Abstract
Background & Aims
We compare the performance of liver surface nodularity (LSN) and liver stiffness measurements (LSM) using transient elastography (TE) for the detection of clinically significant portal hypertension (CSPH) in patients with cirrhosis and hepatocellular carcinoma (HCC).
Methods
All patients with cirrhosis and HCC who underwent computed tomography, LSM and hepatic venous pressure gradient (HVPG) measurements within 30 days between 2015 and 2018 were included. The estimation of CSPH by LSN and LSM, and the LSM-spleen-size-to-platelet ratio score (LSPS) were evaluated and compared.
Results
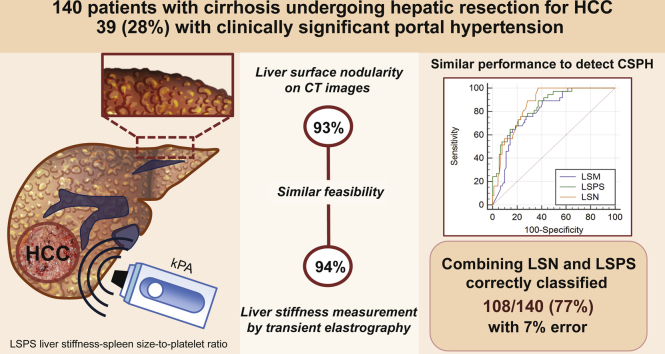
In total, 140 patients were included (109 men [78%], mean age 63 ± 9 years old), including 39 (28%) with CSPH. LSN measurements were valid in 130 patients (93%) and significantly correlated with HVPG (r = 0.68; p <0.001). Patients with CSPH had higher LSN measurements compared with those without [3.1 ± 0.4 vs. 2.5 ± 0.3, p <0.001; area under the receiver operating characteristic (AUROC): 0.87 ± 0.31]. LSM and LSPS were valid in 132 patients (94%) and significantly correlated with HVPG (r = 0.75, p <0.001; AUROC 0.87 ± 0.04 and r = 0.68, p <0.001; AUROC 0.851 ± 0.04, respectively). There was no significant difference in the diagnostic performance between LSN and LSM-LSPS (DeLong, p = 0.28, 0.37, and 0.65, respectively) in patients with both valid tests (n = 122). LSN <2.50 had a 100% negative predictive value for CSPH. A 2-step algorithm combining LSN and LSPS for the diagnosis of CSPH classified 108/140 patients (77%) with an 8% error.
Conclusions
The diagnostic performance and feasibility of LSN measurements were similar to those of LSM for the detection of CSPH in patients with compensated cirrhosis and HCC. Combining LSN and LSPS accurately detected CSPH in >75% of patients. Such a combination could be useful in centres where the HVPG measurement is unavailable.
Lay summary
The diagnostic performance and feasibility of liver surface nodularity was similar to that of liver stiffness measurement (LSM) for the detection of clinically significant portal hypertension in patients with compensated cirrhosis. Thus, liver surface nodularity could be an option for the preoperative detection of clinically significant portal hypertension in patients with hepatocellular carcinoma. Combining liver surface nodularity with LSM-spleen-size-to-platelet ratio score resulted in the accurate detection of clinically significant portal hypertension in >75% of patients, thus limiting the need for HVPG measurements.
Abbreviations: AUROC, area under the receiver operating characteristic; CSPH, clinically significant portal hypertension; HCC, hepatocellular carcinoma; HPV, hepatic venous pressure; HVPG, hepatic venous pressure gradient; LSM, liver stiffness measurements; LSN, liver surface nodularity; LSPS, LSM-spleen-size-to-platelet ratio score; NRI, Net Classification Index Improvement; PHT, portal hypertension; TE, transient elastography
Graphical abstract
Highlights
-
•
Diagnostic performance of LSN is similar to that of LSM for the diagnosis of CSPH in patients with cirrhosis and HCC.
-
•
LSN could be useful for the preoperative detection of CSPH in patients with HCC and compensated cirrhosis.
-
•
Combination of LSN as a first-line non-invasive test and LSPS accurately detected CSPH in >75% of patients.
Introduction
The development of clinically significant portal hypertension (CSPH), defined as a hepatic venous pressure gradient (HVPG) ≥10 mmHg, is a turning point in the progression of chronic liver disease and is associated with the development of gastric and oesophageal varices and the first sign of clinical decompensation in patients with no varices.1 In addition, the assessment of CSPH has a crucial role in the management of patients with cirrhosis and primary liver tumours, especially when major liver resection is the only potential curative treatment. CSPH is associated with an increase in perioperative morbidity of 22–50% in these cases.2 Moreover, European Association for the Study of the Liver and American Association for the Study of Liver Diseases guidelines consider portal hypertension (PHT) to be a major factor in the indication for resection of liver tumours.3,4 Therefore, preoperative screening for CSPH is vital.
Measurement of the HVPG by right hepatic vein catheterisation is the reference technique to assess CSPH. This method is invasive, and can often only be performed in expert centres, limiting its availability. To overcome these limitations, several non-invasive methods have been developed. The most frequently used technique is the liver stiffness measurement (LSM), using transient elastography (TE), which is strongly correlated to HVPG and is useful for the detection of CSPH, including in patients in whom liver resection is indicated.[5], [6], [7] LSM-derived scores, such as the LSM-spleen-size-to-platelet ratio score (LSPS), which combines liver stiffness, spleen diameter and platelet count, have been shown to improve the detection of CSPH.8 Nevertheless, LSM has certain well-known limitations, in particular, the rate of unreliable examinations.9
It was recently shown that the diagnostic performance of the quantification of liver surface nodularity (LSN) on routine computed tomography (CT) was good for detecting CSPH and was better than numerous other non-invasive tests.10,11 The quantification of LSN is easy to obtain and can be retrospectively derived from routine CT, which is especially convenient in patients in whom liver resection is being considered.
Thus, the aim of the present study was to compare the performance of LSM using TE and the quantification of LSN from CT for the diagnosis of CSPH, and to evaluate whether the combination of these 2 techniques could improve the determination of CSPH.
