Abstract
Natural Killer (NK) cells and CD8+ cytotoxic T cells are two types of immune cells that can kill target cells through similar cytotoxic mechanisms. With the remarkable success of chimeric antigen receptor (CAR)-engineered T (CAR-T) cells for treating haematological malignancies, there is a rapid growing interest in developing CAR-engineered NK (CAR-NK) cells for cancer therapy. Compared to CAR-T cells, CAR-NK cells could offer some significant advantages, including: (1) better safety, such as a lack or minimal cytokine release syndrome and neurotoxicity in autologous setting and graft-versus-host disease in allogenic setting, (2) multiple mechanisms for activating cytotoxic activity, and (3) high feasibility for ‘off-the-shelf’ manufacturing. CAR-NK cells could be engineered to target diverse antigens, enhance proliferation and persistence in vivo, increase infiltration into solid tumours, overcome resistant tumour microenvironment, and ultimately achieve an effective anti-tumour response. In this review, we focus on recent progress in genetic engineering and clinical application of CAR-NK cells, and discuss current challenges and future promise of CAR-NK cells as a novel cellular immunotherapy in cancer.
Keywords: Chimeric antigen receptor, NK cells, Immunotherapy, Cancer
Abbreviations: NK, Natural killer; CAR, Chimeric antigen receptor; CAR-T, CAR-engineered T; CAR-NK, CAR-engineered NK; GVHD, graft-versus-host disease; CRS, cytokine release syndrome; TCR, T cell receptor; NCRs, natural cytotoxicity receptors; ITAM, immunoreceptor tyrosine-based activation motif; KIRs, killer cell Ig-like receptors; ADCC, antibody-dependent cell-mediated cytotoxicity; IgG, immunoglobulin G; LAK, lymphokine-activated killer; TIL, tumour-infiltrating lymphocyte; TCR-T, TCR-engineered T; AML, acute myeloid leukaemia; scFv, single-chain variable fragment; TME, tumour microenvironment; TAM, tumour-associated macrophages; ADCP, antibody-dependent cellular phagocytosis; CAR-Ms, macrophages with CARs; PBMCs, peripheral blood mononuclear cells; UCB, umbilical cord blood; iPSCs, induced pluripotent stem cells; HPCs, hematopoietic progenitor cells; PB NK, PBMCs-derived NK; SCF, stem cell factor; VEGF, vascular endothelial growth factor; BMP4, bone morphogenetic protein 4; BaEV-gp, baboon envelop glycoprotein; PB, PiggyBac; SB, sleeping beauty; TIRs, terminal inverted repeats; GMP, good manufacturing practice; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; SBRT, stereotactic body radiotherapy; APCs, antigen-presenting cells
1. Introduction
NK cells account for 5–15% of human peripheral blood leukocytes and are usually identified by expression of CD56 without co-expression of CD3 and T cell receptor (TCR). Human NK cells can be further divided into two subsets depending on CD56 and CD16 (Fcγ receptor III or FcγRIII) expression. The CD56dimCD16high represent 85–95% of peripheral blood NK cells, are developmentally mature and show higher cytotoxic function at baseline, whereas CD56brightCD16low/− NK cells are the minor NK cell subset in the peripheral blood and thought to be developmentally immature NK cells with lower cytotoxicity at baseline. [1]
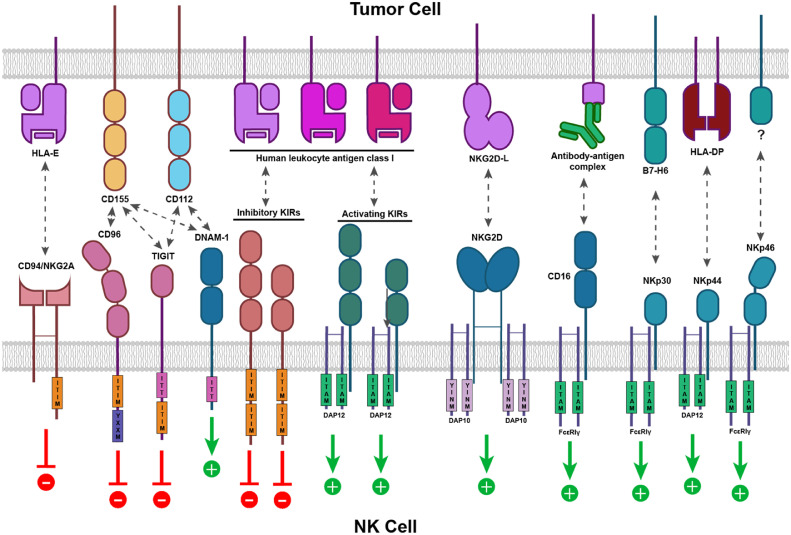
NK cells were originally identified to kill tumour cells non-specifically. [2,3] Since then, they are shown to exhibit potent anti-tumour immunity through multiple mechanisms. NK cells are functionally similar to CD8+ cytotoxic T cells and kill target cells through similar cytotoxic mechanisms, but lack a somatically rearranged and antigen-specific TCR. [4] Activation of NK cell cytotoxic activity is controlled by signals integrated from an array of germline-encoded activating and inhibitory receptors (Fig. 1). Therefore, NK cell killing of a target cell depends on the balance between inhibitory and activating signals. Tumour cells that down regulate expression of human leukocyte antigen (HLA) are more susceptible to the NK cell killing due to a reduced KIR mediated inhibition.
Fig. 1.
Germline-encoded activating and inhibitory receptors control NK cell activation. One of the best-studied activating receptors is NKG2D, a homodimeric receptor that recognises stress-induced ligands MICA, MICB, and ULBP1-6 expressed on damaged, transformed, or otherwise abnormal cells. Upon engaging with its ligand, NKG2D signals through adapter molecule DAP10 to trigger NK activation. NK cells can also be activated through a variety of natural cytotoxicity receptors (NCRs) – such as NKp30, NKp44, and NKp46 - in response to a diverse set of viral, oncogenic, or stress-induced ligands. Upon binding to their respective ligands, NCRs recruit immunoreceptor tyrosine-based activation motif (ITAM)-bearing adapter molecules DAP12, CD3ζ or FcεRIγ, which in turn initiate activation signalling cascades. Immunoglobulin (Ig) superfamily receptor DNAM-1 can activate NK cells via interaction with CD112. Additionally, NK cells can eliminate tumour cells through CD16-mediated ADCC, in which CD16 expressed on NK cells recognises the Fc portion of the IgG bound to the tumour cell surface. Conversely, inhibitory receptors like NKG2A and inhibitory KIRs carry immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their cytoplasmic domains. They provide inhibitory signalling for NK cells when interacting with their ligands (e.g., HLA-E for CD94/NKG2A and certain HLA-A, B, or C allotypes for inhibitor KIRs) expressed by the target cells. Some Ig superfamily receptors, such as CD96 and TIGIT, interact with CD155 or CD112 and transmit inhibitory signals via additional motifs.
NK cells are also the key mediators of the antibody-dependent cell-mediated cytotoxicity (ADCC), in which antibodies bound to the target cells activate NK cell cytotoxicity. [5] NK cells express CD16 that recognize the Fc portion of the immunoglobulin G (IgG) bound to the target cell surface. This interaction signals through ITAM and activate NK cytotoxic function. CD16 is unique in that its engagement is sufficient for inducing NK cell activation, which is not the case for other activating receptors. [6] In addition to their roles as cytotoxic effector cells, activated NK cells also secrete various inflammatory cytokines and chemokines, which in turn recruit and activate other immune cells such as T cells, dendritic cells, and macrophages. [7]
2. Unique advantages of CAR-engineered NK cells
Cellular immunotherapy, also known as adoptive cell therapy, is an innovative treatment approach that aims to harness body's immune system to eliminate cancer. Cellular immunotherapies can be deployed in different ways. For instance, lymphokine-activated killer (LAK) therapy and tumour-infiltrating lymphocyte (TIL) therapy utilize autologous peripheral blood monocytes or naturally occurring tumour-infiltrating T cells, activated and expanded to large numbers ex vivo, [8] and then infused into the same cancer patients. [8] However, these non-genetically-modified LAK or TIL cells have demonstrated only modest benefit in clinical studies. [8] For better recognition and killing of tumour cells, immune cells including T cells, [9,10] NK cells, [11] γδT cells, [12] NKT cells, [13] and even macrophages, [14] are engineered to express antigen-specific T cell receptors (TCRs) or chimeric antigen receptors (CARs). Recently, the approval of CD19-targeted CAR-T cell therapy by the Food and Drug Administration (FDA) is a major milestone in the development of genetically modified cell therapies for cancer and has sparked major interest in developing CAR-NK cells for cancer immunotherapy. [15] The unique biological features of NK cells make them an more attractive source for genetically modified immune cell-based immunotherapy.
