Abstract
Purpose
The aim of this study was to investigate if CT performed in the early disease phase can predict the course of COVID-19 pneumonia in a German cohort.
Method
All patients with RT-PCR proven COVID-19 pneumonia and chest CT performed within 10 days of symptom onset between March 1st and April 15th 2020 were retrospectively identified from two tertiary care hospitals. 12 CT features, their distribution in the lung and the global extent of opacifications were evaluated. For analysis of prognosis two compound outcomes were defined: positive outcome was defined as either discharge or regular ward care; negative outcome was defined as need for mechanical ventilation, treatment on intensive care unit, extracorporeal membrane oxygenation or death. Follow-up was performed until June 19th. For statistical analysis uni- und multivariable logistic regression models were calculated.
Results
64 patients were included in the study. By univariable analysis the following parameters predicted a negative outcome: consolidation (p = 0.034), crazy paving (p = 0.004), geographic shape of opacification (p = 0.022), dilatation of bronchi (p = 0.002), air bronchogram (p = 0.013), vessel enlargement (p = 0.014), pleural effusion (p = 0.05), bilateral disease (p = 0.004), involvement of the upper lobes (p = 0.004, p = 0.015) or the right middle lobe (p < 0.001) and severe extent of opacifications (p = 0.002). Multivariable analysis revealed crazy paving and severe extent of parenchymal involvement to be independently predictive for a poor outcome.
Conclusions
Easy to assess CT features in the early phase of disease independently predicted an adverse outcome of patients with COVID-19 pneumonia.
Keywords: COVID-19, SARS-CoV-2, Outcome, Prognostic factors, Pneumonia, Computed tomography
1. Introduction
In December 2019 a novel coronavirus meanwhile named SARS-CoV-2 was isolated in several patients with acute severe lower respiratory tract illness in Wuhan, Hubei Province, China [1]. The corresponding symptoms were summarized under the term coronavirus disease 2019 (COVID-19). The standard of reference for the diagnosis of COVID-19 is RT-PCR. Initially restricted to Wuhan, the infection spread rapidly reaching the status of a pandemic in March 2020 [2]. Meanwhile over 10 million cases have been confirmed worldwide, among them 194910 in Germany, as of June 29, 2020 [3]. The majority of patients suffering from COVID-19 have no or only mild symptoms and restitute quickly. However, 15 % develop severe pneumonia, and 5% critical disease including acute respiratory distress syndrome (ARDS), septic shock and multi organ failure, eventually leading to death [4].
Several studies reporting the morphology of COVID-19 pneumonia in chest computed tomography (CT) have been published [[5], [6], [7], [8], [9], [10], [11]]. CT features of COVID-19 pneumonia seem to be amazingly similar for many patients. CT morphology can be so suggestive that radiologists might be able to distinguish COVID-19 from pneumonias caused by other viruses [[12], [13], [14], [15]]. However, there are non-infectious differential diagnoses like in particular organizing pneumonia which can look very similar to COVID-19 pneumonia. Regarding sensitivity of CT studies consistently report very high numbers (circa. 95 %) with some of them reporting even higher sensitivities of initial CT as compared to initial RT-PCR [[17], [18], [19]]. Apart from this CT plays an important role for quantification of parenchymal involvement, detection of complications (like pulmonary embolism or superinfection), detection of COVID-19 pneumonia as an “incidental” finding and even for triage in case of constrained resources. Hence, CT has emerged to a widely used tool in the care of patients with suspected or proven COVID-19.
Only few studies evaluated CT features which may allow prediction of course of disease [[20], [21], [22], [23], [24]]. Most of these studies described Chinese cohorts. However, it has to be considered that the course of disease and patient outcome are influenced by many parameters. Among them host features (which again are not only influenced by individual factors but also by ethnicity) and viral genome variability due to mutations might affect outcome. Also organization and resources of the respective health care system determines patient outcome. Thus, evaluation of the prognostic value of CT in a European cohort seems worthwhile. So far there is only one study from Italy linking the amount of parenchymal involvement with patient outcome [25]. Italian hospitals, however, have been intermittently overwhelmed by patient number with consecutive constraints for patient care. Germany so far has been in the fortunate situation to be able to provide sufficient resources at any time.
The aim of this study was to investigate if CT performed in the early disease phase can predict the course of COVID-19 pneumonia and patient outcome in a German cohort.
2. Material and methods
2.1. Study population
This retrospective study was approved by the institutional ethics committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was waived.
The inclusion criteria were consecutive adult patients (≥18 years old) with RT-PCR positive for SARS-CoV-2 and a chest CT performed within 10 days of symptom onset between March 1st and April 15th 2020. Exclusion criteria were a negative result of RT-PCR for SARS-CoV-2 and non-diagnostic CT, for example due to motion-artifacts. Patients were identified by means of a full-text database query of all CT-scans performed between March 1st and April 15th 2020 using the term “*COVID*” and *SARS* in the Radiological Information System (RIS, Nexus.medRIS, Version 8.42, Nexus, Villingen-Schwenningen, Germany). Patient characteristics (age, gender, comorbidities), symptoms, date of symptom onset, RT-PCR results and patient outcome were extracted from electronic patient records. All patients had at least one CT scan of the chest. In case of several scans per patient the first CT performed per patient was included into the analysis. Patients were followed until June 19th 2020.
