Abstract
Entada africana is used in non-conventional medicine for the management of liver ailments. A fraction, designated EaF10 (methylene chloride/methanol 90:10, v/v) with promising hepatoprotective activity has been isolated. Since the mechanisms underlying EaF10 hepatoprotective action remain unknown, this study was undertaken to investigate the anti-hepatotoxic mechanism of the fraction against carbon tetrachloride (CCl4)-induced hepatotoxicity and its antioxidant properties. Antioxidant activities of EaF10 were assessed through four chemical antioxidant assays and its anti-hepatotoxic effect evaluated in vivo and in vitro by post-treatment (25 or 100 mg/Kg) or co-treatment (6.25–100 μg/mL) in CCl4-intoxicated mice and normal human liver cells line L-02 hepatocytes respectively; and biochemical and molecular parameters assessed respectively by spectrophotometry, and by quantitative real-time polymerase chain reaction and western blot analysis. EaF10 exhibited strong antioxidant activities correlated with its polyphenol content. Serum levels of alanine/aspartate aminotransferase (AST/ALT) and nitrite oxide, liver contents of glutathione (GSH) protein carbonylation and malondialdehyde (MDA), liver activities of catalase (CAT), glutathione-S-transferase (GST) and superoxide dismutase (SOD) and cell viability showed the anti-hepatotoxic effect of EaF10, supported by histopathological observations. The fraction decreased the protein level of Cytochrome P450 2E1 (CYP2E1) and Kelch-like ECH-associated protein-1 (Keap-1), induced nuclear translocation of Nuclear factor-erythroid 2-related factor-2 (Nrf2) coupled to an increase of the mRNA levels of CAT, SOD1 and GST in CCl4-intoxicated L-02 hepatocytes. These findings evidenced that the studied plant fraction possesses a strong antioxidant capacity and prevents CCl4-induced hepatotoxicity, likely through inhibition of CYP2E1 and activation of the Nrf2 signaling pathway.
Keywords: Entada africana, Fraction EaF10, Carbon tetrachloride, Anti-hepatotoxic, CYP2E1, Nrf2, Natural product chemistry, Pharmaceutical chemistry, Pharmaceutical science, Biochemistry, Molecular biology, Toxicology
Entada africana; Fraction EaF10; Carbon tetrachloride; Anti-hepatotoxic; CYP2E1; Nrf2; Natural product chemistry; Pharmaceutical chemistry; Pharmaceutical science; Biochemistry; Molecular biology; Toxicology
1. Introduction
In Central and West Africa, Entada africana (Fabaceae), is used in non-conventional medicine to treat many diseases, such as malaria, syphilis, wound healing, skin infections and liver disorders [1, 2, 3]. Modern pharmacological approaches on E. africana reported several biological activities: antimicrobial, antiplasmodial, antiproliferative and antioxidant activities [2, 4, 5]. In addition, investigations from our research group revealed the anti-inflammatory properties of the methylene chloride/methanol (MCM) (1:1, v/v) barks extract of E. africana on raw 267.7 macrophages and microglia cells through inhibition of nuclear factor (NF)-κB and down regulation of the expression of pro-inflammatory cytokines [6, 7]. This crude extract also exhibited anti-hepatitis C virus (HCV) activity in vitro on HCV replicon cell lines via induction of antiviral proteins: heme oxygenase-1 and 2′-5′ oligoadenylate synthetase-3 [8]. Moreover, antioxidant and hepatoprotective properties of MCM barks crude extract of E. africana in primary rat hepatocyte and HC-04 cell line have been described; and a fraction (methylene chloride/methanol 90:10, v/v) from this MCM barks crude extract, named EaF10, with highly propitious activities has been isolated [9, 10]. However, the mechanisms underlying EaF10 hepatoprotection are yet to be demonstrated. Thus, understanding of the hepatoprotective molecular mechanism of EaF10 fraction may be useful to support the development of phyto-pharmaceuticals from E. africana against toxic hepatitis.
Carbon tetrachloride (CCl4), a potent hepatotoxin, is one of the best characterized models of xenobiotics-induced liver damage for evaluation of the hepatoprotective effect of herbal medicine [11]. Pathological mechanisms of CCl4-hepatotoxicity involve the biotransformation of CCl4 by the cytochrome P450 system into reactive metabolites which initiate lipid membrane peroxidation, disruption of liver cells membrane and organelles leading to necrosis of hepatocytes [12, 13]. In addition, overproduction of reactive oxygen species (ROS) such as O2˙, HO˙ and NO˙ upon activation of liver resident macrophages (Kupffer cells), likely by free radicals, exacerbate the oxidative stress and exacerbate CCl4-hepatotoxicity [14]. Therefore, interventions which may inhibit metabolic activation of CCl4, prevent or scavenge ROS overproduction could be benefit to protect the liver from xenobiotics injury.
Several cytochrome P450 isoforms can metabolize CCl4 and the cytochrome P450 2E1 (CYP2E1) isoform has been recognized for its preponderant role in the activation of several xenobiotics [15, 16], implying that it can be regarded as therapeutic target to prevent hepatotoxicity. In the cell, generated ROS are eliminated by the antioxidant enzymes such as superoxide dismutase (SOD), glutathione-s-transferase (GST) and catalase (CAT); which genes are transcriptionally upregulated in the liver by the transcription factor nuclear factor erythroid 2-related factor-2 (Nrf2) [17], suggesting that activation of Nrf2 may be an alternative to attenuate or prevent toxin-induced liver injury [18, 19, 20, 21].
To elucidate the mechanisms underlying hepatoprotective action of the fraction EaF10, we aimed at determining its phytochemical composition and antioxidant properties, and studying the involvement of CYP2E1 and Nrf2 signaling pathways to the anti-hepatotoxic activity mechanism of the fraction using CCl4-induced hepatotoxicity as model of liver oxidative damage.
2. Materials and methods
2.1. Reagents
Carbon tetrachloride (CCl4) was purchased from Macklin (Shanghai, China); Thiazolyl Blue Tetrazolium Bromide, α-Keto-glutaric Acid, L-Alanine, Thiobabituric Acid, Trichloroacetic Acid, 2ʹ-7ʹ-Dichlorodihydrofluorescein diacetate (H2DCFDA), silymarin and ascorbic acid (vitamin C) were purchased from Sigma-Aldrich (St Louis, USA); M-PER Mammalian Protein Extraction Reagent, NE-PER® Nuclear and Cytoplasmic Proteins Extraction Kit, Halt protease inhibitor cocktail EDTA-Free 100X, Pierce bicinchoninic acid (BCA) Proteins Assay Kit, SuperSignal West Pico Chemiluminescent Substrate were purchased from Thermo Fisher Scientific (Rockford, USA). Rabbit polyclonal anti-CYP2E1 antibody (1:1500 dilution) was purchased from Sino Biological Inc (Beijing, China); Rabbit polyclonal anti-Nrf2, Keap-1, and Lamin-B antibodies (all 1:1000 dilution) were purchased from Beijing Biosynthesis Biotechnology CO., LTD (Beijing, China); Mouse monoclonal anti-βactin antibody (1:5000 dilution) and horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG AP-linked secondary antibodies (1:2000 dilution) were purchased from Santa Cruz Biotechnoloy (Ca., USA); TRIzol® Reagent was purchased from Ambion Lifes Technologies (Carlsbad, California, USA); First-Strand cDNA Synthesis Kit was purchased from Promega (Madison, USA); iTaq Universal SYBR Green Supermix Kit was purchased from Bio-Rad Laboratories (Ca., USA); All primers of the genes of interest were synthetized by TSINGKE Biological Technology Company (Beijing, China). The others reagent used were of analytical grade.
2.2. Preparation and sub-fractionation of E. africana active fraction and High Performance Liquid Chromatography (HPLC) analysis of active sub-fractions
Stem bark of E. africana was collected in July 2018 in Foumban (West Cameroon). The plant name was checked with http://www.theplantlist.org and the botanical identification was done at the Cameroon National Herbarium, where voucher specimen is kept under the reference number 52661 YA. The active fraction of E. africana (EaF10: methylene chloride/methanol 90:10, v/v) was prepared as reported by [9]. Briefly, 500 g of barks powder of E. africana were extracted by maceration at room temperature with 2L of methylene chloride/methanol solvent system (1:1, v/v) during 48h. The suspension was filtered using Whatman paper N°1. The filtrate obtained was concentrated under reduced pressure using a rotary evaporator to yield 44 g of crude extract. Forty g of this crude extract were fractionated by flash chromatography over silica gel (70–230 mesh, Merck). Elution was done with a gradient of increasing polarity in the methylene chloride/methanol solvent system 100:0 v/v to 0:100 v/v; affording five fractions: methylene chloride/methanol 100:0, v/v (EaFc); methylene chloride/methanol 95:5, v/v (EaF5); methylene chloride/methanol 90:10, v/v (EaF10); methylene chloride/methanol 75:25, v/v (EaF25); methylene chloride/methanol 0:100, v/v (EaFm). Thirteen g of EaF10 (the most active fraction) were obtained, and 10 g of this fraction were further fractionated over silica gel 60 (particle size 40–63μm) column chromatography (column: d x h = 3.5 × 65 cm) coupled to thin layer chromatography (TLC) and seven sub-fractions namely EaF10sf1 (55mg), EaF10sf2 (47mg); EaF10sf3 (19mg); EaF10sf4 (51mg), EaF10sf5 (66mg), EaF10sf6 (31mg) and EaF10sf7 (58mg) were obtained and evaluated for their hepatoprotective activity in vitro. Finally, the most active sub-fractions along with EaF10 were analyzed by HPLC-ACN (Acetonitrile)-Standard-Method and their phytochemical components detected at 254nm as described by Kouam et al. [22].
2.3. Phytochemical and chemical antioxidant properties of EaF10
2.3.1. Phytochemicals, total polyphenols and flavonoids contents analysis
2.3.1.1. Phytochemicals screening
Preliminary phytochemical screening to detect different class of plant secondary metabolites (triterpenes, saponines, sugars, alkaloids, flavomoids and polyphenols) were conducted according to the reported method [23].
2.3.1.2. Determination of total polyphenols and flavonoids contents
Total polyphenols and flavonoids content determination were performed according to the Folin–Ciocalteu and aluminum chloride methods respectively as described by Dhar et al. [24]. Briefly, for polyphenols content, 50 μL of test sample (1 mg/mL in methanol), 2.4 mL distilled water and 200 μL of Folin–Ciocalteu's reagent (1/10) were mixed and incubated at 25 °C for 5min. Then, 200 μL of Na2CO3 20 % were added and the reaction mixture was incubated at 25 °C for 60min and the absorbance read at 765 nm. Gallic acid was used to establish a calibration curve and the total polyphenols content was expressed as milligrams of Gallic acid equivalent (GAE) per gram of extract.
For Flavonoids content, 25 μL of test sample was mixed with 4.975 mL of aluminum tri-chloride (AlCl3) 2% and incubated for 30 min in the dark, the absorbance of yellowish solution obtained was read at 420 nm. A calibration curve established with Quercetin allowed to express flavonoid content as milligrams of Quercetin equivalent (QE) per gram of extract.
2.3.2. Chemical antioxidant properties study
For the following assays, EaF10 and ascorbic acid used as positive control were tested in triplicate at the final concentrations of 1.5625; 3.125; 6.25; 12.5; 25; 50 and 100 μg/mL.
