Highlights
-
•
Vaginal masses after hysterectomy for benign indications should be investigated.
-
•
Opportunistic salpingectomies may decrease incidence of epithelial ovarian cancers.
-
•
Consider regular pelvic exams for women with asymptomatic prolapsed fallopian tubes.
Abstract
Background
The differential diagnosis for women who present with a vaginal mass after undergoing a hysterectomy is dependent on the indication, type and timing of the hysterectomy. The differential diagnosis includes cervical dysplasia, malignancy, nabothian cysts, prolapsed endocervical polyp/fibroid, abscess, hematoma, granulation tissue, or dehiscence with organ evisceration.
Case
We introduce a case of a woman who presented with a vaginal apex mass and had a remote history of a total hysterectomy for an unknown indication. She was ultimately diagnosed with high grade serous carcinoma of a prolapsed fallopian tube.
Conclusion
This is the first reported case of serous carcinoma of a prolapsed fallopian tube and highlights the importance of maintaining a wide differential diagnosis for women who present with vaginal apex masses.
1. Introduction
The differential diagnosis for women who present with a mass at the apex of the vagina is dependent on the indication, type and timing of hysterectomy. Occasionally, the indication for the hysterectomy and whether or not the cervix was retained remains unclear even after a history and physical examination. Among women who have undergone hysterectomy with preservation of the cervix, the differential diagnosis of a mass at the apex of the vagina includes cervical dysplasia, cervical cancer, nabothian cyst, or a prolapsed endocervical polyp, fibroid or other malignancy. After a recent total hysterectomy, etiologies include abscess, hematoma, granulation tissue or dehiscence with organ evisceration. Reported organs to prolapse through a vaginal apex dehiscence include bowel, omentum, fallopian tube and appendix (Cardosi et al., 1999). Women who present with a distant history of a total hysterectomy for benign indications have a more limited differential diagnosis of a vaginal apex mass which include a de novo vaginal carcinoma, vaginal dysplasia or a prolapsed fallopian tube.
We present a case of a woman with a history of a total hysterectomy for an unknown indication who presented with a vaginal apex mass which was ultimately diagnosed as high grade serous carcinoma of the fallopian tube. The presumed etiology of this presentation is the development of high grade serous carcinoma in a prolapsed fallopian tube, a phenomenon that to the authors’ knowledge has not yet been reported.
2. Case
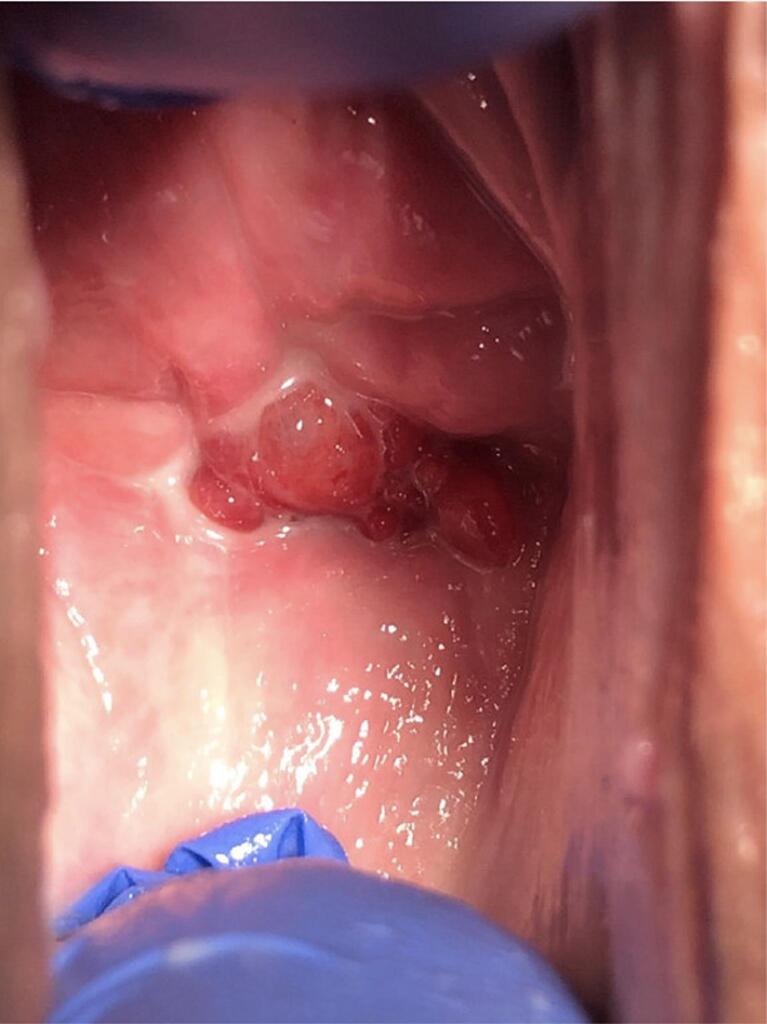
The patient is a 46-year-old G6P3033 woman with a past medical history of hypertension and depression who presented for a routine gynecology visit with symptoms of mixed urinary incontinence. The patient had not seen a gynecologist for over five years, had a negative review of systems and had no family history of malignancy. She reported a history of a hysterectomy ten years prior for a “precancerous condition” at an outside institution for which no records were available. She reported being sexually active and monogamous with her male partner and reported a history of chlamydia two years prior for which she received treatment. On pelvic exam, the patient was found to have stage 1 anterior wall vaginal prolapse and a 3 cm irregularly shaped, friable, verrucous like “cherry red” growth of the posterior vaginal apex which was mildly tender to palpation (Image 1). A vaginal Papanicolaou smear was obtained and resulted as “negative for intraepithelial lesion or malignancy” and negative for high risk HPV by PCR. The patient underwent a transvaginal ultrasound two weeks later which demonstrated a soft tissue density with internal vascularity occupying the vaginal apex with bright echoes. Three weeks from her initial presentation, a biopsy of the vaginal apex was obtained which demonstrated high grade serous carcinoma involving fragments of fallopian tube fimbriae with chronic salpingitis. Immunostains from the pathology sample demonstrated positive p53, p16, WT-1, PAX-8, and ER expression. The patient was then referred to a gynecologic oncologist. She experienced a delay in care due to complex family and social issues and was seen six months later by a gynecologic oncologist. At this time, she was found to have a 3 cm by 3 cm mass at the vaginal apex without palpable adnexal or parametrial involvement.