2.2. CT protocol
The patients underwent CT scans at two tertiary care hospitals. Chest CTs were performed on two different scanners (16 slice Somatom Sensation 16, 128-slice Definition FLASH, all Siemens Healthcare, Erlangen, Germany). All CT acquisitions were obtained in supine position during end-inspiration. Intravenous contrast material was administered at the discretion of the radiologist considering the individual study indication. Automatic tube voltage selection was applied with a reference tube voltage of 120 kV. Tube current was regulated by an automatic tube current modulation technique with the reference mAs being 40−110. Collimation width was 0.625 mm–0.75 mm. Multiplanar reformations (MPR) were reconstructed in the axial plane with a slice thickness of 0.75–1.5 mm (56 CTs) and 2–4 mm (9 CTs) in lung kernel and with a slice thickness of 1−5 mm in soft tissue kernel. Additional sagittal and coronal MPRs were reconstructed with a slice thickness of 1–3 mm using lung and soft tissue kernel. The pictures were sent to a picture archiving and communication system (PACS, Syngo Imaging, Siemens, Erlangen, Germany).
2.3. Image analysis
Images were analyzed in consensus by two radiologists. The radiologists were blinded to clinical data, laboratory data and patient status. The Fleischner Society definition of CT features were applied when appropriate [26]. The following parameters were analyzed: ground-glass-opacities (GGO, hazy increased opacity of lung with preservation of bronchial and vascular margins), consolidation (homogeneous increase in pulmonary parenchymal attenuation that obscures the margins of vessels and airway walls), crazy-paving pattern (thickened interlobular septa and intralobular lines superimposed on a background of ground-glass opacity), cavities (gas-filled space within consolidation), bronchial dilatation (dilated -with respect to the accompanying pulmonary artery non-tapering bronchus; the term “dilatation” instead of “-ectasis” was intentionally used in order to express that the pathology might be reversible), vessel dilatation (diameter of vessel within or near opacifications clearly larger compared to vessels of the same generation in healthy lung tissue), shape of opacification (round vs curvilinear/band-like vs geographic = opacification outlines the shape of multiple adjacent pulmonary lobules, sharply marginated), margin of opacification (unsharp vs at least to some extent sharp), lung lobes affected. Moreover, distribution of opacifications in the axial plane (predominantly peripheral vs predominantly central and predominantly anterior vs predominantly posterior vs diffuse), lymphadenopathy (diameter >10 mm in short axis) and subjective estimation of extent of parenchymal opacifications (0–33 %: mild, 34–66 %: moderate, 67–100 %: severe) were analyzed.
2.4. Definition of endpoint
Two compound outcomes were defined as the endpoint: positive outcome was defined as either discharge or regular ward care (= group 1); negative outcome was defined to be need for intubation or need for admission to intensive care unit or need for extracorporeal membrane oxygenation (ECMO) or death (group 2).
2.5. Statistical analysis
Age is presented as mean (standard deviation) and all categorical variables as absolute and relative frequencies. Univariable logistic regression models were used to analyze the predictive value of all parameters (demographic data, clinical data, comorbidities and CT findings) on the endpoint (positive vs negative outcome). Furthermore, a multivariable logistic regression model analyzing only the CT findings was calculated. At first, all significant CT findings were added to a model followed by a manual stepwise backward elimination until only predictor variables with p < 0.1 were left. A second multivariable model was built the same way including characteristics of parenchymal involvement. Due to quasi-separated data regarding the bronchial dilatation, the penalized likelihood method by Firth [27] was used to reduce the bias of the maximum likelihood estimates. A multivariable model including all CT findings, all grades of parenchymal involvement and further patient related parameters was not feasible due to the limited amount of patients and events. Odds ratios and corresponding 95 %-confidence intervals (95 %-CI) were calculated as effect estimates for all logistic regression models. A p-value <0.05 was considered statistically significant. All analyses were performed using R, version 3.6.1 (The R Foundation for Statistical Computing) and SAS, version 9.4 (The SAS institute, Cary, NC.).
3. Results
3.1. Patient demographics and clinical features
64 patients fulfilled the inclusion criteria. The study population included 62 % male (n = 40) and 38 % female (n = 24) patients. Age ranged from 21 to 88 years with a mean of 57.2 years (SD 14.5). Clinical symptoms were fever in 47 patients (75 %), cough in 48 patients (79 %), dyspnea in 41 patients (68 %) and fatigue in 34 patients (72 %). Less frequently seen were gastrointestinal complaints (n = 12; 27 %) and taste dysfunction (n = 11; 25 %). Most common comorbidities were high blood pressure (n = 23, 37 %), diabetes (n = 8, 12 %) and coronary heart disease (n = 6, 9%). Prevalence of obesity and smoking history could not be determined because of frequent lack of corresponding data in patient charts. 9 patients (14 %) deceased during the observation period. Patient characteristics are presented in Table 1 .
Table 1.
Patient characteristics (n = 64).
| patient characteristics | patients (n = 64) |
|---|---|
| age (years) | 57.2 (SD 14.5) |
| time between symptom onlapse and first CT scan (days) | 7 (IQR 5−7.25) |
| gender | |
| male | 40 (62 %) |
| female | 24 (38 %) |
| symptoms | |
| fevera (>37.5 °C) | 47 (75 %) |
| cougha | 48 (79 %) |
| dyspneaa | 41 (68 %) |
| fatiguea | 34 (72 %) |
| taste dysfunctiona | 11 (25 %) |
| gastrointestinala | 12 (27 %) |
| comorbidities | |
| diabetesa | 8 (12 %) |
| cardiac failurea | 3 (5%) |
| coronary heart diseasea | 6 (9%) |
| COPDa | 2 (3%) |
| asthmaa | 3 (5%) |
| high blood pressurea | 23 (37 %) |
| outcome | |
| positive (group 1) | 31 (48 %) |
| negative (group 2) | 33 (52 %) |
| death | 9 (14 %) |
COPD = chronic obstructive pulmonary disease.
Occasional missing values were counted as patients without symptoms/comorbidities.
