Abstract
The UPF3B-dependent branch of the nonsense-mediated RNA decay (NMD) pathway is critical for human cognition. Here, we examined the role of UPF3B in the olfactory system. Single-cell RNA-sequencing (scRNA-seq) analysis demonstrated considerable heterogeneity of olfactory sensory neuron (OSN) cell populations in wild-type (WT) mice, and revealed that UPF3B loss influences specific subsets of these cell populations. UPF3B also regulates the expression of a large cadre of antimicrobial genes in OSNs, and promotes the selection of specific olfactory receptor (Olfr) genes for expression in mature OSNs (mOSNs). RNA-seq and Ribotag analyses identified classes of mRNAs expressed and translated at different levels in WT and Upf3b-null mOSNs. Integrating multiple computational approaches, UPF3B-dependent NMD target transcripts that are candidates to mediate the functions of NMD in mOSNs were identified in vivo. Together, our data provides a valuable resource for the olfactory field and insights into the roles of NMD in vivo.
Research organism: Mouse
Introduction
Nonsense-mediated RNA decay (NMD) is a conserved pathway originally discovered by virtue of its ability to degrade aberrant RNAs harboring premature termination codons (PTCs) and thus protect cells from truncated, potentially toxic, dominant-negative proteins (Chang et al., 2007; Conti and Izaurralde, 2005; Kurosaki et al., 2019; Lykke-Andersen and Jensen, 2015; Palacios, 2013). Subsequently, it was discovered that NMD degrades subsets of normal RNAs, with loss or disruption of NMD leading to dysregulation of 5–20% of the normal transcriptome in species spanning the phylogenetic scale (Chan et al., 2007; He et al., 2003; Mendell et al., 2004). This discovery raised the possibility that the function of NMD extends beyond quality control. This notion has been supported by scores of subsequent studies showing that NMD factors are critical for many fundamental processes, including development, differentiation, cell proliferation, the integrated stress response, the unfolded protein response, and autophagy (Chang et al., 2007; Karam et al., 2015; Kurosaki et al., 2019; Nasif et al., 2018).
NMD is well-studied at the biochemical level, with over 15 proteins known to be involved in this pathway (Chang et al., 2007; Kurosaki et al., 2019). Three of these proteins—UPF1, UPF2, and UPF3—are present in all eukaryotes and considered to be the core NMD factors (Conti and Izaurralde, 2005). UPF1 is an RNA helicase that forms a complex with the adaptor proteins UPF2 and UPF3. In vertebrates, UPF3 is encoded by two paralogs: UPF3A (also called ‘UPF3’) and UPF3B (also called ‘UPF3X’). UPF3A serves as a weak NMD factor and NMD repressor, while UPF3B is a NMD branch-specific factor that stimulates NMD (Chan et al., 2007; Shum et al., 2016). UPF3B directly binds to the exon-junction complex (EJC), a large multi-subunit complex recruited to RNAs just upstream of exon-exon junctions after RNA splicing (Woodward et al., 2017). The EJC triggers NMD when allowed to interact with other NMD factors. Evidence suggests that EJCs are displaced by the ribosome during the pioneer round of translation, and thus only EJCs deposited downstream of the stop codon defining the main open-reading frame (ORF) are able to elicit NMD (Dostie and Dreyfuss, 2002). Ribosomes would also be predicted to displace the last EJC when the termination codon resides ~50 nucleotides or less upstream of the last exon-exon junction, based on the length of the EJC and ribosome footprints. This has led to the ‘−50-nt boundary rule,’ an empirically verified dictum which states that only in-frame stop codons further than ~50 nt upstream of the last exon-exon junction elicit NMD (Nagy and Maquat, 1998). While there are exceptions to this −50-nt boundary rule (Carter et al., 1996), it reliably predicts a large proportion of EJC-dependent NMD target mRNAs (Boehm et al., 2014; Gehring et al., 2005; Hurt et al., 2013). NMD can be triggered by other molecular signals in addition to downstream EJCs. For example, long 3’-untranslated regions (UTRs) and short ORFs upstream of the main ORF (uORFs) can, in some cases, trigger NMD in an EJC-independent manner (Barrett et al., 2012; Bühler et al., 2006; Chang et al., 2007; Hurt et al., 2013; Kebaara and Atkin, 2009; Rebbapragada and Lykke-Andersen, 2009).
While considerable progress has been made in understanding the molecular features that elicit NMD, we are still largely in the dark with regard to which transcripts are targeted for rapid decay. It is critical to define such NMD target mRNAs in order to begin to unravel the molecular mechanisms by which NMD influences biological processes. A particularly large gap in the field is the identity of NMD targets in vivo.
Many lines of evidence suggests that NMD is not a single linear pathway but instead consists of several branches, each of which depends on different factors and promotes the decay of different sets of transcripts (Chan et al., 2007; Gehring et al., 2005; Mabin et al., 2018). In this report, we focus on the UPF3B-dependent branch of NMD, which has been shown to be important for the nervous system. Pedigree analysis of numerous families harboring mutations in the UPF3B gene have demonstrated that both nonsense and missense mutations cause intellectual disability in humans (Nguyen et al., 2014; Tarpey et al., 2007). Humans with UPF3B mutations also commonly have autism, schizophrenia, and/or attention-deficit/hyperactivity disorder (Nguyen et al., 2014; Tarpey et al., 2007). To understand the underlying mechanism for these behavioral defects, we generated Upf3b-deficient mice (Huang et al., 2011; Karam et al., 2015). These Upf3b-null mice suffer from specific learning and memory deficits, including fear-conditioned learning, and thus replicate some aspects of the behavioral defects in UPF3B-deficient humans (Huang et al., 2018). In part, these behavioral defects may stem from abnormal neural connectivity, as cortical pyramidal neurons from Upf3b-null mice undergo impaired dendritic spine maturation in vivo (Huang et al., 2018). Furthermore, cultured UPF3B-depleted neural cells have subtle dendrite outgrowth defects (Jolly et al., 2013), and expression of UPF3B mutants reduces neurite branching (Alrahbeni et al., 2015). The behavioral defects in Upf3b-null mice may also result from neural differentiation and/or maturation defects that were uncovered using loss-of-function approaches in neural precursor cells in vitro, or by forced expression of UPF3B mutants in cell lines in vitro (Alrahbeni et al., 2015; Huang et al., 2018; Jolly et al., 2013).
In this communication, we examine the role of UPF3B in the olfactory system, a useful model for studying neural development and function. There is also considerable clinical interest in the olfactory system, as olfactory defects predict the later onset of numerous CNS disorders, including Parkinson’s and Alzheimer’s disease (Doty, 2012). Olfactory dysfunction also strongly associates with autism (Rozenkrantz et al., 2015). The olfactory epithelium (OE) retains a life-long capacity for neurogenesis and harbors a robust regeneration system that responds to injury (Whitman and Greer, 2009). Importantly, the olfactory system is much simpler than the CNS. Mature olfactory sensory neurons (mOSNs) develop via a relatively simple linear pathway involving horizontal basal cells (HBCs), globose basal cells (GBCs), and immature olfactory sensory neurons (iOSNs). Both HBCs and GBCs are stem cells, but the two types have different roles (Schwob et al., 2017). HBCs are reserve stem cells, as they are normally quiescent and only undergo proliferative expansion in response to OE injury (Peterson et al., 2019). In contrast, GBCs are a heterogeneous cell population that consists of constitutively active stem cells as well as progenitors (Schwob et al., 2017). Lineage-tracing analysis and single-cell RNA sequencing (scRNA-seq) analysis have shown that after proliferative expansion, HBCs and GBCs give rise to iOSNs, which are responsible for undergoing maturation (Fletcher et al., 2017). Of note, iOSNs share markers with another OSN stage called ‘immediate neural precursors (INPs).” Given the ambiguity of the nomenclature, we will refer to cells with either INP or iOSN characteristics as iOSNs. iOSNs ultimately differentiate into mOSNs, which send an axon to neurons in the glomeruli region of the olfactory bulb, relaying olfactory information from the outside world to the CNS. mOSNs recognize odorants through chemosensory receptors, including olfactory receptors (OLFRs), members of the G-protein-coupled receptor super-family, as well as trace amine–associated receptors, guanylate cyclases, and members of the membrane-spanning 4-pass A gene family (Bear et al., 2016; Saraiva et al., 2019).
To gain insight into the nature of the cells in the OE and their developmental relationships, recent studies have performed transcriptome profiling using whole OE, pools of sorted OSNs, single OSNs, or single OE cells (Fletcher et al., 2017; Ibarra-Soria et al., 2014; Saraiva et al., 2015; Saraiva et al., 2019; Tan et al., 2015). These studies have revealed new OE cell subsets, inferred the developmental pathways of both OSN and non-neural OE cells, defined classes of genes exhibiting enriched expression and unique patterns of expression in different OE subsets, and revealed the expression patterns and dynamics of OLFRs during OSN development and in individual mOSNs. These studies have also advanced our understanding of mammalian olfaction evolution.
In this study, we ascertain whether the NMD factor, UPF3B, has roles in the olfactory system. Using scRNA-seq and RNA-seq analyses, we obtained evidence that UPF3B influences the frequency of specific OSN subsets, broadly suppresses the expression of immune genes in OSNs, and shapes the Olfr gene repertoire. We also identified high-confidence NMD target mRNAs in vivo that are candidates to act downstream of UPF3B in mOSNs. As part of our analysis, we also provide new cellular and molecular information on WT OSNs and their development in vivo. Our findings in Upf3b-null NMD-deficient mice introduce a useful biological system to understand the role of RNA metabolism in neurons, and our scRNA-seq, RNA-seq, and RiboTag datasets are new resources that can be used by the olfactory field.
Results
UPF3B-regulated genes in mOSNs
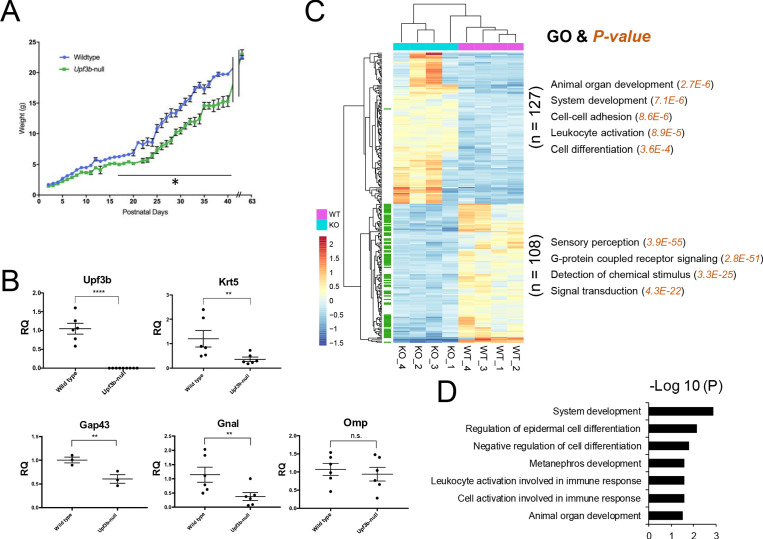
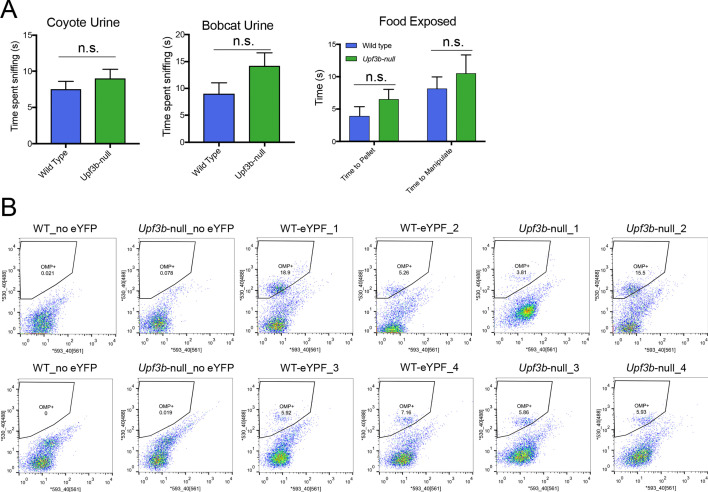
To assess whether NMD-deficient Upf3b-null mice have an olfactory defect, we measured their weight during their growth phase. This follows from the fact that newborn mice are blind and therefore depend on the olfactory system to initiate milk suckling for survival (Logan et al., 2012). We quantified the weight of Upf3b-null and littermate WT mice and found that Upf3b-null mice have a statistically significant postnatal weight deficit (p<0.05; Figure 1A). The weight deficit occurs soon after birth, becomes progressively worse during postnatal development, and is corrected after reaching adulthood. This specific pattern of weight loss is characteristic of mice harboring a partial olfactory defect (Riera et al., 2017). In contrast, newborn mice that completely lack sense of smell are incapable of sensing their source of milk and die soon after birth (Hongo et al., 2000). As further evidence that Upf3b-null mice have an olfactory defect, we found that HBC, iOSN and mOSN marker genes (Krt5, Gap43, and Gnal, respectively) exhibited significantly decreased expression in Upf3b-null as compared to WT OE (Figure 1B). We followed up by testing Upf3b-null mice for evidence for specific olfactory deficits and observed trends but did not observe statistically significant effects (Figure 1—figure supplement 1A), providing further evidence of a partial olfactory defect.
Figure 1. Identification of UPF3B-regulated genes and NMD target genes in the olfactory system.
(A) The weight of Upf3b-null vs. WT (wild type) mice at the indicated time points. Upf3b-null mice gain weight slowly during postnatal development but then reach the weight of WT mice at the last time point (9 weeks), a pattern indicative of a partial olfactory defect. *, p<0.05. (B) qPCR analysis of olfactory marker genes in Upf3b-null and WT OE (n = 6). **, p<0.01; ****, p<0.0001. (C) Heatmap of genes differentially expressed in mOSNs from Upf3b-null (KO) vs. WT mice (four biological replicates from each are shown). Row names labeled as green are Olfr genes. Right, the most statistically significant GO terms associated with upregulated genes (top) and downregulated genes (bottom) after Upf3b loss. (D) A list of most statistically enriched GO terms associated with the 52 high-confidence UPF3B-dependent NMD target mRNAs we identified in mOSNs.
Figure 1—figure supplement 1. Upf3b-null mice behavior and purified mOSNs.
Figure 1—figure supplement 2. UPF3B-regulated genes in mOSNs.
