Abstract
Eggshell thickness is important for physiological, ecological, and ecotoxicological studies on birds; however, empirical eggshell thickness measurements for many species and regions are limited. We measured eggshell thickness at the equator and the egg poles for 12 avian species and related eggshell thickness to egg morphometrics, embryonic development, egg status, and mercury contamination. Within an egg, eggshells were approximately 5.1% thicker at the equator than the sharp pole of the egg, although this difference varied among species (0.6%–9.8%). Within Forster's tern (Sterna forsteri), where eggshell thickness was measured at 5 equally spaced positions along the longitude of the egg, eggshell thickness changed more rapidly near the sharp pole of the egg compared to near the blunt pole of the egg. Within species, eggshell thickness was related to egg width and egg volume for six of the 12 species but was not related to egg length for any species. Among species, mean eggshell thickness was strongly related to species mean egg width, egg length, egg volume, and bird body mass, although species mean body mass was the strongest predictor of species mean eggshell thickness. Using three species (American avocet [Recurvirostra americana], black‐necked stilt [Himantopus mexicanus], and Forster's tern), whose nests were carefully monitored, eggshell thickness (including the eggshell membrane) did not differ among viable, naturally abandoned, dead, or failed‐to‐hatch eggs; was not related to total mercury concentrations of the egg content; and did not decrease with embryonic age. Our study also provides a review of all existing eggshell thickness data for these 12 species.
Keywords: avian, body mass, egg length, egg measurements, egg width, fresh wet weight
We measured eggshell thickness at the egg equator and the egg poles for 12 avian species, and related eggshell thickness to egg morphometrics, embryonic development, egg status, and mercury contamination. Our study also provides a review of all existing eggshell thickness data for these 12 species.
1. INTRODUCTION
Egg morphology has been the subject of many physiological (Ar, Paganelli, Reeves, Greene, & Rahn, 1974; Rahn & Ar, 1980; Rahn, Parisi, & Paganelli, 1982), ecological (Maurer, Russell, & Cassey, 2010; Rahn & Paganelli, 1988, 1989), and ecotoxicological studies (Cooke, 1973; Hickey & Anderson, 1968; Ratcliffe, 1970), yet empirical data for eggshell thickness are limited for many avian species and regions. For example, eggshell thickness can influence egg physiology, specifically gas exchange, because the diffusive properties of gas through pores in the eggshell relate to the ratio of pore length (eggshell thickness) to pore radius (Rahn, Paganelli, & Ar, 1987). Ecologically, eggshell thickness may vary as a result of factors including maternal age (Massaro & Davis, 2006), egg‐laying order (Castilla, Herrel, Robles, Malone, & Negro, 2010), egg mass (Castilla, Herrel, et al., 2010), and egg pigmentation (Gosler, Higham, & Reynolds, 2005). Some of these factors vary enough within and among clutches that they could cause marked differences in eggshell thicknesses among eggs; however, it is unclear whether this variation would result in an eggshell thickness that could influence whether or not an egg would hatch. Eggshell thickness also is an important egg measurement for ecotoxicological studies because it can be directly influenced by contaminant exposure (Cooke, 1973; Hickey & Anderson, 1968) and eggshell thickness is a necessary component in the accurate calculation of egg contaminant concentrations (Herzog, Ackerman, Eagles‐Smith, & Hartman, 2016).
In birds, embryonic development can influence the thickness of the eggshell, with the calcite eggshell thinning as the embryo develops (Ancel & Girard, 1992; Balkan, Karakaş, & Biricik, 2006; Castilla, Herrel, et al., 2010; Finnlund, Hissa, Koivusaari, Merila, & Nuuja, 1985; Orłowski & Hałupka, 2015; Orłowski, Hałupka, Klimczuk, & Sztwiertnia, 2016; Orłowski, Merta, et al., 2019; Santolo, Byron, & Ohlendorf, 2016). Consequently, hatched eggs have thinner eggshells than freshly laid eggs simply due to embryonic development, as calcium is mobilized from the mammillary tips within the calcite eggshell and into the interior of the egg for embryonic growth (Karlsson & Lilja, 2008; Orłowski & Hałupka, 2015). However, as the calcite eggshell thins during development, eggshell membranes may increase in thickness (Castilla, Van Dongen, et al., 2010) and become less tightly attached to the calcite eggshell (Finnlund et al., 1985). Consequently, the thickness of the combined eggshell and membrane may not change as a result of embryonic development, and most studies examining eggshell thickness in wild birds include the membrane in the measurement of the eggshell.
Contaminant exposure, particularly to organochlorine pesticides, can decrease eggshell thickness and influence egg survival (Cooke, 1973; Hickey & Anderson, 1968). It is possible that other environmental contaminants, such as mercury, may influence eggshell thickness as well, but only a few studies have examined this in bird eggs (Blus, Heath, Gish, Belisle, & Prouty, 1971; Hargreaves, Whiteside, & Gilchrist, 2011; Heinz, 1979; King, Custer, & Quinn, 1991; Lundholm, 1995; Rodriguez‐Navarro, Gaines, Romanek, & Masson, 2002; Stoewsand, Anderson, Gutenmann, Bache, & Lisk, 1971).
In ecotoxicological studies, eggshell thickness influences the estimation of an egg's contaminant concentration (Herzog et al., 2016). The ideal reporting metric of contaminant concentrations in avian eggs is the calculation of fresh wet weight (fww) of the egg (Ackerman, Herzog, & Schwarzbach, 2013), a calculation that typically uses estimates of egg density, egg volume, and fresh egg mass. Estimating these measurements without removing the eggshell can result in a 6%–13% underestimate of egg contaminant concentrations (Herzog et al., 2016). Consequently, the calculation of contaminant concentrations in egg contents can be improved by estimating and subsequently excluding the thickness of the eggshell (Herzog et al., 2016). The common allometric equations to estimate eggshell thickness use egg length, egg width, eggshell mass, or whole egg mass (Ar et al., 1974; Khurshid, Farooq, Durrani, Sarbiland, & Chand, 2003; Maurer et al., 2010; Morrison & Kiff, 1979; Osborne & Winters, 1977; Ratcliffe, 1970) and are derived from large, multispecies datasets, although their accuracy has not been well validated for individual eggs (Ancel & Girard, 1992; Maurer, Portugal, & Cassey, 2012). Additionally, eggshell thickness may be estimated using bird body mass (Birchard & Deeming, 2009). The main equation to predict eggshell thickness from egg mass was derived from Schönwetter (1960–1992); this equation can be misused because it was derived using estimates of eggshell thickness from other equations and was not empirically based (Maurer et al., 2012). Furthermore, egg mass decreases by as much as 15% during embryonic development (Brown, 1976; Drent, 1970; Westerskov, 1950) and egg mass can also decrease as a result of desiccation from environmental exposure. Therefore, predictive equations based on egg mass (Ar & Rahn, 1985; Osborne & Winters, 1977; Rahn & Paganelli, 1989) will only be accurate for freshly laid eggs because the relationship between eggshell thickness and egg mass changes after the time point when the egg was laid. Thus, eggshell measurements are needed within and among species to test and improve upon allometric relationships for estimating eggshell thickness (using egg morphometrics or bird mass) that are not based on egg mass and are accurate for individual species.
We used 12 avian species to provide empirical eggshell thickness measurements in relation to egg morphometrics, embryonic development, egg status at the time of collection, and mercury contamination. The methodology we used provided more precise and repeatable eggshell thickness measurements than prior studies that used analog micrometers (Santolo, 2018), and these eggshell thicknesses can be applied in other ecological, physiological, and toxicological studies. Specifically, we examined the following: (a) eggshell thickness at multiple positions on the egg; (b) the relationship between eggshell thickness and egg morphometrics (length, width, and volume) both within and among species, as well as the relationship between species mean eggshell thickness and species mean bird body mass; (c) whether eggshell thickness decreases with embryonic development; (d) whether there are differences in eggshell thickness related to the egg status at the time of collection (normally developing eggs, eggs naturally abandoned by parents, dead embryos in eggs from nests where no sibling eggs hatched, and dead embryos in eggs from nests where sibling eggs hatched); and (e) whether eggshell thickness is related to egg content mercury concentrations. Eggshell thickness in relationship to mercury contamination was chosen because few studies have examined the effects of mercury on eggshell thickness and mercury concentrations were analyzed for related contaminant studies.
2. METHODS
2.1. Sample collection
We salvaged and collected eggs from 12 avian species, representing 6 families from 4 orders (Table 1), as part of related contaminant studies during 2014–2018 (Peterson & Ackerman, 2020). Eggs of 11 species were from multiple sites within San Francisco Bay and the Central Valley in California (USA), some Caspian tern (Hydroprogne caspia) eggs were from the Potholes Reservoir in Washington State (USA), and wood duck (Aix sponsa) eggs were from Fallon, Nevada (USA). In the field, eggs were placed in egg cartons and kept in small coolers with wet ice until they were transported back to the laboratory. Eggs were stored in a refrigerator (2°C) until processing.
TABLE 1.
Eggshell thickness was measured for 12 avian species from 4 orders and 6 families
| Order | Family | Common name | Scientific name | Mean female body mass (g) |
|---|---|---|---|---|
| Anseriformes | Anatidae | Mallard | Anas platyrhynchos | 1,095 |
| Anatidae | Wood duck | Aix sponsa | 647 | |
| Charadriiformes | Charadriidae | Western snowy plover | Charadrius nivosus nivosus | 42 |
| Laridae | Black skimmer | Rynchops niger | 254 | |
| Laridae | California gull | Larus californicus | 599 | |
| Laridae | California least tern | Sternula antillarum browni a | 44 | |
| Laridae | Caspian tern | Hydroprogne caspia | 670 | |
| Laridae | Forster's tern | Sterna forsteri | 136 | |
| Recurvirostridae | American avocet | Recurvirostra americana | 340 | |
| Recurvirostridae | Black‐necked stilt | Himantopus mexicanaus | 169 | |
| Pelecaniformes | Ardeidae | Great egret | Ardea alba | 883 |
| Suliformes | Phalacrocoracidae | Double‐crested cormorant | Phalacrocorax auritus albociliatus b | 1,831 |
Mean female body mass was obtained from published studies (Ackerman, Hartman, et al., 2013; Ackerman et al., 2008; Bluso et al., 2006; Delnicki & Reinecke, 1986; Dunning, 2008; Herring et al., 2008, 2010b; Page et al., 2009; Robinson et al., 1999).
Available body mass measurements were for the subspecies Sternula antillarum athalassos.
Available body mass measurements were for the subspecies Phalacrocorax auritus auritus.
2.2. Eggshell processing
First, the exterior of each egg was cleaned with deionized water, swabbed with isopropyl alcohol, rinsed with deionized water, and allowed to dry. Before egg dissection, length (±0.01 mm) and width (±0.01 mm) were measured using digital calipers (Mitutoyo, Aurora, Illinois, USA) and whole egg mass (±0.01 g) was obtained with a digital balance (Ohaus Adventurer™ Pro AV212, Ohaus Corporation). We then cut an approximately 15 mm diameter circle at the blunt end of each egg using stainless‐steel scissors, removed the blunt end of the eggshell, and transferred the egg contents into a sterile polypropylene jar. The blunt pole of the eggshell was removed and discarded during egg processing for some eggs, prior to the development of this specific study. Embryos were aged to the nearest whole day (Ackerman & Eagles‐Smith, 2010), and the egg contents were prepared for determination of mercury (Ackerman, Eagles‐Smith, Herzog, & Hartman, 2016). Most eggs (68.8%) were identified as fertile and aged to at least 1 day in incubation (mean 7.8 ± 5.2 days; interquartile range 4–11 days; range 1–27 days). Additionally, 3.2% were identified as fresh and fertile (day 0 of incubation). The remaining eggs were either infertile or embryonic age could not be determined. After egg dissection, eggshells were stored in a freezer at −20°C.
Prior to processing of eggshells and measurement of eggshell thickness, eggshells were removed from the freezer and allowed to warm to room temperature. The outside of the eggshell was reexamined to determine whether there was any remaining exogenous material that needed to be removed. Then, we rinsed the inside of eggshells with a mild detergent (Alconox) and used a cotton swab to wipe out the inside. If necessary, a small stainless‐steel spatula was gently used to dislodge any contents adhered to the inside of the eggshell that could not be dislodged with a cotton swab. After any remaining egg contents were dislodged, the inside was rinsed multiple times with deionized water. Eggshell membranes were not removed. We recorded the condition of the ultrathin outermost eggshell membrane, closest to the egg contents (Simkiss, 1961), because that membrane occasionally becomes detached from the rest of the eggshell during dissection and it is almost always absent from the blunt pole as it peels away from the eggshell in the blunt pole region as the air cell expands during embryonic development. The main inner eggshell membrane (Simkiss, 1961) was present in all eggshells. Once cleaned, eggshells were placed in a drying oven for 24 hr at 40°C and stored in a desiccator until they were measured.
2.3. Eggshell thickness measurements
We measured eggshell thickness at 3 positions on each eggshell when possible: equator, sharp pole, and blunt pole (Figure 1). We measured eggshell thickness using a Magna‐Mike® 8600 Hall effect thickness gauge (Olympus Scientific Solutions Americas Corporation) with a 1.58 mm magnetic measurement ball. We measured the minimum thickness of the eggshell and membrane as the ball was rolled across the inside of the eggshell at three measurement positions: the equator, the sharp pole, and the blunt pole, following the methods of Santolo (2018). At the equator measuring position, we slowly rotated the egg 3–5 times over the measurement ball to make sure the entire equator was sampled. At the sharp and blunt pole measuring positions, the measurement ball was rolled around in a small circle to capture the entire end of the eggshell. Our method measured across maculated (pigment spots) and plain sections of eggshells. Because some studies showed differences in eggshell thickness between pigmented and unpigmented sections (Gosler et al., 2005), our method captured the thinnest spot at that measurement position on the eggshell, which may have represented a pigmented section. If only a portion of the eggshell was intact at the equator, we measured as much of the eggshell as possible and recorded the percent of the eggshell area that was sampled. We excluded measurements from sections of eggshell that had mold on them and any eggshells where the main inner eggshell membrane was removed or was visibly separating from the calcite portion of the eggshell. The thickness gauge was calibrated at the start of every day of measurement and any time when the machine was inactive for more than 1 hr.
FIGURE 1.