3.2. CT findings
51 CTs (80 %) were acquired without contrast agent, 13 CTs (20 %) were contrast enhanced. Ground glass opacities (97 %, n = 62) and consolidation (77 %, n = 49) were the most commonly observed patterns. Crazy paving was identified in 25 % of the scans (n = 16). Bronchial dilatation was seen in 14 % (n = 9), air bronchogram in 67 % (n = 43) and vessel enlargement in 48 % (n = 31) of CTs. Pleural effusion was observed in 20 % (n = 13) and lymphadenopathy in 28 % (n = 18) of CTs.
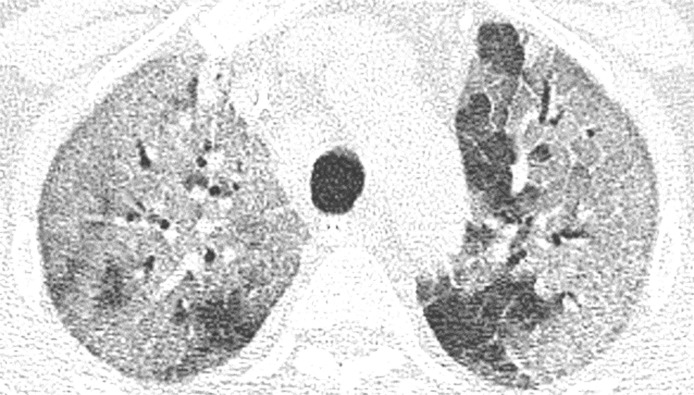
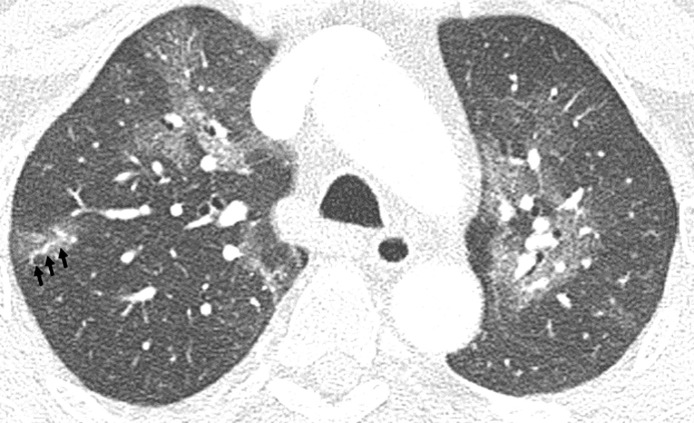
Most of the lesions were at least to some extent sharply marginated (84 %, n = 54). In 30 % of CTs (n = 19) opacifications were curvilinear shaped, round in 61 % (n = 39) and geographic in 41 % (n = 26). In the vast majority of CTs, lesions were found bilaterally (89 %, n = 57). The right lower lobe (95 %, n = 61) and the left lower lobe (92 %, n = 59) were most often affected. Distribution in the axial plane was predominantly peripheral (n = 47, 73 %) and posterior (83 %, n = 53). In 41 % (n = 26) of CTs extent of opacifications was mild (Fig. 1 ), in 36 % (n = 23) moderate (Fig. 2 ) and in 23 % (n = 15) severe (Fig. 3 ).
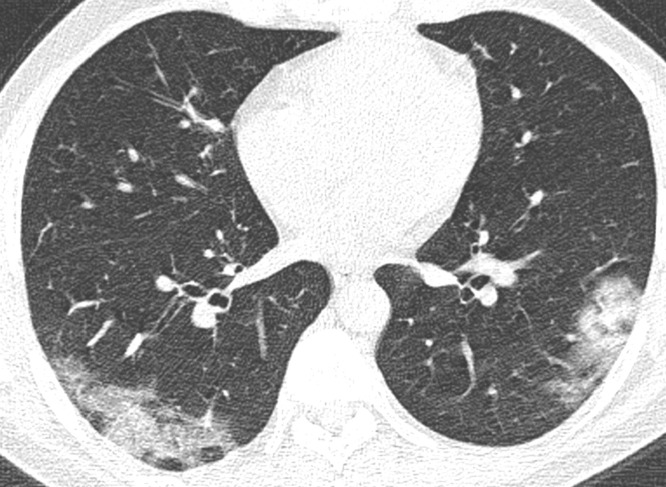
Fig. 1.
Mild extent of opacifications. Axial reconstruction of a CT in lung window showing mild affection with ground glass opacities and minor consolidation in the periphery of both lower lobes.
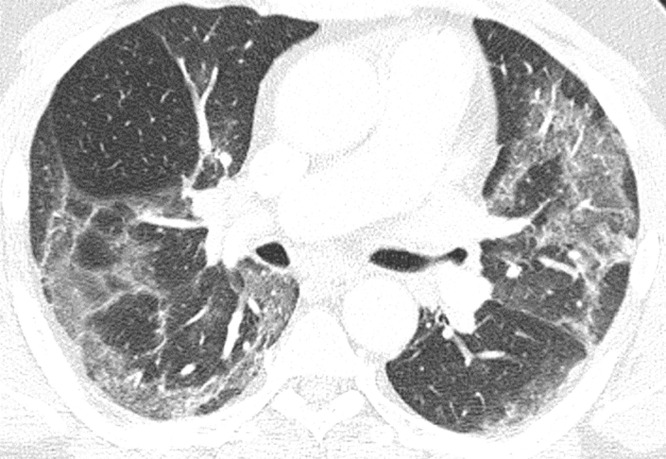
Fig. 2.
Moderate extent of opacifications. Axial reconstruction of a CT in lung window showing moderate affection with ground glass opacities in the upper and lower lobes of both lungs.
