Abstract
Control of helminth parasites is a key challenge for human and veterinary medicine. In the absence of effective vaccines and adequate sanitation, prophylaxis and treatment commonly rely upon anthelmintics. There are concerns about the development of drug resistance, side-effects, lack of efficacy and cost-effectiveness that drive the need for new classes of anthelmintics. Despite this need, only three new drug classes have reached the animal market since 2000 and no new classes of anthelmintic have been approved for human use. So where are all the anthelmintics? What are the barriers to anthelmintic discovery, and what emerging opportunities can be used to address this? This was a discussion group focus at the 2019 8th Consortium for Anthelmintic Resistance and Susceptibility (CARS) in Wisconsin, USA. Here we report the findings of the group in the broader context of the human and veterinary anthelmintic discovery pipeline, highlighting challenges unique to antiparasitic drug discovery. We comment on why the development of novel anthelmintics has been so rare. Further, we discuss potential opportunities for drug development moving into the 21st Century.
Keywords: Anthelmintics, Helminths, Drug discovery, Nematodes, Challenges, Opportunities
Graphical abstract
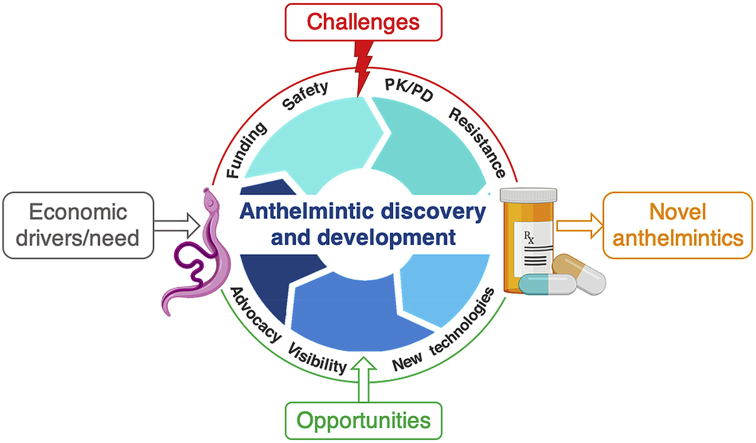
key challenges and opportunities identified by the 2019 CARS meeting for anthelmintic discovery in the context of the overall drug development process.
1. Introduction
Anthelmintics are drugs used for the treatment and control of infections of parasitic nematodes, trematodes and cestodes in animals and humans. The lack of effective vaccines and inadequate sanitation in some endemic regions has limited our ability to break the life cycles of these parasites. Instead, treatment and prophylaxis has had to rely on a limited number of chemical classes of anthelmintics (Table 1). The frequent use of these anthelmintics has led to concerns about the development of anthelmintic resistance in helminths of companion and production animals, with reports of resistance to multiple classes a clear threat to our existing control strategies. The evidence from veterinary medicine and reports of reduced anthelmintic efficacy in human helminths also raise concerns about the risk of resistance in mass drug administration efforts for humans (Geerts and Gryseels, 2000). New chemical classes with novel mechanisms of action are required to overcome the threat of resistance but, despite the urgent need for innovation, development of novel compounds for all helminths has been slow. Since 2000, only three new compounds have reached the animal market: the cyclic octadepsipeptide, emodepside (Harder and von Samson-Himmelstjerna, 2001); the aminoacetonitrile, monepantel (Kaminsky et al., 2008); and the spiroindole, derquantel (Woods et al., 2012). These products have not yet reached the human market.
Table 1.
List of drug classes and anthelmintics available for treating nematodes, trematodes and cestodes discovered pre- and post-2000.
| Drug Class | Nematode (roundworm) | Trematode (fluke) | Cestode (tapeworm) | |
|---|---|---|---|---|
|
Classes discovered pre-2000 |
Benzimidazoles | Albendazole | Albendazole | Albendazole Fenbendazole |
| Febantel | Triclabendazole | Oxfendazole | ||
| Fenbendazole | ||||
| Flubendazole | ||||
| Mebendazole | ||||
| Oxfendazole | ||||
| Oxibendazole | ||||
| Thiabendazole | ||||
| Salicylanilides | Closantel | Closantel | Closantel | |
| Rafoxanide | Rafoxanide | Rafoxanide | ||
| Disophenol | Oxyclozanide | Niclosamide | ||
| Nitroscanate | Resorantel | |||
| Nitroscanate | ||||
| Pyrazinoisoquinolones | Oxamniquine | Epsiprantel | ||
| Praziquantel | Praziquantel | |||
| Sulphonamide | Clorsulon | |||
| Imidazothiazole/Tetrahydropyrimidines | Levamisole | |||
| Morantel | ||||
| Pyrantel | ||||
| Oxantel | ||||
| Organophosphates | Dichlorvos | |||
| Haloxon | ||||
| Napthalofos | ||||
| Macrocylic lactones | Abamectin | |||
| Doramectin | ||||
| Eprinomectin | ||||
| Ivermectin | ||||
| Moxidectin | ||||
| Milbemycin oxime | ||||
| Selamectin | ||||
| Other | Piperazine | |||
| Diethylcarbamazine | ||||
| Doxycycline (anti-Wolbachia) | ||||
| Melarsomine | ||||
| Nitroxynil |
||||
| Post-2000 | Cyclic octadepsipeptide | |||
| Aminoacetonitrile derivatives | Nitroxynil | |||
| Spiroindole | Derquantel |
Why are new compounds so scarce and what can we do to change that? An expert group at the 8th Consortium discussed this topic for Anthelmintic Resistance and Susceptibility (CARS) meeting held on July 6, 2019 in Madison, Wisconsin, United States, in advance of the 27th International conference for the World Association for Veterinary Parasitology (WAAVP). Here we summarize these discussions within the context of the overall anthelmintic discovery and development process, identifying unique challenges for anthelmintics and opportunities for advancing discovery into the 21st Century.
2. Anthelmintic discovery and development
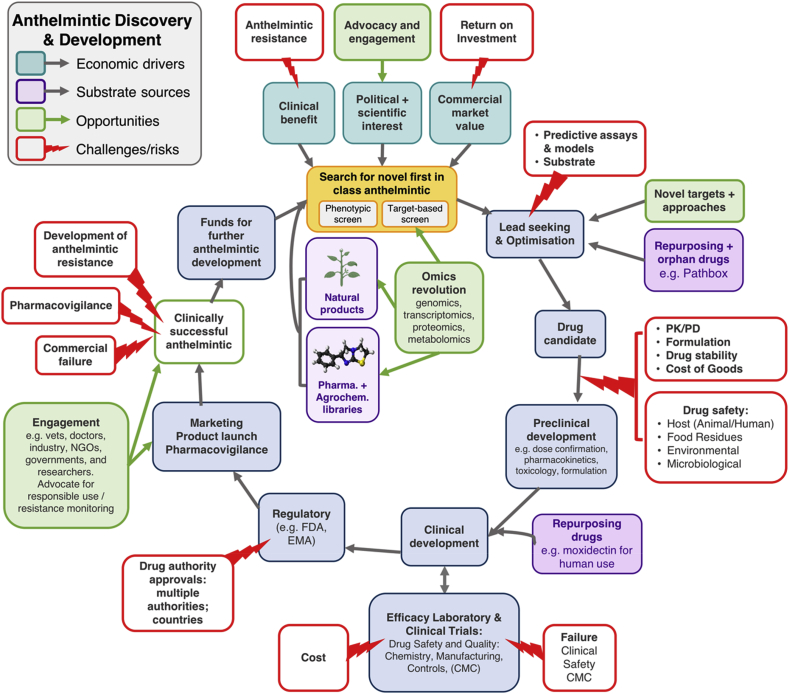
There are a multitude of drivers, processes and hurdles that impact anthelmintic discovery and development as illustrated in Fig. 1. A compound (or class) will typically undergo numerous rounds of optimization to improve potency, safety, pharmacokinetics and pharmacodynamics and formulation. The manufacturing process must be developed, optimized for cost-effectiveness and tightly controlled, according to regulatory standards. Finally, a drug must pass the regulatory hurdles for registration and ongoing pharmacovigilance. Here we discuss some of the unique challenges in anthelmintic discovery raised as part of the CARS discussion.
