Abstract
Background
Tumour necrosis factor (TNF) inhibitors are used in the treatment of certain autoimmune diseases but given the role of TNF in tumour biology and atherosclerosis, such therapies may influence the risk of cancer and cardiovascular disease. We conducted a Mendelian randomization study to explore whether TNF levels are causally related to cardiovascular disease and cancer.
Methods
Single-nucleotide polymorphisms associated with TNF levels at genome-wide significance were identified from a genome-wide association study of 30 912 European-ancestry individuals. Three TNF-associated single-nucleotide polymorphisms associated with higher risk of autoimmune diseases were used as instrumental variables. Summary-level data for 14 cardiovascular diseases, overall cancer and 14 site-specific cancers were obtained from UK Biobank and consortia.
Findings
Genetically-predicted TNF levels were positively associated with coronary artery disease (odds ratio (OR) 2.25; 95% confidence interval (CI) 1.50, 3.37) and ischaemic stroke (OR 2.27; 95% CI 1.50, 3.43), and inversely associated with overall cancer (OR 0.54; 95% CI 0.42, 0.69), breast cancer (OR 0.51; 95% CI 0.39, 0.67), and colorectal cancer (OR 0.20; 95% CI 0.09, 0.45). There were suggestive associations of TNF with venous thromboembolism (OR 2.18; 95% CI 1.32, 3.59), endometrial cancer (OR 0.25; 95% CI 0.07, 0.94), and lung cancer (OR 0.45; 95% CI 0.21, 0.94).
Interpretation
This study found evidence of causal associations of increased TNF levels with higher risk of common cardiovascular diseases and lower risk of overall and certain cancers.
Key Words: Cancer, Cardiovascular disease, Tumour necrosis factor
Research in context.
Evidence before this study
Tumour necrosis factor (TNF) is a pro-inflammatory cytokine secreted primarily by immune cells. It is involved in a broad range of both homoeostatic and pathophysiological processes, such as immunity, inflammation, cell proliferation, apoptosis and lipid metabolism. As such, anti-TNF agents have become cornerstone in the treatment of autoimmune inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease. However, the potential therapeutic, or even deleterious, effects of targeting TNF in other inflammatory conditions, such as cardiovascular disease and cancer remains equivocal. Mendelian randomization (MR) is an epidemiological approach using genetic variants as instrumental variables for an exposure to strengthen the causal inference in an exposure-outcome association by reducing residual confounding and reverse causality.
Added value of this study
In the present MR study, we provided the first causal evidence of positive associations of TNF levels with atherothrombotic disease (coronary artery disease and ischaemic stroke) and venous thromboembolism. Furthermore, we revealed inverse associations of TNF levels with risk of overall cancer and several site-specific cancers (colorectal, breast, endometrial, and lung cancers). We confirmed that higher TNF levels were strongly associated with established TNF-driven diseases (rheumatoid arthritis and inflammatory bowel disease) which added strong support to the validity of the genetic instrument used and the reliability of our findings.
Implications of the all the available evidence
This study reveals evidence of causal associations of increased TNF levels with higher risk of common cardiovascular diseases and lower risk of overall and certain cancers. These results may inform decisions concerning potential benefits and risks of TNF inhibitor therapy. In detail, clinicians need to assess potential increased cancer risk derived from anti-TNF therapy usage especially amongst individuals with inherited or acquired high risk of cancer, and in addition, may use anti-TNF medicine as a potential prevention approach for people with excessive cardiovascular risk and a potential treatment strategy for patients with impaired cardiovascular condition. The study also indicates that randomized controlled trials are warranted to verify our findings and comprehensively evaluate the benefits and risks of anti-TNF therapy in populations with different health conditions.
Alt-text: Unlabelled box
1. Introduction
Tumour necrosis factor (TNF) is a pro-inflammatory cytokine secreted primarily by immune cells. It is involved in a broad range of both homoeostatic and pathophysiological processes, such as immunity, inflammation, cell proliferation, apoptosis and lipid metabolism [1], [2], [3]. As such, anti-TNF agents have become cornerstone in the treatment of autoimmune inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease. However, the potential therapeutic, or even deleterious, effects of targeting TNF in other inflammatory conditions, such as cardiovascular disease (CVD) and cancer remains equivocal.
Atherosclerosis is a chronic inflammatory disease of the arterial wall, driven by immune cells and cytokines at all stages, and, TNF-deficient mice have reduced plaque size [4]. This is likely of importance in humans as TNF levels post-myocardial infarction are a strong predictor of recurrent events [5]. Furthermore, multiple observational studies have shown that TNF inhibition reduces atherosclerosis and cardiovascular events when administered to patients with rheumatoid arthritis [6]. Whether this benefit is also conferred in the general population, rather than patients suffering from conditions characterized by enhanced TNF activity, is poorly understood. Similarly, the role of TNF in heart failure remains equivocal. Although epidemiologically, TNF levels are predictive of heart failure mortality [7], a clinical trial in heart failure patients observed a higher hospitalization rate in the group receiving 10 mg/kg infliximab (anti-TNF) compared with the placebo group [8]. The potentially causal role of TNF in heart failure, atherosclerosis in a range of vascular beds and other cardiovascular diseases therefore need to be investigated.
Cancer is characterized by uncontrolled cell proliferation and survival. As a pro-inflammatory cytokine, TNF can promote all stages of carcinogenesis including survival, angiogenesis, and metastasis. TNF levels are raised in multiple cancer types, are reduced by chemotherapy and the reduction is associated with patient outcomes [9]. TNF inhibition may therefore be a potential cancer therapy. However, there have been multiple reports of increased risk of certain malignancies such as squamous cell cancer [10] in patients treated with anti-TNF agents. This may relate to the paradoxical tumour-suppressive effects of TNF, such as cytotoxicity. Thus, TNF and anti-TNF therapies may both have carcinogenic benefits and risks in different cancer types and the causal role of the cytokine in the development of a wide range of site-specific cancers warrants further evaluation.