Image 1.
Speculum exam demonstrating the lesion at the vaginal apex.
She subsequently underwent a robot assisted bilateral oophorectomy, resection of fallopian tube remnants, omentectomy, pelvic and para-aortic lymph node dissection and radical upper vaginectomy. Intra-operatively, the left ovary and fallopian tube with associated tumor were seen extending into the vaginal apex. The right fallopian tube and ovary appeared normal. There was suspicion for extra-pelvic disease based on the appearance of sub-centimeter hypopigmented lesions on the omentum. The ureters were skeletonized and mobilized laterally and the uterine artery was identified and transected at the origin of the internal iliac artery. A vaginal sizer was placed into the vagina to aid in the creation of the bladder flap and upper vaginectomy. The entire specimen including the upper third of the vagina, bilateral parametrium, bilateral adnexa and associated tumor were removed intact through the vagina. There was no evidence of residual disease at the end of the case. Pathologic staging revealed Stage IC2 fallopian tube high grade serous carcinoma based on fallopian tube surface involvement with all other specimens negative for disease. She was recommended to undergo germline genetic testing and three cycles of adjuvant chemotherapy with carboplatin and paclitaxel followed by active surveillance. After one cycle of chemotherapy she declined further treatment due to treatment toxicity and did not proceed with germline genetic testing. At the time of this report she is ten months from surgical staging without evidence of recurrence.
3. Discussion
Fallopian tube prolapse is a rare hysterectomy complication reported to occur in 0.1% of hysterectomies and is most common after vaginal hysterectomy (Fan et al., 2006). Common presentations from a systematic review of patients with fallopian tube prolapse include pain, dyspareunia, post coital bleeding and an incidental finding of a red friable mass at the vaginal apex (Ouldamer et al., 2013). Of 51 patients with fallopian tube prolapse, none were expectantly managed, 45 (88%) were treated surgically, six (12%) were treated with silver nitrate and no diagnoses of carcinoma were reported. This is the first reported case of a fallopian tube prolapse which was ultimately diagnosed as fallopian tube high grade serous carcinoma.
The fallopian tube has become widely recognized as the source of the majority of clinically recognized “ovarian cancer” based on pre-clinical models and precursor lesions seen in pathologic evaluation of normal appearing tubes in women who undergo ovarian cancer surgery and risk reducing surgery (Perets and Drapkin, 2016). The practice of removing the fallopian tubes at the time of a hysterectomy for benign indications in the absence of a specific indication is referred to as an “opportunistic salpingectomy” and was recently addressed in a 2019 ACOG committee opinion as a safe means of reducing ovarian cancer risk (ACOG Committee Opinion No. 774, 2019). Level 1 evidence is lacking regarding the degree of ovarian cancer risk reduction in low risk women who undergo salpingectomy as evidenced by a recent Cochrane review, and evaluating the survival benefit of opportunistic salpingectomy is challenging given the large numbers of women who would be required to be studied to prove a survival benefit (van Lieshout et al., 2019). Despite this lack of level 1 evidence, a population-based retrospective cohort study shows a 35% decreased risk of ovarian cancer among women who have undergone salpingectomy compared to those who have not and the practice of opportunistic salpingectomy is widely accepted (Falconer et al., 2015).
