Abstract
Objective
To evaluate the predictive value of Computed Tomography Angiography (CTA) measurements of the RVOT for transcatheter valve sizing.
Background
Transcatheter pulmonary valve replacement (TPVR) provides an alternative to surgery in patients with right ventricular outflow tract (RVOT) dysfunction. We studied 18 patients who underwent catheterization for potential TPVR to determine whether CT imaging can be used to accurately predict implant size.
Methods
Cases were grouped by RVOT characteristics: native or transannular patch (n = 8), conduit (n = 5) or bioprosthetic valve (n = 5). TPVR was undertaken in 14/18 cases, after balloon-sizing was used to confirm suitability and select implant size. Retrospective CT measurements of the RVOT (circumference-derived (Dcirc) and area-derived (Darea) diameters) were obtained at the level of the annulus, bioprosthesis or conduit. Using manufacturer sizing guidance, a valve size was generated and a predicted valve category assigned: (1) <18 mm, (2) 18–20 mm, (3) 22–23 mm, (4) 26–29 mm and (5) >29 mm. Predicted and implanted valves were compared for inter-rater agreement using Cohen’s kappa coefficient.
Results
The median age of patients was 37 years old (IQR: 30–49); 55% were male. Diagnoses included: Tetralogy of Fallot (12/18), d-Transposition repair (3/18), congenital pulmonary stenosis (2/18) and carcinoid heart disease (1/18). Measurements of Darea (κ = 0.697, p < 0.01) and Dcirc (κ = 0.540, p < 0.01) were good predictors of implanted valve size. When patients with RVOT conduits were excluded, the predictive accuracy improved for Darea (κ = 0.882, p < 0.01) and Dcirc (κ = 0.882, p < 0.01).
Conclusions
CT measurement of the RVOT, using Darea or Dcirc, can predict prosthetic valve sizing in TPVR. These measurements are less predictive in patients with conduits, compared to those with a native RVOT or pulmonic bioprosthesis.
Condensed abstract
We studied 18 patients who underwent catheterization for TPVR to determine whether CT imaging could be used to accurately predict implant size. Retrospective RVOT measurements were used to generate a predicted valve size, which was compared with implanted valve size for inter-rater agreement. Measurements of Darea (κ = 0.697, p < 0.01) and Dcirc (κ = 0.540, p < 0.01) were good predictors of implanted valve size. When cases with RVOT conduits were excluded, the predictive accuracy improved for Darea (κ = 0.882, p < 0.01) and Dcirc (κ = 0.882, p < 0.01). CT measurement of the RVOT can accurately predict prosthetic valve sizing in TPVR. These measurements are less predictive in patients with conduits.
Abbreviations: Darea, Area-derived Diameter; Dcirc, Circumference-derived Diameter; RVOT, Right Ventricular Outflow; TPVR, Transcatheter Pulmonary Valve Replacement; TAVR, Transcatheter Aortic Valve Replacement
1. Introduction
Over the last 15 years, transcatheter pulmonary valve implantation (TPVR) has emerged as a minimally invasive approach to treating right ventricular outflow tract (RVOT) dysfunction. Currently, TPVR is one of the most commonly performed procedures among adult congenital heart disease (ACHD) patients, often in the context of significant stenosis or regurgitation in the right ventricular outflow tract following surgery undertaken in infancy. If left untreated, these patients suffer the sequelae of right ventricular failure; progressive RV dilatation, reduced exercise tolerance, unstable arrhythmias and increasing the risk of cardiac death [1]. Since the first inhuman implantation by Dr. Bonhoeffer in 2000 [2], multiple modifications have been introduced and TPVR is now widely accepted as an alternative approach to open heart surgery. It has been shown to both improve patients’ hemodynamic parameters [3] and functional capacity [4], whilst avoiding the need for open cardiac surgery in patients with prohibitively high operative risk after multiple previous sternotomies [5].
Suitability for TPVR is determined by several factors, which include: (a) RVOT morphology, (b) compliance and distensibility of the RVOT, and (c) coronary artery anatomy. Careful pre-procedural planning is vital to the success of TPVR. Assessment includes detailed echocardiographic evaluation of the ventricular size and function, characterization of the RVOT morphology, spectral Doppler analysis of the degree of RVOT stenosis and/or regurgitation and assessment of the extent of tricuspid regurgitation (TR). Cross sectional imaging using CT or CMR with 3D reconstruction [6] is key to evaluating factors which influence the suitability for TPVR; understanding of the RVOT morphology, degree of calcification, the proximity of the coronary arteries to the pulmonary annulus and distensibility of the RVOT are required pre-requisites for the procedure.
In the context of Transcatheter Aortic Valve replacement (TAVR), contrast-enhanced CMR and CT have been established as essential tools in sizing the aortic annulus prior to the procedure, as well as providing important information regarding concomitant cardiac disease and the vascular anatomy. Prior to the TAVR procedure, measurements of the annular size are now routinely obtained from CT imaging. These measurements are referenced to existing valve manufacturer guidance for selection of the appropriate size of bioprosthesis. In a multi-center prospective study, implementation of a CT annular sizing algorithm prior to all TAVR procedures was shown to reduce the incidence of para-valvular leak and peri-procedural complications, when compared to an integrated strategy of echocardiographic and angiographic assessment alone [7]. At present, implant sizing in the context of TPVR is based mainly on the estimation of RVOT dimensions obtained from catheter balloon-sizing during the catheterization procedure, rather than reliance on standardized measurements from prior cardiac imaging.