Utilizing genetic variants as instrumental variables for an exposure (e.g., TNF levels), Mendelian randomization (MR) can improve the causal inference of an exposure-outcome association [11]. It minimizes potential methodological limitations, such as confounding and reverse causality. The rationale for diminished bias in MR studies is that genetic variants are randomly assorted and fixed at conception and therefore largely independent of confounders and cannot be modified by disease development [11].
Here, we aimed to evaluate the CVDs and cancers that are causally associated with TNF levels and which could be targeted with TNF-modifying therapies. We conducted a two-sample Mendelian randomization study to explore the associations of genetically predicted TNF levels with risk of 14 CVDs, overall cancer, and 14 site-specific cancers. To validate the instrumental variables, we assessed whether genetically predicted TNF levels were associated with higher risk of rheumatoid arthritis and inflammatory bowel disease.
2. Methods
2.1. Study design
This is a two-sample MR study design based on summary-level data. An MR analysis depends on the assumptions that the genetic variants: [1] are strongly associated with the exposure (the relevance assumption); [2] are not associated with confounders of the exposure-outcome relationship (the independence assumption); and [3] have an effect on the outcome through the exposure only and not through any other causal pathway (the exclusion restriction assumption) [11]. This MR study has been approved by the Swedish Ethical Review Authority.
2.2. Instrumental variable selection and outcome sources
A meta-analysis of genome-wide association studies (GWASs) of 25 cohorts encompassing 30 912 European-descent individuals identified four single-nucleotide polymorphisms (SNPs) associated with TNF levels at genome-wide significance (P<5 × 10−8) (Table 1) [12]. To ensure that the relevance assumption is likely to be satisfied, we used rheumatoid arthritis [13] and inflammatory bowel disease [14] as positive controls to select SNPs (Supplementary Table 1). A genetic instrument containing rs10744774, rs3184504 and rs7182229 was associated with an expected increased odds of rheumatoid arthritis and inflammatory bowel disease. The TNF-raising allele of rs2857602 was associated with lower odds of these autoimmune diseases and was regarded as an unreliable instrumental variable for TNF. We therefore used three SNPs (rs10744774, rs3184504 and rs7182229) as instrumental variables for TNF levels in the primary analysis; all four SNPs were used in a supplementary analysis. The two SNPs on chromosome 12 (rs10744774 and rs3184504) were in modest linkage disequilibrium (r2 = 0.18) based on 1000 G reference panel. The genotype associations with TNF levels were adjusted for age2, sex, body mass index, and study-specific variables such as genetic principal components and relatedness [12].
Table 1.
Detailed information of instrumental variables for TNF levels.
| rsID | Chr | Position (hg19) | Nearby gene | EA | NEA | EAF | Beta | SE | P | Included in main analysis |
| rs2857602 | 6 | 31,533,378 | LTA | G | A | 0.38 | 0.032 | 0.006 | 3.30 × 10−12 | No |
| rs10744774 | 12 | 112,090,022 | BRAP | A | C | 0.83 | 0.044 | 0.007 | 6.94 × 10−11 | Yes |
| rs3184504 | 12 | 111,884,608 | SH2B3 | T | C | 0.48 | 0.030 | 0.005 | 3.96 × 10−10 | Yes |
| rs7182229 | 15 | 58,765,183 | LIPC | T | G | 0.11 | 0.050 | 0.009 | 1.07 × 10−9 | Yes |
Chr indicates chromosome; EA; effect allele; EAF, effect allele frequency; NEA, non-effect allele; SE, standard error; TNF, tumour necrosis factor. Rs2857602 was not included in the main analysis since the TNF-increasing allele was associated with lower odds of rheumatoid arthritis and inflammatory bowel disease.
Fourteen CVDs, overall cancer, and 14 site-specific cancers were included as outcomes in this MR study (Table 2). Summary-level data for outcomes were obtained from UK Biobank [15] and genetic consortia [16], [17], [18], [19], [20], [21], [22]. Rs2857602 was not available in the consortia datasets of coronary artery disease and stroke and was replaced by a proxy (rs2844484, r2=1). From UK Biobank, we included CVDs and cancers with at least 1000 cases to ensure sufficient statistical power to detect moderate to strong associations. The SNP-outcome associations in UK Biobank and most consortia were adjusted for age, sex, and genetic principal components. Detailed information of included outcomes is displayed in Table 2.
Table 2.
Characteristics of included studies or consortia of inflammatory diseases, cardiovascular diseases, and cancers.
