Abstract
Mitochondria are dynamic organelles that have essential metabolic activity and are regarded as signalling hubs with biosynthetic, bioenergetics and signalling functions that orchestrate key biological pathways. However, mitochondria can influence all processes linked to oncogenesis, starting from malignant transformation to metastatic dissemination. In this review, we describe how alterations in the mitochondrial metabolic status contribute to the acquisition of typical malignant traits, discussing the most recent discoveries and the many unanswered questions. We also highlight that expanding our understanding of mitochondrial regulation and function mechanisms in the context of cancer cell metabolism could be an important task in biomedical research, thus offering the possibility of targeting mitochondria for the treatment of cancer.
Keywords: Mitochondria, Cancer, Metabolism, Calcium, ROS
Keywords: Abbreviations: 2-DG, 2-deoxyglucose; 18F-FDG, 2-[18F]fluoro-2-deoxy-d-glucose; α-KG, alpha-ketoglutarate; α-KGDH, alpha-ketoglutarate dehydrogenase; AGEs, advanced glycation products; AML, acute myeloid leukemia; AMPK, AMP-activated protein kinase; ASS1, argininosuccinate synthase 1; ATP, adenosine triphosphate; BAP1, BRCA1-associated protein 1; Ca2+, calcium ion; CQ, chloroquine; D-2-HG, D-2-hydroxyglutarate; DCA, dichloroacetate; EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; ER, endoplasmic reticulum; ETC, electron transport chain; FAD, flavin adenine dinucleotide; FH, fumarate hydratase; GLS, glutaminase; HIF-1, hypoxia-inducible factor-1; HSP90, heat shock protein 90; IDH, isocitrate dehydrogenase; IP3R3, inositol 1,4,5-trisphosphate receptor 3; KDMs, JmjC domain-containing demethylases; KEAP1, kelch-like ECH-associated protein 1; LDHA, lactate dehydrogenase A; LOF, loss-of-function; MAMs, mitochondria associated membranes; MCU, mitochondrial calcium uniporter; MCUR1, mitochondrial Ca2+ regulator 1; MEF2, myocyte enhancer factor 2; MICU1, mitochondrial calcium uptake 1; mPTP, mitochondrial permeability transition pore; MPT, mitochondrial permeability transition; mROS, mitochondrial ROS; mtDNA, mitochondrial DNA; mTOR, mammalian target of rapamycin; NADPH, nicotinamide adenine dinucleotide phosphate; Nf-kb, nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor (erythroid-derived 2) factor 2; NSAIDs, nonsteroidal anti-inflammatory drugs; OCR, oxygen consumption rate; OXPHOS, oxidative phosphorylation; PC, prostate cancer; PDC, pyruvate dehydrogenase complex; PDH, pyruvate dehydrogenase; PDKs, pyruvate dehydrogenase kinase; PDT, photodynamic therapy; PHD, prolyl hydroxylase; PET, positron emission tomography; PI3K, phosphatidylinositol 3-kinase; PML, promyelocytic leukaemia protein; PTEN, phosphatase and tensin homologue; PTK2B, protein tyrosine kinase 2 beta; ROS, reactive oxygen species; SDH, succinate dehydrogenase; TCA, tricarboxylic acid; TFBM2, mitochondrial transcription factor B2; TRAP-1, TNF receptor associated protein; VK3, vitamin K3
1. Introduction
Cancer is a multifaceted disease in which several alterations occur at the genomic, epigenomic, transcriptomic, proteomic and/or metabolic levels.
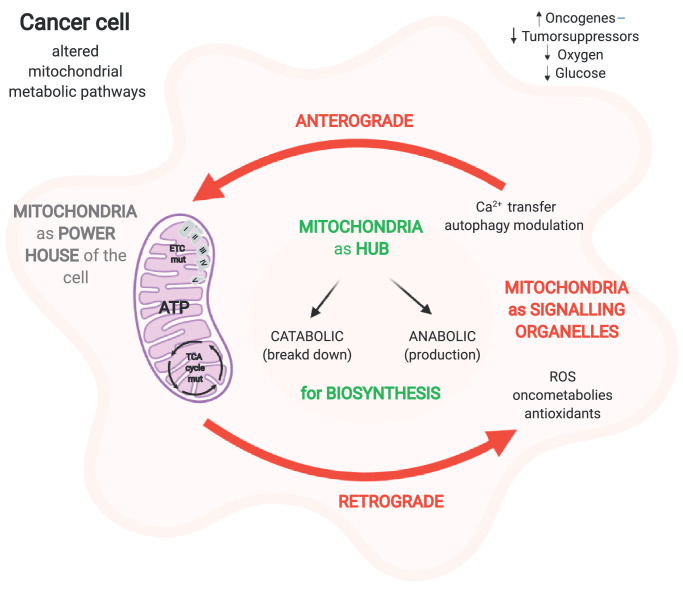
In 1924, Otto Warburg first described that cancer cells exhibit an altered metabolism, metabolizing glucose anaerobically, even in the presence of oxygen, with an associated increase in lactate production, for survival and growth [1]. Today, altered metabolism is considered a main hallmark of tumorigenesis, as it can regulate important processes that are associated with proliferation, migration and invasion. Metabolic profile remodelling is fundamental for the survival of tumour cells in a hostile environment with limited nutrients, low oxygen levels and immune surveillance and for supporting accelerated cell proliferation and enhancing other biological functions of tumour cells. Despite that the Warburg effect provides the rationale for a major clinical modality used for detecting cancer cells, positron emission tomography (PET), which uses 2-[18F]fluoro-2-deoxy-d-glucose (18F-FDG) as a tracer, a single metabolic programme is clearly not sufficient to completely define the altered metabolism in tumours. Tumour cells require not only energy to sustain their replication but also neosynthesized macromolecules to improve their fitness and maintain the redox balance. It has been hypothesized that tumours not only exhibit glucose-dependent metabolism but also take advantage of alternative oxidizable substrates, such as glutamine, serine and fatty acids (which act as anaplerotic sources for tricarboxylic acid (TCA) cycle intermediates). Furthermore, there is increasing evidence that cells in the tumour microenvironment can affect cancer cell behaviour, providing energy substrates and thus contributing to the metabolic demand of cancer cells [2]. Consistent with this view, functional mitochondria are essential for tumour growth [3, 4] mostly due to their biosynthetic role rather than their proenergetic features [5]. To fulfil the metabolic demand of cancer cells, mitochondria act at multiple levels by i) altering the production of ATP and NADPH (bioenergetics), ii) converting the diverse nutrients available into fundamental building blocks required for cell growth (biosynthesis) and iii) continuously communicating with the rest of the cell by receiving anterograde signals and transmitting retrograde signals [6]. Therefore, beyond their energetic role, mitochondria provide cancer cells with a platform that controls the production and release of reactive oxygen species (ROS), oncoproteins and oncometabolites, modulates calcium (Ca2+) homeostasis and autophagic processes; executes cell death; and influences metabolism via both cancer cell-intrinsic and cancer cell-extrinsic mechanisms [7] (Fig. 1). From this perspective, we herein want to highlight recent developments targeting the features of altered mitochondria in cancer cells subjected to a range of current or future treatments.
Fig. 1.
Overview of the central role of mitochondria in cell metabolism. Mitochondria, powerhouses of the cell, are regarded as signalling organelles that receive signals from the cytosol and coordinate responses to determinate the cell's fate (see text for further details). "Created with BioRender.com."
2. Mitochondria-dependent regulation of malignant energetic status
Mitochondria can influence malignant transformation and tumour progression, increasing the plasticity of cancer cells and governing several mechanisms to address tough environmental conditions.