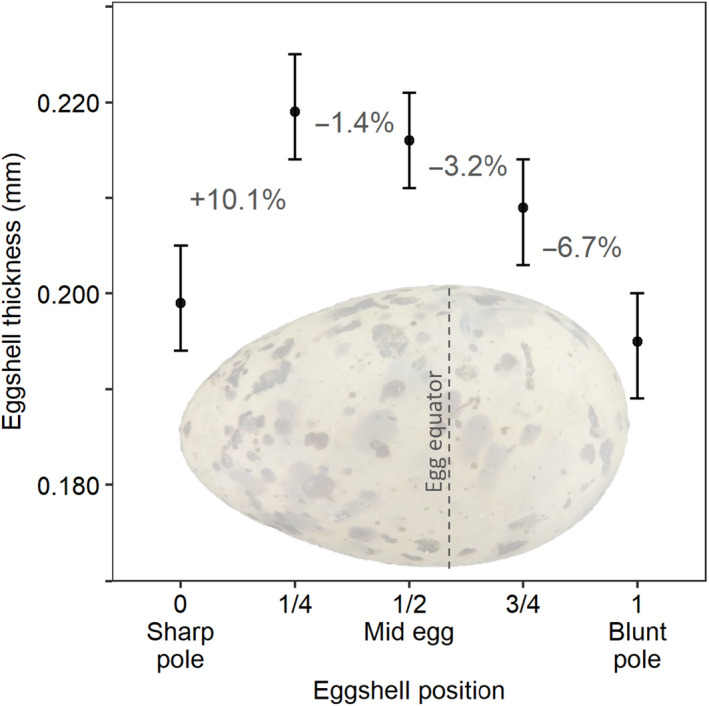
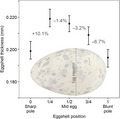
Least squares mean eggshell thickness (±95% CI) at 5 different equally spaced positions on Forster's tern eggshells (n = 40). Percentages represent the percent thicker or thinner each location is relative to the measurement location directly to the left, moving from the sharp pole toward the blunt pole. Note that the ½ egg was measured at the midpoint of the egg and not the widest part of the egg (which is called the egg equator; shown with a dashed line) that is typically closer to the blunt pole than the sharp pole
To examine more specifically whether and how eggshell thickness changed longitudinally from the sharp to the blunt pole of the eggshell, we conducted a separate experiment where we measured eggshell thickness at 5 positions (Figure 1) on a subset of Forster's tern (Sterna forsteri) eggshells (n = 40) where the blunt pole eggshell piece was retained after egg dissection. The sharp pole and blunt pole were measured in the same way as described above, but we also measured eggshell thickness at 3 additional and equally spaced regions on the egg at the ¼ egg (i.e., halfway between the sharp pole and the midpoint of the egg), the ½ egg (i.e., the midpoint of the egg), and the ¾ egg (i.e., halfway between the midpoint of the egg and the blunt pole). The ½ egg was measured at the midpoint of the egg and not the egg equator, which is defined as the widest part of the egg and typically the equator is closer to the blunt pole than the sharp pole of the egg (Figure 1). Each of the 5 positions on an individual eggshell was measured within the same calibration period (<15 min). We used the same protocol described above, turning the eggshell 3–5 times over the measurement ball around each longitudinal section.
2.4. Mercury determination
Eggs were analyzed for total mercury (THg) using a Nippon Instruments MA‐3000 Direct Mercury Analyzer (Nippon Instruments Corporation) at the U.S. Geological Survey Dixon Field Station Environmental Mercury Laboratory, following U.S. Environmental Protection Agency Method 7473 (U.S. Environmental Protection Agency, 2000). This method uses an integrated sequence of drying, thermal decomposition, catalytic conversion, and then amalgamation, followed by atomic absorption spectroscopy. Dried and homogenized egg aliquots were weighed to the nearest 0.00001 g prior to analysis (Mettler Toledo XS105). Egg THg concentrations (µg/g) are reported as fresh wet weight (fww), following Ackerman, Herzog, et al. (2013) as modified by Herzog et al. (2016) to exclude the thickness of the measured eggshell from each egg.
Standard measures of quality assurance were used, including determination of THg concentrations in certified reference materials (DORM‐4, DOLT‐4, DOLT‐5, IAEA‐407, TORT‐3; National Research Council Canada and International Atomic Energy Agency), determination of THg concentrations in internal laboratory reference materials, matrix spikes, continuing calibration verifications, duplicates, and system and method blanks in each run of samples. The mean (±SD) relative percent difference of duplicate samples was 2.7 ± 2.8% (n = 290). Recoveries were 100.2 ± 2.8% (n = 368) for certified reference materials, 99.9 ± 2.1% (n = 328) for calibration verifications, and 100.6 ± 3.5% (n = 322) for matrix spikes.
2.5. Statistical analyses
We examined the influence of species, measurement position, egg morphometrics (egg length, egg width, and egg volume) and bird body mass, embryo age, egg status at the time of collection, and egg THg content on eggshell thickness using a combination of weighted regression, mixed effects linear models, and general linear models. All analyses were performed in the statistical program R (R Core Team, 2019).
2.5.1. Eggshell thickness among species and within individual eggs
We examined whether there were differences in eggshell thickness within and among species at specific egg measurement positions within individual eggshells. First, we compared the eggshell thickness values from 5 positions in the detailed study of Forster's tern eggs with a linear mixed effects model using the lme4 package (Bates et al., 2015). In the model, we included measurement position as a fixed effect and eggshell identification as a random effect. We used the Kenward–Roger approximation for degrees of freedom and tested for significance with F tests generated from the afex package (Singmann, Bolker, & Westfall, 2015). We examined differences in model‐generated least squares mean eggshell thicknesses among the five eggshell measurement positions with a Tukey honest significant difference adjustment.
Using data from multiple species (n = 12), we ran two linear mixed effects models with species, measurement position on the eggshell, and a species × measurement position interaction as fixed effects and eggshell identification nested within nest identification as random effects. The first model included eggshells with paired equator and sharp pole measurements, and the second model included eggshells that had an equator, sharp pole, and blunt pole measurement.
We examined whether there was a consistent difference in eggshell thickness between the sharp pole and the equator within and among species using two approaches. First, we examined the relationship between the sharp pole and the equator using a general linear model with individual eggshell thickness measurements and an equator eggshell thickness × species interaction. Second, we examined the linear relationship among species, using a weighted regression on species mean values of each measurement. We calculated weights as the natural log of the sample size to reduce the weight on higher sample sizes such that species with more samples were weighted only slightly more than species with fewer samples. We then calculated residuals for all individual eggshell measurements from the regression equation generated using the species means. Finally, we used the mean and 95% CI of the residuals to determine whether the residuals for each species fell above, included, or were below zero. If the 95% CI of the residuals for a species included zero, that would suggest that the mean eggshell thickness at the sharp pole for that species was within the range of what would be expected based on the eggshell thickness at the equator. Conversely, if the 95% CI of the residuals for a species was entirely above zero, that would suggest that the eggshell thicknesses at the sharp pole for that species were thicker than would be expected based on the eggshell thickness at the equator. If the 95% CI of the residuals for a species was entirely below zero, that would suggest that the eggshell thicknesses at the sharp pole for that species were thinner than would be expected based on the eggshell thickness at the equator.
2.5.2. Eggshell thickness versus egg morphometrics and bird body mass
We quantified the relationship between eggshell thickness at the equator and egg morphometrics (egg length, width, and volume) within and among species. First, we examined the relationship between the eggshell thickness at the equator and either the egg length, egg width, or egg volume (in separate models), using a general linear model with equator eggshell thickness measurements for individual eggs and an equator eggshell thickness × egg morphometric measurement interaction. We then ran individual models for each species with more than 10 samples. Egg volume was calculated using an egg shape coefficient (Kv), egg length, and egg width (egg volume = Kv × egg length × egg width2; Hoyt, 1979). Second, to qualitatively compare the relationship between the eggshell thickness at the equator and egg morphometrics within versus among species, we quantified the linear relationship among species (length, width, and volume in separate models), using a weighted regression on species mean values, with weights calculated as the natural log of the sample size to reduce the weight on higher sample sizes such that species with more samples were weighted only slightly more than species with fewer samples. We then calculated residuals for all individual eggshells from the regression equation generated using species means and used these residuals to determine whether the mean residual value for each species fell above, included, or was below zero, using the mean and 95% CI for each species. If the 95% CI of the residuals for a species included zero, that would suggest that the mean eggshell thickness at the equator pole for that species was within the range of what would be expected based on the egg length or width. Conversely, if the 95% CI of the residuals for a species was entirely above zero, that would suggest that the eggshell thickness at the equator for that species was thicker than would be expected based on the egg length or width. If the 95% CI of the residuals for a species was entirely below zero, that would suggest that the eggshell thickness at the equator for that species was thinner than would be expected based on the egg morphometric measurements.
We also quantified the relationship between species mean eggshell thickness at the equator and species mean bird body mass. For bird masses, we used published mean female body masses (Ackerman, Hartman, et al., 2013; Ackerman, Takekawa, Bluso, Yee, & Eagles‐Smith, 2008; Bluso, Ackerman, Takekawa, & Yee, 2006; Delnicki & Reinecke, 1986; Dunning, 2008; Herring, Ackerman, Eagles‐Smith, & Takekawa, 2010; Herring, Gawlik, & Beerens, 2008; Page, Stenzel, Warriner, Warriner, & Paton, 2009; Robinson, Reed, Skorupa, & Oring, 1999; Table 1). We transformed bird mass (log10) prior to analysis because we did not expect bird body mass to scale linearly with eggshell thickness (Birchard & Deeming, 2009). We used AICc (corrected for small sample sizes) to compare regression models with different predictors of eggshell thickness.
2.5.3. Eggshell thickness versus embryo age
We examined whether eggshell thickness measured at the equator and sharp pole decreased with embryonic development, using a subset of normally developing eggs of American avocet (Recurvirostra americana), black‐necked stilt (Himantopus mexicanus), and Forster's tern that were collected during weekly nest monitoring. We used a general linear model with fixed effects for species, embryo age (in days), and a species × embryo age interaction. We did not include nest identification as a random effect because we had only 1 normally developing egg from each nest.
2.5.4. Eggshell thickness versus egg status
For a subset of American avocet, black‐necked stilt, and Forster's tern eggs that were sampled during weekly nest monitoring visits, the status of each egg was categorized upon collection as active, abandoned, dead, or failed to hatch (Herring, Ackerman, & Eagles‐Smith, 2010). Active eggs were normally progressing in nests that were actively being incubated, whereas abandoned eggs were from nests where the parents naturally had abandoned the nest. Eggs classified as dead contained dead embryos and had stopped progressing normally in nests while they were still being incubated and no sibling eggs in the clutch hatched. Failed‐to‐hatch eggs contained dead embryos and also did not hatch but were from nests where other sibling eggs in the clutch successfully hatched. We ran two separate mixed effects linear models to compare eggshell thickness at either the equator or the sharp pole with egg status, species, embryo age, an egg status × species interaction, and nest identification as a random effect. Nest identification was included as a random effect, because some nests with dead, abandoned, or failed‐to‐hatch eggs had multiple eggs salvaged.
2.5.5. Eggshell thickness versus egg content mercury
To examine whether eggshell thickness was related to MeHg exposure, we used the subset of normally progressing American avocet, black‐necked stilt, and Forster's tern eggs. We determined THg concentration in the egg as a proxy for MeHg since 96% of the Hg in bird eggs is in the MeHg form (Ackerman, Herzog, et al., 2013). We used two general linear models, one for the equator eggshell thickness and one for the sharp pole eggshell thickness, with fixed effects for the egg content THg concentration (fww), species, embryo age, and an egg content THg concentration × species interaction. We did not include nest identification as a random effect because only 1 normally developing egg in this dataset was from each nest.
3. RESULTS
3.1. Eggshell thickness among species
We observed a 190% difference in mean eggshell thickness at the equator between the species with the thinnest eggshells (California least tern [Sternula antillarum browni]: 0.144 mm) and the thickest eggshells (double‐crested cormorant [Phalacrocorax auratus albociliatus]: 0.418 mm; Figure 2). Similarly, we observed a 181% difference in mean eggshell thickness at the sharp pole between the species with the thinnest eggshells (California least tern: 0.140 mm) and the thickest eggshells (double‐crested cormorant: 0.394 mm). The range in observed eggshell thickness varied within species but was far more extensive among species; 92.6% of the variance in eggshell thickness at the equator occurred among species compared to 7.4% within species. Similarly, 87.0% of the variance in eggshell thickness at the sharp pole occurred among species compared to 13.0% within species.
FIGURE 2.
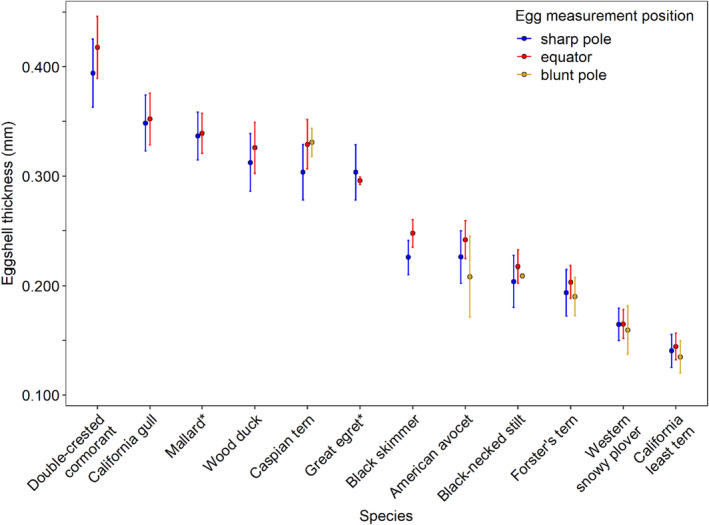
Arithmetic mean eggshell thickness (±SD) at the egg equator, sharp pole, and blunt pole for 12 different avian species (raw data). Asterisks indicate species with <10 eggs measured. Refer to Table 2 for sample sizes and egg length and egg width measurements
3.2. Eggshell thickness varies within individual eggs
3.2.1. Eggshell thickness at 5 equally spaced positions on the egg
We began with a detailed study of eggshell thickness among 5 equally spaced positions on Forster's tern eggs (n = 40). Eggshells were thickest at the ¼ egg and ½ egg and thinnest at the poles (F 4,156.0 = 31.96, p < .001; Figure 1). The increase in eggshell thickness was greater between the sharp pole and the ¼ egg than between the blunt pole and the ¾ egg (Figure 1). Furthermore, average eggshell thickness was similar at the ¼ egg and the ½ egg (t = 1.29, p = .70), whereas eggshell thickness at the ¾ egg was 3.2% thinner than the ½ egg (t = 2.70, p = .059). For this set of eggshells, the ½ egg was 10.8% thicker than the blunt pole (t = 8.06, p < .001) and 8.5% thicker than the sharp pole (t = 6.32, p < .001), and the sharp pole was similar in thickness to the blunt pole (t = 1.74, p = .42).
3.2.2. Eggshell thickness at the equator and poles
When we considered all 12 species, avian eggshells generally were thickest at the equator and thinner at the sharp and blunt poles. On average, eggshells were 5.1% thicker at the equator than the sharp pole when there were at least 10 eggshells measured from a species (n = 10 species; Table 2), although mean differences between eggshell thickness at the equator and sharp pole within a species varied from 0.6% to 9.8% (F 11,2500.0 = 19.46, p < .001; Figure 2). Western snowy plover (Charadrius nivosus nivosus) was the only species with ≥10 eggshells measured where there was no detectable difference between the eggshell thickness at the equator and the sharp pole (t = 0.19, p = .85, 0.6% difference in mean values; all other species t ≥ 2.32, p ≤ .02). Black skimmer (Rynchops niger) had the greatest difference between the eggshell thickness at the equator and the sharp pole (9.8%), followed by Caspian tern (8.3%), American avocet (7.1%), double‐crested cormorant (6.1%), black‐necked stilt (5.9%), Forster's tern (4.6%), wood duck (4.5%), California least tern (2.9%), and California gull (Larus californicus; 1.1%).
TABLE 2.