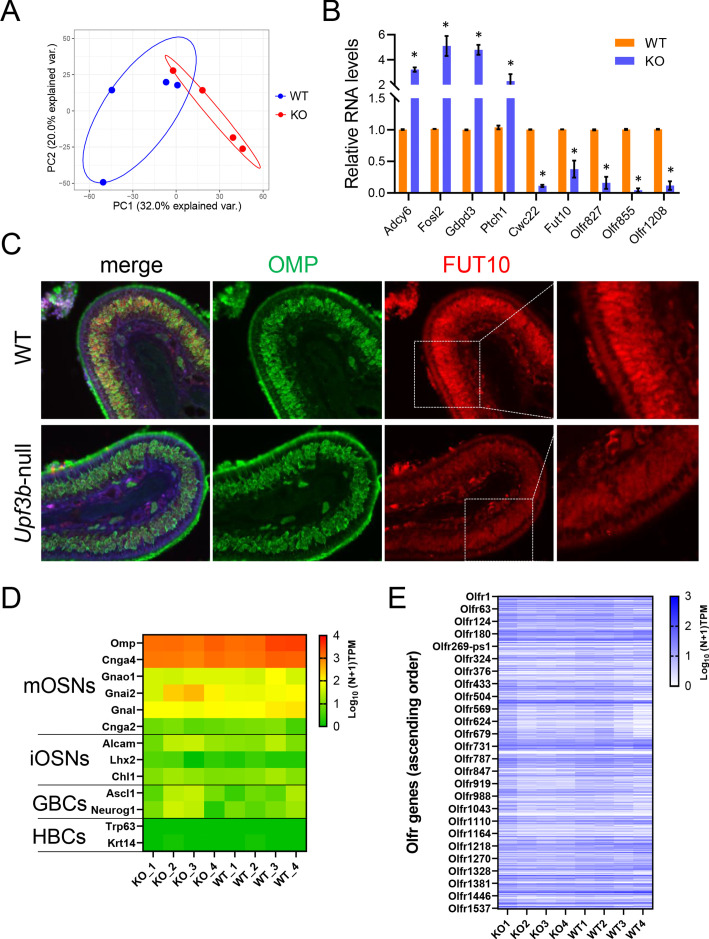
Given that mOSNs are the functional units of the OE, we next focused our attention on these cells. We identified UPF3B-regulated genes in mOSNs by performing RNA-seq analysis on FACS-purified mOSNs (YFP+ cells) from R26-eYFP; Omp-Cre mice (Figure 1—figure supplement 1B). Four samples were analyzed from each genotype (Figure 1—figure supplement 2A and Supplementary file 1). The expression of OSN precursor/OSN canonical markers are shown in Figure 1—figure supplement 2D. RNA-seq analysis identified 235 differentially expressed genes between Upf3b-null and WT mOSNs (q < 0.05) (Figure 1C and Supplementary file 2). We validated our RNA-seq analysis by qPCR analysis and immunofluorescence (Figure 1—figure supplement 2B,C).
Among the 127 upregulated genes were several involved in neurogenesis, including Lrp2, Hk2, Notch2, Gdf11, Fos, Ptch1, and Spry2. Gene ontology (GO) analysis revealed enrichment for ‘organ/system development,’ ‘cell-cell adhesion,’ ‘leukocyte activation,’ and ‘cell differentiation/proliferation’ functions (Figure 1C). In contrast, the 108 downregulated genes were most enriched for GO functions associated with olfaction: ‘sensory perception,’ ‘G-proteins,’ ‘detection of chemical stimulus,’ and ‘signal transduction.’ Indeed, we found that the majority (78 out of 108) of these significantly downregulated genes are Olfr genes (marked in green in Figure 1C; the expression of all Olfr genes in Upf3b-null and control mOSNs is shown in Figure 1—figure supplement 2E and Supplementary file 1). We follow-up on this surprising finding below. Other genes downregulated in Upf3b-null mOSNs include those involved in CNS synaptic transmission (Slc17a6), chromatin remodeling (Chd1), and sensory neuronal plasticity (Cwc22) (Supplementary file 2).
Identification of NMD target mRNAs in mOSNs
NMD is thought to influence biological systems by virtue of its ability to promote the decay of specific subsets of mRNAs (Lykke-Andersen and Jensen, 2015). As described in the introduction, there is dearth of knowledge regarding the identity of such NMD target RNAs, particularly in cells in their normal in vivo context. Our RNA-seq analysis of purified mOSNs from Upf3b-null and WT mice provided an opportunity to identify in vivo direct NMD targets. Because NMD is a negative regulatory pathway (it degrades its targets), the 127 RNAs upregulated in Upf3b-null mOSNs are candidates to be direct NMD targets. Among them, we found that 73 had at least one of the well-established molecular features known to elicit NMD, including an exon-exon junction >50 nt downstream of the main ORF (dEJ) (Table 1; see the Introduction for an explanation of NMD-inducing features [NIFs]). Thus, these 73 mRNAs are strong candidates to be UPF3B-dependent NMD target mRNAs in mOSNs.
Table 1. UPF3B-dependent NMD target mRNAs in mOSNs.
| Symbol | log2FC (KO/WT) | Padj | dEJ | uORF | 3'UTR length | Symbol | log2FC (KO/WT) | Padj | dEJ | uORF | 3'UTR length |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prelid3a | 1.099967 | 0.003745 | YES | NO | 1572 | Fmo2 | 2.02039 | 0.014815 | NO | NO | 2411 |
| 1700025G04Rik | 0.662926 | 0.012989 | NO | YES | 8870 | Gab2 | 0.98414 | 0.003018 | NO | NO | 3927 |
| 6030419C18Rik | 0.73232 | 0.036112 | NO | YES | 55 | Gdf11 | 1.429234 | 0.005353 | NO | NO | 2811 |
| 9330159F19Rik | 0.542375 | 0.017617 | NO | YES | 3408 | Gldn | 2.115908 | 0.045841 | NO | NO | 2970 |
| Adcy6 | 2.587005 | 0.002078 | NO | YES | 2356 | Hk2 | 2.296045 | 0.033161 | NO | NO | 2285 |
| Cdh24 | 1.560901 | 0.001303 | NO | YES | 121 | Lbh | 1.417311 | 0.024315 | NO | NO | 2498 |
| Fam84b | 0.719841 | 0.001704 | NO | YES | 3969 | Luc7l | 0.492061 | 8.19E-05 | NO | NO | 3738 |
| Inpp5f | 1.178064 | 0.043839 | NO | YES | 949 | Map3k9 | 0.79841 | 0.021555 | NO | NO | 1029 |
| Lrp2 | 2.504276 | 0.008534 | NO | YES | 1305 | Msrb3 | 1.668851 | 0.033388 | NO | NO | 2972 |
| Mafg | 0.577538 | 0.046713 | NO | YES | 4167 | Neurl3 | 1.966306 | 0.00546 | NO | NO | 1763 |
| Plxnc1 | 2.322167 | 0.048567 | NO | YES | 2320 | Notch2 | 1.68375 | 0.047733 | NO | NO | 2917 |
| Prdm4 | 0.420203 | 0.027945 | NO | YES | 1160 | Plekha5 | 0.608634 | 0.004216 | NO | NO | 3461 |
| Ptch1 | 0.768864 | 0.01088 | NO | YES | 3205 | Rab43 | 1.033148 | 0.0151 | NO | NO | 3737 |
| Ptger2 | 3.032221 | 0.035664 | NO | YES | 1825 | Rac2 | 3.029035 | 0.038392 | NO | NO | 2319 |
| Sash3 | 2.352656 | 0.033245 | NO | YES | 1309 | Raver2 | 1.921185 | 0.027779 | NO | NO | 1892 |
| Serpinb11 | 1.991555 | 0.002719 | NO | YES | 468 | Rflnb | 0.755198 | 0.017617 | NO | NO | 2716 |
| Snx33 | 1.512032 | 0.012417 | NO | YES | 1258 | Sik1 | 2.027635 | 1.48E-06 | NO | NO | 2035 |
| Zfp36 | 1.802697 | 0.025165 | NO | YES | 774 | Slc38a6 | 1.21151 | 0.025847 | NO | NO | 1512 |
| Agap2 | 1.264604 | 0.00099 | NO | NO | 1357 | Slc5a1 | 2.563582 | 0.00527 | NO | NO | 1868 |
| Aox2 | 1.36834 | 0.018035 | NO | NO | 1640 | Swap70 | 1.863436 | 0.009993 | NO | NO | 2169 |
| Atp10d | 3.315656 | 0.017617 | NO | NO | 2384 | Tgm2 | 2.395934 | 0.042993 | NO | NO | 1399 |
| Bhlhe40 | 1.435423 | 0.000192 | NO | NO | 1593 | Themis2 | 3.496025 | 0.015464 | NO | NO | 1053 |
| Btg2 | 1.281148 | 0.000173 | NO | NO | 2199 | Tmprss2 | 2.167673 | 0.005867 | NO | NO | 1456 |
| Cybrd1 | 2.372842 | 0.002733 | NO | NO | 4269 | Tob2 | 0.667082 | 0.001453 | NO | NO | 2459 |
| Cyth4 | 2.162221 | 0.045105 | NO | NO | 1455 | Ywhag | 0.644673 | 0.017707 | NO | NO | 2586 |
| Ermn | 1.686519 | 0.005793 | NO | NO | 2641 | Zcchc6 | 0.512078 | 0.003018 | NO | NO | 1346 |
Given that NMD degrades its target RNAs, this predicts that its targets should be stabilized after inactivation of UPF3B. Thus, we measured the stability of the 127 mRNAs upregulated in Upf3b-null mOSNs using a method that infers RNA stability based on pre-mRNA and steady-state mRNA levels (Alkallas et al., 2017). This method revealed that 82 of 127 upregulated genes encode mRNAs stabilized in Upf3b-null mOSNs as compared to WT mOSNs (Supplementary file 2). Of these 82 stabilized and upregulated mRNAs, 52 have at least 1 of the 3 well-established NIFs (Table 1), and thus we classified these 52 mRNAs as high-confidence mOSN NMD targets. The statistically enriched GO biological functions of the proteins encoded by these 52 mRNAs are listed in Figure 1D.
To determine whether these high-confidence NMD target mRNAs correspond to known NMD targets, we assembled a list of likely mouse NMD substrates defined by previous studies (Supplementary file 3). To qualify to be in this list, the RNA must have at least one known NMD-inducing feature (NIF) (Palacios, 2013) and experimental evidence from at least one assay that it is an NMD substrate (e.g. high UPF1 occupancy or upregulation and/or stabilization in response to NMD-factor depletion). We found that 11 of these previously defined likely mouse NMD target mRNAs overlapped with the 52 high-confidence targets identified in our study: Atp10d, Lbh, Slc38a6, Tgm2, Notch2, Ywhag, Luc7l, Ptch1, 1700025G04Rik, Ptger2, and Msrb3. Of note, it is not surprising that only a proportion of the upregulated mRNAs we identified in NMD-deficient mOSNs are previously known NMD targets, as NMD target mRNAs can be tissue-, cell type-, and NMD factor-specific (Huang et al., 2011). The list of previously defined candidate NMD targets that we compared with were defined in non-neuronal tissues and cell lines made deficient in NMD by knocking down or eliminating factors other than UPF3B (Supplementary file 3).
The mOSN transcriptome and translome
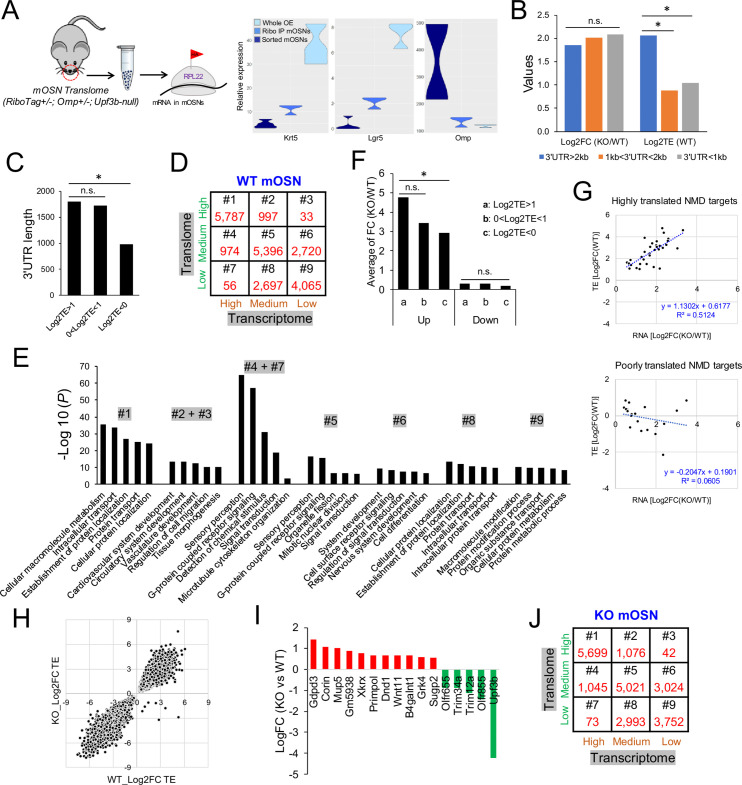
We next determined the translation rate of mRNAs in mOSNs, both as a resource for the field and to address the relationship of NMD with translation in vivo. We assayed the translation rate of mRNAs in mOSNs using RiboTag mice, which express an epitope-tagged ribosomal protein, RPL22HA, which is incorporated into actively translating ribosomes specifically in cells expressing CRE (Sanz et al., 2009). Immunoprecipitation (IP) of the cell lysates of interest with an HA antibody purifies the ribosome-associated mRNAs (Figure 2A, left) with an efficiency associated with polysome density (Hornstein et al., 2016). To examine ribosome density specifically in WT mOSNs, we isolated RiboTag-labeled mRNA from the OE of RiboTag; Omp-Cre mice and performed RNA-seq analysis. As a validation of cell-type specificity, we found that IP of OE lysates with the HA antisera enriched for the mOSN marker, Omp, whereas these lysates were depleted of the HBC and GBC markers, Krt5 and Lgr5, respectively (Figure 2A, right). We then elucidated inferred translation efficiency (TE) for all expressed mRNAs in mOSNs – the ‘mOSN translome’ – by calculating the ratio of the IP signal from the RiboTag mice OE lysates over mOSN steady-state mRNA level, the latter determined as described above (Supplementary file 2).
Figure 2. The mOSN translome and NMD.
(A) Left, strategy used to define the mOSN translome. Right, RNAseq analysis of the expression of gene markers for HBCs (Krt5), GBCs (Lgr5), and mOSNs (Omp) in the indicated samples. (B) Average inferred translation efficiency (TE) of mOSN mRNAs with the indicated 3’UTR length ranges. *, p<0.05. (C) Average 3’UTR length of mOSN mRNAs with the indicated range of TE values. *, p<0.05. (D) mOSN mRNAs from WT mice stratified by steady-state mRNA level (transcriptome) and TE. The number of genes in each category is indicated. (E) Top enriched GO terms associated with the different categories of genes defined in (D). (F) Analysis of upregulated mRNAs (candidate NMD targets) and downregulated mRNAs (indirect targets) are shown on the left and right, respectively. The average shift in expression in Upf3b-null mOSNs relative to WT mOSNs is shown for mOSNs binned by TE (a and c have the highest and lowest TE values, respectively). *, p<0.05. (G) Scatter plot of the 52 high-confidence mOSN NMD targets, showing TE vs. NMD magnitude (upregulation in Upf3b-null mOSNs). Both values are log2-transformed. (H) Scatterplot showing the TE of mRNAs in Upf3b-null vs. WT mOSNs. (I) mRNAs exhibiting significantly altered TE in response to Upf3b loss. (J) mOSN mRNAs from Upf3b-null (KO) mice stratified by steady-state mRNA level (transcriptome) and TE. The number of cells in each category is indicated.