Materials and methods
Study population
This retrospective study, together with a protocol review, was approved by the institutional review board (CERIM, France) and the requirement for written informed consent was waived. The study cohort included all patients with histologically proven compensated cirrhosis and HCC who underwent measurement of HVPG during the work-up for liver surgery between January 2015 and July 2018 at Beaujon University Hospital (Clichy, France). Patients underwent HVPG and TE measurements of the liver on the same day, and a preoperative abdominal contrast-enhanced CT examination within 30 days before or after the HVPG measurement. At our centre, tumour size per se does not represent a contraindication to surgery and liver resection is considered in patients with cirrhosis with large-sized lesions only if: (i) a future liver remnant of at least 40% (with adequate inflow, outflow, and biliary drainage) is secured. This can require a combination of preoperative transarterial chemoembolisation and preoperative liver volume modulation using the portal vein with or without hepatic vein embolisation; and (ii) no CSPH (as evaluated using HPVG or indirect signs) is present.
Clinical data, results of laboratory tests performed within 1 week before the HVPG measurement, and hemodynamic data were prospectively collected. Patients with a prior history of surgery, without a CT scan performed within 30 days before surgery, and those with portal vein thrombosis were not included. It is important to note that 48 patients included in the present study were part of a population included in a study previously published by our group.10
Analysis derived from CT images
LSN quantification was performed on portal venous phase CT images using semiautomated CT software (LSN Software, version 0.88; Liver Nodularity llc) by 2 abdominal radiologists (AS and RS) blinded to clinical data and HVPG results. Radiologists drew a region of interest of 1–2 cm in diameter across the margin of the left liver surface on portal venous phase CT images. The software automatically detected the liver edge compared with adipose tissue on the selected section and on up- and downward continuous sections by propagating the painted region of interest. The software automatically generated a smooth polynomial line to mimic the expected normal liver surface. The distance between the detected liver margin and the polynomial line was measured on a pixel-by-pixel basis, expressed in tenths of a millimetre. A series of 10 valid margin measurements were obtained according to previous studies.[10], [11], [12] The software calculated the arithmetical mean of the measurements. Fewer than 10 valid measurements were arbitrarily considered to be invalid measurements.
Additional CT-derived data, including the size and location of HCC lesions, along with the largest spleen diameter, were analysed and collected.
LSM by TE and derived scores
TE was performed by hepatology nurses with an experience of >500 procedures, according to the instructions provided by the manufacturer. Reliable LSM were defined as the median value of 10 consecutive measurements with a success rate >60% and an interquartile range <30% of the median of measurements. The measurement was expressed in kilopascals (kPa). LSPS, which combines LSM, platelet count, and spleen diameter was calculated as described previously: LSM (by TE in kPa) × spleen diameter (cm)/platelet ratio (109/L).8
HVPG
HVPG measurements were performed under local anaesthesia as previously described.13 A catheter was introduced under ultrasound guidance using the Seldinger technique, and a 7-French balloon catheter was then inflated in the right or median hepatic vein to measure the wedged HVP. Occlusion was then confirmed by injection of 5–10 ml of iodinated contrast. The free HVP was then obtained. Finally, HVPG was calculated as the difference between wedged and free HVPs.
Statistical analysis
Continuous variables are expressed as means and standard deviations or medians and ranges, and were compared using the Student t test or Mann-Whitney U test, when appropriate. Gaussian distribution was tested using the Kolmogorov-Smirnov test. Comparisons between categorical variables were performed using the Chi-square test or the Fisher exact test, when appropriate. Correlations were computed with Pearson's correlation coefficient. The discriminative value of LSN and LSM, either alone or in combination, for the identification of CSPH was assessed by measuring the area under the receiver operating characteristic (AUROC) curve provided with the corresponding standard deviation. Optimal cut-off values were identified using the Youden index and associated sensitivities, specificities, and positive and negative predictive values (with 95% confidence intervals [CI]) were determined. Comparison between AUROCs was performed by using the DeLong method.14 Net Reclassification Index Improvement (NRI) analysis was performed for LSN and LSPS, using LSM as a reference.15 NRI quantifies how well a new model correctly reclassifies subjects, provides separated indices for patients with and without CSPH, and estimates the overall proportion of correct reclassifications in each group.
The performance of non-invasive methods to confirm or exclude CSPH was assessed using previously published cut-off values for the LSN score (exclude <2.50 and rule in ≥3.10) and for LSPS (rule out <1.08 and rule in ≥2.06) and TE (rule out <13.6 kPa and rule in ≥21.0 kPa).8,10,14 Analyses were performed both on an intention-to-diagnose (including the entire cohort) and per-protocol (including only patients with valid LSM and LSN score) basis. All tests were 2-sided and a p value <0.05 was considered to be statistically significant. Statistical analyses and figures were performed by using the SPSS statistical package software (version 24.0; SPSS Inc., Chicago, Ill, USA), GraphPad Prism software (version 7; GraphPad Software, La Jolla, Ca, USA), and Tableau V10.2.0 (Tableau Software Inc, Seattle, WA, USA).
Results
Study population
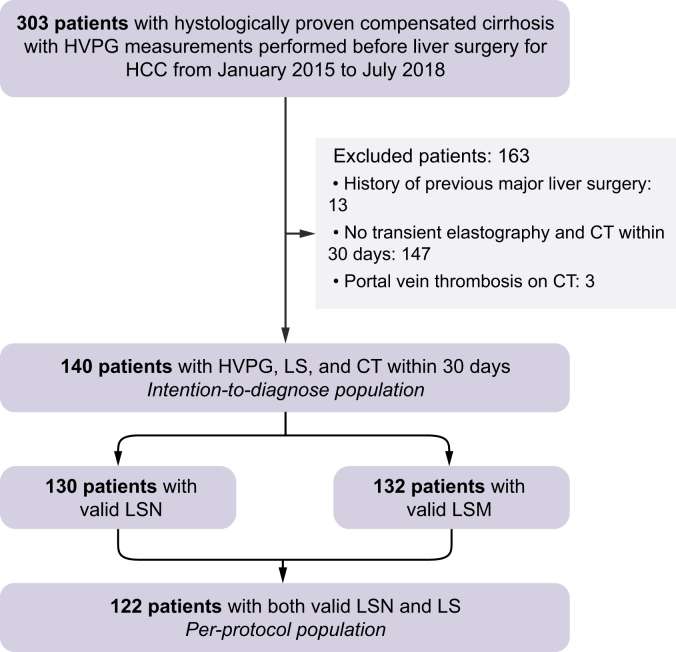
The flowchart of the population is provided in Fig. 1. In total, 140 patients underwent both LSN and LSM (109 men [78%], mean age 63 ± 9 years old), including 39 (28%) with CSPH. Most patients had chronic viral hepatitis infection (50 with hepatitis C virus [36%]; 30 with hepatitis B virus [21%]) or alcoholic cirrhosis (47 [34%]).