| Outcome | Source | Cases | Controls | Sample size | Population |
| Inflammatory disease | |||||
| Rheumatoid arthritis | GARNET consortium | 29 880 | 73 758 | 103 638 | Mix |
| Inflammatory bowel disease | UK IBD consortium | 25 042* | 34 915 | 59 957 | European |
| Cardiovascular disease | |||||
| Cerebrovascular disease | |||||
| Overall stroke | MEGASTROKE consortium | 67 162 | 454 450 | 521 612 | Mix |
| Overall stroke | UKBB | 9652 | 357 991 | 367 643 | European |
| Any ischaemic stroke | MEGASTROKE consortium | 60 341 | NA | NA | Mix |
| Any ischaemic stroke | UKBB | 3554 | 364 089 | 367 643 | European |
| Large artery stroke | MEGASTROKE consortium | 6688 | 146 392 | 153 080 | Mix |
| Small vessel stroke | MEGASTROKE consortium | 11 710 | 192 662 | 204 372 | Mix |
| Cardioembolic stroke | MEGASTROKE consortium | 9006 | 204 570 | 213 576 | Mix |
| Intracerebral haemorrhage | UKBB | 1064 | 366 579 | 367 643 | European |
| Subarachnoid haemorrhage | UKBB | 1084 | 366 559 | 367 643 | European |
| Heart and valvular disease | |||||
| Coronary artery disease | CARDIoGRAMplusC4D consortium | 60 801 | 123 504 | 184 305 | Mix |
| Coronary artery disease | UKBB | 24 531 | 343 112 | 367 643 | European |
| Heart failure | UKBB | 7382 | 387 652 | 395 034 | European |
| Atrial fibrillation | AFGen | 65 446 | 522 000 | 587 446 | Mix |
| Atrial fibrillation | UKBB | 16 945 | 350 698 | 367 643 | European |
| Abdominal aortic aneurysm | UKBB | 1094 | 366 549 | 367 643 | European |
| Aortic valve stenosis | UKBB | 2244 | 365 399 | 367 643 | European |
| Vessel disease | |||||
| Peripheral artery disease | UKBB | 3415 | 364 228 | 367 643 | European |
| Venous thromboembolism | UKBB | 15 602 | 352 041 | 367 643 | European |
| Cancer | |||||
| Bladder cancer | UKBB | 2588 | 365 055 | 367 643 | European |
| Breast cancer | BCAC | 122 977 | 105 974 | 228 951 | Mix |
| Breast cancer ER- | BCAC | 21 468 | NA | NA | Mix |
| Breast cancer ER+ | BCAC | 69 501 | NA | NA | Mix |
| Breast cancer | UKBB | 13 666 | 353 977 | 198 838 | European |
| Cervical cancer | UKBB | 1928 | 365 715 | 198 838 | European |
| Colorectal cancer | UKBB | 5486 | 362 157 | 367 643 | European |
| Endometrial cancer | UKBB | 1520 | 366 123 | 198 838 | European |
| Head-neck cancer | UKBB | 1615 | 366 028 | 367 643 | European |
| Kidney cancer | UKBB | 1310 | 366 333 | 367 643 | European |
| Leukaemia | UKBB | 1403 | 366 240 | 367 643 | European |
| Lung cancer | ILCCO | 11 348 | 15,861 | 27 209 | European |
| Melanoma | UKBB | 4869 | 362 774 | 367 643 | European |
| Non-Hodgkin's lymphoma | UKBB | 2296 | 365 347 | 367 643 | European |
| Ovarian cancer | UKBB | 1520 | 366 123 | 198 838 | European |
| Ovarian cancer | OCAC | 22 406 | 40 941 | 63 347 | Mix |
| Overall cancer | UKBB | 75 037 | 292 606 | 367 643 | European |
| Pancreatic cancer | UKBB | 1264 | 366 379 | 367 643 | European |
| Prostate cancer | PRACTICAL | 79 194 | 61 112 | 140 306 | European |
| Prostate cancer | UKBB | 7872 | 359 771 | 168 748 | European |
AFGen indicates Atrial Fibrillation Consortium; BCAC, Breast Cancer Association Consortium; CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics; GARNET, Genetics and Allied research in Rheumatic diseases Networking; ILCCO, The International Lung Cancer Consortium; NA, not available; OCAC, The Ovarian Cancer Association Consortium; PRACTICAL, The Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome consortium; UKBB, UK Biobank; UK IBD consortium, UK Inflammatory Bowel Disease Genetics Consortium.
Includes Crohn's disease and ulcerative colitis.
2.3. Pleiotropy assessment
To evaluate whether the exclusion restriction assumption is likely to hold, possible pleiotropic associations of the instrumental variables with other phenotypes were assessed by searching a database of human genotype-phenotype associations (PhenoScanner V2) (http://www.phenoscanner.medschl.cam.ac.uk/). One or more of the SNPs related to TNF were associated with autoimmune diseases (coeliac disease, rheumatoid arthritis, and type 1 diabetes), various immune and blood cells, haemoglobin levels, hypothyroidism, diastolic blood pressure, total and low-density lipoprotein cholesterol, and height (Supplementary Table 2).
2.4. Statistical analysis
The inverse-variance weighted method with adjustment for correlations amongst the SNPs [23] was used to analyse the associations of TNF with CVD and cancer outcomes in the main analysis. A matrix of correlations amongst used SNPs was added into the traditional inverse-variance weighted model, thereby diminishing the effects of linkage disequilibrium [23]. All odds ratios (ORs) and 95% confidence intervals (CIs) of the outcomes were expressed per one unit increase in natural log of TNF (pg/ml). We calculated the statistical power using a web-tool and results of the power analyses are presented in Supplementary Table 3 [24]. To account for multiple testing, we deemed associations with p values below 1.7 × 10−3 (where p = 0.05/29 (29 outcomes)) as strong evidence of causal associations. Associations with p values below 0.05 but above 1.7 × 10−3 were treated as suggestive evidence of associations. All analyses were two-sided and performed using TwoSampleMR and MendelianRandomization packages in R 3.6.0.
2.5. Role of funders
The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication.