First, compared to the CAR-T cells, superior safety of the unmodified NK cells and more recently CAR-NK cell immunotherapy has been shown in several clinical settings. The risk of on-target/off-tumour toxicity to normal tissues is relatively low due to a limited lifespan of CAR-NK cells in the circulation. [16] Allogeneic CAR-NK cell infusions have reduced risk for graft versus host disease (GVHD). [17] Cytokine release syndrome (CRS) and neurotoxicity [18] are less likely to occur in CAR-NK immunotherapy [11] partly due to a different spectrum of the secreted cytokines: activated NK cells usually produce IFN-γ and GM-CSF, [19] whereas CAR-T cells predominantly induce cytokines, such as IL-1a, IL-1Ra, IL-2, IL-2Ra, IL-6, TNF-α, MCP-1, IL-8, IL-10, and IL-15, that are highly associated with CRS and severe neurotoxicity. [20]
Second, besides killing tumour target cells in the CAR-dependent manner, CAR-NK cells can potentially eliminate cancer cells in a CAR-independent manner. CAR-NK cells still possess their natural cytotoxic activity against tumour cells [21] and can be activated through CAR-independent mechanism, such as NCRs, NKG2D, co-stimulatory receptor DNAM-1 (CD226), and certain activating KIRs (KIR2DS1, KIR2DS4 and KIR2DL4). [22,23] Moreover, NK cells can eliminate tumour cells through CD16-mediated ADCC. [24] Thus, CAR-modified NK cells may be able to efficiently eradicate a heterogeneous tumour in which some tumour cells do not express CAR-targeted antigen, via both CAR-dependent and NK cell receptor-dependent mechanisms.
Third, the reduced risk for alloreactivity and thus GVHD even with allogeneic NK cells [17] potentially allows CAR-NK cells to be generated from multiple sources, including NK92 cell lines, peripheral blood mononuclear cells (PBMCs), umbilical cord blood (UCB), and induced pluripotent stem cells (iPSCs). [25] For instance, homogeneous NK92 cells provide an “off-the-shelf” CAR-modified product for clinical applications, while the CAR-NK cells derived from genetically modified human iPSCs can be produced in a homogeneous and clinically scalable manner, and have demonstrated potent anti-tumour activity and proliferative capacity in a preclinical setting. [26] Thus, CAR-NK cells can provide an “off-the-shelf” product, eliminating the need for a personalised and patient-specific product that plagues current CAR-T cell therapies.
3. Generation of CAR-NK cells
3.1. Sources of NK cells
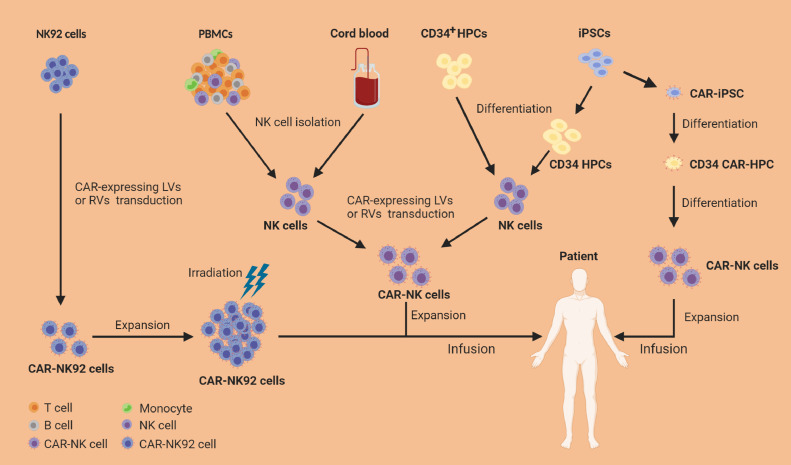
Unlike T cells, NK cells have the reduced risk for GVHD, [27], [28], [29] and thus open the possibility to produce “off-the-shelf” allogeneic cell therapy products, which could be prepared in advance and readily available for multiple patients on demand. Currently, clinical-grade NK cells can be manufactured in a large scale from multiple sources including NK92 cell line, PBMCs, UBC, CD34+ hematopoietic progenitor cells (HPCs), and iPSCs (Fig. 2).
Fig. 2.
Sources and manufacturing of CAR-NK cell products. NK92 cell line has been extensively used as a source of CAR-NK cells because CAR-NK92 cells can expand indefinitely in vitro and have reduced sensitivity to repeated freeze/thaw cycles. As malignant cells from a NK cell lymphoma, NK92 cells engineered with CAR must receive lethal irradiation before their infusion into patients. Primary NK cells can be isolated directly from peripheral blood mononuclear cells (PBMCs) of healthy donors or umbilical cord blood (UCB) using NK cell isolation kit. Isolated primary NK cells can be activated, genetically engineered with CAR-expressing vectors (e.g., lentivirus [LVs] or retrovirus [RVs]), and then expanded in NK cell-specific expansion media with cytokines for GMP-grade clinical application. NK cells also can be differentiated from CD34+ hematopoietic progenitor cells (HPCs) using a cocktail of cytokines in culture; the resulting NK cells are engineered with CAR and then expanded in vitro before infusion. Recently, induced pluripotent stem cells (iPSCs) have become an attractive source of CAR-NK cells for “off-the-shelf” CAR-NK cell products attributing to their unlimited proliferative capacity. iPSCs can differentiate into CD34+ HPCs and then NK cells. Importantly, iPSCs can be genetically engineered with CAR-expressing vectors and the resulting CAR-iPSCs then can be differentiated into CAR-HPCs and finally into CAR-NK cells.
Majority of current clinical trials with CAR-NK cells use NK92 cell line because of its unlimited proliferation ability in vitro and likely reduced sensitivity to repeated freeze/thaw cycles. [30] These properties provide distinct advantages to manufacture “off-the-shelf” CAR-NK products for clinical use, with less manufacturing time and lower cost. However, NK92 cells being a tumour cell line have inherent drawbacks including potential tumorigenicity risk, lack of CD16 and NKp44 expression, and loss of in vivo expansion potential due to lethal irradiation needed before their infusion, [31] making them unlikely to be an ideal cell source for CAR-NK cell therapy approaches.