Fig. 1.
Flowchart of population study.
HCC, hepatocellular carcinoma; HVPG, hepatic venous pressure gradient; LSM, liver stiffness measurement; LSN, liver surface nodularity.
The LSN score was reliable in 130/140 (93%) patients. LSN measurements were unsuccessful in 10 patients because of patient-related issues in 8 (insufficient adipose tissue to delineate the liver surface in 3 patients and HCC leading to left lobe capsular bulging in 5), or software-related issues (errors in reading the Digital Imaging and Communications in Medicine header with the LSN software in 2 patients). LSN measurements took a mean 165 ± 55 s to perform. LSM was reliable in 132/140 patients (94%). Overall, 122/140 patients (87%) had reliable LSN and LSM measurements [91 men (75%), mean age 63 ± 10 years old], including 36 (30%) with CSPH, and constituted the per-protocol cohort. The characteristics of the intention-to-diagnose and per-protocol populations are provided in Table 1 and Supplemental Table S1, respectively. The characteristics of the excluded patients are provided in Supplemental Table S2.
Table 1.
Intention-to-diagnose study population.
| Total (N = 140) | No CSPH (n = 101) | CSPH (n = 39) | p value | |
|---|---|---|---|---|
| Gender (%) | ||||
| Male | 109 (78) | 76 (75) | 33 (85) | 0.23 |
| Female | 31 (22) | 25 (25) | 6 (15) | 0.23 |
| Mean age ± SD | ||||
| Overall | 63 ± 9 | 63 ± 10 | 63 ± 9 | 0.91 |
| Male | 62 ± 10 | 62 ± 10 | 62 ± 9 | 0.87 |
| Female | 67 ± 7 | 67 ± 7 | 67 ± 4 | 0.94 |
| BMI (kg/m2) | 28.9 ± 6.9 | 29.5 ± 8.2 | 27.2 ± 3.9 | 0.10 |
| Child-Pugh score (%) | ||||
| A | 133 (95) | 97 (96) | 36 (93) | 0.40 |
| B | 7 (5) | 4 (4) | 3 (7) | |
| Cause of cirrhosis∗ | ||||
| Hepatitis C virus infection | 50 (36) | 31 (31) | 19 (49) | 0.05 |
| Hepatitis B virus infection | 30 (21) | 21 (21) | 9 (23) | 0.76 |
| Alcohol | 47 (34) | 35 (35) | 12 (31) | 0.67 |
| Non-alcoholic fatty liver disease | 25 (18) | 19 (19) | 6 (15) | 0.64 |
| Duration between HPVG-LSM and CT (days) | 15 ± 11 | 15 ± 12 | 14 ± 10 | 0.41 |
| HVPG (mmHg) | 8 ± 4 | 6 ± 2 | 14 ± 3 | <0.001 |
| Hepatocellular carcinoma | ||||
| Mean size ±SD (mm) | 51 ± 27 | 57 ± 28 | 38 ± 19 | <0.001 |
| Right liver | 98 (70) | 70 (70) | 28 (72) | 0.84 |
| Left liver | 42 (30) | 31 (30) | 11 (28) | |
| Barcelona Clinic Liver Cancer stage | ||||
| Stage 0 | 26 (19) | 14 (14) | 12 (31) | 0.022 |
| Stage A | 114 (81) | 87 (86) | 27 (69) | |
| Laboratory tests | ||||
| INR | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | <0.001 |
| Serum creatinine (μmol/L) | 80 ± 18 | 80 ± 20 | 78 ± 13 | 0.59 |
| Serum bilirubin (μmol/L) | 14 ± 6 | 14 ± 7 | 15 ± 5 | 0.56 |
| Platelet count (G/L) | 193 ± 58 | 211 ± 59 | 146 ± 38 | <0.001 |
| Serum albumin (g/L) | 35 ± 7 | 36 ±6 | 33 ± 7 | 0.004 |
| Serum AST (× ULN) | 1.8 ± 1.1 | 1.6 ± 1.0 | 1.8 ± 1.4 | 0.35 |
| Serum ALT (× ULN) | 1.6 ± 1.2 | 1.6 ± 1.3 | 1.5 ± 1.0 | 0.60 |
| γ-Glutamyl transferase (× ULN) | 3.5 ± 2.9 | 3.3 ± 3.0 | 3.8 ± 3.9 | 0.42 |
| Alkaline phosphatase (× ULN) | 1.2 ± 0.7 | 1.1 ± 0.6 | 1.6 ± 1.1 | 0.002 |
| Non-invasive tests | ||||
| Liver surface nodularity | 2.68 ± 0.4 | 2.50 ± 0.3 | 3.14 ± 0.4 | <0.001 |
| Largest spleen size (cm) | 11.5 ± 1.6 | 11.1 ± 1.4 | 12.6 ± 1.5 | <0.001 |
| Platelets/spleen size | 17.7 ± 6.5 | 20.0 ± 6.4 | 12.2 ± 4.0 | <0.001 |
| LSM kPa median (range) | 14.2 (3.3–75.0) | 10.6 (3.3–75.0) | 29.5 (8.8–75.0) | <0.001 |
| LSPS | 1.78 ± 1.50 | 1.03 ± 0.76 | 3.62 ± 2.21 | <0.001 |
Values are expressed as mean and standard deviation unless otherwise specified and statistical analysis was performed using Student t test, Fisher exact test and Chi2 test.
