Highlights
-
•
Age and fibrocalcific carotid plaques are associated with lower total brain volume.
-
•
Fibrocalcific carotid plaques are associated with lower gray matter volumes.
-
•
White matter volumes are associated with the extent of carotid atherosclerosis.
-
•
Grey and white matter likely have differential susceptibility to processes.
Keywords: Carotid atherosclerosis, Brain volumes, Brain atrophy, Cardiovascular risk factors, Vulnerable plaque, Brain magnetic resonance imaging
Abbreviations: TBV, total brain volume; GM, gray matter; WM, white matter; CV, cardiovascular; CC-IMT, common carotid intima media thickness; CEUS, contrast enhanced ultrasound; TPA, total plaque area; GFR, glomerular filtration rate
Abstract
Background and aims
Extent of subclinical atherosclerosis has been associated with brain parenchymal loss in community-dwelling aged subjects. Identification of patient-related and plaque-related markers could identify subjects at higher risk of brain atrophy, independent of cerebrovascular accidents. Aim of the study was to investigate the relation between extent and characteristics of carotid plaques and brain atrophy in asymptomatic patients with no indication for revascularization.
Methods and results
Sixty-four patients (aged 69 ± 8 years, 45% females) with carotid stenosis <70% based on Doppler flow velocity were enrolled in the study. Potential causes of cerebral damage other than atherosclerosis, including history of atrial fibrillation, heart failure, previous cardiac or neurosurgery and neurological disorders were excluded. All subjects underwent carotid computed tomography angiography, contrast enhanced ultrasound for assessment of plaque neovascularization and brain magnetic resonance imaging for measuring brain volumes. On multivariate regression analysis, age and fibrocalcific plaques were independently associated with lower total brain volumes (β = −3.13 and β = −30.7, both p < 0.05). Fibrocalcific plaques were also independently associated with lower gray matter (GM) volumes (β = -28.6, p = 0.003). On the other hand, age and extent of carotid atherosclerosis were independent predictors of lower white matter (WM) volumes.
Conclusions
WM and GM have different susceptibility to processes involved in parenchymal loss. Contrary to common belief, our results show that presence of fibrocalcific plaques is associated with brain atrophy.
1. Introduction
Atherosclerotic disease of the carotid arteries has a high prevalence in aged population. It has been reported that up to 5% of asymptomatic women and 12% of asymptomatic men over 80 years of age have a moderate carotid plaque (stenotic diameter of 50–70%) [1]. Apart from the risk of ischemic stroke, that in this population is relatively low with a reported annual incidence between 0.35 and 1.30% [2], the presence of increased common carotid intima-media thickness (CC-IMT) and carotid artery stenosis have been associated to cognitive decline [3], and reduced brain volumes [4].
The reduction of cerebral volumes, including total brain volume (TBV), gray matter (GM), white matter (WM) volumes, are considered imaging-based markers of processes that leads to cognitive impairment in several neurological conditions, including Alzheimer’s disease [5], epilepsy [6], multiple sclerosis [7], and eventually cerebrovascular (CV) diseases [8], [9]. The process of brain parenchymal loss may thus be considered by itself a marker of brain frailty, eventually even before any clinical manifestation [10], [11]. Cerebral hypoperfusion could be a factor linking cardiovascular morbidities with accelerated brain volumetric changes. Also the presence of CV risk factors is associated with lower cerebral blood flow [12]. CC-IMT is commonly used as marker of atherosclerotic burden in relation to brain volumes, even if it is a debated marker of CV risk [13], however a few studies have assessed the association of extension and characteristics of carotid atherosclerotic plaques and brain volumes [14].
The increasing prevalence of age-related disorders of the brain such as mild cognitive impairment and dementia need studies to identify novel strategies to hamper the progression of brain ageing [3].
Carotid atherosclerotic plaques are common among elderly individuals [1], and their etiological role in the development of ischemic stroke is well established [2]. More recently, carotid atherosclerosis has been associated with other forms of brain damage, including WM hyperintensities and silent brain infarcts [14], [15], [16]. Different studies suggest that presence of subclinical atherosclerosis, i.e. increased CC-IMT, or non-obstructive plaques may be associated with brain atrophy, in particular decreased total brain volume and GM volume [3], [17], [18], [19]. Furthermore, there is initial evidence that high-grade stenosis or bilateral disease is associated with reduction in brain volumes [19]. To date, however, no study has explored the relation between features of plaque vulnerability and extent of atherosclerosis on the one hand and cerebral atrophy on the other. Such relation may be of particular interest in patients with carotid stenosis of intermediate severity, i.e. <70%, in whom the prognostic significance of such plaques has been the subject of an intense debate [20]. Results from the AGES-Reykjavik Study in 2430 community-dwelling subjects aged 75 ± 5 years, showed that subclinical atherosclerosis (increased CC-IMT) was associated with reduced total brain and GM volumes, but not with WM volumes at 5 years follow up [3].
Aim of this prospective study was to investigate the relation between extent and characteristics of carotid plaques and brain atrophy assessed with multimodality imaging. The patient population consisted of asymptomatic patients with no other potential causes of cerebral damage and no indication for carotid revascularization.
2. Methods
2.1. Study population
The study population consists of the baseline cohort of a prospective study to assess carotid artery plaque features in relation with subclinical cerebral damage (Imaging della PLAcca Carotidea, IMPLAC study, ClinicalTrials.gov registration number NCT03333330 and EudraCT number 2012-000648-83) [21]. The study was approved by the Ethics Committee of the San Raffaele Hospital (date of approval January 30th, 2012, protocol name IMPLAC). In compliance with the Declaration of Helsinki and Good Clinical Practice, all subjects provided written informed consent to take part in the study.
