Abstract
Background
Little is known about the arterial complications and hypercoagulability associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We sought to characterize our experience with arterial thromboembolic complications in patients with hospitalized for coronavirus disease 2019 (COVID-19).
Methods
All patients admitted from March 1 to April 20, 2020, and who underwent carotid, upper, lower and aortoiliac arterial duplex, computed tomography angiogram or magnetic resonance angiography for suspected arterial thrombosis were included. A retrospective case control study design was used to identify, characterize and evaluate potential risk factors for arterial thromboembolic disease in SARS-CoV-2 positive patients. Demographics, characteristics, and laboratory values were abstracted and analyzed.
Results
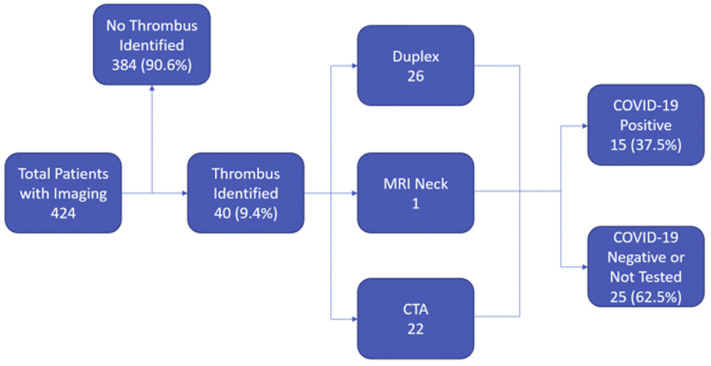
During the study period, 424 patients underwent 499 arterial duplex, computed tomography angiogram, or magnetic resonance angiography imaging studies with an overall 9.4% positive rate for arterial thromboembolism. Of the 40 patients with arterial thromboembolism, 25 (62.5%) were SARS-CoV-2 negative or admitted for unrelated reasons and 15 (37.5%) were SARS-CoV-2 positive. The odds ratio for arterial thrombosis in COVID-19 was 3.37 (95% confidence interval, 1.68-6.78; P = .001). Although not statistically significant, in patients with arterial thromboembolism, patients who were SARS-CoV-2 positive compared with those testing negative or not tested tended to be male (66.7% vs 40.0%; P = .191), have a less frequent history of former or active smoking (42.9% vs 68.0%; P = .233) and have a higher white blood cell count (14.5 vs 9.9; P = .208). Although the SARS-CoV-2 positive patients trended toward a higher the neutrophil-to-lymphocyte ratio (8.9 vs 4.1; P = .134), creatinine phosphokinase level (359.0 vs 144.5; P = .667), C-reactive protein level (24.2 vs 13.8; P = .627), lactate dehydrogenase level (576.5 vs 338.0; P = .313), and ferritin level (974.0 vs 412.0; P = .47), these differences did not reach statistical significance. Patients with arterial thromboembolic complications and SARS-CoV-2 positive when compared with SARS-CoV-2 negative or admitted for unrelated reasons were younger (64 vs 70 years; P = .027), had a significantly higher body mass index (32.6 vs 25.5; P = .012), a higher d-dimer at the time of imaging (17.3 vs 1.8; P = .038), a higher average in hospital d-dimer (8.5 vs 2.0; P = .038), a greater distribution of patients with clot in the aortoiliac location (5 vs 1; P = .040), less prior use of any antiplatelet medication (21.4% vs 62.5%; P = .035), and a higher mortality rate (40.0% vs 8.0%; P = .041). Treatment of arterial thromboembolic disease in COVID-19 positive patients included open thromboembolectomy in six patients (40%), anticoagulation alone in four (26.7%), and five (33.3%) did not require or their overall illness severity precluded additional treatment.
Conclusions
Patients with SARS-CoV-2 are at risk for acute arterial thromboembolic complications despite a lack of conventional risk factors. A hyperinflammatory state may be responsible for this phenomenon with a preponderance for aortoiliac involvement. These findings provide an early characterization of arterial thromboembolic disease in SARS-CoV-2 patients.
Keywords: Thromboembolism, SARS-CoV-2, Hyperinflammatory
Article Highlights.
-
•
Type of Research: Single-center, retrospective cohort study
-
•
Key Findings: Out of the study cohort of patients with acute thromboembolism 22 were negative for severe acute respiratory syndrome associated with coronavirus disease-19 (SARS-CoV-2) and 15 were positive for SARS-CoV-2. The SARS-CoV-2 positive patients had a significantly higher d-dimer at the time of imaging (17.3 vs 1.8; P = .038), a higher average in hospital d-dimer (8.5 vs 2.0; P = .038), and a greater distribution of patients with clot in the aortoiliac location (5 vs 1; P = .040).
-
•
Take Home Message: Patients with SARS-CoV-2 are at risk for acute arterial thromboembolic complications despite a lack of conventional risk factors, a hyperinflammatory state may be responsible for this phenomenon with a preponderance for aortoiliac involvement.