AST/ALT, aspartate aminotransferase/alanine aminotransferase; BMI, body mass index; CSPH, clinically significant portal hypertension; CT, computed tomography; HVPG, hepatic venous pressure gradient; INR, international normalised ratio; LSM, liver stiffness measurement; LSPS, liver stiffness measurement-spleen-size-to-platelet ratio; ULN, upper limit of normal values.
Some patients had several causes of cirrhosis.
Performance of LSN, LSM and LSPS for assessment of CSPH in the per-protocol cohort
Table 2 and Fig. 2 describe the diagnostic performance of LSN, LSM, and LSPS for the diagnosis of CSPH. The mean LSN score in the per-protocol cohort was 2.7 ± 0.4. The score was higher in patients with CSPH than in those without, resulting in an AUROC of 0.87 ± 0.31 (95% CI: 0.80–0.93) for the detection of CSPH. The diagnostic performance of LSN was also assessed in the 92 patients who were not included in the previous study by our group, resulting in a similar diagnostic performance10 (Supplemental Table S3).
Table 2.
Performance of liver surface nodularity, liver stiffness measurement, and LSPS for the diagnosis of clinically significant portal hypertension in the per-protocol population and in patients with HCC based on Milan Criteria.
| Criterion | AUROC | Cut-off values | Se (%) (95%CI) | Sp (%) (95%CI) | PPV (%) (95%CI) | NPV (%) (95%CI) | LR+ (%) (95%CI) | LR- (%) (95%CI) | No. correctly classified | |
|---|---|---|---|---|---|---|---|---|---|---|
| Per-protocol population (n = 122) | ||||||||||
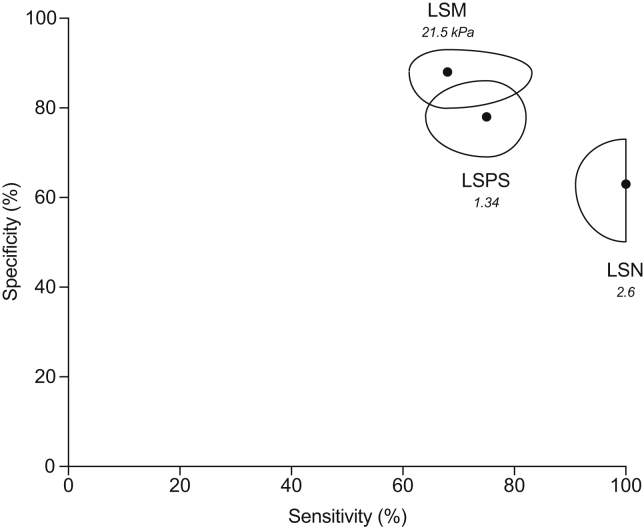
| LSN | 0.871 ± 0.03 | Optimal∗ | >2.57 | 100 (91–100) | 63 (50–73) | 54 (44–61) | 100 (93–100) | 2.70 (1.82–3.70) | 0 (0–0.18) | 90/122 (74%) |
| Se ≥90% | >2.60 | 94 (77–99) | 66 (52–74) | 54 (41–67) | 97 (88–99) | 2.64 (1.0–3.81) | 0.09 (0.01–0.44) | 91/122 (75%) | ||
| Sp ≥90% | >2.96 | 60 (40–77) | 90 (81–95) | 70 (51–85) | 84 (75–91) | 6.00 (5.00–15.4) | 0.44 (0.24–0.74) | 98/122 (80%) | ||
| LSM | 0.825 ± 0.04 | Optimal∗ | >21.5 kPa | 68 (61–83) | 88 (80–93) | 70 (62–81) | 87 (78–94) | 3.09 (3.05–11.9) | 0.36 (0.18–0.49) | 97/122 (80%) |
| Se ≥90% | >13.7 kPa | 91 (77–98) | 63 (52–73) | 49 (37–62) | 93 (83–98) | 2.46 (1.60–3.63) | 0.14 (0.03–0.44) | 85/122 (70%) | ||
| Sp ≥90% | >33.0 kPa | 46 (29–63) | 91 (83–96) | 64 (43–82) | 79 (70–87) | 5.44 (4.14–15.8) | 0.59 (0.39–0.86) | 93 /122 (76%) | ||
| LSPS | 0.851 ± 0.04 | Optimal∗ | >1.34 | 75 (64–82) | 78 (69–86) | 59 (50–67) | 88 (81–94) | 3.40 (2.06–5.86) | 0.32 (0.21–0.52) | 95/122 (78%) |
| Se ≥90% | >0.88 | 91 (77–98) | 60 (49–71) | 49 (36–61) | 94 (85–99) | 2.28 (1.51–3.38) | 0.15 (0.03–0.47) | 84/122 (69%) | ||
| Sp ≥90% | >2.29 | 57 (39–74) | 91 (83–96) | 70 (51–85) | 84 (75–91) | 6.33 (2.29–18.5) | 0.47 (0.27–0.73) | 98/122 (80%) | ||
| Patients with HCC within Milan Criteria (n = 83) | ||||||||||
| LSN | 0.866 ± 0.04 | Optimal∗ | >2.57 | 100 (89–100) | 65 (52,77) | 63 (49–75) | 100 (90–100) | 2.22 (1.85–4.35) | 0 (0–0.21) | 65/83 (78%) |
| Se ≥90% | >2.66 | 92 (79–97) | 62 (47–75) | 67 (54–78) | 90 (75–97) | 2.42 (1.49–3.88) | 0.13 (0.04–0.45) | 63/83 (76%) | ||
| Sp ≥90% | >3.10 | 55 (38–71) | 90 (79–95) | 77 (65–86) | 77 (57–90) | 5.50 (1.81–14.2) | 0.50 (0.31–0.78) | 64/83 (77%) | ||
| LSM | 0.880 ± 0.04 | Optimal∗ | >21.0 kPa | 65 (47–79) | 90 (79–95) | 81 (69–89) | 80 (61–91) | 6.5 (2.23–15.8) | 0.39 (0.22–0.67) | 67/83 (81%) |
| Se ≥90% | >10.0 kPa | 90 (74–97) | 53 (40–66) | 51 (39–65) | 90 (75–97) | 1.91 (1.23–2.20) | 0.40 (0.05–0.90) | 55/83 (66%) | ||
| Sp ≥90% | >21.0 kPa | 65 (47–79) | 90 (79–95) | 81 (69–89) | 80 (61–91) | 6.5 (2.23–15.8) | 0.39 (0.22–0.67) | 67/83 (81%) | ||
| LSPS | 0.886 ± 0.04 | Optimal∗ | >1.38 | 74 (57–86) | 90 (79–95) | 82 (64–92) | 85 (73–92) | 7.40 (2.71–17.2) | 0.32 (0.15–0.54) | 69/83 (83%) |
| Se ≥90% | >0.86 | 92 (78–97) | 61 (46–74) | 64 (51–76) | 90 (75–97) | 2.24 (1.44–3.73) | 0.13 (0.04–0.48) | 61/83 (73%) | ||
| Sp ≥90% | >1.38 | 74 (57–86) | 90 (79–95) | 82 (64–92) | 85 (73–92) | 7.40 (2.71–17.2) | 0.32 (0.15–0.54) | 69/83 (83%) | ||
95% CI, 95% confidence interval; AUROC, area under receiver operating characteristic; LR+/−, positive/negative likelihood ratios; LSM, liver stiffness measurement; LSN, liver surface nodularity; LSPS, liver stiffness measurement-spleen-size-to-platelet ratio; PPV/NPV, positive/negative predictive value; Se/Sp, sensitivity/specificity.
