Summary
Moist wound healing is a method of retaining moisture to increase migration of epithelial cells and synthesis of collagen. One of the liquids that can be used is 0.1% betaine-polyhexanide solution. This study aimed to determine the effect of frequency of dressing replacement with 0.1% betaine-polyhexanide solution on the formation of collagen and epithelial tissue in albino wistar rats with grade IIA burns. The study used the randomized posttest-only control group design to investigate within a period of 13 days 25 male rats, which were divided into 5 groups, each comprising 5 rats. The groups included the control group and the treatment groups with dressing changes every 12 hours, 24 hours, 36 hours, and 48 hours. The variables measured in this study were the epithelium and collagen percentages. One-way ANOVA test revealed a significant difference in the synthesis of collagen with a p-value of 0.002 (p < 0.05) but no significant difference in the formation of epithelial tissue with a p-value of 0.561 (p > 0.05). The highest score was found in the group with a dressing change every 24 hours. It can be concluded that the treatment of grade IIA burns with 0.1% betainepolyhexanide solution once every 24 hours can optimize the formation of collagen and epithelial tissue.
Keywords: betaine-polyhexanide solution, collagen, epithelial tissue, burns, frequency of dressing replacement
Abstract
Le maintien de l’humidité locale a pour but de promouvoir la migration des cellules épithéliales et la synthèse du collagène. Un des liquides utilisables est la solution de bétaïne- polyhexanide (BPH) à 0,1% (Prontosan® en France, NDRLF). Cette étude a pour but d’évaluer l’effet de la fréquence de changement d’un pansement à la bétaïne- polyhexanide 0,1% sur la formation de collagène et de tissu épithélial après brûlure du 2ème degré superficiel chez le rat Wistar albinos. Il s’agit d’une étude randomisée avec mesure en fin de traitement (sur une période de 13 jours) chez 5 groupes de 5 rats, un groupe contrôles et 4 groupes avec pansement toutes les 12, 24, 36 et 48 h, les variables évaluées étant l’épithélialisation et la densité de collagène. En ANOVA unilatéral, on observe une différence significative de la synthèse de collagène (p = 0,002) et pas de différence d’épithélialisation. La plus grande différence est observée lorsque le pansement est changé toutes les 24 h, qui semble donc être la fréquence optimale de changement des pansements à la BPH.
Introduction
Burns are injuries to the skin caused by direct contact with high-temperature sources such as fire, hot water, electricity, chemicals and radiation that can result in skin damage. If a burn is not treated properly, it can cause life-threatening complications. Deaths from burns have been reported, nearly all of which were categorized as grade II and III burns.1,2 In addition, 60% of the burns were caused by home accidents.3,4 Appropriate treatments are thus required for burns to prevent complications. One of such treatments is burn care.
One of the important points in dressing replacement is to keep the wound moist. This can be achieved by the moist wound healing method that can increase epithelialization by 30-50% and collagen synthesis by 50%.5-8 One of the liquids that can be used for moist wound healing is 0.1% betainepolyhexanide solution as agent for wound cleansing and debridement and provider of a moist environment for wounds.9 In addition, there are several things to consider in wound care, such as the presence of wound infections, type of bandage used, wet cotton dressing, and duration of wound closure that affects the frequency of wound dressing.10 The results of previous studies have shown that compressing with 0.1% betaine-polyhexanide solution at 80% moist can help with the healing process of grade IIA burns. The frequency of replacement of compresses was to be investigated in this study. In general, wound care in hospitals uses conventional dressing with a replacement every 12-48 hours. Given this, it is necessary to find out the ideal frequency of dressing replacement to optimize healing of burns. The purpose of this study was to determine whether the frequency of replacement of dressing with 0.1% betaine-polyhexanide solution affects the formation of collagen and epithelial tissue in albino wistar rats with grade IIA burns.
