to short-term hypoxia. J. Pharm. Pharmacol. 1996;48(5):505–509. doi: 10.1111/j.2042-7158.1996.tb05962.x. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Garrud P., Rawlins J.a., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nader M.A., El-Agamy D.S., Suddek G.M. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm. Res. (Seoul) 2010;33(4):637–643. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- Namikawa K., Asakura M., Minami T., Okazaki Y., Kadota E., Hashimoto S. Toxicity of cisplatin to the central nervous system of male rabbits. Biol. Trace Elem. Res. 2000;74(3):223–235. doi: 10.1385/BTER:74:3:223. [DOI] [PubMed] [Google Scholar]
- Onaolapo O.J., Onaolapo A.Y., Akanmu M.A., Olayiwola G. Foraging enrichment modulates open field response to monosodium glutamate in mice. Ann. Neurosci. 2015;22(3):162–170. doi: 10.5214/ans.0972.7531.220306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M., Atalik K.E.N., Yerlikaya F.H., Demir E.A. Curcumin alleviates cisplatin-induced learning and memory impairments. Neurobiol. Learn. Mem. 2015;123:43–49. doi: 10.1016/j.nlm.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Pace A., Giannarelli D., Galie E., Savarese A., Carpano S., Della Giulia M., Pozzi A., Silvani A., Gaviani P., Scaioli V. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology. 2010;74(9):762–766. doi: 10.1212/WNL.0b013e3181d5279e. [DOI] [PubMed] [Google Scholar]
- Prasad S.N. Mitigation of acrylamide-induced behavioral deficits, oxidative impairments and neurotoxicity by oral supplements of geraniol (a monoterpene) in a rat model. Chem. Biol. Interact. 2014;223:27–37. doi: 10.1016/j.cbi.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Ragheb A., Attia A., Eldin W.S., Elbarbry F., Gazarin S., Shoker A. The protective effect of thymoquinone, an anti-oxidant and anti-inflammatory agent, against renal injury: a review. Saudi J. Kidney Dis. Transplant. 2009;20(5):741–752. [PubMed] [Google Scholar]
- Rekha K.R., Selvakumar G.P., Sethupathy S., Santha K., Sivakamasundari R.I. Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson’s disease. J. Mol. Neurosci. 2013;51(3):851–862. doi: 10.1007/s12031-013-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharm. 2005;5(13-14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Sayeed M.S.B., Asaduzzaman M., Morshed H., Hossain M.M., Kadir M.F., Rahman M.R. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J. Ethnopharmacol. 2013;148(3):780–786. doi: 10.1016/j.jep.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Shabani M., Nazeri M., Parsania S., Razavinasab M., Zangiabadi N., Esmaeilpour K., Abareghi F. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology. 2012;33(5):1314–1321. doi: 10.1016/j.neuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Soubh A.A., Abdallah D.M., El-Abhar H.S. Geraniol ameliorates TNBS-induced colitis: involvement of Wnt/β-catenin, p38MAPK, NFκB, and PPARγ signaling pathways. Life Sci. 2015;136:142–150. doi: 10.1016/j.lfs.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Tekeoglu I., Dogan A., Ediz L., Budancamanak M., Demirel A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res.: Int. J. Devoted Pharmacol. Toxicol. Eval. Natural Product Derivatives. 2007;21(9):895–897. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- Tiwari M., Kakkar P. Plant derived antioxidants–geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitro. 2009;23(2):295–301. doi: 10.1016/j.tiv.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Turan M., Cayir A., Cetin N., Suleyman H., Turan I.S., Tan H. An investigation of the effect of thiamine pyrophosphate on cisplatin-induced oxidative stress and DNA damage in rat brain tissue compared with thiamine: thiamine and thiamine pyrophosphate effects on cisplatin neurotoxicity. Hum. Exp. Toxicol. 2014;33(1):14–21. doi: 10.1177/0960327113485251. [DOI] [PubMed] [Google Scholar]
- Vieira A., Heidor R., Cardozo M., Scolastici C., Purgatto E., Shiga T., Barbisan L., Ong T., Moreno F. Efficacy of geraniol but not of β-ionone or their combination for the chemoprevention of rat colon carcinogenesis. Braz. J. Med. Biol. Res. 2011;44(6):538–545. doi: 10.1590/s0100-879x2011007500037. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhao S., Su M., Sun L., Zhang S., Wang D., Liu Z., Yuan Y., Liu Y., Li Y. Geraniol improves endothelial function by inhibiting NOX-2 derived oxidative stress in high fat diet fed mice. Biochem. Biophys. Res. Commun. 2016;474(1):182–187. doi: 10.1016/j.bbrc.2016.04.097. [DOI] [PubMed] [Google Scholar]
- Waseem M., Parvez S. Mitochondrial dysfunction mediated cisplatin induced toxicity: modulatory role of curcumin. Food Chem. Toxicol. 2013;53:334–342. doi: 10.1016/j.fct.2012.11.055. [DOI] [PubMed] [Google Scholar]
- Wilson A.J., Saskowski J., Barham W., Yull F., Khabele D. Thymoquinone enhances cisplatin-response through direct tumor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015;8(1):46. doi: 10.1186/s13048-015-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank A.J., Grisold W. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- Wood J.W., Bas E., Gupta C., Selman Y., Eshraghi A., Telischi F.F., Van De Water T.R. Otoprotective properties of mannitol against gentamicin induced hair cell loss. Otol. Neurotol. 2014;35(5):e187–e194. doi: 10.1097/MAO.0000000000000342. [DOI] [PubMed] [Google Scholar]
- Yang T.H., Young Y.H., Liu S.H. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J. Nutr. Biochem. 2011;22(9):886–894. doi: 10.1016/j.jnutbio.2010.08.009. [DOI] [PubMed] [Google Scholar]