2.3.2.1. 2,2-Diphenyl-Picryl-Hydrazyl (DPPH) radical scavenging assay
The reported method of Moyo et al. [25] was used. In brief, 50 μL of tested sample (EaF10 or ascorbic acid) were mixed with 3.1 mL of DPPH solution (40 μg/mL in methanol) to achieve the indicated concentration. Then, the mixture was incubated at room temperature in the dark for 30 min, and the absorbance measure at 517 nm against the blank. Control samples were prepared with the same volume without plant extract nor ascorbic acid. The percentage of DPPH scavenging was calculated according to the following equation (E):
| DPPH Scavenging Activity (%) = 100 × [Acontrol -Asample]/Acontrol |
where:
Acontrol: absorbance of control; Asample: absorbance of sample.
2.3.2.2. Hydroxyl (HO°) radical scavenging assay
The assay was performed as previously described by [26]. The reaction mixture consisted of 0.7 mL of FeSO4 (3 mM), 1 mL of H2O2 (1 mM), 1 mL of distilled water, 50 μL of the test sample, and 0.4 mL of sodium salicylate (10 mM). The mixture was then incubated at 37 °C for 1 h, and the absorbance recorded at 562 nm against blank containing all reagents except sodium salicylate. The scavenging activity was calculated based on the above equation (E).
2.3.2.3. Nitric oxide (NO°) radical scavenging assay
The reported method of Ebrahimzadeh et al. [27] was used. The reaction mixture in phosphate buffer (1.6 mL, pH 7.4; 0.1 M) consisted of 1 mL sodium nitroprussiate 10 mM and 50 μL of test sample at the desired concentration. After incubation (25 °C, 2 h), 0.5 mL of Griess reagent (equal volume of 0.1% napthyl-ethylenediamine dihydrochloride and 1% sulphanylamide in 2.5% phosphoric acid) was added and the absorbance of the mixture recorded 30 min later at 546 nm. The NO˙ scavenging activity was calculated based on the above equation (E).
2.3.2.4. Inhibition of mice liver lipid peroxidation assay
The thiobarbituric acid method described by Su et al. [26] was used. FeCl2–H2O2 was used to induce lipid peroxidation in mice liver homogenate. Each test sample (50 μL) was mixed with 1 mL of a 10 % liver homogenate, and then, 50 μL of FeCl2 (0.5 mM) and 50 μL of H2O2 (0.5 mM) were added. The mixture was incubated at 37 °C for 60 min, and then, 1 mL of trichloroacetic acid (15%) and 1 mL of thiobarbituric acid (0.67 %) were added, and the mixture was heated to 100 °C for 15 min. After centrifugation (3000 g, 5 min, 4 °C), the absorbance of the supernatant was read at 532 nm. The percentage of inhibition was calculated according to the equation (E).
Different EC50/IC50 values were automatically determined using GraphPad Prism 5.03 software by non-linear regression (Log [inhibitor] vs. response). Then, correlation between total polyphenol content and antioxidant activity of EaF10 were determined by linear regression. Polyphenols content in each tested concentrations (1.5625; 3.125; 6.25; 12.5; 25; 50 and 100 μg/mL) were determined. Then, for each chemical antioxidant model, the activity of EaF10 at each tested concentration was plotted against the total polyphenol content at the same concentration using Microsoft Excel 2013, and the coefficient r2 value was deduced from the graph.
2.4. In vitro hepatoprotective activity studies of EaF10 and its sub-fractions
2.4.1. Cells and culture conditions
The normal human liver cell line L-02 (Cell Bank, Type Culture Collection of Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) were used. Cells were cultured in 100mm dish and maintained in high glucose Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, L-glutamine (2 mM), penicillin (100 IU/mL), streptomycin (100 μg/mL) and amphotericin B (0.25 μg/mL) in an atmosphere of 5 % CO2 at 37 °C.
2.4.2. Experimental design
Silymarin was used as hepatoprotective reference compound. 20% DMSO (20% in phosphate buffer saline [PBS]) was used to dissolve and prepare the initial concentrations CCl4, silymarin, EaF10 and its sub-fractions. L-02 Hepatocytes (density ≈ 2.105 cells/ml) in triplicate were plated into 12-well plate designated as control, CCl4, standard and test (reference or plant sample + CCl4). After 24h of incubation. Medium was replaced with fresh medium and cells were incubated in absence (control group) or in presence of CCl4 (CCl4 group), or in presence of CCl4 and silymarin or plant sample (test groups) for different time points (6; 24 and 36 h) depending on the downstream analysis. Briefly, 10 μL of each initial concentration were added to fresh medium to achieve the final desired concentration in a final volume of 1000 μL. In each group of cells, treatments were as follows:
-
-
Test groups: 980 μL fresh medium +10 μL CCl4 + 10 μL plant sample/silymarin.
-
-
CCl4 group: 980 μL fresh medium+ 10 μL CCl4 + 10 μL 20% DMSO.
-
-
Control group: 980 μL fresh medium +10 μL 20% DMSO +10 μL 20% DMSO.
Overall, 20 μL of 20% DMSO were mixed with 980 μL of fresh medium to achieve the final volume of 1000 μL with a dilution factor of 50. Thus, in test group, CCl4 groups as well as control group, the final concentration of DMSO given to the cells was 0.4%.
2.4.3. Determination of the toxic concentration of CCl4 to be used
Cells were treated with CCl4 at the final concentration of 0; 5; 10; 15; 20; 25 and 30 mM. 36 h later, cell viability was assessed using 3-(4, 5-dimethylthiosol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide kit (MTT; Sigma-Aldrich) as indicated by the manufacturer's instructions and the cell membrane integrity was evaluated by measuring alanine aminotransferase (ALT) activity released into the incubation medium [28]. Cell viability was expressed as percentage of control and the half toxic concentration (TC50) of CCl4 was determined using concentration-response curve and used as toxic concentration for the next experiments.
2.4.4. Hepatoprotective activity screening of plant sub-fractions and concentration-response study of the selected active sub-fractions
In the hepatoprotective activity screening, silymarin, EaF10 and its different sub-fractions were tested at the final concentration of 100 μg/mL. L-02 hepatocytes were seeded at the density of 2.105 cells/mL and incubated for 24h; and afterwards, medium was changed. Then, cells were treated with plant samples or silymarin and CCl4 at the predetermined concentration for 36h and cell viability and membrane integrity were assessed as abovementioned. In the hepatoprotective dose-response study, silymarin, EaF10 and the most active sub-fractions of EaF10 selected, were tested at the final concentration of 6.25; 12.5; 25; 50 and 100 μg/mL; and cell viability and membrane integrity were also assessed.
2.4.5. Measurement of intracellular ROS level and lipid membrane peroxidation
Level of intracellular ROS was determined as described by [29]. In brief, cells were treated with 20 μM H2DCFDA, CCl4 and selected active sub-fractions or silymarin at the determined concentrations for 36 h. Afterward, supernatant was collected and cells were washed with PBS and lysed in a lysis buffer. Then, cell lysates were centrifuged (10,000g, 5 min, 4 °C) and 100 μL of lysate was used to measure the fluorescence at excitation 485 ± 20 nm, emission 525 ± 20 nm in a black wall with clear bottom 96-well plate using a spectrophotometer (SpectraMax M5, Molecular Devices) and relative level of ROS was expressed as percentage of control. Lipid membrane peroxidation was evaluated in the cellular supernatant as previously described [9].
2.4.6. Determination of glutathione (GSH) content
L-02 hepatocytes were incubated with CCl4 and active sub-fractions or silymarin for 36 h. Then, cells were harvested, lysed and centrifuged (10,000g, 5 min, 4 °C). The supernatant was collected and the GSH concentration was determined as previously described [30].
2.4.7. Protein extraction, subcellular fractionation and western blot analysis
L-02 hepatocytes were treated without CCl4 or simultaneously with CCl4 and active sub-fractions or silymarin at the determined concentration and incubated for different time points. Afterwards, total proteins were extracted using M-PER Mammalian Protein Extraction Reagent (Thermo Scientific) containing 0.2 % Halt protease inhibitor cocktail EDTA-Free 100X (Thermo Scientific); cytosolic and nuclear protein were extracted using NE-PER® Nuclear and Cytoplasmic Proteins Extraction Kit (Thermo Scientific); In each sample, protein concentration was determined with Pierce BCA Proteins Assay Kit (Thermo Scientific).
For western blot analysis, approximately 50 μg of protein in Laemmli loading buffer was separated through 12 % Sodium-Dodecyl-Sulfate Poly-Acrylamide Gel Electrophoresis (SDS-PAGE) and electro-transferred into a Nitrocellulose Blotting Membrane (GE Healthcare, Life Science, Germany). The membrane was blocked with 5% w/v dehydrated skimmed milk in Tris-Buffered Saline Tween-20 (TBST: 10mM Tris-HCl; 150mM NaCl; 0.05% tween-20; pH 7.6); incubated overnight at 4 °C with primary antibodies, rinsed, and then incubated for 1h at 25 °C with horseradish peroxidase-conjugated secondary antibodies. Membrane was stained with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and detected by Enhanced Chemiluminescent Method which combines MicroChemi Unit and GelCapture Software. Densitometry analysis of the protein bands was performed using ImageJ Software.
2.4.8. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Cells were exposed to CCl4 or treated simultaneously with CCl4 and active sub-fractions or silymarin at the determined concentration and incubated for 36 h. Then, total RNA was extracted from cells using TRIzol® Reagent (Ambion, Lifes Technologies) according to the manufacturer's instruction. The concentration and purity of RNA were determined spectrophotometrically from A260/A280 and A260/A230 ratio using a ND-2000 NanoDrop (Thermo Scientific). A total of 1μg RNA was converted to cDNA using oligo (dT) and Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT, Promega, Woods Hollow Road Madison, USA) as directed by the manufacturer.
Real-time qPCR was performed in Applied Biosystems 7500 System using iTaq™ Universal SYBR® Green Supermix (Bio-RAD Laboratories, Ca, USA) according to the manufacturer's instruction. Relative expression of target genes was normalized to the endogenous gene (GAPDH) used as internal control, analyzed by the 2−ΔΔCT method using GenEX Software and given as ratio compared to the control group. In addition, the PCR products were analyzed on 1% agarose gel electrophoresis. All primers of interest genes were synthetized by TSINGKE Biological Technology Company (Beijing, China). Their sequences are as follows: CAT: forward: AGGCCAGTCCTGACAAAATG, reverse: GAATCTCCGCACTTCTCCAG; SOD1: forward. GAAGGTGTGGGGAAGCATTA, reverse: ACATTGCCCAAGTCTCCAAC; GST: Forward. TTGGCCTCCTGTATTCCTTG, reverse, AGCCAACTGGATGCTGAGTT and GAPDH: Forward. CGACCACTTTGTCAAGCTCA, reverse, AGGGGTCTACATGGCAACTG.
2.5. In vivo anti-hepatotoxic activity studies of EaF10
2.5.1. Animals and treatments
Male Swiss Albino mice (Mus musculus, 30±5g) were obtained from the Animal house of the Laboratory of Pharmacology and Toxicology (University of Yaoundé 1). The animals were kept in plastic cage, supplied with standard laboratory diet and water ad libitum with a 12h light-dark cycle. All animals received humane care in compliance with the National Institutes of Health guidelines on animal care and all procedures were approved by the Institutional Joint Review Board for Animals and Humans Bioethics of the University of Yaounde I-Cameroon.