Fig. 1.
An overview of the anthelmintic discovery and development process, identifying economic drivers, challenges for anthelmintic discovery and opportunities to further the field.
2.1. Economic drivers
The economic drivers need to be significant to support the costly process of drug discovery, which has been estimated to be $50–100 million for animal health products (Yarborough, 2016) and over U.S.$2.5 billion for human drugs (DiMasi et al., 2016). Drivers for anthelmintic discovery include: the clinical need for anthelmintics in humans and animals; the value of the market and return on investment for a new anthelmintic (commercial success, primarily in Animal Health (AH)); and the political awareness and scientific interest for new drug discovery and development from industry, governments, non-governmental organizations (NGOs) and academia. These drivers provide the impetus for development of novel compounds across the scientific, technical, and legislative landscapes of discovery (Swinney and Anthony, 2011; Eder et al., 2014; Moffat et al., 2017).
Throughout the anthelmintic development process, the overall market value, clinical benefit and final cost of goods must be considered. Following World War 2 and the challenges of tropical parasitic diseases encountered by the military, government, academic and industrial laboratories discovered a range of antimalarial and anti-schistosomal drugs for human diseases (Beaumier et al., 2013). However, due to the significant costs of developing human anthelmintic medicines, subsequent research has been driven by veterinary commercial needs and the majority of human anthelmintics have been leveraged from AH pharmaceutical research (Aziz et al., 1982; Woods et al., 2007; Kyne et al., 2019). Ongoing collaborations between industry and academia continue to tackle human helminth infections with existing animal anthelmintics, including the companion animal anthelmintic, emodepside, which is currently being evaluated for onchocerciasis in humans (Karpstein et al., 2019).
Consequently, the economic drivers of the AH industry have been-and continue to be—critical to the overall success of novel anthelmintic discovery; however, different factors affect the commercial viability of discovery for different species in AH. While the owners of companion animals are often willing to pay a substantial price to protect their pets from parasites, producers and livestock owners must be far more cost-conscious. Given the significant additional hurdles for registration of production animal anthelmintics for food safety (see Section 2.4 Regulatory Challenges for Animal and Human Health) and the requirements for larger amounts of drug per animal, developing low-cost, practical, and long-action drugs are key challenges for livestock anthelmintics.
Primarily human parasitic diseases such as schistosomiasis however, lack the animal health economic drivers to support this approach. The cost of developing an entirely novel anthelmintic for human use would be enormous: a high-risk project, with no financial means of returning the investment. Here, product development partnerships (PDPs) will be essential in bridging this gap, enabling donors to provide funding and pharma and academia to provide technical expertise on projects. PDPs for anthelmintic discovery are being led through organizations like Drugs for Neglected Diseases Initiative (DNDi: www.dndi.org) and the World Intellectual Property Research Organization (WIPO) Re:Search consortium (Ramamoorthi et al., 2014). In addition to PDPs, the possibility of leveraging military partnerships, as was done for early anthelmintics by the Rockefeller foundation for hookworm treatment, was raised in the discussion as a source of future human anthelmintic funding and research; building on the already considerable support for vector-borne diseases like malaria and leishmaniasis, together with other communicable diseases that threaten military personnel (Ratto-Kim et al., 2018).
Discussions at the CARS meeting identified the lack of investment in basic biology, which is a fundamental consideration in the discovery of novel anthelmintics, highlighting the urgent need for stimulating political awareness and funding schemes for greater financial support. One opportunity is to influence representation by parasitologists on government grant awarding committees (such as the National Institutes of Health (NIH) in the United States; National Health and Medical Research Council (NMRC) and Australian Research Council (ARC) in Australia; Biotechnology and Biological Sciences Research Council (BBSRC) in the United Kingdom and the European Union research funding initiatives) bringing the need for anthelmintics to the forefront of funding discussions. The growth and stability of federal support for “curiosity-driven” molecular helminthology research is another important goal for such advocacy. Basic research that is not explicitly motivated by translational endpoints is a major driving force for discoveries that prove useful in unforeseen biomedical contexts (Botstein, 2012). The unique adaptations of parasitic worms that govern their coevolutionary interactions with mammals are fertile ground for novel biological insights. The study of helminths has already led to a fundamentally better understanding of human immunology (Jackson et al., 2009) and to bio-inspired medical devices (Yang et al., 2013). It is reasonable to assume that exploring the basic biology of parasitic worms will also illuminate new paths to anthelmintic discovery and parasite control. Advocacy for basic helminthology research also serves as an acknowledgement of the role of serendipity in pharmaceutical development (Campbell, 2005). We recognized that in addition to investment in basic biology, the successful basic biology needs to be taken up and used to develop effective products that are safe and used appropriately in the field. This too, requires investment and collaborative efforts across disciplines and academic and industry organizations.
2.2. Screening strategies for anthelmintics
Optimal approaches to screening remain an ongoing discussion in anthelmintic discovery. Anthelmintic discovery has traditionally been empirical, with large-scale screening performed using phenotypic whole organism assays with living parasites. Assay readouts include motility, larval development and lethality. The advantage of this approach is that assays are target-blind and may identify novel compound classes that would be missed in a single target approach. However, one of the key challenges in anthelmintic discovery is that whole organism screening is often, but not exclusively (Marcellino et al., 2012), low-throughput, resource-intensive, costly and requires specialist training. By necessity, parasitic helminths must be passaged through a host, maintained in vivo and subsequently isolated for testing. As such, the relevant species or life-stages may be difficult or even impossible to obtain. This substantially raises the costs for anthelmintic discovery relative to antibiotics—bacteria can be maintained far more cheaply, grown, and assayed much more rapidly. Ruminant parasites are especially costly, with only a few successful rodent models available to reduce these costs. History also teaches us that whole organism in vitro screens are not necessarily predictive of clinical efficacy, adding another layer of difficulty. For example, the macrocyclic lactones (MLs) are the primary drugs for prevention of disease caused by the canine heartworm Dirofilaria immitis. MLs only show activity against isolated microfilaria and larvae in vitro at vastly higher concentrations than needed for clinical efficacy in an infected animal (Blair et al., 1982). Similarly, for the human worm Onchocerca volvulus, vastly higher concentrations of ivermectin are required for in vitro activity against microfilaria than are achieved by the approved doses for human use (Laing et al., 2017). A strictly rational approach using phenotypic whole-organism motility screens would not necessarily have identified this chemical class as a candidate for filarial worm control.