3. Results
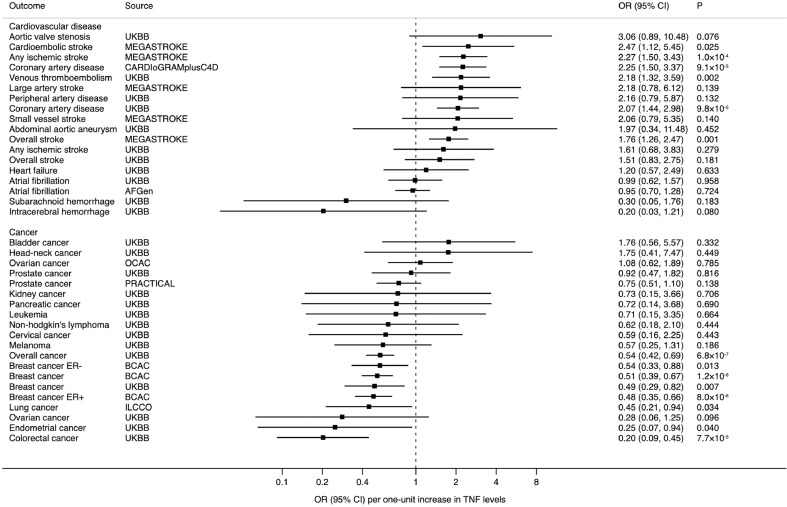
The associations of TNF levels instrumented by three SNPs with the CVD and cancer outcomes are displayed in Fig. 1 and Fig. 2. Genetically higher TNF levels were associated with higher odds of coronary artery disease and ischaemic stroke and lower odds of overall, colorectal, and breast cancer. For one unit increase in natural log-transformed TNF levels, the ORs were 2.25 (95% CI, 1.50, 3.37) for coronary artery disease, 2.27 (95% CI, 1.50, 3.43) for ischaemic stroke, 0.54 (95% CI, 0.42, 0.96) for overall cancer, 0.51 (95% CI, 0.39, 0.67) for breast cancer, and 0.20 (95% CI, 0.09, 0.45) for colorectal cancer. Results for coronary artery disease and breast cancer were similar in UK Biobank and consortia. There was weak evidence of association between TNF levels and ischaemic stroke in UK Biobank. Genetically predicted TNF levels showed a suggestive positive association with risk of venous thromboembolism (OR 2.18, 95% CI 1.32, 3.59) and inverse associations with risk of endometrial cancer (OR 0.25, 95% CI 0.07, 0.94) and lung cancer (OR 0.45, 95% CI 0.21, 0.94). Genetically predicted TNF levels were not associated with the other studied cardiovascular diseases and site-specific cancers in the main analysis. In the supplementary analysis, using four SNPs, there was some evidence of inverse associations of genetically-predicted TNF levels with intracerebral haemorrhage (OR, 0.19; 95% CI, 0.04, 0.92), colorectal cancer (OR, 0.23; 95% CI, 0.09, 0.60), and ovarian cancer (OR, 0.23; 95% CI, 0.06, 0.91) (Supplementary figure 1).
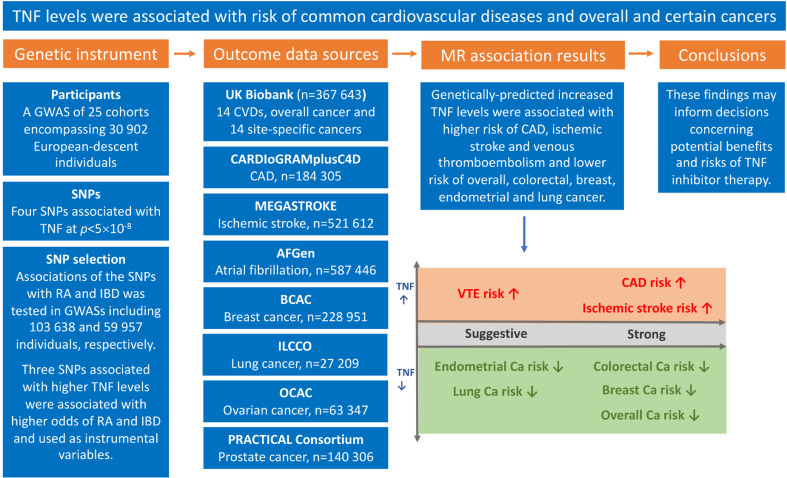
Fig. 1.
Overview of this MR study, including genetic instrument and data sources used, results, and conclusions. AFGen indicates Atrial Fibrillation Consortium; BCAC, Breast Cancer Association Consortium; Ca, cancer; CAD, coronary artery disease; CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics; IBD, inflammatory bowel disease; ILCCO, International Lung Cancer Consortium; GWAS, genome-wide association study; MR, Mendelian randomization; OCAC, The Ovarian Cancer Association Consortium; PRACTICAL, Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome consortium; RA, rheumatoid arthritis; SNPs, single-nucleotide polymorphisms; TNF, tumour necrosis factor; VTE, venous thromboembolism.
Fig. 2.
Associations of genetically higher TNF levels with cardiovascular diseases and cancers. AFGen indicates Atrial Fibrillation Consortium; BCAC, Breast Cancer Association Consortium; CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics; CI, confidence interval; ILCCO, International Lung Cancer Consortium; NA, not available; OCAC, The Ovarian Cancer Association Consortium; OR, odds ratio; PRACTICAL, Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome consortium; TNF, tumour necrosis factor; UKBB, UK Biobank.
4. Discussion
In the present MR study, we provided the first causal evidence of positive associations of TNF levels with atherothrombotic disease (coronary artery disease and ischaemic stroke) and venous thromboembolism. Furthermore, we revealed inverse associations of TNF levels with risk of overall cancer and several site-specific cancers (colorectal, breast, endometrial, and lung cancers). We confirmed that higher TNF levels were strongly associated with established TNF-driven diseases (rheumatoid arthritis and inflammatory bowel disease) which added strong support to the validity of the genetic instrument used and the reliability of our findings.
4.1. Primary findings in cardiovascular disease
A correlation between a TNF-related SNP and CVD was found in a study with 587 patients, which showed an association between the TNFA rs1800629 gene variant and cardiovascular complications in patients with rheumatoid arthritis albeit confined within individuals carrying the rheumatoid shared epitope [25]. The present study comprehensively examined associations of TNF levels with most common CVDs amongst a general population and revealed positive associations of TNF levels with atherothrombotic disease and venous thromboembolism.