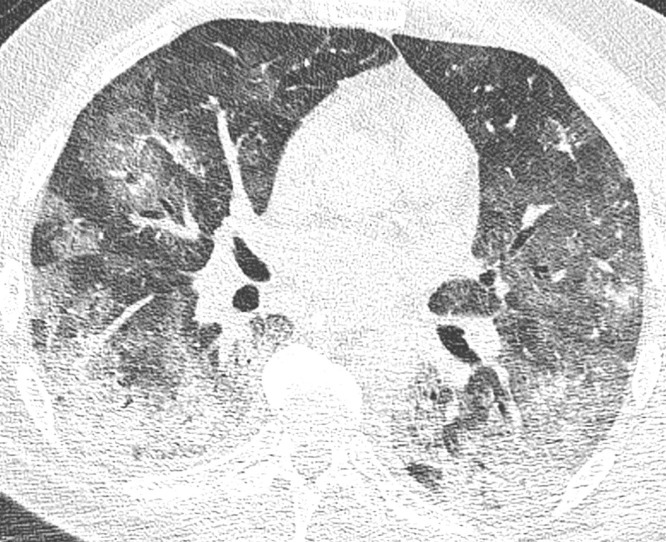
Fig. 3.
Severe extent of opacifications. Axial reconstruction of a CT in lung window showing severe affection with posterior consolidation and adjacent ground glass opacities. Also note the air bronchogram.
CT findings are presented in Table 2 .
Table 2.
Frequency of CT features.
| CT findings | patients (n = 64) |
|---|---|
| CT signs | |
| consolidation | 49 (77 %) |
| GGO | 62 (97 %) |
| crazy paving | 16 (25 %) |
| round shape of opacification | 39 (61 %) |
| sharp margin of opacification | 54 (84 %) |
| geographic shape of opacification | 26 (41 %) |
| curvilinear/bandlike opacification | 19 (30 %) |
| bronchial dilatation | 9 (14 %) |
| air bronchogram | 43 (67 %) |
| cavitation | 0 |
| peripheral vessel enlargement | 31 (48 %) |
| pleural effusion | 13 (20 %) |
| lymphadenopathy | 18 (28 %) |
| distribution | |
| bilateral | 57 (89 %) |
| unilateral | 5 (8%) |
| right upper lobe | 57 (89 %) |
| right middle lobe | 54 (84 %) |
| right lower lobe | 61 (95 %) |
| left upper lobe | 52 (81 %) |
| left lower lobe | 59 (92 %) |
| predominantly peripheral | 47 (73 %) |
| predominantly central | 0 |
| diffuse | 25 (39 %) |
| predominantly anterior | 2 (3%) |
| predominantly posterior | 53 (83 %) |
| extent of lung involvement | |
| mild | 26 (41 %) |
| moderate | 23 (36 %) |
| severe | 15 (23 %) |
| contrast agent | |
| enhanced scans | 13 (20 %) |
| unenhanced scans | 51 (80 %) |
3.3. Univariable analysis for identification of prognostic relevant CT features
As outlined in the Material and Methods section patients were divided into two groups depending on outcome: Group 1 (care on regular ward or discharge) included 31 patients (48 %). Group 2 (mechanical ventilation/ICU/ECMO/death) included 33 patients (52 %).
3.3.1. Demographic data, clinical data and comorbidities
Dyspnea (OR 3.39, 95 %-CI:1.1, 11.38, p = 0.038) was the only clinical parameter that statistically significantly influenced patient outcome. No comorbidity influenced course of disease. Results are presented in Table 3 .
Table 3.
Univariable logistic regression to analyze the predictive value of demographic data, clinical data and comorbidities on the primary endpoint (positive vs. negative outcome).
| parameter | group 1 (n = 31) | group 2 (n = 33) | odds ratio | p-value | |
|---|---|---|---|---|---|
| age | 55.4 (SD 14.3) | 58.85 (SD 14.66) | 1.02 (95 %-CI:0.98,1.05) | 0.341 | |
| time between symptom onlapse and first CT scan (days) | 7 (IQR 5−8.5) | 7 (IQR 5−7) | 0.89 (95 %-CI:0.71,1.1) | 0.296 | |
| gender | |||||
| male | 17 (42 %) | 23 (58 %) | |||
| female | 14 (58 %) | 10 (42 %) | 0.53 (95 %-CI:0.19,1.46) | 0.222 | |
| symptoms | |||||
| fever* | |||||
| no | 6 (38 %) | 10 (62 %) | |||
| yes | 25 (53 %) | 22 (47 %) | 0.53 (95 %-CI:0.16,1.66) | 0.282 | |
| cough* | |||||
| no | 6 (46 %) | 7 (54 %) | |||
| yes | 23 (48 %) | 25 (52 %) | 0.93 (95 %-CI:0.26,3.21) | 0.91 | |
| dyspnea* | |||||
| no | 13 (68 %) | 6 (32 %) | |||
| yes | 16 (39 %) | 25 (61 %) | 3.39 (95 %-CI:1.1,11.38) | 0.038 | |
| fatigue* | |||||
| no | 5 (38 %) | 8 (62 %) | |||
| yes | 17 (50 %) | 17 (50 %) | 0.63 (95 %-CI:0.16,2.27) | 0.48 | |
| gastrointestinal* | |||||
| no | 16 (48 %) | 17 (52 %) | |||
| yes | 5 (42 %) | 7 (58 %) | 1.32 (95 %-CI:0.35,5.27) | 0.686 | |
| taste dysfunction* | |||||
| no | 18 (55 %) | 15 (45 %) | |||
| yes | 4 (36 %) | 7 (64 %) | 2.1 (95 %-CI:0.53,9.34) | 0.301 | |
| comorbidities | |||||
| diabetes* | |||||
| no | 27 (48 %) | 29 (52 %) | |||
| yes | 4 (50 %) | 4 (50 %) | 0.93 (95 %-CI:0.2,4.29) | 0.925 | |
| cardiac failure* | |||||
| no | 29 (48 %) | 32 (52 %) | |||
| yes | 2 (67 %) | 1 (33 %) | 0.45 (95 %-CI:0.02,4.97) | 0.527 | |
| coronary heart disease* | |||||
| no | 28 (48 %) | 30 (52 %) | |||
| yes | 3 (50 %) | 3 (50 %) | 0.93 (95 %-CI:0.16,5.41) | 0.936 | |
| COPD* | |||||
| no | 31 (50 %) | 31 (50 %) | |||
| yes | 0 (0%) | 2 (100 %) | n.c. | 0.493# | |
| asthma* | |||||
| no | 30 (49 %) | 31 (51 %) | |||
| yes | 1 (33 %) | 2 (67 %) | 1.94 (95 %-CI:0.18,42.94) | 0.598 | |
| high blood pressure* | |||||
| no | 21 (52 %) | 19 (48 %) | |||
| yes | 9 (39 %) | 14 (61 %) | 1.72 (95 %-CI:0.61,5) | 0.308 | |
COPD = chronic obstructive pulmonary disease.