2.1. Regulation of mitochondrial ROS in cancer metabolism
Mitochondria are the major source of intracellular ROS, as approximately 1–2% of molecular oxygen (O2) used in oxidative phosphorylation (OXPHOS) can be converted to anion superoxide (O2−). Additionally, other mitochondrial enzymes, such as pyruvate dehydrogenase (PDH), α-ketoglutarate-dehydrogenase (α-KGDH), acyl-CoA dehydrogenase and glycerol-3-phosphate dehydrogenase, are involved in ROS generation [8]. In normal cells, the levels of mitochondrial ROS (mROS) are carefully regulated and play a role in several cellular processes, including differentiation, autophagy, and metabolic adaption [8, 9]. Oncogene activation, tumour suppressor loss, cancer-inducing mutations in TCA cycle enzymes and hypoxia lead to the production of abnormal mROS levels that, as retrograde signals, sustain cancer cells [10]. mROS influence all steps of oncogenesis from tumour initiation to proliferation and metastasis by inducing the accumulation of nuclear or mitochondrial DNA (mtDNA) mutations and by directly affecting multiple biological processes, such as cell proliferation, apoptosis resistance and metabolic reprogramming [8]. Furthermore, mROS can activate different potentially oncogenic signalling pathways, such as the epidermal growth factor receptor (EGFR) signalling pathway [11] or the Akt/NF-κB mitochondrial transcription factor B2 (TFBM2)-dependent signalling pathway [12], known to be correlated with cancer proliferation. mROS can trigger different signal transduction cascades associated with metastatic dissemination [13, 14] including protein tyrosine kinase 2 beta (PTK2B) and Src signalling [15, 16]. Conversely, several studies demonstrated that oxidative stress limits metastatic dissemination in melanoma and lung cancer [17], [18], [19].
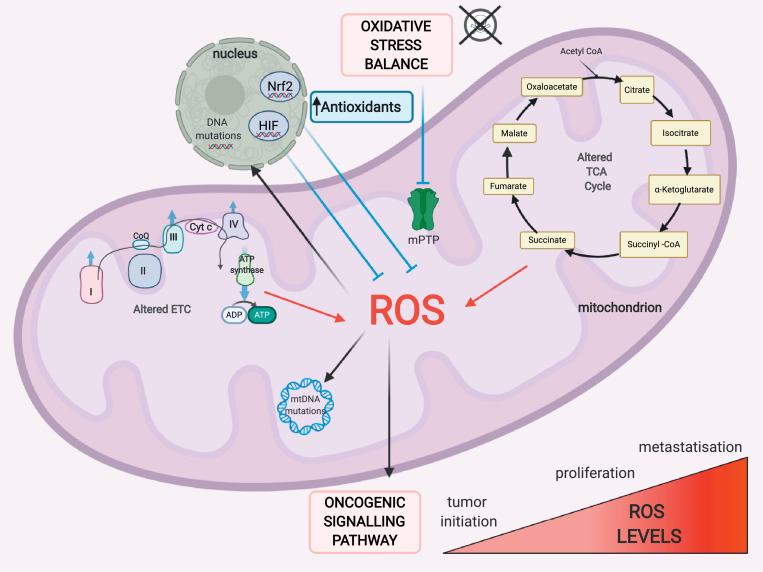
Tumour cells express high levels of antioxidant proteins to prevent ROS accumulation and possibly drive cancer cells toward a proliferative state, avoiding ROS-driven mitochondrial permeability transition (MPT)-regulated cell death (Fig. 2). A good and well-defined balance between mROS generation and ROS scavenging allows cancer cells to remain in the tumorigenic range of ROS levels. In this respect, nuclear factor (erythroid-derived 2) factor 2 (Nrf2) is a transcriptional factor known to be the master regulator of genes that mitigate oxidative stress by binding to antioxidant response elements in gene promoters. mROS exposure induces the prompt activation of Nrf2 through degradation of its allosteric inhibitor, kelch-like ECH-associated protein 1 (KEAP1). Although Nrf2 was originally deemed a tumour suppressor, recent findings have revealed its protumoral function that not only confers oxidative stress resistance but also controls ROS production via NADPH oxidase [20] and directly activates cancer-associated metabolic pathways [21], [22], [23]. Therefore, constitutive stabilization and activation of Nrf2 have been associated with poor prognosis in several types of cancer [24].
Fig. 2.
Crosstalk between redox homeostasis and metabolism in cancer cells. Finely tuned reactive oxygen species (ROS) generation and scavenging are two aspects fundamental to cancer cells. Cancer cells are characterized by high levels of ROS that can impact tumour initiation, proliferation, survival and metastasis. To compensate for the higher rate of mitochondrial ROS (mROS) production, tumour cells express high levels of antioxidants to avoid ROS-driven mitochondrial permeability transition (MPT)-regulated cell death. Nrf2: nuclear respiratory factor 2, HIF-1: hypoxia-inducible factor-1. "Created with BioRender.com."
Hypoxia-mediated mROS generation results in hypoxia-inducible factor-1 (HIF-1) activation, which in turn leads to a metabolic shift from OXPHOS to glycolysis by increasing the expression of glycolytic enzymes to facilitate tumorigenesis and metastasis [25]. However, the association between HIF-1 and mROS appears quite complex, as mROS overproduction stimulates HIF-1, but glycolytic programme activation alleviates oxidative stress in a compensatory manner. Consistent with this view, in several types of cancer, HIF-1 decreases mROS production, promotes tumour growth [26] and facilitates the survival of metastatic cells [27], [28], [29].
Altogether, these considerations indicate that the functional role of mROS may vary depending on the type and stage of cancer but preferentially suggest that mROS activate different signalling pathways towards protumoral metabolic reprogramming. Thus, targeting mROS and antioxidant systems could be beneficial as anticancer therapy.
2.2. Deregulation of mitochondrial metabolism generates tumour-related proteins and oncometabolites used for cancer progression
Mitochondrial alterations are characterized by the accumulation of metabolites due to dysfunctional catabolic and anabolic processes. These can be signatures of certain pathological stages that are also involved in signalling and disease phenotype establishment [30]. Deregulated mitochondrial metabolism can originate from not only somatic mtDNA mutations but also defects in nuclear-encoded mitochondrial enzymes. Specifically, the mitochondrial enzymes whose mutations are considered protumorigenic in many cancer types are either part of the OXPHOS or TCA cycle machinery but are also involved in other biosynthesis pathways.