Given that 3’UTR length has been shown to influence translation rates in cultured cells (Spies et al., 2013), we examined the relationship of 3’UTR length and TE in mOSNs in vivo. We found that mOSN mRNAs harboring 3’UTRs of >2 kb have much higher average TE than mOSN mRNAs harboring shorter 3’UTRs (Figure 2B). Highly translated mOSN mRNAs have an average 3’UTR length of ~1.8 kb, while lowly translated mOSN mRNAs have an average 3’UTR length of only ~0.9 kb (Figure 2C).
To assess the potential functional relevance of translation, we binned WT mOSN mRNAs into three groups: high (top 30%), medium (middle 40%), and low (bottom 30%) (Supplementary file 2). We also binned WT mOSN mRNAs into three groups based on their steady-state level (Supplementary file 2), allowing us to place mOSN mRNAs into the nine categories shown in Figure 2D. GO analysis revealed that category #1—which is mRNAs expressed at high level that are also highly translated—encode proteins that tend to function in ‘metabolism,’ ‘intercellular transport,’ and ‘catabolism’ (Figure 2E). Categories #2 and #3—which are also highly translated mRNAs but less well expressed at the RNA level than category #1—encode proteins with strikingly different functions: ‘development,’ ‘cell migration,’ and ‘morphogenesis’ (Figure 2E). Category #6—which is lowly expressed and modestly translated mRNAs—encode proteins involved in ‘signal transduction,’ ‘differentiation,’ and ‘development,’ including ‘nervous system development’ (Figure 2E). The categories with most Olfr genes—#4 and #5—are also only moderately translated (Figure 2E). Upf3b-null mOSNs had similar numbers of mRNAs in the nine categories as WT mOSNs (compare Figure 2J with Figure 2D), indicative of UPF3B not altering the mOSN transcriptome and translome globally. Rather, UPF3B influences specific mRNAs, as described above for the mOSN transcriptome, and below for the mOSN translome.
The relationship between NMD and translation in vivo
NMD is a translation-dependent pathway, based on protein-synthesis inhibitor and transfection experiments in immortalized cell lines (Belgrader et al., 1993; Carter et al., 1995; Karousis and Mühlemann, 2019). Our mOSN transcriptome and translome data from Upf3b-null and WT mice provided an opportunity to address the relationship of NMD with translation in vivo. Given that higher translation rates allow for a higher frequency of stop codon recognition, it follows that higher translation rates might drive stronger NMD. This predicts that more highly translated mOSN mRNAs will have a higher NMD response than lowly translated mOSN mRNAs. To test this, we binned mRNAs statistically upregulated in Upf3b-null mOSNs into three groups stratified by TE. The most highly translated group was statistically more upregulated (i.e., had stronger NMD) than the least translated group (Figure 2F, left). As a negative control, we examined downregulated mRNAs (as these would not be direct NMD targets) and found no statistical difference between degree of downregulation and TE (Figure 2F, right).
To further examine whether high translation rate is associated with strong NMD magnitude, we binned the 52 high-confidence NMD substrates we defined above into two groups: those with little or no translation and those with high translation (cut-off: log2TE > 1). We then independently plotted these two sets of mRNAs in terms of TE and NMD magnitude (i.e. the degree of upregulation in Upf3b-null mOSNs relative to WT mOSNs). The results show that the high-translation group exhibited a correlation between their inferred translation rate and NMD magnitude (R2 = 0.5; Figure 2G). In contrast, the low-translation group of mRNAs exhibited no correlation between their translation rate and NMD magnitude (R2 = 0.06; Figure 2G). Together, these results support that NMD is translation-dependent in vivo and that its magnitude tends to be enhanced for highly translated mRNAs.
Our mOSN translome data also allowed us to assess the reciprocal question: does Upf3b influence translation in vivo? When we plotted the TE of mRNAs when expressed in Upf3b-null mOSNs vs. when expressed in WT mOSNs, we found that the vast majority of mRNAs were similarly translated in both genetic backgrounds, as measured by RiboTag analysis (Figure 2H, Supplementary file 2). Only 16 mOSN mRNAs migrated off the diagonal and thus had a significant change in TE as a result of Upf3b loss (Figure 2H,I).
Identification of OE cell clusters
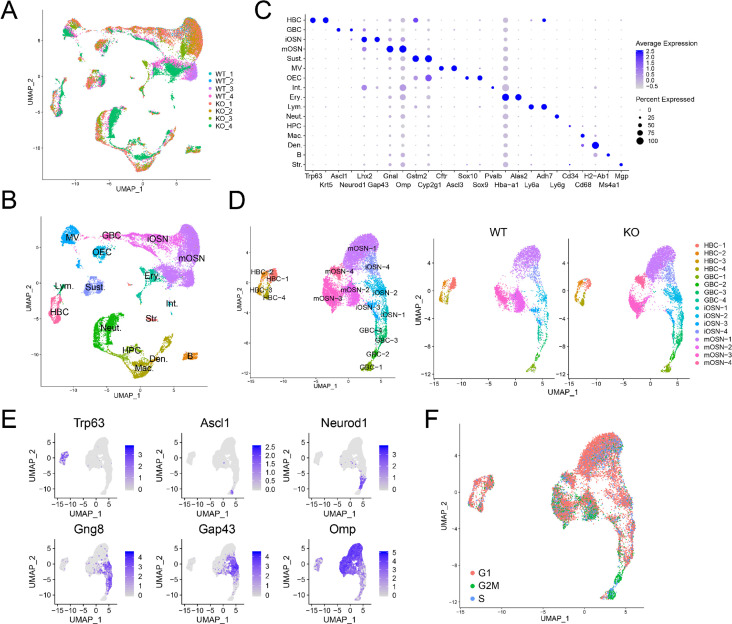
To determine whether UPF3B influences the cellular composition of the OE, we performed scRNA-seq analysis on dissociated OE cells from 4 Upf3b-null and 4 WT mice. After filtering out poor quality cells, 25,165 cells remained for subsequent analysis. Biological replicates exhibited similar cell distributions (Figure 3A). Using a nonlinear dimensionality-reduction technique—uniform manifold approximation and projection (UMAP)—we identified cell clusters corresponding to 16 known cell types in the OE (Figure 3B). Some of the gene markers used to define these cell clusters are shown in Figure 3C. Genes exhibiting enriched expression in each of the 16 cell types are listed in Supplementary file 4.
Figure 3. Identification of OE cell subsets using scRNAseq analysis.
(A) UMAP plot of OE cells from 4 Upf3b-null (KO) and 4 WT mice analyzed by scRNAseq. (B) Same UMAP plot as is in (A), showing the identity of the different cell clusters. (C) Dotplot depicting the expression of gene markers in the cell clusters defined in (B). (D) Left, UMAP plot of reclustered OSN precursors/OSNs defined in (A). Right, genotype information. (E) Same UMAP plot as in (D), showing the expression of stage-specific markers. (F) Same UMAP plot as in (D), showing inferred cell-cycle phase based on the expression of a large set of G2/M- and S-phase genes (Kowalczyk et al., 2015).
Re-clustering of OSN precursors/OSNs (HBCs, GBCs, iOSNs, and mOSNs) revealed several cell sub-clusters within each of these four stages (Figure 3D,E). The identification of these sub-clusters suggested that each of these developmental stages exhibit considerable heterogeneity, at least at the transcriptome level. Genes exhibiting enriched expression in each sub-cluster are shown in Supplementary file 4.
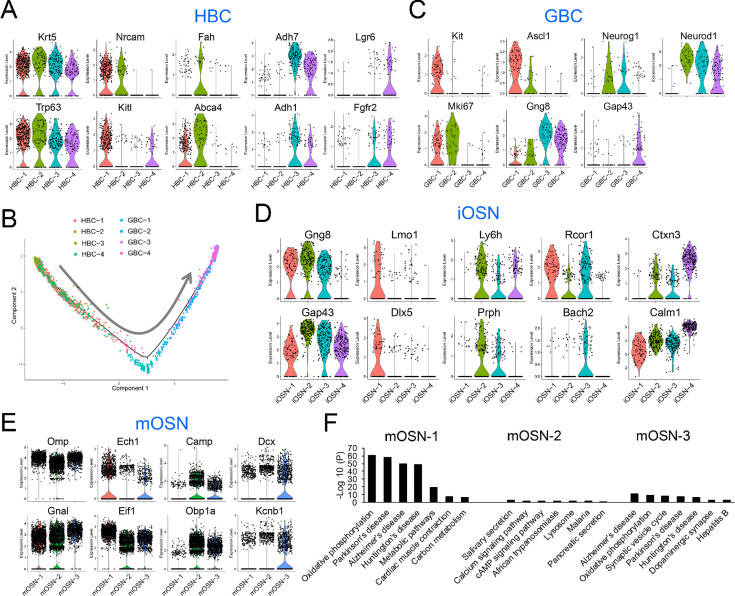
HBC are known to be reserve stem cells, while GBCs consist of active stem cells and progenitors (Schwob et al., 2017). Consistent with this, cell-cycle analysis showed that all four HBC sub-clusters primarily contain quiescent cells, while the GBC sub-clusters have many cells that are proliferating (Figure 3F). All four HBC sub-clusters express similar levels of well-established HBC markers, including Krt5 and Trp63 (Figure 4A). These HBC sub-clusters are each uniquely marked by novel gene markers that we identified (Figure 4A and Supplementary file 4).
Figure 4. HBC, GBC, iOSN and mOSN heterogeneity.
(A) Violin plots showing the expression of selective gene markers in the four indicated HBC sub-clusters in WT mice. (B) Monocle trajectory analysis of the HBC and GBC sub-clusters we identified. The arrow indicates the inferred direction of differentiation. (C–E) Violin plots showing the expression of selective gene markers in the indicated GBC, iOSN, and mOSN sub-clusters in WT mice. (F) The most statistically enriched signaling pathways in the mOSN-1,–2, and −3 sub-clusters.
GBCs also segregated into four sub-clusters (Figure 3D), which is consistent with past studies demonstrating that GBCs are heterogeneous (Cau et al., 1997; Manglapus et al., 2004; Schwob et al., 2017). Monocle pseudotime trajectory analysis suggested that these four sub-clusters have a linear developmental relationship, with GBC-1 the most immature, GBC-2 more advanced, and GBC-3 and −4 the most advanced (Figure 4B). Consistent with this developmental trajectory, both GBC-1 and GBC-2 express the early GBC marker Ascl1 (Cau et al., 1997; Manglapus et al., 2004). GBC-1 is likely to be more primitive than GBC-2, based on the frequent and high expression of the later GBC markers Neurog1 and Neurod1 (Cau et al., 1997; Manglapus et al., 2004) in the latter, not the former (Figure 4C). While GBC-3 and −4 are clearly GBCs based on the expression of several GBC markers (e.g. Neurog1 and Neurod1), they also express iOSN markers (e.g. Gng8 and Gap43 [Iwema and Schwob, 2003; Figure 4C]), consistent with GBC-3 and -4 being iOSN precursors and hence advanced GBCs. iOSNs also segregated into several cell sub-clusters that each express unique genes (Figure 4D). These iOSN sub-clusters follow a ‘linear’ pattern as depicted by the UMAP algorithm (Figure 3D), consistent with them representing sequential developmental states, each with unique transcriptomes.
Most WT mOSNs segregated into three different cell clusters (Figure 3D), each of which preferentially express different genes (Figure 4E). GO and KEGG signaling pathway analyses indicated that these three mOSN sub-clusters are enriched for different functions and signaling pathways, respectively (Figure 4F; Supplementary file 4).
OSN molecular pathways
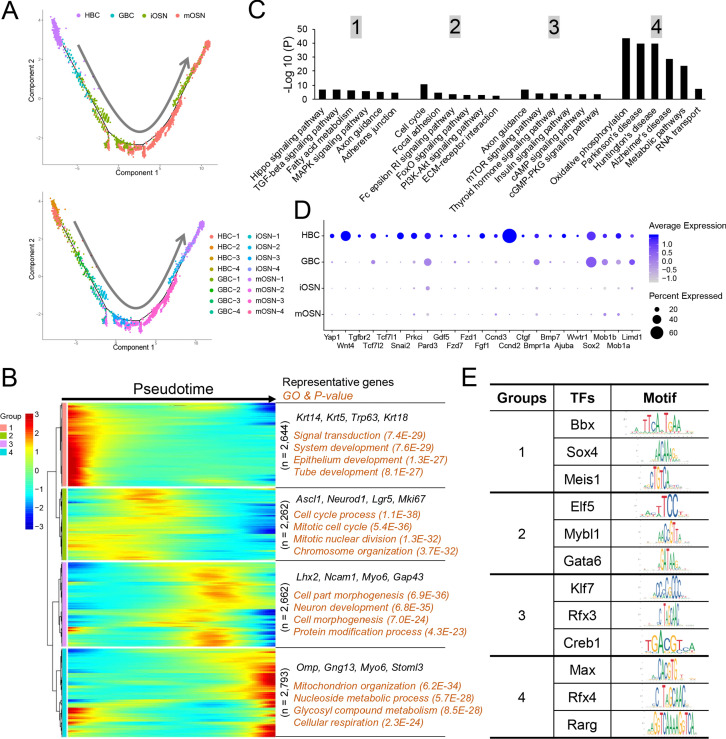
Monocle pseudotime analysis of the OSN precursor/OSN cell clusters indicated that they follow a HBC→GBC→iOSN→mOSN trajectory (Figure 5A), consistent with previous studies (Fletcher et al., 2017; Schwob et al., 2017; Tepe et al., 2018). To define candidate molecular events occurring during OSN development, we identified genes whose expression is statistically enriched along this pseudotime trajectory (Supplementary file 4). This analysis identified 4 distinct patterns of gene expression dynamics that we named groups 1 to 4 (Figure 5B). Group-1 genes are dominated by genes expressed transiently in HBCs, including the previously defined HBC-marker genes Trp63, Krt5, and Krt14. Group-1 genes are statistically enriched for ‘signal transduction’ and various ‘development’ categories (Figure 5B). Group-2 genes contain GBC genes; indeed the GBC markers Ascl1, Neurod1, and Lgr5 are enriched in group 2. ‘Cell cycle process’ is statistically enriched (Figure 5B), consistent with the fact that GBCs undergo self-renewal and proliferative expansion. Group-3 genes are mainly expressed in iOSNs, include the well-established iOSN marker genes Lhx2, Ncam1, and Gap43. ‘Neuron development’ is enriched in group 3 (Figure 5B), consistent with the fact that iOSNs are undergoing the final stages of development prior to becoming mature neurons. Group-4 genes are mainly expressed in mOSNs; enriched GO categories include ‘mitochondrion organization,’ ‘metabolism,’ and ‘cellular respiration’.