Human PBMCs are an important source of primary NK cells, which have been used in numerous clinical trials. Using NK cell isolation kits, a sufficient number of NK cells can be easily isolated directly from PBMCs of a healthy donor, and then stimulated and expanded in NK cell-specific expansion media with cytokines for preclinical or good manufacturing practice (GMP)-grade clinical application. [32] Activated PBMC-derived CAR-NK cells expressing a wide range of activating receptors can be administered without irradiation, which allows them to expand in vivo. PBMCs-derived NK (PB NK) cells, up to 90% of which are CD56dimCD16+ NK cells, typically exhibit a mature phenotype, with increased cytotoxicity and reduced proliferative capacity. [33] Due to the lack of GVHD, PB NK cells can be isolated from matched or HLA-mismatched donors, [34], [35], [36] which provide more choices for possible donors and thus enhances the quality of the final product. Similarly, NK cells can also be isolated from UCB in the same manner. UCB banks provide the advantage of selecting donors with certain HLA types and specific NK receptor profiles. However, low numbers of UCB NK cells due to the limited volume of a single UCB unit, pose a major obstacle to obtain sufficient numbers of NK cells for clinical use. Additionally, compared to PB NK cells, UCB NK cells exhibit a less mature phenotype and lower cytotoxicity against tumour cells, with decreased expression of certain adhesion molecules, CD16, KIRs, perforin and granzyme B, and higher expression of inhibitory molecules such as NKG2A. [37] However, PBMC- and UCB-derived CAR-NK cells are not derived from a homogeneous source, making it difficult to standardise products. [38]
The differentiation of NK cells from CD34+ HPCs is another approach for obtaining large numbers of mature NK cells for clinical applications. CD34+ HPCs can be isolated from bone marrow, embryonic stem cells, mobilized PB, or UCB, and then expanded and differentiated into mature NK cells using a cocktail of cytokines in a culture system. [39] The resulting CD56+CD3− NK cells are mostly similar to PB NK cells, expressing activating NK cell receptors and exhibiting potent cytotoxicity against leukaemia cells in vitro and in vivo. [40] More recently, iPSCs have become an attractive source of CAR-NK cells attributing to their unlimited proliferative capacity. [41] Compared to differentiated NK cells, iPSCs can be more efficiently engineered to stably express a CAR. CAR-engineered iPSCs can be cultured in media containing stem cell factor (SCF), vascular endothelial growth factor (VEGF), and bone morphogenetic protein 4 (BMP4) to differentiate into hematopoietic progenitor cells, which are then stimulated with IL3, IL-15, IL-7, SCF, and FLT3L in culture medium to differentiate into CAR-NK cells. [42] The resulting CAR-NK cells can be harvested and expanded in the presence of irradiated mIL-21 expressing artificial antigen presenting cells. [42] In this process, only one single CAR-engineered iPSC cell is sufficient to differentiate into a large number of highly homogeneous CAR-NK cell product for clinical use. However, iPSCs derived NK cells, similar to UCB NK cells, are usually characterized by an immature phenotype, with lower KIR and CD16 expression and higher NKG2A expression compared to PB NK cells. [43], [44], [45] Nevertheless, iPSC NK cells expressing NK-tailored CAR instead of conventional CAR designed for T cells have demonstrated potent anti-tumour activity in vitro and in vivo, and hence provide a renewable source for an “off-the-shelf” CAR-NK cell products. [42]
3.2. CAR constructs
So far, most CAR-NK cell studies use CAR constructs designed for CAR-T cells. Recently, novel CAR constructs have been designed for NK cells specifically. However, these different CAR constructs exhibited varying effects on cytotoxicity and cytokine production in NK cells. [46,47] Table 1 summarises the components of different CAR constructs currently used in CAR-NK cells. Imai et al. reported that a second-generation anti-CD19 CAR containing 4-1BB co-stimulatory domain (scFv-CD8TM-4-1BB-CD3ζ) could be expressed in primary NK cells, which overcame inhibitory signals and induced NK cell specific killing of CD19+ acute lymphoblastic leukaemia (ALL). [48] CAR construct containing CD28 co-stimulatory domain has also been developed to direct against HER2, [49] EGFR or EGFR variant III, [50] and CS1. [51] Although these 4-1BB/CD28-containing CARs that were originally used in T cells can exert anti-tumour activities when used in NK cells, NK cells with 2B4 (an NK-specific co-stimulatory domain) containing CAR were reported to acquire significantly enhanced cytotoxic activity against tumour cells, induced rapid proliferation, increased cytokine production, and decreased apoptosis compared to transduced NK cells bearing the conventional 4-1BB containing CAR,[52] indicating that NK cell-specific activating signalling affects the CAR performance. Additionally, CAR constructs with different signalling domains (including CD3ζ, DAP10, and DAP12) induced distinct anti-tumour activities in primary NK cells or NK92 cell line: CAR with CD3ζ signalling domain significantly outperformed the one containing DAP10 signalling domain, [48] while DAP12-based CAR in turn was superior to a CAR containing CD3ζ signalling domain. [53,54] To optimize CAR construct for NK cells, Li and colleagues expressed ten different anti-mesothelin CARs in NK92 cells (Table 1), and evaluated their effects in improving NK cells-mediated killing. [42] They found that only three CARs (CAR4, CAR7, and CAR9) that contained an NKG2D TM domain and 2B4 co-stimulatory domain exhibited the greatest increase in anti-tumour activity. A possible mechanism for improved performance of these NKG2D TM and 2B4 containing CARs is their ability to activate signalling by recruitment of endogenous DAP10 under the condition of mesothelinhigh A1847 stimulation.
Table 1.
Summary of the most common CAR constructs used in CAR-NK cells
| Target antigen | Target cells | CAR construct | Vector | NK cell source | Authors |
|---|---|---|---|---|---|
| CD19 | CD19+ Raji cell lines and primary leukaemia cells | scFv-CD8TM-4-1BBIC-CD3ζS | RV⁎ | Umbilical cord blood NK cells | E Liu et al. [58] |
| EGFR HER-2 CS1 |
Human glioblastoma cell lines SKOV-3 ovarian cancer cells IM9 and L363 multiple myeloma cell lines |
scFv-CD28TM+IC-CD3ζS | LV⁎⁎ RV LV |
NK92 cell line Primary NK cells from PBMCs NK92 and NKL cell lines |
Han J et al. [50] Anna Kruschinski et al. [49] Chu J et al. [51] |
| EGFRvIII |
U87-MGEGFRvIII | scFv-DAP12TM+IC | LV | YTS NK cell line | Nadja Müller et al.[119] |
| CD5 | T-cell malignant cells | scFv-CD8TM-2B4IC-CD3ζS | LV | NK92 cell line |
Yingxi Xu et al. [52] |
| CD19, GD2 | CD19+ leukaemia or GD2+ neuroblastoma cell lines | scFv-2B4TM+IC-CD3ζS | RV | PBMCs | Bianca Altvater et al. [59] |
| CD123 | CD123+ AML cell line KG1a and primary AML blasts | scFv-CD28TM+IC-4-1BBIC-CD3ζS | RV | Primary NK cells from PBMCs | Stephan Kloss et al.[60] |
| Mesothelin | K562meso and human ovarian cancer A1847 cells | T-CAR: scFv-CD28TM-CD28IC-4-1BBIC-CD3ζS CAR1: scFv-CD16TM-2B4IC-CD3ζS CAR2: scFv-NKp44TM-DAP10IC-CD3ζS CAR3: scFv-NKp46TM-2B4IC-CD3ζS CAR4: scFv-NKG2DTM-2B4IC-CD3ζS CAR5: scFv-NKG2DTM-4-1BBIC-CD3ζS CAR6: scFv-NKG2DTM-2B4IC-DAP12IC-CD3ζS CAR7: scFv-NKG2DTM-2B4IC-DAP10IC-CD3ζS CAR9: scFv-NKG2DTM-4-1BBIC-2B4IC-CD3ζS CAR10: scFv-NKG2DTM-CD3ζS |
Transposon transfection | human iPSC-derived NK cells or NK92 cells |
Ye Li et al. [42] |
RV: retrovirus
LV: lentivirus
3.3. CAR-gene transfer in NK cells
One of the major barriers for NK cell-based immunotherapy approaches has been the lack of an efficient gene transfer method in the primary NK cells. Many recent studies have shown successful transduction of expanded NK cells with retroviral vectors, with efficiency ranging from 27% to 52% after a single round of transduction. [55,56] A recent report showed that retroviral transduction of ex vivo expanded NK cells with genes coding for either secreted IL-15 or membrane bound IL-15 (mIL-15) resulted in a high transduction efficiency in the 70% range. [57] Due to this high-efficiency of gene transfer in NK92 and activated primary NK cells, retrovirus has been extensively used to generate CAR-NK cells in recent preclinical and clinical studies. [11,49,[58], [59], [60] However, the insertional mutagenesis and deleterious impact on the viability of primary NK cells related to the retroviral transduction are some of the major limitations of this approach in a clinical setting. [61]