Atherothrombotic disease is a chronic inflammatory disease of the arterial wall and has been shown to be TNF-driven. A possible positive association of TNF with ischaemic stroke [26] has been reported. In addition, TNF inhibition in patients with rheumatoid arthritis improves important correlates of CVD such as carotid intimal-medial thickness and aortic stiffness [27], and has been shown to reduce the risk of overall cardiovascular events [28], myocardial infarction and stroke in rheumatoid arthritis patients [6]. However, the putative role of TNF in driving this may differ in the general population and patients with inflammatory arthropathies subject to systemic inflammation, medications known to drive CVD such as nonsteroidal anti-inflammatory drugs and steroids and more abundant traditional risk factors. Our findings support previous research for TNF driving atherothrombosis and extend it to the general population. Importantly, targeting inflammation using the biological therapy has previously been successful. Canakinumab, which neutralizes IL1B, reduced recurrent cardiovascular outcomes in patients with a high inflammatory burden in a clinical trial even though the results of this trial were substantially lower than expected [29]. The underlying mechanism for TNF-driven atherothrombosis could be via a variety of proposed mechanisms, including favourable effects on circul1ating lipids, insulin resistance, endothelial dysfunction, leucocyte recruitment, oxidative stress, vasodilation or coagulation [30]. The observed positive association of genetically-predicted TNF levels with venous thromboembolism is not found in traditional observational studies showing no association [31,32], but the precision was low in those studies. However, a recent longitudinal cohort study based on the German register RABBIT revealed that anti-TNF agents decreased the risk of serious venous thromboembolism events compared to csDMARDs medicine [33], which is in line with our finding. Venous thromboembolism differs in pathology from arterial, which is driven by the atherosclerotic process. Even though inflammation and the innate immune system have an important role in venous thromboembolism, the link between TNF and thrombogenesis remains unclear. On one hand, TNF has been proposed to promote a pro-coagulant state. On the other hand, a recent study in mice found an essential role in the resolution of venous thrombus through the TNF receptor (TNF-Rp55) in intrathrombotic macrophages with no effect on coagulation [34].
4.2. Primary findings in cancer
With regard to overall cancer risk, randomized controlled trials and observational studies assessing the effect of TNF inhibitor treatment, primarily in rheumatoid arthritis and inflammatory bowel disease patients, have yielded inconclusive results [35,36]. This may relate to the complexities of such studies with rare cancer outcomes, short follow-up, high patient exclusions and the potential of reverse causation with the neoplastic process itself affecting levels of inflammatory mediators. Furthermore, there may be confounding from the underlying inflammatory disease or concomitant treatments such as non-steroidal anti-inflammatory drugs or disease-modifying anti-rheumatic drugs. Both a study with a long 10-year follow up [36], and a large meta-analysis of 6 randomized controlled trials [37] demonstrated it, although the latter themselves have been reported to increase cancer risk themselves [37]. The present MR study found an inverse association between TNF levels and overall cancer in UK Biobank, but we cannot exclude that the observed association might be driven by several site-specific cancers contributing a large proportion of cancer cases, such as breast cancer (18%) and colorectal cancer (7%). In any case, our MR study, which avoids many of the aforementioned limitations of previous studies, provides evidence that anti-TNF therapies may promote the development of some cancer types.
Previous studies of TNF levels in relation to risk of colorectal cancer are inconsistent. Carcinogenic effects have been suggested by a clinical study of 30 colorectal cancer patients in which the TNF gene was significantly overexpressed in cancerous tissue compared with adjacent normal colorectal tissue [38]. Although this does not provide causal evidence, in genetic studies polymorphisms of TNF have been associated with colorectal cancer risk [39]. Conversely, register-based studies have detected both no difference [36], and, an increased colorectal cancer risk [40], compared to untreated patients. Epidemiological data on TNF levels in relation to risk of breast, lung and endometrial cancer are also conflicting and scarce. A genetic study showed that TNFA−308 A allele was associated with a lower risk of breast cancer amongst European populations [41]. However, several register-based and cohort studies have found no association between TNF levels and breast cancer risk [42] or a reduced risk of breast cancer with TNF inhibitor use [36]. The protective effect of anti-TNF therapy observed on breast cancer risk may have been confounded by unmeasured effects of non-steroidal anti-inflammatory drugs [43] and other synthetic disease-modifying anti-rheumatic drugs [44] in patients with autoimmune diseases. Endometrial cancer risk was increased in women with elevated pre-diagnostic concentrations of TNF in a case-control study with 270 cases and 518 controls [45]. However, there was no association between TNF and endometrial cancer in a prospective study [46].
4.3. Clinical implications
Increased risk of cardiovascular disease with genetically predicted high TNF level sheds light on the usage of anti-TNF medicine as a potential prevention approach for people with excessive risk of CVD and a potential treatment strategy for patients with impaired cardiovascular condition. In addition, clinicians need to assess the potential increased CVD risk derived from TNF therapy especially amongst individuals with inherited or acquired high risk of CVD. With regard to the observed protective effect of TNF on cancer, our study reveals two important clinical considerations. Firstly, it suggests recombinant TNF therapy as a potential therapy in such cancers, in particular colorectal and breast cancers. Phase 2 trials of recombinant TNF across a range of cancer types have so far not proven successful in causing tumour responses [47] and associated with significant toxicity [48]. An exception is the use of local TNF administered locally by isolated limb perfusion treatments in melanoma and sarcoma [47], or in isolated hepatic perfusion for treatment of liver metastasis [49] which have demonstrated that TNF alone or in combination to cause large response rates of up to 80%. Such studies have been focused on advanced and metastatic cancers for which prognoses are poor and a significant tumour response would be unlikely. Future studies should assess the tumour responses in patients with earlier-staged disease and in combination therapies. The second important clinical implication of the inverse associations observed between TNF levels and cancer risk relates to the use of anti-TNF biological therapies, which are highly effective and ingrained in guidelines for the management of conditions such as rheumatoid arthritis and inflammatory bowel disease. Such therapies have previously been associated with concerns regarding cancer risk, particularly lymphoma [50] and non-melanoma skin cancer [51]. In line with the general consensus, we do not demonstrate a significant association with these cancer types.
