Abstract
This study aimed to determine if cardiac troponin I (cTnI) is an independent predictor of clinical outcomes and whether higher values are associated with worse clinical outcomes in Covid-19 patients. This case-series study was conducted at Phoebe Putney Health System. Participants were confirmed Covid-19 patients admitted to our health system between March 2, 2020 and June 7, 2020. Data were collected from electronic medical records. Patients were divided into 2 groups: with and without elevated cTnI. The cTnI were further divided in 4 tertiles. Multivariable logistic regression analysis was performed to adjust for demographics, baseline comorbidities, and laboratory parameters including D-dimer, ferritin, lactate dehydrogenase, procalcitonin and C-reactive protein. Out of 309 patients, 116 (37.5%) had elevated cTnI. Those with elevated cTnI were older (59.9 vs. 68.2 years, p <0.001), and more likely to be males (53.5% vs. 36.3%, p = 0.003). Elevated cTnI group had higher baseline comorbidities. After multivariable adjustment, overall mortality was significantly higher in elevated cTnI group (37.9% vs. 11.4%, odds ratio:4.45; confidence interval:1.78 to 11.14, p <0.001). Need for intubation, dialysis, and intensive care unit (ICU) transfer was higher in elevated cTnI group. Among those with elevated cTnI, mortality was 23.2% for 50th percentile, 48.4% for 75th percentile, and 55.2% for 100th percentile. Similarly, further increase in cTnI was associated with a higher need for intubation, dialysis, and ICU transfer. In conclusion, myocardial injury occurs in significant proportion of hospitalized Covid-19 patients and is an independent predictor of clinical outcomes, with higher values associated with worse outcomes.
Introduction
Coronavirus disease 2019 (Covid-19) has caused a global pandemic resulting in thousands of deaths worldwide.1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent for coronavirus disease 2019 (Covid-19), infects cells through spike (S) protein binding to human angiotensin-converting enzyme 2 (ACE2) receptors, found primarily in the alveolar lung cells, heart, and gastrointestinal tract.2 Cardiac injury reflected by elevated cardiac troponin I (cTnI) concentrations among infected patients has been shown in multiple studies, and was also shown to be a predictor of mortality.3, 4, 5 Among those with elevated cTnI, it is unknown whether higher values are associated with a further increase in mortality. Thus, the primary objectives of our study are: (i) to determine if elevated cTnI is an independent predictor of mortality in hospitalized Covid-19 patients, and (ii) whether incremental cTnI values are associated with further increase in mortality in the same cohort. The secondary objectives of our study are: (i) to determine if elevated cTnI is an independent predictor for need for mechanical ventilation, dialysis, and transfer to intensive care unit (ICU) in hospitalized Covid-19 patients, and (ii) whether incremental cTnI values are associated with further increase in those outcomes in the same cohort.
Methods
This study was a case-series conducted at Phoebe Putney Health System (PPHS), the largest community health system in Southwest Georgia, serving 42 counties and approximately 815,000 population. The PPHS institutional review board approved the study and waived the requirement for informed consent due to minimal risk. The original study showing demographics and baseline characteristics for this study population is published somewhere else.6 Confirmed Covid-19 positive patients were admitted to any of the 3 Phoebe Putney hospitals between March 2, 2020 and June 7, 2020, inclusive of those dates. Patients who had at least 1 cTnI value during hospitalization, and had an outcome were included in the analysis. The outcome was defined as discharged alive or death during hospitalization. Hospitalized patients who did not have an outcome by the end of the study period or did not have at least 1 cTnI measurement during hospitalization were not included in the analysis. The patients transferred to another hospital (due to the hospital being at full capacity or need for treatment not available at our facility) were not included as well.