Between April 2012 and November 2015 we screened 235 asymptomatic subjects referred to Neurophisiology and Vascular Surgery Outpatients Clinics at Ospedale San Raffaele, Milan, Italy, for routine carotid evaluation who had a documented carotid stenosis of intermediate severity, i.e. <70% according to Doppler flow measurement [22], and were not considered for carotid revascularization. Stringent selection criteria were applied (Table 1) in order to limit the potential confounding effect of causes of brain damage not related to atherosclerosis. The population of subjects fulfilling inclusion criteria and providing informed consent consisted of 67 subjects. Three subjects were excluded from the analysis due to inadequate brain MRI scans, preventing a reliable quantification of brain volumes. Thus, the final population included in the analysis consisted of 64 subjects. Upon enrollment, every individual underwent physical examination with blood pressure measurement and a detailed medical history was taken to identify CV risk factors and current treatment. A global estimate of CV risk was taken using 10-year Framingham risk score [23]. A centralized measure of glycaemia, total, low-density lipoprotein (LDL), high-density lipoprotein (HDL) cholesterol and triglycerides was obtained using a colorimetric method using the Cobas Mira Plus analyzer (Horiba, ABX, France), as previously described [24]. Two patients refused blood draw for cholesterol evaluation.
Table 1.
Exclusion criteria for participation in the study.
| Exclusion criteria for the IMPLAC study |
|---|
| Age < 18 or >85 years |
| Contraindications to CTA (eGFR < 30 mL/min; history of allergic reaction to iodinated contrast media) |
| Pregnancy or child-bearing potential |
Specific contraindication to MRI:
|
| Dementia |
| Life expectancy less than study follow up (18 months) |
| History of drug abuse, alcohol abuse or any psychiatric or social condition which may contraindicate the participation to a clinical study |
| Vertebral artery occlusion |
| Previous revascularization of the carotid artery |
| Current anti-coagulation |
| Atrial fibrillation not necessitating anticoagulation |
| Known PFO necessitating anti-platelet treatment |
| Previous cerebrovascular accidents |
| Previous infections to the CNS |
| Previous surgery to the CNS |
| History of anoxic damage to the CNS |
| Previous cardiac surgery or positioning of intracardiac devices (excluded coronary stents) |
| History of autoimmune vasculitis |
2.2. Carotid ultrasound and CEUS
Ultrasound carotid evaluation and CEUS were performed on the same day using a dedicated equipment (Logiq S8, GE Healthcare, Little Chalfort, UK) and 7-MHz linear probe (7L, GE Healthcare, Little Chalfort, UK), as previously described [24], [25]. Briefly, for each patient, CC-IMT was measured semi-automatically by averaging 350 points on common carotid far wall starting 1 cm from the carotid bifurcation. The highest value between right and left carotid was registered. Doppler measures were taken at each visible plaque in the internal carotid artery (ICA), common carotid artery (CCA) and carotid bulb. Carotid stenosis was estimated on the basis of peak systolic velocity [22]. The plaque determining the highest degree of stenosis was considered the index plaque and was subsequently characterized according to its echogenicity in one of five classes of progressively increasing greyscale as previously described [26]. For subsequent analysis, plaques belonging to classes I-III were considered lipid-rich while plaques in classes IV-V were considered fibrocalcific. TPA was measured on longitudinal B-mode images to evaluate global atherosclerotic burden as previously described [27]. Briefly, TPA represents the sum of maximum longitudinal cross-sectional areas of all plaques identified in ICA, CCA, carotid bulb and external carotid artery (ECA) bilaterally. The number of carotid artery segments, defined according to Rotterdam Study criteria [28], [29], involved by the atherosclerotic process was also recorded [24]. Plaque neovascularization was assessed offline on CEUS recorded loops by three independent operators (MM, FM and EA) as previously described [24], [30]. Each lesion was graded as CEUS- or CEUS + depending on absence or presence of contrast signal reaching the plaque core, representing intense plaque neovascularization. The total number of neovascularized plaques per patient was recorded as a semi-quantitative measure of neoangiogenic activity. Five patients did not undergo CEUS, due to concerns regarding potential allergic reactions (n = 3) and a history of mildly increased pulmonary artery pressure on previous echocardiograms (n = 2).
2.3. CTA
CTA of the carotid arteries was performed using a 64-slice CT scanner (VCT Lightspeed, GE Healthcare, Milwaukee, WI, USA). Fifty ml of non-ionic, iso-osmolar contrast material (Visipaque 320, Iodixanol, 320 mg Iodine/mL-GE Healthcare, Milwaukee, WI, USA) at 37 °C were administered through a 20 or 18-gauge catheter into a peripheral vein at a rate of 5 mL/s, followed by 50 mL of saline. Contrast administration was performed using a dual-shot injector (Nemoto Kyorindo, Tokio, Japan). The helical acquisition was initiated after the bolus reached the aortic arch using a visual bolus tracking. Data were acquired from the aortic arch to the vertex. The section thickness was 0.6 mm, pitch was 0.9 mm/rot, field of view was set as large. Tube voltage and current were 120 kV and 250 mA, respectively. The average absorbed radiation dose per patient was approximately 0.68 mSv. The degree of stenosis in CCA, carotid bulb and ICA were analyzed according to both NASCET and ECST criteria [31], [32]. The axial data and multiplanar reconstruction (MPR) were used to determine the grade of stenosis, to calculate the plaque volume and the composition of the plaque, defined on the basis of plaque density in HU. For each index plaque, the presence of a microcalcification and the degree of positive remodeling were recorded [33], [34]. Five patients did not undergo CTA due to specific contraindications to the administration of iodinated contrast material, in particular 3 subjects had a recent history of allergic reactions to drugs while 2 subjects were diagnosed chronic kidney disease after enrolment in the study.
2.4. MRI
Brain axial fluid-attenuated inversion recovery (FLAIR) and high-resolution three-dimensional T1-weighted images were acquired in all subjects using a 1.5 T scanner (ACHIEVA, Philips) and a standardized protocol of acquisition. Using 3D T1-weighted images, NBV, WMV and GMV were measured using the SIENAx software50. To avoid a possible bias in image segmentation due to the presence of T1-hypointense lesions, a binarized lesion mask from FLAIR lesions was created and transformed to the original space of the 3D T1-weighted image. After visual inspection and an eventual manual editing, WM lesions were refilled using the tool in FSL 5.0.5 software [35].