Figure 5. Gene groups exhibiting distinct expression dynamics during OSN development.
(A) Monocle pseudotime trajectory analysis of the indicated cell clusters and sub-clusters from WT mice defined in Figure 3B (top) and Figure 3D (bottom), respectively. (B) Heatmap depicting the expression pattern of the four gene groups we defined, each with a unique expression pattern, as defined by the trajectory timeline shown in (A), upper. Top: pseudotime directions; right: the number of differentially expressed genes in each group and representative biological processes and P-values. (C) The most statistically enriched signaling pathways corresponding to each of the four gene groups defined in (B). (D) Dot plot showing genes related to the Hippo signaling pathway are primarily expressed in HBCs. (E) Transcription factor genes exhibiting the most statistically enriched expression in each gene group defined in (B). Target sequences predicted by the ENCODE database are indicated.
KEGG signaling pathway analysis revealed that genes involved in different signaling pathways are enriched in each of the 4 groups (Figure 5C). For example, Hippo pathway genes are enriched in group 1 (Figure 5D), raising the possibility this signaling pathway may be important for maintaining HBC stem cells in the quiescent state or eliciting their activation in response to insults.
We also screened for transcription factors preferentially expressed at different stages of OSN development. We identified 209, 178, 169, and 135 transcription factor genes exhibiting enriched expression in groups 1, 2, 3 and 4, respectively (Supplementary file 4). The top 3 transcription factors in each group and their DNA-binding specificity are shown in Figure 5E.
UPF3B impacts HBCs and mOSNs
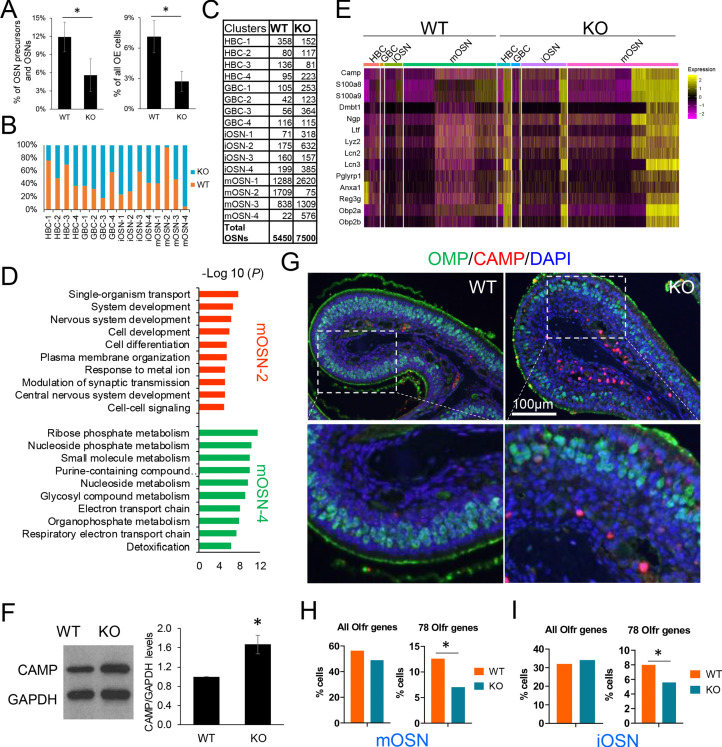
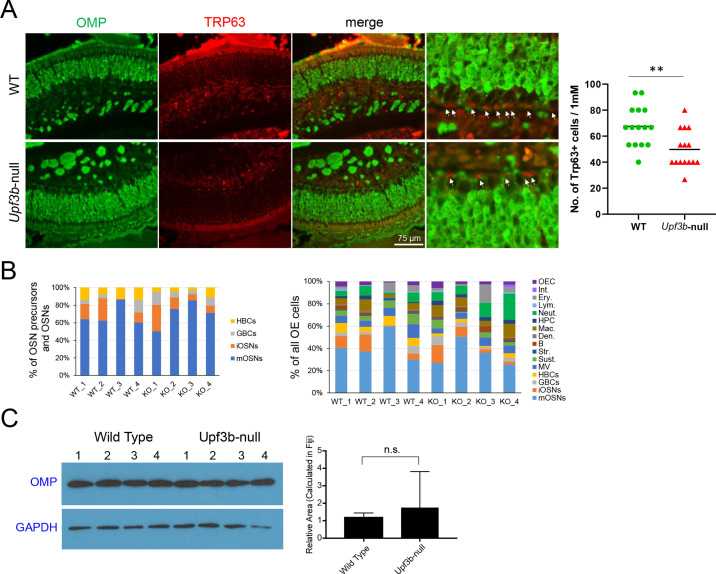
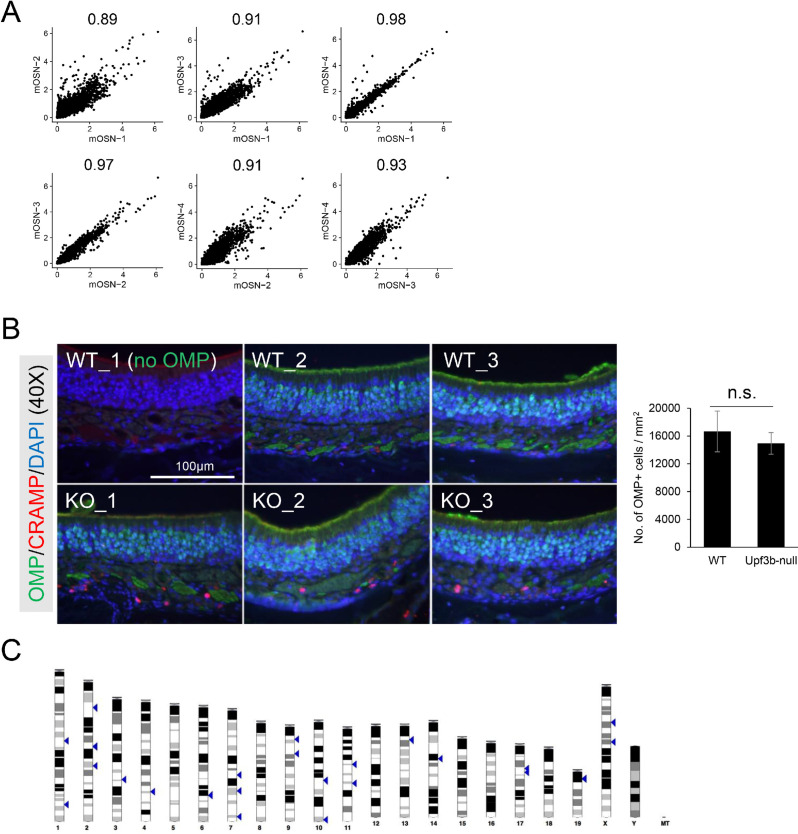
The array of UPF3B-dependent NMD targets we identified in mOSNs (Figure 2) raised the possibility that UPF3B has roles in mOSNs and possibly OSN precursors. To assess this, we first determined whether loss of UPF3B impacts the frequency of HBCs, GBCs, iOSNs, and mOSNs. scRNA-seq analysis revealed that there was a significant reduction in the frequency of HBCs in Upf3b-null mice relative to WT mice, when compared to either all OSN precursors/OSNs or all OE cells (p<0.05; Figure 6A). As validation, IHC staining with the HBC marker, TRP63, showed that the density of TRP63+ cells was significantly less in Upf3b-null OE than WT OE (Figure 6—figure supplement 1A). This effect appeared to be specific, as we observed no significant difference in the relative proportion of GBCs, iOSNs, and mOSNs between Upf3b-null and WT mice (Figure 6—figure supplement 1B). However, we cannot rule out that the variability among the four samples for each genotype might have obscured a subtle change in the fraction of GBCs, iOSNs, or mOSNs in Upf3b-null mice. This variability might either be the result of biological differences between individual mice or differences in dissection and/or cell dissociation. However, as further evidence that the overall frequency of mOSNs was not affected in Upf3b-null mice, the mOSN marker, OMP, was similarly expressed (at both the RNA and protein levels) in OE from Upf3b-null and WT mice, as assessed in OE preparations obtained from different mice (but of the same genotypes) than those used for scRNA-seq analysis (Figure 1B and Figure 6—figure supplement 1C).
Figure 6. UPF3B shapes olfactory neurogenesis.
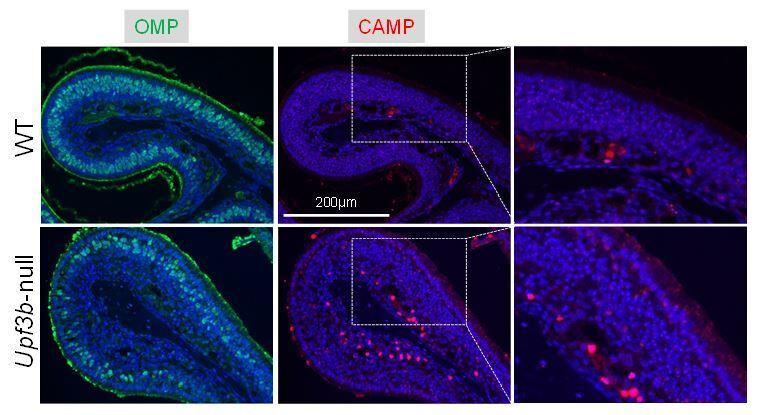
(A) The fraction of HBCs per all OSN precursors/OSNs (HBCs, GBCs, iOSNs and mOSNs) (left) or all OE cells (right), in Upf3b-null (KO) and WT mice, as determined by scRNA-seq analysis. *, p<0.05. (B) The percentage of cells from the indicated cell sub-clusters in Upf3b-null (KO) and WT mice, as determined by scRNAseq analysis. (C) Cell number in each cell sub-cluster, as defined in Figure 3D. (D) Most statistically enriched GO terms in the mOSN-2 and −4 sub-clusters. (E) Heatmap depicting the expression pattern of anti-microbial genes in the indicated cell subsets. (F) Left: Western blot analysis of endogenous CAMP protein level in the OE from Upf3b-null (KO) and WT mice. Right: quantification of CAMP level normalized against GAPDH (n = 3). *, p<0.05. (G) IF analysis of adult mouse OE sections co-stained with antisera against CAMP (red) and OMP (green). Nuclei were stained with DAPI (blue). (H, I) The percentage of mOSNs (H) and iOSNs (I) in our scRNAseq datasets that express Olfr genes. Left, all known Olfr genes. Right, the 78 Olfr genes significantly downregulated in Upf3b-null mice, based on RNAseq analysis (Figure 1C). *, p<0.05.
Figure 6—figure supplement 1. Impact of UPF3B loss on OE cell subsets.
Figure 6—figure supplement 2. mOSN subsets and UPF3B-regulated genes.
Our identification of HBC, GBC, iOSN, and mOSN sub-clusters (Figure 3D) gave us an opportunity to elucidate whether UPF3B has a role in this unexpected heterogeneity. Despite no significant effect on the mOSN stage as a whole (Figure 6—figure supplement 1B), we observed a striking increase in the frequency of 1 of the 4 mOSN sub-clusters—mOSN-4—in Upf3b-null mice (Figure 6B,C). This sub-cluster represented <1% of all OSNs in most WT mice and increased by an average of 25-fold in Upf3b-null mice (p<0.05). Conversely, Upf3b-null mice had an almost complete loss of another mOSN sub-cluster—mOSN-2—a sub-cluster that was populated by many cells in most WT mice (Figure 6B,C). While this reduction failed to reach statistical significance because of variability between samples (p=0.24), it is supported by the independent tSNE plots we generated for Upf3b-null vs. WT OSNs (Figure 3D). Together these results raise the possibility that a ‘mOSN subset switch’ occurs in Upf3b-null mice. Pearson correlation analysis showed that mOSN-2 and −4 sub-clusters are less related to each other in expression profile than they are to the other two mOSN sub-clusters (Figure 6—figure supplement 2A). Indeed, these two mOSN sub-clusters have remarkably distinct molecular characteristics (Figure 6D and Supplementary file 4). Thus, the simultaneous loss and acquisition of these mOSN subsets in Upf3b-null mice has the potential to alter olfaction.
UPF3B shapes the OLFR repertoire and suppresses immune gene activation
To define genes that are candidates to act downstream of NMD in different OSN cell populations, we used our scRNA-seq datasets to identify genes differentially expressed in the Upf3b-null vs. WT cell clusters (Supplementary file 4). This revealed that a major category of Upf3b-regulated genes in OSNs are immune genes, including a large fraction of genes encoding antimicrobial proteins (Supplementary file 5). This was intriguing, as it raised the possibility that OSNs not only normally function in olfaction but also in defense against microbes, a reasonable possibility given that the OE is direct contact with the outside environment. The expression of anti-microbial genes was not confined to the mature neurons in the OE (i.e. mOSNs), as we found that most of these immune-defense genes were also expressed and upregulated in Upf3b-null mice at the HBC, GBC, and iOSNs stages (Figure 6E). More than half (48 out of 88) of these upregulated mRNAs encoding immune-related proteins harbor at least one NIF (Supplementary file 5). This suggests that many of these mRNAs encoding immune system factors are directly targeted for decay by the NMD pathway. This has interesting potential physiological consequences, as described in the Discussion.
Among the antimicrobial genes expressed and upregulated in Upf3b-null OSN precursors and OSNs was Camp (also known as ‘Cramp’), which encodes a member of the cathelicidin family of antimicrobial peptides that has an important role in the defense against microbial infections, and functions in cell chemotaxis, immune mediator induction, and inflammatory response regulation (Zhang and Gallo, 2016). To further assess its regulation, we performed western blot analysis with a validated anti-CAMP antiserum, which showed that CAMP protein is expressed in the OE and is upregulated in Upf3b-null mice (Figure 6F). As further evidence, immunofluorescence analysis detected modest anti-CAMP staining in OE cells (as well as strong staining in the in the lamina propria), both of which were increased in Upf3b-null mice (Figure 6G and Figure 6—figure supplement 2B).
The other major category of genes that we discovered are regulated by Upf3b in the OE is Olfr genes. As described above, our RNA-seq analysis of purified mOSNs from Upf3b-null and WT mice revealed that the majority of genes downregulated in response to Upf3b loss are Olfr genes (Figure 1C). In total, we identified 78 Olfr genes significantly downregulated in Upf3b-null mOSNs (Figure 1C). We considered the possibility that these 78 Olfr genes are regulated by Upf3b through a common local cis-acting regulatory sequence, but against this hypothesis, we found that these 78 Olfr genes are widely chromosomally distributed (Figure 6—figure supplement 2C).