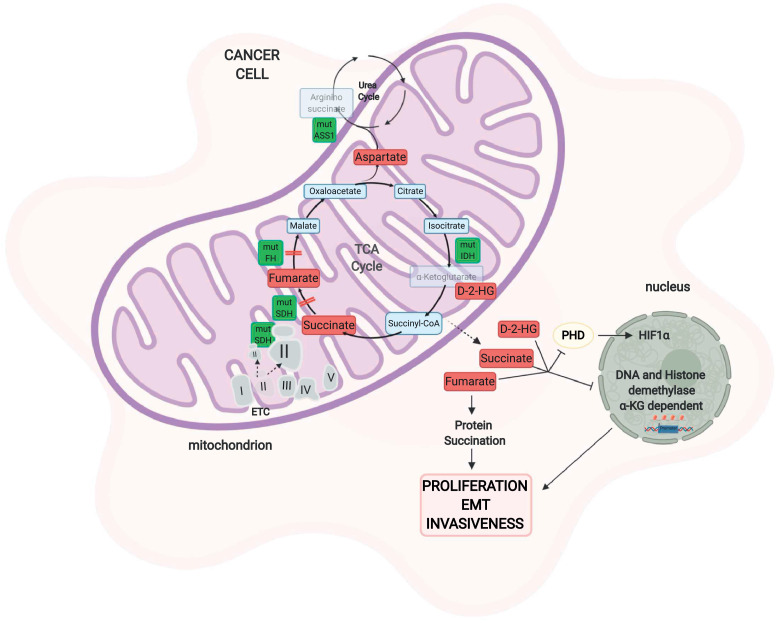
Located in the inner mitochondrial membrane, succinate dehydrogenase (SDH), also known as respiratory complex II, is involved in the conversion of succinate into fumarate. In the cancer setting, SDH is generally hit by a loss-of-function (LOF) mutation that leads to the accumulation of succinate, primarily in the mitochondria and then leaks into the cytoplasm. In contrast, recent findings have highlighted the overexpression of this enzyme in prostate cancer (PC) patients; indeed the oxidation of succinate into fumarate can sustain impaired mitochondrial metabolism through OXPHOS rewiring, supporting the TCA cycle and promoting ATP production [31]. However, the mechanism underlying this process remains elusive. LOF mutation in this enzyme has been associated with gastrointestinal stromal tumors, pheochromocytoma, paraganglioma, neuroblastoma, breast cancer, renal carcinoma and thyroid tumours [32]. The excess succinate in turn inhibits prolyl hydroxylase (PHD), an oxygen-dependent hydroxylase able to regulate HIF-1 expression depending on oxygen homeostasis, consequentially resulting in the stabilization and activation of HIF-1α under normoxic conditions [33]. Thus, defects in this enzyme create a pro-oncogenic pseudohypoxic state. The accumulation of succinate has also been linked to changes in the epigenetic regulation of gene expression through the inhibition of α-ketoglutarate (α-KG)-dependent histone and DNA demethylases [34, 35], thus mastering gene expression rewiring to promote cancer cell proliferation.
Fumarate hydratase (FH) is an enzyme of the TCA cycle and converts fumarate to malate; it is nuclear-encoded and localized at mitochondrial matrix. The FH LOF mutation lowers the respiratory rate due to loss of TCA cycle function and increases lactate production, thus supporting the glycolytic metabolic switch. FH has been found to be mutated mainly in hereditary leiomyomatosis renal cell carcinoma [32]. Similar to SDH, the oncogenic activity of FH relies mainly on the accumulation of fumarate, which, like succinate, induces the stabilization of HIF-1α through the inhibition of PHD [36], inhibits α-KG-dependent histone and DNA methylation, and regulates epigenetic modifications [35]. Fumarate can also bind covalently to cysteine residues of proteins in a reaction called succination, modulating their function. It has been reported that succination occurs preferentially on proteins involved in redox signalling; indeed, KEAP1 is succinated, thus abrogating Nrf2 inhibition and promoting proliferative gene expression [37, 38]. Moreover, PHD is not the only target of fumarate accumulation, as recent research demonstrates that in FH−/− models, the accumulation of fumarate correlates with the inhibition of miR200 and the consequential activation of epithelial-to-mesenchymal transition (EMT)-related genes [39]. Thus, both oncometabolites can competitively inhibit α-KG–dependent dioxygenases as JmjC domain-containing demethylases (KDMs), changing the DNA methylation state of histones and CpG islands near gene promoters and favouring the transcription of proliferative genes through the PHD-HIF1 axis [40] .
SDH and FH could be considered tumour suppressors since the accumulation of fumarate and succinate promotes cell proliferation, generally pointing to the same cell targets. Nevertheless, these oncometabolites are mutated and exert specific functions in different cancer types, for example protein succination by fumarate or SDH-mediated OXPHOS rewiring in PC patients [36] (Fig. 3).
Fig. 3.
Mitochondrial enzyme mutations and cancer metabolism. Mutations in enzymes of the TCA cycle or other metabolic pathways as well as components of the electron transport chain alter the metabolome in response to altered mitochondrial metabolism. Mutations in SDH can lead to two different outcomes. The loss of SDH causes succinate accumulation that inhibits PHD, stabilizes HIF1, and inhibits α-KG-dependent histone and DNA demethylases, leading to the activation of proliferative pathways. On the other hand, SDH overexpression can sustain mitochondrial cancer metabolism due to the conversion of succinate into fumarate, which sustains the TCA cycle. Mutations in fumarate hydratase, which increase fumarate concentrations, lead to the inhibition of PHD and histone demethylases, promoting proliferation. Fumarate is also involved in a cysteine post-transcriptional modification, called succination. Isocitrate dehydrogenase (IDH) mutations in cancer help the generation of a neomorphic enzyme that converts isocitrate into 2-hydroxyglutarate (2-HG), a metabolite that exerts its oncogenic effect on PHD and epigenetic regulation. Epigenetic silencing of the urea cycle enzyme argininosuccinate synthase 1 (ASS1) leads to the accumulation of aspartate, which elicits tumorigenesis. SDH: succinate dehydrogenase, PHD: prolyl hydroxylase, HIF-1: hypoxia-inducible factor-1, IDH: isocitrate dehydrogenase, D-2-HG: D-2-hydroxyglutarate, ASS1: argininosuccinate synthase. "Created with BioRender.com."
Other enzymes in the TCA cycle also display mutations that support tumour proliferation and survival. Of the three isoforms of isocitrate dehydrogenase (IDH) present in mammals, IDH1, IDH2, and IDH3, the first two are homodimeric NADP+-dependent enzymes, while IDH3 is a heterodimeric NAD+-dependent enzyme. If defects in SDH and FH are characterized by LOF mutations, IDH1 and IDH2 exhibit mono-allelic gain-of-function mutations that result in neomorphic enzyme activity, where α-KG is converted to its R-enantiomer, D-2-hydroxyglutarate (D-2-HG), instead of L-2-hydroxyglutarate [41]. D-2-HG accumulation has been linked to colon cancer, gliomas, acute myeloid leukemia (AML) and osteosarcoma [32] and to DNA hypermethylation and broad epigenetic alterations associated with proliferative pathway activation. In addition, D-2-HG inhibits the enzymatic activity of complex V and alters gene expression in tumour cells [42]. Similar to fumarate and succinate, D-2-HG also has an effect on PHD, α-KG-dependent dioxygenases, and histone demethylases [32], mastering the downregulation of genes involved in the suppression of metastasis and promoting EMT and invasiveness [43]. However, the effect of 2-HG on HIF-1 and PHD is controversial. Some studies demonstrate that 2-HG inhibits HIF-1 through the activation of PHD, while others state that 2-HG functions oppositely. This might be linked to a tumour-dependent or cell-dependent role of IDHs and needs further investigations [32].
In addition to mitochondrial oncometabolites, increasing evidence supports alteration in cytosolic enzymes, which are strictly intertwined with mitochondrial metabolism, that supply anabolic substrates for mitochondrial metabolism. One example is the argininosuccinate synthase (ASS1).
ASS1 participates in the urea cycle, and its defects promote a decrease in argininosuccinate production, leading to the accumulation of aspartate and the subsequent increase in cancer cell proliferation [44]. Amino acids sustain cell growth and provide for the massive consumption of glucose.
Given the latest evidence, it is possible to hypothesize that mitochondria, through these signalling molecules named oncometabolites, can modulate the metabolic flux either within itself or in the cytoplasm through the direct regulation of gene expression or other metabolic pathways. This signalling function of mitochondria is strictly related to the modulation of cancer cell metabolism, which supports many cancer-related functions, such as cell proliferation, migration and resistance to death. Thus, the characterization of these tumour-related proteins and their products, the oncometabolites, could represent an intriguing option for novel cancer therapy.