Although CT imaging has been shown to be a useful tool in planning for TPVR [7], [8], there is no current consensus on which specific measurements of the RVOT provide the most reliable information regarding patient suitability for TPVR and which valve size they may require. Given the heterogeneity of patients referred for TPVR, standardizing measurements of the RVOT that are reliably reproducible is a challenge. It is therefore conceivable that patients are being deemed unsuitable for TPVR, and hence not referred for catherization, on the basis of CT measurements which have yet to be validated for this purpose. As this rapidly advancing field of intervention continues to expand, it is vital that we understand how best to predict the suitability of patients that could benefit from TPVR; not only to avoid unnecessary attempts at TPVR in a patient with an outflow tract that is too large for safe intervention, but also to avoid referral for surgical replacement in patients where a percutaneous approach could have been considered.
We performed a retrospective analysis of 18 patients who underwent catherization for TPVR. In 14 of these cases, the patients received TPVR. In the remaining 4 cases, the attempt at TPVR was abandoned after performing catheter balloon-sizing of the RVOT, as the dimensions were too large to safely proceed (>29 mm). Through analysis of these 18 cases, we aim to establish whether CT measurements of the RVOT obtained prior to TPVR can be used to accurately determine the suitability of a patient for potential TPVR and whether CT assessment of the RVOT can ‘predict’ a valve size that matches that which was implanted in the cases where TPVR was undertaken successfully.
2. Methods
2.1. Study population
Using University of California San Francisco cardiology department database, we identified 26 patients who underwent cardiac catherization for potential TPVR between September 2014 and May 2018. Subjects include those with previous surgical or transcatheter interventions to the RVOT. 8 patients were excluded from the study; 7 patients did not have CT imaging as part of their pre-procedural planning (CMR was performed instead) and 1 patient whose imaging could not be analysed due to artefactual distortion. Of the remaining 18 patients, TPVR was successfully performed in 14 patients. In the remaining 4 cases, valve deployment was not attempted as RVOT diameter assessed by balloon-sizing was thought to be too large for safe deployment. Information regarding demographic variables and the details of patients’ underlying cardiac diagnoses were obtained through review of our hospital electronic medical records database Table 1.
Table 1.
Patient demographics and study participants prior to catherization procedure.
| Study Participants (n = 18) | |
|---|---|
| Demographics | |
| Age (yrs), median ± IQR | 37 ± 30–49 |
| Female, n (%) | 8 (44.4) |
| Weight (kg), median ± IQR | 77.3 ± 60–83 |
| Height (cm), median ± IQR | 165 ± 155–176 |
| NYHA Classification, n (%) | |
| I | 8 (44) |
| II | 7 (39) |
| III | 3 (17) |
| IV | 0 (0) |
| Existing RVOT classification, n (%) | |
| Native or Transannular Patch | 8 (44) |
| Bioprosthetic valve | 5 (28) |
| RV-PA Conduit | 5 (28) |
| Underlying Cardiac Diagnosis, n (%) | |
| Tetralogy of Fallot | 12 (67) |
| Congenital Pulmonary Stenosis | 2 (11) |
| d-Transposition of the Great Arteries | 3 (17) |
| Carcinoid Heart Disease | 1 (5) |
| Predominant nature of RVOT lesion, n (%) | |
| Stenotic | 4 (22) |
| Regurgitant | 11 (61) |
| Mixed | 3 (17) |
2.2. TPVR Procedure and catheter hemodynamics
Prior to referral for TPVR, all patients were evaluated by the multi-disciplinary adult congenital heart team to establish their suitability for TPVR. A transfemoral approach was performed in all patients in the study. Hemodynamic evaluation was performed during cardiac catheterization prior to attempted valve deployment including measuring the right atrial pressures, right ventricular pressures, peak to peak gradient across the RVOT, and pulmonary artery pressure. Angiographic evaluation of the RVOT morphology and degree of pulmonary regurgitation was then performed. Patients who underwent the procedure received transfemoral implantation of an Edwards Sapien® bioprosthesis, Sapien XT or S3, (Edwards Lifesciences INC., Irvine, CA) of either 23-mm, 26-mm, or 29-mm diameter, or a Medtronic Melody® valve (Medtronic Inc., Minneapolis, MN, USA) of either 18-mm, 20-mm or 22-mm diameter. The RV-PA was pre-stented, prior to valve implantation, in 8 of the 14 cases where TPVR was undertaken Table 2.
Table 2.