Occasional missing values were counted as patients without symptoms, #p-value of Fishers exact test due to quasi separated data.
3.3.2. CT findings
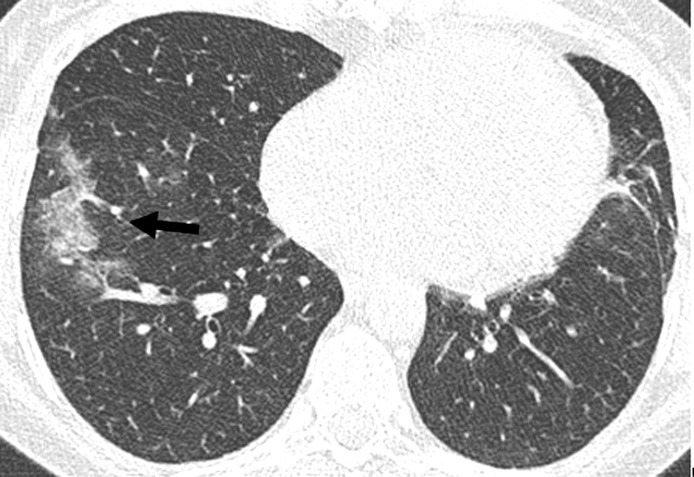
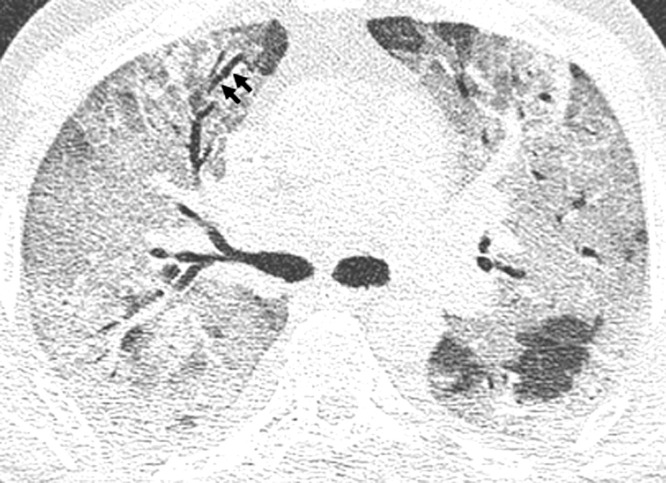
Restriction of opacifications to one side (unilateral) (OR n.c., p = 0.022) was the only parameter correlated with a favorable outcome (Fig. 4 ). Several CT features predicted an adverse outcome: consolidation (OR 3.99, 95 %-CI:1.18,16.06, p = 0.034), crazy paving (OR 10.68, 95 %-CI:2.6,73.12, p = 0.004) (Fig. 5 ), geographic shape of opacification (OR 3.45, 95 %-CI:1.23, 10.35, p = 0.022), bronchial dilatation (OR n.c., p = 0.002) (Fig. 6 ), air bronchogram (Figs. 3, 5 and 6) (OR 4.22, 95 %-CI:1.41, 13.94, p = 0.013), pleural effusion (OR 4.06, 95 %-CI:1.09,19.71, p = 0.05) and vessel enlargement (OR 3.67, 95 %-CI:1.34, 10.71, p = 0.014) (Fig. 7 ). Also, bilateral lesions (OR n.c., p = 0.004), involvement of one of the upper lobes (right upper lobe: OR n.c., p = 0.004, left upper lobe OR 7.38, 95 %-CI:1.73, 51.29, p = 0.015) or of the right middle lobe (OR n.c., p < 0.001) were significantly more often seen in group 2. Moreover, extent of parenchymal opacifications of more than 66 % of lung volume predicted an unfavourable course of disease (OR 14.62, 95 %-CI:3.13, 108.96, p = 0.002) (Fig. 3). Results are presented in Table 4 .
Fig. 4.
Unilateral disease. Axial reconstruction of a CT in lung window demonstrating unilateral ground glass opacifications in the right lower lobe (arrow). Note also incidental scaring in the lingual segment.
Fig. 5.
Crazy paving. Axial reconstruction of a CT in lung window demonstrating extensive crazy paving in a patient with severe disease extent. Also note the air bronchogram.
Fig. 6.
Bronchial dilatation. Axial reconstruction of a CT in lung window showing a dilated bronchus within ground glass opacities (arrows). Also note the air bronchogram.
Fig. 7.
Vessel enlargement. Axial reconstruction of a CT in lung window demonstrating a dilated vessel within a focal ground glass opacity (arrows).
Table 4.