Materials and methods
Design
This study is a true experimental laboratory study. The research sample was divided into 1 control group and 4 treatment groups. The control group (K) consisted of rats with grade IIA burns receiving burn care with the wounds being irrigated and compressed using normal saline (NS) solution with a dressing replacement every 24 hours. Meanwhile, the treatment 1 group (P1) consisted of rats with grade IIA burns receiving wound care in which the wounds were irrigated with NS solution and compressed with 0.1% betaine-polyhexanide solution (Prontosan®) with a dressing replacement every 12 hours. The treatment 2 group (P2), treatment 3 group (P3), and treatment 4 group (P4) consisted of rats with grade IIA burns irrigated with NS solution and compressed using 0.1% betaine-polyhexanide solution with a dressing replacement every 24, 36 and 48 hours, respectively. Wound care was administered to the rats for 13 days. On the 13th day, the rats were sacrificed and the skin tissues were taken for histological examination. Ethical clearance for this study was granted by the Ethics Committee for Health Research of the Faculty of Medicine, Universitas Brawijaya.
Sample
This study used 25 male wistar strain rats (Rattus novergicus). The rats used as sample were healthy rats weighing 200-250 grams and aged 2.5-3 months. The rats were allotted to different groups, each containing 5 rats, by simple random sampling. The rats were singly housed with free access to standard chow diet and water before and after the burn procedure. The rats were not restrained after the procedure. Ambient temperature and humidity were not recorded.
Preparation of grade IIA burns
The rats were anesthetized using ketamine. Then, the back area was shaved 3 x 3 cm2 in size and disinfected. Next, a gauze wrapping of 2 x 2 x 2 cm3 styrofoam was put in boiling water for 3 minutes, and then it was stamped on the shaved skin for 30 seconds. The duration was obtained from our preliminary study, which compared different time lengths of hot gauze stamp soaking to produce grade IIA scalds, and confirmed through histopathological assessment. After the gauze was stamped, the resultant scalds (or burn wounds) were cooled down by putting a gauze compress soaked in room tempera ture and normal salinity to prevent the spread of burns. Further treatment was given to each group.
Treatment of grade IIA burns
Treatment of grade IIA burns was carried out for 13 days. Wound care was given to each group. In group K, wound care and dressing replacement was done every 24 hours, whereas in P1, P2, P3 and P4, it was done every 12, 24, 36 and 48 hours, respectively. Based on the results of our exploratory study, the 0.1% betaine-polyhexanide liquid compress using a 7 x 10 cm2, 0.25 grams gauze would produce 80% moisture if given a volume of 1 cc, which was effective in helping with the healing process of grade II burns. The 80% moisture was validated with MD010 Moisture Meter. Wound care in all groups was carried out by aseptic technique, whereby the wound was covered with gauze to prevent infection, although cultures were not taken from the burns.
Histopathological examination
After the treatment period was completed at the end of the study, the rats were sacrificed, and the wound and its surrounding healthy tissue was harvested. The wound tissue specimens from the control and treatment groups were collected and stored in a buffered formaldehyde solution, after which the specimens entered the dehydration and impregnation stage. Then, the tissue was embedded in solid paraffin, and the middle of the wound where the wound length was greatest was longitudinally cut 4μm thick and stained with hematoxylin and eosin staining. Histopathology was used to observe the amount of collagen, the distance of the two wound edges, and the epithelial tissue formed. For collagen observation, we used Optika Vision Pro (Microscopy) software, and the epithelial tissue formed was observed using OLYMPUS XC10 photo microscope and OlyVia (Viewer for Imaging Application) software with a 10x or 40x magnification.11,12 After that, the results of collagen and epithelial tissue measurements were calculated. The calculation of collagen count was done using the following formula:13

Meanwhile, the modified calculation of re-epithelialization used the following formula:14

Statistical analysis
The test was performed statistically for collagen count and epithelial length. The steps of comparative and correlative hypothesis testing were as follows: data normality test, homogeneity of variance test, one-way ANOVA test, and post-hoc test (Tukey HSD test) with a significance level of 0.05 (p < 0.05) and 95% confidence level (α = 0.05). These tests used the SPSS program.