To induce liver injury, CCl4 diluted in corn oil (1:3, v/v) was administered by intraperitoneal injection to animals at 1mL/Kg b.w. as described by H.-L. Huang et al. [31]. Silymarin and the plant fraction EaF10 were dissolved in 1% carboxylmethylcellulose (CMC) and given orally to animals at 10 mL/Kg b.w. One hour after corn oil or CCl4 injection, animal groups were treated thrice at 1 h, 12 h and 24 h with 1% CMC, silymarin or EaF10, respectively at the doses shown in the following experimental design. After one week of acclimation, thirty (30) mice were divided into five groups of 6 animals each: (1) Normal control group (corn oil + CMC); (2) CCl4-intoxicated group (CCl4 + CMC); (3) Positive control group (CCl4 + Silymarin (100 mg/Kg b.w)); (4) Experimental group 1 (CCl4 + EaF10 (25 mg/Kg b.w)) and (5) Experimental group 2 (CCl4 + EaF10 (100 mg/Kg b.w). Animals were anesthetized and sacrificed 24 h after the last administration of silymarin or EaF10, and blood and liver tissue collected.
2.5.2. Assessment of liver function markers
To assess hepatotoxicity, serum activities of alanine (ALT) and aspartate (AST) aminotransferases were measured. Blood samples collected were kept at room temperature for 60min. Serum were then obtained by centrifugation (3000g, 15min, 4 °C) and the activities of ALT and AST were determined according to the reported method of Reitman and Frankel [21].
2.5.3. Assessment of oxidative stress parameters
Oxidative stress was evaluated by measuring serum level of nitric oxide (NO), liver contents of lipid peroxidation and protein carbonyl.
2.5.3.1. Measurement of NO level
The level of NO in the serum was determined by the colorimetric method based on Griess reaction as previously described [32]. Serum samples were diluted four times with distilled water and deproteinized by adding 1/20 volume of zinc sulfate (300 g/L) to a final concentration of 15 g/L. After centrifugation (10,000g; 5min; 25 °C), 500μL of the supernatant was mixed with 500 μL of Griess reagent (equal volume of 0.1% napthylethylenediamine dihydrochloride and 1% sulphanylamide in 2.5% phosphoric acid) and incubated at room temperature for 15 min. Finally, the absorbance of the sample was read at 540nm; and the level of NO was determined using the calibration curve established with sodium nitrite (0.5–20 μM).
2.5.3.2. Estimation of lipid peroxidation end product and protein carbonyl content
The lipid peroxidation in 10% liver tissue homogenate (in Tris-HCl buffer 20 mM; KCl 150 mM pH 7.4 containing 0.2% Halt protease inhibitor cocktail EDTA-Free 100X) in terms of malondialdehyde (MDA) formation was measured as previously described by Njayou et al. (2016). The absorbance of thiobarbituric acid reactive substance (TBARS) formed as end product, was read at 532 nm and the concentration was determined by using its molar extinction coefficient of MDA (εMDA = 1.56 × 105 M−1.Cm−1).
Protein carbonyl contents were determined as described elsewhere [33]. The samples were treated with an equal volume of 1% (w/v) 2,4-DNPH in 2N HCl and incubated for 60 min at room temperature. One third volume of 20% trichloroacetic acid (TCA) was then added for precipitate formation which was collected by centrifugation (10 000g, 4 °C, 5 min). After extraction (3 times with ethanol/ethylacetate, v/v) and dissolution in 8M guanidine hydrochloride (in 133mM Tris solution containing 13mM EDTA). The absorbance was measured at 365nm and the results were expressed as nmol of DNPH incorporated/mg protein using the molar extinction coefficient of aliphatic hydrazones (22 × 103 M−1.Cm−1). Protein content is liver tissues homogenate was determined by the Biuret Method [34] using BSA as standard.
2.5.4. Assessment of enzymatic and non-enzymatic antioxidant defense systems
2.5.4.1. Superoxide dismutase (SOD) activity measurement
Measurement of SOD activity was performed as described by Ghosh et al. [35]. Assay mixture consisted 1.2 mL sodium pyrophosphate buffer (50mM; pH 8.3), 100 μL phenazine methosulfate (186μM), 300μL nitroblue tetrazolium (300 μM), 200 μL NADH (720 μM), appropriately diluted volume of liver homogenate (10 μg of protein) and distilled water in total volume of 3 mL. Reaction was started by addition of NADH. After incubation (30 °C, 90s), the reaction was stopped by addition of 1mL glacial acetic acid. The reaction mixture was then stirred vigorously and shaken with 4mL of n-butanol. The mixture was allowed to stand for 10 min at room temperature; and centrifuged (3000g, 5min, 25 °C). Finally, absorbance of the chromogen in butanol layer was measured at 560 nm. One unit of enzyme activity was defined as enzyme concentration required to inhibit absorbance of chromogen production by 50% per minute and SOD activity was expressed as specific activity in Unit/min/mg protein.
2.5.4.2. Catalase (CAT) activity measurement
CAT activity was determined based on its ability to convert hydrogen peroxide (H2O2) into water and molecular oxygen. The assay was performed as described by Aebi, [36]. Briefly, 1mL phosphate buffer (50mM; pH 7.2) was mixed to 990μL H2O2 (10mM) solution and 10μL of liver homogenate was added. The decrease of the absorbance of H2O2 was monitored at 240nm and recorded at 20s and 80s. The CAT activity was then calculated by using the following formula: CAT Activity (Unit/min/mg of protein) = (2.3033/ΔT) × (logA1/A2)/Qprotein where A1 is the absorbance at 20s; A2 is the absorbance at 80s; ΔT is the variation in time (1min) and Qprotein is the quantity of proteins (mg) in liver homogenate.
2.5.4.3. Glutathione-S-Transferase (GST) activity measurement
GST activity was assayed based on the conjugation of GSH with 1-chloro-2,4-dinitrobenzene (CDNB) as described by Habig et al. [37]. The reaction was carried out at room temperature and monitored at 340nm for 5min. A blank was run in the absence of enzyme source. In brief, 20μL of liver homogenate was mixed with 2mL potassium phosphate buffer (0.1M; pH 6.5) containing 5mM GSH, 1mM CDNB and 1mM EDTA. The enzyme activity was calculated using the molar extinction coefficient (9.6 × 10−3 M−1.Cm−1). One unit enzyme was defined as enzyme concentration required to yield 1μmol product formation per minute and GST activity was expressed as specific activity in μmole/min/mg protein.
2.5.4.4. Estimation of reduced glutathione (GSH) content
GSH content was assayed according to Ellman, [23]. Twenty microliters of liver homogenate were added to 3 mL of Ellman's reagent (0.05 mM DTNB in phosphate buffer 0.1 M pH 6.5). After homogenization, the mixture was maintained at room temperature for 60 min and the optical density was read at 412 nm. The glutathione concentration was determined by using its molar extinction coefficient (εGSH = 13,600 M−1.Cm−1).
2.5.5. Histological examination
Liver portion were fixed in 10% formaldehyde solution, then dehydrated and embedded in paraffin. Paraffin sections (4–5 μm) were obtained by sectioning embedded fragments on a rotary microtome. The sections were mounted on glass slides and stained with hematoxylin and eosin for visualization of histological changes under optic microscope (X-100).
2.5.6. Statistical analysis
Results are presented as mean ± standard deviation (for in vitro study, three independent experiments in triplicate). Comparisons between the mean values of various treatments groups were analyzed by one-way analysis of variance (ANOVA) followed by the Bonferroni's post hoc test whenever significant differences were observed between the variances. Comparisons were made between untreated group (control group) and intoxicated group (CCl4-intoxicated group), and between CCl4-intoxicated group and treated groups (CCl4 + EaF10, active sub-fractions or silymarin). Differences between compared groups were considered significant for p < 0.05. Analyses were performed using Prism 5.03 statistical software (Graph Pad Inc.).
3. Results
3.1. Phytochemicals composition, total polyphenol and flavonoid contents and HPLC fingerprint of EaF10 and its active sub-fractions
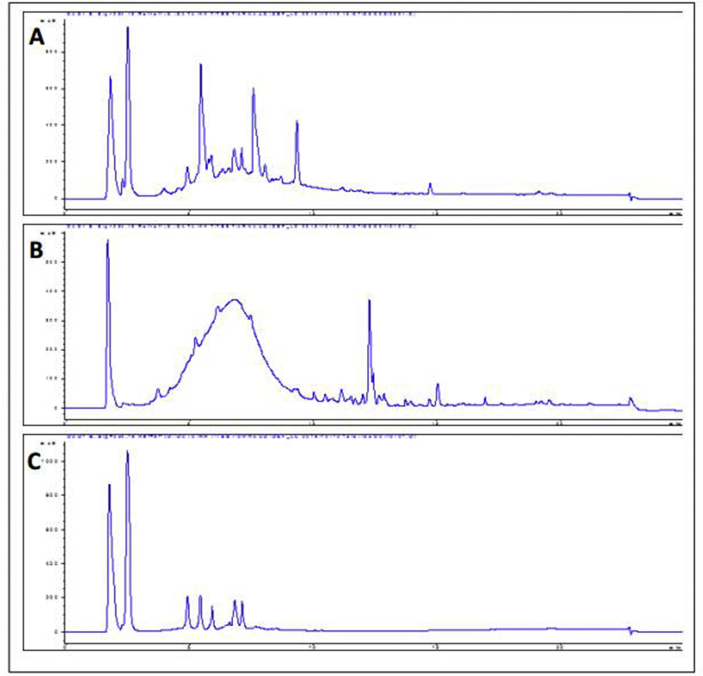
As shown in Table 1, EaF10 fraction of E. africana, revealed the presence of polyphenols and flavonoids among others classes of phytochemical compounds detected. The total polyphenol and flavonoid contents were 39.105 ± 2.306 mg GAE/g of extract and 17.083 ± 3.120 mg QE/g of extract, respectively. Representative HPLC chromatogram Figure 1 of EaF10 and its active sub-fractions EaF10sf1 and EaF10sf2 are presented in Figure 1A, B and C respectively. Several peaks with UV absorbance at 254nm were detected, likely indicating the presence of aromatic compounds.
Table 1.
Phytochemical composition, total polyphenol and flavonoid content of Eaf10.
| EaF10 | Class of secondary metabolites tested positive | Polyphenol content (mg GAE/g of extract) | Flavonoid content (mg QE/g of extract) |
|---|---|---|---|
| Polyphenols, flavonoids, triterpenes, saponines, sugars | 39.105 ± 2.306 | 17.083 ± 3.120 |
EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; mg GAE/g of extract: milligrams of Gallic acid equivalent per gram of extract; mg QE/g of extract: milligrams of Quercetin equivalent per gram of extract. Values are means ± SD of three independents experiments in triplicate.
Figure 1.
HPLC chromatogram of EaF10 and its active sub-fractions. A, B and C: chromatogram of EaF10, EaF10sf1 and EaF10sf2 respectively analyzed by HPLC-ACN-Standard-Method. Eclipse XDB-C8 column (9.4 × 250 mm, 5 μm particle size); mobile phase: (A) water and (B) acetonitrile; elution condition: B, 0–15 min, increasing gradient from 0 to 30 % B, 15–20 min, linear gradient 100 % B; 20–25 min, linear gradient 30 % B; flow rate: 1 mL/min; injection volume: 5 μL. EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; EaF10sf1: sub-fraction 1 of EaF10; EaF10sf2: sub-fraction 2 of EaF10.