Univariable logistic regression to analyze the predictive value of CT features on the primary endpoint (positive vs. negative outcome).
| CT findings | group 1 (n = 31) | group 2 (n = 33) | odds ratio | p-value |
|---|---|---|---|---|
| GGO | ||||
| no | 2 (100 %) | 0 (0%) | ||
| yes | 29 (47 %) | 33 (53 %) | n.c. | 0.231# |
| consolidation | ||||
| no | 11 (73 %) | 4 (27 %) | ||
| yes | 20 (41 %) | 29 (59 %) | 3.99 (95 %-CI:1.18,16.06) | 0.034 |
| crazy paving | ||||
| no | 29 (60 %) | 19 (40 %) | ||
| yes | 2 (12 %) | 14 (88 %) | 10.68 (95 %-CI:2.6,73.12) | 0.004 |
| round shape of opacification | ||||
| no | 9 (36 %) | 16 (64 %) | ||
| yes | 22 (56 %) | 17 (44 %) | 0.43 (95 %-CI:0.15,1.2) | 0.114 |
| sharp margin of opacification | ||||
| no | 6 (60 %) | 4 (40 %) | ||
| yes | 25 (46 %) | 29 (54 %) | 1.74 (95 %-CI:0.45,7.47) | 0.429 |
| geographic shape of opacification | ||||
| no | 23 (61 %) | 15 (39 %) | ||
| yes | 8 (31 %) | 18 (69 %) | 3.45 (95 %-CI:1.23,10.35) | 0.022 |
| curvilinear/bandlike opacification | ||||
| no | 22 (49 %) | 23 (51 %) | ||
| yes | 9 (47 %) | 10 (53 %) | 1.06 (95 %-CI:0.36,3.16) | 0.911 |
| bronchial dilatation | ||||
| no | 31 (56 %) | 24 (44 %) | ||
| yes | 0 (0%) | 9 (100 %) | n.c. | 0.002# |
| air bronchogram | ||||
| no | 15 (71 %) | 6 (29 %) | ||
| yes | 16 (37 %) | 27 (63 %) | 4.22 (95 %-CI:1.41,13.94) | 0.013 |
| cavitation | ||||
| no | 31 (48 %) | 33 (52 %) | n.c. | n.c. |
| vessel enlargment | ||||
| no | 21 (64 %) | 12 (36 %) | ||
| yes | 10 (32 %) | 21 (68 %) | 3.67 (95 %-CI:1.34,10.71) | 0.014 |
| pleural effusion | ||||
| no | 28 (55 %) | 23 (45 %) | ||
| yes | 3 (23 %) | 10 (77 %) | 4.06 (95 %-CI:1.09,19.71) | 0.050 |
| lymphadenopathy | ||||
| no | 24 (52 %) | 22 (48 %) | ||
| yes | 7 (39 %) | 11 (61 %) | 1.71 (95 %-CI:0.57,5.41) | 0.341 |
| unilateral | ||||
| no | 26 (44 %) | 33 (56 %) | ||
| yes | 5 (100 %) | 0 (0%) | n.c. | 0.022# |
| bilateral | ||||
| no | 7 (100 %) | 0 (0%) | ||
| yes | 24 (42 %) | 33 (58 %) | n.c. | 0.004# |
| right upper lobe | ||||
| no | 7 (100 %) | 0 (0%) | ||
| yes | 24 (42 %) | 33 (58 %) | n.c. | 0.004# |
| right middle lobe | ||||
| no | 10 (100 %) | 0 (0%) | ||
| yes | 21 (39 %) | 33 (61 %) | n.c. | <0.001# |
| right lower lobe | ||||
| no | 3 (100 %) | 0 (0%) | ||
| yes | 28 (46 %) | 33 (54 %) | n.c. | 0.108# |
| left upper lobe | ||||
| no | 10 (83 %) | 2 (17 %) | ||
| yes | 21 (40 %) | 31 (60 %) | 7.38 (95 %-CI:1.73,51.29) | 0.015 |
| left lower lobe | ||||
| no | 4 (80 %) | 1 (20 %) | ||
| yes | 27 (46 %) | 32 (54 %) | 4.74 (95 %-CI:0.65,95.75) | 0.175 |
| peripheral | ||||
| no | 6 (35 %) | 11 (65 %) | ||
| yes | 25 (53 %) | 22 (47 %) | 0.48 (95 %-CI:0.14,1.48) | 0.210 |
| central | ||||
| no | 31 (48 %) | 33 (52 %) | n.c. | n.c. |
| diffuse | ||||
| no | 21 (54 %) | 18 (46 %) | ||
| yes | 10 (40 %) | 15 (60 %) | 1.75 (95 %-CI:0.64,4.95) | 0.281 |
| predominantly anterior | ||||
| no | 30 (48 %) | 32 (52 %) | ||
| yes | 1 (50 %) | 1 (50 %) | 0.94 (95 %-CI:0.04,24.42) | 0.964 |
| predominantly posterior | ||||
| no | 5 (45 %) | 6 (55 %) | ||
| yes | 26 (49 %) | 27 (51 %) | 0.87 (95 %-CI:0.22,3.21) | 0.828 |
| extent of lung involvement | ||||
| mild | 18 (69 %) | 8 (31 %) | reference | |
| moderate | 11 (48 %) | 12 (52 %) | 2.45 (95 %-CI:0.78,8.17) | 0.132 |
| severe | 2 (13 %) | 13 (87 %) | 14.62 (95 %-CI:3.13,108.96) | 0.002 |
| contrast agent | ||||
| enhanced scans | 7 (54 %) | 6 (46 %) | ||
| unenhanced scans | 24 (47 %) | 27 (53 %) | ||
n.c.: not computable due to quasi separated data.
p-value of Fishers exact test due to quasi separated data.