2.5. Statistical analysis
All continuous variables were tested for normality using Shapiro-Wilk normality test. Normally distributed variables are expressed as mean ± standard deviation (SD), while non-normal variables or median [interquartile range (Q1-Q3)]. Categorical variables are summarized as absolute frequency (percentage). Group differences were tested using unpaired t test or Mann-Whitney U test as appropriate. Spearman’s rank correlation coefficient was used to assess statistical dependence. A p < 0.05 was considered signifcant. A multivariate regression analysis was then performed to identify factors that independently influence the brain volumes. All variables significantly associated with brain volumes were included in the multivariable model. If more than one variable included in the model were believed to reflect an analogous disease process, subsequent sensitivity analysis excluding one of the variables were performed. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, USA), IBM SPSS Statistics 20 (IBM, Armonk, USA) or R v3.1.2.
3. Results
3.1. Study population and carotid plaques analysis
Mean age of the study population was 69 ± 8 years, and 45% of the participants in the study were females (Table 2). Median CC-IMT was 0.83 (0.75–1.03) mm and median total plaque area (TPA) was 0.80 (0.59–1.17) cm2. The median number of segments involved by the atherosclerotic process was 4 (3–5) per patient, and median number of contrast-enhanced ultrasound (CEUS) + atherosclerotic plaques was 1 (0–2) per patient. Median total plaque volume measured on computed tomography angiography (CTA) images was 300 (171–576) mm3. According to Doppler velocity the index plaque determined a stenosis of 50–70% in 26 (41%) subjects and <50% in the remaining 38 (59%) individuals. On CTA images, the median stenosis severity was 30% (10–40%) when North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria were used whilst stenosis severity was 60% (40–70%) when European Carotid Surgery Trial (ECST) criteria were applied. Index plaques were lipid-rich according to ultrasound B-mode greyscale in 64% of subjects and fibrocalcific in 36%. Median plaque density on CTA was 300 (109–557) Hounsfield’s Unit (HU). The index plaque was CEUS+ in 49% of subjects. Nine subjects (15%) had evidence of a plaque ulcer on CTA images, while 24 (41%) had evidence of luminal calcification. Median positive remodeling was 0.50 (0.36–0.70). Plaque ultrasound and CTA characteristics are summarized in S1 and S2 Table, respectively.
Table 2.
Population characteristics. *n = 57, seven patients did not have recent blood tests.
| Patients (n = 64) | |
|---|---|
| Demographic Characteristics | |
| Age, years | 69 ± 8 |
| Female, n (%) | 28 (45) |
| Cardiovascular Risk Factors | |
| Family history of coronary artery disease, n(%) | 22 (34) |
| Family history of stroke, n(%) | 8 (13) |
| Systemic arterial hypertension, n(%) | 49 (77) |
| Resistant hypertension, n(%) | 10 (16) |
| Hypercholesterolemia, n(%) | 46 (72) |
| Type 2 diabetes mellitus, n(%) | 16 (25) |
| Current smoker, n(%) | 28 (44) |
| Previous smoker, n(%) | 11 (27) |
| Body mass index (kg/cm2) | 25 ± 4 |
| Framingham risk score (%) | 12 (6–19) |
| High cardiovascular risk n(%) | 35 (55) |
| Previous acute coronary syndrome, n (%) | 12 (19) |
| Laboratory Parameters* | |
| White blood cells, 109/L | 7.2 (6–9) |
| Haemoglobin, g/dL | 14 (13–15) |
| AST, UI/L | 20 (16–25) |
| ALT, UI/L | 20 (15–27) |
| Platelets, 109/L | 205 (160–253) |
| Total cholesterol, mg/dL | 174 (155–196) |
| LDL cholesterol, mg/dL | 104 (85–122) |
| HDL cholesterol, mg/dL | 43 (38–50) |
| Triglyceridemia, mg/dL | 128 (95–161) |
| Glycemia, mg/dL | 99 (88–128) |
| Creatinine, mg/dL | 0.85 (0.73–1.04) |
| eGFR, mL/min | 72 (58–96) |
| Medical Therapy | |
| ACE inhibitors, n (%) | 20 (31) |
| Angiotensin Receptor Blockers, n (%) | 19 (30) |
| β-blockers, n(%) | 26 (41) |
| Calcium antagonists, n(%) | 16 (25) |
| Diuretics, n (%) | 13 (20) |
| Others vasodilators, n(%) | 4 (6) |
| Number of anti-hypertensive agents | 2 (1–5) |
| Statins, n(%) | 39 (61) |
| Antiplatelet agent - n(%) | 39 (61) |
| Oral diabetes medications, n(%) | 13 (20) |
| Insulin therapy, n (%) | 2 (3) |
3.2. Brain volumes
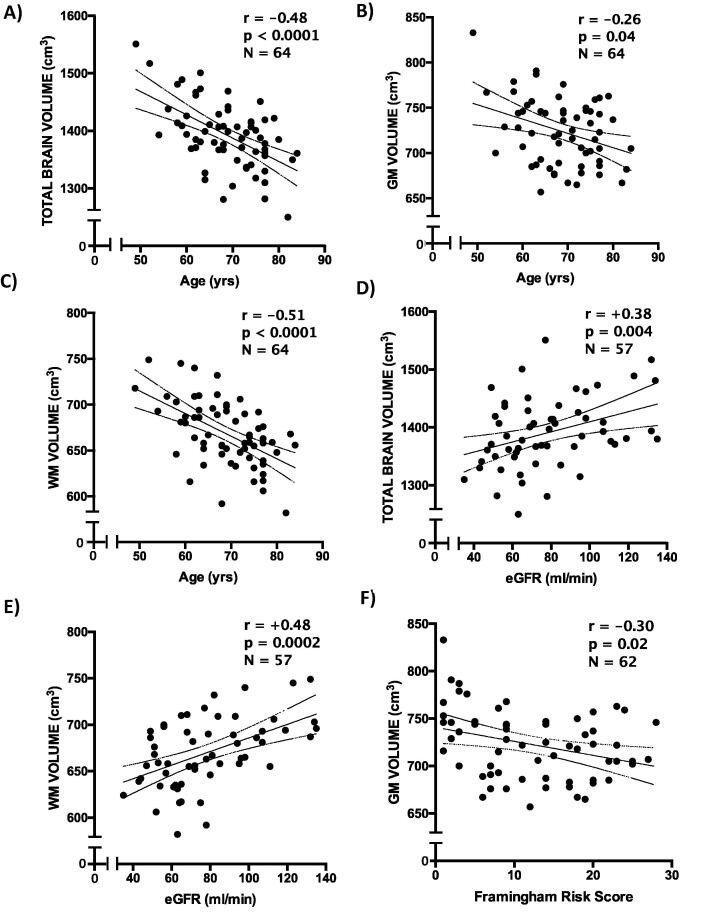
Average normalized brain volume (NBV) was 1392 ± 58 mL, GM volume (GMV) was 724 ± 36 mL and WM volume (WHV) was 669 ± 36 mL. Fig. 1 shows brain volume analysis of two representative subjects. No statistically significant sex-related differences in NBV (p = 0.38), GMV (p = 0.07) and WMV (p = 0.74) were found. Age correlated significantly with NBV (r = -0.482, p < 0.0001), GMV (r = -0.256, p = 0.04) and WMV (r = -0.515, p < 0.0001) (Fig. 2, panels A-C).