Sample size (n) for egg morphometric data (egg length and egg width) salvaged and collected from 12 avian species between 2014 and 2018 throughout western North America
| Species | n | Egg length (mm) ± SD (range) | Egg width (mm) ± SD (range) | Equator n | Eggshell thickness at equator (mm) ± SD (range) | Sharp pole n | Eggshell thickness at sharp pole (mm) ± SD (range) | Blunt pole n | Eggshell thickness at blunt pole (mm) ± SD (range) |
|---|---|---|---|---|---|---|---|---|---|
| American avocet | 844 | 49.19 ± 2.20 (41.31–56.34) | 34.13 ± 1.11 (24.71–39.84) | 843 | 0.242 ± 0.017 (0.156–0.297) | 773 | 0.226 ± 0.024 (0.152–0.298) | 6 | 0.208 ± 0.037 (0.146–0.252) |
| Black‐necked stilt | 204 | 43.31 ± 1.76 (38.88–49.34) | 30.99 ± 0.85 (27.86–33.16) | 201 | 0.217 ± 0.015 (0.184–0.259) | 179 | 0.204 ± 0.024 (0.143–0.261) | 1 | 0.209 |
| Black skimmer | 11 | 47.78 ± 2.95 (42.90–51.91) | 34.59 ± 1.06 (31.79–35.59) | 11 | 0.247 ± 0.013 (0.229–0.273) | 11 | 0.226 ± 0.015 (0.206–0.251) | 0 | NA |
| California gull | 175 | 65.02 ± 2.87 (55.66–72.10) | 45.29 ± 1.43 (41.22–48.60) | 175 | 0.352 ± 0.024 (0.260–0.419) | 162 | 0.348 ± 0.026 (0.275–0.419) | 0 | NA |
| California least tern | 340 | 30.70 ± 1.28 (25.55–35.62) | 22.43 ± 0.70 (20.30–24.34) | 332 | 0.144 ± 0.012 (0.109–0.177) | 249 | 0.140 ± 0.015 (0.095–0.201) | 80 | 0.135 ± 0.015 (0.091–0.182) |
| Caspian tern | 62 | 63.32 ± 2.44 (57.93–68.46) | 43.76 ± 1.27 (40.53–47.02) | 62 | 0.329 ± 0.022 (0.270–0.381) | 60 | 0.303 ± 0.025 (0.217–0.353) | 10 | 0.331 ± 0.013 (0.315–0.355) |
| Double‐crested cormorant | 90 | 60.97 ± 2.61 (55.26–67.34) | 39.02 ± 1.44 (34.04–42.22) | 88 | 0.418 ± 0.029 (0.353–0.467) | 89 | 0.394 ± 0.031 (0.305–0.473) | 0 | NA |
| Forster's tern | 1,103 | 42.82 ± 1.78 (28.03–47.91) | 30.11 ± 0.89 (21.75–33.54) | 1,085 | 0.203 ± 0.015 (0.155–0.257) | 946 | 0.194 ± 0.021 (0.116–0.307) | 176 | 0.190 ± 0.018 (0.138–0.250) |
| Great egret | 3 | 59.67 ± 1.50 (57.95–60.67) | 40.47 ± 1.76 (38.51–41.92) | 3 | 0.296 ± 0.004 (0.292–0.299) | 3 | 0.303 ± 0.025 (0.281–0.331) | 0 | NA |
| Mallard | 2 | 56.70 ± 1.98 (55.30–58.10) | 40.94 ± 0.86 (40.33–41.55) | 2 | 0.339 ± 0.018 (0.326–0.352) | 2 | 0.337 ± 0.022 (0.321–0.352) | 0 | NA |
| Western snowy plover | 35 | 30.67 ± 0.99 (28.81–32.98) | 22.25 ± 0.89 (19.54–23.80) | 34 | 0.165 ± 0.013 (0.133–0.191) | 27 | 0.165 ± 0.015 (0.126–0.189) | 9 | 0.159 ± 0.022 (0.122–0.186) |
| Wood duck | 39 | 49.90 ± 2.07 (43.19–54.60) | 38.04 ± 1.59 (32.64–40.59) | 39 | 0.326 ± 0.023 (0.273–0.377) | 38 | 0.312 ± 0.026 (0.255–0.377) | 0 | NA |
Sample size and raw data on minimum eggshell thickness (including the main inner eggshell membrane) measurements at the equator, sharp pole, and blunt pole. Sample sizes differ because egg morphometric measurements were not obtained for all eggs and some eggshells were not measured at all three egg positions. NA indicates when no measurements were taken at that measurement position for that species.
From the second model, when eggshell thickness was measured at the blunt pole, equator, and sharp pole on at least 10 eggshells from a species (n = 2 species: Forster's tern and California least tern), eggshells were, on average, 6.8% thicker at the equator than at the blunt pole (8.4% and 5.1%, respectively; all t ≥ 2.49, p ≤ .013). The comparison of eggshell thickness between the poles was less clear, with Forster's tern eggshells 5.3% thicker at the sharp pole (t = 6.94, p < .001) but no clear difference observed between eggshell thickness at the poles for California least tern (mean sharp pole 2.2% thicker; t = 0.93, p = .35).
3.2.3. Relationship between sharp pole and equator eggshell thickness within species
When individual eggshell thickness measurements (Table 2) and an equator eggshell thickness × species interaction were included in the model, eggshell thickness at the sharp pole was generally related to eggshell thickness at the equator, although there were some differences among species (F 11,2488 = 2.52, p = .004). Within the global model, American avocet had a slope coefficient of 0.76 and, statistically, all but two species with more than 10 samples had similar slopes (all t ≤ 1.6, all p ≥ .11; slope range: 0.73–0.90). Caspian tern had a shallower slope coefficient than American avocet (t = 3.51, p < .001; slope = 0.37) and black‐necked stilt had a steeper slope coefficient than American avocet (t = 2.30, p = .02; slope = 1.00). When models were run individually, eggshell thickness at the sharp pole was significantly related to eggshell thickness at the equator for all 10 species (all F ≥ 7.05, all p ≤ .015, all R 2 ≥ .11; Figure 3a; Table 3).
FIGURE 3.
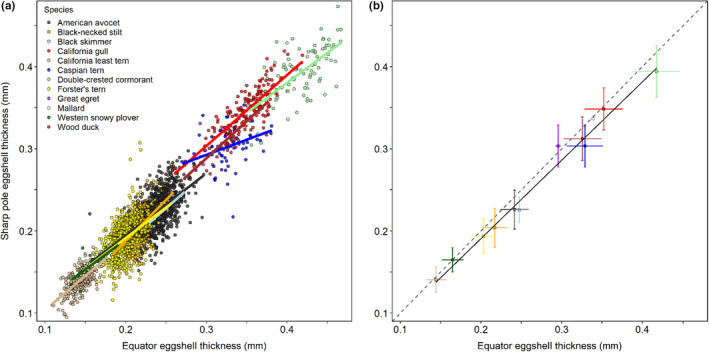
Eggshell thickness at the sharp pole was related to eggshell thickness at the equator during 2014–2018. (a) Individual eggshell measurements are shown with slopes for each species from individual species regressions. (b) Mean (± SD) eggshell thickness values (raw data) are shown for each species on top of the among‐species regression based on the species’ mean values. The solid line is the regression line (sharp pole thickness [mm] = (0.95300 × equator eggshell thickness [mm]) + 0.00029), shown for the range of the data means in the present study, and the dashed line indicates a theoretical 1:1 relationship
TABLE 3.
Slope and intercept values for individual species regressions between sharp pole eggshell thickness (mm) and equator eggshell thickness measurements (mm) for 10 individual species
| Species | Slope | Intercept | Fdf | R 2 | p‐Value |
|---|---|---|---|---|---|
| American avocet | 0.75863 | 0.04271 | F 1,769 = 336.95 | .30 | <.001 |
| Black‐necked stilt | 1.00255 | −0.01266 | F 1,174 = 93.23 | .35 | <.001 |
| Black skimmer | 0.85613 | 0.01369 | F 1,9 = 8.98 | .50 | .015 |
| California gull | 0.86379 | 0.04451 | F 1,160 = 298.63 | .65 | <.001 |
| California least tern | 0.83616 | 0.02009 | F 1,245 = 189.26 | .44 | <.001 |
| Caspian tern | 0.36835 | 0.18224 | F 1,58 = 7.05 | .11 | .010 |
| Double‐crested cormorant | 0.73191 | 0.08782 | F 1,85 = 63.74 | .43 | <.001 |
| Forster's tern | 0.74602 | 0.04220 | F 1,927 = 349.22 | .27 | <.001 |
| Western snowy plover | 0.73016 | 0.04372 | F 1,24 = 28.32 | .54 | <.001 |
| Wood duck | 0.89982 | 0.01872 | F 1,36 = 66.24 | .65 | <.001 |
3.2.4. Relationship between sharp pole and equator eggshell thickness among species
When mean values were used for each species, eggshell thickness at the sharp pole was strongly related to eggshell thickness at the equator (R 2 = .99; Figure 3b). The slope of the relationship was 0.95, indicating that eggshell thickness at the sharp pole was consistently 95% of the thickness at the equator (sharp pole eggshell thickness = eggshell thickness at the equator × 0.95300 + 0.00029). The 95% CI of the residuals for all species except mallard (Anas platyrhynchos), where only 2 eggshells were measured, included zero, indicating that there were no species where the mean relationship between eggshell thickness at the sharp pole and the equator differed from expected.
3.3. Eggshell thickness versus egg morphometrics and bird body mass
The relationship between eggshell thickness at the equator and egg length, egg width, or egg volume was stronger among species than within species. Among species, mean species body mass was the best predictor of species mean eggshell thickness, better than species mean egg length, egg width, or egg volume.
3.3.1. Individual species comparisons
The slope of the relationship between eggshell thickness and egg morphometrics within each species did not differ among species for length (F 11,2844 = 0.67, p = .77; Figure 4) but varied among individual species for width (F 11,2844 = 1.84, p = .042; Figure 4) and for volume (F 11,2720 = 2.93, p < .001). Within each species, eggshell thickness was not related to egg length for any species (all F ≤ 3.50, all p ≥ .06; slope coefficients from −0.00198 to 0.00172), but eggshell thickness was related to egg width in 6 species (all F ≥ 8.54, p ≤ .005, slope coefficients from 0.00247 to 0.00713) but not the remaining 4 species (all F ≤ 2.83, p ≥ .09; Table 4). Within each of these six species (American avocet, black‐necked stilt, California least tern, double‐crested cormorant, Forster's tern, and wood duck), a 10 mm increase in egg width predicted an increase in eggshell thickness at the equator from 0.025 to 0.071 mm. Similarly, within each species, eggshell thickness was related to egg volume in American avocet, black‐necked stilt, California least tern, double‐crested cormorant, Forster's tern, and wood duck (all F ≥ 6.15, p ≤ .018) but not in the remaining 4 species (all F ≤ 3.31, p ≥ .07).
FIGURE 4.
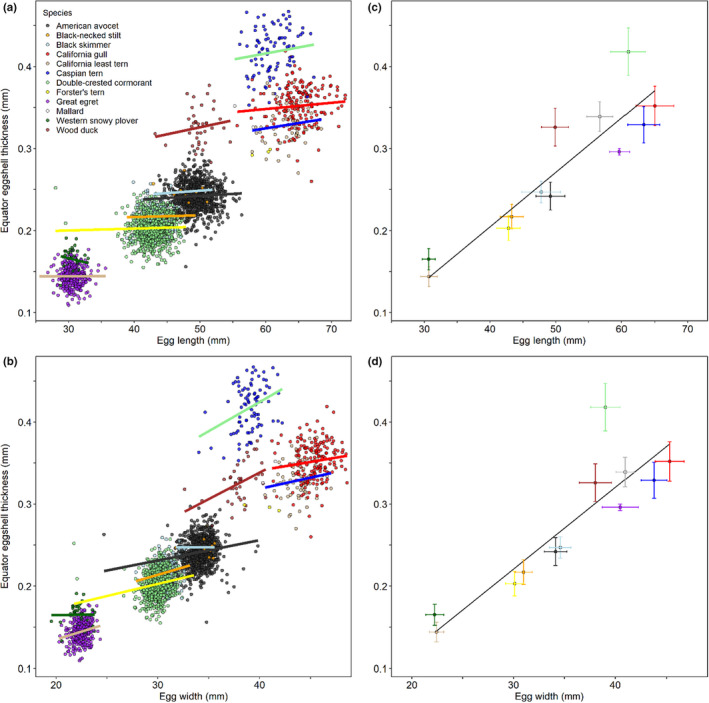
Eggshell thickness at the egg equator as a function of egg length and egg width within and among 12 avian species during 2014–2018. (a) Individual eggs’ equator eggshell thicknesses as a function of egg length within each species (slopes for each species from individual species regressions). (b) Individual eggs’ equator eggshell thicknesses as a function of egg width within each species (slopes for each species from individual species regressions). (c) Arithmetic mean (± SD) eggshell thicknesses and egg lengths (raw data) are shown for each species on top of the among‐species regression (equator eggshell thickness [mm] = (0.00664 × egg length [mm]) – 0.06082) based on the species’ mean values. (d) Arithmetic mean (± SD) eggshell thicknesses and egg widths (raw data) are shown for each species on top of the among‐species regression (equator eggshell thickness [mm] = (0.00999 × egg width [mm]) – 0.07884) based on the species’ mean values
TABLE 4.
Slope and intercept values for individual species regressions between equator eggshell thickness (mm) and egg length (mm) or egg width (mm) for 10 individual species
| Species | Egg length | Egg width | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | Fdf | R 2 | p | Slope | Intercept | Fdf | R 2 | p | |
| American avocet | 0.0005094 | 0.21660 | F 1,840 = 3.50 | <.01 | .06 | 0.00247 | 0.15728 | F 1,840 = 21.65 | .03 | <.001 |
| Black‐necked stilt | 0.0002068 | 0.20836 | F 1,199 = 0.11 | <.01 | .74 | 0.00362 | 0.10513 | F 1,199 = 8.54 | .04 | .004 |
| Black skimmer | 0.0005810 | 0.21971 | F 1,9 = 0.17 | .02 | .69 | −0.00004 | 0.24889 | F 1,9 < 0.01 | <.01 | .99 |
| California gull | 0.0007571 | 0.30291 | F 1,173 = 1.47 | <.01 | .23 | 0.00210 | 0.25683 | F 1,173 = 2.83 | .02 | .09 |
| California least tern | 0.0000569 | 0.14267 | F 1,328 = 0.01 | <.01 | .92 | 0.00382 | 0.05854 | F 1,328 = 15.45 | .04 | <.001 |
| Caspian tern | 0.0012902 | 0.24736 | F 1,60 = 1.21 | .02 | .28 | 0.00270 | 0.21086 | F 1,60 = 1.44 | .02 | .23 |
| Double‐crested cormorant | 0.0014791 | 0.32730 | F 1,86 = 1.53 | .02 | .22 | 0.00713 | 0.13931 | F 1,86 = 12.24 | .12 | <.001 |
| Forster's tern | 0.0002329 | 0.19323 | F 1,1,079 = 0.84 | <.01 | .36 | 0.00296 | 0.11402 | F 1,1,079 = 34.26 | .03 | <.001 |
| Western snowy plover | −0.0019776 | 0.22567 | F 1,32 = 0.72 | .02 | .40 | 0.00006 | 0.16376 | F 1,32 < 0.01 | <.01 | .98 |
| Wood duck | 0.0017174 | 0.24015 | F 1,37 = 0.87 | .02 | .36 | 0.00648 | 0.07923 | F 1,37 = 8.94 | .19 | .005 |
3.3.2. Among species
Among species, the mean eggshell thickness at the equator increased with mean egg length (R 2 = .85; Figure 4), mean egg width (R 2 = .80; Figure 4), and mean egg volume (R 2 = .79). Across species, an increase in mean egg length of 10 mm predicted an increase in eggshell thickness at the equator of 0.066 mm (eggshell thickness = 0.00664 × egg length–0.06082). Mean egg length for each of the 12 species ranged from approximately 30 mm to 65 mm (Table 2), resulting in a predicted difference of 0.232 mm in the eggshell thickness between the shortest and longest species’ eggs represented by this study. Mean egg width for the 12 species ranged from approximately 22 mm to 45 mm, resulting in a predicted eggshell thickness increase of 0.230 mm between the narrowest and widest species’ eggs in this study (eggshell thickness = 0.00999 × egg width–0.07884; Figure 4).