3.2. Chemical antioxidant activities of EaF10 in free-cell system
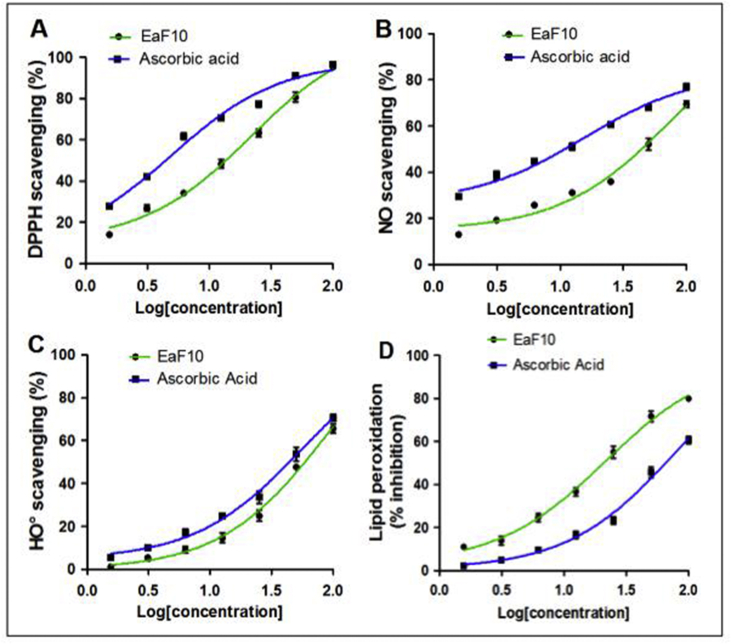
Figure 2 depicts the concentration-dependent activities of EaF10 and ascorbic acid, in the four chemical antioxidant models tested, whereas Table 2 presents their EC50/IC50 values. In DPPH, NO˙ and HO˙ scavenging assays, the antioxidant activities of EaF10 (EC50 range 21.72–89.93 μg/mL) were lower, compared to that of ascorbic acid (EC50 range 5.04–78.45 μg/mL) (Table 2). However, in lipid peroxidation inhibition assay, the fraction EaF10 showed strong antioxidant activity (IC50: 21.45 μg/mL) compared to ascorbic acid (IC50: 78.54 μg/ml).
Figure 2.
Chemical antioxidant activities of EaF10. (A): DPPH radical scavenging assay; (B): Nitrite oxide radical scavenging assay, (C): Hydroxyl radical scavenging assay; (D) Inhibition of rat liver peroxidation assay; EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana. Values are means ± SD of three independent experiments in triplicate.
Table 2.
Half efficient (EC50)/inhibition (IC50) concentration in different chemical antioxidant model and correlation coefficient between antioxidant activities and polyphenol content of EaF10.
| Parameters | Chemical antioxidant models |
|||||||
|---|---|---|---|---|---|---|---|---|
| Effect of EaF10 fraction |
Effect of Ascorbic acid |
|||||||
| DPPH | NO | HO | LP | DPPH | NO | HO | LP | |
| EC50/IC50 (μg/mL) | 21.72 ± 1.15 | 73.87 ± 1.31 | 89.93 ± 1.25 | 21.45 ± 1.16 | 5.04 ± 1.20 | 15.63 ± 1.20 | 55.76 ± 1.23 | 78.54 ± 1.25 |
| Correlation coefficient r2 | 0.831 | 0.938 | 0.953 | 0.805 | / | / | / | / |
EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; DPPH: DPPH radical scavenging assay; NO: Nitrite oxide radical scavenging assay, HO: Hydroxyl radical scavenging assay; LP: Inhibition of rat liver peroxidation assay; EC50/IC50: Concentration of EaF10 necessary to achieve 50% of activity. Values are means ± SD of three independent experiments in triplicate./: none available.
In addition, the all r2 (correlation coefficient of EaF10 between total polyphenol content and antioxidant activity) were found to be ranged from 0.805 to 0.953 in the four chemical antioxidant models studied.
3.3. Effect of EaF10 and its active sub-fractions on CCl4-induced hepatotoxicity in vitro
3.3.1. Effect of CCl4 on cell viability and loss of membrane integrity in L-02 hepatocytes
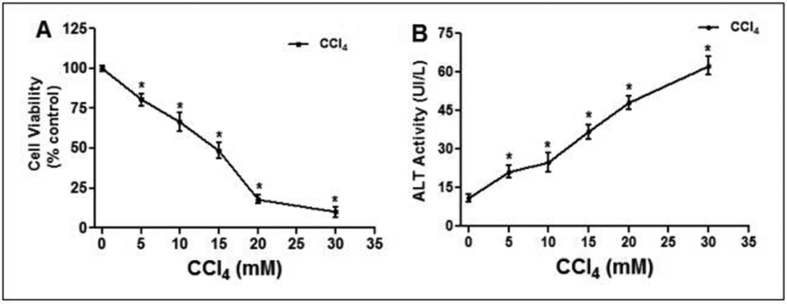
A concentration-dependent assay was used to evaluate the toxicity of CCl4 (concentration range from 5 to 30 mM) in L-02 hepatocytes. As shown in Figure 3, CCl4 significantly (p˂0.05) decreased cell viability (Figure 3A) and increased cellular ALT activity leakage into the incubation medium (Figure 3B) in concentration-dependent manner. The TC50 was 14.67 ± 3.55 mM; accordingly, 15 mM of CCl4 was used as toxic concentration for the following experiment.
Figure 3.
Concentration-dependent effect of CCl4 on cell viability and membrane integrity in L-02 hepatocytes. Cells were treated with different concentration of CCl4 (5–30mM) for 36h. (A) Cell viability indicating dose-effect of CCl4-toxicity; (B) loss of membrane integrity indicated by ALT activity found in the culture medium. Values are means ± SD of three independent experiments in triplicate; ∗P<0.05, compared to control (0mM).
3.3.2. Hepatoprotective activity screening and concentration-dependent protective effect of EaF10 and its active sub-fractions against CCl4-induced toxicity in L-02 hepatocytes
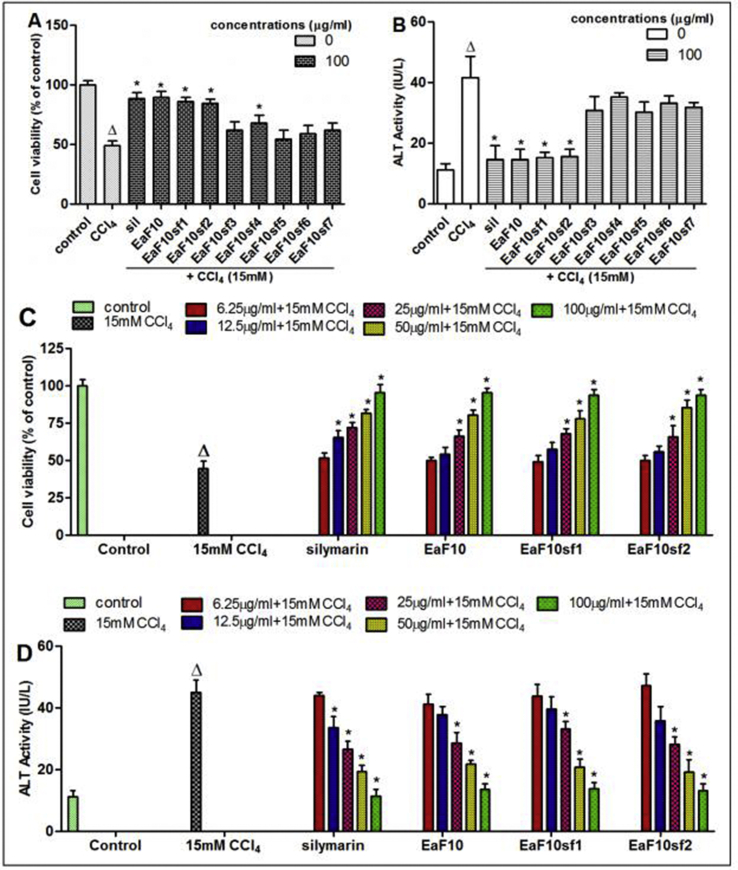
The sub-fractions of EaF10 were screened for their anti-hepatotoxic properties against CCl4-induced cell death. The plant sub-fractions EaF10sf1 and EaF10sf2 as well as fraction EaF10 significantly (p˂0.05) maintained cell viability (Figure 4A) and inhibited the release of ALT (Figure 4B) from the cell. These effects were not obviously different from that observed in silymarin-treated cells. Consequently, the abovementioned sub-fractions were selected as the most active and used for the latter part of the study.
Figure 4.
Hepatoprotective screening activity and concentration-dependent protective effect of EaF10 and its selected active sub-fractions. L-02 cells were treated without or with CCl4 (15mM), or co-treated with CCl4 and EaF10 sub-fractions or silymarin (100 mg/mL) for 36h. After treatment, cell viability (A) and ALT activity (B) leakage into the incubation medium were determined. Cells were treated without or with CCl4 (15mM), or co-treated with CCl4 and selected active sub-fractions of EaF10 or silymarin (6.25; 12.5; 25; 50 and 100 μg/mL) for 36h. C and D: Concentration-dependent effect of active sub-fractions on cell viability and ALT activity leakage into the incubation medium respectively. Values are means ± SD of three independent experiments in triplicate; ΔP<0.05 significantly different compared to control group. ∗P˂0.05 significantly different compared to CCl4-intoxicated group. Sil: silymarin; EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; EaF10sf1: sub-fraction 1 of EaF10; EaF10sf2: sub-fraction 2 of EaF10; EaF10sf3: sub-fraction 3 of EaF10; EaF10sf4: sub-fraction 4 of EaF10; EaF10sf5: sub-fraction 5 of EaF10; EaF10sf6: sub-fraction 6 of EaF10; EaF10sf7: sub-fraction 7 of EaF10.
Simultaneous treatment of L-02 hepatocytes with CCl4 and the selected active sub-fractions or silymarin at different concentrations resulted in a concentration-dependent protective and inhibitory effects on cell viability and ALT leakage, as depicted in Figure 4C and D, respectively. At the concentration of 100 μg/mL, cell viability remained practically unaltered, as compared to untreated cells. Thus, this concentration has been considered as optimum for the subsequent experiments in this part of the study.
3.3.3. Effect of EaF10 and its active sub-fractions on CYP2E1 expression in CCl4-intoxicated L-02 cells
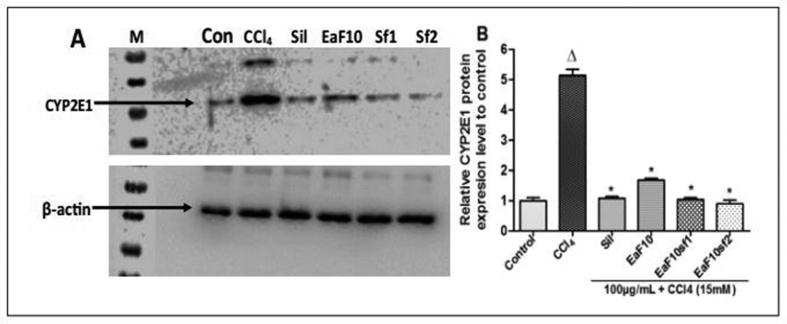
Figure 5 illustrates the effect of EaF10 and its active sub-fractions on the expression of CYP2E1 analyzed by western blot in L-02 cells after CCl4-intoxication. Incubation of L-02 hepatocytes in the presence of CCl4 (15 mM) alone lead to overexpression of CYP2E1 protein which was significantly (p˂0.05) downregulated when cells were co-treated with EaF10 and its active sub-fractions (Figure 5A and B). This effect was comparable to that observed in silymarin co-treated cells.