4.4. Strengths, limitations and caveats
The MR design, which diminishes confounding and reverse causality, was the major strength of this study. Additionally, we comprehensively assessed the causal associations of TNF levels with a broad range of CVD and cancer outcomes. Data were mainly extracted from individuals of European ancestry, except for a few outcomes with a small portion of individuals of non-European ancestry. Moreover, the SNP-exposure and SNP-outcome estimates were adjusted for principal components for ancestry. Thus, population stratification bias is unlikely to have had an essential effect on our results. On the other hand, this population confinement, on a certain degree, compromised the generalizability of the study results to other populations, such as Asians, African Americans, etc. A major limitation is that the number of cases was few for some CVDs and site-specific cancers, which resulted in low precision of the estimates. Thus, we may have missed weak associations.
The results of this MR study should be interpreted in light of the pleiotropic effects of TNF, which plays a role in a wide range of biological processes, such as immunity, inflammation, apoptosis, lipid metabolism, and coagulation [1], [2], [3]. Although the observed associations of genetically higher TNF levels with increased risk of CVD (particularly atherosclerotic- and thrombotic-related CVDs) and lower risk of cancer are biologically plausible, we cannot entirely rule out that our results might have been affected by horizontal pleiotropy. For example, three of the four SNPs were associated with hypothyroidism, potentially reflecting autoimmune thyroiditis. The possible role of hypothyroidism in mediating (vertical pleiotropy) or biasing (horizontal pleiotropy) the results are unclear. In addition, even though the instrumental variables used were validated using two inflammatory diseases as positive controls, our findings need to be interpreted with caution given that the excluded SNP may have influences on inflammation in an opposite pathway or atherosclerosis only. Based on current findings, a comparative effect on cardiovascular system and carcinogenesis of anti-TNF therapies and treatments established on other biological mechanisms cannot be determined. Thus, the study provides limited evidence on drug selection in rheumatic disease treatment. Considering high risk of certain malignancies in individuals with rheumatic disease [52], randomized controlled trials are warranted to verify our findings and comprehensively evaluate the benefits and risks of anti-TNF therapy in populations with different health conditions, even though TNF levels of most included participants were in the healthy range [12].
Conclusions
This MR study found evidence of causal associations of increased TNF levels with higher risk of coronary artery disease, ischaemic stroke, and venous thromboembolism and decreased risk of overall, colorectal, breast, endometrial, and lung cancer. Along with previous observational studies [6,10], the present study strengthened the evidence that TNF inhibitors might reduce the risk of common cardiovascular events but increase risk of overall and certain cancers. These results may inform decisions concerning potential benefits and risks of TNF inhibitor therapy.
Author contributions
Study design: S.Y., P.C., M.B., M.V., S.K., A.M.M., A.L., S.B., S.C.L.; data acquisition and analysis: S.Y., A.M.M., S.B., S.C.L.; figures and writing S.Y.; reviewing and editing: S.Y., P.C., M.B., M.V., S.K., A.M.M., A.L., A.L., S.B., S.C.L.
Declaration of Competing Interests
SCL reports grants from Swedish Research Council (Vetenskapsrådet; grant no. 2019-00977), grants from Swedish Research Council for Health, Working Life and Welfare (Forte; grant no. 2018-00123), grants from Swedish Heart-Lung Foundation (Hjärt-Lungfonden; grant no. 20190247), during the conduct of the study. AMM reports grants from EC-Innovative Medicines Initiative (BigData@Heart), during the conduct of the study; .All other authors declare no conflicts of interest.
Acknowledgments
Acknowledgements
Summary-level data for CVDs and cancers were obtained from the UK Biobank and genetic consortia, including Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics consortium, MEGASTROKE consortium, Atrial Fibrillation Consortium, Breast Cancer Association Consortium, Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome consortium, International Lung Cancer Consortium, and Ovarian Cancer Association Consortium. The analyses of UK Biobank data were conducted under application 29202. The authors thank all investigators for sharing these data. The list of investigators of the MEGASTROKE consortium is available at http://megastroke.org/authors.html and funding of the MEGASTROKE project are specified at megastroke.org/acknowledgements.html.
Funding sources
Funding for this study came from the Swedish Research Council (Vetenskapsrådet; Grant No. 2019-00977), the Swedish Research Council for Health, Working Life and Welfare (Forte; Grant No. 2018-00123), and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden; Grant No. 20190247). Maria Bruzelius is supported by funds from Stockholm county council. Siddhartha Kar is supported by a Cancer Research UK programme grant, the Integrative Cancer Epidemiology Programme (C18281/A19169). Amy M. Mason is supported by EC-Innovative Medicines Initiative (BigData@Heart). Stephen Burgess is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant No. 204623/Z/16/Z). The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication. SCL had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data Sharing Statement
Data from UK Biobank can be obtained via application (https://www.ukbiobank.ac.uk/). Analyses of UK Biobank data were performed under application 29202. Summary-level data from genetic consortia are available online: Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics consortium (http://www.cardiogramplusc4d.org/), MEGASTROKE consortium (http://www.megastroke.org/), Atrial Fibrillation Consortium (https://www.afgen.org/), Breast Cancer Association Consortium (http://bcac.ccge.medschl.cam.ac.uk/), Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome consortium (http://practical.icr.ac.uk/blog/), International Lung Cancer Consortium (https://ilcco.iarc.fr/), and Ovarian Cancer Association Consortium (http://ocac.ccge.medschl.cam.ac.uk/).