3.4. Multivariable analysis for identification of prognostic relevant CT features
Multivariable logistic regression including significant CT findings and distribution patterns from univariable analysis was performed to identify independent parameters for prediction of outcome. In order to avoid overfitting of the model and to keep the number of events per variable (EPV) large enough, the number of parameters included into the multivariable analysis was restricted as described in Material and Methods. Thus, two multivariable analyses were performed by grouping the parameters according to clinical considerations. Independent predictors for a negative outcome were crazy paving (OR 8.9, 95 %-CI: 1.64, 48.1, p = 0.011) and involvement of more than 66 % of lung parenchyma (OR 6.04, 95 %-CI: 1.12, 32.6, p = 0.034). Results are presented in Table 5a, Table 5b .
Table 5a.
Multivariable logistic regression to analyze the predictive value of significant CT features on the primary endpoint (positive vs. negative outcome).
| parameter | odds ratio* | p-value* |
|---|---|---|
| crazy paving | 8.9 (95 %-CI: 1.64, 48.1) | 0.011 |
| bronchial dilatation | 23.5 (95 %-CI: 0.81, 684) | 0.066 |
| consolidation | 4.52 (95 %-CI: 0.98, 20.9) | 0.054 |
Odds ratios and p-values were estimated by the penalized likelihood method by Firth.
Table 5b.
Multivariable logistic regression to analyze the predictive value of significant characteristics of parenchymal involvement on the endpoint (positive vs. negative outcome).
| parameter | odds ratio* | p-value* |
|---|---|---|
| involvement right middle lobe | 19.1 (95 %-CI: 0.89, 410) | 0.060 |
| extent of disease | ||
| mild | reference | |
| moderate | 1.33 (95 %-CI: 0.38, 4.68) | 0.311 |
| severe | 6.04 (95 %-CI: 1.12, 32.6) | 0.034 |
Odds ratios and p-values were estimated by the penalized likelihood method by Firth.
4. Discussion
Prognosis of COVID-19 pneumonia is highly variable. While circa 80 % of patients show only mild or even no symptoms, 20 % suffer from severe or even critical disease and eventually die [4]. Identification of factors predicting prognosis already in the early phase of disease would enable physicians to direct patients into optimal therapeutic pathways. Health care resources could be employed precisely where they are needed most. CT plays a major role in the care of patients infected with SARS-CoV-2. Few studies investigated if CT might be helpful in predicting prognosis [[20], [21], [22], [23], [24]]. However, most of the studies are from China. Evaluation of European cohorts in this regard is scarce [25]. Considering that outcome is influenced by many factors (eg health care resources, organization and preparedness of health care systems, host factors and viral factors influenced for example by genomic variations) we sought to investigate CT features predicting patient prognosis in a German cohort.
For this we evaluated a cohort of 64 patients with RT-PCR proven COVID-19 pneumonia treated at two tertiary care centers in Regensburg, Southern Germany. We restricted the analysis to CTs performed in the early disease phase (within 10 days of symptom onset). CT morphology of the presented cohort was in accordance with previously described features. A combination of bilateral GGO and consolidation located in the periphery of the posterior segments predominantly of the lower lobes was most often seen and seems to be characteristic for COVID-19 pneumonia. For identification of prognostic parameters patients were divided in two groups (group 1 with favorable outcome defined as care on regular ward or discharge, group 2 with unfavorable outcome defined as need for mechanical ventilation, admission to ICU, ECMO therapy or death). None of the analyzed demographic parameters and no comorbidity influenced patient outcome. In particular neither age nor male gender (which has been reported to be a risk factor by other groups) were of prognostic relevance in our cohort [22,[28], [29], [30]]. Regarding clinical symptoms, patients presenting with dyspnea at admission had a significantly worse outcome compared to patients without dyspnea. Also, univariable analysis identified several CT parameters which were associated with an adverse outcome, namely consolidation, crazy paving, geographic shape of opacities, dilatation of bronchi, air bronchogram, vessel enlargement and the presence of pleural effusions. Moreover, involvement of both lungs as well as the upper lobes and the right middle lobe were predictive for an unfavorable course of disease. Also, amount of opacifications more than 66 % of lung volume correlated highly significant with an adverse outcome. Conversely, unilateral disease predicted a benign course of disease. These findings are in accordance with the results of other studies. Several groups from China also reported crazy paving, bronchial dilatation, air bronchogram and extent of opacifications to be associated with an adverse outcome [22,21,24,23,31].
At first glance the presented results and data from literature suggest that outcome of patients suffering from COVID-19 pneumonia might be predicted by several different CT features. But, none of the other authors performed a multivariable analysis. We suspected that the significance of some features can be linked to the extent of parenchymal involvement: COVID-19 pneumonia initially manifests in form of predominant ground glass opacities and possibly minor consolidation in the periphery of the lower lobes. When opacifications increase in extent they gradually involve more segments. Thus, seemingly prognostic parameters like affection of the upper lobes and the right middle lobe might merely indicate increasing disease extent. Likewise it is not surprising that pleural effusion was significantly more prevalent in group 2. Also, there might be a link between crazy paving and geographic shape of opacifications both of which are well known CT features of acute respiratory distress syndrome (ARDS) which is seen in severe cases of COVID-19 pneumonia. To test for our hypothesis we performed a multivariable regression analysis. The results revealed that in our cohort crazy paving was the only CT feature that independently predicted an unfavorable course of disease. The differential diagnosis of crazy paving is wide and includes pulmonary edema and ARDS [32,33]. Both phenomena are thought to be the major pathophysiological events in severe COVID-19 pneumonia [34]. Thus, crazy paving might be an easy to assess surrogate parameter for severe lung injury by COVID-19 pneumonia. The presence of consolidation and bronchial dilatation did not reach statistical significance but showed a tendency for predicting a poor outcome. Bronchial dilatation or bronchiectasis can also be linked to ARDS being a consequence of fibroproliferative processes in the surrounding lung tissue during the proliferative, second phase [35,36]. Hence, bronchial dilatation might have been a surrogate for such a tissue response with an immanent adverse outcome. Another strong independent predictor for a poor outcome was severity of parenchymal involvement even though extent of parenchymal involvement was assessed subjectively.