There are more than 1000 olfactory receptor (Olfr) genes in mice (Zhang and Firestein, 2002). Individual OSNs select a single Olfr gene for expression from this large repertoire (Chess et al., 1994; Malnic et al., 1999; Serizawa et al., 2003). This unique mechanism raised the possibility that rather than regulating Olfr expression per se, Upf3b might instead influence the decision whether or not specific Olfr genes are selected to be the dominant receptors in mOSNs. In other words, Upf3b might increase the probability that these 78 Olfr genes that are selected to be expressed in individual mOSNs. If selected less often in Upf3b-null OSNs, these 78 Olfr genes would appear to be downregulated, but would instead be expressed in fewer OSN. To address this model, we made use of our scRNA-seq datasets. We found that 490 of 3887 mOSNs in WT mice (13%) express one of these 78 Olfr genes as the dominant Olfr gene. In contrast, only 328 of 4654 mOSNs in Upf3b-null mice (7%) express one of these 78 Olfr genes as the dominant Olfr gene (Figure 6H). In contrast, when all known Olfr genes were considered as a group, there was no significant difference in the percentage of Olfr genes selected in Upf3b-null vs. WT mOSNs (Figure 6H). Likewise, we found that these 78 Olfr genes were under-represented in Upf3b-null iOSNs as compared with WT iOSNs (5.6% vs 8.0%, p<0.05) (Figure 6I). Together, these results support a model in which UPF3B promotes the selection of these 78 Olfr genes to be the dominant Olfr gene expressed in individual mOSNs, a model we elaborate on in the Discussion.
Discussion
NMD factors have been shown to have numerous roles in the development and function of neurons (Jaffrey and Wilkinson, 2018). As described in the Introduction, the NMD factor examined in our study – UPF3B – has been shown to be necessary for normal cognition in humans and its loss is associated with several neuro-developmental disorders (Nguyen et al., 2014; Tarpey et al., 2007). While the precise roles of UPF3B in behaviors is not known, it has been shown that UPF3B is critical for both neural differentiation and mature neuronal functions (Alrahbeni et al., 2015; Huang et al., 2018; Jolly et al., 2013). In addition to UPF3B, other NMD genes are likely to have roles in the nervous system (Jaffrey and Wilkinson, 2018). For example, copy-number gain and loss of several genes encoding proteins involved in NMD—including UPF2, UPF3A, SMG6, RBM8A, EIF4A3, and RNPS1—are statistically significantly associated with neural-developmental disorders in humans (Nguyen et al., 2013). Mutations in RBM8A have been shown to cause TAR syndrome, which can cause cognitive dysfunction (Jaffrey and Wilkinson, 2018). In mice, loss of a single copy of Rbm8a or other EJC genes (Magoh or Eif4e) causes microcephaly and severe neural defects (Mao et al., 2017). In worms, flies and mice, genetic perturbation of other NMD genes causes neural defects, including synaptic and axon guidance defects (Colak et al., 2013; Giorgi et al., 2007; Long et al., 2010; Zheng et al., 2012). Two recent studies revealed that conditional loss of the NMD gene, Upf2, in specific neural populations in mice causes a variety of intriguing defects, including aberrant behavior, spine density, and synaptic plasticity (Johnson et al., 2019; Notaras et al., 2019). Together, these studies make a strong case that NMD has roles in the CNS.
Here, we report the first investigation of the role of NMD in the olfactory system. One of our major findings was that Upf3b loss causes shifts in gene expression in OSNs. One major class of genes impacted by Upf3b is the Olfr genes. These genes have evolved to allow recognition of the large array of odors encountered by higher organisms. In mice, there are >1000 Olfr genes, each of which encode a G-coupled receptor that binds to a restricted set of odorants (Godfrey et al., 2004; Zhang and Firestein, 2002). In order to interpret the information from a given odor, it is critical that only a single OLFR be expressed in each mOSN. This is accomplished by a novel gene regulatory mechanism that selects only a single Olfr gene to be expressed in any given mOSN (Chess et al., 1994; Malnic et al., 1999; Serizawa et al., 2003). While the underlying mechanism for this ‘one‐neuron‐one‐receptor’ rule is not fully understood, a prevailing model is that a stochastic mechanism drives a single Olfr to become dominate transcriptionally, a decision that is reinforced by feedback mechanisms (Dalton et al., 2013; Lewcock and Reed, 2004; Serizawa et al., 2004; Serizawa et al., 2005).
The first indication that UPF3B might have a role in the selection of Olfr genes came from our RNA-seq analysis, which revealed that the majority of genes expressed at lower level in Upf3b-null mOSNs are Olfr genes. In total, we found that 78 Olfr genes are statistically downregulated in Upf3b-null mOSN. To address mechanism, we performed scRNA-seq analysis and found that these 78 Olfr genes are rarely represented as the dominant genes in individual mOSNs in Upf3b-null mice. This defect was also present at the iOSN stage, suggesting that Upf3b is involved directly or indirectly in determining which Olfr gene are selected for dominant expression during OSN development.
A caveat is the OE contains zones enriched for mOSNs expressing particular sets of OLFRs (Miyamichi et al., 2005; Ressler et al., 1994), and thus even though we made an effort to dissect the entire OE for RNA-seq analysis, it is possible that there is zonal heterogeneity in the samples we analyzed. To reduce this potential bias, we pooled dissociated OE cells from 3 mice for FACS sorting. Confidence that the 78 Olfr genes are regulated by Upf3b comes from the reproducibility of the regulation in independent samples (Figure 1C) and validation by qPCR (Figure 1—figure supplement 2B). Furthermore, our single-cell RNA-seq analysis (which analyzed samples different from those analyzed by RNA-seq) verified the regulation of these 78 Olfr genes (Figure 6H).
How might Upf3b influence the selection of this particular set of Olfr genes? Given that UPF3B is a NMD factor, it could promote the decay of an mRNA encoding a repressor that acts to regulate the selection of these 78 Olfr genes for dominant expression. To test this model, we screened genes exhibiting significantly upregulated expression in Upf3b-null OSNs for those that encode factors known to regulate Olfr gene expression or have binding sites in Olfr promoters (Clowney et al., 2011; Dalton et al., 2013; Hirota and Mombaerts, 2004; Markenscoff-Papadimitriou et al., 2014; McIntyre et al., 2008; Michaloski et al., 2006; Wang et al., 1997). This screen identified two genes—Mafg and Irf8—that fulfilled this criteria. Both encode transcriptional repressors (Igarashi et al., 1994; Salem et al., 2014) that bind O/E consensus sites found in Olfr gene promoters (Michaloski et al., 2006). Thus, Mafg and Irf8 are candidates to act directly downstream of NMD in a regulatory circuit that suppresses the transcription of these 78 Olfr genes. Mafg is a member of the Maf subfamily of basic leucine-zipper transcription factor genes that encode small proteins containing a B-ZAP DNA-binding domain, but lack a transactivation domain, and thus members of this family dimerize to form transcriptional repressors (Igarashi et al., 1994). MAFG is best known for its ability to regulate globin transcription in erythroid cells; our results raise the possibility that MAFG also functions in OSNs to regulates Olfr genes. IRF8 regulates the development hematopoietic cells; its expression in OSNs raises the possibility that this transcription factor also functions in OSNs.
Our findings support a model in which IRF8 and MAFG normally subtly repress the transcription of a subset of Olfr genes in OSNs to fine-tune their expression. Our evidence suggests that IRF8 and MAFG are encoded by NMD target mRNAs, so when NMD is disrupted, these transcriptional repressors are overexpressed, leading to reduced expression of their Olfr gene targets in developing OSNs. Thus, NMD deficiency would be expected to reduce the probability that these particular Olfr genes will be chosen to be the ‘dominant Olfr gene’ in individual mOSNs, which is precisely what we observed in Upf3b-null mice.
A non-mutually exclusive possibility is that Upf3b dictates the selection of Olfr genes by influencing OSN development. In support, several of the genes we found were regulated by Upf3b have been reported to play essential roles in neurogenesis, including Lrp2, Hk2, Notch2, Gdf11, Fos, Ptch1, Spry2, and Cwc22. Upf3b could also indirectly influence the Olfr repertoire by differentially affecting the survival of OSNs harboring different OLFRs. In support, we found that Upf3b loss upregulates Fos, which is associated with OSN apoptosis (Michel et al., 1994).
The other major class of genes regulated by Upf3b in OSNs is antimicrobial genes. This finding, coupled with our finding that OSNs constitutively express these anti-microbial genes (albeit at low levels), suggests that OSNs function not only in olfaction but also in defense against microbes in the bronchial airways. In support, a recent study showed that inflammation causes OSNs to switch from a role in olfaction to immune defense (Chen et al., 2019). This raises the interesting possibility that loss of Upf3b triggers OE inflammation, which, in turn, diverts OSNs from functioning in olfaction to immune defense, thereby causing deficient olfaction. In support, another recent study reported that NMD disruption causes neuro-inflammation in the central nervous system (Johnson et al., 2019). In particular, this study found that Upf2 conditional knockout in the murine forebrain leads to immune infiltration, coupled with deficits in memory, synaptic plasticity, social, and vocal communication (Johnson et al., 2019). Importantly, they found that anti-inflammatory agents partially rescued many of these deficits, indicating that the inflammation is at least partially responsible for the neural defects in these Upf2-conditional knockout mice. It will be intriguing to determine whether humans with UPF3B mutations also suffer from neuro-inflammation and whether this is responsible for their intellectual disability.
Our finding that loss of UPF3B upregulates a very large number of immune-related genes in OSNs, over half of which encode mRNAs that have NIFs and thus may be direct NMD targets (Supplementary file 5), raises the possibility that this ‘immune induction’ response to NMD inhibition is physiologically important. In this regard, it is notable that some viruses have been shown to inhibit NMD, and, in turn, NMD can inhibit viral infection (Wachter and Hartmann, 2014; Wada et al., 2018). Coupled with our data, these findings raise the intriguing possibility that the reason that OSNs express high levels of antimicrobial genes in response to NMD inhibition is because this provides a means to cope with infectious agents, particularly those that inhibit NMD as a means to avoid the antiviral actions of NMD.
In addition to NMD inhibition directly upregulating mRNAs encoding immune factors in OSNs, we identified candidate intermediary factors that may act in a circuit to achieve the same aim. In particular, we identified three mRNAs—Notch2, Bhlhe40, and Rac2—which are high-confidence NMD targets in mOSNs (Table 1) that encode factors previously shown to regulate the expression of many genes encoding inflammatory mediators and antimicrobial proteins (Dooley et al., 2009; Jarjour et al., 2019; Shang et al., 2016).
Our scRNA-seq analysis indicated that Upf3b impacts the steady-state frequency of specific OSN precursor and OSN cell subsets. We found that Upf3b-null mice have decreased numbers of HBCs, suggesting that UPF3B promotes the maintenance of these reserve stem cells. This effect appeared to be specific, as we observed no significant effects on GBCs, which also serve as olfactory stem cells, but unlike HBCs, function to generate new mOSNs constitutively (Schwob et al., 2017). We also observed that Upf3b-null mice acquired a specific group of mOSNs harboring a unique transcriptome that are hardly present in WT mice. This mOSN-4 sub-cluster is enriched for many genes, such as Tuba1a, Nsg1, Chchd10, Eml2, Ubb, and Gldc, which suggests that Upf3b normally represses these genes. It remains to be determined whether the aberrant over-expression of these genes causes aberrant mOSN function. We also found that Upf3b-null mice largely lack a mOSN sub-cluster—mOSN-2—that we found contained large numbers of cells in most WT mice. The mOSN-2 sub-cluster is likely to be functional, as genes enriched in this sub-cluster include Pten, App, Cnga2, Nrp2, Ncam1, Adcy3, Gnal, Atf5, and Gfy (Supplementary file 4), all of which are known to be essential for olfactory epithelium development and/or olfaction. This reciprocal shift in these two mOSN sub-clusters in Upf3b-null mice raises the possibility that UPF3B loss converts the mOSN-2 sub-cluster into the mOSN-4 sub-cluster. This remains to be determined, as does the physiological consequences of these shifts in mOSN sub-populations. Another important area for future investigation is to determine whether these cell-subset alterations in Upf3b-null mice are cell autonomous or non-cell autonomous.
As described in the Introduction, few direct NMD target RNAs have previously been defined in vivo. Our study fills this gap by identifying high-confidence NMD target mRNAs in mOSNs in vivo. Many of the NMD targets we identified in mOSNs have long 3’UTRs, raising the possibility that mOSNs have a predilection for degrading mRNAs with this particular NIF. By analogy, evidence suggests that mRNAs harboring long 3’UTRs are also preferentially targeted for destruction by NMD in male germ cells (Bao et al., 2016). Several of the NMD target mRNAs that we identified in mOSNs are good candidates to have roles in OSN development. For example, Gdf11 functions in negative-feedback control of OE neurogenesis; Lrp2 promotes the proliferation of neural precursor cells in the subependymal zone of the olfactory bulb; and Notch2 is required for maintaining sustentacular cell function in the OE (Gajera et al., 2010; Kawauchi et al., 2009; Rodriguez et al., 2008). Other NMD target mRNAs that we identified, including Ptch1 and Hk2, encode proteins known to be important for the development of neurons outside of the olfactory system (Iulianella and Stanton-Turcotte, 2019; Zheng et al., 2016).
Our study provides a useful resource for the olfactory field. For example, our scRNA-seq analysis identified putative new OSN precursor and OSN cell subsets. While we do not know the significance of this heterogeneity, the genes differentially expressed by the sub-clusters we identified suggests functional relevance. For example, the genes differentially expressed by the 4 cell-sub clusters we identified for both GBCs and iOSNs suggested that these sub-clusters represent distinct developmental stages. Our results are consistent with Fletcher et al., who demonstrated that that the 1 GBC and 4 INP/iOSN sub-clusters they identified follow a linear developmental pattern (Fletcher et al., 2017). Our genome-wide determination of mOSN mRNA expression levels and ribosome occupancy (i.e. translation rates) will be useful for future studies to determine how transcription, translation, and other post-transcriptional processes coordinate to regulate the expression of large sets of genes in mature neurons in vivo. We divided mOSN-expressed mRNAs into nine categories based on steady-state mRNA level and ribosome occupancy, allowing dissection of common functions encoded by similarly regulated mRNAs. Given that translation is a highly energy-consuming process (Lynch and Marinov, 2015), it is likely that there has been strong selection pressure for many mRNAs to be translated inefficiently. Indeed, we found that modestly translated mRNAs encode many key mOSN proteins, including receptors, signaling factors, and developmental regulators.