The average eggshell thickness for most species fell within the expected range for eggshell thickness at the equator, predicted from egg length or egg width, with zero included in the 95% CI of the residuals for all individual eggs from all species except double‐crested cormorant, wood duck, and great egret (Ardea alba). Double‐crested cormorant had thicker eggshells at the equator than would be predicted based on the length or width of the egg, and wood duck eggshells also were thicker at the equator than would be predicted based on the length, but not the width, of the egg. Great egret had thinner eggshells than would be predicted based on the length, but not the width, of the egg. The estimated species mean eggshell thickness, using the equation with species mean egg length, ranged from 17.7% lower (double‐crested cormorant) to 13.2% higher (great egret) than the mean measured eggshell thickness. Wood duck (16.9% lower), snowy plover (15.4% lower), Forster's tern (10.3% higher), American avocet (9.5% higher), Caspian tern (9.4% higher), and mallard (6.8% lower) had a predicted mean eggshell thickness that was more than 6.0% higher or lower than the mean measured eggshell thickness. The remaining four species had a predicted mean eggshell thickness that was within 6% of the mean measured eggshell thickness.
The predictive equations using egg morphometrics did not perform well for individual eggs; 47.4% of individual eggs had eggshell thicknesses predicted from egg length that were more than 10% thicker or thinner than the observed eggshell thickness (range 27.3% to 88.6% of eggs within individual species, excluding great egret where the three eggshells were >10% thinner than the predicted eggshell thickness). Similarly, 43.3% of individual eggs had eggshell thicknesses predicted from egg width that were more than 10% thicker or thinner than the observed eggshell thickness (range 25.8% to 100.0% of eggs within individual species, excluding mallard where the 2 eggshells were within 10% of predicted eggshell thickness).
Among species, an increase in log10 body mass of 10% predicted an increase in eggshell thickness at the equator of 0.016 mm (R 2 = .92; p < .001). Mean bird mass for the 12 species ranged from approximately 42 g to 1,831 g, resulting in a predicted eggshell thickness increase of 0.261 mm between the birds with the smallest and largest mean body mass in this study (eggshell thickness [mm] = 0.15918 × log10(bird body mass [g])–0.12689). The estimated species mean eggshell thicknesses, using this equation, ranged from 20.6% lower (western snowy plover) to 15.5% higher (great egret) than the mean measured eggshell thicknesses. American avocet (14.0% higher), California gull (10.5% lower), California least tern (6.3% lower), and double‐crested cormorant (6.2% higher) had a predicted mean eggshell thickness that was more than 6.0% higher or lower than the mean measured eggshell thickness. The remaining six species had a predicted mean eggshell thickness that was within 6% of the mean measured eggshell thickness.
We compared the four different models to predict species mean eggshell thickness from species mean egg morphometric measurements (egg length, width, or volume) or bird body mass, and log10(bird body mass) was the best predictor. The AICc value of −42.1 for log10(bird body mass) was more than a ΔAICc of 2 from the models using egg length (ΔAICc = 5.0), egg width (ΔAICc = 8.3), or egg volume (ΔAICc = 8.8).
3.4. Eggshell thickness versus embryo age
We did not find support for a decrease in the eggshell thickness (including the eggshell membrane) with increasing embryonic development for a subset of normally developing American avocet, black‐necked stilt, and Forster's tern eggs (mean embryo age 7.0 ± 3.9 days; interquartile range 4–10 days; range 0–23 days), after removing the nonsignificant species × embryo age interaction term (F 2,1198 = 1.52, p = .22). Instead, we observed a positive, although biologically small, increase in eggshell thickness at the equator with embryonic age (F 1,1200 = 5.85, p = .016), after accounting for differences among species (F 2,1200 = 980.87, p < .001). However, the variability of eggshell thickness at the equator within a species was far greater than any effect of embryonic age. For example, after excluding the 5% thinnest and the 5% thickest eggshell measurements, eggshell thickness measurements at the equator ranged 0.055 mm for American avocet, 0.050 mm for black‐necked stilt, and 0.049 mm for Forster's tern. In contrast, the eggshell thickness at the equator increased by 0.0066 mm during a standard 24‐day incubation period (mean incubation duration is 22 days for American avocets, 23 days for black‐necked stilt, and 24 days for Forster's tern), which is approximately 3.3% of the average equator eggshell thickness for Forster's tern. For American avocet, 0.0066 mm is 2.7% of the average equator eggshell thickness and it is 3.0% of the average equator eggshell thickness for black‐necked stilt. For the model assessing eggshell thickness at the sharp pole, we did not detect any change in eggshell thickness at the sharp pole with embryonic age (F 1,1160 = 0.09, p = .77) when we accounted for differences among species (F 2,1160 = 318.57, p < .001), after removing the nonsignificant species × embryo age interaction term (F 2,1158 = 0.26, p = .77).
For embryos older than 7 days (mean 10.6 ± 2.5 days, interquartile range: 9–12 days; range: 8–23 days), after removing the nonsignificant species × embryo age interaction term (F 2,513 = 0.28, p = .76), we did not find support for any change in eggshell thickness (including the eggshell membrane) at the equator with increasing embryonic development (F 1,515 = 0.03, p = .86), after accounting for differences among species (F 2,515 = 419.15, p < .001). Of note, only 3.5% of eggs had embryos that were in the final quarter of embryonic development. For the sharp pole in eggshells with embryos older than 7 days, after removing the nonsignificant species × embryo age interaction term (F 2,490 = 0.21, p = .81), we did not find support for any change in eggshell thickness (including the eggshell membrane) at the equator with increasing embryonic development (F 1,492 = 0.32, p = .57), after accounting for differences among species (F 2,492 = 146.31, p < .001).
3.5. Eggshell thickness versus egg status
For a subset of American avocet, black‐necked stilt, and Forster's tern eggs that were sampled during weekly nest monitoring visits, the status of each egg was categorized upon collection as active, abandoned, dead, or failed to hatch. Embryo ages were 7.0 ± 3.9 days (interquartile range 4–10 days) for eggs from active nests (active egg status), 8.8 ± 6.0 days (interquartile range 3–14 days) for eggs that were naturally abandoned by the parents (abandoned egg status), 12.1 ± 7.2 days (interquartile range 3–18 days) for eggs with dead embryos where no sibling eggs hatched from the nest (dead egg status), and 11.6 ± 6.9 days (interquartile range 5–18 days) for eggs with dead embryos where sibling eggs hatched from the nest (failed‐to‐hatch egg status). At the egg equator, after removing the nonsignificant egg status × species interaction term (F 6,1015.0 = 1.64, p = .13), eggshell thickness did not differ among egg status (F 3,1281.4 = 0.32, p = .81; Figure 5) after accounting for species (F 2,1438.8 = 1,089.71, p < .001) and embryo age (F 1,1497.4 = 5.05, p = .02). Similarly, at the sharp pole, after removing the nonsignificant egg status × species interaction term (F 6,990.0 = 0.84, p = .54) and accounting for species (F 2,1340.1 = 1,047.1, p < .001) and embryo age (F 1,1394.6 = 2.45, p = .12), there was no detectable effect of egg status (F 3,1141.0 = 0.68, p = .57) on eggshell thickness.
FIGURE 5.
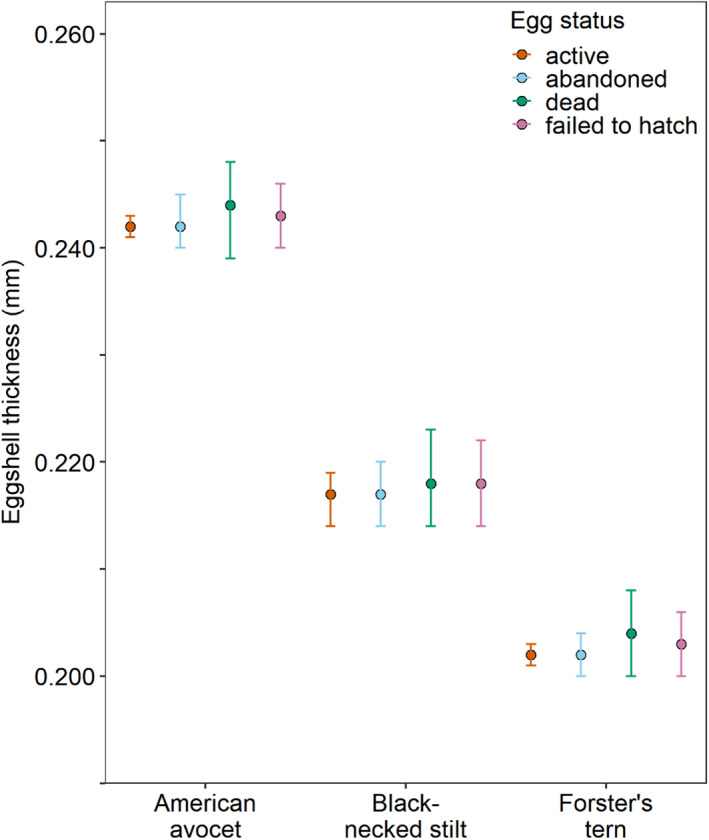
Eggshell thickness at the egg equator (model‐generated least squares mean ± 95% CI) did not relate to the status of the egg at the time of collection for three avian species (American avocet [Recurvirostra americana], black‐necked stilt [Himantopus mexicanus], and Forster's tern [Sterna forsteri]) during 2014–2018. Active eggs were normally progressing in nests that were actively being incubated, whereas abandoned eggs were from nests where the parents had naturally abandoned the nest. Eggs classified as dead contained dead embryos and had stopped progressing normally in nests while they were still being incubated and no sibling eggs in the clutch hatched. Failed‐to‐hatch eggs contained dead embryos and also did not hatch but were from nests where other sibling eggs in the clutch successfully hatched
3.6. Eggshell thickness versus egg content THg
We did not detect a relationship between eggshell thickness and egg content THg concentrations. At the equator, after removing the nonsignificant egg content THg concentration × species interaction term (F 2,1187 = 0.36, p = .70), eggshell thickness was not related to the egg content THg concentration (fww) (F 1,1189 = 1.67, p = .20; Figure 6), after accounting for species (F 2,1189 = 532.24, p < .001) and embryo age (F 1,1189 = 5.39, p = .02). Similarly, at the sharp pole, after removing the nonsignificant egg content THg concentration × species interaction term (F 2,1138 = 0.05, p = .95), eggshell thickness was not related to the egg content THg concentration (fww) (F 1,1140 = 0.12, p = .73), after accounting for species (F 2,1140 = 164.60, p < .001) and embryo age (F 1,1140 = 0.07, p = .79).
FIGURE 6.
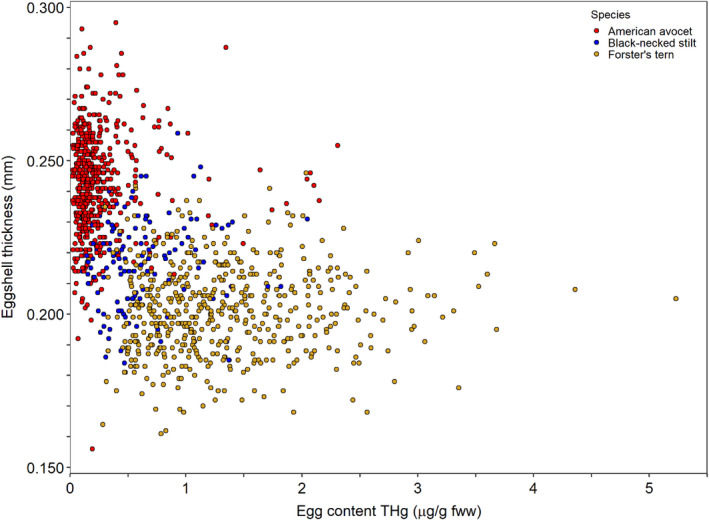
Eggshell thickness at the egg equator was not related to total mercury (THg) concentrations in the egg contents, quantified using fresh wet weight (fww), for three avian species (American avocet [Recurvirostra americana], black‐necked stilt [Himantopus mexicanus], and Forster's tern [Sterna forsteri]) during 2014–2018
4. DISCUSSION
We present eggshell thickness measurements for an additional two avian species in North America (California gull and mallard) that have not been previously measured with the eggshell membrane attached, and we compare eggshell thickness for another 10 species to previous literature (Table 5). Many of the previously measured species were initially sampled to test for effects of organochlorine contaminants on eggshell thickness (Anderson & Hickey, 1972; Blus & Prouty, 1979; Burger, Viscido, & Gochfeld, 1995; Gress, Risebrough, Anderson, Kiff, & Jehl, 1973; Henny, Blus, & Prouty, 1982; King, Flickinger, & Hildebrand, 1978; Postupalsky, 1997; Roberts, 1997). For the 10 species that had been previously measured, the mean eggshell thicknesses were similar to previous studies in North America with some slight differences among studies (Table 5). For example, mean American avocet and black‐necked stilt eggshells were 10.0% and 3.8% thicker, respectively, in the present study than previous mean eggshell thickness measurements for the same species in Utah (Henny, Anderson, & Crayon, 2008), although the ranges of eggshell thicknesses observed in the two studies were similar. Black skimmer eggshells were, on average, 5.6% to 10.3% thicker in the present study than in southern California (Roberts, 1997; Santolo, 2018), but were ≥42.3% thinner than the mean of the most recently sampled eggshells (1990s) of black skimmer from the east coast of the United States (Burger et al., 1995). Forster's tern eggshell thickness in the present study was similar to Forster's tern eggshell thickness from other regions in California (Grant, 1982; Roberts, 1997; Santolo, 2018). However, comparisons of eggshell thickness measurements with previous studies are complicated by the potential influence of geography and subspecies on eggshell thickness in addition to local contamination by DDT and other chlorinated pesticides that might decrease eggshell thickness (Anderson & Hickey, 1972). Many previous studies that examined eggshell thickness in birds were conducted specifically to look for eggshell thinning as a result of environmental contamination. Furthermore, our methodology of using newer technology (a Hall effect thickness gauge) provides a more precise and repeatable thickness measurement than analog micrometers that have been used in many previous studies (Santolo, 2018). Additionally, the Hall effect thickness gauge reduces the potential for the eggshell membrane to be compressed while obtaining the eggshell thickness measurement.
TABLE 5.