Our study has limitations. CT scans were performed at the discretion of the referring physician. Hence, there might be a bias towards seriously ill patients. Both recruiting hospitals were tertiary care institutions with one of them being the regional center for critically ill patients. Thus, the proportion of patients with moderately to severely involved lung parenchyma was relatively high. Differences in treatment were not systematically analyzed.
5. Conclusion
We investigated the prognostic relevance of chest CT performed in the early phase of disease for 64 patients from Germany suffering from RT-PCR proven COVID-19 pneumonia. Independent predictors for an adverse outcome were crazy paving and severe parenchymal involvement. These are easy to asses CT parameters. Hence, CT might be a valuable modality to predict the course of disease and to help clinicians to adapt patient management accordingly.
CRediT authorship contribution statement
Stefanie Meiler: Investigation, Data curation, Writing - original draft, Visualization. Jan Schaible: Investigation, Data curation. Florian Poschenrieder: Conceptualization, Methodology, Project administration. Gregor Scharf: Software. Florian Zeman: Software, Formal analysis, Visualization. Janine Rennert: Methodology, Investigation. Benedikt Pregler: Investigation. Henning Kleine: Investigation. Christian Stroszczynski: Resources, Supervision. Niels Zorger: Resources, Supervision. Okka W. Hamer: Conceptualization, Methodology, Validation, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. WHO Announces COVID-19 Outbreak a Pandemic.http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (Accessed 2 May 2020) [Google Scholar]
- 3.Johns Hopkins University & Medicine . 2020. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)https://coronavirus.jhu.edu/map.html (Accessed 29 June 2020) [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Caruso D., Zerunian M., Polici M., Pucciarelli F., Polidori T., Rucci C., et al. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020 doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan Y.-N., Qin J. Pre- and posttreatment chest CT findings: 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:21. doi: 10.1148/radiol.2020200323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han R., Huang L., Jiang H., Dong J., Peng H., Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 9.Kong W., Agarwal P.P. Chest imaging appearance of COVID-19 infection. Radiol.: Cardiothorac. Imaging. 2020;2:e200028. doi: 10.1148/ryct.2020200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamer O.W., Salzberger B., Gebauer J., Stroszczynski C., Pfeifer M. CT-Morphologie von COVID-19: Fallbeispiel und Literaturübersicht. Fortschr. Röntgenstr. 2020;192:386–392. doi: 10.1055/a-1142-4094. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Wei R., Rao G., Zhu J., Song B. Characteristic CT findings distinguishing 2019 novel coronavirus disease (COVID-19) from influenza pneumonia. Eur. Radiol. 2020;30:4910–4917. doi: 10.1007/s00330-020-06880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M., Zeng W., Wen Y., Zheng Y., Lv F., Xiao K. COVID-19 pneumonia: CT findings of 122 patients and differentiation from influenza pneumonia. Eur. Radiol. 2020;30:1–7. doi: 10.1007/s00330-020-06928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo L., Luo Z., Jia Y., Zhou C., He J., Lyu J., Shen X. CT differential diagnosis of COVID-19 and non-COVID-19 in symptomatic suspects: a practical scoring method. BMC Pulm. Med. 2020;20:129. doi: 10.1186/s12890-020-1170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L., et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabatabaei S.M.H., Talari H., Moghaddas F., Rajebi H. Computed tomographic features and short-term prognosis of coronavirus disease 2019 (COVID-19) pneumonia: a single-center study from Kashan, Iran. Radiol.: Cardiothorac. Imaging. 2020;2:e200130. doi: 10.1148/ryct.2020200130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K., Wu J., Wu F., Guo D., Chen L., Fang Z., Li C. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol. 2020;55:327–331. doi: 10.1097/RLIK.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 23.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyu P., Liu X., Zhang R., Shi L., Gao J. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: identifying critical cases based on CT characteristics. Invest. Radiol. 2020;55:412–421. doi: 10.1097/RLI.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombi D., Bodini F.C., Petrini M., Maffi G., Morelli N., Milanese G., et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 27.FIRTH D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27. doi: 10.2307/2336755. [DOI] [Google Scholar]
- 28.Yu M., Xu D., Lan L., Tu M., Liao R., Cai S., et al. Thin-section chest CT imaging of coronavirus disease 2019 pneumonia: comparison between patients with mild and severe disease. Radiol.: Cardiothorac. Imaging. 2020;2:e200126. doi: 10.1148/ryct.2020200126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng Y., Wu P., Lu W., Liu K., Ma K., Huang L., et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabatabaei S.M.H., Talari H., Moghaddas F., Rajebi H. Computed tomographic features and short-term prognosis of coronavirus disease 2019 (COVID-19) pneumonia: a single-center study from Kashan, Iran. Radiol.: Cardiothorac. Imaging. 2020;2:e200130. doi: 10.1148/ryct.2020200130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wever W., Meersschaert J., Coolen J., Verbeken E., Verschakelen J.A. The crazy-paving pattern: a radiological-pathological correlation. Insights Imaging. 2011;2:117–132. doi: 10.1007/s13244-010-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi S.E., Erasmus J.J., Volpacchio M., Franquet T., Castiglioni T., McAdams H.P. "Crazy-paving" pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23:1509–1519. doi: 10.1148/rg.236035101. [DOI] [PubMed] [Google Scholar]
- 34.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnham E.L., Janssen W.J., Riches D.W.H., Moss M., Downey G.P. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur. Respir. J. 2014;43:276–285. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]