2.3. Mitochondrial calcium: a critical hub for cancer cell metabolism?
Mitochondrial Ca2+ signalling is intimately linked to cell growth and metabolism and constitutes a prosurvival mechanism by activating multiple components of the TCA cycle (IDH, α−KGDH and PDH) and thus feeding electron transport chain (ETC) and ATP production [45, 46]. This signalling pathway also represents fundamental machinery capable of initiating apoptosis through the opening of the mitochondrial permeability transition pore (mPTP) and the release of cytochrome c, thus representing a key program for cell fate decisions [47, 48].
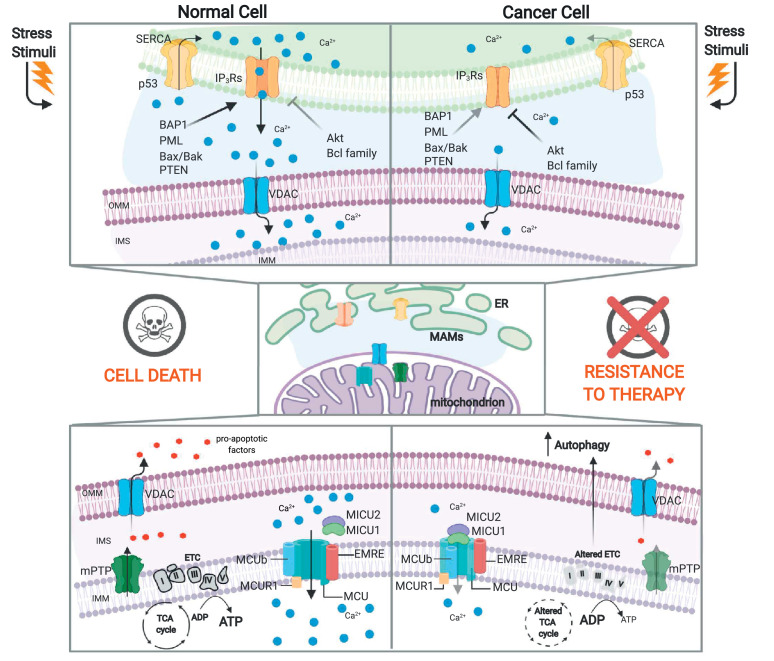
Mitochondria are strictly connected to endoplasmic reticulum (ER, the main intracellular Ca2+ store) through specialized domains called mitochondria-associated membranes (MAMs) (as reviewed in [49, 50]). Several tumour suppressors, such as promyelocytic leukaemia protein (PML), phosphatase and tensin homologue (PTEN), p53 and BRCA1-associated protein 1 (BAP1), and oncogenes, such as AKT and members of BCL-2 family, which reside at MAMs, are able to modulate the ER-mitochondria Ca2+ connection and thus critically regulate cell death [51], [52], [53], [54], [55] after exposure to some cancer therapeutics that act, at least in part, by evoking ER Ca2+ discharge [56, 57]. These observations support the general idea that reducing ER-mitochondria Ca2+transfer could help tumorigenic cells avoid apoptosis and resist chemotherapy in specific types or stages of cancer (Fig. 4).
Fig. 4.
Crosstalk between Ca2+ homeostasis and metabolism in cancer cells. Mitochondrial Ca2+can play a dual role in regulating the energetic status of cancer cells. Oncogene activation or tumour suppressor loss modulates ER-mitochondrial Ca2+ transfer to allow escape from apoptosis and resistance to chemotherapy. The mitochondrial calcium uniporter (MCU) complex is composed of the pore-forming subunit MCU (the channel that allows Ca2+ accumulation into the mitochondrial matrix) and its regulators, EMRE and MCUb, all located at the inner mitochondrial membrane (IMM); the mitochondrial calcium uptake (MICU) family members (MICU1–3) are located in the intermembrane space (IMS) and regulate the opening/closing of the complex. The MCU complex can exert both pro- and antineoplastic effects, leading to an altered energetic metabolic status in cancer cells (see text for further details). IMM: inner mitochondrial membrane, MICU: mitochondrial calcium uptake, IMS: intermembrane space, OMM: outer mitochondrial membrane, PTEN: phosphatase and tensin homologue, BAP1: BRCA1-associated protein 1, PML: promyelocytic leukaemia protein; IP3Rs: inositol 1,4,5-trisphosphate receptors, SERCA: sarco-endoplasmic reticulum ATPase, mPTP: mitochondrial permeability transition pore, ER: endoplasmic reticulum, MAMs: mitochondria-associated membranes. "Created with BioRender.com."
In this respect, reduced mitochondrial Ca2+ uptake is still able to activate the TCA cycle, smoothing the production of reducing equivalents that feed into the ETC and maintaining enough ATP for cellular needs [58].
However, the few studies that have directly assessed the intrinsic role of mitochondrial Ca2+ in the regulation of the cancer cell energetic status have depicted a complex scenario that mainly involves aberrant expression or function of the mitochondrial calcium uniporter (MCU) complex [59] (Fig. 4). Although the reduction in mitochondrial Ca2+ accumulation protects cancer cells from death induced by some Ca2+-dependent chemotherapeutic agents [60, 61], the MCU-dependent mitochondrial Ca2+elevation has been associated with invasion, metastasis and poor prognosis which occur by stimulating TCA cycle activity and increasing the NADH/NAD+ ratio [14, 62]. Consistent with this view, AMPK-mediated MCU activation permits a rapid mitochondrial Ca2+ transient that boosts mitochondrial respiration during mitosis and promotes cell cycle progression [63]. Moreover, downregulation of mitochondrial calcium uptake 1 (MICU1), known to negatively regulate MCU and function as a gatekeeper of mitochondrial Ca2+ uptake, has been correlated with poor prognosis in breast and hepatocellular carcinomas [14, 64] and AKT-mediated MICU1 instability correlates with high Ca2+ levels, aberrant mROS production and tumour progression [65]. Overall, these observations suggest that MCU complex activation permits Ca2+entry and sustains the energetic status of cancer cells needed for their proliferation. However, cancerous cells can take advantage of inhibition induced by MICU1 overexpression rather than activation of mitochondrial Ca2+ accumulation, mainly driving glycolysis for ATP production [66]. The correlation between low MCU complex activity and the switch to glycolytic metabolism is also evident in MCU−/− mice that display a low oxygen consumption rate (OCR) and a high PDH phosphorylation level, which correlate with an increased serum lactate level [67]. Nonetheless, mitochondria respond to deprivation of TCA cycle metabolites by increasing MICU1 levels, which in turn alters mitochondrial Ca2+ and protects the cells from Ca2+overload and death [68].
Taken together, these findings reveal that alterations in the organization and activity of the MCU complex could represent critical scenarios contributing to the remodelling of the metabolic profile and to the aggressiveness of certain types of cancer (as elegantly reviewed in [58]).
A recent factor that deserves attention is the tumour suppressor BAP1, which has been shown to increase the amount of Ca2+ released from the ER into the cytosol and mitochondria, promoting apoptosis [54] and increasing aerobic glycolysis [69]. Although the exact mechanisms responsible for the distinctive metabolic signature of BAP1 mutations have not yet been identified, deregulation of intracellular Ca2+ signalling could be a good candidate.