There are however potential opportunities to expand and nuance whole-organism screening to include phenotypes that may be more predictive of in vivo drug efficacy with new technologies (see Aulner et al. (2019) for a review). The move towards automated imaging and processing pipelines enhances the throughput relative to traditional manual microscopy, as exemplified by screening systems such as Worminator and INVAPP (Storey et al., 2014; Partridge et al., 2018) and freely available analysis toolkits incorporating machine learning (Wahlby et al., 2012; Hakim et al., 2018). Discussions proposed greater utilisation of high-content imaging platforms, which can offer deeper insight into parasite morphological states, complex patterns of movement, or vital dye staining dynamics in response to drug exposure. Additionally, in vitro organoid screens that incorporate co-culture with host immune cells may better model relevant interactions at the drug-parasite-host interface (Duque-Correa et al., 2020). Some of the ‘cryptic’ phenotypes revealed by these lines of work can be paired to computational pipelines, including unsupervised or semi-supervised machine learning techniques, that allow for parasite phenotyping divorced from preconceived notions of how a novel antiparasitic should act. Progress on this front can conceivably improve the predictive power of in vitro whole-organism screening and should be an area of further research.
Today, there is increasing emphasis on target-based drug screening approaches, to improve throughput and leverage new technologies for identifying substrate. The major caveat of this approach is that compounds must be able to bio-accumulate in parasites to demonstrate efficacy, whereas small molecules with activity in target-based screens do not necessarily have the physicochemical properties to achieve this (Zhou et al., 2014; Lanusse et al., 2016). Despite this additional hurdle, target-based screening can be valuable in supporting lead optimization and understanding structure activity relationships, including optimizing for parasite selectivity over the host (Geary et al., 2009; Woods and Knauer, 2010). While this approach can improve throughput and enable medicinal chemistry, to be optimal it requires in-depth knowledge of the basic biology of the parasite—an area of research which has been underfunded for helminths. Advances in ‘omics’, such as genomics, transcriptomics, proteomics and metabolomics are facilitating rational target discovery in pathways and mechanisms considered vitally important for the parasite. However, these approaches must be validated with functional biology experiments. For example, the glutamate-gated chloride (GluCl) channel is the target of macrocyclic lactones in nematodes (Martin et al., 1992; Hibbs and Gouaux, 2011; Althoff et al., 2014). However, in filarial larvae, ML-responsive channels appear to only be expressed in muscles surrounding the excretory-secretory pore (Moreno et al., 2010) and unlikely to play the same role in locomotion and motility for filarial worms as it does for other nematodes (Wolstenholme et al., 2016). Current publications indicate that the immune system of the host and the host-parasite interaction may play a major role (Vatta et al., 2014; Wolstenholme et al., 2016), which may be related to the effect of MLs on secretion of immune modulatory products, but this still has to be demonstrated. Hence, it is clear that no single approach suits all parasites or all discovery purposes. Ultimately, we are in favor of more research into fundamental parasite biology, which can inform model and assay development to build more predictive assays for the identification and progression of better anthelmintics.
2.3. The challenges of preclinical development of drug candidates
Efficacy in vitro is only the first hurdle—a compound must also have appropriate safety, stability, solubility, desirable pharmacokinetics and—dynamics and in vivo efficacy to progress to clinical development. Anthelmintics represent unique challenges in all of these areas. Safety is a key priority in any drug discovery program, and any compound may prove harmful to a patient, due to both on- and off-target effects. As eukaryotes, parasites share significant homology with their hosts making selectivity and safety more difficult to achieve than for antibiotics against bacteria. Counter-screening for unwanted mechanisms of action and adverse side effects can and should be built early into the discovery process to help identify unsuitable compounds well before they are administered to an animal. An emerging opportunity is the use of machine-learning, analyzing large compound libraries to predict adverse reactions for new combinations of molecular structures, which could help reduce the time involved in producing and testing analogues (Gao et al., 2017; Dey et al., 2018).
Some helminth diseases require drugs with life-stage specificity to achieve patient safety, for example, L3/L4 larval-specific therapies are required for the filarial nematode D. immitis (canine heartworm) where rapid death of microfilariae and the adult life-stage can result in pulmonary thromboembolism or anaphylaxis and prove rapidly fatal for the dog (Keith et al., 1983; Hoch and Strickland, 2008; Bowman and Atkins, 2009). For the human filarial worm O. volvulus, which causes river blindness, the rapid death of microfilaria induced by diethylcarbamazine can lead to severe adverse reactions with increased ocular lesions and systemic inflammation (Bryceson et al., 1977; Greene et al., 1985). Consequently, ivermectin is preferred as a safer alternative; however, ivermectin similarly rapidly kills Loa microfilaria leading to severe neurological adverse reactions with high L. loa microfilaraemia (Gardon et al., 1997). Consequently millions of people are left untreated, hampering efforts to eliminate onchocerciasis (Gebrezgabiher et al., 2019). Hence, in some cases it is necessary to find drugs with life-stage and even species selectivity to improve patient outcomes. In dogs, an additional issue with the mdr-1 mutation in some breeds leads to toxicity of macrocyclic lactones and other drugs, limiting their use at higher doses (Dowling, 2006) and requiring additional safety testing for canine drugs.
Beyond safety, a new anthelmintic should be active against existing resistant isolates and optimally be active against a broad spectrum of parasites, especially in AH. Farmers and pet owners want to reduce animal dosing as much as possible, so long-lasting efficacy against a spectrum of parasites is preferred. Likewise, for human helminths, minimal dosing is preferred to ease the logistics of mass drug administration. In both cases, a product that is stable with a long shelf life is needed. Once all these factors are established, the compound may be administered to an animal—where we then encounter further challenges in anthelmintic discovery. The pharmacokinetics and pharmacodynamics (PK/PD) of the compound are critical in achieving efficacy of anthelmintics; the compound must be absorbed and distributed to the tissue where the parasite resides within the host, without being transformed to an inactive metabolite or excreted before acting on the parasite. There is a complex relationship between the anthelmintic drug concentration in the plasma, gastrointestinal tract and the concentration found in different species of nematode parasite that has to be overcome (Cowan et al., 2017). An exception can occur in gastrointestinal worm drug discovery, as orally administered compounds in principle do not need to be absorbed into the bloodstream for activity against the helminths, potentially avoiding systemic toxicities with poorly absorbed compounds. Of course, this does not account for situations where there may be advantages to enabling reduced drug dose due to intestinal reabsorption of drugs excreted via the bile (Alvarez-Bujidos et al., 1998). It is also unclear whether a product for blood-feeding nematodes (such as hookworm species) needs to be available in the blood at therapeutic levels for efficacy (Garcia-Bustos et al., 2019). Furthermore, given that many worms are found in tissues other than the gastrointestinal tract, the desire for a broad-spectrum anthelmintic may negate this advantage. Formulation and delivery technologies to overcome these issues are available, but may not be economically feasible for each indication and target species (Pritchard et al., 2003; Datta and Grant, 2004; Porter et al., 2007).
2.4. Regulatory challenges for animal and human health
Ultimately, a successful new anthelmintic drug must pass regulatory hurdles for safety, efficacy and manufacturing. In contrast to other indication areas, the efficacy for anthelmintics is required to be as high as 90%, based on Veterinary International Committee on Harmonization (VICH) guidelines, and may be required to be up to 100% for parasites such as D. immitis or Echinococcus granulosus. Effectiveness below 90% may be adequate when the claimed parasites do not have any other effective treatment.