Data were collected from the electronic medical records (Meditech and Athena Health). Data collection included demographics, insurance, baseline comorbidities, tobacco use, alcohol use, illicit drug use, home medications, symptoms on presentation, vitals, laboratory test results, initial electrocardiogram, the severity of presenting illness, treatment course (intensive care unit [ICU] care, need for mechanical ventilation and dialysis), length of stay (LOS), and outcomes (discharged alive or death). The comorbidities included hypertension (HTN), diabetes mellitus (DM), coronary artery disease (CAD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), asthma, chronic kidney disease (CKD), cancer, immunosuppression, and chronic liver disease. All comorbidities except immunosuppression were adjudicated based on the 10th version of the International Classification of Diseases (ICD-10). Patients were considered immunosuppressed if they had been on chronic steroids or other immunosuppressive therapy. The highest values of cTnI, D-dimer, lactate dehydrogenase (LDH), ferritin, procalcitonin, and C-reactive protein (CRP) were used for analysis. Severe Covid-19 was defined if ARDS, septic shock, or severe pneumonia was present on admission. A decision to intubate or transfer to ICU was at the discretion of the attending physician. The standardized laboratory value of the upper limit of the 99th percentile of normal cTnI is 0.05 ng/ml at our institution, and any value above this was considered elevated in this study. The decision to order cTnI was at the discretion of the treating physician. Accordingly, patients were divided into 2 groups: with and without elevated troponin.
We utilized Statistical Analysis Software (SAS) version 9.4 (Cary, NC) for all the statistical analyses. Categorical variables were expressed in numbers and percentages and analyzed using the Pearson chi-square test. Continuous variables were expressed in mean ± standard deviation and analyzed using the Student's T-test. Hierarchical multivariable logistic regression analysis was performed to adjust for variation in baseline characteristics. In this model, we included age, gender, race, body mass index, comorbidities, presenting illness severity, D-dimer, LDH, procalcitonin, ferritin, and CRP. C-statistics was above 0.7 for all the models to be acceptable. To determine if the higher value of cTnI was associated with worse outcomes, we divided cTnI values in 4 groups: normal values (<0.05 ng/ml), 0 to 50th percentile (0.05 to 0.22 ng/ml), 50 to 75th percentile (0.23 to 0.73 ng/ml) and 75 to 100th percentile (>0.73 ng/ml).
Results
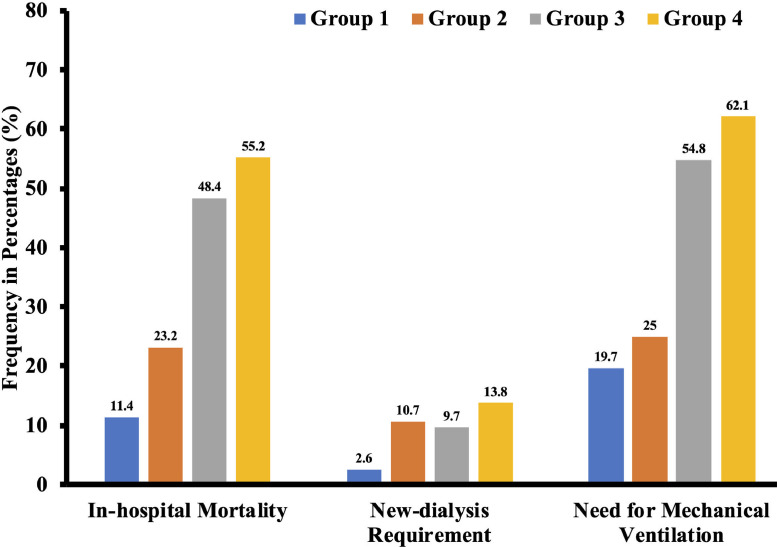
A total of 635 consecutive patient records were reviewed, out of which cTnI was measured at least once during the hospitalization in 309 patients. Out of 309 total patients, 116 (37.5%) had elevated cTnI. Those with elevated cTnI were older (59.9 vs. 68.2 years, p <0.001), and more likely to be males (53.5% vs. 36.3%, p = 0.003). Majority of the patients were African Americans in both groups (∼ 87%). Patients with elevated cTnI were more likely to have hypertension (93.1% vs. 79.3%, p = 0.001), coronary artery disease (16.4% vs. 4.7%, p <0.001), congestive heart failure (31.9% vs. 14.5%, p <0.001), chronic kidney disease (33.6% vs. 9.8%, p <0.001), and dialysis (17.3% vs. 1.8%, p <0.001) at baseline (Table 1 ). They also had higher D-dimer, procalcitonin, ferritin, and C-reactive protein levels (Table 1). After multivariable adjustment, overall mortality was significantly higher in elevated cTnI group (37.9% vs. 11.4%, odds ratio {OR}:4.45; confidence interval {CI}:1.78 to 11.14, p <0.001). The need for mechanical ventilation (42.2% vs. 19.7%, OR:5.18; CI: 2.12 to 12.69, p <0.001), and transfer to ICU (50% vs. 26.9%, OR:4.95; CI:2.19 to 11.17, p <0.001) were also significantly higher in elevated cTnI group (Table 1). With higher cTnI levels, the mortality increases further. Among those with elevated cTnI, mortality was 23.2% for 50th percentile, 48.4% for 75th percentile, and 55.2% for 100th percentile. The need for new dialysis, mechanical ventilation, and ICU care was significantly higher in patients with higher cTnI (Figure 1 ).