Figure 5.
Inhibitory effect of EaF10 active sub-fractions on CYP2E1 protein expression. L-02 cells were treated without or with CCl4 (15mM), or co-treated with CCl4 and EaF10 active sub-fractions or silymarin (100 mg/mL) for 6h. After treatment, total proteins were extracted from cells and CYP2E1 expression was determined by western blotting. β-actin was used as loading control. Each blot represents one of three independent experiments. A and B: effect of active sub-fraction on CYP2E1 expression and densitometry analysis of blots respectively. Values are means ± SD of three independent experiments in triplicate. ΔP<0.05 significantly different compared to control group. ∗P˂0.05 significantly different compared to CCl4-intoxicated group. M: molecular weight marker; Con: control group; Sil: silymarin; EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; EaF10sf1: sub-fraction 1 of EaF10; EaF10sf2: sub-fraction 2 of EaF10.
3.3.4. Effect of EaF10 and its active sub-fractions on CCl4-induced oxidative stress in L-02 hepatocytes
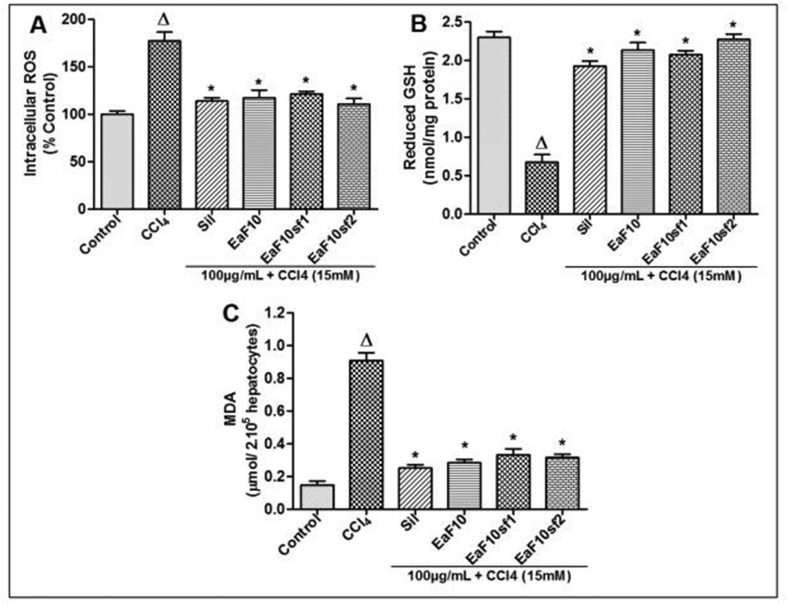
The effect of EaF10 and its active sub-fractions on intracellular ROS generation, cellular GSH content and MDA formation was assessed after intoxication of cells with CCl4. As shown in Figure 6A, B and C, incubation of L-02 hepatocytes in presence of CCl4 alone significantly (p˂0.05) increased intracellular ROS, decreased GSH content and increased MDA formation respectively, as compared to untreated cells. In contrast, co-treatment of cells with EaF10, its active sub-fractions or silymarin significantly (p˂0.05) inhibited excessive generation of ROS (Figure 6A), rescued GSH content (Figure 6B) and inhibited MDA production (Figure 6C), compared to CCl4-intoxicated cells.
Figure 6.
Protective effect of EaF10 and its active sub-fractions against CCl4-induced ROS overproduction, GSH depletion and lipid peroxidation in L-02 hepatocytes. L-02 cells were treated without or with CCl4 (15mM), or co-treated with CCl4 and EaF10 active sub-fractions or silymarin (100 mg/mL) for 36h. After treatment, intracellular ROS level (A), cellular GSH content (B) and MDA concentrations (B) in the incubation medium were measured. Values are means ± SD of three independent experiments in triplicate. ΔP<0.05 significantly different compared to control group. ∗P˂0.05 significantly different compared to CCl4-intoxicated group. Sil: silymarin; EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; EaF10sf1: sub-fraction 1 of EaF10; EaF10sf2: sub-fraction 2 of EaF10.
3.3.5. Effect of EaF10 and active sub-fractions on nuclear translocation of Nrf2 in CCl4-intoxicated L-02 cells
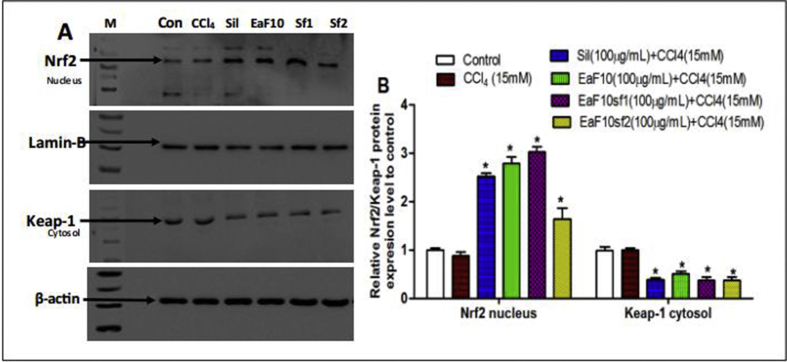
The capacity of EaF10 and its active sub-fractions to induce the nuclear translocation of Nrf2 in CCl4-intoxicated hepatocytes was analyzed by immunoblotting. As presented in Figure 7, simultaneous treatment of L-02 hepatocytes with CCl4 and EaF10, its active sub-fractions or silymarin (100 μg/mL) increased the Nrf2 protein level in the nucleus by up-to 3.1-fold at 24h (Figure 7A and B) after treatment, as compared to untreated cells. There was no obvious change in nuclear Nrf2 protein level between untreated cells and CCl4-intoxicated cells. Interestingly, in the cytosol of EaF10, active sub-fractions or silymarin co-treated cells, a significant (p˂0.05) decrease of the expression of Keap-1 (Figure 7A and B), a repressor of Nrf2 activation, was observed.
Figure 7.
Up-regulating effect of EaF10 active sub-fractions on the nuclear translocation of Nrf2 in CCl4-intoxicated cells. L-02 cells were treated without or with CCl4 (15mM), or co-treated with CCl4 and EaF10 active sub-fractions or silymarin (100 mg/mL) for 24h. After treatment, Nrf2 level was detected into the nuclear fraction and Keap-1 level was detected into the cytosolic fractions by western blotting. Lamin-B and β-actin were used as loading control respectively for the nuclear and cytosolic fraction. Each blot represents one of three independent experiments. A and B: effect of active sub-fraction on Nuclear Nrf2 and cytosolic Keap-1 protein expression level; and densitometry analysis of blots respectively. Values are means ± SD of three independent experiments in triplicate. ΔP<0.05 significantly different compared to control group. ∗P˂0.05 significantly different compared to CCl4-intoxicated group. M: molecular weight marker; Con: control group; Sil: silymarin; EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; EaF10sf1: sub-fraction 1 of EaF10; EaF10sf2: sub-fraction 2 of EaF10.
3.3.6. Effect of EaF10 and active sub-fractions on the mRNA expression level of antioxidant enzymes in CCl4-intoxicated L-02 cells
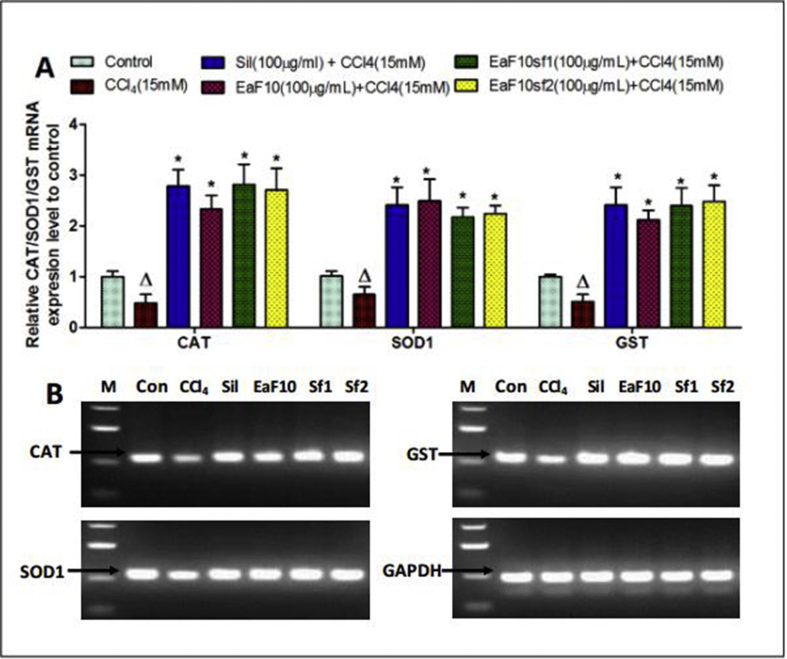
Figure 8 depicts the effect of EaF10 and its active sub-fractions on the mRNA expression level of the antioxidant enzymes (CAT, SOD1 and GST) in CCl4-intoxicated cells, quantified by qRT-PCR (Figure 8A), followed by agarose gel electrophoresis analysis of PCR product (Figure 8B). Incubation of L-02 hepatocytes in presence of CCl4 (15mM) alone during 36h significantly (p˂0.05) decreased the mRNA expression levels of CAT, SOD1 and GST. In EaF10, active sub-fractions or silymarin co-treated cells, the mRNA expression levels of these antioxidant enzymes were increased by up-to 2.9-fold as compared to untreated cells.
Figure 8.
Up-regulating effect of EaF10 active sub-fractions on the mRNA expression level of antioxidant enzymes in CCl4-intoxicated cells. L-02 cells were treated without or with CCl4 (15mM), or co-treated with CCl4 and EaF10 active sub-fractions or silymarin (100 mg/mL) for 36h. After treatment, total RNA was extracted from cells and relative mRNA expression level (A) of CAT, SOD1and GST were determined by qRT-PCR. GAPDH was used as internal control. (B): Agarose gel electrophoresis analysis of qRT-PCR product showing up-regulating effect of EaF10 active sub-fractions. Values are means ± SD of three independent experiments in triplicate. ΔP<0.05 significantly different compared to control group. ∗P˂0.05 significantly different compared to CCl4-intoxicated group. M: molecular weight marker; Con: control group; Sil: silymarin; EaF10: methylene chloride/methanol (90:10, v/v) fraction of E. africana; EaF10sf1: sub-fraction 1 of EaF10; EaF10sf2: sub-fraction 2 of EaF10.
3.4. Effect of EaF10 on CCl4-induced hepatotoxicity in vivo
3.4.1. Effect of EaF10 on CCl4-induced hepatic injury
To evaluate the effects of EaF10 on liver injury induced by CCl4 in mice, the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined. As presented in Table 3, the levels of ALT and AST activities in the serum were significantly (p˂0.05) increased in CCl4-intoxicated group, compared to normal control group. After treatment of mice with EaF10 (25 mg/Kg b.w or 100 mg/Kg b.w), or silymarin (100 mg/Kg b.w), a significant reduction in serum levels of ALT and AST activities, as compared to the CCl4-intoxicated mice was observed.
Table 3.