Footnotes
Funding: Funding for this study came from the Swedish Research Council (Vetenskapsrådet; grant no. 2019–00977), the Swedish Research Council for Health, Working Life and Welfare (Forte; grant no. 2018–00,123), and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden; grant no. 20190247). Siddhartha Kar is supported by a Cancer Research UK programme grant, the Integrative Cancer Epidemiology Programme (C18281/A19169). Amy M. Mason is supported by EC-Innovative Medicines Initiative (BigData@Heart). Stephen Burgess is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant no. 204623/Z/16/Z).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102956.
Appendix. Supplementary materials
References
- 1.Waters J.P., Pober J.S., Bradley J.R. Tumour necrosis factor and cancer. J Pathol. 2013;230(3):241–248. doi: 10.1002/path.4188. [DOI] [PubMed] [Google Scholar]
- 2.Popa C., Netea M.G., van Riel P.L., van der Meer J.W., Stalenhoef A.F. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48(4):751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Page M.J., Bester J., Pretorius E. The inflammatory effects of TNF-alpha and complement component 3 on coagulation. Sci Rep. 2018;8(1):1812. doi: 10.1038/s41598-018-20220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branen L., Hovgaard L., Nitulescu M., Bengtsson E., Nilsson J., Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 5.Ridker P.M., Rifai N., Pfeffer M., Sacks F., Lepage S., Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg J.D., Furer V., Farkouh M.E. Cardiovascular safety of biologic therapies for the treatment of RA. Nat Rev Rheumatol. 2011;8(1):13–21. doi: 10.1038/nrrheum.2011.168. [DOI] [PubMed] [Google Scholar]
- 7.Mann D.L., Deswal A., Bozkurt B., Torre-Amione G. New therapeutics for chronic heart failure. Annu Rev Med. 2002;53:59–74. doi: 10.1146/annurev.med.53.082901.104004. [DOI] [PubMed] [Google Scholar]
- 8.Chung E.S., Packer M., Lo K.H., Fasanmade A.A., Willerson J.T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107(25):3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 9.Michalaki V., Syrigos K., Charles P., Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90(12):2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raaschou P., Simard J.F., Asker Hagelberg C., Askling J. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262. doi: 10.1136/bmj.i262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess S., Thompson S.G. Chapman and Hall/CRC Press; London, UK: 2015. Mendelian randomization: methods for using genetic variants in causal estimation. [Google Scholar]
- 12.Prins B.P. University of Groningen; Groningen, Netherland: 2016. Inflammatory biomarker genomics: from discovery to causality [dissertation] [Google Scholar]
- 13.Okada Y., Wu D., Trynka G., Raj T., Terao C., Ikari K. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lange K.M., Moutsianas L., Lee J.C., Lamb C.A., Luo Y., Kennedy N.A. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49(2):256–261. doi: 10.1038/ng.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michailidou K., Lindstrom S., Dennis J., Beesley J., Hui S., Kar S. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551(7678):92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher F.R., Al Olama A.A., Berndt S.I., Benlloch S., Ahmed M., Saunders E.J. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–936. doi: 10.1038/s41588-018-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., McKay J.D., Rafnar T., Wang Z., Timofeeva M.N., Broderick P. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet. 2014;46(7):736–741. doi: 10.1038/ng.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikpay M., Goel A., Won H.H., Hall L.M., Willenborg C., Kanoni S. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik R., Chauhan G., Traylor M., Sargurupremraj M., Okada Y., Mishra A. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50(4):524–537. doi: 10.1038/s41588-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragam K.G., Chaffin M., Levinson R.T., McDermott G., Choi S.H., Shoemaker M.B. Phenotypic refinement of heart failure in a national biobank facilitates genetic discovery. Circulation. 2019;139:489–501. doi: 10.1161/CIRCULATIONAHA.118.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelan C.M., Kuchenbaecker K.B., Tyrer J.P., Kar S.P., Lawrenson K., Winham S.J. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017;49(5):680–691. doi: 10.1038/ng.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S., Scott R.A., Timpson N.J., Davey Smith G., Thompson S.G. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–552. doi: 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brion M.J., Shakhbazov K., Visscher P.M. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Rodríguez L., González-Juanatey C., Palomino-Morales R., Vázquez-Rodríguez T.R., Miranda-Filloy J.A., Fernández-Gutiérrez B. TNFA -308 (rs1800629) polymorphism is associated with a higher risk of cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis. 2011;216(1):125–130. doi: 10.1016/j.atherosclerosis.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Cui G., Wang H., Li R., Zhang L., Li Z., Wang Y. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. J Neuroinflammation. 2012;9:235. doi: 10.1186/1742-2094-9-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maki-Petaja K.M., Hall F.C., Booth A.D., Wallace S.M., Yasmin Bearcroft PW. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114(11):1185–1192. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 28.Sattin M., Towheed T. The effect of TNFalpha-inhibitors on cardiovascular events in patients with rheumatoid arthritis: an updated systematic review of the literature. Curr Rheumatol Rev. 2016;12(3):208–222. [PubMed] [Google Scholar]