Patient demographics and study participants prior to catherization procedure. *Denotes statistical significance (p < 0.05). † Two-staged procedure. ‡One patient death unrelated to procedure; one patient lost to follow-up.
| Patients who underwent TPVR (n = 14) | Prior to TPVR | Post TPVR |
|---|---|---|
| Echocardiographic Variables (mmHg, median ± IQR) | ||
| IVC | 16.5 ± 15.5–19.5 | 17.5 ± 15.8–18 |
| RA pressure | 3 ± 3–4.5 | 3 ± 3–3 |
| Peak TR velocity | 3 ± 2.5–3.9 | 2.5 ± 2.2–3.5 |
| RVOT mean gradient | 19 ± 10–36.3 | 10 ± 9–20* |
| RVOT peak gradient | 37.5 ± 18.5–69.3 | 19 ± 15–33* |
| PR grade | Severe | None/trace * |
| TR grade | Mild | Mild |
| Baseline Catheter Hemodynamics (mmHg, median ± IQR) | ||
| RVSP | 55 ± 40–62 | |
| PASP | 30 ± 25–37 | |
| Peak RV-PA gradient | 19 ± 11–31 | |
| RVEDP | 12 ± 11–13 | |
| Mean RAP | 11 ± 7–15 | |
| Mean PAP | 18 ± 15–24 | |
| Aortic systolic pressure | 113 ± 101–122 | |
| Right Ventricle(RV): Aortic (Ao) pressure ratio | 0.45 ± 0.32–0.61 | |
| Manufacturer of implanted valve, n (%) | ||
| Edwards Sapien S3 | 5 (36) | |
| Edwards XT | 1 (7) | |
| Melody | 8 (57) | |
| Complications or Events | ||
| Within 48 h of procedure | 1 (7) † | |
| Within 1 year follow-up | 2 (14) ‡ | |
2.3. Methods of valve size selection during TPVR
All TPVR procedures were performed by a single experienced congenital interventional cardiologist. The method of valve size selection varied depending on whether the patient had undergone previous surgical or transcatheter intervention to the RVOT. In patients with a transannular patch or native outflow tract, serial balloon sizes are deployed and inflated until the desired ‘waist diameter’ is observed through fluoroscopic assessment of the balloon’s shape. As these patients tend to have more compliant outflow tracts, depending on operator preference, the valve size that is selected can be up to one size larger than the observed balloon waist to reduce the risk of device embolization and paravalvular regurgitation. In cases where a bioprosthesis already exists in the pulmonary position (valve-in-valve), the balloon was inflated to estimate the internal diameter of the prosthetic valve along with published bioprosthetic inner valve diameter as per the manufacturer to select an appropriate valve size. In patients with a pre-existing conduit, the conduit size was confirmed from the operative note to choose the first sizing balloon. If necessary, subsequent larger diameter balloons are utilized until the desired ‘waist diameter’ is observed. The subsequent pre stent and valve size was chosen based on the size of the balloon used and the waist measured (usually at least 2 mm greater than the measured waist). Repeated hemodynamic and angiographic assessment is used to confirm the position of the deployed valve and assess for any sign of paravalvular regurgitation and coronary compression. The valve sizes chosen by the transcatheter operator were retrospectively assigned as the ‘implanted’ valve size for the purposes of subsequent analysis.
2.4. Echocardiography
Prior to the implantation procedure, all patients underwent 2-dimensional and Doppler echocardiographic examination using transthoracic echocardiography using Philips EPIQ ultrasound system (Philips Ultrasound Ltd, Bothell, WA, USA). In all patients, spectral and colour Doppler evaluation of the right ventricular outflow tract for grading of stenosis and/or regurgitation was performed. Right atrial pressure was estimated based on IVC diameter and inspiratory collapse. Peak TR velocity was reported and RVSP was estimated utilizing the modified Bernoulli equation with the addition of RA pressure. Repeat echocardiographic assessments, performed between 1 and 23 months post-procedure, were analysed to evaluate whether TPVR had resulted in an improvement in echocardiographic parameters. Follow-up echocardiographic assessment was available in 12 of the 14 cases were TPVR was performed Table 2. A single operator (AK) was responsible for the measurement of all echocardiographic data in order to limit inter-observer variation.
2.5. CT protocols and image acquisition
The CT chest images were acquired in preparation for TPVR implantation, some of which were performed specifically to analyse suitability for TPVR and others of which were performed without this specific indication. As such, CT protocols were utilized, as appropriate to the clinical situation. Of the 18 cases, 13 were performed as cardiac -gated CT angiogram (CTA), and 5 were performed without cardiac gating. Field of view, slice thickness, radiation dose parameters, and volume of intravenous contrast administered varied based upon the clinical indication. Contrast enhanced CT or CTA was performed using Iohexol 350 mg/ml (Omnipaque, Novaplus®, GE Healthcare, Milwaukee, WI, USA), with 3–5 ml/sec injection rate, with approximately 100 to 150 cc of contrast administered. Multiple scanners were used at our institution to acquire the exams, including Lightspeed VCT (GE Healthcare, Milwaukee, WI, USA), Discovery CT750 HD (GE Healthcare, Milwaukee, WI, USA), Lightspeed 7.X/VCT (GE Healthcare, Milwaukee, WI, USA), and Revolution (GE Healthcare, Milwaukee, WI, USA). Two external CT examinations were also analysed which were performed on a LightSpeed VCT (GE Healthcare, Milwaukee, WI, USA) and SOMATOM Sensation 64 (Siemens Healthcare, Erlangen, Germany). All scanners had at least 64 MDCT capabilities. At our Institution, CTs were performed based upon the subsequently described parameters. Retrospectively gated CTAs (8 patients): retrospective cardiac gating with ECG and weight-based dose modulation, slice thickness of 0.625 mm, field of view of 25 cm or entire chest, and 100 kV. Images were reconstructed at 10% intervals throughout the cardiac cycle, although the diastolic phase at approximately 70% of the R-R interval was used for analysis to be consistent with the prospectively gated CTAs. Additional images were reconstructed at 40% of the R-R interval for assessment of agreement between diastolic and systolic measurements in this subgroup. Bolus tracking technique was used for contrast bolus timing, with region of interest on the descending aorta. Prospectively gated CTAs (5 patients): prospective cardiac gating and ECG dose modulation, slice thickness of 0.625 mm, field of view of 25 cm, and 120 kV. Images were reconstructed at 75% of the R-R interval, with additional phases reconstructed when motion artefact was present. Bolus tracking was used for contrast bolus timing, with region of interest on the ascending aorta. Pulmonary embolism CT (3 patients): slice thickness 1.25 mm, automatic tube current modulation, and 120 kV. Bolus tracking was used for contrast bolus timing, with region of interest on the pulmonary artery or left atrium. Routine chest CT with contrast (2 patients): slice thickness of 1.25 mm, automatic tube current modulation, and 120 kV. Scans were performed after a fixed 60 s IV contrast delay.
