Abstract
The cardiopulmonary nematodes Angiostrongylus vasorum, Crenosoma vulpis, Capillaria aerophila and Aelurostrongylus abstrusus, are a cause of concern in the scientific and veterinary community, potentially causing significant disease in domestic animals. To investigate the potential of wild carnivores as reservoir hosts to these parasites, a total of 1041 animals from seven regions of Denmark were sampled: 476 raccoon dogs (Nyctereutes procyonoides), 367 red foxes (Vulpes vulpes), 123 American mink (Neovison vison), 31 beech martens (Martes foina), 30 Eurasian otters (Lutra lutra) and 14 polecats (Mustela putorius). Hearts and lungs were collected and examined for cardiopulmonary parasites. Capillaria aerophila was identified using morphology, whereas A. vasorum and C. vulpis were identified by a duplex real-time PCR, and A. abstrusus by conventional PCR. This is the first Danish report of A. vasorum and C. vulpis infections in raccoon dogs, mink and polecats, and of C. aerophila in raccoon dogs and beech martens. In addition, this is the first time A. vasorum and C. vulpis have been identified in wild animals from the island of Bornholm, just as it is the first report of C. vulpis in American mink, and C. vulpis and A. vasorum in polecats in Europe. The prevalence of A. vasorum appears to have increased in red foxes in Denmark compared to previous studies, while C. vulpis and C. aerophila prevalences are lower. Our data show that several wild carnivores can serve as reservoir hosts for A. vasorum, C. vulpis and C. aerophila in Denmark, and that A. vasorum appears more abundant than previously reported. It is speculated that the A. vasorum increase might relate to increased snail abundance, which may be due to a rise in mean yearly temperatures in Denmark.
Keywords: Cardiopulmonary nematodes, Necropsy, Molecular analysis, Red foxes, Raccoon dogs, American mink, Eurasian otters, Beech martens
Graphical abstract
Highlights
-
•
The first report of C. vulpis in American mink.
-
•
The first report of C. vulpis and A. vasorum in polecats.
-
•
The first report of A. vasorum in raccoon dogs, mink, and polecats from Denmark.
-
•
The first report of C. vulpis in raccoon dogs, mink, and polecats from Denmark.
-
•
The first report of C. aerophila in raccoon dogs and beech martens from Denmark.
1. Introduction
The cardiopulmonary nematodes Angiostrongylus vasorum, Crenosoma vulpis, Capillaria aerophila (synonym Eucoleus aerophilus) and Aelurostrongylus abstrusus, have gained increased attention since recent introduction into previously non-endemic areas (Demiaszkiewicz et al., 2014; Hurníková et al., 2013; Jolly et al., 2015; Simin et al., 2014). Canine angiostrongylosis can be severe and even fatal (Conboy, 2009; Koch and Willesen, 2009), whereas C. vulpis and C. aerophila are less pathogenic, typically characterized by chronic cough (Burgess et al., 2008; Conboy, 2009; Reilly et al., 2000; Rinaldi et al., 2007), therefore updated knowledge of parasite distribution is of particular importance to veterinary clinicians.
Angiostrongylus vasorum and C. vulpis commonly infect red foxes (Vulpes vulpes) and domestic dogs (Canis familiaris), though infection of several wild canids and mustelids are possible (Anderson, 2000; Traversa et al., 2010). Capillaria aerophila are identified from red foxes, wild mustelids, domestic cats (Felis catus) and dogs (Otranto et al., 2015; Traversa and Cesare, 2014), and occasionally humans (Lalošević et al., 2013). Aelurostrongylus abstrusus commonly infect domestic cats (Giannelli et al., 2017), and feral felids (Conboy, 2009; Elsheikha et al., 2016; Penagos-Tabares et al., 2018). In Spain, Aelurostrongylus spp. has been documented in American mink (Neovison vison) (Martínez-Rondán et al., 2017), suggesting that mink can act as reservoir hosts. With few exceptions, in Denmark A. vasorum, C. vulpis and C. aerophila are only documented from red foxes (Koch and Willesen, 2009; Saeed et al., 2006; Willingham et al., 1996); a badger (Meles meles) infected with C. aerophila (Dietz et al., 1998), an Eurasian otter (Lutra lutra) infected with A. vasorum (Madsen et al., 1999), and feral cats infected with C. aerophila (Olsen et al., 2015). Aelurostrongylus abstrusus infections have been reported in domestic and feral cats from Denmark (Olsen et al., 2015).
Previous Danish studies on cardiopulmonary nematodes in wildlife are based solely on morphological identification (Al-Sabi et al., 2014; Saeed et al., 2006; Willingham et al., 1996). However, accurate morphological identification can be problematic due to fragmented nematodes, poor organ quality as animals have been shot in the thoracic region, confounding debris obscuring larvae and partial decomposition of the carcasses and thus the parasites. Moreover, the previous Danish publications includes only a single wildlife species. Therefore, the current role of wildlife as reservoir hosts for cardiopulmonary nematodes in Denmark is unknown. In addition, several Danish veterinary clinics report increasing incidence of canine angiostrongylosis outside the metropolitan area of Copenhagen and North Zealand (personal communication). North Zealand, including Copenhagen, has been considered a hyperendemic focus of A. vasorum in red foxes and domestic dogs for decades (Bolt et al., 1992; Saeed et al., 2006). The increase in canine angiostrongylosis outside the hyperendemic area might relate to an increased prevalence in wildlife over the past decade as observed for A. vasorum in Great Britain (Taylor et al., 2015) and Switzerland (Gillis-Germitsch et al., 2020).
Red foxes, raccoon dogs (Nyctereutes procyonoides) and American mink (hereafter named mink, as European mink (Mustela lutreola) have never been recorded in Denmark) are abundant wildlife species in Denmark and could contribute to substantial contamination with environmental stages of cardiopulmonary nematodes. Red foxes are native to Denmark and the most abundant species distributed throughout the country (apart from the island of Bornholm and some smaller islands) with a population size of approx. 40,000–60,000 individuals (Asferg et al., 2013). The raccoon dog is an invasive species in Denmark. It was first recorded in 1980, and the population has increased significantly since then to approx. 2000–3000 individuals (The Environmental Protection Agency, n.d.). Mink were originally imported to Denmark for fur production and kept in captivity. Today, mink can be found in the wild all over Denmark due to escapees from farms (Pagh et al., 2019), but the population size is unknown. Eurasian otters, beech martens (Martes foina) and polecats (Mustela putorius) are believed to be less common.
The aim of this study was to investigate the potential of six Danish wild carnivore species as reservoir hosts for A. vasorum, C. vulpis, C. aerophila, and A. abstrusus in seven different Danish regions.