Eggshell thickness measurements (mm) from the present study (raw data) and the literature
| Species | Collection location | Collection year | Sample size | Mean eggshell thickness (mm) ± SD, SE or CL (range) | Position of measurement on egg | Eggshell membrane included in measurement (yes/no/not specified) | Measurement method | Reference |
|---|---|---|---|---|---|---|---|---|
| American avocet | California | 1895–1936 | 19 | 0.249 ± 0.006SD | Equator | Yes | Magna‐Mike 8600 HETG | Santolo et al. (2016) |
| Salton Sea, California | 1975–1978 | 7 | 0.236 ± 0.005SD | Not specified | Yes | Micrometer | Grant (1982) | |
| Nevada | 1991 | 32 | 0.262 ± 0.022 (0.220–0.310) | Not specified | Yes | Screw‐type caliper | Ackerman, Hartman, et al. (2013) | |
| Utah | 2004 | 3 | 0.220 ± 0.027SD | Equator | Yes | Modified Starrett micrometer | Henny et al. (2008) | |
| Newport Bay, California | 2004–2005 | 13 | 0.243 ± 0.020SD | Equator | Yes | Modified Starrett micrometer | Santolo et al. (2016) | |
| Great Salt Lake, Utah | 2010 | 6 | 0.228 ± 0.019SD | Not specified | Yes | Starrett micrometer | Cavitt, Linford, and Wilson (2010) | |
| Great Salt Lake, Utah | 2011 | 10 | 0.254 ± 0.021SD | Not specified | Yes | Starrett micrometer | Cavitt and Wilson (2011) | |
| Newport Bay, California | 2013–2016 | 37 | 0.237 | Equator | Yes | Magna‐Mike 8600 HETG | Santolo (2018) | |
| Newport Bay, California* | 2013–2016 | 37 | 0.231 | Equator | Yes | Modified Starrett micrometer | Santolo (2018) | |
| California | 2014–2018 | 843 | 0.242 ± 0.017SD (0.156–0.297) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Black‐necked stilt | Utah | Pre‐DDT a | 40 | 0.205 ± 0.013SE | Not specified | Not specified | Micrometer | Henny, Blus, and Hulse (1985) |
| California | 1893–1930 | 56 | 0.214 ± 0.012SD | Equator | Yes | Magna‐Mike 8600 HETG | Santolo et al. (2016) | |
| Salton Sea, California | 1975–1978 | 22 | 0.224 ± 0.017SD | Not specified | Yes | Micrometer | Grant (1982) | |
| Carson Lake, Nevada | 1980 | 10 | 0.217geo | Equator | Not specified | Micrometer | Henny et al. (1985) | |
| Carson Lake, Nevada | 1981 | 10 | 0.203geo | Equator | Not specified | Micrometer | Henny et al. (1985) | |
| Carson Lake, Nevada | 1982 | 10 | 0.209geo | Equator | Not specified | Micrometer | Henny et al. (1985) | |
| Carson Lake, Nevada | 1983 | 10 | 0.217geo | Equator | Not specified | Micrometer | Henny et al. (1985) | |
| California | 1985–2007 | 5 | 0.236 ± 0.015SD | Equator | Yes | Starrett micrometer | Mora, Brattin, Baxter, and Rivers (2011) | |
| Nevada | 1991 | 18 | 0.234 ± 0.022 (0.210–0.280) | Not specified | Yes | Screw‐type caliper | Robinson et al. (1999) | |
| Utah | 2004 | 12 | 0.209 ± 0.011SD | Equator | Yes | Modified Starrett micrometer | Henny et al. (2008) | |
| Newport Bay, California | 2004–2005 | 13 | 0.214 ± 0.017SD | Equator | Yes | Modified Starrett micrometer | Santolo et al. (2016) | |
| Great Salt Lake, Utah | 2010 | 5 | 0.210 ± 0.027SD | Not specified | Yes | Starrett micrometer | Cavitt et al. (2010) | |
| Newport Bay, California | 2013–2016 | 36 | 0.208 | Equator | Yes | Magna‐Mike 8600 HETG | Santolo (2018) | |
| Newport Bay, California* | 2013–2016 | 36 | 0.199 | Equator | Yes | Modified Starrett micrometer | Santolo (2018) | |
| San Francisco Bay, California | 2014–2018 | 201 | 0.217 ± 0.015SD (0.184–0.259) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Black skimmer | Texas b | Pre‐1931 c | 28 | 0.24 ± 0.004SE | Not specified | Yes | Micrometer | White, Mitchell, and Swineford (1984) |
| Texas | Pre‐1943 c | 28 | 0.249 ± 0.004SE | Not specified | Yes | Micrometer | King et al. (1978) | |
| South Carolina | Pre‐1947 | 241 | 0.229 ± 0.001SE | Not specified | Yes | Micrometer | Blus and Stafford (1980) | |
| South Carolina | 1969 | 10 | 0.217 ± 0.004SE | Equator | Yes | Micrometer | Blus and Stafford (1980) | |
| Galveston Bay, Texas | 1970 | 48 | 0.244 ± 0.002SE (0.22–0.28) | Equator | Yes | Micrometer | King and Krynitsky (1986) | |
| New York | 1970s | 31 | 0.366 ± 0.010SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| South Carolina | 1971 | 26 | 0.238 ± 0.004SE | Equator | Yes | Micrometer | Blus and Stafford (1980) | |
| South Carolina | 1972 | 11 | 0.218 ± 0.004SE | Equator | Yes | Micrometer | Blus and Stafford (1980) | |
| South Carolina | 1973 | 21 | 0.224 ± 0.004SE | Equator | Yes | Micrometer | Blus and Stafford (1980) | |
| South Carolina | 1974 | 12 | 0.227 ± 0.005SE | Equator | Yes | Micrometer | Blus and Stafford (1980) | |
| South Carolina | 1975 | 23 | 0.221 ± 0.003SE | Equator | Yes | Micrometer | Blus and Stafford (1980) | |
| Corpus Christi, Texas | 1978 | 12 | 0.21 ± 0.003SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Corpus Christi, Texas | 1979 | 40 | 0.22 ± 0.002SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Port Mansfield, Texas | 1979 | 24 | 0.23 ± 0.003SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Laguna Vista, Texas | 1979 | 22 | 0.23 ± 0.003SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Corpus Christi, Texas | 1980 | 19 | 0.24 ± 0.003SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Port Mansfield, Texas | 1980 | 21 | 0.23 ± 0.003SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Laguna Vista, Texas | 1980 | 20 | 0.22 ± 0.003SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Galveston Bay, Texas | 1980 | 57 | 0.235 ± 0.003SE (0.21–0.28) | Equator | Yes | Micrometer | King and Krynitsky (1986) | |
| New York | 1980s | 45 | 0.363 ± 0.005SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| New Jersey | 1980s | 16 | 0.351 ± 0.010SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| Galveston Bay, Texas | 1981 | 41 | 0.245 ± 0.002SE (0.20–0.29) | Equator | Yes | Micrometer | King and Krynitsky (1986) | |
| Corpus Christi, Texas | 1981 | 15 | 0.23 ± 0.004SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Port Mansfield, Texas | 1981 | 13 | 0.22 ± 0.003SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Laguna Vista, Texas | 1981 | 15 | 0.22 ± 0.005SE | Equator | Yes | Micrometer | White et al. (1984) | |
| Galveston Bay, Texas | 1982 | 48 | 0.238 ± 0.002SE (0.21–0.26) | Equator | Yes | Micrometer | King and Krynitsky (1986) | |
| Texas | 1984 | ≥41 | 0.240 | Equator | Yes | Micrometer | King et al. (1991) | |
| Laguna Vista, Texas | 1984 | 80 | 0.24 ± 0.01SD | Equator | Yes | Micrometer | Custer and Mitchell (1987) | |
| Not specified | Pre‐1985 | 5 | 0.200 | Not specified | No | Ball‐point caliper | Ar and Rahn (1985) | |
| New York | 1990s | 49 | 0.546 ± 0.005SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| New Jersey | 1990s | 13 | 0.428 ± 0.008SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| San Diego Bay, California | 1991 | 6 | 0.234 (0.215–0.253) | Equator | Yes | Modified Mitutoyo Micrometer | Roberts (1997) | |
| San Diego Bay, California | 1993–1994 | 22 | 0.229 (0.211–0.258) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| Newport Bay, California | 2013–2016 | 11 | 0.224 | Equator | Yes | Magna‐Mike 8600 HETG | Santolo (2018) | |
| Newport Bay, California* | 2013–2016 | 11 | 0.224 | Equator | Yes | Modified Starrett micrometer | Santolo (2018) | |
| San Francisco Bay, California | 2014–2018 | 11 | 0.247 ± 0.013 (0.229–0.273)SD | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| California gull | San Francisco Bay, California | 2014–2018 | 175 | 0.352 ± 0.024 (0.260–0.419)SD | Equator | Yes | Magna‐Mike 8600 HETG | Present study |
| Caspian tern | Pacific Northwest | 1911–1931 | 8 | 0.34 ± 0.03 | Not specified | Yes | Not specified | Penland (1981) |
| Not specified | 1941–1945 | 5 | 0.346 ± 0.015SD | Equator | Yes | Modified Starrett micrometer | Ohlendorf, Schaffner, Custer, Stafford, and Charles (1985) | |
| Texas | Pre‐1943 | 15 | 0.336 ± 0.005SE | Not specified | Yes | Micrometer | King et al. (1978) | |
| San Francisco Bay, California | Pre‐1945 | 44 | 0.329 ± 0.017SD | Equator | Yes | Modified Starrett micrometer | Parkin (1998) | |
| Not specified | 1960–1964 | 5 | 0.320 ± 0.010SD | Equator | Yes | Modified Starrett micrometer | Ohlendorf et al. (1985) | |
| Grays Harbor, Washington | 1961–1976 | 12 | 0.35 ± 0.03 | Not specified | Yes | Not specified | Penland (1981) | |
| Texas | 1970 | 32 | 0.337 ± 0.003SE | Equator | Yes | Micrometer | King et al. (1978) | |
| San Francisco Bay, California | 1971 | 6 | 0.323 ± 0.013SD | Equator | Yes | Modified Starrett micrometer | Parkin (1998) | |
| San Francisco Bay, California | 1971 | 6 | 0.329 ± 0.012SD | Equator | Yes | Modified Starrett micrometer | Parkin (1998) | |
| Bothnian Bay, Finland | 1977–1978 | 118 | 0.225 ± 0.001SE (0.150–0.258) | Equator | No | Binocular microscope | Pulliainen and Marjakangas (1980) | |
| Lake Michigan, Michigan | 1980 | 11 | 0.324 ± 0.02SD | Not specified | Not specified | Dial micrometer with ball attachment | Struger and Weseloh (1985) | |
| Lake Michigan, Michigan | 1980 | 10 | 0.338 ± 0.02SD | Not specified | Not specified | Dial micrometer with ball attachment | Struger and Weseloh (1985) | |
| Lake Michigan, Michigan | 1980 | 10 | 0.338 ± 0.03SD | Not specified | Not specified | dial micrometer with ball attachment | Struger and Weseloh (1985) | |
| Lake Huron, Ontario, Canada | 1980 | 10 | 0.314 ± 0.02SD | Not specified | Not specified | Dial micrometer with ball attachment | Struger and Weseloh (1985) | |
| Lake Huron, Ontario, Canada | 1980 | 10 | 0.303 ± 0.02SD | Not specified | Not specified | Dial micrometer with ball attachment | Struger and Weseloh (1985) | |
| Lake Huron, Ontario, Canada | 1980 | 9 | 0.318 ± 0.02SD | Not specified | Not specified | Dial micrometer with ball attachment | Struger and Weseloh (1985) | |
| Lake Ontario, Ontario, Canada | 1981 | 10 | 0.329 ± 0.02SD | Not specified | Not specified | Dial micrometer with ball attachment | Struger and Weseloh (1985) | |
| San Diego Bay, California | 1981 | 25 | 0.33 | Equator | Yes | Modified Starrett Micrometer | Ohlendorf et al. (1985) | |
| San Diego Bay, California | 1991 | 1 | 0.339 | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| Great Lakes, Ontario, Canada | 1991 | 108 | 0.332 | Equator | Yes | Starrett micrometer | Ewins et al. (1994) | |
| San Diego Bay, California | 1993–1994 | 9 | 0.329 (0.275–0.358) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| Elkhorn Slough, California | 1994 | 4 | 0.302 ± 0.004SD | Equator | Yes | Modified Starrett micrometer | Parkin (1998) | |
| Elkhorn Slough, California | 1995 | 48 | 0.294 ± 0.008SD | Equator | Yes | Modified Starrett micrometer | Parkin (1998) | |
| Salinas River, California | 1996 | 13 | 0.290 ± 0.003SD | Equator | Yes | Modified Starrett micrometer | Parkin (1998) | |
| California | 2013 | 15 | 0.341 (0.300–0.371) | Equator | Yes | Starrett electronic digital micrometer | Clatterbuck, Lewison, Dodder, Zeeman, and Schiff (2018) | |
| San Francisco Bay, California; Washington | 2017–2018 | 62 | 0.329 ± 0.022SD (0.270–0.381) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Double‐crested cormorant d | ||||||||
| P. a. albociliatus | Pacific Northwest | pre−1947 | 51 | 0.432 ± 0.005SE | Not specified | Yes | Micrometer | Henny et al. (1982) |
| California; Mexico | pre−1947 | 29 | 0.428 ± 0.012CL | Equator | Yes | Micrometer | Anderson and Hickey (1972) | |
| Pacific Northwest | 1949–1953 | 14 | 0.386 ± 0.011SE | Equator | Yes | Micrometer | Henny et al. (1982) | |
| Pacific Northwest | 1954–1958 | 11 | 0.374 ± 0.007SE | Equator | Yes | Micrometer | Henny et al. (1982) | |
| Anacapa, California | 1969 | 7 | 0.38 ± 0.02CL | Equator | Yes | Micrometer | Gress et al. (1973) | |
| Los Coronados, Mexico | 1969 | 6 | 0.30 ± 0.03CL | Equator | Yes | Micrometer | Gress et al. (1973) | |
| San Martin, Mexico | 1969 | 7 | 0.44 ± 0.02CL | Equator | Yes | Micrometer | Gress et al. (1973) | |
| Klamath NWR, Oregon | 1977 | 5 | 0.413 ± 0.033SD | Equator | Yes | Micrometer | Henny, Blus, Thompson, and Wilson (1989) | |
| Oregon | 1979 | 10 | 0.432 ± 0.045SD | Equator | Yes | Micrometer | Henny et al. (1982) | |
| Malheur NWR, Oregon | 1980 | 6 | 0.442 ± 0.024SD | Equator | Yes | Micrometer | Henny et al. (1989) | |
| Grays Harbor, Washington | 1984 | 14 | 0.453 ± 0.063SD | Equator | Yes | Modified bench comparator | Speich et al. (1992) | |
| Puget Sound, Washington | 1984 | 11 | 0.454 ± 0.039SD | Equator | Yes | Micrometer | Henny et al. (1989) | |
| Puget Sound, Washington | 1984 | 36 | 0.408 ± 0.033SD | Equator | Yes | Micrometer | Henny et al. (1989) | |
| Columbia River, Washington | 1990–1995 | 31 | 0.428 ± 0.040SD (0.370–0.530) | Equator | Yes | Dial micrometer | Buck and Sproul (1999) | |
| Columbia River, Washington | 1990–1995 | 37 | 0.416 ± 0.042SD (0.297–0.479) | Equator | Yes | Dial micrometer | Buck and Sproul (1999) | |
| Oregon Coast, Oregon | 1992 | 15 | 0.428 ± 0.040SD (0.370–0.530) | Not specified | Not specified | Not specified | Buck and Sproul (1999) | |
| California | 2013 | 8 | 0.406 (0.328–0.466) | Equator | Yes | Starrett electronic digital micrometer | Clatterbuck et al. (2018) | |
| California | 2016–2018 | 88 | 0.433 ± 0.033SD | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| P. a. auritus | Interior North America | Pre‐1947 | 350 | 0.430 ± 0.003CL | Equator | Yes | Micrometer | Anderson and Hickey (1972) |
| Ontario | 1947 | 6 | 0.440 ± 0.013CL | Equator | Yes | Micrometer | Anderson and Hickey (1972) | |
| Manitoba, Canada | 1952 | 11 | 0.406 ± 0.015SE | Equator | Yes | Micrometer | Anderson and Hickey (1972) | |