In conclusion, our study provides an invaluable set of resources for the olfactory field and identifies a post-transcriptional regulatory pathway that impacts OSNs.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Mus musculus) |
Upf3b | GenBank | Gene ID: 68134 | |
| Genetic reagent (Mus. musculus) | C57BL/6J | Jackson Laboratory | Stock #: 000664 RRID:MGI:3028467 |
|
| Genetic reagent (Mus. musculus) | Upf3b-null mice | PMID:21925383 | RRID:MGI:6110148 | Miles Wilkinson lab |
| Genetic reagent (Mus. musculus) | R26-eYFP mice | PMID:11299042 | Obtained from Dr. Maike Sander (UCSD) | |
| Genetic reagent (Mus. musculus) | Omp-Cre mice | PMID:22057188 | Obtained from Dr. Haiqing Zhao (Johns Hopkins University) | |
| Genetic reagent (Mus. musculus) | RiboTag mice | PMID:19666516 | Obtained from Dr. Paul Ameiux (University of Washington) | |
| Antibody | Rabbit monoclonal anti-OMP (EPR19190) | Abcam | Cat# ab183947 RRID:AB_2858281 |
IF (1:400), WB (1:2000) |
| Antibody | Goat polyclonal anti-OMP | FUJIFILM Wako Chemicals | Cat# 544–10001-WAKO RRID:AB_2315007 |
IF (1:200) |
| Antibody | Rabbit polyclonal anti-CAMP | Generated by Richard L. Gallo laboratory | PMID:11442754 | IF (1:200) |
| Antibody | Rabbit polyclonal anti-FUT10 | Proteintech | Cat#: 18660–1-AP RRID:AB_10641997 |
IF (1:200) |
| Antibody | Donkey anti-Goat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat#: A-11055 RRID:AB_2534102 |
IF (1:1000) |
| Antibody | Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 | Thermo Fisher Scientific | Cat#: A-31572 RRID:AB_162543 |
IF (1:1000) |
| Sequence-based reagent | Fosl2_F | This paper | PCR primers |
CCGCAGAAGGAGAGATGAG
(from IDT) |
| Sequenced-based reagent | Fosl2_R | This paper | PCR primers |
GCAGCTTCTCTGTCAGCTC
(from IDT) |
| Sequence-based reagent | Ptger2_F | This paper | PCR primers |
TGCTCCTTGCCTTTCACAATC
(from IDT) |
| Sequenced-based reagent | Ptger2_R | This paper | PCR primers |
CCTAAGTATGGCAAAGACCCAAG
(from IDT) |
| Sequence-based reagent | Adcy6_F | This paper | PCR primers |
TTCCTGACCGTGCCTTCTC
(from IDT) |
| Sequenced-based reagent | Adcy6_R | This paper | PCR primers |
CACCCCGGTTGTCTTTGC
(from IDT) |
| Sequence-based reagent | Ptch1_F | This paper | PCR primers |
ACCTCCTAGGTAAGCCTCC
(from IDT) |
| Sequenced-based reagent | Ptch1_R | This paper | PCR primers |
CACCCACAATCAACTCCTCC
(from IDT) |
| Sequence-based reagent | Cwc22_F | This paper | PCR primers |
CAGAAGACAGATACACAGAGCAAG
(from IDT) |
| Sequenced-based reagent | Cwc22_R | This paper | PCR primers |
CTCTCTCTCTCTCTCTGCGTTT
(from IDT) |
| Sequence-based reagent | Fut10_F | This paper | PCR primers |
CCAGGGCCTTCCTATTCTACG
(from IDT) |
| Sequenced-based reagent | Fut10_R | This paper | PCR primers |
CTGAATGTGGCCGTATGGTTG
(from IDT) |
| Sequence-based reagent | Gdpd3_F | This paper | PCR primers |
TGATCCGACACTTGCAGGAC
(from IDT) |
| Sequenced-based reagent | Gdpd3_R | This paper | PCR primers |
GCTGTGGGGTAATCGGTCAT
(from IDT) |
| Sequence-based reagent | Olfr827_F | This paper | PCR primers |
TGGGATGGTTCTTCTGGGAA
(from IDT) |
| Sequenced-based reagent | Olfr827_R | This paper | PCR primers |
ACCGTGGAGTAGGAGAGGTC
(from IDT) |
| Sequence-based reagent | Rpl19_F | This paper | PCR primers |
CCTGAAGGTCAAAGGGAATGTG
(from IDT) |
| Sequenced-based reagent | Rpl19_R | This paper | PCR primers |
CTTTCGTGCTTCCTTGGTCTT
(from IDT) |
| Commercial assay or kit | Chromium Single Cell 3' Library and Gel Bead Kit | 10X Genomics | Cat# 120237 | |
| Commercial assay or kit | iScript cDNA synthesis Kit | BioRad | Cat# 170–8891 | |
| Commercial assay or kit | SsoAdvanceD Universal SYBR Green Supermix | BioRad | Cat# 172–5274 | |
| Commercial assay or kit | RNeasy Mini Kit | Qiagen | Cat# 74104 | |
| Software, algorithm | Cell Ranger Version 2.1.1 | 10x genomics | Cell Ranger Version 2.1.1 | |
| Software, algorithm | Seurat (v3.1.5) | Designed by Rahul Satija laboratory | PMID:31178118 | |
| Software, algorithm | Monocle (v2.16.0) | Designed by Cole Trapnell laboratory | PMID:28114287 | |
| Software, algorithm | NIH ImageJ (v1.8.0) | NIH | Version 1.8.0 |
Mice
This study was carried out in strict accordance with the Guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Diego. The protocol was approved by the IACUC at the University of California, San Diego (permit number: S09160). All studies were conducted on adult male mice housed under a 12 hr light:12 hr dark cycle and provided with food and water ad libitum. Of note, we only performed analyses on male mice. Since Upf3b is X-linked gene, we analyzed Upf3b+/y (WT) and Upf3-/y (KO) mice. All mouse strains used for analysis were backcrossed to C57BL/6J for at least eight passages.
Behavioral and weight analyses
To assess the effect of UPF3B loss on mouse weight, 19male pups (nine Upf3b-null and ten WT mice) from Upf3b+/- × WT breeders (6 litters) were assessed, performed as described previously (Tan et al., 2016). For pre-weaning pups, to reduce stress, forceps and gloves were changed frequently between cages.
For the coyote/bobcat urine experiment, 10 male mice (10- to 16 weeks of age) from each genotype were analyzed. Each mouse was placed into a cage for 10 min to acclimatize, a strip of filter paper soaked with coyote urine (Snow Joe) or bobcat urine (Predator Pee) was placed into the cage for 5 min, and the amount of time the mouse was in the vicinity of the filter paper was determined by video recording. Each mouse was tested separately in the absence of humans or other mice in the room.
RNA-seq analysis
For each mOSN sample analyzed, 3 C57BL/6J male mice (8- to 9-weeks old) were pooled. Four replicate samples were analyzed per genotype (Upf3b+/y; Omp-Cre; R26-eYFP and Upf3b-/y; Omp-Cre; R26-eYFP). Cell sorting experiments were performed on two separate days, with two samples sorted per day. The OE was dissected as described (Gong, 2012) and dissociated using the Papain Dissociation System (Worthington) at 37°C for 15 min, followed by extensive trituration. Cells were filtered using a 40‐μm strainer (Falcon). After spinning at 200 g for 5 min, cells were resuspended in Hanks’ balanced salt solution (HBSS) containing 3% FBS (Gibco) but without Ca2+ and Mg2+. The cell suspension was mixed with propidium iodide (final concentration of 1 μg/ml) and the OMP-eYFP+ cells were sorted by flow-cytometry. RNA was isolated from the OMP-eYFP+ cells using TriZOL (Life Technologies), followed by a secondary purification step using a RNeasy column (Qiagen). Total RNA was assessed for quality using an Agilent Bioanalyzer, and samples determined to have an RNA Integrity Number (RIN) of at least 8 or greater were used to generate RNA libraries using Illumina's TruSeq RNA Sample Prep Kit, following the manufacturer's specifications, with the RNA fragmentation time adjusted to 5 min. RNA-seq was performed at the Institute of Genomic Medicine at UCSD. RNA libraries were multiplexed and sequenced with 100 base pair (bp) pair end reads on an Illumina HiSeq4000. The average number of reads per sample ranged from approximately 15 to 22 million reads. Reads were filtered for quality and aligned with STAR (2.5.2b) against Mus musculus release-90, Ensembl genome (GRCm38). The exon counts were aggregated for each gene to build a read count table using SubRead function featureCounts (Liao et al., 2014). Using the exon start/end positions, we extracted the exon sequences from the mm10 mouse genome, and ligated them together in silico for each transcript. For each entry, the entire transcript sequence was subtracted from the known CDS sequence (obtained as above) to identify 3’UTR length. DEGs were defined using DESeq2 (Love et al., 2014) using a threshold q-val of <0.05. The R package program ‘pheatmap’ was used for clustering and to generate heatmap plots. GO analysis was done using database for annotation, visualization and integrated discovery (DAVID), version v6.8. To infer relative RNA stability, we used the REMBRANDTS program (Alkallas et al., 2017) following the tutorial (https://github.com/csglab/REMBRANDTS).
RiboTag analysis
For each mOSN sample analyzed, three C57BL/6J male mice (8 to 9-weeks old) were pooled. Three replicate samples were analyzed per genotype (Upf3b+/y; Omp-Cre; RiboTag and Upf3b-/y; Omp-Cre; RiboTag). The OEs was dissected as described (Gong, 2012), homogenized, washed with HBSS, centrifuged at 16,000 g at 4°C for 10 min, the supernatant was transferred into a new tube and incubated with HA antisera (#16B12; BioLegend, CA) at 4°C for 2.5 hr. Ribosome-bound RNAs were captured on anti-HA agarose beads (Pierce) for 1 hr at 4°C on a tube rotator. RNA libraries were multiplexed and sequenced with 50 bp single-end reads on an Illumina HiSeq4000. RNA sequencing, alignment, and downstream analyses were done as described above for RNA-seq analysis. TE was determined by dividing RiboTag reads by RNA-seq reads. Log2-transformed transcripts per million (TPM) values were used to segregate mRNAs into different categories.
scRNA-seq analysis
Four C57BL/6J male mice (7 to 8-weeks old) per genotype (Upf3b+/y and Upf3b-/y) were used to obtain OE for scRNA-seq analysis. After dissecting the OE as described (Gong, 2012), the cells were dissociated following the 10X Genomics Chromium sample preparation protocol. Briefly, tissue was cut into 1 mm3 pieces and digested in HBSS without Ca2+ and Mg2+ and supplemented with 44 U/ml Dispase (Invitrogen), 1000 U/ml Collagenase type II (Invitrogen) and 10 mg/ml DNaseI (Sigma), for 20 min at 37°C with gentle agitation. The digested tissue was centrifuged at 300 rcf for 5 min and washed in HBSS without Ca2+ and Mg2+. Dissociated cells were resuspended in 3% FBS in PBS. Dead cells were removed using the ClioCell Dead Cell Removal kit (Amsbio) following the manufacturer's instructions. Single cells were resuspended in 0.04% BSA in PBS (w/v) and loaded on the 10x Chromium chip. Cell capturing, and library preparation was carried as per kit instructions (Chromium Single Cell Kit [v2 chemistry]). The resultant libraries were size selected, pooled, and sequenced using 2 × 100 paired-end sequencing protocol on an Illumina HiSeq 4000 instrument. The libraries initially underwent shallow sequencing to access quality and to adjust subsequent sequencing depth based on the capture rate and unique molecular indices (UMI) detected. All sequencing analyses were performed at the Institute of Genomic Medicine at UCSD.
As described previously (Sohni et al., 2019; Tan et al., 2020b; Tan et al., 2020a), de-multiplexed raw sequencing reads were processed and mapped to the mouse genome (mm10) using Cell Ranger software (v2.0) with default parameters. We filtered raw count matrices by excluding cells expressing less than 200 detectably expressed genes and genes expressed in less than 3 cells. Each library was tagged with a library batch ID and combined across independent experiments using the Seurat package (Butler et al., 2018) in R. To check the quality of the single-cell data and to remove multiplets, we performed Seurat-based filtering of cells based on three criteria: number of detected features (nFeature_RNA) per cell, number of UMIs expressed per cell (nCount_RNA) and mitochondrial content, using the following threshold parameters: nFeature_RNA (>500), nCount_RNA (>1,500), and percentage of mitochondrial genes expressed (<0.2%). We used known lineage marker profiles to exclude cell multiplets (cells expressing different lineage markers) and cell-free droplets. Gene expression values were log normalized and regressed by mitochondrial expression (‘percent.mt’) and cell cycle gene expession (‘S.Score’ and ‘G2M.Score’) using the SCTransform function. Batch correction was performed using the JackStraw functions in the Seurat package.
To identify cell clusters, we employed the UMAP algorithm (Becht et al., 2019). The FindMarkers function (a Wilcoxon rank sum test) was used to determine differential gene expression between clusters (set at minimum expression in 25% of cells). The DoHeatmap function was used to generate an expression heatmap for specific cells and features. GO analysis (DAVID v6.8) was done using the top differentially (positively) expressed genes, with a p-adjusted cut off of 0.01.
Single-cell pseudotime trajectories were constructed with the Monocle two package (v2.10.1) (Qiu et al., 2017) according to the provided documentation (http://cole-trapnell-lab.github.io/monocle-release/). UMI counts were modeled as a negative binomial distribution. The ordering genes were identified as having high dispersion across cells (mean_expression >= 0.01; dispersion_empirical >= 1). The discriminative dimensionality reduction with trees (DDRTree) method was used to reduce data to two dimensions. Differentially expressed genes were identified and used for dynamic trajectory analysis (NO discovery rate [FDR]<0.01) to order cells in pseudotime. The plot_pseudotime_heatmap function was used to generate heatmaps.
NIF analysis
To define NIFs, Refseq-defined transcripts were first converted into Ensemble transcript IDs and their sequences were obtained using the UCSC Table Browser. NIFs were identified in these transcripts using an algorithm written in Python 2.7, Zenith.py, created by the Wilkinson laboratory. Only transcripts with a detectable 5’UTR and 3’UTR were considered. A transcript was defined as harboring a dEJ if it contained at least one exon-exon junction ≥50 nt downstream of the stop codon terminating the main ORF. A transcript was defined as harboring an uORF if the following criteria were met: (i) the ORF is in the 5’ UTR, (ii) the start codon and surrounding nts are in a context known to initiate translation (a purine at the −3 position or a guanine at the +4 position, relative to the A in the AUG initiation codon [+1]) (Kozak, 1986), (iii) the ORF is ≥30 nt long, and (iv) the ORF does not overlap with the main ORF (to reduce the probability that translation could be re-initiated, thereby allowing the transcript to escape NMD).
Immunofluorescence analysis
Adult mice were anesthetized and perfused with 4% paraformaldehyde (PFA; Sigma). OE was dissected and fixed in 4% PFA at 4°C for 24 hr, then transferred to 70% ethanol. After embedding in paraffin, 5 µM sections were prepared, deparaffinized 2 times in xylene, followed by serial dilutions of ethanol. Unmasking was performed with IHC-TekTM epitope retrieval solution using a steamer (IHCWORLD) for 40 min. Blocking was performed by incubating with 5% serum (from the species that the secondary antibody was raised in) for 1 hr at room temperature. The sections were then incubated overnight with the primary antibody (goat polyclonal OMP, rabbit anti-CAMP [Gallo et al., 1997]) at 4°C and incubated with secondary antibody (Donkey anti-Goat IgG [H+L] conjugated with Alexa Fluor 488 or Donkey anti-Rabbit IgG [H+L] conjugated with Alexa Fluor 555) for 1 hr at room temperature. The nuclei were counterstained with DAPI, and a coverslip was placed over the sections with mounting medium.