To maximise the Youden index.
Fig. 2.
Plot of sensitivities and specificities associated with LSM, LSN and LSPS for the diagnosis of CSPH in the 122 patients with available LSN, LSM and LSPS.
Potatoids are centered by couples of sensitivities and specificities associated with optimal diagnostic cut-off values for each test. The cut-off values are indicated in italic. Their shapes represent the associated 95% CIs. CSPH, clinically significant portal hypertension; LSM, liver stiffness measurement; LSN, liver surface nodularity; LSPS, LSM-spleen-size-to-platelet ratio score.
Median LSM was 14.1 (range 3.2–75.0) kPa. It was higher in patients with CSPH than in those without, resulting in an AUROC of 0.83 ± 0.04 (95% CI: 0.75–0.89) for the detection of CSPH.
The mean LSPS value was 1.80 ± 1.50 and was higher in patients with CSPH than in those without, resulting in an AUROC of 0.85 ± 0.04 (95% CI: 0.78–0.91).
Pairwise comparison of AUROCs of LSN, LSM, and LSPS for the diagnosis of CSPH showed no significant difference (p = 0.28–0.65). Results were found to be consistent when focusing on the 83 patients with HCC based on Milan Criteria (Table 2).
Using LSM as the reference for patient classification, the overall NRI for LSN and LSPS was 0.05 and −0.01, respectively. The NRI of patients with CSPH was 0.25 and 0.06 for LSN and LSPS, respectively. The NRI of patients without CSPH was −0.20 and −0.07 for LSN and LSPS, respectively.
Although tumour location (i.e. right or left liver) did not significantly influence LSN, LSM or LSPS values in the overall per-protocol population (p = 0.06–0.53; Supplemental Table S4), median LSM values tended to be higher when HCC was located in the right liver lobe (p = 0.06). Therefore, we performed a more detailed analysis (presented in Supplemental Fig. S1 and Table S4) and found that, in the 13 patients with HCC ≥10 cm, CSPH was correctly assessed in 12 (92%) patients with LSN, and in only 5 (38%) patients with LSM and LSPS (p = 0.011). The other 8 patients were misclassified as having CSPH because of elevated LSM or LSPS values (all >55 kPa or >1.5, respectively). All tumours were located in the right liver (Supplemental Fig. S1). A detailed comparison of LSN, LSM, and LSPS values according to HCC size (i.e. < or ≥10 cm) and location, and to the presence of CSPH is provided in Supplemental Table S4.
Value of LSN, LSM, and LSPS to rule in or rule out CSPH in the intention-to-diagnose cohort
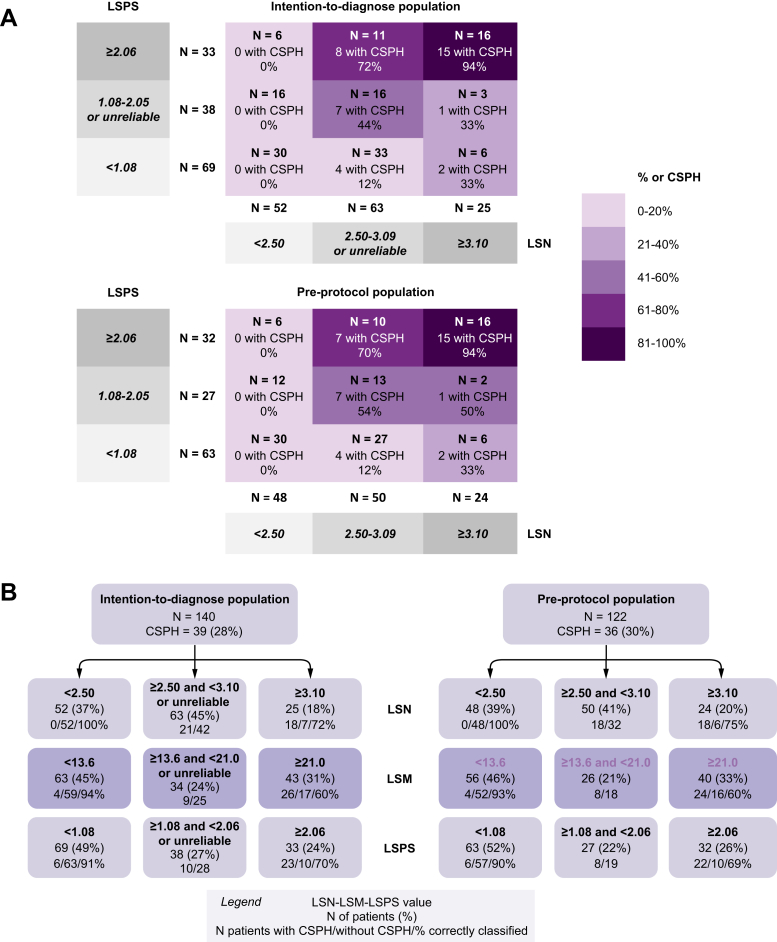
In the intention-to-diagnose cohort, the previously proposed10 LSN cut-off values of <2.5 ruled out the presence of CSPH in 52 patients with a negative predictive value of 100%, and ≥3.10 ruled-in CSPH in 25 patients with a positive predictive value of 72% (18/25 patients with CSPH). A total of 63 patients (45%) had either an unreliable or intermediate LSN score (LSN value ≥2.5 and <3.1, respectively). These rule-in and -out thresholds resulted in 70/140 (50%) correctly classified patients with an error of 9%.