In late 2019, the first reports of human infection with a novel coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged. This pathogen was identified as the causative organism of coronavirus disease 2019 (COVID-19), a highly transmissible, significantly morbid, and potentially fatal disease.1, 2, 3 In addition to the pulmonary impact of this infection, cardiac, renal, and hematologic manifestations have been identified. A recent study has estimated the incidence of a thrombotic complication (arterial or venous) at 31% in intensive care unit patients with COVID-19.4 Evidence is still accumulating with respect to the characterization of the suspected hypercoagulability observed in SARS-CoV-2 infection; however, it may be associated with venous and arterial thromboembolic disease on the basis of excessive inflammation, hypoxia, immobilization, and a diffuse intravascular coagulation (DIC)-like picture.1, 2, 3, 4
The exact relationship between the occurrence of arterial thrombotic events and SARS-CoV-2 remains unclear. We therefore set out to identify and characterize patients in the early stage of the epidemic with arterial thromboembolic disease and its relationship to SARS-CoV-2 infection at our institution.
Methods
Patients 18 years of age or older admitted to Montefiore Medical Center/Albert Einstein College of Medicine from March 1, 2020, through April 20, 2020, and who were evaluated for suspected acute arterial ischemia were included. Records of all arterial imaging studies performed during the study period were reviewed. These studies included computed tomography angiography, magnetic resonance angiography (MRA), and/or arterial duplex. The choice of imaging modality (computed tomography angiography, MRA, or arterial duplex) was obtained at the provider's discretion and was based on the presenting clinical complaints. For patients requiring duplex testing, the majority of these studies were conducted as portable bedside tests. Patients presenting with neurologic events underwent MRA in addition to carotid duplex evaluation as a part of the stroke protocol.
SARS-CoV-2 status was obtained from the medical record. Patients were tested for SARS-CoV-2 based on clinical suspicion and in those requiring surgical treatment as part of their preprocedural evaluation. Nasopharyngeal swab specimens were tested for SARS-CoV-2 by in-house polymerase chain reaction testing. Confirmatory repeat testing was obtained in patients with a high clinical suspicion for COVID-19 and negative initial testing. Patients were excluded if SARS-CoV-2 test results were pending at the time of data abstraction. Patients were divided into one of three groups: SARS-CoV-2 positive, SARS-CoV-2 negative, and patients not tested. The SARS-CoV-2 positive group included patients with a range of COVID-19 symptomatology (mild to severe) as well as those tested as part of routine preoperative preparation. The SARS-CoV-2 negative group included patients who presented with respiratory symptoms or other influenza-like illness symptoms and who were found to have a negative polymerase chain reaction result. Patients admitted for other reasons and without suspicion for COVID-19 were not tested and categorized as such. Of the 25 patients who are SARS-CoV-2 negative or not tested 5 were negative, 19 were not tested (admitted for unrelated reasons), and 1 patient was under investigation. COVID-19 severity was classified as previously described.5
Patients with imaging that confirmed acute thrombosis were further evaluated. SARS-CoV-2 positive patients were compared with the combined SARS-CoV-2 negative and not tested groups. Retrospective chart review abstracted demographic variables including age, race, body mass index (BMI), gender, ethnicity, medical comorbidities, and treatment for further evaluation. The presence of metabolic syndrome was defined as patients with three or more of the following: BMI greater than 30, serum triglyceride level 150 mg/dL or greater within the last 6 months, serum high-density lipoprotein cholesterol level less than 40 mg/dL in men or 50 mg/dL in women within the last 6 months, systolic blood pressure greater than 130 mm Hg or diastolic blood pressure greater than 85 mm Hg, and fasting serum glucose greater than 100 mg/dL. Additional variables assessed included laboratory values particularly on the day of the imaging study such as hemoglobin, blood cell counts, coagulation parameters, cardiac biomarkers, and creatinine; radiologic studies; and operative variables if patients underwent surgery related to the acute thrombosis. d-Dimers were recorded throughout the hospitalization and an average d-dimer for the admission calculated. Values for creatinine phosphokinase (CPK), C-reactive protein (CRP), ferritin, fibrinogen, d-dimer and lactate dehydrogenase were obtained from the closest time point in the admission when not available on the exact day of imaging. The 30-day outcomes were collected at 4 weeks after completion of data collection analysis.
Univariate analysis was conducted with t-tests for continuous and χ 2 tests for categorical variables with nonparametric testing as appropriate. Multivariable analysis was not performed owing to small sample sizes. Values for d-dimer and activated partial thromboplastin time beyond the limit of detection for the assay were imputed at the threshold value. All analysis was conducted in RStudio (version 1.2.1335; The R Foundation, Vienna, Austria) and Microsoft Excel (version 16.0; Microsoft, Redmond, Wash). Missing data were assumed to be missing at random and excluded from the analysis for that variable. The odds ratio for acute arterial thrombosis in COVID-19 was evaluated with a two-tailed Fisher exact test. This study was approved by the Institutional Review Board of Montefiore Medical Center/Albert Einstein College of Medicine with a waiver of informed consent for this observational review (#2020-11452).
Results
Of patients with imaging evidence of arterial thrombosis, 15 (37.5%) were SARS-CoV-2 positive and 25 (62.5%) were SARS-CoV-2 negative or not tested (Fig 1 ). Of the patients without imaging evidence of arterial thrombosis, 359 were SARS-CoV-2 negative or not tested and 58 were SARS-CoV-2 positive. The odds ratio for arterial thrombosis in COVID-19 was 3.37 (95% confidence interval, 1.68-6.78; P = .001).
Fig 1.