Table 1.
Demographics, comorbidities, laboratory parameters, and outcomes in hospitalized Covid-19 patients with and without elevated troponins
| Characteristics | Elevated Troponin |
P Value | |
|---|---|---|---|
| No (N=193) | Yes (N=116) | ||
| Mean age (years) | 59.9±14.0 | 68.2±14.1 | <0.001 |
| Women | 123 (63.7 %) | 54 (46.5 %) | 0.003 |
| Mean Body mass index, kg/m2 | 35.8±10.1 | 33.6±9.8 | 0.06 |
| White | 22 (11.4 %) | 15 (12.9 %) | 0.28 |
| Black | 167 (86.5 %) | 101 (87.1 %) | |
| Others | 4 (2.1 %) | 0 (0) | |
| Hypertension | 153 (79.3 %) | 108 (93.1 %) | 0.001 |
| Coronary artery disease | 9 (4.7 %) | 19 (16.4 %) | <0.001 |
| Congestive heart failure | 28 (14.5 %) | 37 (31.9 %) | <0.001 |
| Chronic obstructive pulmonary disease3 | 20 (10.4 %) | 17 (14.7 %) | 0.26 |
| Asthma | 34 (17.6 %) | 17 (14.7 %) | 0.50 |
| Chronic kidney disease | 19 (9.8 %) | 29 (33.6 %) | <0.001 |
| End stage renal disease (dialysis) | 3 (1.8 %) | 18 (17.3 %) | <0.001 |
| Diabetes | 86 (44.6 %) | 57 (49.1 %) | 0.43 |
| Cancer | 22 (11.4 %) | 12 (10.3 %) | 0.77 |
| Immunosuppression | 9 (4.7 %) | 9 (7.8 %) | 0.26 |
| Chronic liver disease | 2 (1 %) | 3 (2.6 %) | 0.29 |
| Smoking | 29 (15 %) | 28 (24.1 %) | 0.04 |
| Severe Covid-19 disease | 95 (49.2 %) | 59 (50.9 %) | 0.78 |
| Laboratory Values | |||
| D-Dimer (mcg/ml) | 4.8±4.2 | 6.1±5.2 | 0.019 |
| Lactate Dehydrogenase (U/L) | 429±408 | 448±311 | 0.66 |
| Procalcitonin (ng/ml) | 5.1±17.5 | 10.5±25.3 | <0.001 |
| Ferritin (ng/ml) | 1600±2922 | 2983±3605 | <0.001 |
| C-Reactive Protein (mg/L) | 16.1±10.4 | 18.6±10.7 | <0.001 |
| In-hospital Outcomes | |||
| In-hospital Mortality | 22 (11.4 %) | 44 (37.9 %) | <0.001 |
| New Dialysis Requirement | 5 (2.6 %) | 13 (11.2 %) | 0.002 |
| Mechanical Ventilation | 38 (19.7 %) | 49 (42.2 %) | <0.001 |
| Intensive Care Unit Transfer | 52 (26.9 %) | 58 (50.0 %) | <0.001 |
Figure 1.
Outcomes stratified by 4 different groups based on cardiac troponin I level.
Group 1: Normal value < 0.05 ng/ml; Group 2: 0 to 50th percentile and value of cardiac troponin I = 0.05 to 0.22 ng/ml; Group 3: 51 to 75th Percentile and value of cardiac troponin I = 0.22 to 0.73 ng/ml; Group 4: 75th to 100th percentile and value of cardiac troponin I = >0.73 ng/ml.