Results
Histological results of collagen density
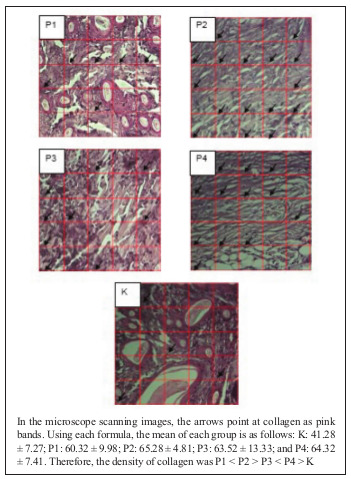
Based on the results of microscope scanning, in P2 and P4, collagen that filled the dermis area could be seen (Fig. 1).
Fig. 1. Histological density of collagen formed in this study.
Histological results of epithelial tissue percentage
Based on the results of microscope scanning, as indicated by arrows (Fig. 2), there was an appearance consisting of a section from the basal layer to the corneum layer minus the keratin layer. It can be concluded that the structure and shape of the epithelium found, as shown in the histological picture in this study, are as described in theory. In P2 and K, there were epithelial tissues that had covered the wound (Fig. 3). The summary of the percentage of epithelial tissue formed in the wound can be seen in Fig. 3 and Table 1.
Fig. 2. Percentage of epithelial tissue formed.

Fig. 3. Mean percentage of epithelial tissue formed.

Table I. Statistical analysis of collagen density and percentage of epithelial tissue formed.

Statistical analysis of the percentage of epithelial tissue formed
The normality and homogeneity tests of collagen and the percentage of epithelial tissue formed showed normal and homogeneous data (p > 0.05). One-way ANOVA test of collagen showed significance with p value = 0.002 (p < 0.05). Meanwhile, one-way ANOVA test of the percentage of epithelial tissue formed showed p value = 0.561 (p < 0.05). One-way ANOVA test results showed no significant difference in the formation of epithelial tissue between the 0.1% betaine-polyhexanide treatment groups and the NS control group. The results of post-hoc collagen test showed that there were significant differences between the control group and the treatment groups. There was a significant increase in the amount of collagen in P1, P2, P3 and P4. As presented in Table I, the lowest mean of collagen was found in K (41.28 ± 7.27), while the highest mean was found in P2 (65.28 ± 4.81). The lowest mean of epithelial tissue formed was found in P4 (65.05 ± 35.08), while the highest mean was found in P2 (84.89 ± 14.93).
Discussion
Collagen
Collagen is the most common protein in the extracellular skin matrix, and it serves to fill the extracellular matrix. Collagen fibres in the wound repair process begin to show up on the 3rd day post-injury around the edges of the wound, especially those mediated by IL-4 exposure from macrophage cells, and will be apparent in number by day 7. Furthermore, in the proliferative phase, collagen begins to be synthesized by the fibroblasts stimulated by TGF-β from macrophages and fibroblasts themselves, especially collagen type III fibres. The collagen deposition seen in this phase is arranged randomly. In the remodelling phase on the 14th day post-injury, collagen type III is being replaced by collagen type I in the form of bands. It has a higher stretch strength, stability and organization.15-17
As the results of this study show, collagen was formed and organized in the form of bands. Based on the descriptive analysis results, there was an inverted U-shaped relationship between the formation of collagen and the frequency of the dressing replacement, with the peak in the 24 hours of 0.1% betaine-polyhexanide solution administration group (P2). This result is in contrast to the studies indicating that the density of collagen increased more rapidly in 14 days of wound care with a dressing replacement every 2 days using hydrocolloids and in treatment of wound wrapped in conventional gauze with a replacement every 2 days and left open for up to 14 days due to mechanical factors because of the attachment between the wound to the conventional gauze which affected the wound healing process.18,19 Moisture on the surface of the lower wound on conventional uncompressed gauze will also affect the synthesis of collagen. However, in this study the dressings consisted of 0.1% betaine-polyhexanide liquid compress, which was able to maintain the moisture with a replacement every 24 hours, allowing the wound to heal faster and not to return to the previous phase. The 0.1% betaine-polyhexanide compress also gave an antibacterial effect on wounds that could help with the process of burn healing, although no measurements were taken in this study. The ability of 0.1% betaine-polyhexanide liquid as an antibacterial agent, as tested in several studies, is better than 2% chlorhexidine and silver sulfadiazine.20-22 It generates less pain than dry environment which can cause dehydrated cells and cell deaths.23,24 Conventional dressing has a high evaporation rate, so the low moisture above the surface of the wound will cause the oxygen pressure inside the wound to be lower.18,25 Then, the process of collagen synthesis becomes slower. This is associated with the importance of oxygen as a co-factor during the hydroxylation of proline and lysine in the process of formation of procollagen. Conversely, if the moisture above the surface of the wound is high, the formation of collagen will be accelerated. This means that the one replacement in every 24 hours frequency is able to maintain maximum humidity, because the evaporation caused by conventional gauze does not occur for a long time and there is no mechanical contact that can damage the granulation tissue, preventing the healing phase from returning to the previous phase.