Compared to retroviral vectors, lentivirus-based transduction represents a safer option because of a lower genotoxicity and insertional mutagenesis. [62] But the efficiency of lentiviral transduction in primary NK cells is low, often requiring multiple rounds of transduction. [61] Recently, Bari et al. reported that lentiviral vectors pseudotyped with a modified baboon envelop glycoprotein (BaEV-gp) exhibited 20-fold or higher transduction efficiency than VSV-G pseudotyped counterparts. [63] Using this transduction method, CD19-CAR was successfully expressed in an average of 70% of the primary human NK cells from different donors, and these CD19-CAR NK cells could efficiently and specifically kill CD19-psositive tumour cells. [63] In our unpublished study, BaEV-gp pseudotyped lentivirus encoding a tumour-specific CAR exhibited nearly 100% transduction efficiency in NK92 cells and 50~80% in activated primary NK cells or iPSC-derived NK cells. Therefore, BaEV-gp lentivirus may serve as a promising vehicle for CAR gene transfer in NK cells. Similarly, lentivirus pseudotyped with envelop protein of Gibbon ape leukaemia virus also efficiently transduce primary NK cells. [64]
Given the challenges with genetic transduction in primary NK cells, transfection methods such as electroporation and lipofection have also been used to deliver exogenous genes into NK cells. Compared to viral transduction, transfection of NK cells is associated with more rapid expression of the transgene with lower level of apoptosis, less variability among individuals, and higher gene transfer efficiency. [61] However, exogenous DNA is usually not integrated into the genome of the target cells, and thus expression of transgene is transient and declines about 3-5 days after transfection. [65] Combination of transfection methods with DNA integration techniques has been developed to generate stable transgene expressing cells. The DNA transposons are mobile DNA elements that can efficiently transpose between vectors and chromosomes via a “cut-and-paste” mechanism. The PiggyBac (PB) and the sleeping beauty (SB) are two most commonly used transposon systems to date, with the highest transposition activity in mammalian cells compared to other transposon systems. [66] PB and SB transposon systems consist of two components: the transposase mediating the “cut-and-paste” function and the DNA vector flanked by two terminal inverted repeats (TIRs). By transfecting CAR-containing plasmid in combination with transposase DNA into iPSCs, Li et al. generated CAR-iPSC-NK cells that stably expressed CAR molecules. [42] Compared with virus vectors, these transposon systems have several advantages including low immunogenicity, increased biosafety, decreased production costs, [67] and capacity to transduce large gene fragment >100 kb in length, [68] making them an attractive option for CAR insertion into NK cell genomes with long-lasting expression. Nevertheless, the applicability of the transposon system in transducing primary NK cells still need to be further modified to surmount the barriers like low transduction efficiency and cytopathic effects of plasmid DNA electroporation on NK cells. [69]
The gene-transduction approaches usually lead to random integration of DNA into the target cell genome, resulting in the potential risk for off-target effects, such as silencing of essential genes or tumour suppressor genes that may trigger cell apoptosis or malignant transformation. [61] CRISPR/Cas9 technique has been developed for targeted gene integration with high on-target specificity. [70] CRISPR/Cas9 system can induce permanent modifications at specific sites of the genome utilizing guide RNAs to direct the Cas9 nuclease to a target site, generate a double-stranded break and then insert a new gene through homology-directed recombination. [71] CRISPR/Cas9 system has already been used to generate allogeneic universal CAR-T cells by replacing the endogenous TCR locus with the CAR construct in T cells. [72] In NK cells, CRISPR/Cas9 system could be used to augment NK cell anti-tumour functions by targeted CAR insertion and stably knock-outing or integrating genes of interest involved in NK cell exhaustion, activation, tolerance, or memory. [73]
3.4. Expansion of CAR-NK cells in vitro
NK cells represent a minor fraction of peripheral blood leukocytes, and thus the generation of sufficient numbers of NK cells remains a major challenge for adoptive immunotherapy in patients. Several cytokines, such as IL-2, IL-15, IL-12, IL-21, and IL-18, have been used to expand primary NK cells in vitro. [74], [75], [76], [77] Nevertheless, NK proliferation in vitro induced by these cytokine is generally limited. [25] Co-culturing with stimulatory cells, such as a Wilms tumour-derived cell line, [78] autologous PBMCs, [79,80] Epstein-Barr virus-transformed lymphoblastoid cells, [81,82] and chronic myelogenous leukaemia-derived cell line K562 cells, [83] can induce rapid and sustained proliferation in NK cells. K562 cells transduced with mIL-15 or 4-1BB ligand (4-1BBL) achieved greater proliferation response than un-transduced K562 cells. [84] Furthermore, K562 cells co-expressing both mIL-15 and 4-1BBL stimulated more efficient NK cell expansion, which has been adapted to produce large numbers of clinical-grade NK cells. [84], [85], [86] However, primary NK cells activated by these feeder cells can eventually became unresponsive to stimulation and undergo senescence after 8-15 weeks of continuous proliferation. [87] Reportedly, K562 cells co-expressing CD64, CD86, 4-1BBL, truncated CD19, and membrane bound IL-21 (mIL-21) can promote log-phase NK cells expansion without evidence of senescence for up to 6 weeks compared to K562 cells with the same four core transgenes and mIL-15. [88] The method has also been adapted to large-scale GMP and recently successfully used to generate large numbers of NK cells or CAR-NK cells for phase 1 and 2 clinical trials. [11,89] K562 feeder cells have to be lethally irradiated before co-culture with NK cells to ensure that no proliferating and viable feeder cells are detectable in the final product. [90]
NK cells expressing mIL-15 showed superior proliferation and survival and had higher cytotoxicity against tumour cells in vitro and in vivo, compared to mock-transduced NK cells. [91] The cis stimulus provided by mIL-15 was even slightly superior to the trans stimulus of an identical mIL-15 expressed on K562 feeder cells. [91] Because of the autocrine signalling, expression of mIL-15 by NK cells could allow NK cell therapy without the potential adverse effects mediated by exogenous cytokine administration. [91] Consistently, in our ongoing study, CAR-NK cells co-expressing mIL-15 had higher expansion (≥4 folds) without the feeder cells than CAR-NK cells without mIL-15 expression. Additionally, we have reported that a brief pre-activation (12–16 h) with cytokine combinations of IL-12, IL-15, and IL-18 induced memory-like NK cells which exhibited enhanced response to cytokine or activating receptor re-stimulation for weeks to months after pre-activation. [92,93] Data from the Phase I clinical trial demonstrated that the cytokine-induced memory-like NK cells exhibited enhanced expansion and robust anti-leukaemia responses in AML patients after adoptive transfer, [92] suggesting that introduction of CAR into these memory-like NK cells could be a promising approach for NK cell-mediated cancer therapy. A recent study has provided the first proof-of-concept for this approach in mice. [94]
4. Clinical applications of CAR-NK cells
Despite the safety and promising clinical efficacy of unmodified allogenic NK cells particularly for AML [92] and the potential to address some of the key challenges associated with CAR-T cells, the CAR-NK cell field has made only limited progress in the clinic so far. Only two trials (NCT01974479, NCT00995137) started before 2016 although many more are ongoing currently. As of June 2020, there are nineteen studies registered in the clinicaltrials.gov evaluating the safety and efficacy of CAR-NK cells in cancer patients (summarised in Table 2), including twelve Phase I/II trials under actively patient recruitment and two completed Phase I/II trials. In most of these trials (11/19), the CAR-NK cells target lineage markers such as CD19, CD22, BCMA, CD33, or CD7 on hematopoietic malignancies. Some of the CAR-NK cells target metastatic solid tumours expressing tumour-associated antigens like HER2, PSMA, mesothelin, ROBO1, or MUC1. An NKG2DL-targeted CAR is being evaluated for solid tumours (NCT03415000) based on the observation that several stress molecules recognized by NKG2D are commonly upregulated in metastatic solid tumours.
Table 2.
CAR-NK cell clinical trials.