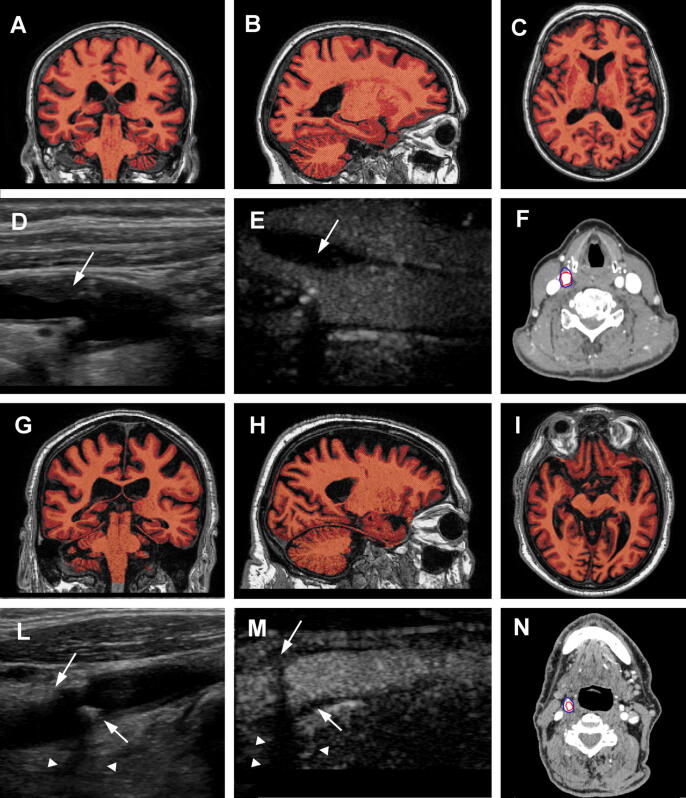
Fig. 1.
Brain volumes of two representative subjects. Panels A-C show respectively a transverse, sagittal and coronal reconstruction of 3D T1-weighted brain scan from the same patient. The subject had a total normalized brain volume of 1451 mL, with 759 mL of grey matter and 692 mL of white matter. Panels D-F show an ultrasound, contrast enhanced ultrasound (CEUS) and computed tomography angiography (CTA) of the right carotid bulb of the same patient. The plaque (white arrows) appears to be lipid rich, as showed by hypoechogenic images in B-mode ultrasound. The plaque also had a lower attenuation in CTA images (274HU). Panels G-I show brain images from a different patient reconstructed at comparable levels. The second subject had more pronounced reduction of cerebral volumes, with a total brain volume of 1341 mL, a grey matter volume of 700 mL and white matte volume of 641 mL. Panels L-N show ultrasound, CEUS and CTA images of the right carotid bifurcation of the second subject. This time the plaque (white arrows) appears markedly fibrocalcific, as demonstrated by posterior acoustic shadows (white arrowheads) in ultrasound images. CTA demonstrates a high attenuation, with a plaque density of 1368HU. In CTA images, the blue line delimitated the contour of the carotid artery, while the blue line showed the lumen of the artery. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Association between cardiovascular risk factors and cerebral volumes. Panels A-C show the significant associations between brain volumes and age; panels D and E the correlation between brain volumes and renal function; panel F the association between grey matter volume and overall cardiovascular risk. WM = white matter; GM = grey matter; eGFR = estimated glomerular filtration rate; FRS = 10-year Framingham risk score.
3.3. CV factors and brain atrophy
Renal function significantly affected total and WM volumes: estimated glomerular filtration rate (eGFR) correlated with NBV (r=+0.378, p = 0.004) and WMV (r=+0.477, p = 0.0002) (Fig. 2, panels D and E). Body mass index (BMI) showed a significant negative correlation with GMV (r = −0.283, p = 0.02). Hypertensive subjects had lower GMV compared with normotensive individuals (715 vs 739 mL, p = 0.03). Global CV risk burden, estimated with 10-year Framingham risk score, showed a modest, albeit significant, inverse correlation with GMV (r = −0.30, p = 0.02) (Fig. 2, panel F). S3 and S4 Tables show all the explored associations.