- 29.Shah S.R., Abbasi Z., Fatima M., Ochani R.K., Shahnawaz W., Asim Khan M. Canakinumab and cardiovascular outcomes: results of the CANTOS trial. J Community Hosp Intern Med Perspect. 2018;8(1):21–22. doi: 10.1080/20009666.2018.1428023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Poll T., Jansen P.M., Van Zee K.J., Welborn M.B., 3rd, de Jong I., Hack C.E. Tumor necrosis factor-alpha induces activation of coagulation and fibrinolysis in baboons through an exclusive effect on the p55 receptor. Blood. 1996;88(3):922–927. [PubMed] [Google Scholar]
- 31.Desai R.J., Pawar A., Weinblatt M.E., Kim S.C. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol. 2019;71(6):892–900. doi: 10.1002/art.40798. [DOI] [PubMed] [Google Scholar]
- 32.Davies R., Galloway J.B., Watson K.D., Lunt M., Symmons D.P., Hyrich K.L. Venous thrombotic events are not increased in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the british society for rheumatology biologics register. Ann Rheum Dis. 2011;70(10):1831–1834. doi: 10.1136/ard.2011.153536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer M., Schneider M., Graessler A., Ochs W., Zink A., Strangfeld A. OP0012 TNF inhibitors are associated with a reduced risk of venous thromboembolism compared to csdmards in RA patientS. Ann Rheum Dis. 2020;79(Suppl 1):8–9. [Google Scholar]
- 34.Saha P., Smith A. TNF-alpha (tumor necrosis factor-alpha) Arterioscler Thromb Vasc Biol. 2018;38(11):2542–2543. doi: 10.1161/ATVBAHA.118.311660. [DOI] [PubMed] [Google Scholar]
- 35.Mariette X., Matucci-Cerinic M., Pavelka K., Taylor P., van Vollenhoven R., Heatley R. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70(11):1895–1904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 36.Mercer L.K., Lunt M., Low A.L., Dixon W.G., Watson K.D., Symmons D.P. Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: results from the British society for rheumatology biologics register for rheumatoid arthritis. Ann Rheum Dis. 2015;74(6):1087–1093. doi: 10.1136/annrheumdis-2013-204851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon D.H., Kremer J.M., Fisher M., Curtis J.R., Furer V., Harrold L.R. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2014;43(4):489–497. doi: 10.1016/j.semarthrit.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Al Obeed O.A., Alkhayal K.A., Al Sheikh A., Zubaidi A.M., Vaali-Mohammed M.A., Boushey R. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J Gastroenterol. 2014;20(48):18390–18396. doi: 10.3748/wjg.v20.i48.18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min L., Chen D., Qu L., Shou C. Tumor necrosis factor-a polymorphisms and colorectal cancer risk: a meta-analysis. PLoS ONE. 2014;9(1):e85187. doi: 10.1371/journal.pone.0085187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dreyer L., Mellemkjaer L., Andersen A.R., Bennett P., Poulsen U.E., Juulsgaard Ellingsen T. Incidences of overall and site specific cancers in TNFalpha inhibitor treated patients with rheumatoid arthritis and other arthritides - a follow-up study from the DANBIO Registry. Ann Rheum Dis. 2013;72(1):79–82. doi: 10.1136/annrheumdis-2012-201969. [DOI] [PubMed] [Google Scholar]
- 41.Shen C., Sun H., Sun D., Xu L., Zhang X., Liu A. Polymorphisms of tumor necrosis factor-alpha and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2011;126(3):763–770. doi: 10.1007/s10549-010-1184-5. [DOI] [PubMed] [Google Scholar]
- 42.Mercer L.K., Davies R., Galloway J.B., Low A., Lunt M., Dixon W.G. Risk of cancer in patients receiving non-biologic disease-modifying therapy for rheumatoid arthritis compared with the UK general population. Rheumatol (Oxford) 2013;52(1):91–98. doi: 10.1093/rheumatology/kes350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry M.B., Gammon M.D., Zhang F.F., Tawfik H., Teitelbaum S.L., Britton J.A. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291(20):2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 44.Parikh-Patel A., White R.H., Allen M., Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001–1010. doi: 10.1007/s10552-009-9298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dossus L., Becker S., Rinaldi S., Lukanova A., Tjonneland A., Olsen A. Tumor necrosis factor (TNF)-alpha, soluble TNF receptors and endometrial cancer risk: the EPIC study. Int J Cancer. 2011;129(8):2032–2037. doi: 10.1002/ijc.25840. [DOI] [PubMed] [Google Scholar]
- 46.Wang T., Rohan T.E., Gunter M.J., Xue X., Wactawski-Wende J., Rajpathak S.N. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol Biomarkers Prev. 2011;20(5):971–977. doi: 10.1158/1055-9965.EPI-10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josephs S.F., Ichim T.E., Prince S.M., Kesari S., Marincola F.M., Escobedo A.R. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med. 2018;16(1):242. doi: 10.1186/s12967-018-1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts N.J., Zhou S., Diaz L.A., Jr., Holdhoff M. Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget. 2011;2(10):739–751. doi: 10.18632/oncotarget.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander H.R., Jr., Bartlett D.L., Libutti S.K. Current status of isolated hepatic perfusion with or without tumor necrosis factor for the treatment of unresectable cancers confined to liver. Oncologist. 2000;5(5):416–424. doi: 10.1634/theoncologist.5-5-416. [DOI] [PubMed] [Google Scholar]
- 50.Geborek P., Bladstrom A., Turesson C., Gulfe A., Petersson I.F., Saxne T. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis. 2005;64(5):699–703. doi: 10.1136/ard.2004.030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amari W., Zeringue A.L., McDonald J.R., Caplan L., Eisen S.A., Ranganathan P. Risk of non-melanoma skin cancer in a national cohort of veterans with rheumatoid arthritis. Rheumatol (Oxford) 2011;50(8):1431–1439. doi: 10.1093/rheumatology/ker113. [DOI] [PubMed] [Google Scholar]
- 52.Turesson C., Matteson E.L. Malignancy as a comorbidity in rheumatic diseases. Rheumatol (Oxford) 2013;52(1):5–14. doi: 10.1093/rheumatology/kes189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.