| No. | NCT | Start Year | Stage | Tumors | Target | NK source | sponsor locations | CAR structure | Gene transfer |
|---|---|---|---|---|---|---|---|---|---|
| Trials completed | |||||||||
| 1 | NCT00995137 | 2009 | I | B-ALL | CD19 | PB-NK | St. Jude Children's Research Hospital, US | ScFv-CD8αTM-CD137-CD3ζ | mRNA electroporation |
| 2 | NCT02944162 | 2016 | I/II | AML | CD33 | NK92 | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | ScFv-CD28-CD137-CD3ζ | LV* |
| Trials actively recruiting | |||||||||
| 1 | NCT01974479 | 2013 | II | B-ALL | CD19 | PB-NK | National University Health System, Singapore | ScFv-CD8αTM-CD137-CD3ζ | mRNA electroporation |
| 2 | NCT02742727 | 2016 | I/II | Lymphoma, leukaemia | CD7 | NK92 | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | ScFv-CD28-CD137-CD3ζ | electroporation |
| 3 | NCT02839954 | 2016 | I/II | Solid tumour | MUCI | NK92 | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | ScFv-CD28-CD137-CD3ζ | LV |
| 4 | NCT02892695 | 2016 | I/II | Lymphoma, leukaemia | CD19 | NK92 | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | ScFv-CD28-CD137-CD3ζ | LV |
| 5 | NCT03056339 | 2017 | I/II | B-lymphoma | CD19 | UCB-NK | MD Anderson, US | iCasp9-ScFv-CD28-CD3ζ-IL-15 | RV⁎⁎ |
| 6 | NCT03383978 | 2017 | I | GBM | HER2 | NK92 | Johann Wolfgang Goethe University Hospital, Germany | ScFv-CD28-CD3ζ | LV |
| 7 | NCT03415100 | 2018 | I | Metastatic solid tumour | NKG2DL | PB-NK | The Third Affiliated Hospital of Guangzhou Medical University, China | ScFv-CD8αTM-CD3ζ; ScFv-CD8αTM-DAP12 | mRNA electroporation |
| 8 | NCT03656705 | 2018 | I | NSCLC | NR | NK92 | Xinxiang medical university, China | NR | RV/LV |
| 9 | NCT03940833 | 2019 | I/II | R/R multiple myeloma | BCMA | NK92 | Asclepius Technology Company Group (Suzhou) Co., Ltd., China | NR | LV |
| 10 | NCT03941457 | 2019 | I/II | Pancreatic Cancer | ROBO1 | NK92 | Asclepius Technology Company Group (Suzhou) Co., Ltd., China | NR | LV |
| 11 | NCT03940820 | 2019 | I/II | Solid tumour | ROBO1 | NK92 | Asclepius Technology Company Group (Suzhou) Co., Ltd., China | NR | LV |
| 12 | NCT04245722 | 2020 | I | B-cell lymphoma, CLL | CD19 | iPSC (FT596) | Fate Therapeutics, San Diego, USA | scFv-NKG2D-2B4-CD3ζ-IL-15/R-hnCD16 | LV |
| Trials not yet recruiting | |||||||||
| 1 | NCT03692767 | Early I | Refractory B-cell lymphoma | CD22 | Unknown | Allife Medical Science and Technology, Beijing, China | |||
| 2 | NCT03690310 | Early I | Refractory B-cell lymphoma | CD19 | Unknown | Allife Medical Science and Technology, Beijing, China | |||
| 3 | NCT03692637 | Early I | Epithelial ovarian cancer | Mesothelin | PB-NK | Allife Medical Science and Technology, Beijing, China | |||
| 4 | NCT03692663 | Early I | Castration-resistant prostate Cancer | PSMA | Unknown | Allife Medical Science and Technology, Beijing, China | |||
| 5 | NCT03824964 | Early I | Refractory B-cell lymphoma | CD19/CD22 | Unknown | Beijing Cancer Hospital, Beijing, China | |||
LV: lentivirus
RV: retrovirus
The clinical results from most of these trials are currently pending, with only three studies published and two of which are very small-scale: NCT02944162 utilized NK92 targeting CD33-expressing AML, [95] while NCT03415100 used primary NK cells targeting NKG2DL in colorectal cancer. [96] Regardless of the small cohort number (n = 3), there are several aspects that merit discussion. First, the NK92 cell line has several advantages as an NK cell source for CAR-NK cells as it is fully characterised, is expandable with maintained cytotoxicity, and is relatively easy to transduce. The clinical safety test by Tang et al [95] along with preclinical studies demonstrating the efficacy provide a solid rationale for further investigating CAR-NK92 therapy and there are at least nine ongoing clinical studies to date. Second, primary NK cells are a preferred cell source with predictable safety, while the study of Xiao et al provided a first glimpse of promising clinical evidence of primary NK cell therapy with transient CAR expression to target metastatic solid tumours. [96] To date there are eight clinical CAR-NK studies focusing on solid malignancies such as brain, prostate, ovarian, pancreatic and lung cancer. Lastly, broad utilization of primary NK cells in CAR-NK products has been largely hindered by their resistance to transduction for stable gene editing and limited capability to expand and persist. At least three out of four studies with primary NK cells used mRNA electroporation to mediate transient CAR expression.
The first large-scale CAR-NK cell trial (NCT03056339) was recently published and is still actively recruiting patients at MD Anderson Cancer Centre. [11] Eleven patients with high risk CD19+ B cell malignancies received an allogeneic UCB-derived CAR-NK product after a standard lymphodepleting treatment. A conventional anti-CD19-CD28-CD3ζ CAR was used for the transduction and the retroviral vector also included an IL-15 gene and a suicidal switch. The manufacturing time of the CAR product was relatively short, within two weeks after each patient's enrolment. Seven out of eleven patients achieved a complete remission, exhibiting a significantly higher early expansion of CAR-NK cells compared to the non-responders. Strikingly, this high response rate was achieved in the absence of serious side effect, such as CRS, neurotoxicity and GVHD - even in KIR-ligand mismatch cases (5/11), shedding light on generating a truly off-the-shelf product by eliminating the need for HLA-matching. The published observation of circulating CAR-NK cells by flow cytometry was limited to the first three weeks, and 5/7 responding patients went on to receive additional treatments because of evidence of progressing disease, [11] though further studies on a larger cohort are required to conclusively address the durability of the CAR-NK cell responses. Overall, this study demonstrates feasibility, initial efficacy and very attractive safety profile of the cord blood CAR-NK cell immunotherapy.
Instead of umbilical cord blood, iPSC is used for an off-the-shelf CAR-NK product (named FT596) to treat CD19-expressing B cell malignancies in a recent Phase I trial sponsored by Fate Therapeutics (NCT04245722). Compared to NCT03056339, where retroviral vector and secreted IL-15 was included in the CAR construct to support in vivo cell expansion, [11,58] FT596 is generated via lentiviral gene transfer and the construct includes a membrane-tethered IL-15/15R complex for autocrine IL-15 signalling to support CAR-NK cell persistence in vivo. Furthermore, FT596 has an NK cell-optimized CAR construct design which includes an NKG2D transmembrane domain and a 2B4 costimulatory domain for superior activation and function. [42] This trial which only started very recently and pending its clinical results, may demonstrate the feasibility and efficacy of iPSC-derived CAR-NK cells as a promising new cancer immunotherapy. Besides safety and efficacy, the viability and potency of the CAR product after freeze/thaw cycles still need to be clinically tested.
5. CAR-NK cell therapy in the near future
The results from the first large-scale trial (NCT03056339) of CAR-NK cells have shown safety and promising clinical activity in patients with CD19+ CLL and B cell lymphoma. [11] As mentioned above, CAR-NK cells have multiple advantages as cancer immunotherapy compared to CAR-T cells. However, like CAR-T cells, CAR-NK cell therapy still has to struggle with several inevitable difficulties, such as loss of targeted antigen, tumour heterogeneity and hostile TME. Therefore, some strategies should be considered to maximise the efficacy of CAR-based NK cell therapy in the future.
5.1. Target antigens for CAR-NK cells
Unlike CAR-T cells, CAR-NK cells can mediate cytotoxic activity against cancer cells in both CAR-dependent and CAR-independent manner. Therefore, a CAR can be developed to induce a moderate activating signal, for example, a CAR without co-stimulatory domain, and thus anti-tumour response of CAR-NK cells could be mediated preferentially by natural killing mechanism of NK cells and weakly by CAR-mediated direct cytotoxicity. NK cells could even be engineered to express a non-signalling CAR that does not induce a direct killing signal but promotes NK cell homing and adhesion to targets, allowing cytotoxicity triggered by natural killing mechanisms of the NK cells, with no or minimal on-target toxic effects to the normal cells expressing the same antigen. In this scenario, a wider range of antigens can be used as CAR-NK cell targets, including antigens like HER2, EGFR, and mesothelin that are preferentially expressed by tumour cells but also maintain a low level in some healthy cells.