3.4. Carotid atherosclerotic burden and cerebral volumes
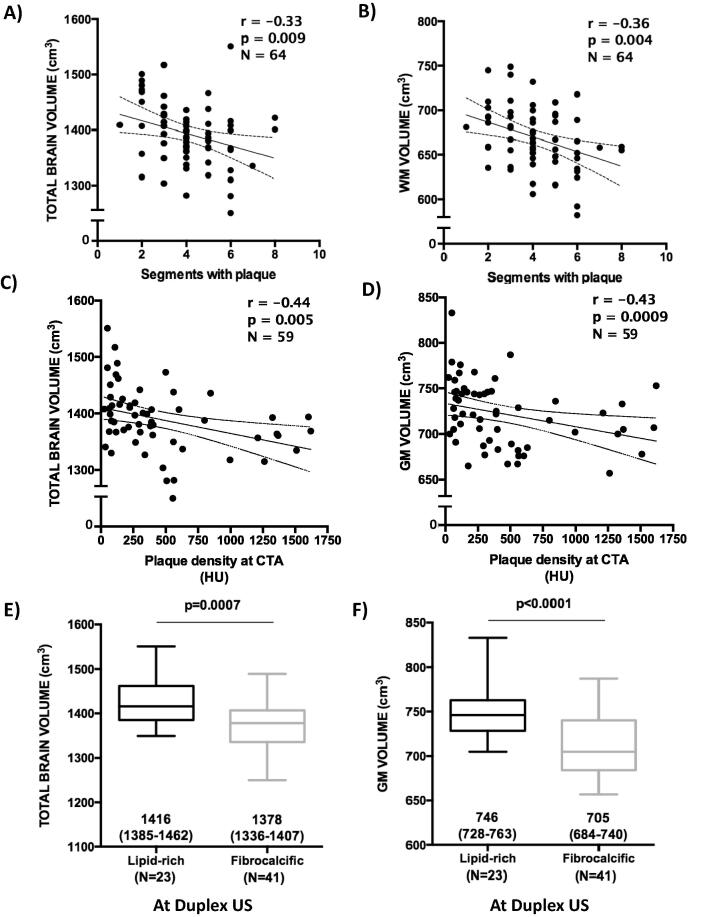
CC-IMT was significantly associated with lower NBV (r = −0.372, p = 0.002) and GMV (r = −0.325, p = 0.009). A higher TPA correlated with lower NBV (r = −0.256, p = 0.04) and GMV (r = -0.302, p = 0.02). The number of segments affected by atherosclerosis was associated with lower NBV (−0.325, p = 0.009) and WMV (−0.358, p = 0.003) (Fig. 3, panels A and B). CTA total plaque volume in the carotid artery was not associated with brain volumes. See S5 Table for all tested associations.
Fig. 3.
Associations between carotid atherosclerosis features and cerebral volumes. Panels A and B show the association between atherosclerotic burden estimated as number of carotid segments involved by the atherosclerotic process and total brain volume and white matter volume. Panels C and D show the significant inverse correlation between plaque density and total and grey matter volumes; the last two panels show the significant association between plaque composition evaluated with ultrasound and total and grey matter volumes. WM = white matter; GM = grey matter; HU = Hounsfield’s unit.
3.5. Index plaque characteristics and brain atrophy
No significant association was found between cerebral volumes and severity of carotid artery stenosis evaluated with ultrasound or CTA. Plaque density at CTA was associated with lower NBV (r = −0.44, p = 0.005) and GMV (r = −0.43, p = 0.0009) (Fig. 3, panels C and D). Lipid-rich plaques evaluated with ultrasound were significantly associated to higher total and GM volumes when compared to fibrocalcific plaques (1416 mL vs 1378 mL, p = 0.0009 and 746 mL vs 705 mL, p < 0.0001) (Fig. 3, panels E and F). A similar finding was observed when plaque composition was determined by CTA (S1 Figure). No other significant association was found. S6 and S7 Tables show all tested associations.
3.6. Multivariate regression analysis
Age, GFR, CC-IMT, TPA, the number of segments affected by atherosclerosis, plaque density at CTA and plaque composition at ultrasound were included in the multivariate model for NBV. Age and plaque density were independent predictors of lower NBV (β = −3.2, p = 0.003 and β = −0.03, p = 0.03, respectively). R2 of the model was 0.52, p < 0.0001 (Table 3). We performed a sensitivity analysis excluding plaque density from the model, since plaque density and plaque composition refer to the same plaque characteristic, albeit with different imaging method. The sensitivity analysis demonstrated that plaque composition was significantly associated with lower NBV (β = −42.3, p = 0.002). The model for GMV included age, BMI, Framingham risk score, hypertension, CC-IMT, plaque density and plaque composition. Only plaque composition emerged as significantly associated with GMV on multivariate analysis (β = −26.9, p = 0.007). The model R2 was 0.44, p = 0.0003. With regard to WMV, we included age, eGFR and the number of carotid segments involved by atherosclerosis in the multivariable model. Age and the number of segments involved by the atherosclerotic process were significantly associated with lower WMV (β = −1.7, p = 0.008 and β = −6.1, p = 0.03, respectively). R2 of the model was 0.41, p < 0.0001.
Table 3.
Multivariate regression analysis. eGFR = estimated glomerular filtration rate; CC-IMT = common carotid intima-media thickness; TPA = total plaque area; N° segments = number of segments involved by atherosclerosis as identified by ultrasound; BMI = body mass index; FRS = 10-years Framingham risk score; HTN = hypertension.