| Ontario | 1959–1961 | 7 | 0.340 ± 0.015CL | Equator | Yes | Micrometer | Anderson and Hickey (1972) | |
| Interior North America | 1965–1967 | 76 | 0.399 ± 0.014CL | Equator | Yes | Micrometer | Anderson and Hickey (1972) | |
| Wisconsin | 1965–1968 | 5 | 0.309 ± 0.010CL | Equator | Yes | Micrometer | Anderson and Hickey (1972) | |
| Ontario, Canada | 1969 | 23 | 0.315 (0.22–0.39) | Equator | Yes | Micrometer | Postupalsky (1997) | |
| Ontario, Canada | 1969 | 39 | 0.340 ± 0.020CL | Equator | Yes | Micrometer | Anderson and Hickey (1972) | |
| Ontario, Canada | 1970 | 39 | 0.317 (0.18–0.41) | Equator | Yes | Micrometer | Postupalsky (1997) | |
| Ontario, Canada | 1970 | 40 | 0.272 (0.17–0.37) | Equator | Yes | Micrometer | Postupalsky (1997) | |
| Ontario, Canada | 1971 | 28 | 0.352 (0.25–0.44) | Equator | Yes | Micrometer | Postupalsky (1997) | |
| Ontario, Canada | 1971 | 40 | 0.303 (0.17–0.40) | Equator | Yes | Micrometer | Postupalsky (1997) | |
| Ontario, Canada | 1972 | 18 | 0.327 ± 0.023SD | Not specified | Not specified | Not specified | Weseloh, Teeple, and Gilbertson (1983) | |
| Ontario, Canada | 1972–1973 | 9 | 0.392 | Equator | Yes | Micrometer | Postupalsky (1997) | |
| Lake Michigan, Michigan | 1977 | 4 | 0.370 | Equator | Yes | Not specified | Heinz, Erdman, Haseltine, and Stafford (1985) | |
| Wisconsin | 1977 | 3 | 0.373 | Equator | Yes | Not specified | Heinz et al. (1985) | |
| Lake Michigan, Michigan | 1978 | 5 | 0.397 | Equator | Yes | Not specified | Heinz et al. (1985) | |
| Lake Michigan, Michigan | 1978 | 1 | 0.427 | Equator | Yes | Not specified | Heinz et al. (1985) | |
| Wisconsin | 1980 | 3 | 0.423 | Equator | Yes | Not specified | Heinz et al. (1985) | |
| Alberta, Canada | 1984–1985 | 127 | 0.44 ± 0.04 | Equator | Yes | Micrometer | Somers, Goski, and Barbeau (1993) | |
| Minnesota, Wisconsin | 1994–1995 | 306 | 0.410 (0.313–0.501) | Equator | Yes | Micrometer | Custer et al. (1999) | |
| Forster's tern | Monterey County, California | 1932–1939 | 60 | 0.192 (0.166–0.215) | Equator | Yes | Magna‐Mike 8600 HETG | Santolo (2018) |
| Texas b | pre−1943 | 26 | 0.219 ± 0.003SE | Not specified | Yes | Micrometer | King et al. (1978) | |
| Texas | 1970 | 41 | 0.218 ± 0.003SE | Equator | Yes | Micrometer | King et al. (1978) | |
| Salton Sea, California | 1975–1978 | 7 | 0.206 ± 0.014SD | Not specified | Yes | Micrometer | Grant (1982) | |
| San Diego Bay, California | 1981 | 3 | 0.206 ± 0.014SD | Equator | Yes | Modified Starrett micrometer | Ohlendorf et al. (1985) | |
| San Diego Bay, California | 1991 | 12 | 0.201 (0.188–0.216) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Diego Bay, California | 1994 | 2 | 0.208 (0.201–0.216) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| Newport Bay, California | 2013–2016 | 27 | 0.198 | Equator | Yes | Magna‐Mike 8600 HETG | Santolo (2018) | |
| San Francisco Bay, California | 2014–2018 | 1,085 | 0.203 ± 0.015SD (0.155–0.257) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Great egret | Florida, South Carolina | Pre‐1943 | 30 | 0.295 ± 0.004SE | Not specified | Yes | Micrometer | King et al. (1978) |
| Not specified | Pre‐1947 | 235 | 0.295 ± 0.003CL | Equator | Yes | Micrometer | Faber, Risebrough, and Pratt (1972) | |
| California | 1969–1970 | 64 | 0.250 ± 0.007CL | Equator | Yes | Micrometer | Faber et al. (1972) | |
| California | 1969–1970 | 13 | 0.272 ± 0.013CL | Equator | Yes | Micrometer | Faber et al. (1972) | |
| California | 1969–1970 | 51 | 0.244 ± 0.008CL | Equator | Yes | Micrometer | Faber et al. (1972) | |
| Texas | 1970 | 113 | 0.282 ± 0.002SE | Equator | Yes | Micrometer | King et al. (1978) | |
| Salton Sea, California | 1985 | 11 | 0.244 ± 0.016SD | Equator | Yes | Modified Starrett micrometer | Ohlendorf and Marois (1990) | |
| Salton Sea, California | 1993 | 29 | 0.282 ± 0.024SD | Not specified | Not specified | Not specified | Bennett (1998) | |
| Utah | 2004 | 12 | 0.289 ± 0.012SD | Equator | Yes | Modified Starrett micrometer | Henny et al. (2008) | |
| California | 2018 | 3 | 0.296 ± 0.004SD (0.292–0.299) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Least tern e | Texas b | Pre‐1943 | 22 | 0.156 ± 0.003SE | Not specified | Yes | Micrometer | King et al. (1978) |
| South Carolina | Pre‐1947 | 61 | 0.152 ± 0.002SE | Equator | Yes | Micrometer | Blus and Prouty (1979) | |
| New Jersey | 1970s | 31 | 0.366 ± 0.012SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| Texas | 1970 | 15 | 0.154 ± 0.004SE | Equator | Yes | Micrometer | King et al. (1978) | |
| South Carolina | 1972 | 11 | 0.145 ± 0.005SE | Equator | Yes | Micrometer | Blus and Prouty (1979) | |
| Massachusetts | 1974 | 12 | 0.13 ± 0.01SD | Equator | Yes | Micrometer | Rahn, Paganelli, Nisbet, and Whittow (1976) | |
| South Carolina | 1974 | 20 | 0.142 ± 0.002SE | Equator | Yes | Micrometer | Blus and Prouty (1979) | |
| South Carolina | 1975 | 15 | 0.149 ± 0.004SE | Equator | Yes | Micrometer | Blus and Prouty (1979) | |
| New Jersey | 1980s | 20 | 0.338 ± 0.013SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| South Dakota | 1989–1991 | 99 | 0.245 ± 0.030SD | Not specified | Yes | Micrometer | Ruelle (1993) | |
| New Jersey | 1990s | 23 | 0.410 ± 0.013SE | Equator | Yes | Micrometer | Burger et al. (1995) | |
| Kansas | 1991–1994 | 16 | 0.160 ± 0.005SE | Equator | Yes | Starrett pocket dial gauge 1010RZ | Koenen and Leslie (1996) | |
| Oklahoma | 1993–1994 | 80 | 0.153 ± 0.002SE | Equator | Yes | Starret pocket dial gauge 1010RZ | Koenen and Leslie (1996) | |
| California least tern (S. a. browni) | California | Pre‐1947 | 32 | 0.154 ± 0.002SE (0.13–0.18) | Not specified | Yes | Dial micrometer | Massey (1972) |
| California | 1970–1971 | 22 | 0.148 ± 0.003SE (0.13–0.18) | Not specified | Yes | Dial micrometer | Massey (1972) | |
| California | 1981–1985 | 16 | 0.151 ± 0.001SE | Not specified | Yes | Federal 35 bench comparator | Boardman (1998) | |
| San Diego Bay, California | 1990–1991 | 21 | 0.149 (0.109–0.174) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Diego Bay, California | 1990–1992 | 28 | 0.148 (0.134–0.156) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Diego Bay, California | 1990–1992 | 27 | 0.144 (0.125–0.166) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Diego Bay, California | 1991 | 2 | 0.152 (0.140–0.164) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Diego Bay, California | 1991 | 22 | 0.149 (0.129–0.180) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Diego Bay, California | 1994 | 4 | 0.150 (0.144–0.158) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| California | 2013 | 55 | 0.145 (0.123–0.169) | Equator | Yes | Starrett electronic digital micrometer | Clatterbuck et al. (2018) | |
| San Francisco Bay, California | 2014–2018 | 332 | 0.144 ± 0.012SD (0.109–0.177) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Mallard | Not specified | pre−1964 | 1 | 0.286 | Not specified | No | Micrometer screw gauge | Tyler (1964) |
| San Francisco Bay, California | 2014 | 2 | 0.339 ± 0.018SD (0.326–0.352) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Snowy plover | Pacific Northwest | pre−1947 | 9 | 0.146 ± 0.003SE | Equator | Yes | Micrometer | Henny et al. (1982) |
| Oregon | 1949–1953 | 15 | 0.151 ± 0.002SE | Equator | Yes | Micrometer | Henny et al. (1982) | |
| Sand Lake, Oregon | 1980 | 1 | 0.163 | Equator | Yes | Micrometer | Henny et al. (1982) | |
| Newport Beach, California | 1991 | 3 | 0.167 (0.165–0.170) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Diego Bay, California | 1993 | 3 | 0.155 (0.144–0.171) | Equator | Yes | Modified Mitutoyo micrometer | Roberts (1997) | |
| San Francisco Bay, California | 2014–2018 | 34 | 0.165 ± 0.013SD (0.133–0.191) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
| Wood duck | Not specified | Pre‐1964 | 1 | 0.243 | Not specified | No | Micrometer screw gauge | Tyler (1964) |
| Not specified | Pre‐1982 | 5 | 0.300 | Equator | Yes | Micrometer | Rahn et al. (1982) | |
| Wisconsin | Pre‐1986 | ≥8 | 0.255 ± 0.004SE (0.196–0.300) | Shell fragments | Not specified | Tubular micrometer | Soulliere (1987) | |
| Nevada | 2014 | 39 | 0.326 ± 0.023SD (0.273–0.377) | Equator | Yes | Magna‐Mike 8600 HETG | Present study | |
Means are assumed to be arithmetic unless otherwise indicated. Standard deviation (SD) standard error (SE) or confidence limit (CL) is indicated using a superscript after the value. Eggs for multiple studies were salvaged and collected from locations known to contain contaminants that could influence eggshell thickness. Most studies that included museum specimens from prior to 1947 state that the researchers compared eggshell thickness between pre‐DDT eggshells from museums and post‐DDT eggshells that were measured at the egg equator. Although most blow‐out holes in museum eggshells are at or near the equator, if eggs from museum collections were measured closer to the blunt end of the egg than the true egg equator it would result in a thinner eggshell measurement (Santolo, 2018).
Pre‐DDT is put for one study where there were no corresponding dates reported.
Eggs from Texas and other southern latitudes were used when possible but it is unclear exactly where the museum eggs were collected.
Measurements may be from the same eggshells.
Double‐crested cormorant include P. a. albociliatus and P. a. auratus subspecies.
Least tern include interior (S. a. athalassos), eastern (S. a. antillarum), and Pacific coast (S. a. browni) subspecies.
Eggshells were typically thickest at the equator and middle portions of the egg and eggshell thickness declined toward each pole, which is consistent with most previous studies (Longcore, Samson, & Whittendale, 1971; Maurer et al., 2012; Orłowski, Siekiera, et al., 2019; Tyler, 1964). Orłowski, Siekiera, et al. (2019) found that the mean eggshell thickness of white stork (Ciconia ciconia) eggs was 4.6% and 7.7% thicker at the equator than at either the sharp pole or blunt pole, respectively. Conversely, a study on Eurasian reed warblers (Acrocephalus scirpaceus) found the blunt end of the egg to be thicker than the equator (Orłowski et al., 2016), as did a study on domestic Peking duck (A. platyrhynchos f. dom) (Balkan et al., 2006), domestic guinea fowl (Numida meleagris galeata) (Ancel & Girard, 1992), and domestic Japanese quail (Coturnix japonica) (Kul & Seker, 2004). The slope of 0.95 we observed for the relationship between eggshell thickness at the sharp pole and the equator indicates that the eggshell thickness at the sharp pole is consistently 95% of the eggshell thickness at the equator. Therefore, when a whole egg is not available and only eggshell fragments can be collected, if the sharp pole can be identified and measured, this relationship may be used to estimate eggshell thickness at the equator.
Physical characteristics of eggs and biological attributes of the clutch may explain variability in eggshell thickness within species. Our use of a minimum eggshell thickness measurement would likely capture the more pigmented and sometimes thinner sections of an eggshell (Gosler et al., 2005), although variation in eggshell thickness with pigmentation has not been supported in all studies (Maurer, Portugal, Boomer, & Cassey, 2011), suggesting that variation in pigmentation within and among some species may complicate interpretations of eggshell thickness. Additionally, the laying order of a clutch may influence the length and width of an egg (Ackerman, Eagles‐Smith, Herzog, Yee, & Hartman, 2016; Penland, 1981); consequently, this may explain some of the observed variability in eggshell thickness within species. Laying order influenced eggshell thickness in falcons (Falco sp.), with the thickest eggshells observed for the first egg laid (Castilla, Herrel, et al., 2010), but a study on passerines (Passeriformes) found no such relationship (Orłowski et al., 2016). In addition, other studies indicate that the overall clutch size influences an individual egg's eggshell thickness, with bigger clutches associated with thinner individual eggshells in passerine birds (Orłowski et al., 2016).
We did not detect a decrease in the average eggshell thickness at the equator or sharp pole of the egg with embryonic development, and we found some support for a small increase in thickness of the eggshell membrane and eggshell when all embryo ages were included. Most studies observed eggshell thinning as a result of advances in embryonic development, when the eggshell membrane was removed from the calcite eggshell (Ancel & Girard, 1992; Castilla, Herrel, et al., 2010; Finnlund et al., 1985; Orłowski & Hałupka, 2015; Santolo, 2018). However, removal of the eggshell membrane can remove some of the mammillary core and result in an underestimate of the eggshell thickness (Simkiss, 1961). Furthermore, eggshell membranes may increase in thickness during embryonic development (Castilla, Van Dongen, et al., 2010; Finnlund et al., 1985). However, when we examined eggshells with embryos older than one week of age, we found no evidence of eggshell thinning with embryonic development and we no longer observed an increase in eggshell and membrane with embryonic age. Of note, only 3.5% of embryos were in the final quarter of development. Detectable eggshell thinning due to embryonic development may not occur until the final quarter of the incubation period, similar to observations in capercaillie (Tetrao urogallus) eggs (Orłowski, Merta, et al., 2019).