The previously proposed LSM cut-off values <13.6 kPa16 ruled out the presence of CSPH in 63 patients with a negative predictive value of 93% (59 without CSPH), and ≥21 kPa ruled in CSPH in 43 patients with a positive predictive value of 60% (26 with CSPH). A total of 34 patients had either an unreliable or intermediate value (LSM value ≥13.6 and <21 kPa). Rule-in and -out thresholds resulted in 85/140 (61%) patients correctly classified, with an error of 20%.
The previously proposed8 LSPS cut-off values <1.08 ruled out the presence of CSPH in 69 patients with a negative predictive value of 91% (63 without CSPH), and ≥2.06 ruled in CSPH in 33 patients with a positive predictive value of 70% (23 with CSPH). A total of 38 patients had an unreliable or intermediate LSPS score (LSPS value ≥1.08 and <2.06). Rule-in and -out thresholds resulted in 86/140 (61%) patients correctly classified, with an error of 15%.
Performance of the combination of LSN and LSPS for estimating CSPH in the intention-to-diagnose cohort
Combining the rule-in and -out thresholds for both LSN and LSPS8,10 successfully stratified patients with an increasing risk of CSPH (Fig. 3). This combination was chosen because LSPS has been shown to outperform LSM alone for patient classification. Briefly, of the 27 patients with both LSN ≥2.50 and LSPS ≥2.06, 23 (85%) had CSPH. By contrast, none of the 52 patients with LSN values <2.50 had CSPH, regardless of LSPS values.
Fig. 3.
Performance of the combination of LSN and LSPS for the diagnosis of CSPH.
(A) Performance in the intention-to-diagnose cohort and per-protocol cohort. (B) Performance of rule-in and rule-out cut-offs of non-invasive variables (LSN, LSM and LSPS) for predicting CSPH. CSPH, clinically significant portal hypertension; LSM, liver stiffness measurement; LSN, liver surface nodularity; LSPS, LSM-spleen-size-to-platelet ratio score.
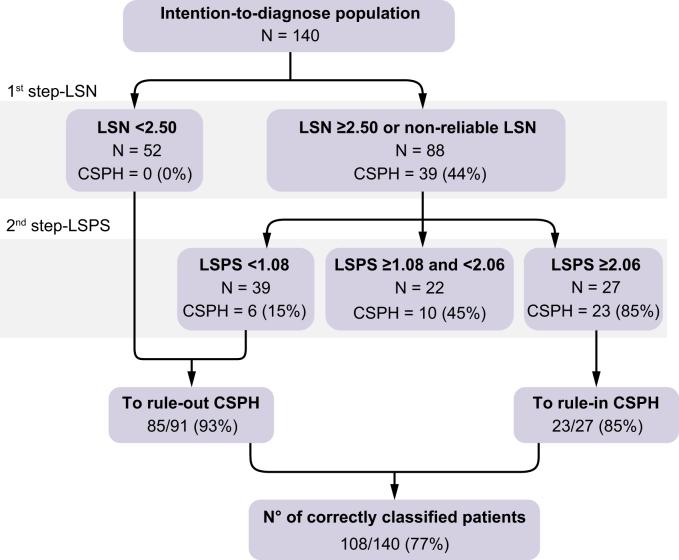
Based on these observations, a 2-step diagnostic algorithm applying first LSN and then LSPS in the subset of 88 patients with either LSN ≥2.5 or an unreliable LSN was designed. In the latter group (n = 88), a LSPS <1.08 ruled-out CSPH in 39 patients with a negative predictive value of 85% (33 patients without CSPH), whereas a LSPS >2.06 ruled in CSPH in 27 patients with a positive predictive value of 85% (23 patients with CSPH). Therefore, applying the LSPS score after the LSN measurement correctly reclassified 56/88 patients (64%). Overall, this 2-step algorithm had an AUROC of 0.874 (95% CI: 0.760–0.949) for the diagnosis of CSPH, and resulted in 108/140 correctly classified patients (77%), with an error of 8% (Fig. 4). Applying this algorithm to the subset of 91/140 patients with HCC based on Milan Criteria correctly classified 65/91 (71%) patients with 10% error (AUROC 0.851; 95%CI: 0.760–0.942). Details of the calibration of this model are provided in the Supplemental Material.
Fig. 4.
Two-step algorithm with LSN as the first step and LSPS as the second step in the intention-to-diagnose cohort to rule in and rule out patients with CSPH.
CSPH, clinically significant portal hypertension; LSN, liver surface nodularity; LSPS, liver stiffness measurement-spleen-size-to-platelet ratio score.
Discussion
The aim of the present study was to compare the diagnostic performance of the quantification of LSN on CT images and LSM measured by TE, either alone or in a derived score, for the diagnosis of CSPH in patients with cirrhosis during the initial preoperative work-up for HCC surgery. Our results showed that the feasibility and diagnostic performance of LSN and LSM are similar. Moreover, the combination of tests correctly classified approximately three-quarters of the patients as with or without CSPH, and had clinically acceptable error rates.