Beyond efficacy and target animal safety, there are several additional regulatory hurdles for livestock regarding how anthelmintics affect food production—such as milk, meat or eggs. To ensure the safety of the consumer, the regulatory body calculates maximum residues levels (MRL). Detectable levels of the anthelmintic must be below the MRL, leading to a withdrawal period defined by the regulatory body which must be adhered to by producers (Delatour et al., 2018). As a result, food safety hurdles must also be factored into the livestock anthelmintic discovery and development process. For example, high lipophilicity is undesirable for dairy cattle anthelmintics as the drug and its metabolites are likely to be eliminated at least in part via the milk, necessitating a long withdrawal period. The high lipophilicity and subsequent long withdrawal period of ivermectin, therefore made it undesirable as an anthelmintic for dairy cattle, despite its effectiveness (Campbell, 2016). In contrast, eprinomectin can be used with a withdrawal period of 0 days for milk in lactating cows, due to its pharmacokinetic properties resulting in a low plasma-milk ratio. Environmental safety is another issue that is becoming increasingly challenging, for both human and animal drug production. Because of the potential impacts of pharmaceutical residues on the aquatic and terrestrial ecosystems, regulatory authorities require an environmental risk assessment (ERA) of all pharmaceutical products before marketing is allowed (Lee and Choi, 2019). For livestock on pastures, the risk is considered even higher, due to a combination of ecotoxicity and persistence in soil/sediment (Liebig et al., 2010; Lumaret et al., 2012), which is an added hurdle for livestock anthelmintics. For both human and animal pharmaceuticals, safety of the manufacturing process is essential, user safety is critical, with occupational exposure values determined following toxicological evaluations.
Finally, some of the most challenging aspects of the drug approval process are the Chemistry, Manufacturing and Controls (CMC) requirements, which ensure the consistency and quality of drug manufacturing.
3. Inspired by nature: Natural products as a source for new anthelmintics
So–accepting the challenges—where then might we look for new anthelmintics? Much of our discussion at CARS centered around natural product anthelmintic discovery. Nature has already proven to be a valuable resource for anthelmintic discovery, exemplified by the discovery and development of avermectins, originally isolated from the bacteria Streptomyces avermitilis in the 1970s (Burg et al., 1979; Egerton et al., 1979; Miller et al., 1979). The subsequent development of the macrocyclic lactone class revolutionized animal and human health to become the predominant class of anthelmintics and save millions of lives. This breakthrough was rewarded with the Nobel Prize in Physiology or Medicine 2015 being divided, one half jointly to William C. Campbell and Satoshi Ōmura “for their discoveries concerning a novel therapy against infections caused by roundworm parasites” (Nobel Media, 2020). Likewise, the emodepside precursor, PF1022 A, was isolated from fungi on camellia plant leaves (Sasaki et al., 1992). Beyond micro-organisms, a broad range of plants and animals produce defensive molecules against predators and parasites, including helminths (Trowbridge, 2014), presenting a huge library of potential biopharmaceuticals for anthelmintic discovery.
It is important to note that successful natural product drugs are primarily single compounds isolated from a natural source, not requiring the use of the whole plant, fungi or bacteria itself. Due to the highly variable characteristics of natural product generation, mixtures of natural products are unsuitable for the tightly regulated drug production process, which has very strict quality assurance requirements (CMC). For instance, compound production in plants can be greatly affected by plant genetics, the environment (rainfall, soil, temperature, pests, pesticides and harvest time) and a host of other factors, resulting in variable quantities of active compounds, plus impurities also being produced in similarly variable quantities. In addition, observations of biological activity in mixtures of natural products can be lost on purification, possibly due to synergistic or additive activity of multiple compounds. Consequently, natural product mixtures are not suitable as drugs; rather, the identification of a single active molecule is critical. However, identification of a bioactive molecule is not trivial. The compound must be isolated, structurally elucidated and produced (either semi-synthetically or recombinantly). Here too the ‘omics’ revolution and biotechnological advances are aiding in identification of natural compounds with microbiome, transcriptome, proteome and metabolome studies. Subsequently, this product must be able to be produced reproducibly and cost-effectively at scale by either natural or synthetic means with quality control and assurance as per regulatory body standards.
Despite the challenges in natural product drug discovery, there has been a rapid increase in the medicinal plant and natural product studies in recent years (Atanasov et al., 2015). A casual PubMed search using the keywords ‘natural product anthelmintic’ yields over two thousand results. However as noted by Garcia-Bustos et al. (2019) there has been a strong bias towards plant extracts with few of the active compounds identified and even more limited follow-up post initial description. The lack of follow-through is a key area of weakness in current anthelmintic discovery; more funding and incentive through industry, academic and stakeholder partnerships is needed to develop these discoveries beyond simple screening. We recommend that there is better communication and expectation management around natural product discovery and the drug development process, particularly regarding regulatory requirements, to manage the observed lack of follow-up. Nonetheless, the identification of numerous anthelmintic compounds from a variety of plants and even more unusual natural resources, such as funnel-web spider venoms (Nixon et al., 2019; Herzig et al., 2020) and marine organisms (Mayer et al., 2009), may because for optimism that the next ivermectin is out there.
4. Challenge of resistance and ongoing management
Anthelmintic resistance underpinned our discovery discussions, as it drives further anthelmintic discovery. Our discussion group noted that we face at least some partial misconceptions around the issue of anthelmintic resistance. Anthelmintic resistance is clearly a severe problem in certain parasites and geographic localizations—for example in Australian livestock production—requiring strong advocacy for anthelmintic management and increased support for anthelmintic discovery to overcome this. In addition to soil-transmitted helminths, there is increasing recognition of the food-borne trematode infections and concerns around the development of resistance to triclabendazole (Fairweather et al., 2020). However, in our communications around resistance we must take care not to confound the discussion about anthelmintic resistance, with the concerns around the use of critically important antibiotics in human and veterinary medicine (Scott et al., 2019). One advantage here is that parasites are typically host-selective and specific, so there are few parasites that are both (1) infectious for both humans and animals; and (2) exposed to anthelmintics in both worlds (Sibley and Hunt, 2003). While there is evidence of horizontal gene transfer between hosts and parasites and retroviral infections in parasites (reviewed by Wijayawardena et al. (2013)), group discussions concluded that based on current understandings it is unlikely resistance would be transferred from animal to human parasites.
Nonetheless, it is clear that we cannot be complacent on the matter of resistance across a number of host and parasite species. Beyond the known issue of resistance in livestock helminths (Waghorn et al., 2006; Geurden et al., 2015), there are issues of reduced efficacy or resistance in companion animal nematodes, notably with regard to macrocyclic lactones in canine heartworms (Wolstenholme et al., 2015; Bourguinat et al., 2017) and concerning new reports of multidrug resistance in canine hookworms (Jimenez Castro et al., 2019). There have also been reports of reduced efficacy of: praziquantel against human Schistosoma infections (Alonso et al., 2006; Crellen et al., 2016); ivermectin against O. volvulus (Osei-Atweneboana et al., 2011); and reduced benzimidazole efficacy against Ascaris lumbricoides (Krücken et al., 2017; Furtado et al., 2019), raising concerns for mass drug administration efforts. In order to monitor development of anthelmintic resistance in human soil-transmitted nematodes because of concerns about the use of mass drug administration and the limited number of drug classes, the StarWorms (STop Anthelmintic Resistant Worms) Project, funded by the Gates Foundation, has been developed (www.starworms.org). The discovery of novel classes and mechanisms of action would be a key opportunity to circumvent this existing resistance. With the introduction of any novel anthelmintic, however, we must carefully manage and monitor for the emergence of resistance to ensure future sustainability of the new anthelmintic class. This requires collaborative efforts between industry, academia, governments, regulatory authorities and clinicians to ensure that owners and producers are accurately informed as to correct usage —and hence, is an opportunity to strengthen our partnerships across all these spheres.