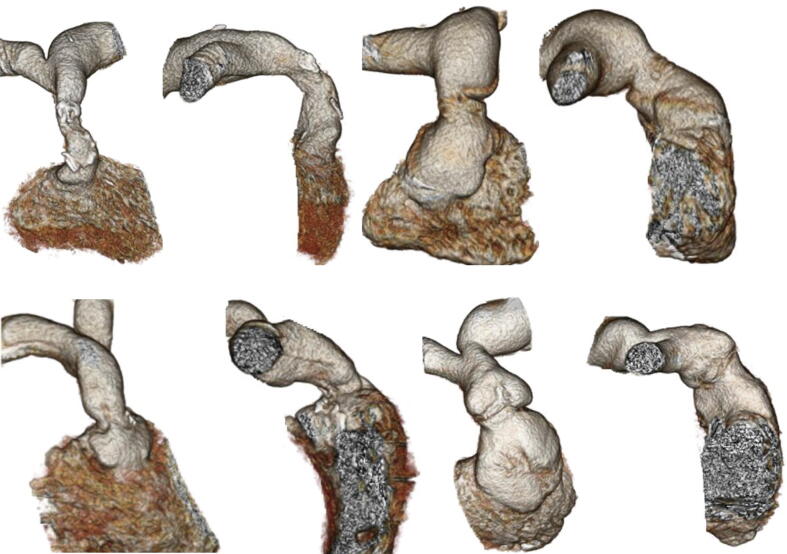
The 3-dimensional RVOT models were reconstructed using Aquarius iNtuition software (Version 4.4., TeraRecon, Inc, San. Mateo, CA, USA). Volume Rendering preset tool of “Angio” for CTA images was used to visualize the blood pool model of the heart, pulmonary artery and other vascular structures. The “FreeROI” tool was used to remove additional structures, leaving the RVOT and associated pulmonary artery and main branches intact (see Fig. 1).
Fig. 1.
Computed Tomography 3-Dimensional models of the RVOT in a selection of our patient cohort demonstrating the anatomical variation between cases where TPVR was successful.
2.6. Achieving RVOT plane and measurements
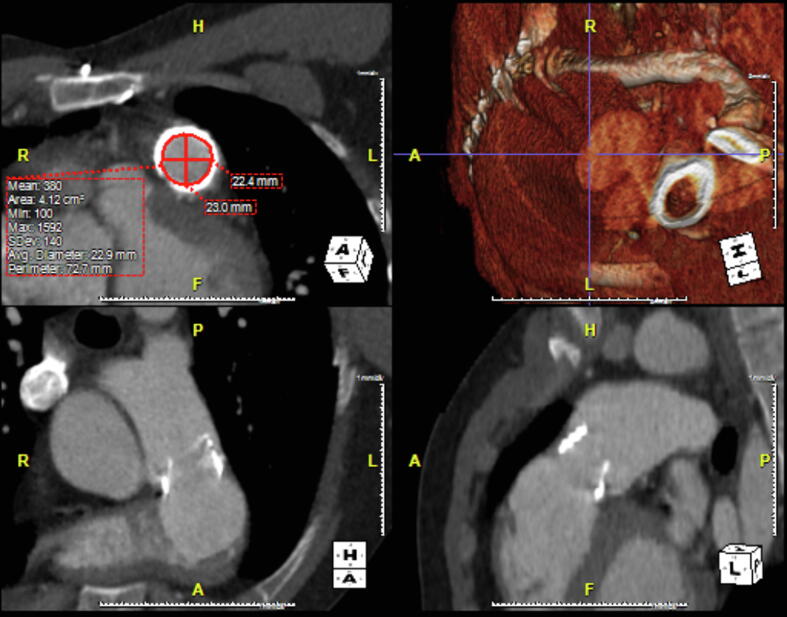
All measurements were conducted by a single operator (LC) blinded to the implanted valve size. Measurements were reviewed by two senior cardiothoracic radiologists (KK, KO), independently and in a blinded fashion, and they have agreed with all the original measurements. Images were viewed and measurements were obtained using Aquarius iNtuition software (Version 4.4.7, TeraRecon, Inc, San. Mateo, CA, USA). To measure the native pulmonary annulus, multiplanar reformation (MPR) was used to create the oblique transverse plane, transecting the basal attachments of the pulmonary valve cusps. The polygonal shape of the annulus was manually traced, using the imaging software to calculate its cross-sectional area (mm2) and perimeter (mm). The operator then measured the minimum (Dmin) and maximum (Dmax) cross-sectional diameters at this level (mm). The area-derived diameter (Darea) was calculated using the cross-sectional area of the pulmonic annulus and the circumference-derived diameter (Dcirc) using the annulus perimeter/circumference. In patients with a surgical conduit, the same method was used to measure the ‘outer to outer’ dimensions of the conduit at its widest portion. In patients with an existing bioprosthesis, the internal dimension of the existing valve (internal diameter) was measured in cross-section (Fig. 2, Fig. 3).