2. Materials and methods
2.1. Study animals
Carcasses from 1041 wild carnivores; raccoon dogs (n = 476), red foxes (n = 367), mink (n = 123), beech martens (n = 31), Eurasian otters (n = 30) and polecats (n = 14) were examined for cardiopulmonary nematodes (Table 1).
Table 1.
Number of animals and organs examined, and number and total percentage of animals positive for cardiopulmonary nematodes (with 95% confidence intervals) per animal species.
| Animal species | Animals |
Lungs | Hearts | Positive |
% positive |
|---|---|---|---|---|---|
| examined | animals | [95% CI] | |||
| Raccoon dog (Nyctereutes procyonoides) | 476 | 473 | 467 | 48 | 10.1 |
| [7.6–13.2] | |||||
| Red fox (Vulpes vulpes) | 367 | 363 | 357 | 82 | 22.3 |
| [18.2–27.0] | |||||
| American mink (Neovison vison) | 123 | 122 | 121 | 18 | 14.6 |
| [9.1–22.4] | |||||
| Beech martens (Martes foina) | 31 | 30 | 28 | 6 | 19.4 |
| [8.1–38.1] | |||||
| Eurasian otters (Lutra lutra) | 30 | 29 | 28 | 1 | 3.3 |
| [0.2–19.1] | |||||
| Polecats (Mustela putorius) | 14 | 14 | 13 | 14 | 100.0 |
| [73.2–100.0] | |||||
| Total | 1041 | 1031 | 1014 | 169 | 16.2 |
| [14.1–18.6] |
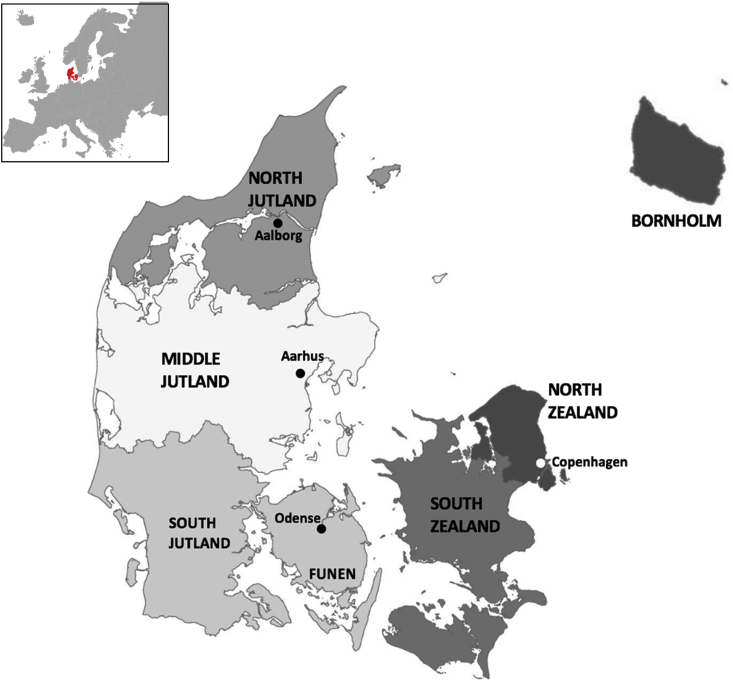
The carcasses were submitted for necropsy between January 2017 and March 2018, as a part of the national wildlife surveillance program. The carnivores were hunted, found dead or euthanized for animal welfare reasons. All animals were transported in sealed plastic bags and subsequently stored at −80 °C for min. 4 days prior to necropsy to inactivate potential zoonotic parasites. The wild carnivores were divided into seven regional groups depending on their origin: North Jutland (NJ), Middle Jutland (MJ), South Jutland (SJ), Funen (FU), North Zealand including Copenhagen (NZ), South Zealand (SZ) and Bornholm (BO) (Fig. 1). Funen, Zealand and Bornholm are all major populated islands, while Jutland is a peninsula extending from north Germany.
Fig. 1.
Map of Denmark showing Denmark's position in Europe and a map of Denmark showing the regional division and the four major cities.
At necropsy, hearts and lungs were collected and stored at −20 °C until further examination. Occasionally, hearts or lungs were unsuitable for examination due to traumatic injury, predation or decomposition. Individual data on regional origin, and gender were recorded when possible.
2.2. Examination of hearts and lungs
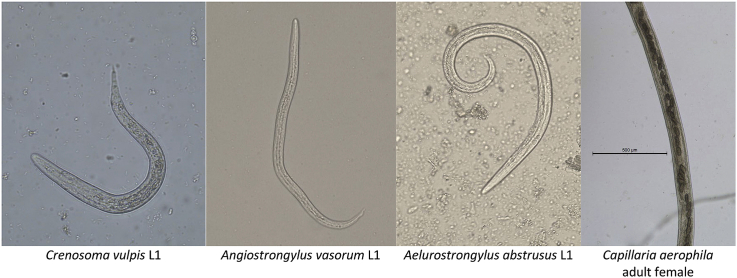
The heart was separated from the lungs by transecting all the arteries and veins proximal to the base of the heart. The ventricles and major blood vessels were opened using scissors and the heart was examined microscopically for adult worms using a stereomicroscope (Leica microsystems GmBH, Germany) at 80–100× magnification. The pulmonary blood vessels, trachea, and bronchial tree were dissected using scissors and inspected macroscopically for adult worms. Parallel incisions were made throughout the lung lobes approximately 1–2 cm apart. Subsequently, the heart and lungs were flushed with 500 mL tap water into a 500 mL conical glass. The fluid was left to sediment for 20 min. After which, the supernatant was removed, and the sediment transferred to microscopic slides, for examination using a light microscope (Leica DM2500 LED optical microsystems GmBH, Germany) at 100–400× magnification. All worms and larvae, except adult worms of C. aerophila, from individual animals were collected in 2 mL eppendorf tubes and stored at −20 °C. Adult worms of C. aerophila were identified morphologically based on their easily identifiable characteristics, as described by Christenson (1935). Samples co-infected with C. aerophila and other cardiopulmonary parasites were subjected to DNA extraction and molecular analysis.
2.3. DNA extraction of parasites
From each positive animal, up to 30 larvae or two adult worms or worm fragments were distributed into 1.5 mL eppendorf tubes, i.e. some samples were distributed into >1 tube and all tubes were analysed for each animal. From a pilot study, it was verified that >30 larvae or >2 adult worms could saturate the DNA extraction columns. Genomic DNA was extracted from all eppendorf tubes using the QIAmp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Extracted DNA was eluted in a final volume of 100 μL AE Buffer (QIAmp DNA Mini Kit, Qiagen, Hilden, Germany) and stored at −20 °C.