5. Opportunities: Putting anthelmintics back on the map
To address the challenges of anthelmintic discovery, we must seek out and apply new opportunities and technologies. The key barrier identified by the discussion group was the lack of funding for basic helminth research and anthelmintic discovery, which in turn raised concerns for recruitment and retention of talented scientists in parasitology research. The low visibility of helminth diseases, among the neglected diseases, was cited as a key contributing factor for the lack of funding. There are examples of public-private partnerships between industry and academia for anthelmintics, such as the recently announced Helminth Elimination Platform (HELP), part of Europe's Horizon 2020 program, where Bayer Animal Health, and the second industry partner Celgene, now part of Bristol-Myers Squibb, give access to their proprietary molecules to be tested for efficacy against different parasitic nematodes. Nonetheless, we agreed that more is needed to engage with end-users of anthelmintics, media and decision makers. If WHO and other stakeholders advocate that the existing drugs are sufficient it is not surprising that the interest in developing novel anthelmintics is modest. There is a clear need to increase the visibility of the field and our researchers. So how can we better engage with our target audiences and increase our visibility and representation?
Our younger researchers advocated for the use of digital communications, to facilitate global reach and didactic conversations. These platforms present an opportunity for researchers to communicate their findings, build community trust, and seek out new collaborations and opportunities worldwide. We recognize and emphasize the importance of publishing in traditional academic journals as a key means of communicating research with the added credibility of peer-review; however, we encourage researchers to consider co-publishing research and opinions on freely accessible digital platforms. One example is The Conversation, where academics communicate their research in short form online articles for the public. The Conversation has wide readership: beginning in Australia in March 2011, and expanded into the United Kingdom in 2013, the United States in 2014, France and then Africa in 2015, Canada in 2017, and Spain in 2018. The Conversation publishes content under a creative commons licence and in 2019, it had a monthly online audience of 10.7 million onsite users. As The Conversation is authored by academics, it is viewed as a reputable source and utilized by policy makers and media—authors on The Conversation are contacted for further interviews by traditional media (radio, television and print media).
Our younger researchers also highlighted the impact of increasing visibility via social media. Social media enables us to ‘put a human face’ to our research, generate large reach and rapidly converse with diverse audiences for science communication (Collins et al., 2016). There is a variety of platforms, including Twitter, Facebook, YouTube, Instagram, ResearchGate, LinkedIn, TikTok and WhatsApp broadcast lists. There has been increasing interest in scientists using social media; a 2015 survey of scientists associated with the American Association for the Advancement of Science (AAAS) found that about half were using social media to discuss science and keep up to date with discoveries (Pew Research Center, 2015). Indeed, Twitter interactions has now overtaken journal-impact factors as a stronger predictor of citation rates in some fields of scientific research (Peoples et al., 2016). We recommend that researchers choose the platform that best suits their target audience (e.g. clinicians, producers or media) and the content they are able to produce (e.g. videos for YouTube; Twitter to facilitate rapid sharing of links to articles). Likewise, it also presents an opportunity for our stakeholders—clinicians, pet owners and producers—to contribute to the conversation, rapidly communicate concerns (such as emerging resistance) and build connections between researchers and end-users. A significant following (>1000 for Twitter) however, is required to reach diverse audiences beyond scientists within the same discipline (Côté and Darling, 2018). When combined with existing platforms, such as parasitology society websites, we can directly share accurate information and trainings for veterinarians, producers and pet-owners, increase our visibility and advocate for evidence-based policy. A good example of this is the American Heartworm Society, which provides resources specifically targeted for both pet-owners and veterinarians on their website (www.heartwormsociety.org/), and promotes this information via Facebook. We also highlight Dr Martin Nielsen's work (University of Kentucky), communicating on evidence-based horse parasite management and anthelmintic use in his award-winning short, plain English videos called ‘Deworm Debunked’ video series shared over Facebook, Twitter and YouTube (Nielsen, 2019). Hence, digital communications provide a strong opportunity for us to communicate directly to anthelmintic users (e.g. veterinarians, farmers and pet owners) and about the importance of anthelmintic research to decision-makers, in addition to our more traditional approaches. As with all new approaches, there does need to be a recognition that there are attendant risks of this more immediate form of communication (Bucchi, 2019). Researchers may find the book Communicating Science Effectively, published by the National Academy of Sciences, Engineering and Medicine (2017) informative regarding science communication benefits, as it summarizes current evidence, strategies and risks for a number of communication styles and platforms, including social media. Regardless of the communications platform selected (be that digital media, conference presentations or academic journal articles), time and training are required for researchers to communicate effectively—providing such training is a further opportunity to build new interdisciplinary collaborations. The balance and exchange, however, between quick and timely individual communication on the one hand and peer-reviewed publications on the other hand is important – there must be reliable sources to enable readers to differentiate trusted information from ideas, hypotheses and, worst case, fake news. Active communication by parasitologists via social media, however, can also support the spread of peer-reviewed information.
The importance of collaborations was emphasized in the CARS discussion group. The group represented diverse career stages, expertise and academic and industry representatives. The subsequent exchanges between creative and enthusiastic young researchers familiar with emerging technologies in science and communications, and highly experienced senior researchers, across disciplines and professions were valuable for encouraging new ideas and collaborations. Future conferences may benefit from integrating similar breakout discussions to facilitate these connections. The nature of anthelmintic discovery requires collaboration and diverse expertise; and ensuring responsible use of new anthelmintics takes a further collective effort. Providing opportunities for developing and maintaining strong cross-disciplinary partnerships will be key for growing our capacity for anthelmintic discovery into the 21st Century.
Declaration of competing interest
The authors are employees of different universities, federal institutions and independent commercial companies as listed below. The opinions expressed by the authors were written in good faith for the scientific benefit.
Acknowledgements
The commentary arose from a larger group discussing this topic that included the authors. Thanks to all participants as well as Anne Lespine and Roger Prichard as part of the organizing team of the CARS meeting, a WAAVP satellite meeting sponsored by Bayer Animal Health. SAN acknowledges support from the Westpac Bicentennial Foundation (Westpac Future Leaders Scholarship). MZ acknowledges support from NIH R01AI151171. RJM acknowledges support from NIH R01AI047194, R21AI138967 and the E. A. Benbrook Foundation for Pathology and Parasitology. The content does not represent the official views of the National Institute of Allergy and Infectious Diseases.