Western blot analysis
OEs were incubated in radioimmunoprecipitation assay (RIPA) buffer (Bio-Rad) supplemented with protease inhibitor cocktail (Sigma) on ice for 30 min, followed by centrifugation at 16,000 g for 15 min at 4°C. The lysates were then transferred to new tubes, and protein level was quantified using the DC Protein Assay kit (Bio‐Rad). Twenty micrograms of the protein samples were separated on an 15% polyacrylamide gel, and Western blot analysis was performed as previously described (Ramaiah et al., 2019). Quantification of the blots was performed using NIH ImageJ (1.8.0).
Acknowledgements
We thank the UCSD Institute for Genomic Medicine for technical support and the San Diego Supercomputer Center for providing data analysis resources. We are grateful to Drs. Maike Sander (UCSD), Haiqing Zhao (Johns Hopkins University), and Paul Ameiux (University of Washington) for providing R26-eYFP, Omp-Cre, and RiboTag mice, respectively.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Miles F Wilkinson, Email: mfwilkinson@health.ucsd.edu.
Didier YR Stainier, Max Planck Institute for Heart and Lung Research, Germany.
Didier YR Stainier, Max Planck Institute for Heart and Lung Research, Germany.
Funding Information
This paper was supported by the following grant:
Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD093846 to Miles F Wilkinson.
Additional information
Competing interests
No competing interests declared.
Author contributions
Resources, Data curation, Formal analysis, Validation, Writing - original draft.
Conceptualization, Data curation.
Formal analysis.
Formal analysis.
Conceptualization.
Data curation.
Data curation.
Data curation.
Data curation.
Supervision.
Supervision.
Supervision.
Conceptualization, Supervision, Funding acquisition, Project administration, Writing - review and editing.
Ethics
Animal experimentation: This study was carried out in strict accordance with the Guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Diego. The protocol was approved by the IACUC at the University of California, San Diego (permit number: S09160).
Additional files
(1) QC metrics, (2) reads count, and (3) TPM values from Upf3b-null (KO) and control (WT) mOSN RNA-seq and RiboTag datasets. (4) The expression of all annotated Olfr genes in Upf3b-null (KO) and control (WT) mOSN samples, based on RNA-seq analysis.
(1) Genes differentially expressed between Upf3b-null and WT mOSNs (as defined by RNA-seq analysis), including their known NIFs. (2) High-confidence NMD targets: transcripts both upregulated and stabilized in Upf3b-null mOSNs. (3) The transcriptome and translome of Upf3b-null and WT mOSNs, based on RNA-seq and RiboTag analyses. (4) Translational efficiency (TE) of RNAs expressed in Upf3b-null and WT mOSNs, as defined by RNA-seq and RiboTag analyses.
(1) Genes exhibiting enriched expression in all OE cell types identified by scRNA-seq analysis. (2) Genes exhibiting enriched expression in HBC, GBC, iOSN, and mOSN sub-clusters identfied by scRNA-seq analysis. (3) Genes exhibiting enriched expression in the 4 temporally distinct gene groups defined by pseudotime trajectory analysis of HBCs, GBCs, iOSNs, and mOSNs. (4) Transcription factor (TF) genes exhibiting enriched expression in the 4 temporally distinct gene groups defined by pseudotime trajectory analysis of HBCs, GBCs, iOSNs, and mOSNs.
(1) Immune genes enriched in Upf3b-null HBC, GBC, iOSN, and mOSN sub-clusters identified by scRNA-seq analysis. (2) NIFs present in immune genes expressed in Upf3b-null OSNs.
Data availability
Sequencing data have been deposited in GEO under accession code GSE146043.
The following dataset was generated:
Tan K, Jones SH, Wilkinson MF. 2020. The role of the NMD factor UPF3B in olfactory sensory neurons. NCBI Gene Expression Omnibus. GSE146043
The following previously published datasets were used:
Hurt JA, Robertson AD, Burge CB. 2013. Global Analyses of UPF1 Binding and Function Reveal Expanded Scope of Nonsense-Mediated mRNA Decay. NCBI Gene Expression Omnibus. GSE41785
Mao H, McMahon JJ, Tsai YH, Wang Z, Silver DL. 2016. Haploinsufficiency for Core Exon Junction Complex Components Disrupts Embryonic Neurogenesis and Causes p53-Mediated Microcephaly. NCBI Gene Expression Omnibus. GSE85576
Mooney CM, Jimenez-Mateos EM, Engel T, Mooney C, Diviney M, Venø MT, Kjems J, Farrell MA, O'Brien DF, Delanty N, Henshall DC. 2017. RNA sequencing of synaptic and cytoplasmic Upf1-bound transcripts supports contribution of nonsense-mediated decay to epileptogenesis. RNA sequencing of synaptic and cytoplasmic Upf1-bound transcripts supports contribution of nonsense-mediated decay to epileptogenesis.
Bao j, Vitting-Seerup K, Waage J, Tang C, Ge Y, Porse BT, Yan W. 2016. UPF2-Dependent Nonsense-Mediated mRNA Decay Pathway Is Essential for Spermatogenesis by Selectively Eliminating Longer 3'UTR Transcripts. NCBI Gene Expression Omnibus. GSE55180
McIlwain DR, Pan Q, Reilly PT, Elia AJ, McCracken S, Wakeham AC, Itie-Youten A, Blencowe BJ, Mak TW. 2010. Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay.
Weischenfeldt J, Damgaard I, Bryder D, Theilgaard-Mönch K. Thoren LA, Nielsen FC, Jacobsen SEW, Nerlov C, Porse BT. 2008. NMD Is Essential for Hematopoietic Stem and Progenitor Cells and for Eliminating By-Products of Programmed DNA Rearrangements. NMD Is Essential for Hematopoietic Stem and Progenitor Cells and for Eliminating By-Products of Programmed DNA Rearrangements.
Thoren LA, Nørgaard GA, Weischenfeldt J, Waage J, Jakobsen JS, Damgaard I, Bergström FC, Blom AM, Borup R, Bisgaard HC, Porse BT. 2010. UPF2 Is a Critical Regulator of Liver Development, Function and Regeneration. UPF2 Is a Critical Regulator of Liver Development, Function and Regeneration.
References
- Alkallas R, Fish L, Goodarzi H, Najafabadi HS. Inference of RNA decay rate from transcriptional profiling highlights the regulatory programs of Alzheimer's disease. Nature Communications. 2017;8:909. doi: 10.1038/s41467-017-00867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrahbeni T, Sartor F, Anderson J, Miedzybrodzka Z, McCaig C, Müller B. Full UPF3B function is critical for neuronal differentiation of neural stem cells. Molecular Brain. 2015;8:33. doi: 10.1186/s13041-015-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Vitting-Seerup K, Waage J, Tang C, Ge Y, Porse BT, Yan W. UPF2-Dependent Nonsense-Mediated mRNA decay pathway is essential for spermatogenesis by selectively eliminating longer 3'UTR Transcripts. PLOS Genetics. 2016;12:e1005863. doi: 10.1371/journal.pgen.1005863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LW, Fletcher S, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cellular and Molecular Life Sciences. 2012;69:3613–3634. doi: 10.1007/s00018-012-0990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear DM, Lassance JM, Hoekstra HE, Datta SR. The evolving neural and genetic architecture of Vertebrate Olfaction. Current Biology. 2016;26:R1039–R1049. doi: 10.1016/j.cub.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E, McInnes L, Healy J, Dutertre C-A, Kwok IWH, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nature Biotechnology. 2019;37:38–44. doi: 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- Belgrader P, Cheng J, Maquat LE. Evidence to implicate translation by ribosomes in the mechanism by which nonsense codons reduce the nuclear level of human triosephosphate isomerase mRNA. PNAS. 1993;90:482–486. doi: 10.1073/pnas.90.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm V, Haberman N, Ottens F, Ule J, Gehring NH. 3' UTR length and messenger ribonucleoprotein composition determine endocleavage efficiencies at termination codons. Cell Reports. 2014;9:555–568. doi: 10.1016/j.celrep.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Bühler M, Steiner S, Mohn F, Paillusson A, Mühlemann O. EJC-independent degradation of nonsense immunoglobulin-mu mRNA depends on 3' UTR length. Nature Structural & Molecular Biology. 2006;13:462–464. doi: 10.1038/nsmb1081. [DOI] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnology. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter MS, Doskow J, Morris P, Li S, Nhim RP, Sandstedt S, Wilkinson MF. A regulatory mechanism that detects premature nonsense codons in T-cell receptor transcripts in vivo is reversed by protein synthesis inhibitors in vitro. Journal of Biological Chemistry. 1995;270:28995–29003. doi: 10.1074/jbc.270.48.28995. [DOI] [PubMed] [Google Scholar]
- Carter MS, Li S, Wilkinson MF. A splicing-dependent regulatory mechanism that detects translation signals. The EMBO Journal. 1996;15:5965–5975. doi: 10.1002/j.1460-2075.1996.tb00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Chan WK, Huang L, Gudikote JP, Chang YF, Imam JS, MacLean JA, Wilkinson MF. An alternative branch of the nonsense-mediated decay pathway. The EMBO Journal. 2007;26:1820–1830. doi: 10.1038/sj.emboj.7601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annual Review of Biochemistry. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- Chen M, Reed RR, Lane AP. Chronic inflammation directs an olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell. 2019;25:501–513. doi: 10.1016/j.stem.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/S0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Clowney EJ, Magklara A, Colquitt BM, Pathak N, Lane RP, Lomvardas S. High-throughput mapping of the promoters of the mouse olfactory receptor genes reveals a new type of mammalian promoter and provides insight into olfactory receptor gene regulation. Genome Research. 2011;21:1249–1259. doi: 10.1101/gr.120162.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak D, Ji SJ, Porse BT, Jaffrey SR. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell. 2013;153:1252–1265. doi: 10.1016/j.cell.2013.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Izaurralde E. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Current Opinion in Cell Biology. 2005;17:316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Dalton RP, Lyons DB, Lomvardas S. Co-opting the unfolded protein response to elicit olfactory receptor feedback. Cell. 2013;155:321–332. doi: 10.1016/j.cell.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley JL, Abdel-Latif D, St Laurent CD, Puttagunta L, Befus D, Lacy P. Regulation of inflammation by Rac2 in immune complex-mediated acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2009;297:L1091–L1102. doi: 10.1152/ajplung.90471.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostie J, Dreyfuss G. Translation is required to remove Y14 from mRNAs in the cytoplasm. Current Biology. 2002;12:1060–1067. doi: 10.1016/S0960-9822(02)00902-8. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in parkinson disease. Nature Reviews Neurology. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Das D, Gadye L, Street KN, Baudhuin A, Wagner A, Cole MB, Flores Q, Choi YG, Yosef N, Purdom E, Dudoit S, Risso D, Ngai J. Deconstructing olfactory stem cell trajectories at Single-Cell resolution. Cell Stem Cell. 2017;20:817–830. doi: 10.1016/j.stem.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, Bachmann S, Christensen EI, Götz M, Kempermann G, Peterson AS, Willnow TE, Hammes A. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. Journal of Cell Science. 2010;123:1922–1930. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. Journal of Biological Chemistry. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- Gehring NH, Kunz JB, Neu-Yilik G, Breit S, Viegas MH, Hentze MW, Kulozik AE. Exon-junction complex components specify distinct routes of nonsense-mediated mRNA decay with differential cofactor requirements. Molecular Cell. 2005;20:65–75. doi: 10.1016/j.molcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Godfrey PA, Malnic B, Buck LB. The mouse olfactory receptor gene family. PNAS. 2004;101:2156–2161. doi: 10.1073/pnas.0308051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q. Culture of mouse olfactory sensory neurons. Current Protocols in Neuroscience. 2012;3:24. doi: 10.1002/0471142301.ns0324s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Li X, Spatrick P, Casillo R, Dong S, Jacobson A. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5' to 3' mRNA decay pathways in yeast. Molecular Cell. 2003;12:1439–1452. doi: 10.1016/S1097-2765(03)00446-5. [DOI] [PubMed] [Google Scholar]
- Hirota J, Mombaerts P. The LIM-homeodomain protein Lhx2 is required for complete development of mouse olfactory sensory neurons. PNAS. 2004;101:8751–8755. doi: 10.1073/pnas.0400940101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Hakuba A, Shiota K, Naruse I. Suckling dysfunction caused by defects in the olfactory system in genetic arhinencephaly mice. Neonatology. 2000;78:293–299. doi: 10.1159/000014282. [DOI] [PubMed] [Google Scholar]
- Hornstein N, Torres D, Das Sharma S, Tang G, Canoll P, Sims PA. Ligation-free ribosome profiling of cell type-specific translation in the brain. Genome Biology. 2016;17:149. doi: 10.1186/s13059-016-1005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Lou CH, Chan W, Shum EY, Shao A, Stone E, Karam R, Song HW, Wilkinson MF. RNA homeostasis governed by cell type-specific and branched feedback loops acting on NMD. Molecular Cell. 2011;43:950–961. doi: 10.1016/j.molcel.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Shum EY, Jones SH, Lou CH, Dumdie J, Kim H, Roberts AJ, Jolly LA, Espinoza JL, Skarbrevik DM, Phan MH, Cook-Andersen H, Swerdlow NR, Gecz J, Wilkinson MF. A Upf3b-mutant mouse model with behavioral and neurogenesis defects. Molecular Psychiatry. 2018;23:1773–1786. doi: 10.1038/mp.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt JA, Robertson AD, Burge CB. Global analyses of UPF1 binding and function reveal expanded scope of nonsense-mediated mRNA decay. Genome Research. 2013;23:1636–1650. doi: 10.1101/gr.157354.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Soria X, Levitin MO, Saraiva LR, Logan DW. The olfactory transcriptomes of mice. PLOS Genetics. 2014;10:e1004593. doi: 10.1371/journal.pgen.1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Kataoka K, Itoh K, Hayashi N, Nishizawa M, Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small maf proteins. Nature. 1994;367:568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- Iulianella A, Stanton-Turcotte D. The hedgehog receptor Patched1 regulates proliferation, neurogenesis, and axon guidance in the embryonic spinal cord. Mechanisms of Development. 2019;160:103577. doi: 10.1016/j.mod.2019.103577. [DOI] [PubMed] [Google Scholar]