Fig. 2.
Cross-sectional measurements of area, perimeter (circumference), long-axis (Dmax) and short- axis (Dmin) for patient with (a) native annulus (b) valve-in-valve and (c) conduit. Note for valve-in-valve case (b), the internal diameter of the existing bioprosthesis is measured. For the conduit case (c), the outer-outer dimension of the conduit, including calcifications, have been measured.
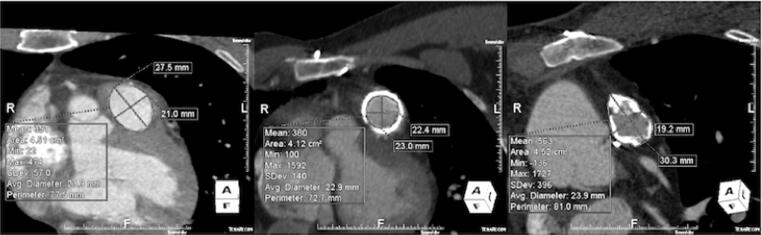
Fig. 3.
Multiplanar reformation (MPR) was used to create a double-oblique transverse plane. In this image the internal diameter of the existing bioprosthesis is manually traced, using the imaging software to calculate its cross-sectional area (mm2) and perimeter (mm). The operator then measured the minimum (Dmin = 22.4 mm) and maximum (Dmax = 23 mm) cross-sectional diameters.
Darea and Dcirc were calculated using the following mathematical formulae:
-
(1)
Darea = 2 × √(Area/π)
-
(2)
Dcirc = Circumference/π
2.7. Method of generating predicted valve size
For each diameter measurement (Dmax, Dmin, Dcirc, Darea), a theoretical ‘predicted’ valve size was generated, based on valve manufacturer guidance (see tables). In a case such that the measured diameter was found to be suitable for either a Melody or a Sapien implant, the predicted valve size was generated using the sizing guidance was the same manufacturer as that selected by the transcatheter operator for the implanted valve. For patients with a pre-existing bioprosthesis, ‘valve-in-valve’ manufacturer sizing guidance was used where available. For each patient, four ‘predicted’ valve sizes were generated using the Dmax, Dmin, Darea and DCirc. Given the significant overlap in the sizes of valves between the two manufacturers, it was decided that each valve would be assigned a numbered category to allow for grouping of similar prosthesis sizes as follows: (1) <18 mm, (2) 18–20 mm, (3) 22–23 mm, (4) 26–29 mm and (5) >29 mm. The predicted valve category was then compared to the category of implanted valve, using each of the diameter measurements (Dmax, Dmin, Darea, Dcirc) Table 3, Table 4.
Table 3.
Sizing tables used to generate a ‘predicted’ valve size, adapted from valve manufacturer guidance for Melody, Edwards Sapien S3 and Edwards XT.
| Valve Manufacturer | CT-derived diameter (mm) | Valve Size (mm) |
|---|---|---|
| Melody |
16–20.1 20.1–22.4 22.4–24.1 |
18 20 22 |
| Edwards S3 |
18.6–20.7 20.7–23.4 23.4–26.4 26.4–29.5 |
20 23 26 29 |
| Edwards S3 Valve-in-valve |
16.5–18.5 18.5–22 22–25 25–28.5 |
20 23 26 29 |
| Edwards XT |
20–23 23–26 26–29 |
23 26 29 |
Table 4.
Kappa coefficient values comparing predicted valve size, derived from selected RVOT diameter, compared to implanted valve size.
| Diameter Measurement | Kappa Coefficient Values (p) |
|
|---|---|---|
| All TPVR cases | Conduit Group Excluded | |
| Dmax | 0.514 (0.08) | 0.328 (0.01) |
| Dmin | 0.071 (0.53) | 0.041 (0.769) |
| Darea | 0.697 (<0.01) | 0.882 (<0.01) |
| Dcirc | 0.540 (<0.01) | 0.882 (<0.01) |
2.8. Statistical analysis
Continuous variables were assumed to have a non-parametric distribution due to the sample size and hence are described as medians with interquartile ranges (IQR). Categorical variables are described by frequencies and percentages. Comparison of paired continuous and categorical variables was performed using the Wilcoxon Matched-pair Signed Rank Test. Cohen’s Kappa coefficient was calculated to determine the degree of agreement between the predicted and implanted valve groups. All analyses were performed using SPSS IBM statistical software.