5.2. “Armored” CAR-NK cells with multiple functions
Using advanced genetic-engineering technology, NK cells can be engineered to co-express other molecules with CARs, which could include cytokines, antibodies, protease, etc., that are able to promote NK cell proliferation, trafficking and penetration into tumour. Additionally, some molecules can potentially reprogram TME, switching immunosuppressive TME to immuno-activating one, which facilitates infiltration of other immune cells and thus facilitate anti-tumour immune responses independent of the CAR-mediated cytotoxicity. Importantly, CAR-NK cells expressing multiple exogenous genes, known as “armored” CAR-NK cells or “NK cell pharmacies” have a potential of providing a unique and safer approach for modulating the local TME with less or no systemic adverse effects. [15]
5.3. Combination of CAR-NK therapy with other therapeutic approaches
Lymphodepleting chemotherapy is regularly administered before infusion of allogeneic NK cells to delay NK cell rejection by the recipient's immune system. Fludarabine and cyclophosphamide, which can preferentially eliminate lymphoid cells, are two most commonly used drugs in the lymphodepleting regimen. [34] In addition to reducing NK cell rejection by the recipient, lymphodepleting chemotherapy might reprogram an immunosuppressive host TME by depleting myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), and thus create a cytokine milieu that favours NK cell expansion after infusion. [32,34,97] Additionally, intensive chemotherapy before NK cell infusion can be used to greatly reduce tumour burden, and thus increase effector-to-target ratio after NK cell infusion. [32] Therefore, chemotherapy remains an important adjuvant therapeutic approach for CAR-NK cell therapy in the future.
Radiation therapy has been used as a predominant treatment modality for most types of cancers in the curative or palliative setting. Emerging evidence supports the idea that radiation therapy, especially stereotactic body radiotherapy (SBRT), can synergize with immunotherapy, such as anti-PD1 or anti-CTLA4 antibody. [98,99] Proposed mechanisms from preclinical studies involve an increased availability of tumour antigens, the release of immunostimulatory cytokines and danger signals, and the destruction of the tumour-supporting stroma—which potentiates the recruitment and activation of antigen-presenting cells (APCs) and subsequently stimulates a tumour-specific immune response, leading to tumour regression in distant non-irradiated lesions (a phenomenon known as ‘abscopal effect’). [100] Additionally, DNA damage by radiotherapy is able to induce the expression of NKG2D ligands on tumour cells, [101,102] which may promote activation of NK cells and enhance their cytotoxicity against tumour cells. Therefore, the combination of CAR-NK cell therapy with local radiotherapy would be an alternative treatment strategy especially for solid tumours.
Immune checkpoint blockade (ICB) by corresponding antibodies (e.g., anti-PD1/PDL1 or anti-CTLA4 antibody) has demonstrated impressive clinical outcomes in many different types of tumours including haematological malignancies and solid tumours. Blockade of PD-1 axis has been demonstrated to significantly enhance the anti-tumour activity of CAR-T cells. [103], [104], [105] However, systemic administration of ICB agents can increase immune-related toxicities. [106] Recent reports have shown that local secretion of anti-PD1 antibodies [107] or scFv [108] at the tumour site by engineered CAR-T cells achieved superior anti-tumour activity and reduced adverse effect compared with systemic therapy. Additionally, CRISPR/Cas9-mediated PD-1 deletion in CD19 CAR-T cells showed enhanced anti-tumour efficacy against CD19+ tumour cells. [109] Like T cells, activated NK cells also expressed some T cell immune checkpoint molecules (e.g., PD-1, CTLA-4, LAG3, and TIM3) that might inhibit NK anti-tumour responses, [110] and their blockade with checkpoint inhibitors enhances NK cell activity. [111], [112], [113] Therefore, using CAR-NK cells in combination with systemic ICB therapy or co-expressing ICB molecules, or with knockout of checkpoint molecule by gene-editing would be another promising approach for CAR-NK cell therapy, especially for patients with solid tumours.
The combination with antibodies targeting different tumour-associated antigens is another strategy to improve the efficacy of CAR-NK cell therapy. NK cells express CD16 and exert ADCC-mediated tumour killing. [24] Promising results have been obtained in patients with neuroblastoma or refractory NHL receiving allogeneic NK cells following administration of anti-GD2 or anti-CD20 antibody. [114], [115], [116] However, cytokine activation or target cell stimulation usually lead to marked decreases in CD16 expression on NK cells, [117] which impairs ADCC-mediated tumour killing. A possible approach is to genetically engineer NK cells to stably express a non-cleavable CD16. An iPSC-derived CAR-NK cell product, FT596, is engineered with three active anti-tumour components including a CD19-CAR, a novel high-affinity and non-cleavable CD16, and an IL-15 receptor fusion protein. Preclinical data showed that FT596 not only induced durable tumour regression and extended survival in humanized mice with CD19+ lymphoma, but also exhibited enhanced killing of the CD20+ lymphoma cells in vivo when combined with the anti-CD20 antibody rituximab, compared to rituximab alone. [118] These data suggest that FT596 possesses multi-antigen targeting function when administered with therapeutic antibodies, and thus effectively overcome tumour resistance due to loss of CD19 antigen. These promising preclinical results make FT596 being investigated in an open-label Phase I clinical trial (NCT04245722).
Additionally, sequential therapy of CAR-NK and CAR-T would be an effective and safe strategy to synergistically and persistently eliminate tumour, especially for patients with high tumour burden. “Off-the-shelf” products of CAR-NK cells can be used immediately to reduce tumour burden before CAR-T cell product (usually requiring 2–4 weeks to produce) can be infused into a patient. A portion of CAR-T cells may survive as memory T cells that retain persistent anti-tumour functionality. Hence, sequential infusion of CAR-NK and CAR-T cells could promote rapid and durable tumour elimination. Additionally, reduced tumour burden resulted from prior CAR-NK cell therapy could potentially lower the risk of CRS and neurotoxicity of subsequent CAR-T cell therapy due to less cytokine release.
6. Conclusion
CAR-NK cells have a great promise as a novel cellular immunotherapy platform against cancer and with a potential for generating “off-the-shelf” products that could be readily available and safe for clinical use. Recent advances in gene manipulation techniques have allowed for the creation of novel CAR-NK cell products with potent anti-tumour activity but mostly non-toxic to normal tissues after infusion into the patients. Multiple strategies, including CRISPR based genetic modifications and inclusion of novel genes to modulate TME in the CAR constructs are some of the key innovations helping to significantly advance the field. With the increasing safety and promising activity in preclinical studies and clinical trials, combined with advanced efforts addressing the remaining challenges, it is expected that CAR-NK cell therapy will continue to evolve and lead to major improvement in the survival of cancer patients with otherwise very limited treatment options.
7. Outstanding questions
To develop CAR-NK cells as safe, potent and “off-the-shelf” cellular immunotherapy for cancer, many aspects of CAR-NK cells and manufacturing processes must be optimized, including:
-
1.
Improving CAR design for optimal NK cell activation and cytotoxicity.
-
2.
Augmenting CAR-NK cell infiltration into solid tumours.
-
3.
Reprograming CAR-NK cells to overcome tumour suppression and escape.
-
4.
Reprograming CAR-NK cells with memory property in vivo for long-term surveillance of tumour.
-
5.
Optimizing the CAR-NK cell's unspecific natural killing function combined with CAR-derived specific killing.
-
6.
Simplifying and optimizing CAR-NK cells manufacturing process.
-
7.
Efficient storage and recovery of “off-the-shelf” CAR-NK cells.
Search strategy and selection criteria
Data for this review were identified by searches of MEDLINE, PubMed, and references from relevant articles using the search terms “NK cell”, “Chimeric antigen receptor”, “Immunotherapy”, and “Cancer”. Only articles published in English between 1975 and 2020 were included.
Originality of figures
The authors confirm originality of Fig. 1 and 2. These figures have not been published previously.
Author contributions
J.C. and R.R. conceived this review, organized and critically revised the manuscript. G.X. did major work of writing the manuscript. H.D., Y.L., and J.D.H made contributions to writing the manuscript. G.X., H.D., Y.L., J.D.H., R.R., and J.C. gave final approval of the version to be published.
Declaration of Competing interest
All of authors declare no competing interests.
Acknowledgements
This work was supported in part by National Institutes of Health Grants CA197605 and NS104315, the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute, the Koch Institute Frontier Research program through the Michael (1957) and Inara Erdei Fund and the Kathy and Curt Mable Cancer Research Fund, Claudia Adams Barr program from Dana-Farber Cancer Institute, and a Helen Gurley Brown Presidential Initiative Award from Dana-Farber Cancer Institute.
Contributor Information
Romee Rizwan, Email: rizwan_romee@dfci.harvard.edu.