The results generated in P4 showed the second highest collagen density after those generated in P2. This is due to the difference in oxygen pressure on the surface of the wound relating to the duration of wound closure and the frequency of dressing replacement that will affect the formation of collagen. Collagen formation itself is associated with the differences in wound surface oxygenation and the mechanical factors at the time of dressing replacement. However, this group had a lower collagen density compared to P2. This is related to the high evaporation in conventional gauze dressings. 18,25 The results in P1 showed that this group had the lowest collagen density in the treatment group. This is likely due to the use of conventional gauze dressing that is considered traumatic and due to the mechanical factors involved at the time of dressing replacement, which will influence the attachment of the wound surface. Then, the wound healing process partly or completely returns to the previous phase, causing the formation of collagen to be slower.
The statistical test of collagen density in P1 to P2, P3 and P4 showed an insignificant difference (P2, p = 0.905; P3, p = 0.979; P4, p = 0.954). This is because the wound superficial moisture produced by the four treatment groups is similar as in the four groups a 0.1% betaine-polyhexanide liquid compress was used. There is no standard for determining the frequency of dressing replacement, but most surgical practitioners perform dressing replacements every 24-48 hours to maintain wound moisture, hygiene and drainage in the wound.18,23,26 One factor in collagen synthesis is the oxygen pressure on the wound surface. When the production of O2 exceeds the amount of oxygen available, the synthesis of collagen will increase. The description above shows that, as in evaporated gauze, high frequency of bandage opening will affect the oxygen pressure on the tissue. The duration of injury exposure to the environment will affect the oxygen pressure on the wound, thus affecting the density of collagen in the wound. The more often the wound is exposed to the external environment, the lower the oxygen pressure on the wound will be, resulting in lower collagen synthesis. So, it can be concluded that the frequencies of dressing replacement of once in 12 hours, 24 hours, 36 hours and 48 hours generate no effect on collagen.18 The results of this study are beneficial for nursing applications in that they inform that the more often dressing is replaced or removed, the more time and gauze is needed. So, the replacement of bandages every 48 hours will increase the effectiveness of healing, time and cost because it can suppress the need for gauze, energy and time for wound care.
Epithelial tissue
There was no statistically significant difference in the formation of epithelial tissue in all treatment groups of 0.1% betaine-polyhexanide liquid with frequencies of once in 12 hours, 24 hours, 36 hours and 48 hours. This is because 0.1% betaine-polyhexanide liquid can keep the wound moist. In wound healing, there is an epithelialization process. Epithelialization is the migration of epithelial cells from the edges of the wound to the centre of the wound. When the epithelium covers the wound, the wound is considered to be healed. Wide or full-thickness wounds require skin grafts because epidermal migration is limited to 3 cm.