References
- Alonso D., Munoz J., Gascon J., Valls M.E., Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am. J. Trop. Med. Hyg. 2006;74:342–344. [PubMed] [Google Scholar]
- Althoff T., Hibbs R.E., Banerjee S., Gouaux E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature. 2014;512:333–337. doi: 10.1038/nature13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Bujidos L., Ortiz A.I., Molina-Martinez I.T., Cubria C., Ordonez D. Pharmacokinetics of intravenous luxabendazole in rabbits: influence of the enterohepatic circulation. Biopharm Drug Dispos. 1998;19:341–347. doi: 10.1002/(sici)1099-081x(199807)19:5<341::aid-bdd110>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., Rollinger J.M., Schuster D., Breuss J.M., Bochkov V., Mihovilovic M.D., Kopp B., Bauer R., Dirsch V.M., Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulner N., Danckaert A., Ihm J., Shum D., Shorte S.L. Next-generation phenotypic screening in early drug discovery for infectious diseases. Trends Parasitol. 2019;35:559–570. doi: 10.1016/j.pt.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Aziz M.A., Diallo S., Diop I.M., Lariviere M., Porta M. Efficacy and tolerance of ivermectin in human onchocerciasis. Lancet. 1982;2:171–173. doi: 10.1016/s0140-6736(82)91026-1. [DOI] [PubMed] [Google Scholar]
- Beaumier C.M., Gillespie P.M., Hotez P.J., Bottazzi M.E. New vaccines for neglected parasitic diseases and dengue. Transl. Res. 2013;162:144–155. doi: 10.1016/j.trsl.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Blair L.S., Williams E., Ewanciw D.V. Efficacy of ivermectin against third-stage Dirofilaria immitis larvae in ferrets and dogs. Res. Vet. Sci. 1982;33:386–387. [PubMed] [Google Scholar]
- Botstein D. Why we need more basic biology research, not less. Mol. Biol. Cell. 2012;23:4160–4161. doi: 10.1091/mbc.E12-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguinat C., Keller K., Xia J., Lepage P., McTier T.L., Woods D.J., Prichard R.K. Genetic profiles of ten Dirofilaria immitis isolates susceptible or resistant to macrocyclic lactone heartworm preventives. Parasites Vectors. 2017;10:504. doi: 10.1186/s13071-017-2428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman D.D., Atkins C.E. Heartworm biology, treatment, and control. Vet. Clin. N. Am. Small Anim. Pract. 2009;39:1127–1158. doi: 10.1016/j.cvsm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bryceson A.D., Warrell D.A., Pope H.M. Dangerous reactions to treatment of onchocerciasis with diethylcarbamazine. Br. Med. J. 1977;1:742–744. doi: 10.1136/bmj.1.6063.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchi M. Facing the challenges of science communication 2.0: quality, credibility and expertise. EFSA J. 2019;17 doi: 10.2903/j.efsa.2019.e170702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg R.W., Miller B.M., Baker E.E., Birnbaum J., Currie S.A., Hartman R., Kong Y.L., Monaghan R.L., Olson G., Putter I., Tunac J.B., Wallick H., Stapley E.O., Oiwa R., Omura S. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 1979;15:361–367. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W.C. Serendipity and new drugs for infectious disease. ILAR J. 2005;46:352–356. doi: 10.1093/ilar.46.4.352. [DOI] [PubMed] [Google Scholar]
- Campbell W.C. Lessons from the history of ivermectin and other antiparasitic agents. Ann. Rev. Anim. Biosci. 2016;4:1–14. doi: 10.1146/annurev-animal-021815-111209. [DOI] [PubMed] [Google Scholar]
- Collins K., Shiffman D., Rock J. How are scientists using social media in the workplace? PLoS One. 2016;11 doi: 10.1371/journal.pone.0162680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté I.M., Darling E.S. Scientists on Twitter: preaching to the choir or singing from the rooftops? FACETS. 2018;3:682–694. [Google Scholar]
- Cowan N., Meier C., Neodo A., Keiser J. Exposure of Heligmosomoides polygyrus and Trichuris muris to albendazole, albendazole sulfoxide, mebendazole and oxantel pamoate in vitro and in vivo to elucidate the pathway of drug entry into these gastrointestinal nematodes. Int. J. Parasitol. Drugs Drug Resist. 2017;7:159–173. doi: 10.1016/j.ijpddr.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellen T., Walker M., Lamberton P.H.L., Kabatereine N.B., Tukahebwa E.M., Cotton J.A., Webster J.P. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 2016;63:1151–1159. doi: 10.1093/cid/ciw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S., Grant D.J. Crystal structures of drugs: advances in determination, prediction and engineering. Nat. Rev. Drug Discov. 2004;3:42–57. doi: 10.1038/nrd1280. [DOI] [PubMed] [Google Scholar]
- Delatour T., Racault L., Bessaire T., Desmarchelier A. Screening of veterinary drug residues in food by LC-MS/MS. Background and challenges. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:632–645. doi: 10.1080/19440049.2018.1426890. [DOI] [PubMed] [Google Scholar]
- Dey S., Luo H., Fokoue A., Hu J., Zhang P. Predicting adverse drug reactions through interpretable deep learning framework. BMC Bioinf. 2018;19:476. doi: 10.1186/s12859-018-2544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMasi J.A., Grabowski H.G., Hansen R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Dowling P. Pharmacogenetics: it's not just about ivermectin in collies. Can. Vet. J. 2006;47:1165–1168. [PMC free article] [PubMed] [Google Scholar]
- Duque-Correa M.A., Maizels R.M., Grencis R.K., Berriman M. Organoids — new models for host-helminth interactions. Trends Parasitol. 2020;36:170–181. doi: 10.1016/j.pt.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder J., Sedrani R., Wiesmann C. The discovery of first-in-class drugs: origins and evolution. Nat. Rev. Drug Discov. 2014;13:577–587. doi: 10.1038/nrd4336. [DOI] [PubMed] [Google Scholar]
- Egerton J.R., Ostlind D.A., Blair L.S., Eary C.H., Suhayda D., Cifelli S., Riek R.F., Campbell W.C. Avermectins, new family of potent anthelmintic agents: efficacy of the B1a component. Antimicrob. Agents Chemother. 1979;15:372–378. doi: 10.1128/aac.15.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather I., Brennan G.P., Hanna R.E.B., Robinson M.W., Skuce P.J. Drug resistance in liver flukes. Int. J. Parasitol. Drugs Drug Resist. 2020;12:39–59. doi: 10.1016/j.ijpddr.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado L.F.V., Medeiros C.d.S., Zuccherato L.W., Alves W.P., de Oliveira V.N.G.M., da Silva V.J., Miranda G.S., Fujiwara R.T., Rabelo É.M.L. First identification of the benzimidazole resistance-associated F200Y SNP in the beta-tubulin gene in Ascaris lumbricoides. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Igata H., Takeuchi A., Sato K., Ikegaya Y. Machine learning-based prediction of adverse drug effects: an example of seizure-inducing compounds. J. Pharmacol. Sci. 2017;133:70–78. doi: 10.1016/j.jphs.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J.F., Sleebs B.E., Gasser R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasites Vectors. 2019;12 doi: 10.1186/s13071-019-3537-1. 306-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardon J., Gardon-Wendel N., Demanga N., Kamgno J., Chippaux J.P., Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Woods D.J., Williams T., Nwaka S. Target identification and mechanism-based screening for anthelmintics: application of veterinary antiparasitic research programs to search for new antiparasitic drugs for human indications. In: Selzer P.M., editor. Antiparasitic and Antibacterial Drug Discovery. Wiley‐VCH Verlag GmbH & Co; Weinheim, Germany: 2009. pp. 1–15. [Google Scholar]