Jianzhu Chen, Email: jchen@mit.edu.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumours. II. characterization of effector cells. Int J Cancer. 1975;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 3.Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukaemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivier E, Raulet DH, Moretta A. Innate or adaptive immunity? the example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JS. At the bedside: adoptive cell therapy for melanoma-clinical development. J Leukoc Biol. 2014;95(6):875–882. doi: 10.1189/jlb.0513293. [DOI] [PubMed] [Google Scholar]
- 9.Rapoport AP, Stadtmauer EA, Binder-Scholl GK. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumour effects in myeloma. Nat Med. 2015;21(8):914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern LA, Jonsson VD, Priceman SJ. CAR T Cell Therapy Progress and Challenges for Solid Tumours. Cancer Treat Res. 2020;180:297–326. doi: 10.1007/978-3-030-38862-1_11. [DOI] [PubMed] [Google Scholar]
- 11.Liu E, Marin D, Banerjee P. 2020. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozenbaum M, Meir A, Aharony Y. Gamma-Delta CAR-T Cells Show CAR-Directed and Independent Activity Against Leukaemia. Front Immunol. 2020;11:1347. doi: 10.3389/fimmu.2020.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Huang W, Heczey A. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin Cancer Res. 2019;25(23):7126–7138. doi: 10.1158/1078-0432.CCR-19-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klichinsky M, Ruella M, Shestova O. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020 doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey SR, Maus MV. Gene editing for immune cell therapies. Nat Biotechnol. 2019;37(12):1425–1434. doi: 10.1038/s41587-019-0137-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Wallace DL, de Lara CM. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupo KB, Matosevic S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. cancers (basel) 2019;11(6) doi: 10.3390/cancers11060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou CK, Turtle CJ. Insight into mechanisms associated with cytokine release syndrome and neurotoxicity after CD19 CAR-T cell immunotherapy. Bone Marrow Transp. 2019;54(Suppl 2):780–784. doi: 10.1038/s41409-019-0602-5. [DOI] [PubMed] [Google Scholar]
- 19.Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019 doi: 10.1093/jnci/djz017. [DOI] [PubMed] [Google Scholar]
- 21.Oei V, Siernicka M, Graczyk-Jarzynka A. Intrinsic Functional Potential of NK-Cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol Res. 2018;6(4):467–480. doi: 10.1158/2326-6066.CIR-17-0207. [DOI] [PubMed] [Google Scholar]
- 22.Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol. 2015;12(3):292–302. doi: 10.1038/cmi.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin. 2015;36(10):1191–1199. doi: 10.1038/aps.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Mishra HK, Walcheck B. Role of ADAM17 as a regulatory checkpoint of CD16A in NK cells and as a potential target for cancer immunotherapy. J Leukoc Biol. 2019;105(6):1297–1303. doi: 10.1002/JLB.2MR1218-501R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 26.Ueda T, Kumagai A, Iriguchi S. Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 2020 doi: 10.1111/cas.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggeri L, Capanni M, Urbani E. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 28.Miller JS, Soignier Y, Panoskaltsis-Mortari A. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 29.Rubnitz JE, Inaba H, Ribeiro RC. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukaemia. J Clin Oncol. 2010;28(6):955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8(4):652–658. [PubMed] [Google Scholar]
- 31.Schönfeld K, Sahm C, Zhang C. Selective inhibition of tumour growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23(2):330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 33.Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JS, Soignier Y, Panoskaltsis-Mortari A. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 35.Curti A, Ruggeri L, Parisi S. Larger size of donor Alloreactive NK Cell Repertoire correlates with better response to nk cell immunotherapy in elderly acute Myeloid Leukaemia patients. Clin Cancer Res. 2016;22(8):1914–1921. doi: 10.1158/1078-0432.CCR-15-1604. [DOI] [PubMed] [Google Scholar]
- 36.Pfefferle A, Huntington ND. You Have got a fast CAR: Chimeric Antigen Receptor NK srapy. Cancers (Basel) 2020;12(3) doi: 10.3390/cancers12030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical Cord Blood Natural Killer Cells, Their Characteristics, and Potential Clinical Applications. Front Immunol. 2017;8:329. doi: 10.3389/fimmu.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54. doi: 10.1016/j.smim.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Spanholtz J, Preijers F, Tordoir M. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. Plos One. 2011;6(6):e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cany J, van der Waart AB, Spanholtz J. Combined IL-15 and IL-12 drives the generation of CD34(+)-derived natural killer cells with superior maturation and alloreactivity potential following adoptive transfer. Oncoimmunology. 2015;4(7) doi: 10.1080/2162402X.2015.1017701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valamehr B, Robinson M, Abujarour R. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Rep. 2014;2(3):366–381. doi: 10.1016/j.stemcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumour Activity. Cell Stem Cell. 2018;23(2):181–192. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knorr DA, Ni Z, Hermanson D. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2(4):274–283. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermanson DL, Bendzick L, Pribyl L. Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. Stem Cells. 2016;34(1):93–101. doi: 10.1002/stem.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saetersmoen ML, Hammer Q, Valamehr B, Kaufman DS, Malmberg KJ. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin Immunopathol. 2019;41(1):59–68. doi: 10.1007/s00281-018-0721-x. [DOI] [PubMed] [Google Scholar]
- 46.Zheng L, Ren L, Kouhi A. A humanized Lym-1 CAR with novel DAP10/DAP12 signalling domains demonstrates reduced tonic signalling and increased anti-tumour activity in B Cell Lymphoma models. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-3417. [DOI] [PubMed] [Google Scholar]
- 47.Kotanides H, Sattler RM, Lebron MB. Characterization of 7A5: A Human CD137 (4-1BB) Receptor Binding Monoclonal Antibody with Differential Agonist Properties That Promotes Antitumor Immunity. Mol Cancer Ther. 2020;19(4):988–998. doi: 10.1158/1535-7163.MCT-19-0893. [DOI] [PubMed] [Google Scholar]
- 48.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kruschinski A, Moosmann A, Poschke I. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A. 2008;105(45):17481–17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J, Chu J, Keung CW. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu J, Deng Y, Benson DM. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Liu Q, Zhong M. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol. 2019;12(1):49. doi: 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao L, Cen D, Gan H. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther. 2019;27(6):1114–1125. doi: 10.1016/j.ymthe.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Töpfer K, Cartellieri M, Michen S. DAP12-based activating chimeric antigen receptor for NK cell tumour immunotherapy. J Immunol. 2015;194(7):3201–3212. doi: 10.4049/jimmunol.1400330. [DOI] [PubMed] [Google Scholar]
- 55.Guven H, Konstantinidis KV, Alici E. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp Hematol. 2005;33(11):1320–1328. doi: 10.1016/j.exphem.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Streltsova MA, Barsov E, Erokhina SA, Kovalenko EI. Retroviral gene transfer into primary human NK cells activated by IL-2 and K562 feeder cells expressing membrane-bound IL-21. J Immunol Methods. 2017;450:90–94. doi: 10.1016/j.jim.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Imamura M, Shook D, Kamiya T. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124(7):1081–1088. doi: 10.1182/blood-2014-02-556837. [DOI] [PubMed] [Google Scholar]
- 58.Liu E, Tong Y, Dotti G. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altvater B, Landmeier S, Pscherer S. 2B4 (CD244) signalling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukaemia and neuroblastoma cells. Clin Cancer Res. 2009;15(15):4857–4866. doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kloss S, Oberschmidt O, Morgan M. Optimization of Human NK Cell Manufacturing: fully automated separation, improved ex vivo expansion using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells. Hum Gene Ther. 2017;28(10):897–913. doi: 10.1089/hum.2017.157. [DOI] [PubMed] [Google Scholar]
- 61.Carlsten M, Childs RW. Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications. Front Immunol. 2015;6:266. doi: 10.3389/fimmu.2015.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papayannakos C, Daniel R. Understanding lentiviral vector chromatin targeting: working to reduce insertional mutagenic potential for gene therapy. Gene Ther. 2013;20(6):581–588. doi: 10.1038/gt.2012.88. [DOI] [PubMed] [Google Scholar]