Epithelialization can be accelerated if the wound moisture can be maintained.27 In the wound to which the basal membrane is intact, epithelialization proceeds more rapidly so that keratinocytes are stimulated to migrate.28-30
Based on previous research, 0.1% betaine-polyhexanide liquid has no toxic risk and low sensitivity or allergic risk. The main indications for using 0.1% betaine-polyhexanide liquid are to decontaminate and excessively exudate, cleanse slough and eschar to prevent biofilm formation, and reduce wound odour. It is also very easy to use, excellent for patient comfort when replacing dressing, and applicable in the long run.27 The selection of dressing in wound care can also affect the wound moisture. Conventional dressing has a high evaporation rate (moisture vapour transmission rate = 68 ± 2), and the duration of wound closure affects low moisture above the wound surface, slowing down the collagen synthesis process.31 If collagen synthesis is inhibited, epithelialization may also be inhibited because to initiate the epithelialization process repair of the underlying tissue will be required. NS solution has a moisturizing property that can help the formation of new blood vessels faster, thus increasing the process of tissue oxygenation and nutrient supply to the wound. Increased oxygenation and supply to the wound can accelerate the epithelialization process.32 However, NS solution has a high evaporation rate, so the dressing becomes dry quickly and the wound moisture becomes low, thereby slowing down the wound healing. In this study, the epithelial tissues formed on the grade IIA burns given NS solution had a mean of 82.54. This suggests that the treatment of grade IIA burns using NS solution promotes epithelialization.
Wound care using gauze with NS compress generally uses wet-dry and wet-moist techniques. The wet-dry technique is used with the aim of debridement, while the wet-moist technique is used to keep moisture on the wound. However, the wetdry technique for debridement is not recommended because it damages healthy tissue. So, for the use of gauze with NS compress, it is recommended that the wet-moist technique is used. If the wet-moist dressing technique is employed, to keep the gauze moist, the gauze should be replaced with NS solution on a regular basis. When the gauze dries, the release of the gauze can cause pain and disturbance to the healing process due to the presence of the dry gauze on the surface of the wound and the presence of granulation tissue attached to the gauze.28 This brings the wound back to the initial phase, and an extension of time is needed for wound healing. In addition, NS also has a high permeability rate for gas and steam, causing the evaporation of oxygen on the surface of the wound to go high and the moisture to go down, thus slowing the process of wound healing, especially epithelialization.32 NS is an isotonic solution. However, by evaporating the water in the saline solution and rendering a hypertonic state, the liquid from the wound will be absorbed into the dressing to replace the water in order to maintain the isotonic state. If it continues for a longer period, the wound can no longer replace it, so the dressing will become dry and allow the existing tissue on the surface to stick to the gauze. Therefore, dressing should be regularly made wet again with NS solution to prevent dryness. This is in contrast to 0.1% betaine-polyhexanide solution, which does not evaporate quickly and can keep the wound moist. The nature of polyhexanide can provide moisture balance in the wound.27 The solution is also very convenient for the patient, and it reduces pain during dressing replacement. Hence, it does not cause stress to the patients, while stress can extend wound healing.33 Moisture in wounds resulting from the use of 0.1% betaine polyhexanide solution can prevent tissue trauma at the time of dressing replacement.27
Descriptively, the replacement of dressing every 24 hours using either 0.1% betaine polyhexanide solution or NS solution tends to result in the most optimal epithelialization process, so both of these solutions can be used to optimize the epithelialization process. However, when NS solution is used, we should always replace the dressing every 24 hours to prevent dryness of dressing due to the rapid evaporation of NS solution. In other words, treatment with NS solution requires more effort in wound care. Meanwhile, the use of 0.1% betaine-polyhexanide allows for a replacement every 12 hours to 48 hours, with the best result generated if replacement is done every 24 hours. Mismatch in microscopic examination results does not necessarily indicate abnormality. This may occur due to several factors, such as the examiners’ lack of accuracy in calculating the number of cells, error in determining the field of view, or error in preparing the specimens. For example, the smear is so thin that it contains only a few cells. In this study, insignificant results can also be attributed to technical error factors, such as the researchers’ poor accuracy in calculating the lengths. This may be associated with the many curvy forms on the specimen, hence requiring a very long time for the calculation. It may also be associated with the time constraints in using the Olyvia software, making it difficult for the researchers to calculate the exact length of the curvy epithelium tissue using the software.