Flowchart demonstrating patient selection based on imaging performed. Duplex includes cervical carotid, upper extremity and lower extremity arterial testing. CTA, computed tomography angiogram (neck, aorta or lower extremity); COVID-19, coronavirus disease 2019; MRI, magnetic resonance imaging (neck).
SARS-CoV-2 positive patients with evidence of arterial thrombosis tended to be younger (64 years of age vs 70 years of age; P = .027) and more frequently male (66.7% vs 40.0%; P = .191). SARS-CoV-2 positive patients were also less frequently smokers (42.9% vs 68%; P = .233). The two groups were relatively equal with respect to race, ethnicity, comorbidities, and the presence of metabolic syndrome. Patients who were SARS-CoV-2 positive had a much lower rate of any antiplatelet use (21.4% vs 62.5%; P = .035). None of the SARS-CoV-2 positive patients had a history of anticoagulant use although three patients in the negative/not tested group were on apixaban chronically for lower extremity bypass maintenance and one was one heparin therapeutic intravenous infusion for a left ventricular assist device (P = .287). SARS-CoV-2 positive patients had a slightly higher history of statin use (64.3% vs 48%; P = .520). Patient presentations and symptoms of arterial thrombosis for both groups were overall similar (Table I ).
Table I.
Demographic factors and clinical characteristics for patients with (coronavirus disease-19 COVID-19)
| Characteristics | No COVID-19 or not tested | COVID-19 positive | P value |
|---|---|---|---|
| No. | 25 | 15 | |
| Age | 70.0 (61.0-80.0) | 64.0 (54.5-65.5) | .027 |
| Male sex | 10 (40.0) | 10 (66.7) | .191 |
| Race | .726 | ||
| Black | 7 (28.0) | 3 (20.0) | |
| White | 3 (12.0) | 3 (20.0) | |
| Other/unknown | 15 (60.0) | 9 (60.0) | |
| Ethnicity | .506 | ||
| Hispanic | 13 (52.0) | 6 (40.0) | |
| Not Hispanic | 10 (40.0) | 6 (40.0) | |
| Unknown | 2 (8.0) | 3 (20.0) | |
| BMI | 25.5 (22.7-30.0) | 32.6 (28.0-32.8) | .012 |
| Metabolic syndrome | 7 (58.3) | 5 (71.4) | .938 |
| History of HTN | 23 (92.0) | 12 (80.0) | .537 |
| History of DM | 14 (56.0) | 8 (53.3) | 1.000 |
| History of HLD | 12 (48.0) | 6 (40.0) | .870 |
| History of smoking | 17 (68.0) | 6 (42.9) | .233 |
| Never | 8 (32.0) | 8 (57.1) | .120 |
| Former | 12 (48.0) | 6 (42.9) | |
| Current | 5 (20.0) | 0 (0.0) | |
| History of CAD | 9 (36.0) | 2 (13.3) | .235 |
| History of PVD | 9 (36.0) | 4 (26.7) | .794 |
| History of COPD | 5 (20.0) | 1 (6.7) | .493 |
| History of CHF | 4 (16.0) | 0 (0.0) | .276 |
| History of CKD | 5 (20.0) | 1 (6.7) | .493 |
| History of DVT/PE | 3 (12.0) | 1 (6.7) | 1.000 |
| History of atrial fibrillation | 1 (4.0) | 0 (0.0) | 1.000 |
ALI, Acute limb ischemia; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DVT, deep venous thrombosis; HLD, hyperlipidemia; HTN, hypertension; PE, pulmonary embolism; PVV, peripheral vascular disease.
Values are median (interquartile range) or number (%). Boldface entries indicate statistical significance.
Patients positive for SARS-CoV-2 had significantly higher d-dimer levels at the time of imaging (217.3 vs 1.8; P = .038) and average in-hospital d-dimer levels (8.5 vs 2.0; P = .043) than SARS-CoV-2 negative or not tested patients (Table II ). SARS-CoV-2 positive patients exhibited a higher white blood cell count (WBC) despite lack of statistical significance (14.5 vs 9.9; P = .208). There were no significant differences in prothrombin time, activated partial thromboplastin time, platelet count, serum creatine, troponin, or fibrinogen. Although the SARS-CoV-2 positive patients trended toward a higher the neutrophil-to-lymphocyte ratio (8.9 vs 4.1; P = .134), CPK level (359.0 vs 144.5; P = .667), CRP level (24.2 vs 13.8; P = .627), lactate dehydrogenase level (576.5 vs 338.0; P = .313), and ferritin level (974.0 vs 412.0; P = .47), these differences did not reach statistical significance.
Table II.