3. Results
Among the 18 patients who underwent catherization for potential TPVR, the median age was 37 years old (interquartile range: 30–49) and 55% were male. Underlying cardiac diagnoses were of Tetralogy of Fallot (12/18), d-Transposition of Great Arteries (d-TGA) with Rastelli repair (3/18), congenital pulmonary stenosis (2/18) and carcinoid heart disease (1/18). TPVR was performed in 77% (14/18) of these cases, where catheter balloon-sizing had confirmed that the dimensions of the RVOT were within the acceptable limit for safe intervention (16–29 mm). Prior to TPVR, right-heart catheter assessment demonstrated a pulmonary artery systolic pressure of 30 mmHg (IQR: 25–37), mean pulmonary artery pressure of 18 mmHg (IQR: 15–24) and peak RV-PA gradient of 19 mmHg (IQR: 11–31). The RV/Ao pressure ratio was 0.45 (IQR: 0.32–0.61), with an aortic systolic pressure of 113 mmHg (IQR: 101–122) and a right ventricular systolic pressure of 55 mmHg (IQR: 40–65). Prior echocardiographic assessment revealed that the degree of pulmonary regurgitation was severe in the majority (8/14) of patients who underwent subsequent TPVR.
In patients who underwent TPVR (14/18), 57% (n = 8) received a Melody bioprosthesis, 36% (n = 5) received a Sapien 3 bioprosthesis and 7% (n = 1) received a Sapien XT bioprosthesis. Post-procedural echocardiographic data was not available for comparison in 2 of the 14 cases were TPVR was performed. Echocardiographic assessment in the remaining patients (12/14) demonstrated a reduction in both peak (38.5 mmHg to 19 mmHg, IQR 18.5–69.3, p < 0.05) and mean (19 mmHg to 10 mmHg, IQR 15–33, p < 0.05) RVOT gradients post TPVR. The median grading of pulmonary regurgitation was also reduced from severe to none/trace (p < 0.05). There were no reported complications related to the TPVR procedure at 1 year follow up. In one case, a two-staged TPVR procedure was undertaken, with an RVOT stent placed before the valve was later implanted. In the year following the procedure, there was one recorded patient mortality related to an underlying malignancy and another patient lost to follow-up.
In the analysis of our 18 patients, Darea was found to be a good predictor of the valve category size (κ = 0.697, p < 0.01), both in cases where patients underwent TPVR (18–20 mm, 22–23 mm or 26–29 mm) and in cases where the RVOT was too large to proceed safely with intervention (>29 mm). Dcirc was also found to be a good predictor in these patients (κ = 0.540, p < 0.01), showing significant agreement between the predicted and implanted valve categories. When patients with RVOT conduits were excluded, so that only patients with a native, patched or existing pulmonic bioprosthesis valve were analysed (13/18), the agreement improved for both Darea and Dcirc (κ = 0.882, p < 0.01, κ = 0.882, p < 0.01 respectively).
However, when Dmax was used to predict valve size category, there was only a moderate and non-statistically significant level of agreement with the implanted valve category (κ = 0.514, p = 0.08). The level of agreement did not improve when patients with conduits were removed from the analysis (κ = 0.328, p < 0.01). Similarly, the predicted valve categories derived from Dmin did not show good agreement with the implanted valves (κ = 0.071p = 0.530), and did not improve when the conduit group was excluded from the analysis (κ = 0.041, p = 0.769).
In the subgroup of patients who underwent a retrospectively gated CT scans (n = 8), there was no significant difference in predictive ability of systolic measurements (Darea: k = 0.130, p = 0.475 and Dcirc: k = 0.467, p = 0.02) than those taken in diastole (Darea: k = 0.636, p < 0.01 and Dcirc: k = 0.636, p < 0.01).
4. Discussion
We retrospectively analysed cross-sectional imaging from 14 patients who underwent successful TPVR in order to determine whether the predicted valve size derived from CT-measurements of the RVOT matched the implanted valve chosen by the transcatheter operator during catherization. Echocardiographic postprocedure assessment of these patients confirmed the success of the TPVR procedure, demonstrating a significant reduction in both the mean and peak RVOT gradients and the degree of pulmonic regurgitation.
Based on our pragmatic approach to valve sizing in our cohort of TPVR patients, CT measurements of Dcirc and Darea both reliably predict the category of implanted valve size for these patients. These measurements were highly predictive in the 13/18 patients with a native RVOT or an existing pulmonic bioprosthesis. This method of assessing annulus size has already been established in the setting of TAVR, where the measurement of the aortic annulus by computed tomography is an important factor when making a decision regarding prosthesis size [9].
Previous studies have shown that the diameter calculated by using the aortic annulus area (Darea) or its perimeter/circumference (Dcirc), sometimes referred to as the ‘average diameter’ or ‘estimated diameter’, is the most reproducible and reliable measurement for prosthesis sizing [9], [10]. It represents the diameter of an idealized circle with either the same area or the same perimeter/circumference as the measured annulus. In the case of a perfectly circular pulmonary annulus, the diameter derived from area (Darea) and from circumference (Dcirc) would be identical. However, as the outflow tract becomes more ovoid in shape or irregular due to calcification, the relationship between these two measurements becomes increasingly disproportionate. The case is also true that as the annulus becomes more ovoid, the maximum (Dmax) and the minimum (Dmin) measured diameters are less reflective of the average annulus diameter, imagined as an idealized circular structure.