2.4. Molecular analysis
2.4.1. Molecular identification of Angiostrongylus vasorum and Crenosoma vulpis
Angiostrongylus vasorum and C. vulpis were identified using a modified method by Schug et al. (2018). In brief, duplex real-time PCT (rt-PCR) was used to target the ribosomal DNA (rDNA) of the internal transcribed spacer 2 (ITS-2) region, with primer and probe sequences listed in Table 2. PCR amplification was carried out in a total volume of 20 μl, containing 0.5 μM of each primer, 0.2 μM of each probe, 10 μL JumpStart™ Taq ReadyMix™ for Quantitative PCR (Sigma-Aldrich), 2.5 mM MgCl2, 2.0 μL DNA, and dH2O. PCR reactions were performed in a Rotor-Gene Q rt-PCR cycler (Qiagen, Hilden, Germany) using the following conditions: 94 °C for 2 min, followed by 45 cycles of 94 °C for 15 s, 55 °C for 60 s, 1 min with fluorescence detection in the green (FAM) and yellow (HEX) channel. DNA from 15 A. vasorum first stage larvae (L1) obtained from a German domestic dog, and DNA from 15 C. vulpis L1 obtained from a Swedish domestic dog were used as positive controls.
Table 2.
Primer and probe sequences used in PCR assays.
| Parasite | Sequence | Partial gene | Base pair length | Reference |
|---|---|---|---|---|
| A. vasorum | 121 | a | ||
| Primer | ||||
| I2F10 (forward) | 5′-CGCATGATGAAAGAATGCTG-3′ | ITS-2 | ||
| I2R9 (reverse) | 5′-GACGACGACGACAACCACT-3′ | ITS-2 | ||
| Probe | ||||
| I2P2 | FAM-ACAACATTGCTTGTCGAACGGCGTT-BHQ1 | |||
| C. vulpis | ~90 | a | ||
| Primer | ||||
| CvITS2f (forward) | 5′-GCATGATATTCGACGATTG-3′ | ITS-2 | ||
| CvITS2r (reverse) | 5′-GTGTGATCTAGTCATGTATAAC-3′ | ITS-2 | ||
| Probe | ||||
| CvITS2p | HEX-CAGCAATGAGAAGACACTATACACAAG-BHQ1 | |||
| A. abstrusus | ~233 | b | ||
| AabFor (forward) | 5′-GTAACAACGATATTGGTACTATG-3′ | ITS-2 | ||
| AabRev (reverse) | 5′-GAACTCCTTCACGTGCTACTCG-3′ | ITS-2 | ||
2.4.2. Molecular identification of Aelurostrongylus abstrusus
Examination for A. abstrusus was performed by conventional PCR according to Traversa and Guglielmini (2008) with minor modifications. The ITS-2 region of ribosomal DNA (~233 base pairs) was amplified using the primers listed in Table 2. PCR amplifications were carried out in a final volume of 50 μL containing: 100 pmol of each primer, 25.0 μL REDTaq ReadyMix (Sigma-Aldrich, Chemie, GmbH), 5.0 μl template DNA, and dH2O. Amplifications were performed using a Biometra T3 Thermocycler using the following conditions: 94 °C for 7 min followed by 40 cycles of 94 °C for 45 s, 50 °C for 45 s, and 72 °C for 45 s, and a final extension for 10 min at 72 °C. DNA from 15 A. abstrusus L1 obtained from a Danish domestic cat was used as a positive control.
Amplicons were electrophoresed in a 2% agarose E-Gel™ (Invitrogen, Thermo Fisher Scientific, Denmark) and lengths were sized by comparison with a 100-bp DNA Ladder (New England BioLabsInc., Ipswich, England).
2.4.3. Efficacy of PCR assays
For each parasite species, the detection limits were established using a duplex dilution series of MQ water spiked with 1, 2, 5, 10, 15, and 30 L1 isolated from the same material that was used for positive controls (see section 2.4.1 and 2.4.2). The rt-PCR consistently detected A. vasorum and C. vulpis DNA in samples containing ≥2 and ≥5 L1, respectively. The conventional PCR consistently detected A. abstrusus in samples containing ≥1 L1.
2.5. Data analysis
The prevalence of cardiopulmonary parasites and 95% confidence intervals were calculated for each carnivore species and separately for their region of origin.
3. Results
Table 1 gives an overview of the wild carnivores included in the study, the number of examined organs and animals positive for cardiopulmonary nematodes. Adult C. aerophila were morphologically identified in 50 carnivores (4.8%), of which 19 were co-infected with other cardiopulmonary parasites. These were subjected to DNA extraction together with other parasites from a further 119 carnivores (n = 138). In total, DNA was successfully extracted from 119/138 samples and determined to belong to A. vasorum and C. vulpis. The remaining 19 samples have been designated as positive for unidentified cardiopulmonary parasites. The prevalences and 95% CI of A. vasorum, C. vulpis, C. aerophila and unidentified nematodes by carnivore species and region of origin are listed in Table 3.
Table 3.
Parasite prevalence (with 95% confidence interval) based on biomolecular identification according to host species and region. NJ = North Jutland, MJ = Middle Jutland, SJ = South Jutland, FU = Funen, NZ = North Zealand, SZ = South Zealand and BO = Bornholm, U = unknown region, DK = Denmark, total countrywide prevalence.