Laboratory values and treatment characteristics for patients with coronavirus disease 2019 (COVID-19)
| No COVID-19 or not tested | COVID-19 positive | P value | |
|---|---|---|---|
| No. | 25 | 15 | |
| WBC, k/μL | 9.9 [8.0-13.1] | 14.5 [8.7-16.7] | .208 |
| Neutrophil count, k/μL | 7.6 [5.5-12.3] | 12.3 [5.5-15.1] | .253 |
| Lymphocyte count, k/μL | 1.5 [1.2-2.1] | 1.1 [0.8-1.7] | .079 |
| Monocyte count, k/μL | 0.8 [0.6-1.0] | 0.7 [0.5-0.8] | .316 |
| Eosinophil count, k/μL | 0.2 [0.1-0.2] | 0.1 [0.0-0.1] | .032 |
| Neutrophil to lymphocyte ratio | 4.6 [2.2-7.3] | 10.1 [4.4-23.6] | .084 |
| Hemoglobin, g/dL | 11.6 [9.0-12.5] | 12.3 [10.8-14.1] | .203 |
| Platelet Count, k/μL | 261.0 [192.0-331.0] | 295.5 [170.5-318.0] | .884 |
| Prothrombin time, seconds | 15.2 [14.3-17.1] | 15.9 [14.7-20.8] | .368 |
| aPTT, seconds | 33.1 [29.5-45.7] | 39.1 [32.0-47.4] | .400 |
| Total bilirubin, mg/dL | 0.4 [0.3-0.8] | 0.6 [0.3-1.3] | .341 |
| SCr at admission, mg/dL | 1.05 [0.86-1.62] | 1.18 [0.77-1.35] | .665 |
| SCr at time of duplex, mg/dL | 1.01 [0.83-1.35] | 1.30 [0.90-2.04] | .247 |
| Troponin, ng/mL | 0.01 [0.01-0.04] | 0.01 [0.01-0.09] | .718 |
| d-Dimer at time of diagnosis, μg/mL | 1.8 [1.1-2.7] | 17.3 [5.5-20.0] | .038 |
| Average in-hospital d-dimer, μg/mL | 2.0 [1.5-2.7] | 8.5 [3.5-17.1] | .043 |
| CRP | 13.8 [9.5-21.6] | 24.2 [9.7-28.1] | .627 |
| CPK | 144.5 [75.5-524.0] | 359.0 [195.0-523.0] | .667 |
| Ferritin | 412.0 [296.5-704.0] | 974.0 [293.5-1311.0] | .470 |
| Fibrinogen | 653.0 [511.0-691.3] | 488.0 [386.0-653.0] | .628 |
| LDH | 338.0 [272.0-512.5] | 576.5 [454.8-744.3] | .313 |
aPTT, Activated partial thromboplastin time; CPK, creatine phosphokinase; CRP, C-reactive protein; LDH, lactate dehydrogenase; SCr, serum creatinine; WBC, white blood cell.
Values are median [interquartile range] unless otherwise indicated. Boldface entries indicate statistical significance.
SARS-CoV-2 positive patients with arterial thrombosis had a higher mortality rate than those who were SARS-CoV-2 negative or not tested (40.0% vs 8.0%; P = .041). There was no difference in length of stay between the two groups with both averaging about 5 days (P = .949), although hospitalizations were ongoing at the time of this writing in 15 of the 40 (37.5%) patients with thrombosis (Table III ). The SARS-CoV-2 positive patients were treated with successful open surgical thrombectomy in six cases (40.0%), anticoagulation alone in four cases (26.7%), or were not treated in five patients (33.3%). Of patients not treated, three were related to the severity of the ischemia at presentation and/or the severity of the COVID-19. Two patients were found to have radial artery thrombosis, one of which was without clinically evident hand ischemia and the other was already anticoagulated with systemic therapeutic heparin infusion for a left ventricular assist device. Amputation was required in three of the patients in the SARS-CoV-2 negative or untested group.
Table III.
Anatomic distribution, treatment details and outcomes for patients with acute arterial thrombosis
| No COVID-19 or not tested | COVID-19 positive | P value | |
|---|---|---|---|
| No. | 25 | 15 | |
| Anatomy | |||
| Carotid/vertebral | 3 (12.0) | 2 (13.3) | 1.000 |
| Aortoiliac | 1 (4.0) | 5 (33.3) | .040 |
| Femoropopliteal | 14 (56.0) | 6 (40.0) | .514 |
| Tibiopedal | 10 (40.0) | 2 (13.3) | .154 |
| Upper extremity | 2 (8.0) | 3 (20.0) | .537 |
| Prior leg bypass | 4 (16.0) | 1 (6.7) | .711 |
| Therapy | .115 | ||
| None | 8 (32.0) | 5 (33.3) | |
| AC only | 5 (20.0) | 4 (26.7) | |
| Surgical | 3 (12.0) | 6 (40.0) | |
| Endovascular | 5 (20.0) | 0 (0.0) | |
| Hybrid | 1 (4.0) | 0 (0.0) | |
| Primary amputation | 3 (12.0) | 0 (0.0) | |
| Hospital LOS, days | 5.0 [3.0-10.5] | 5.0 [5.0-7.3] | .949 |
| Mortality | 2 (8.0) | 6 (40.0) | .041 |
AC, Anticoagulation; COVID-19, coronavirus disease; LOS, length of stay.
Values are number (%) or median [interquartile range]. Boldface entries indicate statistical significance.
There were significantly more SARS-CoV-2 positive patients with aortoiliac involvement compared with the SARS-CoV-2 negative or not tested patients (33.3% vs 4.0%; P = .040). There was a trend toward less tibial/pedal (13.3% vs 40.0%; P = .154) and more upper extremity (20.0% vs 8.0%; P = .537) involvement in the SARS-CoV-2 positive patients when compared with SARS-CoV-2 negative or not tested patients. Carotid and femoropopliteal involvement was relatively equal between the two groups (Table III).