- Gebrezgabiher G., Mekonnen Z., Yewhalaw D., Hailu A. Reaching the last mile: main challenges relating to and recommendations to accelerate onchocerciasis elimination in Africa. Inf. Dis. Pov. 2019;8:60. doi: 10.1186/s40249-019-0567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts S., Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin. Microbiol. Rev. 2000;13:207–222. doi: 10.1128/cmr.13.2.207-222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurden T., Chartier C., Fanke J., di Regalbono A.F., Traversa D., von Samson-Himmelstjerna G., Demeler J., Vanimisetti H.B., Bartram D.J., Denwood M.J. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int. J. Parasitol. Drugs Drug Resist. 2015;5:163–171. doi: 10.1016/j.ijpddr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene B.M., Taylor H.R., Cupp E.W., Murphy R.P., White A.T., Aziz M.A., Schulz-Key H., D'Anna S.A., Newland H.S., Goldschmidt L.P., Auer C., Hanson A.P., Freeman S.V., Reber E.W., Williams P.N. Comparison of ivermectin and diethylcarbamazine in the treatment of onchocerciasis. N. Engl. J. Med. 1985;313:133–138. doi: 10.1056/NEJM198507183130301. [DOI] [PubMed] [Google Scholar]
- Hakim A., Mor Y., Toker I.A., Levine A., Neuhof M., Markovitz Y., Rechavi O. WorMachine: machine learning-based phenotypic analysis tool for worms. BMC Biol. 2018;16 doi: 10.1186/s12915-017-0477-0. 8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder A., von Samson-Himmelstjerna G. Activity of the cyclic depsipeptide emodepside (BAY 44-4400) against larval and adult stages of nematodes in rodents and the influence on worm survival. Parasitol. Res. 2001;87:924–928. doi: 10.1007/s004360100479. [DOI] [PubMed] [Google Scholar]
- Herzig V., Cristofori-Armstrong B., Israel M.R., Nixon S.A., Vetter I., King G.F. Animal toxins — nature's evolutionary-refined toolkit for basic research and drug discovery. Biochem. Pharmacol. 2020:114096. doi: 10.1016/j.bcp.2020.114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs R.E., Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch H., Strickland K. Canine and feline dirofilariasis: prophylaxis, treatment, and complications of treatment. Compend. Contin. Educ. Vet. 2008;30:146–151. quiz 151-142. [PubMed] [Google Scholar]
- Jackson J.A., Friberg I.M., Little S., Bradley J.E. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunol. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez Castro P.D., Howell S.B., Schaefer J.J., Avramenko R.W., Gilleard J.S., Kaplan R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasites Vectors. 2019;12:576. doi: 10.1186/s13071-019-3828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky R., Ducray P., Jung M., Clover R., Rufener L., Bouvier J., Weber S.S., Wenger A., Wieland-Berghausen S., Goebel T., Gauvry N., Pautrat F., Skripsky T., Froelich O., Komoin-Oka C., Westlund B., Sluder A., Maser P. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- Karpstein T., Pasche V., Häberli C., Scandale I., Neodo A., Keiser J. Evaluation of emodepside in laboratory models of human intestinal nematode and schistosome infections. Parasites Vectors. 2019;12:226. doi: 10.1186/s13071-019-3476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith J.C., Jr., Rawlings C.A., Schaub R.G. Treatment of canine dirofilariasis: pulmonary thromboembolism caused by thiacetarsamide--microscopic changes. Am. J. Vet. Res. 1983;44:1272–1277. [PubMed] [Google Scholar]
- Krücken J., Fraundorfer K., Mugisha J.C., Ramünke S., Sifft K.C., Geus D., Habarugira F., Ndoli J., Sendegeya A., Mukampunga C., Bayingana C., Aebischer T., Demeler J., Gahutu J.B., Mockenhaupt F.P., von Samson-Himmelstjerna G. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int. J. Parasitol. Drugs Drug Resist. 2017;7:262–271. doi: 10.1016/j.ijpddr.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyne G.M., Curtis M.P., Keiser J., Woods D.J. Soil-transmitted helminthiasis — challenges with discovery of novel anthelmintics. In: Swinney D., Pollastri Michael, editors. Neglected Tropical Diseases: Drug Discovery and Development. Wiley-VCH; Weinheim: 2019. pp. 227–251. [Google Scholar]
- Laing R., Gillan V., Devaney E. Ivermectin: old drug, new tricks? Trends Parasitol. 2017;33:463–472. doi: 10.1016/j.pt.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanusse C.E., Alvarez L.I., Lifschitz A.L. Gaining insights into the pharmacology of anthelmintics using Haemonchus contortus as a model nematode. Adv. Parasitol. 2016;93:465–518. doi: 10.1016/bs.apar.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Lee D., Choi K. Comparison of regulatory frameworks of environmental risk assessments for human pharmaceuticals in EU, USA, and Canada. Sci. Total Environ. 2019;671:1026–1035. [Google Scholar]
- Liebig M., Fernandez Á.A., Blübaum-Gronau E., Boxall A., Brinke M., Carbonell G., Egeler P., Fenner K., Fernandez C., Fink G., Garric J., Halling-Sørensen B., Knacker T., Krogh K.A., Küster A., Löffler D., Cots M.Á.P., Pope L., Prasse C., Römbke J., Rönnefahrt I., Schneider M.K., Schweitzer N., Tarazona J.V., Ternes T.A., Traunspurger W., Wehrhan A., Duis K. Environmental risk assessment of ivermectin: a case study. Integr. Environ. Assess. 2010;6:567–587. doi: 10.1002/ieam.96. [DOI] [PubMed] [Google Scholar]
- Lumaret J.P., Errouissi F., Floate K., Rombke J., Wardhaugh K. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr. Pharmaceut. Biotechnol. 2012;13:1004–1060. doi: 10.2174/138920112800399257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino C., Gut J., Lim K.C., Singh R., McKerrow J., Sakanari J. WormAssay: a novel computer application for whole-plate motion-based screening of macroscopic parasites. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Kusel J.R., Robertson S.J., Minta A., Haugland R.P. Distribution of a fluorescent ivermectin probe, bodipy ivermectin, in tissues of the nematode parasite Ascaris suum. Parasitol. Res. 1992;78:341–348. [Google Scholar]
- Mayer A.M., Rodriguez A.D., Berlinck R.G., Hamann M.T. Marine pharmacology in 2005-6: marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta. 2009;1790:283–308. doi: 10.1016/j.bbagen.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T.W., Chaiet L., Cole D.J., Cole L.J., Flor J.E., Goegelman R.T., Gullo V.P., Joshua H., Kempf A.J., Krellwitz W.R., Monaghan R.L., Ormond R.E., Wilson K.E., Albers-Schönberg G., Putter I. Avermectins, new family of potent anthelmintic agents: isolation and chromatographic properties. Antimicrob. Agents Chemother. 1979;15:368. doi: 10.1128/aac.15.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J.G., Vincent F., Lee J.A., Eder J., Prunotto M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat. Rev. Drug Discov. 2017;16:531–543. doi: 10.1038/nrd.2017.111. [DOI] [PubMed] [Google Scholar]