|
A. vasorum |
Host species |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Red fox |
Raccoon dog |
Mink |
Beech Marten |
Polecat |
Eurasian otter |
Total |
|||||||||||||||||||||||||||||||||||||||||||||
| No. pos |
% pos |
No. pos |
% pos |
No. pos |
% pos |
No. pos |
% pos |
No. pos |
% pos |
No. pos |
% pos |
No. pos |
% pos |
|||||||||||||||||||||||||||||||||||||||
| /total | [95% CI] | /total | [95% CI] | /total | [95% CI] | /total | [95% CI] | /total | [95% CI] | /total | [95% CI] | /total | [95% CI] | |||||||||||||||||||||||||||||||||||||||
| NJ | 2/88 | 2.3 | 1/47 | 2.1 | 0/10 | – | 0/8 | – | 1/1 | 10.0 | 0/8 | – | 4/162 | 2.5 | ||||||||||||||||||||||||||||||||||||||
| [0.4–8.7] | [0.1–12.7] | [5.5–100] | [0.8–6.6] | |||||||||||||||||||||||||||||||||||||||||||||||||
| MJ | 3/161 | 1.9 | 7/195 | 3.6 | 0/9 | – | 0/16 | – | 2/4 | 5.0 | 0/9 | – | 11/394 | 2.8 | ||||||||||||||||||||||||||||||||||||||
| [0.5–5.8] | [1.6–7.6] | [9.2–90.8] | [1.5–5.1] | |||||||||||||||||||||||||||||||||||||||||||||||||
| SJ | 1/59 | 1.7 | 7/234 | 3.0 | 0/1 | – | 0/4 | – | – | – | 0/10 | – | 7/308 | 2.3 | ||||||||||||||||||||||||||||||||||||||
| [0.09–10.3] | [1.3–6.3] | [1.0–4.8] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| FU | 1/4 | 25.0 | – | – | 0/11 | – | – | – | – | – | 0/1 | – | 1/15 | 6.7 | ||||||||||||||||||||||||||||||||||||||
| [1.3–78.1] | [0.4–34.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| NZ | 6/16 | 37.5 | – | – | 0/2 | – | – | – | – | – | – | – | 6/18 | 33.3 | ||||||||||||||||||||||||||||||||||||||
| [16.3–64.1] | [14.4–58.8] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SZ | 10/27 | 37.0 | – | – | 0/1 | – | 0/2 | – | 4/9 | 44.4 | – | – | 14/39 | 35.9 | ||||||||||||||||||||||||||||||||||||||
| [20.1–57.5] | [15.3–77.3] | [21.7–52.9] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| BO | – | – | – | – | 1/86 | 1.2 | – | – | – | – | – | – | 1/86 | 1.2 | ||||||||||||||||||||||||||||||||||||||
| [0.1–7.2] | [0.06–7.2] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| U | 2/12 | 16.7 | – | – | 0/3 | – | 0/1 | – | – | – | 0/2 | – | 2/18 | 11.1 | ||||||||||||||||||||||||||||||||||||||
| [2.9–49.1] | [2.0–36.1] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 25/367 | 6.8 | 15/476 | 3.2 | 1/123 | 0.8 | 0/31 | - | 7/14 | 5.0 | 0/30 | - | 48/1041 | 4.6 | ||||||||||||||||||||||||||||||||||||||
|
[4.5–10.0] |
[1.8–5.3] |
[0.0–5.1] |
[24.0–76.0] |
[3.5–6.1] |
||||||||||||||||||||||||||||||||||||||||||||||||
| C. vulpisa | Host species | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Region | Red fox | Raccoon dog | Mink | Beech Marten | Polecat | Eurasian otter | Total | |||||||||||||||||||||||||||||||||||||||||||||
| No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | No.pos | % pos | No. pos | % pos | No. pos | % pos | |||||||||||||||||||||||||||||||||||||||
|
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
|||||||||||||||||||||||||||||||||||||||
| NJ | 2/87 | 2.3 | 0/47 | – | 0/10 | – | 0/8 | – | 0/1 | – | 0/7 | – | 2/160 | 1.3 | ||||||||||||||||||||||||||||||||||||||
| [0.4–8.8] | [0.2–4.9] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| MJ | 12/158 | 7.6 | 7/194 | 3.6 | 0/9 | – | 0/15 | – | 0/4 | – | 0/9 | – | 19/389 | 4.9 | ||||||||||||||||||||||||||||||||||||||
| [4.2–13.2] | [1.6–7.6] | [3.0–7.7] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| SJ | 1/59 | 1.7 | 18/232 | 7.8 | 0/1 | – | 0/4 | – | – | – | 0/10 | – | 19/306 | 6.2 | ||||||||||||||||||||||||||||||||||||||
| [0.1–10.3] | [4.8–12.2] | [3.9–9.7] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| FU | 0/4 | – | – | – | 0/11 | – | – | – | – | – | 0/1 | – | 0/16 | |||||||||||||||||||||||||||||||||||||||
| NZ | 3/16 | 18.8 | – | – | 0/2 | – | – | – | – | – | – | – | 3/18 | 1.7 | ||||||||||||||||||||||||||||||||||||||
| [5.0–46.3] | [4.4–42.3] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SZ | 1/27 | 3.7 | – | – | 0/1 | – | 0/2 | – | 1/9 | 11.1 | – | – | 2/39 | 5.1 | ||||||||||||||||||||||||||||||||||||||
| [0.1–20.9] | [0.6–49.3] | [0.9–18.3] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| BO | – | – | – | – | 7/85 | 8.2 | – | – | – | – | – | – | 7/85 | 8.3 | ||||||||||||||||||||||||||||||||||||||
| [3.7–16.8] | [3.7–16.8] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| U | 1/12 | 8.3 | – | – | 0/3 | – | 0/1 | – | – | – | 0/2 | – | 1/18 | 5.6 | ||||||||||||||||||||||||||||||||||||||
| [0.4–40.2] | [0.3–29.4] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 20/363 | 5.5 | 25/473 | 5.3 | 7/122 | 5.7 | 0/30 | – | 1/14 | 7.1 | 0/29 | – | 53/1031 | 5.1 | ||||||||||||||||||||||||||||||||||||||
|
DK |
[3.5–8.5] |
[3.5–7.8] |
[2.5–11.9] |
[0.4–35.8] |
[3.9–6.7] |
|||||||||||||||||||||||||||||||||||||||||||||||
| C. aerophilaa | Host species | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Region | Red fox | Raccoon dog | Mink | Beech Marten | Polecat | Eurasian otter | Total | |||||||||||||||||||||||||||||||||||||||||||||
| No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | |||||||||||||||||||||||||||||||||||||||
|
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
|||||||||||||||||||||||||||||||||||||||
| NJ | 12/87 | 13.8 | 1/47 | 2.1 | 0/10 | – | 0/8 | – | 0/1 | – | 0/7 | – | 13/160 | 8.1 | ||||||||||||||||||||||||||||||||||||||
| [7.6–23.2] | [0.1–12.7] | [4.6–13.8] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| MJ | 17/158 | 10.8 | 3/194 | 1.6 | 0/9 | – | 0/15 | – | 0/4 | – | 0/9 | – | 20/389 | 5.1 | ||||||||||||||||||||||||||||||||||||||
| [6.7–16.9] | [0.4–4.8] | [3.3–8.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| SJ | 7/59 | 11.9 | 5/232 | 2.2 | 0/1 | – | 0/4 | – | – | – | 0/10 | – | 12/306 | 3.9 | ||||||||||||||||||||||||||||||||||||||
| [5.3–23.5] | [0.8–5.2] | [2.1–6.9] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| FU | 0/4 | – | – | – | 0/11 | – | – | – | – | – | 0/1 | – | 0/16 | – | ||||||||||||||||||||||||||||||||||||||
| NZ | 2/16 | 12.5 | – | – | 0/2 | – | – | – | – | – | – | – | 2/18 | 11.1 | ||||||||||||||||||||||||||||||||||||||
| [2.2–39.6] | [2.0–36.1] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SZ | 1/27 | 3.7 | – | – | 0/1 | – | 2/2 | 100 | 0/9 | – | – | – | 3/39 | 7.7 | ||||||||||||||||||||||||||||||||||||||
| [0.2–20.9] | [19.8–100] | [2.0–22.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| BO | – | – | – | – | 0/85 | – | – | – | – | – | – | – | 0/85 | – | ||||||||||||||||||||||||||||||||||||||
| U | 0/12 | – | – | – | 0/3 | – | 0/1 | – | – | – | 0/2 | – | 0/18 | – | ||||||||||||||||||||||||||||||||||||||