The correlation between mitochondrial matrix Ca2+ entry and bioenergetics is also essential for regulation of the autophagic process, a key event that characterizes different malignancies and their resistance to anticancer therapies. Reduction in the mitochondrial Ca2+ concentration by genetic depletion of MCU or mitochondrial Ca2+ regulator 1 (MCUR1), a positive MCU cofactor that resides inside the matrix, results in low ATP levels and activation of autophagy as an alternative mode to counteract the bioenergetic crisis [70]. Accordingly, by maintaining correct ER-mitochondria Ca2+ transfer, the tumour suppressors PML and p53 are critical for the repression of autophagy and thus for blunt cancer development [71, 72]. Cancer cells in which PML or p53 was downregulated displayed low ATP production, AMPK-dependent activation of autophagy, and enhanced resistance to metabolic stress [71, 72]. Indeed, autophagy is also critical to sustain mitochondrial metabolism and promote tumour growth in KRAS-driven lung cancer [73, 74] and BRAF-driven malignancies [75].
Conversely, it has been proposed that cancer cells require basal mitochondrial Ca2+ uptake for survival, and pharmacological inhibition of ER-mitochondria Ca2+transfer diminishes OXPHOS, thereby inducing autophagy; however, this mechanism is insufficient to overcome the energetic derangements [76]. Thus, lowering mitochondrial Ca2+ levels might be conceived as a non-conventional strategy to selectively eradicate cancer cells.
Taken together, these recent findings define the complex correlation between ER-mitochondria Ca2+ fluxes and autophagy, suggesting new molecular targets for the theorization of Ca2+-based anticancer treatments.
3. Mitochondria as promising targets for cancer therapy
The rearrangement of different pathways in cancer cells inevitably exposes some vulnerabilities that may be used in therapeutic strategies. Nevertheless, tumour heterogeneity and the presence of compensatory pathways limit the progress of such therapeutic approaches. To date, numerous drugs have been proposed to attack different functions of the mitochondrial metabolism (bioenergetics, signalling and biosynthesis) for the treatment of cancer (Table 1).
Table 1.
Summary of drugs discussed in the review and their mechanism of action.
| Mitochondria function | Drug | Mechanism of action | Type of tumour tested | Phase | References |
|---|---|---|---|---|---|
| Bioenergetic | Metformin | Inhibition complex I | Breast, prostate, melanoma, ovary, lung | clinical trials | [77], [78], [79]) |
| 2-DG and 2-FDG | competitor for binding hexokinase | lung, prostate, ovary, breast | clinical trials | [82], [83], [84]) | |
| CPI-613 | pdh and kgdh inhibitor | haematological cancers, pancreas | clinical trials | [94], [95], [96], [97]) | |
| BAY 87–2243 | Inhibition complex I | lung, prostate | clinical trials | [85] | |
| IACS-010,759 | Inhibition complex I | AML, CLL | clinical trials | [86] | |
| MitoTam | Inhibition complex I | breast | clinical trials | [87] | |
| MitoVES | Inhibition complex I and II | breast | preclinical | [88] | |
| Lonidamine | Inhibition complex II | lung | clinical trials | [89] | |
| Enasidenib and Ivosidenib | mutant IDH inhibitors | AML | clinical trials | [100] | |
| DCA | PDKs inhibitor | lung, liver | clinical trials | [102] | |
| Gossypol | LDHA inhibitor, NADH competitor | breast, brain, prostate | clinical trials | [81] | |
| diclofenac and lumiracoxib | anti-glycolitic activity | melanoma | preclinical | [90] | |
| VLX600 | ETC inhibitor | colon | clinical trials | [92] | |
| gamitrinib | inhibition HSP90 and TRAP-1 activity | lung, prostate | preclinical | [93] | |
| venetoclax and WEHI-539 | reducing bioenergetic | breast | preclinical | [91] | |
| Signalling | chloroquine | inhibition autophagy | hepatocarcinoma | clinical trials | [105] |
| Tioconazole | blocking autophagy targeting ATG4B | colon | preclinical | [106] | |
| Verteporfin | blocking autophagosome formation | pancreas | clinical trials | [107] | |
| Vitamin K3 | increasing generation of ROS | ovary | clinical trials | [110] | |
| PDT | mitochondrial Ca2+ transfer modulation | lung, liver | clinical trials | [56] | |
| Mipsagargin (G-202) | mitochondrial Ca2+ transfer modulation | glioblastoma, liver | clinical trials | [111] | |
| mitoxantrone and pixantrone | MCU complex inhibition | B-cell non-Hodgkin's lymphoma | clinical trials | [112] | |
| Biosynthesis | GLS inhibitors | reducing glutamine catabolism | breast, Burkitt lymphoma | clinical trials | [115, 116] |
Considering bioenergetics, metformin is able to inhibit complex I of the ETC in mitochondria [77], which suppresses ATP production, disturbs the NAD+/NADH ratio and diminishes oxygen consumption [78, 79]. This induces AMPK activation due to the reduced TCA activity and the consequential strong energetic stress as well as inhibits the mTOR pathway and induces autophagy [78, 79]. Cancer cells compensate for these effects by various mechanisms, including increasing glucose uptake and glycolysis and switching to glutamine utilization. For this reason, a combination of the glycolytic inhibitor 2-deoxyglucose (2-DG) with metformin was proposed for treating cancer. This synergistic combination can significantly reduce the amount of ATP stored and the activation of the proliferative signalling pathway, decreasing the side effects of high-dose treatment with a single drug [80, 81]. In fact, 2-DG has limited therapeutic power in different kinds of cancer, but it can have a synergistic antitumour effect when combined with chemo- or radiotherapy. Specifically, 2-DG, which is a glucose analogue, competes with glucose to bind hexokinase, increases oxidative stress, induces autophagy [82] and increases apoptosis, thus reducing cancer cell growth [83]. However, its limited anticancer efficacy could be due to increased autophagy, which continues to sustain cancer cells. Hence, it is necessary to simultaneously target multiple mitochondrial pathways. Recently, it was demonstrated that 2-fluoro-deoxyglucose (2-FDG) is a more potent glycolytic inhibitor than 2-DG. However, emerging evidence indicates that the cytotoxicity of these two analogues depends on tumour cell growth conditions (anaerobic or aerobic) [84]. Interestingly, other new complex I inhibitors, BAY 87–2243, IACS-010759 and mitochondrially targeted tamoxifen (MitoTam), have been demonstrated to induce cancer cell death and to reduce cell proliferation [85], [86], [87]. Another drug modified to be targeted to mitochondria is vitamin E succinate (MitoVES), which inhibits complex I and, even more drastically, complex II [88], promoting ROS generation and apoptosis of breast cancer cells [89]. Concerning complex II inhibitors, lonidamine (LND; 1-(2,4-dichlorobenzyl)−1H-indazole-3-carboxylic acid) alters the TCA cycle and glutamine metabolism in melanoma cell lines and has been used in combination with other chemotherapeutic agents to improve efficacy and overall response to cancer therapy [90]. Recently, Brummer et al. demonstrated that two nonsteroidal anti-inflammatory drugs (NSAIDs), diclofenac and lumiracoxib, are able to sensitize human melanoma cells to the RAF inhibitor vemurafenib by increasing its antiglycolytic effect and preventing metabolic reprogramming toward OXPHOS [91]. A similar study showed how the BcL2 and BcL-XL inhibitors venetoclax and WEHI-539 combined with 2DG reduced the cellular bioenergetics and abolished the clonogenic potential of breast cancer cells [92]. Another compound, VLX600, is an ETC inhibitor capable of impairing mitochondrial bioenergetics and reducing tumour growth [93], especially in low-glucose conditions. In addition to the direct activity of ETC inhibitors, several studies have demonstrated how reduced mitochondrial protein translation and stability could impact the mitochondrial bioenergetic capacity. In fact, Chae and colleagues demonstrated that gamitrinib, engineered to accumulate in mitochondria, inhibited HSP90 and TRAP-1 ATPase activity (chaperones responsible for ETC protein stability), diminishing tumour cell growth [94]. Among the numerous inhibitors of enzymes involved in mitochondrial metabolism, CPI-613, a novel lipoic acid analogue, targets mitochondrial metabolism in tumour cells. CPI-613 inhibits mitochondrial respiration through PDH and α-KGDH inactivation, resulting in PDH hyperphosphorylation and AMPK activation [95, 96]. Promising results have been obtained in a phase I study in patients with advanced haematological malignancies [97] treated with CPI-613 alone and together with a high dose of the chemotherapeutic drugs cytarabine and mitoxantrone [98]. Moreover, CPI-613 in combination with chemotherapy [99] or with chloroquine (CQ) [100] was able to reduce tumour progression. Once again, these interesting data highlight the importance of targeting different pathways simultaneously.