A study by Schievano et al was able to demonstrate the wide range of RVOT morphologies, sizes and dynamics that develop late after surgical repair of congenital heart disease [11]. Through measurement of the maximum and minimum diameter, perimeter-derived diameter and cross-sectional area along various planes of the RVOT, they were able to demonstrate that the pulmonary annulus was in fact elliptical, not circular, in cross-section and that its shape underwent dynamic transformation during different phases of the cardiac cycle.
Previous studies have shown that the aortic root also undergoes significant change during the cardiac cycle, with the most elliptical deformation seen during diastole [11], [12]. As a consequence, diameter measurements of the aortic annulus, used for prosthesis sizing in TAVR, are typically obtained in systole when the annulus is at its most circular and also its when the aortic annulus is largest. The imaging analysed in our study included both ECG-gated and non-gated exams, with gated exams measured in diastole. Non-gated studies of the heart correlate well with diastolic dimensions of the cardiac chambers, as diastole is much longer than systole [13]. Therefore, we believe it is acceptable to assume the 5 non-gated studies provided measurements in a diastolic phase. Assuming that the RVOT is an elliptical structure, which undergoes its greatest deformation during diastole, such factors could explain why neither Dmax or Dmin were accurate predictors of the implanted bioprosthesis size. Future studies with pulmonary valve annulus measurements in systole are needed to assess if measurements obtained at peak-systole perform better than those in diastole. However, that doesn't diminish our pragmatic study finding that diastolic CT measurements of the RVOT were able to predict final valve size.
In our study, we found that that measurements of Darea and Dcirc were both found to be better predictors of implanted valve size when the 5 patients with existing surgical RV-PA conduits were excluded from our analysis. This appears to imply that these measurements are less reliable predictors of valve size for the surgical conduit cohort when compared to the group of patients with a native/patched RVOT or an existing bioprosthesis valve in situ. Although the number of patients with RV-PA conduits included in our study is too small to reach a definitive conclusion, we postulate that within this group the RVOT is more likely to be calcified and stenotic therefore leading to a non-cylindrical morphology which is less accurately measured using the methods described above. Furthermore, the surgical RV-PA conduit is a long, tubular structure with few anatomical landmarks that can be relied upon to obtain standardised measurements between cases.
There has been recent debate regarding whether Darea or Dcirc is the superior method of estimating aortic annulus diameter size, with the suggestion that Dcirc shows less variation over the cardiac cycle [14]. Further study is required to elucidate which of these parameters is the most reliable measurement of the RVOT in both diastole and systole, especially given the wide range of anatomical variants that exist in this group of patients, and falls beyond the scope of our investigation.
4.1. Study limitations
We conducted a retrospective analysis of contrast-enhanced CT imaging in order to generate predicted valve sizes based on measurements of RVOT diameter. In a real-life setting, the choice of valve size and manufacturer is multi-factorial, dependent the degree of RVOT calcification, availability of various devices and operator preference. Although our method allowed for the grouping of valves from different manufacturers of a similar size (e.g. 22 mm Melody and 23 mm Edwards Sapien S3), we were unable to incorporate these factors into our analysis, relying solely on annular diameters to predict valve size.
The CT imaging that we analysed included both non-gated exams and exams which were obtained with prospective gating in diastole. Whilst the majority of CT scans were performed to establish suitability for TPVR, others were performed without this specific indication. Of the 18 cases, 13 were performed as cardiac-gated CT angiogram (CTA), and 5 were performed without cardiac-gating. Whilst it is traditional to measure the pulmonic valve during systole, owing to the fact that our prospectively-gated scans were performed in diastole, image analysis was performed in the diastolic phase to ensure consistency. Therefore, we were only able to assess the predictive value of diastolic CT measurements for TPVR guidance. As previously discussed, the RVOT is likely to be smaller and more distorted during diastole when compared to systole. The compliance of the RVOT and its behaviour over the cardiac cycle may vary greatly between patients depending on a number of different factors affecting its compliance and elasticity, hence it is difficult to estimate the impact this has had on the accuracy of our measurements. To partially address this uncertainty, we performed a reassuring subgroup analysis of only retrospectively gated CT scans (n = 8), which revealed that measurements obtained in the early systolic phase (were not more predictive than those taken in diastole.
In the study by Schievano et al [11], the majority of patients (10/12) had a native outflow tract, hence the dynamic behaviour of the RVOT in patients with a bioprosthesis or calcified conduit is less well known. It is highly conceivable that the presence of previous surgical intervention, significant conduit calcification or a bioprosthetic implant will all impact the compliance of the RVOT to varying degrees. In a study by Ebel et al [15], contrast-enhanced magnetic resonance angiography (ceMRA) and 3D steady-state free precession (SSFP) during systole and diastole for assessment of the right ventricle outflow tract (RVOT) were performed in 89 post-TOF repair patients who were being considered for percutaneous pulmonic valve implantations. The authors did observe that the maximal RVOT diameter was seen during systole however they concluded that the effective and not the maximum or minimum diameter should be considered for planning.