| Total | 39/363 | 10.7 | 9/473 | 1.9 | 0/122 | 2/30 | 6.7 | 0/14 | – | 0/29 | - | 50/1031 | 4.8 | |||||||||||||||||||||||||||||||||||||||
|
DK |
[7.8–14.5] |
[0.9–3.7] |
[1.2–23.5] |
[3.7–6.4] |
||||||||||||||||||||||||||||||||||||||||||||||||
| Unidenfied lungworms | Red fox | Racoon dog | Mink | Beech Marten | Polecat | Eurasian otter | Total | |||||||||||||||||||||||||||||||||||||||||||||
| No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | No. pos | % pos | |||||||||||||||||||||||||||||||||||||||
|
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
/total |
[95% CI] |
|||||||||||||||||||||||||||||||||||||||
| Total | 9/367 | 2.5 | 4/476 | 0.8 | 0/123 | – | 4/31 | 12.9 | 2/14 | 14.3 | 0/30 | – | 19/1041 | 1.8 | ||||||||||||||||||||||||||||||||||||||
| [1.3–4.6] | [0.3–2.1] | [5.1–28.9] | [4.0–40.0] | [1.2–2.8] | ||||||||||||||||||||||||||||||||||||||||||||||||
In 10 animals, lungs were missing and the heart was the only organ examined. These animals were only examined for A. vasorum.
Angiostrongylus vasorum and C. vulpis were identified from red foxes, raccoon dogs, mink and polecats, while C. aerophila was identified from red foxes, raccoon dogs and beech martens. Otters were negative for all parasite species. None of the carnivores were positive for A. abstrusus.
Co-infections with A. vasorum and C. vulpis were observed in 11 carnivores (six raccoon dogs and five red foxes), four were co-infected with A. vasorum and C. aerophila (one raccoon dog and three red foxes) and three were co-infected with C. vulpis and C. aerophila (three red foxes).
4. Discussion
This study confirmed the occurrence of A. vasorum, C. vulpis and C. aerophila in red foxes, but also provided novel evidence of these parasites from other wildlife species, confirming their potential as reservoir host for domestic animals in Denmark. Thus, we report, for the first time, infection with A. vasorum and C. vulpis in raccoon dogs, mink and polecats, and C. aerophila infection in raccoon dogs and beech martens from Denmark. Additionally, this is the first report of A. vasorum and C. vulpis in wild carnivores from the island of Bornholm. Lastly, this is, to our knowledge, the first report of C. vulpis infection in mink, and C. vulpis and A. vasorum infections in polecats worldwide. Aelurostrongylus abstrusus was absent from all examined animals.
Angiostrongylus vasorum and C. vulpis prevalences in raccoon dogs from Denmark (2.1–3.6% and 3.6–7.8%, respectively) were similar to those observed for red foxes from the same regions (1.7–2.3% and 1.7–7.6%, respectively). Similarity in the regional prevalence of A. vasorum and C. vulpis was observed for both red foxes and raccoon dogs. Both parasites have previously been identified in raccoon dogs in other European countries (Bružinskaitė-Schmidhalter et al., 2012; Laurimaa et al., 2016; Shimalov and Shimalov, 2002; Thiess et al., 2001), and have probably been present in raccoon dogs since they first came to Denmark in 1980. Raccoon dogs are currently only present in Jutland (the peninsula of Denmark) and with a few individuals on the island of Funen. However, the population continuous to increase despite a large effort from local hunters and the authorities to control population numbers. Based on the prevalence and the increasing population size, raccoon dogs might constitute an important reservoir for A. vasorum and C. vulpis infections in domestic animals in Jutland. Fortunately, this may be counteracted by the raccoon dog's behaviour as they tend to defecate in “latrines”, hibernate during winter and avoid human contact (Al-sabi et al., 2009). This may diminish scattered environmental contamination with the parasites near domestic animals, but does not reduce exposure for other wildlife species that may bring the infections closer to domestic animals.
Angiostrongylus vasorum and C. vulpis were recorded in red foxes with regional prevalences of 1.7–37.5% and 1.7–18.8%, respectively (Table 3), demonstrating that these parasites are common in red foxes from Denmark. The red fox abundance is significantly higher than raccoon dogs and polecats, although the true population sizes are unknown. Moreover, red foxes are distributed throughout most of the country, both in rural and urban areas, and are active year-round (Al-sabi et al., 2009). In urban areas, red foxes often defecate in private gardens and city parks, areas where intermediate hosts are common and domestic dogs are walked. Due to behavioural habits, abundance and parasite prevalences, red foxes are most likely the main reservoir host for cardiopulmonary infections in domestic dogs in Denmark.
Polecats also constitute a previously unknown reservoir for A. vasorum and C. vulpis in Denmark with prevalences of 5.0% and 7.1%, respectively. Angiostrongylus vasorum has previously been identified from other small mustelids (Simpson et al., 2016), but neither A. vasorum nor C. vulpis infections in polecats have previously been documented. As only 14 polecats were included in this study, further investigations with larger sample size is needed to investigate the importance of this species as a reservoir host.
We observed C. aerophila in raccoon dogs and beech martens from Denmark for the first time, although with relatively low prevalences in raccoon dogs. Capillaria aerophila was more prevalent in red foxes (10.7%) compared to beech martens (6.7%) and raccoon dogs (1.9%) suggesting that red foxes are the most important reservoir host. In contrast to A. vasorum and C. vulpis, C. aerophila prevalences in red foxes were similar (10.8%–13.8%) in four out of five regions. These findings concur with previous reported prevalences for red foxes in South Jutland and North Zealand (Al-Sabi et al., 2014). Evenly distributed C. aerophila prevalences in different geographical areas have also been reported from other countries (Davidson et al., 2006; Hodžíc et al., 2016; Morgan et al., 2008; Schug et al., 2018). However, prevalences reported in former Danish studies were considerably higher (87.1% and 93.8%, respectively) (Al-Sabi et al., 2014) than in our study, which may reflect differences in sampling procedures (see below). However, fluctuation in C. aerophila prevalences from year to year has been observed in red foxes from Switzerland (Gillis-Germitsch et al., 2020), while it has remained constant in other countries (Morgan et al., 2008; Sréter et al., 2003; Taylor et al., 2015; Tolnai et al., 2015).