LSN was initially developed as a non-invasive test for the diagnosis and prognosis of cirrhosis17,18 with an excellent reproducibility and interobserver agreement.12 This score reflects the major architectural changes that occur during the development of cirrhosis, including progressive liver fibrosis and the formation of regenerative nodules in the liver.19 Our group extended the field of application and showed the high diagnostic performance of LSN for detecting CSPH.10 The present results further validate the diagnostic performance of LSN. Indeed, using the same rule-in and -out thresholds (i.e. LSN <2.50 and ≥3.10, respectively), we correctly classified 50% of patients with a 9% error, which is comparable to the 58% of correctly classified patients and the 5% error in the initial publication.10
Although our previous study also showed that the performance of LSN was better than numerous other non-invasive tests, it was not better compared with LSM or its derived scores. Several studies have reported that the performance of LSM, measured by TE, is good for the detection of CSPH, with sensitivities and specificities ranging between 88% and 98%, and between 50% and 73%, respectively, when applying cut-off values of ∼21 kPa (19.0–21.8 kPa).[20], [21], [22] By contrast, Vizzutti et al. also showed a strong correlation between the absence of PHT and LSM <13.6 kPa (AUROC 0.99).23
LSPS was initially developed for the prediction of high-risk esophageal varices.24 This simple score, using 3 parameters (LSM, spleen size, and platelet count), was proposed to increase the diagnostic performance of these separate parameters, merging different aspects of the pathogenesis of PHT, including fibrosis, vascular congestion, and PHT-induced splenic sequestration. The performance of this score has been shown to be better than that of LSM alone (AUROC 0.95 vs. 0.88, p <0.001) for the detection of CSPH.25 Berzigotti et al. proposed comprehensive rule-in and -out thresholds for both LSM and LSPS in patients with compensated cirrhosis.8 The diagnostic performance and feasibilities in the present study were similar between LSN and LSM or LSPS. Thus, in practice, we believe that the choice of LSN or LSM/LSPS for the diagnosis of CSPH should be based on the context rather than the diagnostic performance.
CT is not routinely recommended in patients with cirrhosis and, thus, LSM/LSPS is more frequently performed. By contrast, in patients with HCC, cross-sectional imaging, especially CT, is systematically performed and results show that LSN is a valuable, easy-to-use tool to estimate CSPH, which can also be determined retrospectively. Although the population in this study only included patients with HCC before resection, we believe that it is also representative of patients with compensated cirrhosis, allowing our results to be extrapolated to this group.
Hepatic resection in patients with cirrhosis and PHT is associated with significantly increased mortality and morbidity.2,7 Thus, a preoperative evaluation of PHT is recommended, and is included in the preoperative work-up of expert teams.26 Although measurement of HVPG by invasive transjugular catheter is the reference method, numerous other non-invasive tests are available, which could lead to confusion and a nonstandardised approach. The advantage of LSN is that it can be quickly and easily quantified on routine abdominal CT scan and, thus, can be used as a first-line triage test, allowing additional, more specific, examinations in selected patients. Moreover, LSN had excellent negative predictive values in the current study. This justifies our proposal of a 2-step algorithm with LSN as the first step. It suggests that patients with low LSN values (i.e. <2.50) do not require additional examinations to exclude CSPH. Applying LSPS to others (i.e. unreliable LSN or LSN ≥2.50) could help to identify patients with suspected (LSPS ≥1.08) or nearly certain (LSPS ≥2.06) CSPH, which would require further testing with an HVPG measurement. This algorithm had good performance, but appeared better at ruling out than at ruling in CSPH. This should be acknowledged before implementing it in practice. Importantly, results were consistent when focusing on patients with HCC based on Milan Criteria only, reinforcing its clinical value.
LSN measurements were found to be unreliable in 7% of cases, which is similar to previous studies.10,17,18 In particular, the presence of exophytic HCC in the anterior left liver lobe resulted in 5 (4%) unreliable LSN evaluations in our study, but did not affect the LSM measurement. By contrast, LSM values were significantly increased in patients with large HCC (i.e. ≥10 cm) located in the right liver, resulting in a significant overestimation of CSPH, whereas LSN was not affected and correctly classified most of these patients. HCC ≥10 cm in the right liver lobe should be added to the list of potential causes of false positives of LSM. This suggests that an LSM-based strategy is unreliable in patients with such large right-sided HCC. Thus, LSN should probably be favoured in these patients.
This single-centre study has certain limitations. First and foremost, its retrospective design comes with possible selection bias. For instance, LSM values were in the lower bound of reported values in patients with cirrhosis, because we included patients with compensated cirrhosis undergoing hepatic resection only. We did not analyse the predictive role of the LSN score for PHT-related complications, such as oesophageal varices bleeding or death and postoperative morbidity or mortality, because this was beyond the scope of the study. Noticeably, our group recently showed that the preoperative LSN value was associated with severe complications, postoperative liver failure, and clinically significant postoperative liver failure in patients with HCC undergoing resection.27 In addition, there was no external validation cohort. Nevertheless, our study further validates the results of Sartoris et al.10 Finally, no sample size calculation was performed.
In conclusion, LSN determined on routine CT scan might be useful for the preoperative detection of CSPH in patients with HCC and compensated cirrhosis. A combination of LSN and LSPS could improve the non-invasive classification of these patients. LSN is probably more relevant in clinical practice as a first-line triage test.
Financial support
The authors have no financial support to declare.
Authors' contributions
AS and RS: acquisition, analysis, and interpretation of data, drafting of the manuscript; PER: study concept and design; acquisition, analysis, and interpretation of data; critical revision of manuscript; VG and EG: acquisition, analysis, and interpretation of data; FC and VV: study concept and design; interpretation of data; drafting and critical revision of manuscript; MR: study concept and design; analysis and interpretation of data; drafting and critical revision of manuscript; MB, LC, FD, DV and OS: critical revision of manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Lucile Dehecq, Cécilia de Freitas, Anissa Messali, Maryse Moinat, Maryvonne Quesseveur, Djalila Rezigue, Marie Santin, Thomas Trion, and Kamal Zekrini for their help in collecting the data and/or in performing LSM.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100147.