Organochlorine compounds, such as DDT (dichlorodiphenyltrichloroethane), were widely used beginning in the 1940s and were found to decrease eggshell thickness and affect egg survival (Cooke, 1973; Hickey & Anderson, 1968). It is possible that Hg within the egg itself, which also indicates Hg within the female developing the egg (Ackerman et al., 2020), may also influence eggshell thickness, but studies are less conclusive for this contaminant. We found that eggshell thickness, measured either at the equator or the sharp pole, was not correlated with egg content THg concentrations. In contrast, eggshells of Japanese quail thinned significantly when fed diets containing known quantities of mercuric chloride (Stoewsand et al., 1971). Additionally, research on mallard eggshells (Heinz, 1979) showed that eggs of game farm mallards fed a diet of 0.5 ppm Hg had slightly thinner eggshells at the equator (6.6%) than mallards fed on a control diet, but this occurred only in the third generation of mallards fed Hg and not in the first two generations fed Hg (Heinz, 1979). Other field studies have indicated more ambiguous results. Previous research on clapper rail (Rallus longitrostris) indicated that eggshells were thinner at a contaminated site than a control site, with Hg the only metal that significantly differed by site (Rodriguez‐Navarro et al., 2002). However, when data were analyzed by site there was no detectable relationship between eggshell thickness and Hg concentrations; consequently, the observed variation in eggshell thickness may have been driven by site characteristics rather than Hg (Rodriguez‐Navarro et al., 2002). Additionally, eggshell thickness and mercury concentrations were not related in eggs of Forster's tern (n = 79), black skimmer (n = 41) (King et al., 1991), or several shorebird species (Hargreaves et al., 2011).
Eggshell thickness was not related to egg status in the subset of the three avian species where we monitored nests weekly (American avocet, black‐necked stilt, and Forster's tern), similar to previous research on least tern (Koenen & Leslie, 1996). Specifically, eggshells from eggs that did not hatch, either from clutches where no eggs hatched (dead egg status) or from clutches where other eggs in the clutch hatched (failed‐to‐hatch egg status), were not thicker or thinner, on average, than eggs that were progressing normally.
Accounting for eggshell thickness can be important in the accurate calculation of contaminant concentrations in avian eggs. Specifically, using estimates of eggshell thickness results in more accurate (6%–13%) fresh wet weight egg contaminant concentrations than when the eggshell is ignored (Herzog et al., 2016). Using measured eggshell thickness will provide the most accurate calculation of fresh wet weight contaminant concentrations for an individual egg. However, measuring eggshell thickness for every egg can be time consuming and costly. If eggshell thickness cannot be measured for individual eggs, we suggest using mean eggshell thickness for the species (Table 2) in the calculation of fresh wet weight contaminant concentrations instead of using multispecies allometric equations to estimate eggshell thickness. For species where mean eggshell thicknesses do not exist in the literature, you could estimate a species mean equator eggshell thickness using the equation with species mean female body mass in the present study (equator eggshell thickness [mm] = (0.15918 × log10(bird body mass) [mg]) – 0.12689), or other available multispecies equations based on other egg morphometrics (Birchard & Deeming, 2009; Maurer et al., 2012). However, individual species can deviate substantially from expected eggshell thicknesses. For example, in the present study, the mean equator eggshell thickness of snowy plover was 26.0% thicker than would be predicted based on the among‐species equation for body mass. Therefore, using multispecies equations to estimate eggshell thickness is a tool that should be employed with caution only in the absence of empirically measured eggshell thickness data for the specific species being studied.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Sarah H. Peterson: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Funding acquisition (supporting); Investigation (lead); Methodology (equal); Project administration (supporting); Supervision (equal); Validation (equal); Visualization (lead); Writing‐original draft (lead). Joshua T. Ackerman: Conceptualization (equal); Data curation (supporting); Formal analysis (supporting); Funding acquisition (lead); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Validation (equal); Visualization (supporting); Writing‐review & editing (equal). Mark P. Herzog: Conceptualization (equal); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (equal); Methodology (equal); Project administration (supporting); Supervision (equal); Validation (equal); Visualization (supporting); Writing‐review & editing (equal). Matthew S. Toney: Conceptualization (supporting); Data curation (supporting); Investigation (supporting); Methodology (supporting); Writing‐review & editing (supporting). Breanne Cooney: Conceptualization (supporting); Data curation (supporting); Investigation (supporting); Methodology (supporting); Writing‐review & editing (supporting). C. Alex Hartman: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (equal); Methodology (equal); Project administration (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐review & editing (equal).
Ethics statement
Research was conducted with the approval of the U.S. Geological Survey Western Ecological Research Center's Animal Care and Use Committee.
ACKNOWLEDGMENTS
We thank Susan Ewing, Chris Nicolai (U.S. Fish and Wildlife Service), Karen Taylor (California Department of Fish and Wildlife), Caitlin Robinson‐Nilsen, Max Tarjan (San Francisco Bay Bird Observatory), and Ken Collis (Real Time Research) for providing eggs; many field technicians who helped with this research; Gary Santolo for advice and discussions on measuring eggshells; M. Peterson for providing comments that improved the manuscript; and two anonymous reviewers for comments that improved the quality of the manuscript. The use of trade, product, or firm names in the publication is for descriptive purposes and does not imply endorsement by the U. S. Government.
Peterson SH, Ackerman JT, Herzog MP, Toney MS, Cooney B, Hartman CA. Avian eggshell thickness in relation to egg morphometrics, embryonic development, and mercury contamination. Ecol Evol. 2020;10:8715–8740. 10.1002/ece3.6570
Funding information
This research was supported by the U.S. Geological Survey Environmental Health Mission Area's Contaminant Biology Program, the U.S. Geological Survey Ecosystems Mission Area, and the U.S. Geological Survey Western Ecological Research Center.
DATA AVAILABILITY STATEMENT
The data in this article are in ScienceBase: https://doi.org/10.5066/P981OW6T.
REFERENCES
- Ackerman, J. T. , & Eagles‐Smith, C. A. (2010). Accuracy of egg flotation throughout incubation to determine embryo age and incubation day in waterbird nests. Condor, 112, 438–446. [Google Scholar]
- Ackerman, J. T. , Eagles‐Smith, C. A. , Herzog, M. P. , & Hartman, C. A. (2016). Maternal transfer of contaminants in birds: Mercury and selenium concentrations in parents and their eggs. Environmental Pollution, 210, 145–154. 10.1016/j.envpol.2015.12.016 [DOI] [PubMed] [Google Scholar]
- Ackerman, J. T. , Eagles‐Smith, C. A. , Herzog, M. P. , Yee, J. L. , & Hartman, C. A. (2016). Egg laying sequence influences egg mercury concentrations and egg size in three bird species: Implications for contaminant monitoring programs. Environmental Toxicology and Chemistry, 35, 1458–1469. 10.1002/etc.3291 [DOI] [PubMed] [Google Scholar]
- Ackerman, J. T. , Hartman, C. A. , Herzog, M. P. , Takekawa, J. Y. , Robinson, J. A. , Oring, L. W. , … Boettcher, R. (2013). American Avocet (Recurvirostra americana) In Poole A. (Ed.), The Birds of North America online. Ithaca, NY: Cornell Lab of Ornithology. [Google Scholar]
- Ackerman, J. T. , Herzog, M. P. , Evers, D. C. , Cristol, D. A. , Kenow, K. P. , Heinz, G. H. , … Hartman, C. A. (2020). Synthesis of maternal transfer of mercury in birds: Implications for altered toxicity risk. Environmental Science and Technology, 54(5), 2878–2891. 10.1021/acs.est.9b06119 [DOI] [PubMed] [Google Scholar]
- Ackerman, J. T. , Herzog, M. P. , & Schwarzbach, S. E. (2013). Methylmercury is the predominant form of mercury in bird eggs: A synthesis. Environmental Science & Technology, 47, 2052–2060. 10.1021/es304385y [DOI] [PubMed] [Google Scholar]
- Ackerman, J. T. , Takekawa, J. Y. , Bluso, J. D. , Yee, J. L. , & Eagles‐Smith, C. A. (2008). Gender identification of Caspian terns using external morphology and discriminant function analysis. Wilson Journal of Ornithology, 120, 378–383. [Google Scholar]
- Ancel, A. , & Girard, H. (1992). Eggshell of the domestic guinea fowl. British Poultry Science, 33, 993–1001. [DOI] [PubMed] [Google Scholar]
- Anderson, D. , & Hickey, J. (1972). Eggshell changes in certain North American birds. Proceedings of the International Ornithological Congress, 15, 514–540. [Google Scholar]
- Ar, A. , Paganelli, C. V. , Reeves, R. B. , Greene, D. G. , & Rahn, H. (1974). The avian egg: Water vapor conductance, shell thickness, and functional pore area. The Condor, 76, 153–158. [Google Scholar]
- Ar, A. , & Rahn, H. (1985). Pores in avian eggshells: Gas conductance, gas exchange and embryonic growth rate. Respiration Physiology, 61, 1–20. [DOI] [PubMed] [Google Scholar]
- Balkan, M. , Karakaş, R. , & Biricik, M. (2006). Changes in eggshell thickness, shell conductance and pore density during incubation in the Peking duck (Anas platyrhynchos f. dom.). Ornis Fennica, 83, 117–123. [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. , Walker, S. , Christensen, R. H. B. , Singmann, H. , … Green, P. (2015). Linear mixed‐effects models using “Eigen” and S4. R package v. 1.1‐9. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://CRAN.R‐project.org/package=lme4 [Google Scholar]
- Bennett, J. (1998). Biological effects of selenium and other contaminants associated with irrigation drainage in the Salton sea area, California 1992‐1994. National Irrigation Water Quality Program, Information Rept. No. 4. [Google Scholar]
- Birchard, G. F. , & Deeming, D. C. (2009). Avian eggshell thickness: Scaling and maximum body mass in birds. Journal of Zoology, 279, 95–101. [Google Scholar]
- Blus, L. J. , Heath, R. G. , Gish, C. D. , Belisle, A. A. , & Prouty, R. M. (1971). Eggshell thinning in the brown pelican: Implication of DDE. BioScience, 21, 1213–1215. [Google Scholar]
- Blus, L. J. , & Prouty, R. M. (1979). Organochlorine pollutants and population status of least terns in South Carolina. The Wilson Bulletin, 91, 62–71. [Google Scholar]
- Blus, L. J. , & Stafford, C. J. (1980). Breeding biology and relation of pollutants to black skimmers and gull‐billed terns in South Carolina. U.S. Fish & Wildl. Serv. Spec. Scient. Rept.‐Wildlife, no. 230. [Google Scholar]
- Bluso, J. D. , Ackerman, J. T. , Takekawa, J. Y. , & Yee, J. L. (2006). Sexing Forster’s terns using morphometric measurements. Waterbirds, 29, 512–517. [Google Scholar]
- Boardman, C. J. (1998). Organochlorine pesticides in California least terns (Sterna antillarum browni). [Google Scholar]
- Brown, W. Y. (1976). Egg specific gravity and incubation in the sooty tern and brown noddy. The Auk, 93, 371–374. [Google Scholar]
- Buck, J. , & Sproul, E. (1999). Organochlorine contaminants in double‐crested cormorants from Lewis and Clark National Wildlife Refuge in the Columbia River Estuary. [Google Scholar]
- Burger, J. , Viscido, K. , & Gochfeld, M. (1995). Eggshell thickness in marine birds in the New York Bight‐1970s to 1990s. Archives of Environmental Contamination and Toxicology, 29, 187–191. [DOI] [PubMed] [Google Scholar]
- Castilla, A. M. , Herrel, A. , Robles, H. , Malone, J. , & Negro, J. J. (2010). The effect of developmental stage on eggshell thickness variation in endangered falcons. Zoology, 113, 184–188. 10.1016/j.zool.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Castilla, A. M. , Van Dongen, S. , Herrel, A. , Francesch, A. , Martínez De Aragón, J. , Malone, J. , & José Negro, J. (2010). Increase in membrane thickness during development compensates for eggshell thinning due to calcium uptake by the embryo in falcons. Naturwissenschaften, 97, 143–151. 10.1007/s00114-009-0620-z [DOI] [PubMed] [Google Scholar]
- Cavitt, J. F. , Linford, M. , & Wilson, N. (2010). Selenium concentration in shorebird eggs at Great Salt Lake Utah 2010 report. [Google Scholar]
- Cavitt, J. F. , & Wilson, N. (2011). Concentration of selenium and mercury in American avocet eggs at the Great Salt Lake, Utah 2011 report. [Google Scholar]
- Clatterbuck, C. A. , Lewison, R. L. , Dodder, N. G. , Zeeman, C. , & Schiff, K. (2018). Seabirds as regional biomonitors of legacy toxicants on an urbanized coastline. Science of the Total Environment, 619–620, 460–469. [DOI] [PubMed] [Google Scholar]
- Cooke, A. S. (1973). Shell thinning in avian eggs by environmental pollutants. Environmental Pollution, 14, 85–152. [Google Scholar]
- Custer, T. W. , Custer, C. M. , Hines, R. K. , Gutreuter, S. , Stromborg, K. L. , Allen, P. D. , & Melancon, M. J. (1999). Organochlorine contaminants and reproductive success of double‐crested cormorants from Green Bay, Wisconsin, USA. Environmental Toxicology and Chemistry, 18, 1209–1217. [Google Scholar]
- Custer, T. , & Mitchell, C. (1987). Organochlorine contaminants and reproductive success of black skimmers in south Texas, 1984. Journal of Field Ornithology, 58, 480–489. [Google Scholar]
- Delnicki, D. , & Reinecke, K. J. (1986). Mid‐winter food use and body weights of mallards and wood ducks in Mississippi. Journal of Wildlife Management, 50, 43–51. [Google Scholar]
- Drent, R. (1970). Functional aspects of incubation in the herring gull. Behaviour Supplement, 17, 1–132. [Google Scholar]
- Dunning, J. B. J. (2008). CRC handbook of avian body masses (2nd ed.). Boca Raton, FL: CRC Press. [Google Scholar]
- Ewins, P. J. , Weseloh, D. V. C. , Norstrom, R. J. , Legierse, K. , Auman, H. J. , & Ludwig, J. P. (1994). Caspian terns on the Great Lakes: Organochlorine contamination, reproduction, diet, and population changes, 1972–91. Occasional Paper No 85 Canadian Wildlife Service.