The A. vasorum prevalence in red foxes was, as expected, considerably higher (37.5%) in North Zealand compared to red foxes from Jutland (1.7–2.3%), which is in line with previous reports in this area (35.9–80.0%) (Al-Sabi et al., 2014; Bolt et al., 1992; Saeed et al., 2006; Willingham et al., 1996). North Zealand has been considered an A. vasorum hyperendemic area for decades (Bolt et al., 1992; Saeed et al., 2006). Originally, A. vasorum was introduced to North Zealand with a domestic dog, which was infected in France (Finnerup, 1983). Thereafter, the parasite seemingly spread to other domestic dogs and red foxes in the area. Unexpectedly, A. vasorum prevalence in red foxes originating from South Zealand was equal to the prevalence in the hyperendemic North Zealand. This new finding indicates that the hyperendemic area has expanded to include all of Zealand. In contrast, A. vasorum prevalence in red foxes from Jutland was significantly lower. This geographical difference between Jutland and Zealand could include variation in intermediate host density and density of the final host. Nevertheless, the prevalence in red foxes originating from Jutland was slightly higher, and prevalence in red foxes originating from South Zealand substantially higher than previously reported for these areas (Al-Sabi et al., 2014; Bolt et al., 1992; Jacobsen, 2007; Saeed et al., 2006; Willingham et al., 1996). Our results are in line with observations from other European countries: For example, A. vasorum prevalence in red foxes in Switzerland has increased 4-fold in 5 years (Gillis-Germitsch et al., 2020), in Hungary, 3.6-fold in 12 years (Sréter et al., 2003; Tolnai et al., 2015), and 2.6-fold in 8 years in Great Britain (Taylor et al., 2015). This increase over time might relate to increased snail abundance. Snail abundance is temperature dependent, e.g. snail egg development requires at least 10 °C for Arion lusitanicus (Kozłowski, 2007), a common intermediate host for A. vasorum (Ferdushy et al., 2009). Moreover, parasite larvae development in the intermediate host is also dependent on temperature which can impact transmission of the parasite (Ferdushy et al., 2010). In Denmark, the mean yearly temperature has increased the past years (Danmarks statistik, 2014). Moreover, foxes do not develop protective immunity against A. vasorum and can be re-infected (Woolsey et al., 2017).
As for A. vasorum, C. vulpis was more prevalent in red foxes from North Zealand (18.8%) compared to those from Jutland (1.7–7.6%). This contrasts findings from a previous Danish study where C. vulpis prevalence in red foxes from South Jutland and Copenhagen metropolitan area (in our study, animals from the Copenhagen metropolitan area is included in the area designated North Zealand) was equal (22.9%) (Al-Sabi et al., 2014). Thus, C. vulpis prevalence in our study is low in South Jutland compared to previous Danish study by Al-Sabi et al. (2014). Likewise, C. vulpis prevalence in red foxes in our study is lower than previously reported in Europe (23.3%–53.8%) (Bružinskaitė-Schmidhalter et al., 2012; Schug et al., 2018). Besides variations in final- and intermediate host abundance, the variation could relate to differences in diagnostic methods (Hodžíc et al., 2016; Morgan et al., 2008). The method used herein for parasite collection has some limitations. Only a moderate amount of water was used to flush the organs compared to other studies, lung lobes were examined with parallel incisions, the trachea and bronchi were not all scraped, and blood clots were not dissolved in water as has previously been reported (Davidson et al., 2006; Hodžíc et al., 2016; Schug et al., 2018). All of which may have reduced the sensitivity of the method leading to the lower prevalences observed for C. vulpis and C. aerophila compared to previous studies.
Aelurostrongylus abstrusus was absent from our study sample population, suggesting that wild carnivores can be considered unimportant as reservoir hosts for this parasite. This confirms that the parasite is rather species-specific (Otranto et al., 2015; Traversa et al., 2010), even though there is a recent report on Aelurostrongylus spp. in mink (Martínez-Rondán et al., 2017).
Mink is the only carnivore present on the island of Bornholm. In total, 8.2% of these was infected with C. vulpis and 1.2% with A. vasorum. Interestingly, mink from other regions were not infected with these parasites. No mink were infected with C. aerophila. As Bornholm is an island, parasites were likely introduced through importation of infected animals, notably domestic dogs. As prevalences were low in mink, their role as reservoir hosts can be considered negligible.
5. Conclusion
Our findings showed for the first time that raccoon dogs, mink, and polecats from Denmark can be infected with A. vasorum and C. vulpis, and that raccoon dogs and beech martens were infected with C. aerophila. Our findings confirm that other wild carnivores in addition to red foxes can act as reservoirs for cardiopulmonary nematodes in domestic animals. Furthermore, prevalences of A. vasorum have increased in red foxes in most areas compared to previous studies, while quantitatively and epidemiologically, the role of these reservoir hosts is unknown.
Declaration of competing interest
None.
Acknowledgements
This work was supported by the Ministry of Environment and Food of Denmark, the Environmental Protection Agency. The authors are grateful to the Institute for Parasitology, University of Veterinary Medicine Hannover, Department of Virology, Immunobiology and Parasitology, National Veterinary Institute of Sweden and Lise-Lotte Christiansen for providing L1 reference material for A. vasorum, C. vulpis and A. abstrusus, respectively.
References
- Al-Sabi M.N.S., Halasa T., Kapel C.M.O. Infections with cardiopulmonary and intestinal helminths and sarcoptic mange in red foxes from two different localities in Denmark. Acta Parasitol. 2014;59:98–107. doi: 10.2478/s11686-014-0214-6. [DOI] [PubMed] [Google Scholar]
- Al-sabi M.N.S., Hammer T., Chriél M., Larsen Enemark H. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012 – a comparative study. Int. J. Parasitol. 2009;2:144–151. doi: 10.1016/j.ijppaw.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. Nematode Parasites of Vertebrates. Their Development and Transmission. CABI Publishing, C.A.B. International; Wallingford, Oxfordshire, United Kingdom.: 2000. The superfamily metastrongyloidea; pp. 153–164. [Google Scholar]
- Asferg T., Madsen A.B., Pagh S. Dansk pattadyratlas. 2013. https://pattedyratlas.lex.dk/Ræv Ræv [in Danish] [WWW Document] accessed 6.9.20.