| Total brain volume | |||
|---|---|---|---|
| Variable | β | CI (95%) | p |
| (Intercept) | 1745.6 | 1556.3; 1934.8 | <0.0001 |
| Age | −3.2 | −5.3; −0.9 | 0.003 |
| eGFR | −0.04 | −0.7; 0.6 | 0.90 |
| CC-IMT | −63.8 | −154.4; 26.8 | 0.16 |
| TPA | 2.2 | −35.3; 3.9 | 0.90 |
| N° of segments | −5.4 | −15.8; 4.9 | 0.29 |
| Plaque density | −0.03 | −0.06; −0.003 | 0.03 |
| Plaque composition | −24.7 | −53,9; 4.5 | 0.09 |
| GM volume | |||
| Variable | β | CI (95%) | p |
| (Intercept) | 895.6 | 794.1; 997.1 | <0.0001 |
| Age | −0.8 | −2.1; 0.5 | 0.22 |
| BMI | −1.2 | −3.4; 1.0 | 0.28 |
| FRS | −0.7 | −2.1; 0.6 | 0.28 |
| HTN | 0.1 | −20.9; 21.3 | 0.98 |
| CC-IMT | −25.0 | −75.9; 25.9 | 0.32 |
| TPA | −9.5 | −30.1; 11.2 | 0.36 |
| Plaque density | −0.01 | −0.03; 0.01 | 0.28 |
| Plaque composition | −26.9 | −46.4; −74 | 0.007 |
| WM volume | |||
| Variable | β | CI (95%) | p |
| (Intercept) | 755.6 | 627.1; 884.0 | <0.0001 |
| Age | −1.7 | −2.9; −0.5 | 0.008 |
| eGFR | 0.3 | −0.04; 0.7 | 0.08 |
| N° segments | −6.1 | −11.6; −0.7 | 0.03 |
4. Discussion
The new finding of our study is the demonstration of a relationship between GM and WM volumes and specific characteristics of the atherosclerotic plaque. Specifically, GMV appears to be associated by plaque composition, with more fibrocalcific plaques associating to reduced volumes, while lower WMV are associated with a more widespread involvement of the carotid artery by atherosclerosis. In keeping with previous literature, we observed an association of GM and WM volumes with age.
At variance with vulnerable atherosclerotic plaques, which have been shown to be associated with higher rates of CV events and with subclinical cerebral damage [27], [33], [36], fibrocalcific plaque composition, identified as high attenuating atherosclerotic lesions on CT or as echogenic plaques on ultrasound, has been associated with low risk of CV events [37], [38]. Indeed, fibrocalcific plaques are widely considered to be stable, low risk, long-standing lesions. Interestingly however, in our study we show a significant association between the presence of fibrocalcific plaques in the carotid arteries and reduced GMV. It is unlikely that the observed association could be mediated by an increased rate of thromboembolic events. Nevertheless, it is important to note that fibrocalcific plaques constitute areas of high arterial wall stiffening [39], and may constitute a surrogate marker of impaired arterial elasticity. The alteration of arterial elastic properties may impact on cerebral circulation, which has been shown to be associated to reduced GMV [40], [41], and TBV [42]. Approximately two thirds of cerebral flow are directed to GM compare to WM. In fact GM has a high metabolic demand, and is more vulnerable than WM to factors that affect regulation of blood flow to the brain [43]. In addition, since arterial stiffening gradually progresses with age [44], the association between fibrocalcific plaques and reduced GMV may be part of a common, multi-system process of biological ageing that goes beyond chronological age.
A high atherosclerotic burden both in the carotid and in the coronary arteries correlates with a higher risk of acute CV events. Indeed, a recent survey on 3398 subjects from the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that coronary artery calcification volume was a strong predictor for incident coronary events [37], while in the BioImage Study, comprising 5808 subjects, a higher carotid artery burden was associated with a higher incidence of CV events [45]. An intriguing hypothesis would be that, in our cohort, WM was affected by asymptomatic cerebral events, which tend to occur with high probability in the deep WM [46], proportionally to carotid involvement by atherosclerosis, leading to a reduction in WMV. However, no sign of stroke, even asymptomatic, was evident on magnetic resonance imaging (MRI) in any of the individuals analyzed. Still, more subtle lesions of vascular origins have been described in the deep WM, including WM hyperintensities, which may contribute to cognitive decline [15].
Among the different predictors of lower cerebral volumes, older age is of particular interest. Indeed, apart from being a major determinant of cardiovascular risk, ageing is well known to foster degenerative changes in the central nervous system, which appear to be more complex and pronounced in the WM [47], [48]. Our findings confirm the association between WMV and age. While a strong effect of age has been reported by other groups on GMV, albeit generally on small and selected groups of subjects with neuropsychiatric conditions, such association did not emerge in our study [49], [50].
The differential association of carotid atherosclerosis extension and composition with WM and GM volumes, respectively, is very interesting. In the AGES-Rejkjavik study the combination of high N-terminal-pro-brain natriuretic peptide (NT-pro-BNP) and CC-IMT were longitudinally associated to loss of TBV and GM volumes, but WM volumes were unaffected [3]. Advanced atherosclerosis could also reflect a global systemic vascular condition that affects not only the large extracranial vessels but also cerebral small vessels [51].
To date, this is the first study to systematically evaluate carotid plaque characteristics in relation to cerebral volumes. Previous studies had indeed indicated that the presence of carotid artery plaque is associated to lower global brain volumes [14], but no mention is made of carotid plaque burden or features. Interestingly, we show that carotid plaque features and CV risk factors account for approximately 50% of brain volumes total variance, as can be inferred by the R2 of our multivariate models.
There are some limitations that need to be acknowledged. First, the limited sample size, which hampers the generalization of our findings. We tried to minimize potential confounding effects by the meticulous selection of the participants. While we believe that applying stringent selection criteria increases the robustness of our findings by greatly reducing the burden of confounders, it does also reduce the external validity of our work. It especially hampers the generalization of our results to a population with more comorbidities, including for example atrial fibrillation or previous stroke, in whom the relative impact of plaque composition on cerebral volumes cannot be extrapolated from the present data. We acknowledge that the cross-sectional nature of this intermediate analysis renders it impossible to draw definite conclusion about the cause-effect relation between carotid atherosclerosis and alterations in cerebral volumes.
5. Conclusions
Age and widespread atherosclerotic involvement of the carotid arteries were associated with lower WMVs, while the presence of fibrocalcific plaques was associated with lower GMVs. While fibrocalcific plaques are regarded as being stable and thus not harmful, their identification may mark a diffuse impairment of vascular function, leading to brain atrophy. No shared predictors of lower WM and GM volumes were identified, indicating that possibly different pathophysiological processes subtend the progressive damage of either of the two.
Acknowledgements
The present work was supported by “Giovane Ricercatore 2009 Grant” from Italian Health Ministry (project code GR-2009-1608780). The Authors have no relation with industry relevant to the present work to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100619.