4.2. Conclusions
Contrast-enhanced CT imaging can provide detailed anatomical information about the RVOT anatomy prior to planned TPVR. To our knowledge, we believe we are one of the first to demonstrate that retrospective CT measurements of the area-derived (Darea) and circumference-derived (Dcirc) diameter of the RVOT can be used to accurately predict the size of a transcatheter valve implanted during TPVR. This method appears to be less accurate in patients with a previous surgical RV-PA conduit, when compared to those with either a native RVOT or an existing pulmonic bioprosthesis. Further studies could test this principle in a larger cohort of patients who have undergone TPVR or integrate this method into a prospective model for selecting suitable candidates for TPVR and choosing the appropriate bioprosthesis size.
Sources of funding
None.
Disclosures
Dr Karen Ordovas holds a Research grant for General Electric and is on the Scientific advisory board for Arterys. Professor Vaikom S Mahadevan is a Proctor for Edwards Life Sciences.
CRediT authorship contribution statement
Lara Curran: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft. Harsh Agrawal: Writing - review & editing. Kimberly Kallianos: Conceptualization, Methodology, Data curation, Writing - review & editing. Ahmed Kheiwa: Conceptualization, Methodology, Data curation, Writing - review & editing. Shezhang Lin: Methodology, Data curation, Writing - review & editing. Karen Ordovas: Conceptualization, Methodology, Formal analysis, Supervision. Vaikom S Mahadevan: Conceptualization, Methodology, Formal analysis, Supervision.
References
- 1.Gatzoulis M.A., Balaji S., Webber S.A. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 2.Bonhoeffer P., Boudjemline Y., Saliba Z. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 3.Coats L., Khambadkone S., Derrick G. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113:2037–2044. doi: 10.1161/CIRCULATIONAHA.105.591438. [DOI] [PubMed] [Google Scholar]
- 4.Lurz P., Nordmeyer J., Giardini A. Early versus late functional outcome after successful percutaneous pulmonary valve implantation: are the acute effects of altered right ventricular loading all we can expect? J. Am. Coll. Cardiol. 2011;57:724–731. doi: 10.1016/j.jacc.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Lurz P., Coats L., Khambadkone S. Percutaneous pulmonary valve implantation: Impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. doi: 10.1161/CIRCULATIONAHA.107.735779. [DOI] [PubMed] [Google Scholar]
- 6.Chung R., Taylor A.M. Imaging for preintervention planning transcatheter pulmonary valve therapy. Circ. Cardiovasc. Imaging. 2014;7(1):182–189. doi: 10.1161/CIRCIMAGING.113.000826. [DOI] [PubMed] [Google Scholar]
- 7.Binder R.K., Webb J.G., Willson A.B. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J. Am. Coll. Cardiol. 2013;30;62(5):431–438. doi: 10.1016/j.jacc.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Jabbour A., Ismail T.F., Moat N. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J. Am. Coll. Cardiol. 2011;58:2165–2173. doi: 10.1016/j.jacc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Gurvitch R., Webb J.G., Yuan R. Aortic annulus diameter determination by multidetector computed tomography: reproducibility, applicability, and implications for transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2011;4:1235–1245. doi: 10.1016/j.jcin.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Lehmkuhl L., Foldyna B., Von Aspern K. Inter-individual variance and cardiac cycle dependency of aortic root dimensions and shape as assessed by ECG-gated multi-slice computed tomography in patients with severe aortic stenosis prior to transcatheter aortic valve implantation: is it crucial for correct sizing? Int. J. Cardiovasc. Imaging. 2013;29:693–703. doi: 10.1007/s10554-012-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schievano S., Capelli C., Young C. Four-dimensional computed tomography: a method of assessing right ventricular outflow tract and pulmonary artery deformations throughout the cardiac cycle. Eur. Radiol. 2011;21:36–45. doi: 10.1007/s00330-010-1913-5. [DOI] [PubMed] [Google Scholar]
- 12.Sucha D., Tuncay V., Prakken N. Does the aortic annulus undergo conformational change throughout the cardiac cycle? A systematic review. Eur. Heart J. Cardiovasc. Imaging. 2015;16(12):1307–1317. doi: 10.1093/ehjci/jev210. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S., Yamada Z., Nishimoto Y. Measurement ofcardiac volume by computed tomography (author’s transl) J. Cardiogr. 1981;11:1273–1281. [PubMed] [Google Scholar]
- 14.Von Aspern K., Foldyna B., Etz C.D. Effective diameter of the aortic annulus prior to transcatheter aortic valve implantation: influence of area-based versus perimeter-based calculation. Int. J. Cardiovasc. Imaging. 2015;31:163. doi: 10.1007/s10554-014-0527-4. [DOI] [PubMed] [Google Scholar]
- 15.Ebel S., Gottschling S., Buzan M.T.A. 3D-assessment of RVOT dimensions prior percutaneous pulmonary valve implantation: comparison of contrast-enhanced magnetic resonance angiography versus 3D steady-state free precession sequence. Int. J. Cardiovasc. Imaging. 2019;35(8):1453–1463. doi: 10.1007/s10554-019-01578-w. [DOI] [PMC free article] [PubMed] [Google Scholar]