Effect of EaF10 on the levels of serum markers related to hepatic dysfunction.
| Name of serum markers | Level of serum markers |
||||
|---|---|---|---|---|---|
| Control | CCl4 | CCl4 + Sil (100mg/Kg) | CCl4 + EaF10 (25mg/Kg) | CCl4 + EaF10 (100mg/Kg) | |
| ALT (IU/L) | 34.48 ± 5.26 | 87.68 ± 7.56a | 40.77 ± 8.42b | 61.36 ± 4.73b | 45.24 ± 8.07b |
| AST (IU/L) | 19.23 ± 6.07 | 69.25 ± 7.56a | 28.61 ± 7.33b | 42.07 ± 5.89b | 27.51 ± 4.73b |
Mice in different groups were treated as indicated in the material and methods section. Hepatic dysfunction related to CCl4-hepatotoxicity was determined by quantifying the serum activities (ALT) and (AST). Values are expressed as means ± SD, n = 6; a values significantly different compared to normal control group (P˂0.05); b values significantly different compared to CCl4-intoxicated group (P˂0.05) using Bonferroni's test. Control: normal control group; CCl4: CCl4-intoxicated group alone; CCl4+Sil (100mg/Kg): CCl4-intoxicated + silymarin (100mg/Kg)-treated group; CCl4 + EaF10 (25mg/Kg): CCl4-intoxicated + EaF10 (25mg/Kg)-treated group; CCl4 + EaF10 (100mg/Kg): CCl4-intoxicated + EaF10 (100mg/Kg)-treated group. ALT: alanine aminotransferase; AST: aspartate aminotransferase.
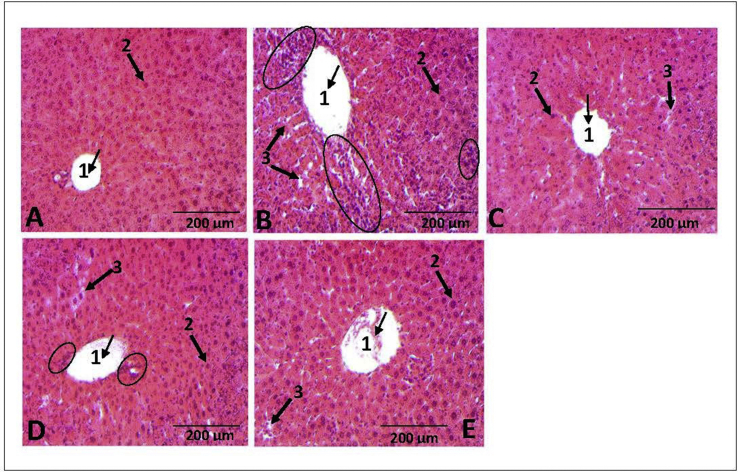
Furthermore, liver tissue sections, stained with hematoxylin-eosin were used to investigate the protective effect of EaF10 on CCl4-induced histopathological alterations. The results are shown in Figure 9. The structure of liver tissues was unaltered in the normal control group (Figure 9A) with hepatocytes in ordered arrangement and no pathological changes area. In contrast, liver tissues in CCl4-intoxicated group were characterized by hepatic lobule impairment, severe hepatocytes necrosis and massive inflammatory cells infiltration around centrolobular vein (Figure 9B). Interestingly, EaF10 treatment effectively reduced the percentage of necrotic areas in the liver with moderated inflammatory cells infiltration and cyto-architecture repair (Figure 9D). Compared to CCl4-intoxicated group, no inflammatory cells infiltration was observed in EaF10 high-dose group (100 mg/Kg b.w; Figure 9E), or silymarin-treated group (100 mg/Kg b.w; Figure 9C).
Figure 9.
Effect of EaF10 on histopathological changes by CCl4 in mice liver tissue stained with hematoxylin-eosin (H-E, X100). Mice in different groups were treated as indicated in the material and methods section. The animals were sacrificed 24h after the last EaF10 or silymarin administration and the liver was removed, fixed and embedded in paraffin. Sections were stained with H-E, X100. (A) Liver section of control mice showing normal morphology: centro-lobular vein (arrow 1) and normal hepatocyte (arrow 2). (B) Liver section CCl4-treated mice showing severe hepatocytes necrosis (arrow 3) with massive inflammatory cell infiltration (circle) around centro-lobular vein (arrow 1). (C) Liver section of mice co-treated with CCl4+silymarin (100mg/Kg, b.w) showing almost normal morphology with mild hepatocytes necrosis (arrow 3) without inflammatory cell infiltration. (D) Liver section of mice co-treated with CCl4+EaF10 (25mg/Kg, b.w) showing mild hepatocytes necrosis (arrow 3) with moderate inflammatory cell infiltration (circle). (E) Liver section of mice co-treated with CCl4+EaF10 (100mg/Kg, b.w) showing almost normal morphology with mild hepatocytes necrosis (arrow 3) without inflammatory cells infiltration.
3.4.2. Effect of EaF10 on CCl4-induced hepatic oxidative stress
To evaluate the effect of EaF10 on CCl4-induced hepatic oxidative stress in mice, the serum level of nitrite oxide (NO), as indicator of ROS production, and protein carbonylation and MDA contents in liver tissue as markers of protein and lipid oxidation respectively, were determined. As shown in Table 4, a significant (p˂0.05) increase of NO, protein carbonylation and MDA levels was observed in mice exposed to CCl4 alone, as compared to control mice. EaF10 (25 mg/Kg or 100 mg/Kg b.w) or silymarin (100 mg/Kg b.w) treatment significantly decreased NO, protein carbonylation and MDA levels compared to CCl4-intoxicated mice.
Table 4.
Effect of EaF10 on the parameters of oxidative stress: serum level of nitrite oxide, and lipid peroxidation end product (MDA) and protein carbonylation content in liver tissues.
| Parameters | Level of oxidative stress parameters |
||||
|---|---|---|---|---|---|
| Control | CCl4 | CCl4 + Sil (100mg/Kg) | CCl4 + EaF10 (25mg/Kg) | CCl4 + EaF10 (100mg/Kg) | |
| NO (μg) | 2.19 ± 0.45 | 8.76 ± 1.05a | 3.29 ± 0.37b | 5.21 ± 0.46b | 3.67 ± 0.55b |
| MDA (nmol/mg protein) | 7.58 ± 1.07 | 23.44 ± 0.81a | 12.71 ± 0.88b | 15.44 ± 0.96b | 10.09 ± 0.41b |
| Protein carbonylation (nmol/mg protein) | 17.06 ± 0.87 | 42.15 ± 1.21a | 23.68 ± 2.55b | 35.55 ± 1.44b | 24.18 ± 3.11b |
Mice in different groups were treated as indicated in the material and methods section. Parameters of oxidative stress related to CCl4-hepatotoxicity was determined by quantifying the serum level of NO, and MDA and protein carbonylation contents in liver tissues. Values are expressed as means ± SD, n = 6; a values significantly different compared to normal control group (P˂0.05); b values significantly different compared to CCl4-intoxicated group (P˂0.05) using Bonferroni's test. Control: normal control group; CCl4: CCl4-intoxicated group alone; CCl4+Sil (100mg/Kg): CCl4-intoxicated + silymarin (100mg/Kg)-treated group; CCl4 + EaF10 (25mg/Kg): CCl4-intoxicated + EaF10 (25mg/Kg)-treated group; CCl4 + EaF10 (100mg/Kg): CCl4-intoxicated + EaF10 (100mg/Kg)-treated group. NO: nitrite oxide; MDA: Malondialdehyde.
3.4.3. Effect of EaF10 on CCl4-induced changes in levels of enzymatic and non-enzymatic antioxidant defense system
Table 5 presents the activities of antioxidant enzymes (CAT, SOD and GST) and non-enzymatic antioxidant content (GSH) in liver tissues of different group of experimental mice. CCl4 exposure alone significantly (p˂0.05) decreased the activities of all the antioxidant enzymes and GSH content, as compared to untreated mice. Treatment of mice with EaF10 (25 mg/Kg or 100 mg/Kg b.w) or silymarin (100 mg/Kg b.w) after CCl4 administration significantly (p˂0.05) prevented these changes and maintained the activities of CAT, SOD and GST as well as GSH content nearly to those present in normal control mice.
Table 5.
Effect of EaF10 on the enzymatic and non-enzymatic antioxidant in the liver.
| Name of the enzymatic/non-enzymatic antioxidant | Level of the enzymatic (activity)/non-enzymatic (content) antioxidant |
||||
|---|---|---|---|---|---|
| Control | CCl4 | CCl4 + Sil (100mg/Kg) | CCl4 + EaF10 (25mg/Kg) | CCl4 + EaF10 (100mg/Kg) | |
| SOD (Unit/min/mg protein) | 72.36 ± 2.47 | 39.44 ± 1.87a | 63.88 ± 2.31b | 57.12 ± 2.58b | 66.29 ± 3.16b |
| CAT (Unit/min/mg protein) | 19.28 ± 1.22 | 6.39 ± 1.53a | 16.83 ± 2.44b | 11.52 ± 2.84b | 17.13 ± 1.59b |
| GST (nmol/min/mg protein) | 0.77 ± 0.03 | 0.21 ± 0.01a | 0.69 ± 0.02b | 0.47 ± 0.01b | 0.70 ± 0.02b |
| GSH (nmol/mg protein) | 25.64 ± 1.41 | 10.76 ± 2.17 a | 21.03 ± 1.54 b | 15.69 ± 2.57 b | 22.74 ± 2.33b |
Mice in different groups were treated as indicated in the material and methods section. Effect of EaF10 on the level of the enzymatic (activity) and non-enzymatic (content) antioxidants in the liver was determined. Values are expressed as means ± SD, n = 6; a values significantly different compared to normal control group (P˂0.05); b values significantly different compared to CCl4-intoxicated group (P˂0.05) using Bonferroni's test. Control: normal control group; CCl4: CCl4-intoxicated group alone; CCl4+Sil (100mg/Kg): CCl4-intoxicated + silymarin (100mg/Kg)-treated group; CCl4 + EaF10 (25mg/Kg): CCl4-intoxicated + EaF10 (25mg/Kg)-treated group; CCl4 + EaF10 (100mg/Kg): CCl4-intoxicated + EaF10 (100mg/Kg)-treated group. SOD: Superoxide dismutase; CAT: Catalase; GST: Glutathione-s-transferase; GSH: Reduced glutathione.
4. Discussion
E. africana is widely used to treat various ailments, among which liver related diseases [3, 38]. Investigations from our research group have led to the isolation of an active fraction, namely EaF10 (fraction methylene chloride/methanol 90:10, v/v) with promising activity against toxic hepatitis [9, 10]. However, the mechanisms underlying this anti-hepatotoxic property are yet to be demonstrated. Hence, in addition to the phytochemical composition and chemical antioxidant properties of EaF10, we showed the ability of this fraction to reverse carbon tetrachloride (CCl4)-induced hepatotoxicity and provided an insight to the mechanism supporting this anti-hepatotoxic effect.