Contributor Information
Enrico Ammirati, Email: ammirati.enrico@hsr.it.
Francesco Moroni, Email: moroni.francesco@hsr.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.de Weerd M., Greving J.P., Hedblad B., Lorenz M.W., Mathiesen E.B., O’Leary D.H. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41(6):1294–1297. doi: 10.1161/STROKEAHA.110.581058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.den Hartog A.G., Achterberg S., Moll F.L., Kappelle L.J., Visseren F.L.J., van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke. 2013;44(4):1002–1007. doi: 10.1161/STROKEAHA.111.669267. [DOI] [PubMed] [Google Scholar]
- 3.Sabayan B., van Buchem M.A., Sigurdsson S., Zhang Q., Meirelles O., Harris T.B. Cardiac and carotid markers link with accelerated brain atrophy: the AGES-Reykjavik study (age, gene/environment susceptibility-Reykjavik) Arterioscler Thromb Vasc Biol. 2016;36(11):2246–2251. doi: 10.1161/ATVBAHA.116.308018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuo J., Liu Y., Liao W., Gu W., Yang S., Tan X. Altered brain volume and its relationship to characteristics of carotid plaques in asymptomatic patients. Medicine (Baltimore). 2018;97(52) doi: 10.1097/MD.0000000000013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack C.R.J., Barnes J., Bernstein M.A., Borowski B.J., Brewer J., Clegg S. Magnetic resonance imaging in Alzheimer’s disease neuroimaging initiative 2. Alzheimers Dement. 2015 Jul;11(7):740–756. doi: 10.1016/j.jalz.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernasconi A., Bernasconi N., Bernhardt B.C., Schrader D. Advances in MRI for “cryptogenic” epilepsies. Nat Rev Neurol. 2011;7(2):99–108. doi: 10.1038/nrneurol.2010.199. [DOI] [PubMed] [Google Scholar]
- 7.Rocca M.A., Battaglini M., Benedict R.H.B., De Stefano N., Geurts J.J.G., Henry R.G. Brain MRI atrophy quantification in MS: from methods to clinical application. Neurology. 2017;88(4):403–413. doi: 10.1212/WNL.0000000000003542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien J.T., Thomas A. Vascular dementia. Lancet (London, England). 2015 Oct;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 9.Lambert C., Benjamin P., Zeestraten E., Lawrence A.J., Barrick T.R., Markus H.S. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain. 2016;139(Pt 4):1136–1151. doi: 10.1093/brain/aww009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgio A., De Stefano N. Clinical use of brain volumetry. J. Magn. Reson. Imaging. 2013;37(1):1–14. doi: 10.1002/jmri.23671. [DOI] [PubMed] [Google Scholar]
- 11.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pase M.P., Grima N.A., Stough C.K., Scholey A., Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke. 2012;43(10):2803–2805. doi: 10.1161/STROKEAHA.112.666727. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz M.W., Polak J.F., Kavousi M., Mathiesen E.B., Volzke H., Tuomainen T.-P. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet (London, England). 2012;379(9831):2053–2062. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroni F., Ammirati E., Magnoni M., D’Ascenzo F., Anselmino M., Anzalone N. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: a systematic review and meta-analysis. Int. J. Cardiol. 2016;223 doi: 10.1016/j.ijcard.2016.08.234. [DOI] [PubMed] [Google Scholar]
- 15.Moroni F, Ammirati E, Rocca MA, Filippi M, Magnoni M, Camici PG. Cardiovascular disease and brain health: Focus on white matter hyperintensities. IJC Hear Vasc. 2018;19. [DOI] [PMC free article] [PubMed]
- 16.Moroni F., Ammirati E., Hainsworth A.H., Camici P.G. Association of White Matter Hyperintensities and Cardiovascular Disease. Circ Cardiovasc Imaging [Internet]. 2020 Aug 1;13(8):e010460. Available from: 10.1161/CIRCIMAGING.120.010460. [DOI] [PubMed]
- 17.Romero J.R., Beiser A., Seshadri S., Benjamin E.J., Polak J.F., Vasan R.S. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manolio T.A., Burke G.L., O’Leary D.H., Evans G., Beauchamp N., Knepper L. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. 1999;19(2):356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 19.Muller M., van der Graaf Y., Algra A., Hendrikse J., Mali W.P., Geerlings M.I. Carotid atherosclerosis and progression of brain atrophy: the SMART-MR study. Ann. Neurol. 2011;70(2):237–244. doi: 10.1002/ana.22392. [DOI] [PubMed] [Google Scholar]
- 20.Spence J.D. Endarterectomy vs. stenting vs. medical therapy. Int. J. Stroke. 2016;11(5):500–501. doi: 10.1177/1747493016643552. [DOI] [PubMed] [Google Scholar]
- 21.Ammirati E., Moroni F., Magnoni M., Rocca M.A., Anzalone N., Cacciaguerra L. Progression of brain white matter hyperintensities in asymptomatic patients with carotid atherosclerotic plaques and no indication for revascularization. Atherosclerosis. 2019;287 doi: 10.1016/j.atherosclerosis.2019.04.230. [DOI] [PubMed] [Google Scholar]
- 22.Grant E.G., Benson C.B., Moneta G.L., Alexandrov A.V., Baker J.D., Bluth E.I. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229(2):340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino R.B.S., Pencina M.J., Massaro J.M., Coady S. Cardiovascular disease risk assessment: insights from framingham. Glob Heart. 2013;8(1):11–23. doi: 10.1016/j.gheart.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ammirati E., Moroni F., Magnoni M., Di Terlizzi S., Villa C., Sizzano F. Circulating CD14+ and CD14highCD16− classical monocytes are reduced in patients with signs of plaque neovascularization in the carotid artery. Atherosclerosis. 2016;255 doi: 10.1016/j.atherosclerosis.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Magnoni M., Ammirati E., Moroni F., Norata G.D., Camici P.G. Impact of cardiovascular risk factors and pharmacologic treatments on carotid intraplaque neovascularization detected by contrast-enhanced ultrasound. J. Am. Soc. Echocardiogr. 2019;32(1) doi: 10.1016/j.echo.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Pourcelot L., Tranquart F., De Bray J.M., Philippot M., Bonithon M.C., Salez F. Ultrasound characterization and quantification of carotid atherosclerosis lesions. Minerva Cardioangiol. 1999;47(1–2):15–24. [PubMed] [Google Scholar]