- Bolt G., Monrad J., Henriksen P., Dietz H.H., Koch J., Bindseil E., Jensen A.L. The fox (Vulpes vulpes) as a reservoir for canine angiostrongylosis in Denmark. Field survey and experimental infections. Acta Vet. Scand. 1992;33:357–362. doi: 10.1186/BF03547302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bružinskaitė-Schmidhalter R., Šarkūnas M., Malakauskas A., Mathis A., Torgerson P.R., Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:120–127. doi: 10.1017/S0031182011001715. [DOI] [PubMed] [Google Scholar]
- Burgess H., Ruotsalo K., Peregrine A.S., Hanselman B., Abrams-Ogg A. Eucoleus aerophilus respiratory infection in a dog with Addison's disease. Can. Vet. J. 2008;49:389–392. [PMC free article] [PubMed] [Google Scholar]
- Christenson R.0. Studies on the morphology of the common fox lungworm, Capillaria aërophila (Creplin, 1839) Trans. Am. Microsc. Soc. 1935;54:145–154. [Google Scholar]
- Conboy G. Helminth parasites of the canine and feline respiratory tract. Vet. Clin. North Am. Small Anim. Pract. 2009;39:1109–1126. doi: 10.1016/j.cvsm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Danmarks statistik . 65 Years in Numbers - Denmark since 2. World War [in Danish] Rosendahls-Schultz; Søborg: 2014. [Google Scholar]
- Davidson R.K., Gjerde B., Vikøren T., Lillehaug A., Handeland K. Prevalence of Trichinella larvae and extra-intestinal nematodes in Norwegian red foxes (Vulpes vulpes) Vet. Parasitol. 2006;136:307–316. doi: 10.1016/J.VETPAR.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Demiaszkiewicz A.W., Pyziel A.M., Kuligowska I., Lachowicz J. The first report of Angiostrongylus vasorum (Nematoda; Metastrongyloidea) in Poland, in red foxes (Vulpes vulpes) Acta Parasitol. 2014;59:758–762. doi: 10.2478/s11686-014-0290-7. [DOI] [PubMed] [Google Scholar]
- Dietz H.H., Andersen T.H., Rattenborg E., Wolstrup C., Henriksen S.A. Diseases diagnosticed in Danish wildlife 1994-1996 [In Danish] Dansk Veterinærtidsskrift. 1998;81:200–206. [Google Scholar]
- Elsheikha H.M., Schnyder M., Traversa D., Cesare A. Di, Wright I., Lacher D.W. Updates on feline aelurostrongylosis and research priorities for the next decade. Parasites Vectors. 2016;9:389. doi: 10.1186/s13071-016-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdushy T., Kapel C.M.O., Webster P., Al-Sabi M.N.S., Grønvold J. The occurrence of Angiostrongylus vasorum in terrestrial slugs from forests and parks in the Copenhagen area, Denmark. J. Helminthol. 2009;83:379–383. doi: 10.1017/S0022149X09377706. [DOI] [PubMed] [Google Scholar]
- Ferdushy T., Kapel C.M.O., Webster P., Al-Sabi M.N.S., Grønvold J.R. The effect of temperature and host age on the infectivity and development of Angiostrongylus vasorum in the slug Arion lusitanicus. Parasitol. Res. 2010;107:147–151. doi: 10.1007/s00436-010-1850-x. [DOI] [PubMed] [Google Scholar]
- Finnerup E. Angiostrongylus vasorum in dogs [In Danish] Dansk Veterinærtidsskrift. 1983;66:338–340. [Google Scholar]
- Giannelli A., Capelli G., Joachim A., Hinney B., Losson B., Kirkova Z., René-Martellet M., Papadopoulos E., Farkas R., Napoli E., Brianti E., Tamponi C., Varcasia A., Margarida Alho A., Madeira de Carvalho L., Cardoso L., Maia C., Mircean V., Mihalca A.D., Miró G., Schnyder M., Cantacessi C., Colella V., Cavalera M.A., Latrofa M.S., Annoscia G., Knaus M., Halos L., Beugnet F., Otranto D. Lungworms and gastrointestinal parasites of domestic cats: a European perspective. Int. J. Parasitol. 2017;47:517–528. doi: 10.1016/j.ijpara.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Gillis-Germitsch N., Tritten L., Hegglin D., Deplazes P., Schnyder M. Conquering Switzerland: emergence of Angiostrongylus vasorum over three decades and rapid regional increase in the fox population contrasts with the stable prevalence of lungworms. Parasitology. 2020;147:1071–1079. doi: 10.1017/S0031182020000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžíc A., Alí A., Klebí I., Kadrí M., Brianti E., Duscher G.G. Red fox (Vulpes vulpes) as a potential reservoir host of cardiorespiratory parasites in Bosnia and Herzegovina. Vet. Parasitol. 2016;223:63–70. doi: 10.1016/j.vetpar.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Hurníková Z., Miterpáková M., Mandelík R. First autochthonous case of canine Angiostrongylus vasorum in Slovakia. Parasitol. Res. 2013;112:3505–3508. doi: 10.1007/s00436-013-3532-y. [DOI] [PubMed] [Google Scholar]
- Jacobsen K.L. University of Copenhagen; Denmark: 2007. Prevalence studies of Crenosoma vulpis and Angiostrongylus vasorum in red foxes (Vulpes vulpes) and dogs (Canis familiaris) in an area of Midjutland, Denmark. [In danish]. Masterthesis. [Google Scholar]
- Jolly S., Poncelet L., Lempereur L., Caron Y., Bayrou C., Cassart D., Grimm F., Losson B. First report of a fatal autochthonous canine Angiostrongylus vasorum infection in Belgium. Parasitol. Int. 2015;64:97–99. doi: 10.1016/j.parint.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Koch J., Willesen J.L. Canine pulmonary angiostrongylosis: An update. Vet. J. 2009;179:348–359. doi: 10.1016/j.tvjl.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Kozłowski J. The distribution, biology, population dynamics and harmfulness of Arion lusitanicus Mabille, 1868 (Gastropoda: pulmonata: Arionidae) in Poland. J. plant Prot. Res. 2007;47:219–230. [Google Scholar]
- Lalošević V., Lalošević D., Capo I., Simin V., Galfi A., Traversa D. High infection rate of zoonotic Eucoleus aerophilus infection in foxes from Serbia. Parasite. 2013;20:3. doi: 10.1051/parasite/2012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurimaa L., Süld K., Davison J., Moks E., Valdmann H., Saarma U. Alien species and their zoonotic parasites in native and introduced ranges: The raccoon dog example. Vet. Parasitol. 2016;219:24–33. doi: 10.1016/j.vetpar.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Madsen A.B., Dietz H.H., Henriksen P., Clausen B. Survey of Danish free living otters Lutra lutra - a consecutive collection and necropsy of dead bodies. IUCN Otter Spec. Gr. 1999;16:65–76. [Google Scholar]