CCl4 remains one of the best characterized models of xenobiotic-induced hepatotoxicity and is commonly used for the screening of anti-hepatotoxic and hepatoprotective potential of natural products and medicinal plant extracts [11, 39, 40]. Several experimental data indicated that CCl4-hepatocicity is evidenced by the abnormally high level of serum hepato-specific enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) [41, 42]. In this study, CCl4 treatment significantly (p˂0.05) increased the serum levels of these hepatic enzymes in mice (Table 3). This change reflected the occurrence of CCl4-induced hepatocellular damages appearing from histopathological data demonstrating disarrangement of hepatic cell architecture with intense hepatocytes necrosis and massive inflammatory cells infiltration in CCl4-intoxicated mice (Figure 9B). Interestingly, a curative treatment 1 h after CCl4 intoxication with EaF10 (25 or 100 mg/Kg) or silymarin (100 mg/Kg) used as hepatoprotective reference agent significantly (p˂0.05) reduced CCl4-induced increase in serum levels of hepatic enzymes (Table 3); suggesting a stabilization of hepatic cell membrane. Consistent with these biochemical findings, histopathological examination showed a reduction or full abrogation of liver abnormities after treatment of mice with EaF10 (25 or 100 mg/Kg) or silymarin (100 mg/Kg) (Figure 9). Similarly, by using normal human hepatic cell line L-02 hepatocytes, which are commonly used as in vitro model in hepatotoxicity studies of various xenobiotics [20, 43], we evaluated the capacity of EaF10 and its active sub-fractions to protect L-02 cells from CCl4-induced oxidative damage. At the determined concentration of 15 mM, CCl4 was toxic for L-02 hepatocytes after 36 h of incubation, decreasing cell viability to about 50% and increasing ALT leakage into the incubation medium (Figure 3). However, co-treatment of cells with EaF10, its active sub-fractions or silymarin dose-dependently prevented cells death and ALT leakage into the incubation medium (Figure 4). These results further confirm that EaF10 is able to stabilize membrane integrity of hepatocytes.
The mechanism of CCl4-induced hepatotoxicity is firstly initiated by its biotransformation by the cytochrome P450 enzymes system to form a highly reactive trichloromethyl (CCl3˙) free radical. The isoform CYP2E1 has been demonstrated to be largely responsible for this metabolic activation [12, 13, 16] and many studies have demonstrated that CCl4-induced hepatotoxicity can be potentiated or inhibited by substances that induce or inhibit CYP2E1 expression [44, 45, 46, 47]. Interestingly, substances that inhibit CYP2E1 may protect cells against CCl4-toxicity [41, 48]. In support of these observations in our study, we investigated whether the cyto-protective effect of EaF10 is related to the modulation of CYP2E1 expression through western blot analysis. We found that overexpression of CYP2E1 protein level induced by CCl4 administration alone was effectively inhibited when L-02 hepatocytes were co-treated with EaF10, its active sub-fractions or sylymarin (Figure 5). These findings suggest that inhibition of CYP2E1 expression by EaF10 could not only protect hepatic cells from CCl4-toxicity, but may also reduce or inhibit metabolic activation of xenobiotics involving this cytochrome P450 isoform.
Following CCl4-biotransformation, the second step involved in CCl4-hepatotoxicity is the activation of Kupfer cells, which is accompanied by excessive generation of reactive oxygen species (ROS) such as O2˙, HO˙ and NO˙ [14]. These ROS, together with CCl3˙ and CCl3OO˙ issues from CCl4 activation, initiate the oxidation of biological molecules such as proteins and lipids leading to the disruption of liver cells membrane and organelle, and subsequently cell death [12, 13]. In our study, high serum level of nitrite oxide (NO) and increase content of MDA and protein carbonylation in liver tissues as biomarker of lipid and protein oxidation respectively, observed in CCl4-intoxicated mice were significantly (p˂0.05) abrogated by the post-treatment of mice with EaF10 or silymarin (Table 4). Similarly, co-treatment of L-02 hepatocytes with EaF10 and its active sub-fractions or silymarin effectively inhibited intracellular ROS overproduction (Figure 6A) and high level of MDA content (Figure 6C) induced by CCl4. These results indicate that EaF10 prevents liver cells from CCl4-induced oxidative injury.
To deal with the harmful consequences of oxidative stress, liver cells possess a defense system consisting of antioxidant and detoxification enzymes such as CAT, SOD and GST; and also antioxidant molecules such as GSH which convert the toxic reactive metabolites of xenobiotic-biotransformation into non-toxic substances, scavenge and/or prevent overproduction of ROS as O2˙, HO˙ and NO˙ which damage the liver [49]. The gene expression of these enzymes is regulated by the transcription factor Nrf2. Located into the cytoplasm, Nrf2 dissociates upon appropriate stimulation, from its suppressor Keap-1 and translocates into the nucleus where it binds to the antioxidant response element and promotes the expression of its target genes [50]. It has been shown the Nrf2 protects liver against several xenobiotics through transcriptional up-regulation of antioxidant enzymes [20, 51]. In our study, immunoblotting analysis showed no obvious change neither in nuclear Nrf2 protein level, nor in cytosolic Keap-1 protein level after 24h of incubation in L-02 hepatocytes treated with CCl4 alone, as compared to untreated cells. In contrast, co-treatment of cells with EaF10, its active sub-fractions or silymarin increased the nuclear protein level of Nrf2 by up-to 3.1-fold concomitantly with a significant (p˂0.05) decrease of cytosolic protein level of Keap-1 (Figure 7). Interestingly, the mRNA level of antioxidant enzymes CAT, SOD1 and GST (Figure 8), target genes of Nrf2, as well as cellular GSH content (Figure 6B), were significantly (p˂0.05) increase in L-02 cells co-treated with EaF10, its active sub-fractions or silymarin, compared to CCl4-intoxicated cells alone. Similarly, a significant (p˂0.05) decrease of the activity of these antioxidant enzymes as well as GSH content, observed in mice liver tissues in response to CCl4 administration alone, was abrogated when mice were treated with EaF10 or silymarin (Table 5). Based on these observations, it can be suggested that EaF10 stimulates Nrf2, which dissociates from Keap-1, then translocates into the nucleus and increases the expression of antioxidant enzymes; and correspondingly, contributes to the protection of liver against CCl4-toxicity. Thus, activation of Nrf2-Keap1 antioxidant defense system appears as a protective mechanism by which EaF10 may protect against CCl4-induced hepatotoxicity.
In the present study, EaF10 was preliminary screened for its phytochemical composition and flavonoid and total polyphenol content determination, in addition to the evaluation of its antioxidant properties in cell-free systems. Firstly, phytochemical screening revealed the presence of polyphenols, flavonoids, triterpenes, saponins and sugars (Table 1) tested positive among others class of secondary metabolites. HPLC-UV fingerprint (Figure 1) indicates that EaF10 is a mixture containing about 6 predominant compounds which can be separated and eluted with a retention time ranging from 2.5-10 min. In addition, the HPLC-UV fingerprint also indicates the presence of aromatic compounds. Since polyphenols and flavonoids contain aromatic group in their structure, the presence of these phytochemical groups of compounds in the fraction EaF10 is confirmed; and quantitatively, the fraction showed high polyphenols and flavonoids contents (Table 1). While assessing the antioxidant activity of plant extracts in cell-free system, more than one assay should be performed, since their antioxidant action can be the combined effect of a mixture of compounds working through different mechanism [52, 53]. Hence, in addition to in vitro lipid peroxidation inhibition assay, the ability of EaF10 to scavenge synthetic DPPH radical, as well as HO˙ and NO˙, two radicals found in biological system, were analyzed. Indeed, these free radicals oxidize and damage polyunsaturated fatty acids present in cell membrane, leading to lipid peroxidation [54]; and free radical scavenging being one of the mechanism by which antioxidants prevent oxidation of biological molecules [55]. In radical-scavenging/inhibitory assays, the low EC50/IC50 values (Table 2) for EaF10 was comparable to that of ascorbic acid, used as reference antioxidant compound and therefore, indicate the strong antioxidant capacity of this fraction. Several reports support the hypothesis that antioxidant activity of plant extracts can be attributed to the presence of polyphenolic compounds which function as free radical scavengers [25, 53, 56]. Accordingly, we analyzed and found a strong and positive correlation between the antioxidant activities of EaF10 and its total polyphenols content (Table 2). Taking all these results together, it may be suggested that the strong antioxidant capacity of EaF10 is due to its higher polyphenolic compounds content and that EaF10 protects liver cells from CCl4-induced oxidative injury through an antioxidant mechanism.
5. Conclusion
The results presented in this study not only demonstrate the strong antioxidant capacity of an active fraction (EaF10) of E. africana, but also support its traditional use to manage liver-related diseases and provide scientific evidence which may support the development of phyto-pharmaceutics from the plant to treat toxin or drugs-induced liver injury. Accordingly, further studies aimed to isolate the active molecules in this fraction, and to conduct in depth toxicological analysis are needed.
Declarations
Author contribution statement
A.F. Kouam: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
B.A. Owona: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
R. Fifen: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
F.N. Njayou: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
P.F. Moundipa: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was co-supported by the International Center for Genetic Engineering and Biotechnology (ICGEB) [grant number S/CMR15-06] and the Organization for the Prohibition of Chemical Weapons (OPCW) [grant number F/4223-2].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the Department of Biochemistry, University of Yaoundé 1 and the Institute of Microbiology, Chinese Academy of Sciences for providing the facilities to carry out this research; and are grateful to Dr. CHUISSEU DJAMEN Pascal for his support and assistance.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary file S1 Uncropped gel of CYP2E1 in Figure 5.
Supplementary file S2 Uncropped gel B-Actin in Figure 5.
Supplementary file S3 Uncropped Gel Nrf2 Figure 7.
Supplementary file S4 Uncropped gel of Lamin-B in Figure 7.
Supplementary file S5 Uncropped gel of Keap-1 in Figure 7.
Supplementary file S6 Uncropped gel of B-Actin in Figure 7.
Supplementary file S7 Uncropped gel of CAT in figure 8.
Supplementary file S8 Uncropped gel of SOD1in figure 8.
Supplementary file S9 Uncropped gel of GST in figure 8.
Supplementary file S10 Uncropped gel of GAPDH in figure 8.