Various mutant IDH inhibitors have been tested in clinical trials to antagonize D-2HG, but only two of these, enasidenib (AG-221) and ivosidenib (AG-120), have been approved for use in treating refractory AML [101]. Furthermore, phase I studies have focused on the safety and tolerance of other mutant IDH inhibitors in different types of cancer, such as glioma, cholangiocarcinoma, and chondrosarcoma. In parallel, other strategies targeting two key enzymes in glucose metabolism, the pyruvate dehydrogenase complex (PDC) and pyruvate dehydrogenase kinase (PDK), which are usually altered in carcinogenesis, are being explored [102]. Dichloroacetate (DCA), a PDK inhibitor and thus a PDC activator, has been demonstrated to reduce cancer cell proliferation. However, its clinical application is limited by its nonspecific activity, low potency and high required doses [103]. As such, DCA derivates have been developed to target the pyruvate-binding pocket and improve the DCA toxicity profile. Another potential antitumour target is lactate dehydrogenase A (LDHA), which converts pyruvate to lactate and is overexpressed in several types of cancer [104]. Different inhibitors have been developed based on different mechanisms of action, but only gossypol, a natural phenol that competes with NADH, has been tested in clinical trials [105]. However, its application is limited due to its interaction with other cellular components involved in different biological activities, resulting in nonspecific toxicity.
Since mitochondria are a fundamental hub for cellular communication and signal transduction, targeting this function could clearly be another promising anticancer strategy. In support of the idea that autophagy plays an active role in drug resistance, substantial evidence suggests that autophagy inhibitors improve chemotherapy efficacy. For instance, CQ has a synergistic effect when combined with sorafenib in treating hepatocellular carcinoma [106]. Additionally, tioconazole, which suppresses autophagy by targeting ATG4B, enhances the efficacy of doxorubicin in colorectal cancer patients [107]. Verteporfin blocks autophagosome formation and sensitizes pancreatic ductal adenocarcinoma to gemcitabine [108].
Several lines of evidence suggest that PI3K/Akt/mTOR-mediated autophagy could be a promising target to enhance the chemosensitivity of tumour cells and avoid drug resistance [109]. Although many inhibitors have been developed and are currently in clinical trials, they have not displayed exciting results in patients because of their effects on mitochondrial reprogramming in cell bioenergetics and trafficking [110]. In line with this focus, although they undoubtedly affect multiple aspects of oncogenesis, PI3K inhibitors are not discussed herein.
Mitochondrial ROS signalling is also considered a valuable target since the imbalance between ROS production and ROS detoxification constitutes the pivotal molecular route contributing to aberrant proliferation and tumour cell survival in several types of cancer. Vitamin K3 (VK3) has the potential to be developed as an antitumour agent since it induces apoptosis by increasing the generation of ROS in ovarian cancer cells [111]. However, many cancer cells benefit from mROS generation through its effects on redox signalling; thus, refined strategies that allow for the specific modulation of mROS must be conceived. Today, several therapeutic approaches are used to modulate mitochondrial Ca2+ transfer by acting on ER Ca2+ release [58]. Among these, photodynamic therapy (PDT) and Mipsagargin (G-202) inducing apoptosis by favouring ER Ca2+ depletion and mitochondrial Ca2+ overload [56, 112]. More recently, new MCU complex inhibitors have been developed, such as mitoxantrone, pixantrone, DS16570511 and ruthenium complex Ru265 [113], [114], [115]. However, their biological activities are not exclusively relay on MCU inhibition and further studies may elucidate the role of mitochondrial Ca2+ and the MCU complex in tumour progression.
As mentioned previously, glutamine is the major carbon source that sustains the TCA cycle and its intermediates. Thus, targeting glutamine catabolism could be an effective strategy to limit cancer cell energy. The GLS inhibitors compound 968 and bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide retard cancer progression in addition to reducing glutamine catabolism [116, 117].
Taken together, these considerations reveal the controversial and multifaceted role of mitochondria in cancer cell metabolism due to the variety of therapeutic targets and the ability of cells to adapt and compensate.
4. Outstanding questions
Substantial evidence has been accumulated regarding how metabolic activities support cancer biology and how altered mitochondrial function is fundamental for tumour progression. Modifications in the mitochondrial metabolic status influence multiple intracellular pathways and contribute to the acquisition of typical malignant traits. The accumulation of oncometabolites deriving from mutated mitochondrial enzymes sustains cancer cells proliferation, so it is reasonable to wonder if mitochondria, through oncometabolites, are able to modulate the metabolic flux.
As already mentioned, SDH plays a dual role in cancer cell mitochondrial metabolism. On the one hand, SDH loss of function generates succinate accumulation, which supports cancer cell proliferation; on the other hand, SDH overexpression in PC patient-derived cells rewires their OXPHOS in favor of succinate oxidation. What can be more advantageous for cancer cells? A rewired OXPHOS or the overproduction of succinate?
Therapeutic approaches currently used target just one mitochondrial pathway and are not always efficacious; thus, could targeting more than one mitochondrial function, avoiding compensatory mechanisms, be the right strategy for effective anticancer therapy? For example, GGTi-2418 stabilizes inositol 1,4,5-trisphosphate receptor 3 (IP3R3), increases Ca2+ signalling and sensitizes tumours to PDT [57]. To date, there is no proof that GGTi-2418 has a direct effect on cancer cell metabolism, but its use in combination with other therapies that target different mitochondrial pathways could be a promising approach for the treatment of several types of cancer. Importantly, defining how mitochondrial Ca2+ signalling could influence the metabolic changes that characterize tumorigenesis may constitute promising candidates for the development of new anticancer drugs.
5. Conclusions
A complete understanding of the pathways allowing tumours to take advantage of altered mitochondrial function to increase their growth rate and invasiveness will be crucial to build an integrated model of the hallmark metabolic features of cancer and to establish effective therapeutic strategies.
Further investigations are required to shed light on all the factors that influence the metabolic profile of cancer cells. Nevertheless, understanding the mechanisms of mitochondrial regulation and function in the metabolism of cancer cells could be used to better define treatment strategies.
Search strategy and selection criteria
Data for this review were identified by searches of MEDLINE, PubMed and references from relevant articles using the search terms “mitochondria”, “metabolism”, “cancer”, “autophagy”, “mitochondrial ROS” and “calcium”.
Preference was given to articles published over the last five years, and each was academically and peer-reviewed.