- Martínez-Rondán F.J., Ruiz De Ybáñez M.R., Tizzani P., López-Beceiro A.M., Fidalgo L.E., Martínez-Carrasco C. The American mink (Neovison vison) is a competent host for native European parasites. Vet. Parasitol. 2017;247:93–99. doi: 10.1016/j.vetpar.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Tomlinson A., Hunter S., Nichols T., Roberts E., Fox M.T., Taylor M.A. Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2008;154:48–57. doi: 10.1016/j.vetpar.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Olsen C.S., Willesen J.L., Pipper C.B., Mejer H. Occurrence of Aelurostrongylus abstrusus (Railliet, 1898) in Danish cats: a modified lung digestion method for isolating adult worms. Vet. Parasitol. 2015;210:32–39. doi: 10.1016/j.vetpar.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Dantas-Torres F., Brianti E., Pfeffer M., Genchi C., Guberti V., Capelli G., Deplazes P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Spec. Issue Spread Parasites with Anim. Movements. 2015;213:24–37. doi: 10.1016/j.vetpar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Pagh S., Petersen H.H., Jensen T.H., Hansen M.S., Madsen S., Kraft D.C.E., Iversen N., Roslev P., Chriel M. Methods for the identification of farm escapees in feral mink (Neovison vison) populations. PloS One. 2019;14 doi: 10.1371/journal.pone.0224559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagos-Tabares F., Lange M.K., Chaparro-Gutiérrez J.J., Taubert A., Hermosilla C. Angiostrongylus vasorum and Aelurostrongylus abstrusus: Neglected and underestimated parasites in South America. Parasites Vectors. 2018;11:208. doi: 10.1186/s13071-018-2765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly G.A.C., Mcgarry J.W., Martin M., Belford C. Crenosoma vulpis, the fox lungworm, in a dog in Ireland. Vet. Rec. 2000;146:764–765. doi: 10.1136/vr.146.26.764. [DOI] [PubMed] [Google Scholar]
- Rinaldi L., Calabria G., Carbone S., Carrella A., Cringoli G. Crenosoma vulpis in dog: first case report in Italy and use of the FLOTAC technique for copromicroscopic diagnosis. Parasitol. Res. 2007;101:1681–1684. doi: 10.1007/s00436-007-0713-6. [DOI] [PubMed] [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Schug K., Krämer F., Schaper R., Hirzmann J., Failing K., Hermosilla C., Taubert A. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in Central Germany. Parasites Vectors. 2018;11:85. doi: 10.1186/s13071-018-2672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimalov V.V., Shimalov V.T. Helminth fauna of the racoon dog (Nyctereutes procyonoides gray, 1834) in belorussian polesie. Parasitol. Res. 2002;88:944–945. doi: 10.1007/s00436-001-0582-3. [DOI] [PubMed] [Google Scholar]
- Simin S., Spasojević Kosić L., Kuruca L., Pavlović I., Savović M., Lalošević V. Moving the boundaries to the South-East: first record of autochthonous Angiostrongylus vasorum infection in a dog in Vojvodina province, northern Serbia. Parasites Vectors. 2014;7:396. doi: 10.1186/1756-3305-7-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson V.R., Tomlinson A.J., Stevenson K., McLuckie J.A., Benavides J., Dagleish M.P. A post-mortem study of respiratory disease in small mustelids in south-west England. BMC Vet. Res. 2016;12:72. doi: 10.1186/s12917-016-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sréter T., Széll Z., Marucci G., Pozio E., Varga I. Extraintestinal nematode infections of red foxes (Vulpes vulpes) in Hungary. Vet. Parasitol. 2003;115:329–334. doi: 10.1016/S0304-4017(03)00217-6. [DOI] [PubMed] [Google Scholar]
- Taylor C.S., Garcia Gato R., Learmount J., Aziz N.A., Montgomery C., Rose H., Coulthwaite C.L., McGarry J.W., Forman D.W., Allen S., Wall R., Morgan E.R. Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology. 2015;142:1190–1195. doi: 10.1017/S0031182015000463. [DOI] [PubMed] [Google Scholar]
- The Environmental Protection Agency Raccoon dog [In Danish] https://mst.dk/natur-vand/natur/artsleksikon/pattedyr/maarhund/ [WWW Document] accessed 5.29.20.
- Thiess A., Schuster R., Nöckler K., Mix H. Helminth findings in indigenous raccoon dogs Nyctereutes procyonoides (Gray, 1843) [In German] Berl. Münchener Tierärztliche Wochenschr. 2001;114:273–276. [PubMed] [Google Scholar]
- Tolnai Z., Széll Z., Sréter T. Environmental determinants of the spatial distribution of Angiostrongylus vasorum, Crenosoma vulpis and Eucoleus aerophilus in Hungary. Vet. Parasitol. 2015;207:355–358. doi: 10.1016/J.VETPAR.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Traversa D., Cesare A. Di. Cardio-pulmonary parasitic nematodes affecting cats in Europe: unraveling the past, depicting the present, and predicting the future. Front. Vet. Sci. 2014;1:11. doi: 10.3389/fvets.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D., Cesare A. Di, Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasites Vectors. 2010;6:62. doi: 10.1186/1756-3305-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D., Guglielmini C. Feline aelurostrongylosis and canine angiostrongylosis: a challenging diagnosis for two emerging verminous pneumonia infections. Vet. Parasitol. 2008;157:163–174. doi: 10.1016/j.vetpar.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Willingham A.L., Ockens N.W., Kapel C.M.O., Monrad J. A helminthological survey of wild red foxes (Vulpes vulpes) from the metropolitan area of Copenhagen. J. Helminthol. 1996;70:259. doi: 10.1017/S0022149X00015509. [DOI] [PubMed] [Google Scholar]
- Woolsey I.D., Webster P., Thamsborg S., Schnyder M., Monrad J., Kapel C.M.O. Repeated inoculations with the lung and heartworm nematode Angiostrongylus vasorum result in increasing larval excretion and worm burden in the red fox (Vulpes vulpes) Int. J. Parasitol. Parasites Wildl. 2017;6:139–145. doi: 10.1016/j.ijppaw.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]