- 27.Spence J.D., Eliasziw M., DiCicco M., Hackam D.G., Galil R., Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33(12):2916–2922. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 28.Bots M.L., Hoes A.W., Koudstaal P.J., Hofman A., Grobbee D.E. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 29.Hollander M., Hak A.E., Koudstaal P.J., Bots M.L., Grobbee D.E., Hofman A. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34(10):2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 30.Magnoni M., Coli S., Marrocco-Trischitta M.M., Melisurgo G., De Dominicis D., Cianflone D. Contrast-enhanced ultrasound imaging of periadventitial vasa vasorum in human carotid arteries. Eur. J. Echocardiogr. 2009;10(2):260–264. doi: 10.1093/ejechocard/jen221. [DOI] [PubMed] [Google Scholar]
- 31.Inzitari D., Eliasziw M., Gates P., Sharpe B.L., Chan R.K., Meldrum H.E. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl. J. Med. 2000;342(23):1693–1700. doi: 10.1056/NEJM200006083422302. [DOI] [PubMed] [Google Scholar]
- 32.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet (London, England). 1998 May;351(9113):1379–87. [PubMed]
- 33.Chen W., Dilsizian V. Targeted PET/CT imaging of vulnerable atherosclerotic plaques: microcalcification with sodium fluoride and inflammation with fluorodeoxyglucose. Curr. Cardiol. Rep. 2013;15(6):364. doi: 10.1007/s11886-013-0364-4. [DOI] [PubMed] [Google Scholar]
- 34.Motoyama S., Sarai M., Harigaya H., Anno H., Inoue K., Hara T. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 2009;54(1):49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 35.Battaglini M., Jenkinson M., De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. 2012 Sep;33(9):2062–2071. doi: 10.1002/hbm.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ammirati E., Moroni F., Magnoni M., Rocca M.A., Messina R., Anzalone N. Relation between characteristics of carotid atherosclerotic plaques and brain white matter hyperintensities in asymptomatic patients. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-11216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Criqui M.H., Denenberg J.O., Ix J.H., McClelland R.L., Wassel C.L., Rifkin D.E. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311(3):271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta A., Kesavabhotla K., Baradaran H., Kamel H., Pandya A., Giambrone A.E. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke. 2015;46(1):91–97. doi: 10.1161/STROKEAHA.114.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czernuszewicz T.J., Homeister J.W., Caughey M.C., Farber M.A., Fulton J.J., Ford P.F. Non-invasive in vivo characterization of human carotid plaques with acoustic radiation force impulse ultrasound: comparison with histology after endarterectomy. Ultrasound Med. Biol. 2015;41(3):685–697. doi: 10.1016/j.ultrasmedbio.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirth M., Pichet Binette A., Brunecker P., Kobe T., Witte A.V., Floel A. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. J. Cereb. Blood Flow Metab. 2017;37(3):814–824. doi: 10.1177/0271678X16641128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S., Wang B., Xie Y., Zhu S., Thomas R., Qing G. Retinotopic changes in the gray matter volume and cerebral blood flow in the primary visual cortex of patients with primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 2015;56(10):6171–6178. doi: 10.1167/iovs.15-17286. [DOI] [PubMed] [Google Scholar]
- 42.de Havenon A., Wong K.-H., Elkhetali A., McNally J.S., Majersik J.J., Rost N.S. Carotid artery stiffness accurately predicts white matter hyperintensity volume 20 years later: a secondary analysis of the atherosclerosis risk in the community study. AJNR Am. J. Neuroradiol. 2019;40(8):1369–1373. doi: 10.3174/ajnr.A6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willie C.K., Ainslie P.N. Cool head, hot brain: cerebral blood flow distribution during exercise. J. Physiol. 2011;589(Pt 11):2657–2658. doi: 10.1113/jphysiol.2011.209668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohn J.C., Lampi M.C., Reinhart-King C.A. Age-related vascular stiffening: causes and consequences. Front Genet. 2015;6:112. doi: 10.3389/fgene.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baber U., Mehran R., Sartori S., Schoos M.M., Sillesen H., Muntendam P. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J. Am. Coll. Cardiol. 2015;65(11):1065–1074. doi: 10.1016/j.jacc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 46.del Valdes Hernandez M.C., Maconick L.C., Munoz Maniega S., Wang X., Wiseman S., Armitage P.A. A comparison of location of acute symptomatic vs. “silent” small vessel lesions. Int. J. Stroke. 2015;10(7):1044–1050. doi: 10.1111/ijs.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H., Yang Y., Xia Y., Zhu W., Leak R.K., Wei Z. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stahon K.E., Bastian C., Griffith S., Kidd G.J., Brunet S., Baltan S. Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J. Neurosci. 2016;36(39):9990–10001. doi: 10.1523/JNEUROSCI.1316-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cropley V.L., Klauser P., Lenroot R.K., Bruggemann J., Sundram S., Bousman C. Accelerated gray and white matter deterioration with age in schizophrenia. Am. J. Psychiatry. 2017;174(3):286–295. doi: 10.1176/appi.ajp.2016.16050610. [DOI] [PubMed] [Google Scholar]
- 50.Ma X., Li Z., Jing B., Liu H., Li D., Li H. Identify the atrophy of alzheimer’s disease, mild cognitive impairment and normal aging using morphometric MRI analysis. Front Aging Neurosci. 2016;8:243. doi: 10.3389/fnagi.2016.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nitkunan A., Lanfranconi S., Charlton R.A., Barrick T.R., Markus H.S. Brain atrophy and cerebral small vessel disease: a prospective follow-up study. Stroke. 2011;42(1):133–138. doi: 10.1161/STROKEAHA.110.594267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.