Author contributions
S.M. and C.G. conceived the article; S.M., M.P., I.G. wrote the first version of the manuscript with constructive input from C.G. and P.P.; S.M. and M.P. prepared display items (with https://biorender.com) under the supervision of C.G. Figures are original and have not been published before. P.P and C.G reviewed and edited the manuscript before submission. All of the authors read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
This work is supported by the Italian Association for Cancer Research (AIRC IG-19803 to C.G. and IG-23670 to P.P.), A-ROSE, Progetti di Rilevante Interesse Nazionale (PRIN20177E9EPY to C.G. and PRIN2017E5L5P3 to P.P.), the Italian Ministry of Health (GR-2013-02356747 to C.G.) and local funds from the University of Ferrara to PP and C.G. PP is grateful to Camilla degli Scrovegni for continuous support. I.G. is supported by a research fellowship (AIRC “Acqua Vitasnella”, ID: 22552). The funders had no role in study design, literature analysis and interpretation, writing of the report.
References
- 1.Liberti M.V., Locasale J.W. The warburg effect: how does it benefit cancer cells. Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane A.N., Higashi R.M., Fan T.W. Metabolic reprogramming in tumors: contributions of the tumor microenvironment. Genes Dis. 2020;7:185–198. doi: 10.1016/j.gendis.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan A.S. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21:81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg F. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajzikova M. Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 2019;29:399–416. doi: 10.1016/j.cmet.2018.10.014. e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porporato P.E., Filigheddu N., Pedro J.M.B., Kroemer G., Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel. Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmstrom K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 10.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 11.Liou G.Y. Mutant KRas-induced mitochondrial oxidative stress in Acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Rep. 2016;14:2325–2336. doi: 10.1016/j.celrep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng X. Over-expression of TFB2M facilitates cell growth and metastasis via activating ROS-Akt-NF-kappaB signalling in hepatocellular carcinoma. Liver Int. 2020 doi: 10.1111/liv.14440. [DOI] [PubMed] [Google Scholar]
- 13.Fu L. SIRT4 inhibits malignancy progression of NSCLCs, through mitochondrial dynamics mediated by the ERK-Drp1 pathway. Oncogene. 2017;36:2724–2736. doi: 10.1038/onc.2016.425. [DOI] [PubMed] [Google Scholar]
- 14.Ren T. MCU-dependent mitochondrial Ca(2+) inhibits NAD(+)/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene. 2017;36:5897–5909. doi: 10.1038/onc.2017.167. [DOI] [PubMed] [Google Scholar]
- 15.Park J.H. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 2016;14:2154–2165. doi: 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porporato P.E. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Le Gal K. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aad3740. 308re308. [DOI] [PubMed] [Google Scholar]
- 18.Piskounova E. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayin V.I. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007653. 221ra215. [DOI] [PubMed] [Google Scholar]
- 20.Kovac S. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850:794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bollong M.J. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature. 2018;562:600–604. doi: 10.1038/s41586-018-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeNicola G.M. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan A.U.H. Mitochondrial complex I activity signals antioxidant response through ERK5. Sci Rep. 2018;8:7420. doi: 10.1038/s41598-018-23884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menegon S., Columbano A., Giordano S. The dual roles of NRF2 in cancer. Trends Mol Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Yu L. Modeling the genetic regulation of cancer metabolism: interplay between glycolysis and oxidative phosphorylation. Cancer Res. 2017;77:1564–1574. doi: 10.1158/0008-5472.CAN-16-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung-Chun Chiu D. Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell Death Dis. 2019;10:934. doi: 10.1038/s41419-019-2155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao T. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci Rep. 2014;4:3793. doi: 10.1038/srep03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza G.L. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36:252–259. doi: 10.15252/embj.201695204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shukla S.K. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32:71–87. doi: 10.1016/j.ccell.2017.06.004. e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan S., Guha M., Kashina A., Avadhani N.G. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim Biophys Acta Bioenerg. 2017;1858:602–614. doi: 10.1016/j.bbabio.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schopf B. OXPHOS remodeling in high-grade prostate cancer involves mtDNA mutations and increased succinate oxidation. Nat Commun. 2020;11:1487. doi: 10.1038/s41467-020-15237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu C.C., Tseng L.M., Lee H.C. Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (Maywood) 2016;241:1281–1295. doi: 10.1177/1535370216641787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selak M.A. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Letouze E. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Xiao M. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannino G., Ciscato F., Masgras I., Sanchez-Martin C., Rasola A. Metabolic plasticity of tumor cell mitochondria. Front Oncol. 2018;8:333. doi: 10.3389/fonc.2018.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam J. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M. The succinated proteome of FH-mutant tumours. Metabolites. 2014;4:640–654. doi: 10.3390/metabo4030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sciacovelli M. Corrigendum: Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;540:150. doi: 10.1038/nature20144. [DOI] [PubMed] [Google Scholar]
- 40.Nowicki S., Gottlieb E. Oncometabolites: tailoring our genes. FEBS J. 2015;282:2796–2805. doi: 10.1111/febs.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan L.B., Gui D.Y., Vander Heiden M.G. Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer. 2016;16:680–693. doi: 10.1038/nrc.2016.85. [DOI] [PubMed] [Google Scholar]
- 42.Reitman Z.J. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A. 2011;108:3270–3275. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crispo F. Metabolic dysregulations and epigenetics: a bidirectional interplay that drives tumor progression. Cells. 2019;8 doi: 10.3390/cells8080798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabinovich S. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527:379–383. doi: 10.1038/nature15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonora M. ATP synthesis and storage. Purinergic Signal. 2012;8:343–357. doi: 10.1007/s11302-012-9305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Giorgi C., Marchi S., Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018;19:713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- 48.Giorgi C., Danese A., Missiroli S., Patergnani S., Pinton P. Calcium dynamics as a machine for decoding signals. Trends Cell Biol. 2018;28:258–273. doi: 10.1016/j.tcb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Giorgi C. Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid Redox Signal. 2015;22:995–1019. doi: 10.1089/ars.2014.6223. [DOI] [PubMed] [Google Scholar]
- 50.Missiroli S. Endoplasmic reticulum-mitochondria Ca(2+) crosstalk in the control of the tumor cell fate. Biochim Biophys Acta Mol Cell Res. 2017;1864:858–864. doi: 10.1016/j.bbamcr.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Rimessi A., Marchi S., Patergnani S., Pinton P. H-Ras-driven tumoral maintenance is sustained through caveolin-1-dependent alterations in calcium signaling. Oncogene. 2014;33:2329–2340. doi: 10.1038/onc.2013.192. [DOI] [PubMed] [Google Scholar]
- 52.Giorgi C. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giorgi C. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 2015;112:1779–1784. doi: 10.1073/pnas.1410723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bononi A. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature. 2017;546:549–553. doi: 10.1038/nature22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bononi A. Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ. 2013;20:1631–1643. doi: 10.1038/cdd.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giorgi C. Intravital imaging reveals p53-dependent cancer cell death induced by phototherapy via calcium signaling. Oncotarget. 2015;6:1435–1445. doi: 10.18632/oncotarget.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuchay S. PTEN counteracts FBXL2 to promote IP3R3- and Ca(2+)-mediated apoptosis limiting tumour growth. Nature. 2017;546:554–558. doi: 10.1038/nature22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchi S., Giorgi C., Galluzzi L., Pinton P. Ca(2+) fluxes and cancer. Mol Cell. 2020;78:1055–1069. doi: 10.1016/j.molcel.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Marchi S. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle. 2019;18:1068–1083. doi: 10.1080/15384101.2019.1612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong Z. MicroRNA-138 and MicroRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med. 2017;195:515–529. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marchi S. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tosatto A. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol Med. 2016;8:569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao H. AMPK-mediated activation of MCU stimulates mitochondrial Ca(2+) entry to promote mitotic progression. Nat Cell Biol. 2019;21:476–486. doi: 10.1038/s41556-019-0296-3. [DOI] [PubMed] [Google Scholar]