- Faber, R. A. , Risebrough, R. W. , & Pratt, H. M. (1972). Organochlorines and mercury in common egrets and great blue herons. Environmental Pollution, 3, 111–122. [Google Scholar]
- Finnlund, M. , Hissa, R. , Koivusaari, J. , Merila, E. , & Nuuja, I. (1985). Eggshells of Arctic terns from Finland: Effects of incubation and geography. The Condor, 87, 79–86. [Google Scholar]
- Gosler, A. G. , Higham, J. P. , & Reynolds, S. J. (2005). Why are birds’ eggs speckled? Ecology Letters, 8, 1105–1113. 10.1111/j.1461-0248.2005.00816.x [DOI] [Google Scholar]
- Grant, G. (1982). Avian incubation: Egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Ornithological Monographs, 30, 1–75. [Google Scholar]
- Gress, F. , Risebrough, R. W. , Anderson, D. W. , Kiff, L. F. , & Jehl, J. R. (1973). Reproductive failures of double‐crested cormorants in southern California and Baja California. Wilson Bulletin, 85, 197–208. [Google Scholar]
- Hargreaves, A. L. , Whiteside, D. P. , & Gilchrist, G. (2011). Concentrations of 17 elements, including mercury, in the tissues, food and abiotic environment of Arctic shorebirds. Science of the Total Environment, 409, 3757–3770. [DOI] [PubMed] [Google Scholar]
- Heinz, G. H. (1979). Methylmercury: Reproductive and behavioral effects on three generations of mallard ducks. Journal of Wildlife Management, 43, 394–401. [Google Scholar]
- Heinz, G. H. , Erdman, T. C. , Haseltine, S. D. , & Stafford, C. (1985). Contaminant levels in colonial waterbirds from Green Bay and Lake Michigan, 1975–80. Environmental Monitoring and Assessment, 5, 223–236. [DOI] [PubMed] [Google Scholar]
- Henny, C. J. , Anderson, T. W. , & Crayon, J. J. (2008). Organochlorine pesticides, polychlorinated biphenyls, metals, and trace elements in waterbird eggs, Salton Sea, California, 2004. Hydrobiologia, 604, 137–149. [Google Scholar]
- Henny, C. J. , Blus, L. J. , & Hulse, C. S. (1985). Trends and effects of organochlorine residues on Oregon and Nevada wading birds, 1979–1983. Colonial Waterbirds, 8, 117–128. [Google Scholar]
- Henny, C. J. , Blus, L. J. , & Prouty, R. M. (1982). Organochlorine residues and shell thinning in Oregon seabird eggs. The Murrelet, 63, 15–21. [Google Scholar]
- Henny, C. J. , Blus, L. J. , Thompson, S. P. , & Wilson, U. W. (1989). Environmental contaminants, human disturbance and nesting of double‐crested cormorants in northwestern Washington. Colonial Waterbirds, 12, 198–206. [Google Scholar]
- Herring, G. , Ackerman, J. T. , & Eagles‐Smith, C. A. (2010). Embryo malposition as a potential mechanism for mercury‐induced hatching failure in bird eggs. Environmental Toxicology and Chemistry, 29, 1788–1794. 10.1002/etc.208 [DOI] [PubMed] [Google Scholar]
- Herring, G. , Ackerman, J. T. , Eagles‐Smith, C. A. , & Takekawa, J. Y. (2010). Sexing California gulls using morphometrics and discriminant function analysis. Waterbirds, 33, 79–85. 10.1675/063.033.0109 [DOI] [Google Scholar]
- Herring, G. , Gawlik, D. E. , & Beerens, J. M. (2008). Sex determination for the great egret and white ibis. Waterbirds, 31, 298–303. [Google Scholar]
- Herzog, M. P. , Ackerman, J. T. , Eagles‐Smith, C. A. , & Hartman, C. A. (2016). It’s what’s inside that counts: Egg contaminant concentrations are influenced by estimates of egg density, egg volume, and fresh egg mass. Ecotoxicology, 25, 770–776. [DOI] [PubMed] [Google Scholar]
- Hickey, J. , & Anderson, D. W. (1968). Chlorinated hydrocarbons and eggshell changes in raptorial and fish‐eating birds. Science, 162, 271–273. [DOI] [PubMed] [Google Scholar]
- Hoyt, D. F. (1979). Practical methods of estimating volume and fresh weight of bird eggs. The Auk, 96, 73–77. [Google Scholar]
- Karlsson, O. , & Lilja, C. (2008). Eggshell structure, mode of development and growth rate in birds. Zoology, 111, 494–502. [DOI] [PubMed] [Google Scholar]
- Khurshid, A. , Farooq, M. , Durrani, F. R. , Sarbiland, K. , & Chand, N. (2003). Predicting egg weight, shell weight, shell thicknesses and hatching chick weight of Japanese quails using various egg traits as regressors. International Journal of Poultry Science, 2(2), 164–167. [Google Scholar]
- King, K. A. , Custer, T. W. , & Quinn, J. S. (1991). Effects of mercury, selenium, and organochlorine contaminants on reproduction of Forster’s terns and black skimmers nesting in a contaminated Texas bay. Archives of Environmental Contamination and Toxicology, 20, 32–40. [DOI] [PubMed] [Google Scholar]
- King, K. A. , Flickinger, E. L. , & Hildebrand, H. H. (1978). Shell thinning and pesticide residues in Texas aquatic bird eggs, 1970. Pesticides Monitoring Journal, 12, 16–21. [PubMed] [Google Scholar]
- King, K. A. , & Krynitsky, A. J. (1986). Population trends, reproductive success, and organochlorine chemical contaminants in waterbirds nesting in Galveston Bay, Texas. Archives of Environmental Contamination and Toxicology, 15, 367–376. [DOI] [PubMed] [Google Scholar]
- Koenen, M. T. , & Leslie, D. M., Jr. (1996). Evaluation of interior least tern eggshell thickness. Colonial Waterbirds, 19, 143–146. [Google Scholar]
- Kul, S. , & Seker, I. (2004). Phenotypic correlations between some external and internal egg quality traits in the Japanese quail (Coturnix coturnix japonica). International Journal of Poultry Science, 3, 400–405. [Google Scholar]
- Longcore, J. R. , Samson, F. B. , & Whittendale, T. W. (1971). DDE thins eggshells and lowers reproductive success of captive black ducks. Bulletin of Environmental Contamination and Toxicology, 6, 485–490. [DOI] [PubMed] [Google Scholar]
- Lundholm, C. E. (1995). Effects of methyl mercury at different dose regimes on eggshell formation and some biochemical characteristics of the eggshell gland mucosa of the domestic fowl. Comparative Biochemistry and Physiology C: Pharmacology Toxicology and Endocrinology, 110, 23–28. [DOI] [PubMed] [Google Scholar]
- Massaro, M. , & Davis, L. S. (2006). The influence of laying date and maternal age on eggshell thickness and pore density in yellow‐eyed penguins. The Condor, 106, 496–505. [Google Scholar]
- Massey, B. W. (1972). Breeding biology of the California least tern. Master of Science Thesis, Department of Biology at California State University.
- Maurer, G. , Portugal, S. J. , Boomer, I. , & Cassey, P. (2011). Avian embryonic development does not change the stable isotope composition of the calcite eggshell. Reproduction, Fertility and Development, 23, 339–345. [DOI] [PubMed] [Google Scholar]
- Maurer, G. , Portugal, S. , & Cassey, P. (2012). A comparison of indices and measured values of eggshell thickness of different shell regions using museum eggs of 230 European bird species. Ibis, 154, 714–724. [Google Scholar]
- Maurer, G. , Russell, D. G. D. , & Cassey, P. (2010). Interpreting the lists and equations of egg dimensions in Schönwetter’s Handbuch Der Oologie. The Auk, 127, 940–947. [Google Scholar]
- Mora, M. A. , Brattin, B. , Baxter, C. , & Rivers, J. W. (2011). Regional and interspecific variation in Sr, Ca, and Sr/Ca ratios in avian eggshells from the USA. Ecotoxicology, 20, 1467–1475. [DOI] [PubMed] [Google Scholar]
- Morrison, M. L. , & Kiff, L. F. (1979). Eggshell thickness in American shorebirds before and since DDT. Canadian Field‐Naturalist, 93, 187–190. [Google Scholar]
- Ohlendorf, H. M. , & Marois, K. C. (1990). Organochlorines and selenium in California night‐heron and egret eggs. Environmental Monitoring and Assessment, 15, 91–104. [DOI] [PubMed] [Google Scholar]
- Ohlendorf, H. M. , Schaffner, F. C. , Custer, T. W. , & Stafford, C. J. (1985). Reproduction and organochlorine contaminants in terns at San Diego Bay. Colonial Waterbirds, 8, 42–53. [Google Scholar]
- Orłowski, G. , & Hałupka, L. (2015). Embryonic eggshell thickness erosion: A literature survey re‐assessing embryo‐induced eggshell thinning in birds. Environmental Pollution, 205, 218–224. [DOI] [PubMed] [Google Scholar]
- Orłowski, G. , Hałupka, L. , Klimczuk, E. , & Sztwiertnia, H. (2016). Shell thinning due to embryo development in eggs of a small passerine bird. Journal of Ornithology, 157, 565–572. [Google Scholar]
- Orłowski, G. , Merta, D. , Pokorny, P. , Łukaszewicz, E. , Dobicki, W. , Kobielski, J. , … Krzywiński, A. (2019). Eggshell resorption, and embryonic mobilization and accumulation of calcium and metals in eggs of wild and captive Capercaillies Tetrao urogallus . Environmental Pollution, 249, 152–162. 10.1016/j.envpol.2019.03.010 [DOI] [PubMed] [Google Scholar]
- Orłowski, G. , Siekiera, J. , Karg, J. , Tobolka, M. , Wuczyński, A. , Kaługa, I. , … Dudzik, E. (2019). Calcium and metals are not evenly distributed in avian eggshells over their longitudinal section. The Auk, 136, 1–14. 10.1093/auk/ukz026 [DOI] [Google Scholar]
- Osborne, D. R. , & Winters, R. (1977). Pre‐1941 eggshell characteristics of some birds. The Ohio Journal of Science, 1, 10–23. [Google Scholar]
- Page, G. W. , Stenzel, L. E. , Warriner, J. S. , Warriner, J. C. , & Paton, P. W. (2009). Snowy plover (Charadrius nivosus) In Poole A. (Ed.), The Birds of North America online. Ithaca, NY: Cornell Lab of Ornithology. [Google Scholar]
- Parkin, J. L. (1998). Ecology of breeding Caspian terns (Sterna caspia) in Elkhorn Slough, California. Department of Biology. [Google Scholar]
- Penland, S. (1981). Natural history of the Caspian tern in Grays Harbor. The Murrelet, 62, 66–72. [Google Scholar]
- Peterson, S. , & Ackerman, J. (2020). Avian Eggshell Thickness 12 Species 2014 to 2018. U.S. Geological Survey Data Release, 10.5066/P981OW6T [DOI] [Google Scholar]
- Postupalsky, S. (1978). Toxic chemicals and cormorant populations in the Great Lakes, Wildlife Services, Wildlife Toxicology Division, MS Report number 40. 25 pages.
- Pulliainen, E. , & Marjakangas, A. (1980). Eggshell thickness in eleven sea and shore bird species of the Bothnian Bay. Ornis Fennica, 57, 65–70. [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R‐project.org/ [Google Scholar]
- Rahn, H. , & Ar, A. (1980). Gas exchange of the avian egg time, structure, and function. American Zoologist, 20, 477–484. [Google Scholar]
- Rahn, H. , & Paganelli, C. V. (1988). Length, breadth, and elongation of avian eggs from the tables of Schönwetter. Journal of Ornithology, 129, 366–369. [Google Scholar]
- Rahn, H. , & Paganelli, C. V. (1989). Shell mass, thickness and density of avian eggs derived from the tables of Schönwetter. Journal of Ornithology, 130, 59–68. [Google Scholar]
- Rahn, H. , Paganelli, C. , & Ar, A. (1987). Pores and gas exchange of avian eggs: A review. Journal of Experimental Zoology Supplement, 1(Suppl.), 165–172. [PubMed] [Google Scholar]
- Rahn, H. , Paganelli, C. V. , Nisbet, I. C. E. , & Whittow, G. C. (1976). Regulation of incubation water loss in eggs of seven species of terns. Physiological Zoology, 49, 245–259. [Google Scholar]
- Rahn, H. , Parisi, P. , & Paganelli, C. V. (1982). Estimating the initial density of birds’ eggs. Condor, 84, 339–341. [Google Scholar]
- Ratcliffe, D. A. (1970). Changes attributable to pesticides in egg breakage frequency and eggshell thickness in some British birds. Journal of Applied Ecology, 7, 67–115. [Google Scholar]
- Roberts, C. A. (1997). Organochlorine contaminants in eggs of tern species and the western snowy plover nesting in San Diego Bay. U.S. Fish and Wildlife Service. [Google Scholar]
- Robinson, J. , Reed, J. , Skorupa, J. , & Oring, L. (1999). Black‐necked stilt (Himantopus mexicanus) In Poole A. (Ed.), The birds of North America online. Ithaca, NY: Cornell Lab of Ornithology,. [Google Scholar]
- Rodriguez‐Navarro, A. B. , Gaines, K. F. , Romanek, C. S. , & Masson, G. R. (2002). Mineralization of clapper rail eggshell from a contaminated salt marsh system. Archives of Environmental Contamination and Toxicology, 43, 449–460. [DOI] [PubMed] [Google Scholar]
- Ruelle, R. (1993). Contaminant evaluation of interior least tern and piping plover eggs from the Missouri River in South Dakota In Proceedings of the Missouri River and its tributaries piping plover and least tern symposium/workshop, Brookings, South Dakota (pp. 159–171). [Google Scholar]
- Santolo, G. M. (2018). A new nondestructive method for measuring eggshell thickness using a non‐ferrous material thickness gauge. The Wilson Journal of Ornithology, 130, 502–509. [Google Scholar]
- Santolo, G. M. , Byron, E. R. , & Ohlendorf, H. M. (2016). Contaminants in sediment, food‐chain biota, and bird eggs from the Newport Bay watershed, Orange County, California. Environmental Monitoring and Assessment, 188, 1–19. [DOI] [PubMed] [Google Scholar]
- Schönwetter, M. , & Meise, W. (1960). Handbuch Der Oologie. Berlin, Germany: Akademie Verlag. [Google Scholar]
- Simkiss, K. (1961). Calcium metabolism and avian reproduction. Biological Reviews, 36, 321–359. [Google Scholar]
- Singmann, H. , Bolker, B. , & Westfall, J. (2015). afex: analysis of factorial experiments. R package v. 0.14‐2. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://CRAN.R‐project.org/package=afex [Google Scholar]
- Somers, J. D. , Goski, B. C. , & Barbeau, J. M. (1993). Accumulation of organochlorine contaminants in double‐crested cormorants. Environmental Pollution, 80, 17–23. [DOI] [PubMed] [Google Scholar]
- Soulliere, G. J. (1987). Distinguishing hooded merganser and wood duck nests by eggshell thickness. The Journal of Wildlife Management, 51, 534. [Google Scholar]
- Speich, S. M. , Calambokidas, J. , Shea, D. W. , Peard, J. , Witter, M. , & Fry, D. M. (1992). Eggshell thinning and organochlorine contaminants in western Washington waterbirds. Colonial Waterbirds, 15, 103–112. [Google Scholar]
- Stoewsand, G. S. , Anderson, J. L. , Gutenmann, W. H. , Bache, C. A. , & Lisk, D. J. (1971). Eggshell thinning in Japanese quail fed mercuric chloride. Science, 173, 1030–1031. [DOI] [PubMed] [Google Scholar]
- Struger, J. , & Weseloh, D. V. (1985). Great Lakes Caspian terns: Egg contaminants and biological implications. Colonial Waterbirds, 8, 142–149. [Google Scholar]
- Tyler, C. (1964). A study of the egg shells of the Anatidae. Journal of Zoology, 142, 547–583. [Google Scholar]
- U.S. Environmental Protection Agency (2000). Method 7473, Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry In Test methods for evaluating solid waste, physical/chemical methods; SW 846, Update IVA. Washington, DC: U.S. Government Printing Office. [Google Scholar]
- Weseloh, D. V. , Teeple, S. M. , & Gilbertson, M. (1983). Double‐crested cormorants of the Great Lakes: Egg laying parameters, reproductive failure, and contaminant residues in eggs, Lake Huron 1972–1973. Canadian Journal of Zoology, 61, 427–436. [Google Scholar]
- Westerskov, K. (1950). Methods for determining the age of game bird eggs. Journal of Wildlife Management, 14, 56–67. [Google Scholar]
- White, D. H. , Mitchell, C. A. , & Swineford, D. M. (1984). Reproductive success of black skimmers in Texas relative to environmental pollutants. Journal of Field Ornithology, 55, 18–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this article are in ScienceBase: https://doi.org/10.5066/P981OW6T.