Supplementary data
References
- 1.Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackl C., Schlitt H.J., Renner P., Lang S.A. Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol. 2016;22:2725–2735. doi: 10.3748/wjg.v22.i9.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galle P.R., Forner A., Llovet J.M., Mazzaferro V., Piscaglia F., Raoul J.L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 5.Augustin S., Millan L., Gonzalez A., Martell M., Gelabert A., Segarra A. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol. 2014;60:561–569. doi: 10.1016/j.jhep.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Chong C.C., Wong G.L., Chan A.W., Wong V.W., Fong A.K., Cheung Y.S. Liver stiffness measurement predicts high-grade post-hepatectomy liver failure: a prospective cohort study. J Gastroenterol Hepatol. 2017;32:506–514. doi: 10.1111/jgh.13503. [DOI] [PubMed] [Google Scholar]
- 7.Berzigotti A., Reig M., Abraldes J.G., Bosch J., Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis; a systematic review and meta-analysis. Hepatology. 2015;61:526–536. doi: 10.1002/hep.27431. [DOI] [PubMed] [Google Scholar]
- 8.Berzigotti A., Seijo S., Arena U., Abraldes J.G., Vizzutti F., García-Pagán J.C. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:2–111. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Schwabl P., Bota S., Salzl P., Mandorfer M., Payer B.A., Ferlitsch A. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int. 2015;35:381–390. doi: 10.1111/liv.12623. [DOI] [PubMed] [Google Scholar]
- 10.Sartoris R., Rautou P.E., Elkrief L., Pollorsi G., Durand F., Valla D. Quantification of liver surface nodularity at CT: utility for detection of portal hypertension. Radiology. 2018;289:698–707. doi: 10.1148/radiol.2018181131. [DOI] [PubMed] [Google Scholar]
- 11.De Vos N., Sartoris R., Cauchy F., Rautou P.E., Vilgrain V., Ronot M. Performance of liver surface nodularity quantification for the diagnosis of portal hypertension in patients with cirrhosis: comparison between MRI with hepatobiliary phase sequences and CT. Abdom Radiol (NY) 2020;45:365–372. doi: 10.1007/s00261-019-02355-y. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt P.J., Malecki K., Kloke J., Lubner M.G. Accuracy of liver surface nodularity quantification on MDCT as a noninvasive biomarker for staging hepatic fibrosis. Am J Roentgenol. 2016;207:1194–1199. doi: 10.2214/AJR.16.16514. [DOI] [PubMed] [Google Scholar]
- 13.Payancé A., Silva-Junior G., Bissonnette J., Tanguy M.5, Pasquet B., Garcia-Pagan J.C. Hepatocyte microvesicle levels improve prediction of mortality in patients with cirrhosis. Hepatology. 2018;68:1508–1518. doi: 10.1002/hep.29903. [DOI] [PubMed] [Google Scholar]
- 14.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 15.Leening M.J.G., Vedder M.M., Witteman J.C.M., Pencina M.J., Steyerberg E.W. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160:122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- 16.Song J., Ma Z., Huang J., Liu S., Luo Y., Lu Q. Comparison of three cut-offs to diagnose clinically significant portal hypertension by liver stiffness in chronic viral liver diseases: a meta-analysis. Eur Radiol. 2018;28:5221–5230. doi: 10.1007/s00330-018-5478-z. [DOI] [PubMed] [Google Scholar]
- 17.Smith A.D., Zand K.A., Florez E., Sirous R., Shlapak D., Souza F. Liver surface nodularity score allows prediction of cirrhosis decompensation and death. Radiology. 2017;283:711–722. doi: 10.1148/radiol.2016160799. [DOI] [PubMed] [Google Scholar]
- 18.Smith A.D., Branch C.R., Zand K., Subramony C., Zhang H., Thaggard K. Liver surface nodularity quantification from routine CT images as a biomarker for detection and evaluation of cirrhosis. Radiology. 2016;280:771–781. doi: 10.1148/radiol.2016151542. [DOI] [PubMed] [Google Scholar]
- 19.Sethasine S., Jain D., Groszmann R.J., Garcia-Tsao G. Quantitative histological-hemodynamic correlations in cirrhosis. Hepatology. 2012;55:1146–1153. doi: 10.1002/hep.24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bureau C., Metivier S., Peron J.M., Selves J., Robic M.A., Gourraud P.A. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261–1268. doi: 10.1111/j.1365-2036.2008.03701.x. [DOI] [PubMed] [Google Scholar]
- 21.Reiberger T., Ferlitsch A., Payer B.A., Pinter M., Schwabl P., Stiff J. Noninvasive screening for liver fibrosis and portal hypertension by transient elastography: a large single center experience. Wien Klin Wochenschr. 2012;124:395–402. doi: 10.1007/s00508-012-0190-5. [DOI] [PubMed] [Google Scholar]
- 22.Kitson M.T., Roberts S.K., Colman J.C., Paul E., Button P., Kemp W. Liver stiffness and the prediction of clinically significant portal hypertension and portal hypertensive complications. Scand J Gastroenterol. 2015;50:462–469. doi: 10.3109/00365521.2014.964758. [DOI] [PubMed] [Google Scholar]
- 23.Vizzutti F., Arena U., Romanelli R.G., Rega L., Foschi M., Colagrande S. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 24.Kim B.K., Han K.H., Park J.Y., Ahn S.H., Kim J.K., Paik Y.H. A liver stiffness measurement-based noninvasive prediction model for high-risk esophageal varices in B-viral cirrhosis. Am J Gastroenterol. 2010;105:1382–1390. doi: 10.1038/ajg.2009.750. [DOI] [PubMed] [Google Scholar]
- 25.Shi K.Q., Fan Y.C., Pan Z.Z., Lin X.F., Liu W.Y., Chen Y.P. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013;33:62–71. doi: 10.1111/liv.12003. [DOI] [PubMed] [Google Scholar]
- 26.Citterio D., Facciorusso A., Sposito C., Rota R., Bhoori S., Mazzaferro V. Hierarchic interaction of factors associated with liver decompensation after resection for hepatocellular carcinoma. JAMA Surg. 2016;151:846–853. doi: 10.1001/jamasurg.2016.1121. [DOI] [PubMed] [Google Scholar]
- 27.Hobeika C., Cauchy F., Sartoris R., Beaufrère A., Yoh T., Vilgrain V. Relevance of liver surface nodularity for preoperative risk assessment in patients with resectable hepatocellular carcinoma. Br J Surg. 2020;107:878–888. doi: 10.1002/bjs.11511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.