The COVID-19-specific severity of disease on presentation was documented (Table IV ). Most of the patients with arterial thrombosis had critically severe disease (60.0%). Three patients (20.0%) were asymptomatic, and one each had a mild, moderate, and severe COVID-19 presentation (6.7% each). There was no observed correlation between the severity of COVID-19 disease and the degree of arterial thrombosis. None of the patients in the study had bleeding complications. The 30-day outcomes resulted in six deaths owing to COVID-19 complications, four major amputations, three patients being discharged to skilled nursing facilities, and one to home.
Table IV.
Coronavirus disease (COVID-19)-related details of the presentation and treatment for patients who tested positive
| Patient | Location | Age | Sex | Race/ethnicity | COVID severity | COVID Rx | Treatment for arterial thrombosis | 30-Day Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | CIA, bypass, tibial | 44 | F | AA/black | Asymptomatic | Aorta/CIA/EIA thrombectomy. Plan for interval tibial bypass thrombectomy. | BKA | |
| 2 | Ulnar | 50 | F | Other, unknown | Critical | HCQ, steroids | AC only. | Expired - COVID |
| 3 | EIA, SFA | 62 | M | White | Critical | Unstable for surgery or AC. | Expired - COVID | |
| 4 | CCA | 56 | M | Other, unknown | Mild | CEA. | DC to SNF | |
| 5 | SCA, axillary, brachial, radial, ulnar | 53 | M | Other, Latino | Critical | HCQ | Subclavian/axillary/brachial thrombectomy and fasciotomy, rethrombosis on postoperative day 2 requiring repeat thrombectomy. | Below elbow amputation |
| 6 | Aorta, CIA, popliteal, tibial | 64 | M | White | Moderate | CIA embolectomy, RLE thrombolysis, RLE 4 compartment fasciotomy. | DC home | |
| 7 | SFA, tibial | 59 | M | Other, Latino | Critical | HCQ, steroids | Heparin only. Unstable for surgery | Expired - COVID |
| 8 | SFA, profunda | 91 | F | Other, Latino | Critical | HCQ, steroids | Heparin only. surgery refused. | Expired - COVID |
| 9 | CFA, SFA, popliteal | 65 | M | Other, Latino | Critical | HCQ | Heparin only. Unstable for surgery. | Expired - COVID |
| 10 | Radial | 66 | M | Other, Latino | Critical | HCQ, azithromycin, steroids | Radial artery partial occlusion without hand ischemia. | DC to SNF |
| 11 | Tibial, pedal | 67 | M | White | Critical | Unstable for surgery or AC. | Expired - COVID | |
| 12 | CIA, profunda, popliteal | 65 | M | Other, Latino | Severe | HCQ, steroids | AC Only. Patient refused surgery owing to the risk of prolonged intubation. Eventually stabilized for surgery and BKA. | BKA |
| 13 | CIA, EIA, profunda, popliteal | 74 | M | Other, Latino | Critical | AC only. Unstable for surgery. | Expired - COVID | |
| 14 | Aorta, CIA | 64 | F | AA/Black | Asymptomatic | HCQ, azithromycin | Aorta/CIA/EIA thrombectomy, fasciotomy. | AKA |
| 15 | ICA | 42 | F | AA/Black | Asymptomatic | Underwent decompressive craniectomy. No treatment directed at arterial occlusion. | DC to SNF |
AKA, Above knee amputation; ARDS, acute respiratory distress syndrome; BKA, below knee amputation; CCA, common carotid artery; CIA, common iliac artery; DC, discharge; EIA, external iliac artery; HCQ, hydroxychloroquine; ICA, internal carotid artery; RLE, right lower extremity; SCA, subclavian artery; SNF, skilled nursing facility; SFA, superficial femoral artery.
Discussion
This is a limited and primarily descriptive review of the early and rather intense experience during a surge of COVID-19 cases in a large, tertiary academic medical center in New York City. This experience occurred during an unprecedented period when our hospital, like others in the region, were caring for patients with proven or suspected COVID-19. State mandates and clinical demand spawned an increase in bed capacity to more than 150% of normal, with staffing of all newly created beds. Many of these units were critical care in nature and the increased demand necessitated redeployment of much of the workforce.
The first documented case of COVID-19 in New York State occurred on March 1, 2020, with the first documented case at our institution on March 11, 2020. During the initial 6 weeks of what proved to be a COVID-19 surge, we were asked to consult on an increasing number of patients with arterial thromboembolic events. Over this brief study period, there existed variability in the availability and reliability of SARS-CoV-2 diagnostic testing, posing clinicians with challenges in developing treatment strategies.
SARS-CoV-2 was originally characterized as a respiratory pathogen whose effects were primarily pulmonary in nature. Over time, the impact of the virus on other systems has become increasingly recognized and some authors speculate as to whether one of, if not the, major underlying etiology of this systemic pathology is a disordered coagulation system either from direct viral effect or the body's own inflammatory mediators. It has been postulated that patients with COVID-19 develop viral hyperinflammation, which makes them more susceptible to complications such as acute respiratory distress syndrome and hypercoagulability. Preliminary reports suggest that hemostatic abnormalities, including DIC, occur in patients affected by COVID-19. Although some, but not all, laboratory findings appear similar to sepsis-associated DIC, COVID-19-induced coagulopathy (CIC) seems to be more prothrombotic than hemorrhagic. It has been postulated that CIC may be an uncontrolled immunothrombotic response to COVID-19, and there is growing evidence of venous and arterial thromboembolic events in these critically ill patients. Additionally, the hypoxia, severe inflammatory response, critical illness, and underlying traditional risk factors may all predispose patients to thromboembolic events.6, 7, 8, 9, 10 It is possible that the 15 COVID-19 patients with arterial thromboembolic complications in our series had CIC.