Conclusion
Statistically, there was a significant difference of collagen density between the 0.1% betainepolyhexanide treatment groups and the NS control group, whereas regarding epithelial tissue formation there was no significant difference. In other words, the frequency of compression change using 0.1% betaine-polyhexanide solution influenced only the density of collagen in grade IIA burns and not the formation of epithelial tissue. Descriptively, the highest collagen and epithelial tissue results were in the 0.1% betaine-polyhexanide treatment group with a replacement every 24 hours, and the second highest was in the treatment group with a replacement every 48 hours for collagen and in the control group with a replacement every 24 hours for epithelial tissue. Therefore, it can be concluded that the treatment of grade IIA burns with a replacement every 24 hours can optimize the collagen formation and epithelialization processes. The authors recommend measurements of epithelial thickness in wound care using 0.1% betaine-polyhexanide compress with a replacement every 12 hours, 24 hours, 36 hours and 48 hours. It is recommended that future studies should use a secondary gauze that can fixate the primary gauze and conduct moisture measurements of the NS control group with frequencies of one replacement in 12 hours, 24 hours, 36 hours and 48 hours against the evaporation rate of 0.1% betaine-polyhexanide and NS solutions. Further research suggestions include examinations on wound care using 0.1% betaine-polyhexanide compress with replacements every 12 hours, 24 hours, 36 hours and 48 hours on for burns of other degrees of severity, replacement frequency of once in > 48 hours, and efficiency of 0.1% betaine-polyhexanide and NS solutions in grade IIA burns.
References
- 1.Martina NR, Wardhana A. Mortality analysis of adult burn patients. Jurnal Plastik Rekonstruksi. 2013;2(2):96–100. [Google Scholar]
- 2.Suza DED, Asrizal A. Relationship between nutritional status, burn degree, and treatment with burn injury healing process in patients at hospital in Medan city, Indonesia. International Journal of Nursing and Health Services (IJNHS) 2019;2(4):389–396. [Google Scholar]
- 3.Rismana E, Bunga O, Prasetyawan Y, Rosidah I. Efektivitas khasiat pengobatan luka bakar sediaan gel mengandung fraksi ekstrak pegagan berdasarkan analisis hidroksiprolin dan histopatologi pada kulit kelinci. Indonesian Bulletin of Health Research. 2013;41(1):45–60. [Google Scholar]
- 4.Duan W-q, Xu X-w, Cen Y, Xiao H-t. Epidemiologic investigation of burn patients in Sichuan province, China. Med Sci Mon Int Med J Exp Clin Res. 2019;25:872. doi: 10.12659/MSM.912821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutting KF. Wound dressings: 21st century performance requirements. J Wound Care. 2010;19(Sup):4–9. [Google Scholar]
- 6.Ose MI, Utami PA, Damayanti A. Efektivitas perawatan luka teknik balutan wet-dry dan moist wound healing pada penyembuhan ulkus diabetik. Journal of Borneo Holistic Health. 2018;1(1):101–112. [Google Scholar]
- 7.Sjamsuhidajat R. EGC. Jakarta: 2011. Buku Ajar Ilmu Bedah. [Google Scholar]
- 8.Weller C, Team V. Advanced textiles for wound care. Elsevier; 2019. Interactive dressings and their role in moist wound management. pp. 105–134. [Google Scholar]
- 9.Ciprandi G, Ramsay S, Budkevich L, Strack A. A retrospective systematic data review on the use of a polihexanide-containing product on burns in children. J Tissue Viability. 2018;27(4):244–248. doi: 10.1016/j.jtv.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Aditya S, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care. 2014;3(8):511–529. doi: 10.1089/wound.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtin C, Nolan JC, Conlon R, Deneweth L. A physiologically relevant 3D collagen-based scaffold–neuroblastoma cell system exhibits chemosensitivity similar to orthotopic xenograft models. Acta Biomaterialia. 2018;70:84–97. doi: 10.1016/j.actbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Pospíšilová E, Černochová D, Lichnovská R, Erdösová B. Application and evaluation of teaching practical histology with the use of virtual microscopy. Diagnostic Pathology. 2013;8(1):S7. [Google Scholar]