- Iwema CL, Schwob JE. Odorant receptor expression as a function of neuronal maturity in the adult rodent olfactory system. The Journal of Comparative Neurology. 2003;459:209–222. doi: 10.1002/cne.10583. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Wilkinson MF. Nonsense-mediated RNA decay in the brain: emerging modulator of neural development and disease. Nature Reviews Neuroscience. 2018;19:715–728. doi: 10.1038/s41583-018-0079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour NN, Schwarzkopf EA, Bradstreet TR, Shchukina I, Lin CC, Huang SC, Lai CW, Cook ME, Taneja R, Stappenbeck TS, Randolph GJ, Artyomov MN, Urban JF, Edelson BT. Bhlhe40 mediates tissue-specific control of macrophage proliferation in homeostasis and type 2 immunity. Nature Immunology. 2019;20:687–700. doi: 10.1038/s41590-019-0382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Stoica L, Liu Y, Zhu PJ, Bhattacharya A, Buffington SA, Huq R, Eissa NT, Larsson O, Porse BT, Domingo D, Nawaz U, Carroll R, Jolly L, Scerri TS, Kim HG, Brignell A, Coleman MJ, Braden R, Kini U, Jackson V, Baxter A, Bahlo M, Scheffer IE, Amor DJ, Hildebrand MS, Bonnen PE, Beeton C, Gecz J, Morgan AT, Costa-Mattioli M. Inhibition of Upf2-Dependent Nonsense-Mediated decay leads to behavioral and neurophysiological abnormalities by activating the immune response. Neuron. 2019;104:665–679. doi: 10.1016/j.neuron.2019.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly LA, Homan CC, Jacob R, Barry S, Gecz J. The UPF3B gene, implicated in intellectual disability, autism, ADHD and childhood onset schizophrenia regulates neural progenitor cell behaviour and neuronal outgrowth. Human Molecular Genetics. 2013;22:4673–4687. doi: 10.1093/hmg/ddt315. [DOI] [PubMed] [Google Scholar]
- Karam R, Lou CH, Kroeger H, Huang L, Lin JH, Wilkinson MF. The unfolded protein response is shaped by the NMD pathway. EMBO Reports. 2015;16:599–609. doi: 10.15252/embr.201439696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karousis ED, Mühlemann O. Nonsense-Mediated mRNA decay begins where translation ends. Cold Spring Harbor Perspectives in Biology. 2019;11:a032862. doi: 10.1101/cshperspect.a032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Kim J, Santos R, Wu HH, Lander AD, Calof AL. Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development. 2009;136:1453–1464. doi: 10.1242/dev.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebaara BW, Atkin AL. Long 3'-UTRs target wild-type mRNAs for nonsense-mediated mRNA decay in Saccharomyces cerevisiae. Nucleic Acids Research. 2009;37:2771–2778. doi: 10.1093/nar/gkp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk MS, Tirosh I, Heckl D, Rao TN, Dixit A, Haas BJ, Schneider RK, Wagers AJ, Ebert BL, Regev A. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Research. 2015;25:1860–1872. doi: 10.1101/gr.192237.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator Codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Popp MW, Maquat LE. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nature Reviews Molecular Cell Biology. 2019;20:406–420. doi: 10.1038/s41580-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewcock JW, Reed RR. A feedback mechanism regulates monoallelic odorant receptor expression. PNAS. 2004;101:1069–1074. doi: 10.1073/pnas.0307986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Current Biology. 2012;22:1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AA, Mahapatra CT, Woodruff EA, Rohrbough J, Leung HT, Shino S, An L, Doerge RW, Metzstein MM, Pak WL, Broadie K. The nonsense-mediated decay pathway maintains synapse architecture and synaptic vesicle cycle efficacy. Journal of Cell Science. 2010;123:3303–3315. doi: 10.1242/jcs.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen S, Jensen TH. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nature Reviews Molecular Cell Biology. 2015;16:665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- Lynch M, Marinov GK. The bioenergetic costs of a gene. PNAS. 2015;112:15690–15695. doi: 10.1073/pnas.1514974112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabin JW, Woodward LA, Patton RD, Yi Z, Jia M, Wysocki VH, Bundschuh R, Singh G. The exon junction complex undergoes a compositional switch that alters mRNP structure and Nonsense-Mediated mRNA decay activity. Cell Reports. 2018;25:2431–2446. doi: 10.1016/j.celrep.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/S0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. The Journal of Comparative Neurology. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- Mao H, Brown HE, Silver DL. Mouse models of Casc3 reveal developmental functions distinct from other components of the exon junction complex. RNA. 2017;23:23–31. doi: 10.1261/rna.058826.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markenscoff-Papadimitriou E, Allen WE, Colquitt BM, Goh T, Murphy KK, Monahan K, Mosley CP, Ahituv N, Lomvardas S. Enhancer interaction networks as a means for singular olfactory receptor expression. Cell. 2014;159:543–557. doi: 10.1016/j.cell.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JC, Bose SC, Stromberg AJ, McClintock TS. Emx2 stimulates odorant receptor gene expression. Chemical Senses. 2008;33:825–837. doi: 10.1093/chemse/bjn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nature Genetics. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- Michaloski JS, Galante PA, Malnic B. Identification of potential regulatory motifs in odorant receptor genes by analysis of promoter sequences. Genome Research. 2006;16:1091–1098. doi: 10.1101/gr.5185406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D, Moyse E, Brun G, Jourdan F. Induction of apoptosis in mouse [correction of rat] olfactory neuroepithelium by synaptic target ablation. Neuroreport. 1994;5:1329–1332. [PubMed] [Google Scholar]
- Miyamichi K, Serizawa S, Kimura HM, Sakano H. Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. Journal of Neuroscience. 2005;25:3586–3592. doi: 10.1523/JNEUROSCI.0324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E, Maquat LE. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends in Biochemical Sciences. 1998;23:198–199. doi: 10.1016/S0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- Nasif S, Contu L, Mühlemann O. Beyond quality control: the role of nonsense-mediated mRNA decay (NMD) in regulating gene expression. Seminars in Cell & Developmental Biology. 2018;75:78–87. doi: 10.1016/j.semcdb.2017.08.053. [DOI] [PubMed] [Google Scholar]
- Nguyen LS, Kim HG, Rosenfeld JA, Shen Y, Gusella JF, Lacassie Y, Layman LC, Shaffer LG, Gécz J. Contribution of copy number variants involving nonsense-mediated mRNA decay pathway genes to neuro-developmental disorders. Human Molecular Genetics. 2013;22:1816–1825. doi: 10.1093/hmg/ddt035. [DOI] [PubMed] [Google Scholar]
- Nguyen LS, Wilkinson MF, Gecz J. Nonsense-mediated mRNA decay: inter-individual variability and human disease. Neuroscience & Biobehavioral Reviews. 2014;46 Pt 2:175–186. doi: 10.1016/j.neubiorev.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M, Allen M, Longo F, Volk N, Toth M, Li Jeon N, Colak D. UPF2 leads to degradation of dendritically targeted mRNAs to regulate synaptic plasticity and cognitive function. Molecular Psychiatry. 2019;21:5. doi: 10.1038/s41380-019-0547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios IM. Nonsense-mediated mRNA decay: from mechanistic insights to impacts on human health. Briefings in Functional Genomics. 2013;12:25–36. doi: 10.1093/bfgp/els051. [DOI] [PubMed] [Google Scholar]
- Peterson J, Lin B, Barrios-Camacho CM, Herrick DB, Holbrook EH, Jang W, Coleman JH, Schwob JE. Activating a reserve neural stem cell population in Vitro Enables Engraftment and Multipotency after Transplantation. Stem Cell Reports. 2019;12:680–695. doi: 10.1016/j.stemcr.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nature Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah M, Tan K, Plank TM, Song HW, Dumdie JN, Jones S, Shum EY, Sheridan SD, Peterson KJ, Gromoll J, Haggarty SJ, Cook-Andersen H, Wilkinson MF. A microRNA cluster in the Fragile-X region expressed during spermatogenesis targets FMR1. EMBO Reports. 2019;20:e46566. doi: 10.15252/embr.201846566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada I, Lykke-Andersen J. Execution of nonsense-mediated mRNA decay: what defines a substrate? Current Opinion in Cell Biology. 2009;21:394–402. doi: 10.1016/j.ceb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A molecular dissection of spatial patterning in the olfactory system. Current Opinion in Neurobiology. 1994;4:588–596. doi: 10.1016/0959-4388(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Riera CE, Tsaousidou E, Halloran J, Follett P, Hahn O, Pereira MMA, Ruud LE, Alber J, Tharp K, Anderson CM, Brönneke H, Hampel B, Filho CDM, Stahl A, Brüning JC, Dillin A. The sense of smell impacts metabolic health and obesity. Cell Metabolism. 2017;26:198–211. doi: 10.1016/j.cmet.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Sickles HM, Deleonardis C, Alcaraz A, Gridley T, Lin DM. Notch2 is required for maintaining sustentacular cell function in the adult mouse main olfactory epithelium. Developmental Biology. 2008;314:40–58. doi: 10.1016/j.ydbio.2007.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenkrantz L, Zachor D, Heller I, Plotkin A, Weissbrod A, Snitz K, Secundo L, Sobel N. A mechanistic link between olfaction and autism spectrum disorder. Current Biology. 2015;25:1904–1910. doi: 10.1016/j.cub.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem S, Langlais D, Lefebvre F, Bourque G, Bigley V, Haniffa M, Casanova JL, Burk D, Berghuis A, Butler KM, Leahy TR, Hambleton S, Gros P. Functional characterization of the human dendritic cell immunodeficiency associated with the IRF8(K108E) mutation. Blood. 2014;124:1894–1904. doi: 10.1182/blood-2014-04-570879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. PNAS. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva LR, Ibarra-Soria X, Khan M, Omura M, Scialdone A, Mombaerts P, Marioni JC, Logan DW. Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Scientific Reports. 2015;5:18178. doi: 10.1038/srep18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva LR, Riveros-McKay F, Mezzavilla M, Abou-Moussa EH, Arayata CJ, Makhlouf M, Trimmer C, Ibarra-Soria X, Khan M, Van Gerven L, Jorissen M, Gibbs M, O'Flynn C, McGrane S, Mombaerts P, Marioni JC, Mainland JD, Logan DW. A transcriptomic atlas of mammalian olfactory mucosae reveals an evolutionary influence on food odor detection in humans. Science Advances. 2019;5:eaax0396. doi: 10.1126/sciadv.aax0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, Hewitt Coleman J. Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. Journal of Comparative Neurology. 2017;525:1034–1054. doi: 10.1002/cne.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2088–2094. doi: 10.1126/science.1089122. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends in Genetics. 2004;20:648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Sakano H. Negative feedback regulation ensures the one neuron-one receptor rule in the mouse olfactory system. Chemical Senses. 2005;30:i99–i100. doi: 10.1093/chemse/bjh133. [DOI] [PubMed] [Google Scholar]
- Shang Y, Smith S, Hu X. Role of notch signaling in regulating innate immunity and inflammation in health and disease. Protein & Cell. 2016;7:159–174. doi: 10.1007/s13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum EY, Jones SH, Shao A, Dumdie J, Krause MD, Chan WK, Lou CH, Espinoza JL, Song HW, Phan MH, Ramaiah M, Huang L, McCarrey JR, Peterson KJ, De Rooij DG, Cook-Andersen H, Wilkinson MF. The antagonistic gene paralogs Upf3a and Upf3b govern Nonsense-Mediated RNA decay. Cell. 2016;165:382–395. doi: 10.1016/j.cell.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, Hsieh TC, Rabah R, Hammoud SS, Vicini E, Wilkinson MF. The neonatal and adult human testis defined at the Single-Cell level. Cell Reports. 2019;26:1501–1517. doi: 10.1016/j.celrep.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies N, Burge CB, Bartel DP. 3' UTR-isoform choice has limited influence on the stability and translational efficiency of most mRNAs in mouse fibroblasts. Genome Research. 2013;23:2078–2090. doi: 10.1101/gr.156919.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Li Q, Xie XS. Olfactory sensory neurons transiently express multiple olfactory receptors during development. Molecular Systems Biology. 2015;11:844. doi: 10.15252/msb.20156639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Wang Z, Zhang Z, An L, Tian J. IVF affects embryonic development in a sex-biased manner in mice. Reproduction. 2016;151:443–453. doi: 10.1530/REP-15-0588. [DOI] [PubMed] [Google Scholar]
- Tan K, Song HW, Wilkinson MF. Single-cell RNAseq analysis of testicular germ and somatic cell development during the perinatal period. Development. 2020a;147:dev183251. doi: 10.1242/dev.183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Song HW, Thompson M, Munyoki S, Sukhwani M, Hsieh TC, Orwig KE, Wilkinson MF. Transcriptome profiling reveals signaling conditions dictating human spermatogonia fate in vitro. PNAS. 2020b;117:17832–17841. doi: 10.1073/pnas.2000362117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey PS, Raymond FL, Nguyen LS, Rodriguez J, Hackett A, Vandeleur L, Smith R, Shoubridge C, Edkins S, Stevens C, O'Meara S, Tofts C, Barthorpe S, Buck G, Cole J, Halliday K, Hills K, Jones D, Mironenko T, Perry J, Varian J, West S, Widaa S, Teague J, Dicks E, Butler A, Menzies A, Richardson D, Jenkinson A, Shepherd R, Raine K, Moon J, Luo Y, Parnau J, Bhat SS, Gardner A, Corbett M, Brooks D, Thomas P, Parkinson-Lawrence E, Porteous ME, Warner JP, Sanderson T, Pearson P, Simensen RJ, Skinner C, Hoganson G, Superneau D, Wooster R, Bobrow M, Turner G, Stevenson RE, Schwartz CE, Futreal PA, Srivastava AK, Stratton MR, Gécz J. Mutations in UPF3B, a member of the nonsense-mediated mRNA decay complex, cause syndromic and nonsyndromic mental retardation. Nature Genetics. 2007;39:1127–1133. doi: 10.1038/ng2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepe B, Hill MC, Pekarek BT, Hunt PJ, Martin TJ, Martin JF, Arenkiel BR. Single-Cell RNA-Seq of mouse olfactory bulb reveals cellular heterogeneity and Activity-Dependent molecular census of Adult-Born neurons. Cell Reports. 2018;25:2689–2703. doi: 10.1016/j.celrep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter A, Hartmann L. NMD: nonsense-mediated defense. Cell Host & Microbe. 2014;16:273–275. doi: 10.1016/j.chom.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Wada M, Lokugamage KG, Nakagawa K, Narayanan K, Makino S. Interplay between coronavirus, a cytoplasmic RNA virus, and nonsense-mediated mRNA decay pathway. PNAS. 2018;115:E10157–E10166. doi: 10.1073/pnas.1811675115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Tsai RY, Reed RR. The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. The Journal of Neuroscience. 1997;17:4149–4158. doi: 10.1523/JNEUROSCI.17-11-04149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Progress in Neurobiology. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LA, Mabin JW, Gangras P, Singh G. The exon junction complex: a lifelong guardian of mRNA fate. Wiley Interdisciplinary Reviews: RNA. 2017;8:e1411. doi: 10.1002/wrna.1411. [DOI] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nature Neuroscience. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Gallo RL. Antimicrobial peptides. Current Biology : CB. 2016;26:R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Zheng S, Gray EE, Chawla G, Porse BT, O'Dell TJ, Black DL. PSD-95 is post-transcriptionally repressed during early neural development by PTBP1 and PTBP2. Nature Neuroscience. 2012;15:381–388. doi: 10.1038/nn.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M, Gage FH, Hunter T. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife. 2016;5:e13374. doi: 10.7554/eLife.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]