Our early experience with arterial thrombosis during the COVID-19 crisis demonstrates several interesting features. The distribution of lesions, at least those presenting symptomatically, are primarily in large vessels such as the aortoiliac vessels, often without obvious underlying atherosclerotic changes. Arterial thromboses were seen in SARS-CoV-2 positive patients that ranged from mild to severe in intensity. Patients with arterial thrombosis who were SARS-CoV-2 positive had significantly higher d-dimer levels, BMI, were younger, and less often on antiplatelet medications as compared with patients who were SARS-CoV-2 negative or not tested. In addition, we noticed that SARS-CoV-2 positive patients with arterial thromboembolic complications exhibited a trend toward higher WBC count and neutrophil-to-lymphocyte ratio, which may indicate an increased inflammatory response. Other markers of increased inflammation such as CRP and CPK were also greater in the SARS-CoV-2 positive group when compared with their negative counterparts, but these differences did not reach statistical significance. All patients with acute arterial ischemia are placed on a heparin drip upon diagnosis.
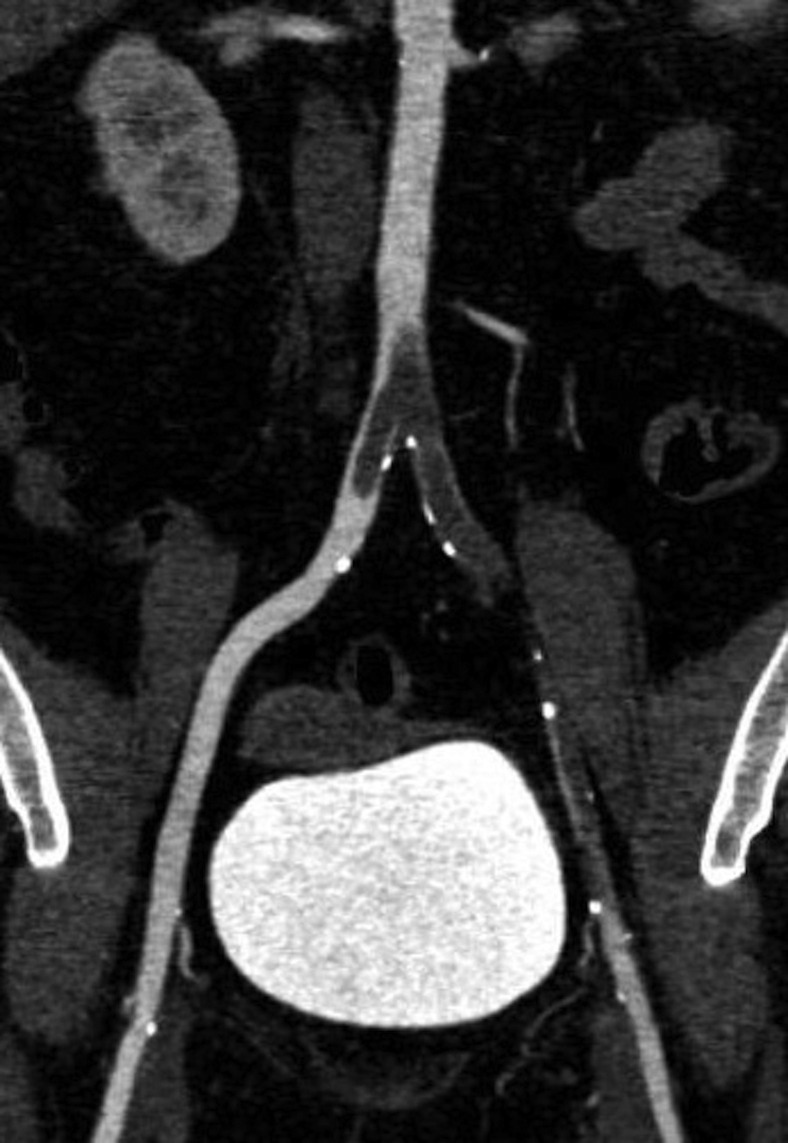
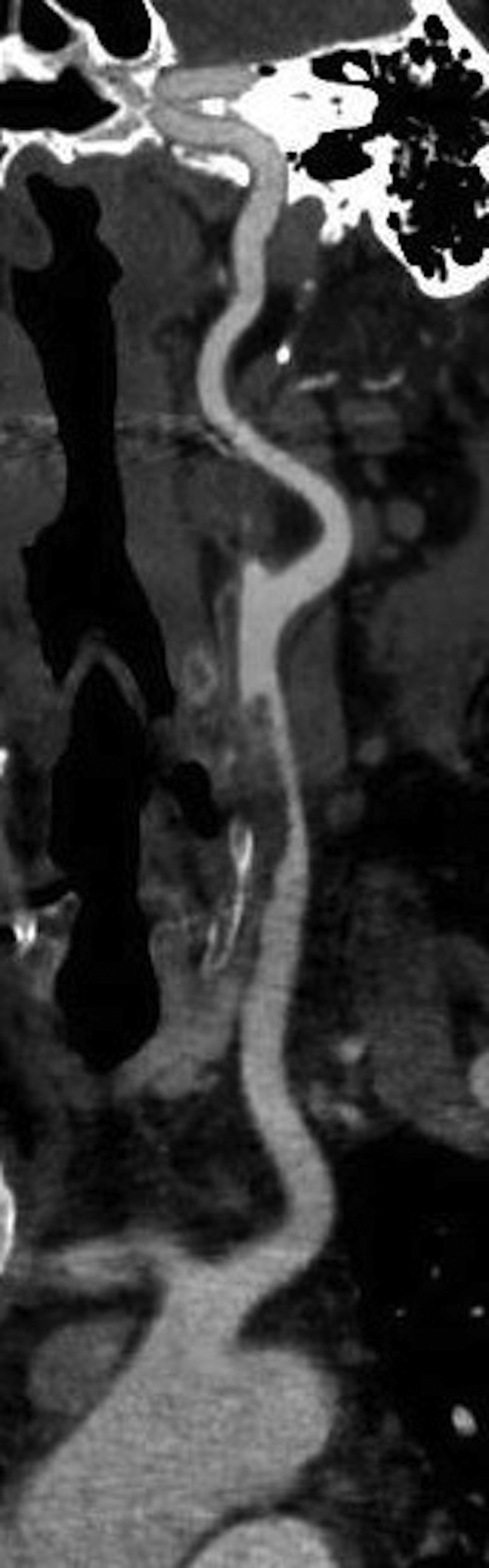
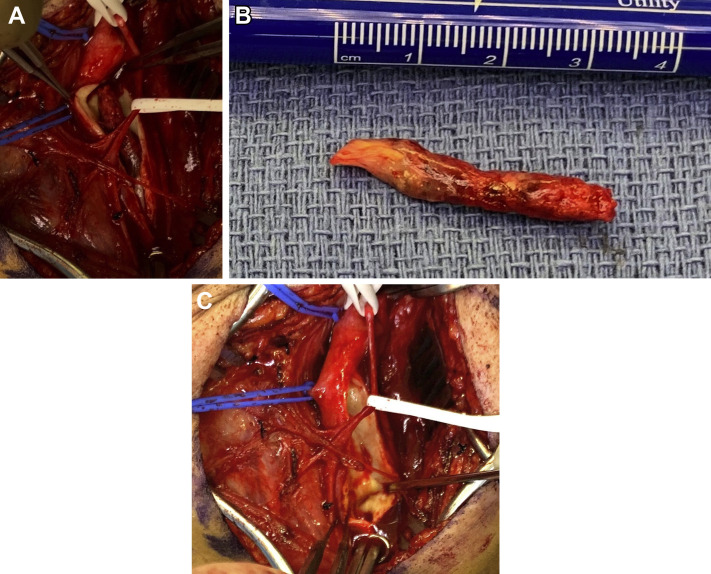
We did not observe a typical cardioembolic pattern in the SARS-CoV-2 positive group, but rather a picture that was more consistent with in situ thrombosis. The arterial thrombotic events seemed out of proportion to the degree of underlying atherosclerotic disease. Imaging of a patient with aortoiliac involvement showed eccentric thrombus adherent to a locally thickened arterial wall (Fig 2 ). A similar finding on computed tomography angiogram was observed in a patient with carotid involvement (Fig 3 ). This pattern is consistent with the proposed mechanism of endothelial damage, dysregulation, and hypercoagulability seen in SARS-CoV-2 infection.11 Direct observation of an organized thrombus in the common carotid artery of a patient who presented