Discussion
Our results indicate that more than one-third of the patients hospitalized with Covid-19 have evidence of cardiac injury, assessed by elevated cTnI, and is an independent predictor of mortality. The prevalence of myocardial injury in our study was higher than reported in studies from China. These studies reported a prevalence ranging from 7% to 28% compared to 37.5% in our study.3, 4, 5 But a recent study from New York showed a similar prevalence of myocardial injury in hospitalized Covid-19 patients.7 There is evidence to suggest acute myocardial injury at hospital admission is also associated with increased in-hospital mortality in Covid-19.8 However, ours is the first study to demonstrate increased mortality with higher cTnI levels. In our study, the mortality was 5 times higher for patients with troponins in the highest quartile compared to those with normal troponin values. Similar to previous studies, we found that patients with myocardial injury were older, had a higher prevalence of the cardiovascular disease, and had higher inflammatory markers.3, 4, 5 , 7 Possible mechanisms of myocardial injury in Covid-19 include direct damage to the cardiomyocytes, systemic inflammation, myocardial interstitial fibrosis, interferon -mediated immune response, exaggerated cytokine response by Type 1 and 2 helper T cells, coronary plaque destabilization, oxygen supply-demand mismatch, microembolic infarcts, hyperadrenergic state, and pulmonary embolism.9 , 10 Based on the Fourth Universal Definition of Myocardial Infarction, there were very few patients who met the strict criteria for acute myocardial infarction.11 Although we could not ascertain the etiology of myocardial injury in all the patients, the majority were believed to have a noncoronary mediated mechanism. This remains an ongoing challenge and none of the previous studies have been able to identify the etiology of myocardial injury in Covid-19 patients.
The decision to measure cTnI was at the discretion of the treating physician and hence there is a selection bias based on cTnI measurement. Since we used electronic medical records, some data elements might not be accurately captured. We did not have electrocardiograms and echocardiograms in all patients at the time of troponin measurement to correlate with troponin elevations.
Myocardial injury is present in a significant proportion of hospitalized Covid-19 patients. Even though respiratory symptoms are the most common presentation, cTnI is a relatively cheap test to risk-stratify patients based on their cTnI level which has prognostic value and is independently associated with higher mortality as shown in this study. Future studies should explore the etiology as well as best management strategies in Covid-19 patients with evidence of myocardial injury.
Authors' contribution
Priyank Shah: Conceptualization, data curation, formal analysis, methodology, writing original draft, review and editing. Rajkumar Doshi: Data curation, formal analysis, writing original draft, review and editing. Avantika Chenna: writing original draft, review and editing. Robin Owens: data curation, review and editing. Abigail Cobb: data curation, review and editing. Holly Ivey: data curation, review and editing. Sarah Newton: data curation, review and editing. Kelly McCarley: data curation, review and editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would sincerely like to thank Jennifer Hill and Krista Barfield for their diligent efforts in data abstraction.
Footnotes
Source of Funding: None.
Conflicts of Interest: None.
Statement of Authorship: All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) Situation Report – 151. Available athttps://www.who.int/docs/default-source/coronaviruse/situation-reports/20200619-covid-19-sitrep-151.pdf?sfvrsn=8b23b56e_2 (Accessed on June 20, 2020).
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens T, Herrler G, Wu N, Nitsche A, Muller M, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardio. 2020 doi: 10.1001/jamacardio.2020.0950. e200950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. e201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie SF, Yu M, Xie T, Yang F, Wang H, Wang Z, Li M, Gao X, Lv B, Wang S, Zhang X, He S, Qiu Z, Liao Y, Zhou Z, Cheng X. Cardiac troponin I is an independent predictor for mortality in hospitalized patients with coronavirus disease 2019. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah P, Owens J, Franklin J, Mehta A, Heymann W, Sewell W, Hill J, Barfield K, Doshi R. Demographics, comorbidities, and outcomes in hospitalized covid-19 patients in rural Southwest Georgia. Annals of Medicine. 2020:1–7. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lala A, Johnson KW, Januzzi JL, Russak A, Paranjpe I, Richter F, Zhao S, Somani S, Vleck T, Vaid A, Chaudhry F, De Freitas J, Fayad Z, Pinney S, Levin M, Charney A, Bagiella E, Narula J, Glicksberg B, Nadkarni G, Mancini D, Fuster V. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni W, Yang X, Liu J, Bao J, Li R, Xu Y, Guo W, Hu Y, Gao Z. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020;76(1):124–125. doi: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert J, Jaffe A, Chaitman B, Bax J, Morrow D, White H. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]