- 63.Bari R, Granzin M, Tsang KS. A Distinct Subset of Highly Proliferative and Lentiviral Vector (LV)-Transducible NK Cells Define a Readily Engineered Subset for Adoptive Cellular Therapy. Front Immunol. 2019;10:2001. doi: 10.3389/fimmu.2019.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomás HA, Mestre DA, Rodrigues AF, Guerreiro MR, Carrondo M, Coroadinha AS. Improved GaLV-TR Glycoproteins to Pseudotype Lentiviral Vectors: Impact of Viral Protease Activity in the Production of LV Pseudotypes. Mol Ther Methods Clin Dev. 2019;15 doi: 10.1016/j.omtm.2019.08.001. 1–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Floch V, Audrezet MP, Guillaume C. Transgene expression kinetics after transfection with cationic phosphonolipids in hematopoietic non adherent cells. Biochim Biophys Acta. 1998;1371(1):53–70. doi: 10.1016/s0005-2736(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 66.Kim A, Pyykko I. Size matters: versatile use of PiggyBac transposons as a genetic manipulation tool. Mol Cell Biochem. 2011;354(1-2):301–309. doi: 10.1007/s11010-011-0832-3. [DOI] [PubMed] [Google Scholar]
- 67.Vargas JE, Chicaybam L, Stein RT, Tanuri A, Delgado-Canedo A, Bonamino MH. Retroviral vectors and transposons for stable gene therapy: advances, current challenges and perspectives. J Transl Med. 2016;14(1):288. doi: 10.1186/s12967-016-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rostovskaya M, Fu J, Obst M. Transposon-mediated BAC transgenesis in human ES cells. Nucleic Acids Res. 2012;40(19):e150. doi: 10.1093/nar/gks643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monjezi R, Miskey C, Gogishvili T. Enhanced CAR T-cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia. 2017;31(1):186–194. doi: 10.1038/leu.2016.180. [DOI] [PubMed] [Google Scholar]
- 70.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32(4):347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Georgiadis C, Preece R, Nickolay L. Long Terminal Repeat CRISPR-CAR-Coupled "Universal" T Cells Mediate Potent Anti-leukaemic Effects. Mol Ther. 2018;26(5):1215–1227. doi: 10.1016/j.ymthe.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang RS, Shih HA, Lai MC, Chang YJ, Lin S. Enhanced NK-92 Cytotoxicity by CRISPR Genome Engineering Using Cas9 Ribonucleoproteins. Front Immunol. 2020;11:1008. doi: 10.3389/fimmu.2020.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naume B, Gately M, Espevik T. A comparative study of IL-12 (cytotoxic lymphocyte maturation factor)-, IL-2-, and IL-7-induced effects on immunomagnetically purified CD56+ NK cells. J Immunol. 1992;148(8):2429–2436. [PubMed] [Google Scholar]
- 76.Carson WE, Fehniger TA, Haldar S. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99(5):937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagner J, Pfannenstiel V, Waldmann A. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front Immunol. 2017;8:676. doi: 10.3389/fimmu.2017.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harada H, Watanabe S, Saijo K, Ishiwata I, Ohno T. A Wilms tumor cell line, HFWT, can greatly stimulate proliferation of CD56+ human natural killer cells and their novel precursors in blood mononuclear cells. Exp Hematol. 2004;32(7):614–621. doi: 10.1016/j.exphem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 79.Kloss S, Oberschmidt O, Morgan M. Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells. Hum Gene Ther. 2017;28(10):897–913. doi: 10.1089/hum.2017.157. [DOI] [PubMed] [Google Scholar]
- 80.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumour regression. Clin Cancer Res. 2011;17(19):6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berg M, Lundqvist A, McCoy PJ. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumour cells. Cytotherapy. 2009;11(3):341–355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Granzin M, Stojanovic A, Miller M, Childs R, Huppert V, Cerwenka A. Highly efficient IL-21 and feeder cell-driven ex vivo expansion of human NK cells with therapeutic activity in a xenograft mouse model of melanoma. Oncoimmunology. 2016;5(9) doi: 10.1080/2162402X.2016.1219007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phillips JH, Lanier LL. A model for the differentiation of human natural killer cells. Studies on the in vitro activation of Leu-11+ granular lymphocytes with a natural killer-sensitive tumour cell, K562. J Exp Med. 1985;161(6):1464–1482. doi: 10.1084/jem.161.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujisaki H, Kakuda H, Shimasaki N. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lapteva N, Durett AG, Sun J. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14(9):1131–1143. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujisaki H, Kakuda H, Imai C, Mullighan CG, Campana D. Replicative potential of human natural killer cells. Br J Haematol. 2009;145(5):606–613. doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Denman CJ, Senyukov VV, Somanchi SS. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. Plos One. 2012;7(1):e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ciurea SO, Schafer JR, Bassett R. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130(16):1857–1868. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimasaki N, Coustan-Smith E, Kamiya T, Campana D. Expanded and armed natural killer cells for cancer treatment. Cytotherapy. 2016;18(11):1422–1434. doi: 10.1016/j.jcyt.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 91.Imamura M, Shook D, Kamiya T. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124(7):1081–1088. doi: 10.1182/blood-2014-02-556837. [DOI] [PubMed] [Google Scholar]
- 92.Romee R, Rosario M, Berrien-Elliott MM. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukaemia. Sci Transl Med. 2016;8(357):123r–357r. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romee R, Schneider SE, Leong JW. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gang M, Marin ND, Wong P. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood. 2020 doi: 10.1182/blood.2020006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang X, Yang L, Li Z. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukaemia. Am J Cancer Res. 2018;8(6):1083–1089. [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao L, Cen D, Gan H. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther. 2019;27(6):1114–1125. doi: 10.1016/j.ymthe.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Theelen W, Peulen H, Lalezari F. Effect of Pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumour response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT Phase 2 randomized clinical trial. Jama Oncol. 2019 doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie G, Gu D, Zhang L, Chen S, Wu D. A rapid and systemic complete response to stereotactic body radiation therapy and pembrolizumab in a patient with metastatic renal cell carcinoma. Cancer Biol Ther20171–05. [DOI] [PMC free article] [PubMed]
- 100.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev. 2006;214:130–142. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 103.Cherkassky L, Morello A, Villena-Vargas J. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumour-mediated inhibition. J Clin Invest. 2016;126(8):3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2(10):e26286. doi: 10.4161/onci.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chong EA, Melenhorst JJ, Lacey SF. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refuelling the CAR. Blood. 2017;129(8):1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramos-Casals M, Brahmer JR, Callahan MK. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suarez ER, Chang DK, Sun J. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7(23):34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rafiq S, Yeku OO, Jackson HJ. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumour efficacy in vivo. Nat Biotechnol. 2018;36(9):847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rupp LJ, Schumann K, Roybal KT. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumour efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lanuza PM, Pesini C, Arias MA, Calvo C, Ramirez-Labrada A, Pardo J. Recalling the biological significance of immune checkpoints on NK Cells: a chance to overcome LAG3, PD1, and CTLA4 inhibitory pathways by adoptive NK Cell transfer? Front Immunol. 2019;10:3010. doi: 10.3389/fimmu.2019.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsu J, Hodgins JJ, Marathe M. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128(10):4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lanuza PM, Pesini C, Arias MA, Calvo C, Ramirez-Labrada A, Pardo J. Recalling the Biological Significance of Immune Checkpoints on NK Cells: A Chance to Overcome LAG3, PD1, and CTLA4 Inhibitory Pathways by Adoptive NK Cell Transfer? Front Immunol. 2019;10:3010. doi: 10.3389/fimmu.2019.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oyer JL, Gitto SB, Altomare DA, Copik AJ. PD-L1 blockade enhances anti-tumour efficacy of NK cells. Oncoimmunology. 2018;7(11) doi: 10.1080/2162402X.2018.1509819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Federico SM, McCarville MB, Shulkin BL. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin Cancer Res. 2017;23(21):6441–6449. doi: 10.1158/1078-0432.CCR-17-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Modak S, Le Luduec JB, Cheung IY. Adoptive immunotherapy with haploidentical natural killer cells and Anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: Results of a phase I study. Oncoimmunology. 2018;7(8) doi: 10.1080/2162402X.2018.1461305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bachanova V, Sarhan D, DeFor TE. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother. 2018;67(3):483–494. doi: 10.1007/s00262-017-2100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romee R, Foley B, Lenvik T. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121(18):3599–3608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.FT596-Fate Therapeutics. https://fatetherapeutics.com/pipeline/immuno-oncology-candidates/ft596/. Accessed 26 Dec 2019.
- 119.Muller N, Michen S, Tietze S. Engineering NK Cells Modified with an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1alpha-secreting Glioblastoma. J Immunother. 2015;38(5):197–210. doi: 10.1097/CJI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]