- 13.Ashkani-Esfahani S, Imanieh M, Khoshneviszadeh M, Meshksar A. The healing effect of arnebia euchroma in second degree burn wounds in rat as an animal model. Iranian Red Crescent Medical Journal. 2012;14(2):70. [PMC free article] [PubMed] [Google Scholar]
- 14.Low QE, Drugea IA, Duffner LA, Quinn DG. Wound healing in MIP-1alpha(-/) and MCP-1(-/-) mice. A J Pathol. 2001;159(2):457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granick MS, Téot L. Surgical wound healing and management. CRC Press. 2012 [Google Scholar]
- 16.Hawkins HK, Finnerty CC. Total Burn Care. Fourth Edition. London,: W.B. Saunders; 2012. Pathophysiology of the burn scar; Chapter 46; pp. 507–516.e3. [Google Scholar]
- 17.Schultz GS, Ladwig G, Wysocki A. Extracellular matrix: review of its roles in acute and chronic wounds. World Wide Wounds. 2005;2005:1–18. [Google Scholar]
- 18.Novriansyah R. Perbedaan kepadatan kolagen di sekitar luka insisi tikus wistar yang dibalut kasa konvensional dan penutup oklusif hidrokoloid selama 2 dan 14 Hari: Diponegoro University. 2008 [Google Scholar]
- 19.Rao KS, Ravi K, Chandra KR. A comparative study between collagen dressing and conventional dressing in case of superficial and second degree superficial burns. IOSR Journal of Dental and Medical Sciences. 2017;16(12):11–15. [Google Scholar]
- 20.Machuca J, Lopez-Rojas R, Fernandez-Cuenca F, Pascual A. Comparative activity of a polyhexanide–betaine solution against biofilms produced by multidrug-resistant bacteria belonging to high-risk clones. J Hosp Infect. 2019;103(1):e92–e96. doi: 10.1016/j.jhin.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Wattanaploy S, Chinaroonchai K, Namviriyachote N, Muangman P. Randomized controlled trial of polyhexanide/betaine gel versus silver sulfadiazine for partial-thickness burn treatment. Int J Low Extrem Wounds. 2017;16(1):45–50. doi: 10.1177/1534734617690949. [DOI] [PubMed] [Google Scholar]
- 22.López-Rojas R, Fernández-Cuenca F, Serrano-Rocha L, Pascual A. In vitro activity of a polyhexanide–betaine solution against high-risk clones of multidrug-resistant nosocomial pathogens. Enfermedades infecciosas y microbiologia clinica (English ed) 2017;35(1):12–19. doi: 10.1016/j.eimc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Hess CL, Howard MA, Attinger CE. A review of mechanical adjuncts in wound healing: hydrotherapy, ultrasound, negative pressure therapy, hyperbaric oxygen, and electrostimulation. Ann Plast Surg. 2003;51(2):210–218. doi: 10.1097/01.SAP.0000058513.10033.6B. [DOI] [PubMed] [Google Scholar]
- 24.Haesler E, White W. Minimising wound-related pain: a discussion of traditional wound dressings and topical agents used in low-resource communities. Wound Practice & Research: Journal of the Australian Wound Management Association. 2017;25(3):138. [Google Scholar]
- 25.Kamoun EA, Kenawy E-RS, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8(3):217–233. doi: 10.1016/j.jare.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry P, Potter P. Buku ajar fundamental keperawatan: konsep, proses, dan praktik. EGC. 2007 [Google Scholar]
- 27.Bradbury S, Fletcher J. Prontosan made easy. Wounds International. 2011;2:1–6. [Google Scholar]
- 28.Black J, Hawks J. Saunders Elsevier. St Louis: 2009. Medical-Surgical Nursing: Clinical Management for Positive Outcomes. [Google Scholar]
- 29.Kozier B, Erb G, Berman A, Snyder S. EGC. Jakarta: 2010. Fundamental keperawatan konsep, proses, & praktik. [Google Scholar]
- 30.Townsend CM, Beauchamp RD, Evers BM, Mattox KL. Elsevier Saunders. Philadelphia, PA: 2016. Sabiston Textbook Of Surgery: The Biological Basis of Modern Surgical Practice. [Google Scholar]
- 31.Pastar I, Stojadinovic O, Yin NC, Ramirez H. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas S. Medetec. Cardiff,: 2010. Surgical Dressings and Wound Management. [Google Scholar]
- 33.Upton D, Andrews A. The impact of stress at dressing change in patients with burns: a review of the literature on pain and itching. Wounds. 2014;26(3):77–82. [PubMed] [Google Scholar]