with stroke supports this theory, in addition to computed tomography scans in patients with aortoiliac thrombosis (Supplementary Fig, online only). At the time of operation, we found the thrombus to be organized and moderately adherent to the common carotid arterial wall over a distance of approximately 1 cm with an approximately a 3-cm tail of organized thrombus. The arterial wall adjacent to the attachment site was thickened and inflamed Supplementary Fig, A, online only). On removing the thrombus, there was no gross evidence of endothelial damage (Supplementary Fig, C, online only).
Fig 2.
Computed tomography angiography (CTA) demonstrating thrombus in the abdominal aorta.
Fig 3.
Computed tomography angiography (CTA) demonstrating thrombus in the left common carotid artery.
Supplementary Fig (online only).
A, Intraoperative picture demonstrating acute thrombus in the common carotid artery. B, Gross specimen representing thrombus extracted from common carotid artery. C, Common carotid artery after specimen removal showing no endothelial injury.
The majority of arterial emboli originate in the heart and travel to the extremities, with the lower extremities being affected more frequently than the upper extremities and carotid arteries. Thromboemboli typically lodge where there is an acute narrowing of the artery, such as an atherosclerotic plaque or a vessel branch point.12, 13, 14, 15 We saw instances of this anatomic distribution in the SARS-CoV-2 negative group. In a recent series of 29 non-COVID patients with acute aortoiliac occlusion the etiology was felt to be aortoiliac thrombosis in 22 cases, embolic occlusion in 2, and indeterminate in 5.15 All of the COVID-19 positive patients treated surgically for thromboemboli were done so with open operations. No patients were treated with endovascular only or pharmacologic thrombolysis owing to the severity of their comorbid disease and thrombus burden. In addition, the organized nature of the thrombus on gross review, calls into question the efficacy of pharmacologic thrombolytics.