- 64.Hall D.D., Wu Y., Domann F.E., Spitz D.R., Anderson M.E. Mitochondrial calcium uniporter activity is dispensable for MDA-MB-231 breast carcinoma cell survival. PLoS One. 2014;9:e96866. doi: 10.1371/journal.pone.0096866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchi S. Akt-mediated phosphorylation of MICU1 regulates mitochondrial Ca(2+) levels and tumor growth. EMBO J. 2019;38 doi: 10.15252/embj.201899435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakraborty P.K. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun. 2017;8:14634. doi: 10.1038/ncomms14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan X. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nemani N. Mitochondrial pyruvate and fatty acid flux modulate MICU1-dependent control of MCU activity. Sci Signal. 2020;13 doi: 10.1126/scisignal.aaz6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bononi A. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 2017;24:1694–1704. doi: 10.1038/cdd.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomar D. MCUR1 is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell Rep. 2016;15:1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Missiroli S. PML at mitochondria-associated membranes is critical for the repression of autophagy and cancer development. Cell Rep. 2016;16:2415–2427. doi: 10.1016/j.celrep.2016.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tasdemir E. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo J.Y. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 2016;30:1704–1717. doi: 10.1101/gad.283416.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo J.Y. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27:1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strohecker A.M., White E. Targeting mitochondrial metabolism by inhibiting autophagy in BRAF-driven cancers. Cancer Discov. 2014;4:766–772. doi: 10.1158/2159-8290.CD-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cardenas C. Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Rep. 2016;15:219–220. doi: 10.1016/j.celrep.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 77.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000:607–614. 348 Pt 3. [PMC free article] [PubMed] [Google Scholar]
- 78.Wheaton W.W. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andrzejewski S., Gravel S.P., Pollak M., St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue C. Targeting P-glycoprotein expression and cancer cell energy metabolism: combination of metformin and 2-deoxyglucose reverses the multidrug resistance of K562/Dox cells to doxorubicin. Tumour Biol. 2016;37:8587–8597. doi: 10.1007/s13277-015-4478-8. [DOI] [PubMed] [Google Scholar]
- 81.Ben Sahra I. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 82.Zhang D. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355:176–183. doi: 10.1016/j.canlet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Wang H. Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell Int. 2016;16:9. doi: 10.1186/s12935-016-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurtoglu M., Maher J.C., Lampidis T.J. Differential toxic mechanisms of 2-deoxy-D-glucose versus 2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells. Antioxid Redox Signal. 2007;9:1383–1390. doi: 10.1089/ars.2007.1714. [DOI] [PubMed] [Google Scholar]
- 85.Molina J.R. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med. 2018;24:1036–1046. doi: 10.1038/s41591-018-0052-4. [DOI] [PubMed] [Google Scholar]
- 86.Rohlenova K. Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2(high) Breast Cancer. Antioxid Redox Signal. 2017;26:84–103. doi: 10.1089/ars.2016.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schockel L. Targeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growth. Cancer Metab. 2015;3:11. doi: 10.1186/s40170-015-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong L.F. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J Biol Chem. 2011;286:3717–3728. doi: 10.1074/jbc.M110.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan B. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Cancer. 2015;15:401. doi: 10.1186/s12885-015-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo L. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J Biol Chem. 2016;291:42–57. doi: 10.1074/jbc.M115.697516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brummer C. Metabolic targeting synergizes with MAPK inhibition and delays drug resistance in melanoma. Cancer Lett. 2019;442:453–463. doi: 10.1016/j.canlet.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Lucantoni F., Dussmann H., Llorente-Folch I., Prehn J.H.M. BCL2 and BCL(X)L selective inhibitors decrease mitochondrial ATP production in breast cancer cells and are synthetically lethal when combined with 2-deoxy-D-glucose. Oncotarget. 2018;9:26046–26063. doi: 10.18632/oncotarget.25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun. 2014;5:3295. doi: 10.1038/ncomms4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chae Y.C. Control of tumor bioenergetics and survival stress signaling by mitochondrial HSP90s. Cancer Cell. 2012;22:331–344. doi: 10.1016/j.ccr.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zachar Z. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl) 2011;89:1137–1148. doi: 10.1007/s00109-011-0785-8. [DOI] [PubMed] [Google Scholar]
- 96.Stuart S.D. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab. 2014;2:4. doi: 10.1186/2049-3002-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pardee T.S. A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res. 2014;20:5255–5264. doi: 10.1158/1078-0432.CCR-14-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pardee T.S. A phase I study of CPI-613 in combination with high-dose cytarabine and mitoxantrone for relapsed or refractory acute myeloid leukemia. Clin Cancer Res. 2018;24:2060–2073. doi: 10.1158/1078-0432.CCR-17-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alistar A. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18:770–778. doi: 10.1016/S1470-2045(17)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Egawa Y., Saigo C., Kito Y., Moriki T., Takeuchi T. Therapeutic potential of CPI-613 for targeting tumorous mitochondrial energy metabolism and inhibiting autophagy in clear cell sarcoma. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Golub D. Mutant isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front Oncol. 2019;9:417. doi: 10.3389/fonc.2019.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jha M.K., Suk K. Pyruvate dehydrogenase kinase as a potential therapeutic target for malignant gliomas. Brain Tumor Res Treat. 2013;1:57–63. doi: 10.14791/btrt.2013.1.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W., Zhang S.L., Hu X., Tam K.Y. Targeting tumor metabolism for cancer treatment: is Pyruvate Dehydrogenase Kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11:1390–1400. doi: 10.7150/ijbs.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sheng S.L. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. doi: 10.1111/j.1742-4658.2012.08748.x. [DOI] [PubMed] [Google Scholar]
- 105.Rani R., Kumar V. Recent update on Human Lactate Dehydrogenase Enzyme 5 (hLDH5) inhibitors: a promising approach for cancer chemotherapy. J Med Chem. 2016;59:487–496. doi: 10.1021/acs.jmedchem.5b00168. [DOI] [PubMed] [Google Scholar]
- 106.Shi Y.H. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–1172. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- 107.Liu P.F. Drug repurposing screening identifies tioconazole as an ATG4 inhibitor that suppresses autophagy and sensitizes cancer cells to chemotherapy. Theranostics. 2018;8:830–845. doi: 10.7150/thno.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Donohue E. The autophagy inhibitor verteporfin moderately enhances the antitumor activity of gemcitabine in a pancreatic ductal adenocarcinoma model. J Cancer. 2013;4:585–596. doi: 10.7150/jca.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Z. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol. 2020;104:575–587. doi: 10.1007/s00253-019-10257-8. [DOI] [PubMed] [Google Scholar]
- 110.Caino M.C. PI3K therapy reprograms mitochondrial trafficking to fuel tumor cell invasion. Proc Natl Acad Sci U S A. 2015;112:8638–8643. doi: 10.1073/pnas.1500722112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xia M.H. p62 Suppressed VK3-induced oxidative damage through Keap1/Nrf2 pathway in human ovarian cancer cells. J Cancer. 2020;11:1299–1307. doi: 10.7150/jca.34423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mahalingam D. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br J Cancer. 2016;114:986–994. doi: 10.1038/bjc.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arduino D.M. Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol Cell. 2017;67:711–723. doi: 10.1016/j.molcel.2017.07.019. e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kon N. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discov. 2017;3:17045. doi: 10.1038/cddiscovery.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woods J.J. A selective and cell-permeable Mitochondrial Calcium Uniporter (MCU) inhibitor preserves mitochondrial bioenergetics after hypoxia/reoxygenation injury. ACS Cent Sci. 2019;5:153–166. doi: 10.1021/acscentsci.8b00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le A. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J.B. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]