References
- 1.Maiga A., Diallo D., Fane S., Sanogo R., Paulsen B.S., Cisse B. A survey of toxic plants on the market in the district of Bamako, Mali: traditional knowledge compared with a literature search of modern pharmacology and toxicology. J. Ethnopharmacol. 2005;96:183–193. doi: 10.1016/j.jep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Karou S.D., Tchacondo T., Ouattara L., Anani K., Savadogo A., Agbonon A., Attaia M.B., de Souza C., Sakly M., Simpore J. Antimicrobial, antiplasmodial, haemolytic and antioxidant activities of crude extracts from three selected Togolese medicinal plants. Asian Pac. J. Trop. Med. 2011;4:808–813. doi: 10.1016/S1995-7645(11)60199-5. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf A.J., Abdullahi M.I. The phytochemical and pharmacological actions of Entada africana Guill. & Perr. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cioffi G., Dal Piaz F., De Caprariis P., Sanogo R., Marzocco S., Autore G., De Tommasi N. Antiproliferative triterpene saponins from Entada africana⊥. J. Nat. Prod. 2006;69:1323–1329. doi: 10.1021/np060257w. [DOI] [PubMed] [Google Scholar]
- 5.Ifemeje J., Egbuna C., Udedi S., Iheukwumere H. Phytochemical and in vitro antibacterial evaluation of the ethanolic extract of the stem bark of Entada africana guill. & perr and sarcocephalus latifolus. Int. J. Biochem. Res. Rev. 2014;4:584–592. [Google Scholar]
- 6.Ayissi Owona B., Njayou N.F., Laufer S., Moundipa P.F., Schluesener H.J. A fraction of stem bark extract of Entada africana suppresses lipopolysaccharide-induced inflammation in RAW 264.7 cells. J. Ethnopharmacol. 2013;149:162–168. doi: 10.1016/j.jep.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Owona B.A., Njayou N.F., Laufer S.A., Schluesener H.J., Moundipa P.F. Entada africana fraction CH2Cl2/MEOH 5% inhibits inducible nitric oxide synthase and pro-inflammatory cytokines gene expression induced by lipopolysaccharide in microglia. BMC Compl. Alternative Med. 2013;13 doi: 10.1186/1472-6882-13-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galani Tietcheu B.R., Sass G., Njayou N.F., Mkounga P., Tiegs G., Moundipa P.F. Anti-hepatitis C virus activity of crude extract and fractions of Entada africana in genotype 1b replicon systems. Am. J. Chin. Med. 2014;42:853–868. doi: 10.1142/S0192415X14500542. [DOI] [PubMed] [Google Scholar]
- 9.Njayou F.N., Kouam A.F., Simo B.F.N., Tchana A.N., Moundipa P.F. Active chemical fractions of stem bark extract of Khaya grandifoliola C.DC and Entada africana Guill. et Perr. synergistically protect primary rat hepatocytes against paracetamol-induced damage. BMC Compl. Alternative Med. 2016;16 doi: 10.1186/s12906-016-1169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Njayou F.N., Amougou A.M., Fouemene Tsayem R., Njikam Manjia J., Rudraiah S., Bradley B., Manautou J.E., Fewou Moundipa P. Antioxidant fractions of Khaya grandifoliola C.DC. and Entada africana Guill. et Perr. induce nuclear translocation of Nrf2 in HC-04 cells. Cell Stress Chaperones. 2015;20:991–1000. doi: 10.1007/s12192-015-0628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke H., Williams C.D., McGill M.R., Xie Y., Ramachandran A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem. Toxicol. 2013;55:279–289. doi: 10.1016/j.fct.2012.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brattin W.J., Glende E.A., Recknagel R.O. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J. Free Radic. Biol. Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 13.Recknagel R.O., Glende E.A., Dolak J.A., Waller R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhu W., Fung P.C.W. The roles played by crucial free radicals like lipid free radicals, nitric oxide, and enzymes NOS and NADPH in CCl4-induced acute liver injury of mice. Free Radic. Biol. Med. 2000;29:870–880. doi: 10.1016/s0891-5849(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 15.Jeong H.G. Inhibition of cytochrome P450 2E1 expression by oleanolic acid: hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol. Lett. 1999;105:215–222. doi: 10.1016/s0378-4274(99)00004-1. [DOI] [PubMed] [Google Scholar]
- 16.Zangar R.C., Benson J.M., Burnett V.L., Springer D.L. Cytochrome P450 2E1 is the primary enzyme responsible for low-dose carbon tetrachloride metabolism in human liver microsomes. Chem. Biol. Interact. 2000;125:233–243. doi: 10.1016/s0009-2797(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 17.Bataille A.M., Manautou J.E. Nrf2: a potential target for new therapeutics in liver disease. Clin. Pharmacol. Ther. 2012;92:340–348. doi: 10.1038/clpt.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong Y., Yan W., Chen S., Sun C., Zhang J. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol. Sin. 2010;31:1421–1430. doi: 10.1038/aps.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang T., Huang Z., Chan J.Y., Zhang D.D. Nrf2 protects against As(III)-induced damage in mouse liver and bladder. Toxicol. Appl. Pharmacol. 2009;240:8–14. doi: 10.1016/j.taap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang C., Zheng Z., Shi L., Sheng Y., Wei H., Wang Z., Ji L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016;91:236–246. doi: 10.1016/j.freeradbiomed.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Xu W., Hellerbrand C., Köhler U.A., Bugnon P., Kan Y.-W., Werner S., Beyer T.A. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab. Invest. 2008;88:1068–1078. doi: 10.1038/labinvest.2008.75. [DOI] [PubMed] [Google Scholar]
- 22.Kouam A.F., Yuan F., Njayou F.N., He H., Tsayem R.F., Oladejo B.O., Song F., Moundipa P.F., Gao G.F. Induction of Mkp-1 and nuclear translocation of Nrf2 by limonoids from Khaya grandifoliola C.DC protect L-02 hepatocytes against acetaminophen-induced hepatotoxicity. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadood A. Phytochemical analysis of medicinal plants occurring in local area of mardan. Biochem. Anal. Biochem. 2013;2 [Google Scholar]
- 24.Dhar P., Bajpai P.K., Tayade A.B., Chaurasia O.P., Srivastava R.B., Singh S.B. Chemical composition and antioxidant capacities of phytococktail extracts from trans-Himalayan cold desert. BMC Compl. Alternative Med. 2013;13 doi: 10.1186/1472-6882-13-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyo M., Ndhlala A.R., Finnie J.F., Van Staden J. Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem. 2010;123:69–76. [Google Scholar]
- 26.Su X.-Y., Wang Z.-Y., Liu J.-R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009;117:681–686. [Google Scholar]
- 27.Ebrahimzadeh M.A., Nabavi S.M., Nabavi S.F., Bahramian F., Bekhradnia A.R. Antioxidant and free radical scavenging activity of H. Officinalis L. var. Angustifolius, V. Odorata, B. Hyrcana and C. Speciosum. Pak. J. Pharm. Sci. 2010;7 [PubMed] [Google Scholar]
- 28.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Ji L., Liu T., Chen Y., Wang Z. Protective mechanisms of N -acetyl-cysteine against pyrrolizidine alkaloid clivorine-induced hepatotoxicity. J. Cell. Biochem. 2009;108:424–432. doi: 10.1002/jcb.22269. [DOI] [PubMed] [Google Scholar]
- 30.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Huang H.-L., wang Y.-J., Zhang Q.-Y., Liu B., Wang F.-Y., Li J.-J., Zhu R.-Z. Hepatoprotective effects of baicalein against CCl 4 -induced acute liver injury in mice. World J. Gastroenterol. 2012;18:6605. doi: 10.3748/wjg.v18.i45.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang G.-J., Deng J.-S., Chiu C.-S., Liao J.-C., Hsieh W.-T., Sheu M.-J., Wu C.-H. Hispolon protects against acute liver damage in the rat by inhibiting lipid peroxidation, proinflammatory cytokine, and oxidative stress and downregulating the expressions of iNOS, COX-2, and MMP-9. Evid. base Compl. Alternative Med. 2012;2012:1–12. doi: 10.1155/2012/480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida K., Stadtman E.R. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J. Biol. Chem. 1993;268:6388–6393. [PubMed] [Google Scholar]
- 34.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 35.Ghosh J., Das J., Manna P., Sil P.C. Cytoprotective effect of arjunolic acid in response to sodium fluoride mediated oxidative stress and cell death via necrotic pathway. Toxicol. In Vitro. 2008;22:1918–1926. doi: 10.1016/j.tiv.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Aebi H. Methods Enzymol. Academic Press; 1984. [13] Catalase in vitro; pp. 121–126. [Google Scholar]
- 37.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 38.Baidoo M.F., Asante-Kwatia E., Mensah A.Y., Sam G.H., Amponsah I.K. Pharmacognostic characterization and development of standardization parameters for the quality control of Entada africana Guill. & Perr. J. Appl. Res. Med. Aromat. Plants. 2019;12:36–42. [Google Scholar]
- 39.Cai Z., Lou Q., Wang F., Li E., Sun J., Fang H., Xi J., Ju L. 2015. N-acetylcysteine Protects against Liver Injure Induced by Carbon Tetrachloride via Activation of the Nrf2/HO-1 Pathway; p. 8. [PMC free article] [PubMed] [Google Scholar]
- 40.Go J., Kim J., Koh E., Song S., Sung J., Lee H., Lee Y., Lim Y., Hong J., Hwang D. Protective effect of gallotannin-enriched extract isolated from galla rhois against CCl4-induced hepatotoxicity in ICR mice. Nutrients. 2016;8:107. doi: 10.3390/nu8030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeong S.C., Kim S.M., Jeong Y.T., Song C.H. Hepatoprotective effect of water extract from Chrysanthemum indicum L. flower. Chin. Med. 2013;8:7. doi: 10.1186/1749-8546-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao J., Liong E.C., Ling M.-T., Ching Y.-P., Fung M.-L., Tipoe G.L. S-allylmercaptocysteine reduces carbon tetrachloride-induced hepatic oxidative stress and necroinflammation via nuclear factor kappa B-dependent pathways in mice. Eur. J. Nutr. 2012;51:323–333. doi: 10.1007/s00394-011-0217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao F., Li Y., Luo L., Xie Y., Zeng M., Wang A., Chen H., Zhong C. Role of mitochondrial electron transport chain dysfunction in Cr(VI)-Induced cytotoxicity in L-02 hepatocytes. Cell. Physiol. Biochem. 2014;33:1013–1025. doi: 10.1159/000358672. [DOI] [PubMed] [Google Scholar]
- 44.Allis J.W., Brown B.L., Simmons J.E., Hatch G.E., McDonald A., House D.E. Methanol potentiation of carbon tetrachloride hepatotoxicity: the central role of cytochrome P450. Toxicology. 1996;112:131–140. doi: 10.1016/0300-483x(96)03366-5. [DOI] [PubMed] [Google Scholar]
- 45.Day B.J., Carlson G.P., Denicola D.B. Potentiation of carbon tetrachloride-induced hepatotoxicity and pneumotoxicity by pyridine. J. Biochem. Toxicol. 1993;8:11–18. doi: 10.1002/jbt.2570080104. [DOI] [PubMed] [Google Scholar]
- 46.Hwang Y.P., Choi C.Y., Chung Y.C., Jeon S.S., Jeong H.G. Protective effects of puerarin on carbon tetrachloride-induced hepatotoxicity. Arch. Pharm. Res. 2007;30:1309–1317. doi: 10.1007/BF02980272. [DOI] [PubMed] [Google Scholar]
- 47.Lee K.J., Choi J.H., Jeong H.G. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem. Toxicol. 2007;45:2118–2125. doi: 10.1016/j.fct.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Lim D.-W., Kim H., Park J.-Y., Kim J.-E., Moon J.-Y., Park S.-D., Park W.-H. Amomum cardamomum L. ethyl acetate fraction protects against carbon tetrachloride-induced liver injury via an antioxidant mechanism in rats. BMC Compl. Alternative Med. 2016;16 doi: 10.1186/s12906-016-1121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 50.Niture S.K., Kaspar J.W., Shen J., Jaiswal A.K. Nrf2 signaling and cell survival. Toxicol. Appl. Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S., Zou L., Li L., Wu T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PloS One. 2013;8 doi: 10.1371/journal.pone.0053662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chanda S., Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr. J. Microbiol. Res. 2009:16. [Google Scholar]
- 53.Wong S., Leong L., Williamkoh J. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. [Google Scholar]
- 54.Battu G.R., Ethadi S.R., Veda Priya G., Swathi Priya K., Chandrika K., Venkateswara Rao A., Reddy S.O. Evaluation of antioxidant and anti-inflammatory activity of Euphorbia heyneana Spreng. Asian Pac. J. Trop. Biomed. 2011;1:S191–S194. [Google Scholar]
- 55.Amarowicz R., Pegg R.B., Rahimi-Moghaddam P., Barl B., Weil J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. [Google Scholar]
- 56.Quideau S., Deffieux D., Douat-Casassus C., Pouységu L. Plant polyphenols: chemical properties, biological activities, and Synthesis. Angew. Chem. Int. Ed. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]