- Moreno Y., Nabhan J.F., Solomon J., Mackenzie C.D., Geary T.G. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20120–20125. doi: 10.1073/pnas.1011983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering and Medicine, Division of Behavioral and Social Sciences and Education, Commitee on the Science of Science Communication: A Research Agenda . National Academies Press (US); Washington, DC: 2017. Communicating Science Effectively: a Research Agenda. [PubMed] [Google Scholar]
- Nielsen M.K. Deworm debunk. YouTube. 2019. https://www.youtube.com/channel/UC_pDqB6sRvi1XJ8AHUapPCw Available at:
- Nixon S.A., Saez N.J., Herzig V., King G.F., Kotze A.C. The antitrypanosomal diarylamidines, diminazene and pentamidine, show anthelmintic activity against Haemonchus contortus in vitro. Vet. Parasitol. 2019;270:40–46. doi: 10.1016/j.vetpar.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Nobel Media The Nobel prize in Physiology or medicine 2015. 2020. https://www.nobelprize.org/prizes/medicine/2015/summary/ Available at:
- Osei-Atweneboana M.Y., Awadzi K., Attah S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Neglected Trop. Dis. 2011;5:e998. doi: 10.1371/journal.pntd.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge F.A., Brown A.E., Buckingham S.D., Willis N.J., Wynne G.M., Forman R., Else K.J., Morrison A.A., Matthews J.B., Russell A.J., Lomas D.A., Sattelle D.B. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018;8:8–21. doi: 10.1016/j.ijpddr.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples B.K., Midway S.R., Sackett D., Lynch A., Cooney P.B. Twitter predicts citation rates of ecological research. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center . 2015. How Scientists Engage the Public. [Google Scholar]
- Porter C.J., Trevaskis N.L., Charman W.N. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- Pritchard J.F., Jurima-Romet M., Reimer M.L., Mortimer E., Rolfe B., Cayen M.N. Making better drugs: decision gates in non-clinical drug development. Nat. Rev. Drug Discov. 2003;2:542–553. doi: 10.1038/nrd1131. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi R., Graef K.M., Dent J. WIPO Re:Search: accelerating anthelmintic development through cross-sector partnerships. Int. J. Parasitol. Drugs Drug Resist. 2014;4:220–225. doi: 10.1016/j.ijpddr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto-Kim S., Yoon I.K., Paris R.M., Excler J.L., Kim J.H., O'Connell R.J. The US military commitment to vaccine development: a century of successes and challenges. Front. Immunol. 2018;9:1397. doi: 10.3389/fimmu.2018.01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Takagi M., Yaguchi T., Miyadoh S., Okada T., Koyama M. A new anthelmintic cyclodepsipeptide, PF1022A. J. Antibiot. (Tokyo) 1992;45:692–697. doi: 10.7164/antibiotics.45.692. [DOI] [PubMed] [Google Scholar]
- Scott H.M., Acuff G., Bergeron G., Bourassa M.W., Gill J., Graham D.W., Kahn L.H., Morley P.S., Salois M.J., Simjee S., Singer R.S., Smith T.C., Storrs C., Wittum T.E. Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019;1441:8–16. doi: 10.1111/nyas.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C.H., Hunt S.Y. Drug resistance in parasites: can we stay ahead of the evolutionary curve? Trends Parasitol. 2003;19:532–537. doi: 10.1016/j.pt.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Storey B., Marcellino C., Miller M., Maclean M., Mostafa E., Howell S., Sakanari J., Wolstenholme A., Kaplan R. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: "The Worminator. Int. J. Parasitol. Drugs Drug Resist. 2014;4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney D.C., Anthony J. How were new medicines discovered? Nat. Rev. Drug Discov. 2011;10:507–519. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- Trowbridge A.M. Evolutionary ecology of chemically mediated plant-insect interactions. In: Monson R.K., editor. Ecology and the Environment. Springer New York; New York, NY: 2014. pp. 143–176. [Google Scholar]
- Vatta A.F., Dzimianski M., Storey B.E., Camus M.S., Moorhead A.R., Kaplan R.M., Wolstenholme A.J. Ivermectin-dependent attachment of neutrophils and peripheral blood mononuclear cells to Dirofilaria immitis microfilariae in vitro. Vet. Parasitol. 2014;206:38–42. doi: 10.1016/j.vetpar.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Waghorn T.S., Leathwick D.M., Rhodes A.P., Lawrence K.E., Jackson R., Pomroy W.E., West D.M., Moffat J.R. Prevalence of anthelmintic resistance on sheep farms in New Zealand. N. Z. Vet. J. 2006;54:271–277. doi: 10.1080/00480169.2006.36710. [DOI] [PubMed] [Google Scholar]
- Wahlby C., Kamentsky L., Liu Z.H., Riklin-Raviv T., Conery A.L., O'Rourke E.J., Sokolnicki K.L., Visvikis O., Ljosa V., Irazoqui J.E., Golland P., Ruvkun G., Ausubel F.M., Carpenter A.E. An image analysis toolbox for high-throughput C. elegans assays. Nat. Methods. 2012;9:714–716. doi: 10.1038/nmeth.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardena B.K., Minchella D.J., DeWoody J.A. Hosts, parasites, and horizontal gene transfer. Trends Parasitol. 2013;29:329–338. doi: 10.1016/j.pt.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Evans C.C., Jimenez P.D., Moorhead A.R. The emergence of macrocyclic lactone resistance in the canine heartworm, Dirofilaria immitis. Parasitology. 2015;142:1249–1259. doi: 10.1017/S003118201500061X. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Maclean M.J., Coates R., McCoy C.J., Reaves B.J. How do the macrocyclic lactones kill filarial nematode larvae? Invertebr. Neurosci. 2016;16:7. doi: 10.1007/s10158-016-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D.J., Knauer C.S. Discovery of veterinary antiparasitic agents in the 21st Century: a view from industry. Int. J. Parasitol. 2010;40:1177–1181. doi: 10.1016/j.ijpara.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Woods D.J., Lauret C., Geary T. Anthelmintic discovery and development in the animal health industry. Expet Opin. Drug Discov. 2007;2:S25–S33. doi: 10.1517/17460441.2.S1.S25. [DOI] [PubMed] [Google Scholar]
- Woods D.J., Maeder S.J., Robertson A.P., Martin R.J., Geary T.G., Thompson D.P., Johnson S.S., Conder G.A. Discovery, mode of action, and commercialization of derquantel. In: Caffery C.R., Selzner P.M., editors. Parasitic Helminths: Targets, Screens, Drugs, and Vaccines. Wiley & Blackwell; Weinhem, Germany: 2012. pp. 297–307. [Google Scholar]
- Yang S.Y., O'Cearbhaill E.D., Sisk G.C., Park K.M., Cho W.K., Villiger M., Bouma B.E., Pomahac B., Karp J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Comms. 2013;4:1702. doi: 10.1038/ncomms2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarborough C. Why animal health is the next big area. Life Science Connect. 2016. www.lifescienceleader.com/doc/why-animal-health-is-the-next-big-growth-area-0001 Available at:
- Zhou X., Deng J.N., Hummel B.D., Woods D.J., Collard W.T., Hu S.X., Zaya M.J., Knauer C.S., Thompson D.P., Merritt D.A., Lorenz J.K., Marchiondo A.A. Development of an in vitro screen for compound bioaccumulation in Haemonchus contortus. J. Parasitol. 2014;100:848–855. doi: 10.1645/14-556.1. [DOI] [PubMed] [Google Scholar]