There remains much to learn about the SARS-CoV-2 and the disease it causes. In addition to an early lack of comprehensive testing for both active disease as well as prior infection, the reliability of many of these tests have been called into question. False-negative rates ranging from 20% to as high as 50% for diagnostic tests have been quoted and, more recently, it has been suggested that antibody tests may not be as indicative of prior infection as first believed.11 , 16 Whether these are issues with the tests themselves or a peculiarity of the virus is unclear. We do not know whether the virus can remain dormant and “reactivate” in an individual nor whether individuals develop immunity and if so to what degree and for how long. We also do not know whether the impact of inflammatory changes and possible endothelial damage will resolve with convalescence or whether the changes may persist and lead to symptomatic vascular disease in the future at an undetermined time point. If the latter is the case, we will need to anticipate this eventuality and investigate therapies to help delay or prevent these occurrences.
There are several limitations to our study in addition to the small retrospective nature over a short time period. The number of cases is small and the significance of P values should be viewed with caution. We also do not have baseline studies that fully delineate the degree of underlying atherosclerotic disease in these patients. Evaluation of administrative inpatient consult records identify at least seven patients with clinically suspected arterial ischemia during the study period who were deemed too unstable for imaging. Other patients who were in extremis and not candidates for intervention may not have come to our attention. These two patient subsets were not included in the dataset and could represent a significant number that would impact study results and generalizability. Outcomes related to SARS-CoV-2 positive patients with arterial thromboembolic complications were not examined and compared with SARS-CoV-2 positive patients without clinically detected arterial thromboemboli. Because d-dimer is not a routine serum test for patients without suspected COVID-19, many patients did not have a d-dimer on the day of imaging. including 7 in the SARS-CoV-2 positive group and 21 in the SARS-CoV-2 negative/not tested group. Some patients did not have d-dimer testing during their hospitalization including 1 in the SARS-CoV-2 positive group and 21 in the SARS-CoV-2 negative/not tested group. Finally, the impact of the surge, in terms of both physical space and personnel placed tremendous pressure on our vascular lab and diagnostic radiology testing services. Diagnostic services were strained and used judiciously. Before SARS-CoV-2 testing capabilities increased, many patients with a clinical picture consistent with SARS-CoV-2 infection tested negative or were not tested, impacting capture within our study.6 Most important, several of the patients who were in the SARS-CoV-2 negative group and in whom there was a strong clinical suspicion for the disease may have had false-negative tests, even on repeat testing.16 , 17
Conclusions
Patients with SARS-CoV-2 infection are at risk for developing significant acute arterial thromboembolic complications. In addition, the arterial thromboembolic complications seen in patients with SARS-CoV-2 seem to be more frequently seen in large vessel distributions and associated with little or no underlying atherosclerosis or associated risk factors suggestive of in situ arterial thrombosis. SARS-CoV-2 positive patients with arterial thromboemboli demonstrated significantly higher d-dimer levels and greater BMI, and exhibited a trend toward elevated WBC and neutrophil-to-lymphocyte ratio. This hyperinflammatory state may produce a transient hypercoagulable milieu and favor the formation of arterial thromboemboli. These findings provide an early description of the characteristics of arterial thromboembolic disease in SARS-CoV-2 patients.
Author contributions
Conception and design: JEI, ECL
Analysis and interpretation: JEI, IK, ANH, CC, ECL
Data collection: ANH, CC, DBJ, HA, HB, JS
Writing the article: JEI, IK, ECL
Critical revision of the article: JEI, IK, ANH, CC, DBJ, HA, HB, JD, ECL
Final approval of the article: JEI, IK, ANH, CC, DBJ, HA, HB, JD, ECL
Statistical analysis: IK, ANH
Obtained funding: Not applicable
Overall responsibility: JEI
Footnotes
Author conflict of interest: I.K. owns Doximity stock and consults/advises for eHealth Connect, Medline, and Empire BCBS. The remaining authors have no conflicts to disclose.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvascsurg.org.
Appendix (online only).
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Hak M.J., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombos Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020 Apr 3 doi: 10.1002/jmv.25819. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan B.E., Chong V.C.L., Chan S.S.W., Hsiang G., Guan K., Lime E., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 10.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cukeret A., et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oudkerk M., Büller H.R., Kuijpers D., van Es N., Oudkerk S.F., McLoud T.C., et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology. 2020 Apr 23 doi: 10.1148/radiol.2020201629. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creager M.A., Kaufman J.A., Conte M.S. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366:2198–2206. doi: 10.1056/NEJMcp1006054. [DOI] [PubMed] [Google Scholar]
- 13.Abbott W.M., Maloney R.D., McCabe C.C., Lee C.E. Wirthlin LS Arterial embolism: a 44 year perspective. Am J Surg. 1982;143:460–464. doi: 10.1016/0002-9610(82)90196-9. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell M.E., Carpenter J.P. In: UptoDate. Eidt J.F., Mills J.L., Hoekstra J., editors. March 2020. Clinical features and diagnosis of acute lower extremity ischemia. [Google Scholar]
- 15.Crawford J.D., Perrone K.H., Wong V.W., Mitchell E.L., Azarbal A.F., Liem T.M., et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59:1044–1050. doi: 10.1016/j.jvs.2013.10.080. [DOI] [PubMed] [Google Scholar]
- 16.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long C., Xu H., Shen Q., Zhang X., Fan B., Wang C., et al. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]