Concerns over the effect of salpingectomy on ovarian reserve and sexual function have been raised. In a recent systematic review, no differences in anti-mullerian hormone levels were observed in a comparison of women before and after salpingectomy in addition to women with and without salpintectomy (Mohamed et al., 2017). A non-randomized prospective trial is currently being launched to demonstrate non-inferiority of salpingectomy to salpingo-oopherectomy for ovarian cancer risk reduction among BRCA1 carriers and also includes survey data on estrogen deprivation symptoms and sexual dysfunction which will be important to inform the risks of salpingectomy (NRG Oncology, 2020).
Our patient undeniably would have benefitted from an opportunistic salpingectomy at the time of her hysterectomy. Access to care may have also contributed to this presentation; had she had regular annual visits with a gynecologist, her underlying pathology might have been identified earlier which might have changed her clinical outcome. Regular gynecologic care could also have solidified the pathogenesis of this malignancy as it cannot be confirmed that the prolapsed fallopian tube occurred prior to the development of serous carcinoma. The appearance of the mass without erosion suggests a prolapsed fallopian tube but this cannot be known with certainty. Despite the lack of evidence that pelvic exams have clinical benefit in asymptomatic women, this patient is an example of a person who could have benefitted from a timelier diagnosis of her fallopian tube prolapse. Her presentation also demonstrates the importance of immediate biopsy for any suspicious vaginal apex masses, as this ultimately triaged her to see a gynecologic oncologist.
Treatment planning for this patient was individualized and incorporated principles of more than one gynecologic malignancy. Surgical staging was performed according to the staging system of a tubal serous carcinoma, yet included a dissection similar to a radical trachelectomy to assure the specimen was removed intact as a complete gross resection. Ultimately, pathologic staging and adjuvant treatment recommendations were derived from the histologic site of disease and were separated from the anatomic location from which the pathology was found.
In reporting the first case of high grade serous carcinoma of a prolapsed fallopian tube, we demonstrate a novel diagnosis for a woman who presents with a vaginal apex mass. We present a patient who was successfully surgically staged and ultimately declined adjuvant chemotherapy after receiving one cycle.
Précis
This case highlights the utility in maintaining a broad differential diagnoses for vaginal masses after hysterectomy.
Informed consent
This patient has provided informed consent for publication of her case.
Credit of authorship contribution statement
Tyler J. Woodard: Writing - original draft. Benjamin Margolis: Writing - review & editing. Sarah Lee: Writing - review & editing. Ghadir Salame: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cardosi R.J., Hoffman M.S., Roberts W.S., Spellacy W.N. Vaginal evisceration after hysterectomy in premenopausal women. Obstetr. Gynecol. 1999;94(5):859. doi: 10.1016/s0029-7844(99)00530-x. [DOI] [PubMed] [Google Scholar]
- Fan Q.B., Liu Z.F., Lang J.H., Sun D.W., Leng J.H., Zhu L., Liu N. Clinical analysis of fallopian tube prolapse after hysterectomy. Zhonghua fu chan ke za zhi. 2006;41(7):449–451. [PubMed] [Google Scholar]
- Ouldamer L., Caille A., Body G. Fallopian tube prolapse after hysterectomy: a systematic review. PloS One. 2013;8(10):e76543. doi: 10.1371/journal.pone.0076543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets R., Drapkin R. It's totally tubular… riding the new wave of ovarian cancer research. Cancer Res. 2016;76(1):10–17. doi: 10.1158/0008-5472.CAN-15-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOG Committee Opinion, No. 774 Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet. Gynecol. 2019;133(4):e279–e284. doi: 10.1097/AOG.0000000000003164. [DOI] [PubMed] [Google Scholar]
- Van Lieshout L.A., Steenbeek M.P., De Hullu J.A., Vos M.C., Houterman S., Wilkinson J., Piek J.M. Hysterectomy with opportunistic salpingectomy versus hysterectomy alone. Cochrane Database Syst. Rev. 2019;8 doi: 10.1002/14651858.CD012858.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer H., Yin L., Grönberg H., Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J. Natl Cancer Inst. 2015;107(2):dju410. doi: 10.1093/jnci/dju410. [DOI] [PubMed] [Google Scholar]
- Mohamed A.A., Yosef A.H., James C., Al-Hussaini T.K., Bedaiwy M.A., Amer S.A.K. Ovarian reserve after salpingectomy: a systematic review and meta-analysis. Acta obstetricia et gynecologica Scandinavica. 2017;96(7):795–803. doi: 10.1111/aogs.13133. [DOI] [PubMed] [Google Scholar]
- NRG Oncology, 2020. A study to compare two surgical procedures in women with BRCA1 mutations to assess reduced risk of ovarian cancer. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04251052 (accessed July 26th 2020).