Abstract
Objective
To describe the clinical and epidemiological characteristics of hospitalized children with multisystem inflammatory syndrome in children (MIS-C) in Santiago, Chile.
Methods
This was an observational study of children with MIS-C (May 1 to June 24, 2020), in three pediatric hospitals in Santiago. Demographic characteristics and epidemiological data, medical history, laboratory tests, cardiology evaluations, treatment, and clinical outcomes were analyzed.
Results
Twenty-seven patients were admitted (median age 6, range 0–14 years). Sixteen of the 27 (59%) required intensive care unit admission; there were no deaths. Seventy-four percent had no comorbidities, and the median number of days of symptoms before admission was 4 (range 2–9 days). Gastrointestinal symptoms were the most frequent, and inflammatory markers were increased at admission. A recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was detected in 82% of cases. The severe group showed significantly lower hemoglobin and albumin levels, decreased platelet counts, and higher d-dimer during disease evolution. Echocardiography showed abnormalities (myocardial, pericardial, or coronary) in 12 patients (46%) during their hospital stay. Anti-inflammatory treatment (immunoglobulin and/or corticosteroids) was prescribed in 24 patients. MIS-C appeared in clusters weeks after the peak of SARS-CoV-2 cases, especially in the most vulnerable areas of Santiago.
Conclusions
This study describes the first series (n = 27) of children with MIS-C in a Latin American country, showing favorable clinical outcomes. Education and alerts are required for clinical teams to establish an early diagnosis and prompt treatment.
Keyword: MIS-C multisystem inflammatory syndrome in children, SARS-CoV-2, COVID-19
Introduction
A novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was described in December 2019 in China (Zhu et al., 2020). This virus has spread rapidly to almost every country in the world, causing more than 12 million confirmed cases of coronavirus disease 2019 (COVID-19) and over 500,000 deaths as of the first week of July 2020 (Coronavirus Resource Center, 2020). Severe pneumonia with acute respiratory failure is the most common adverse outcome of SARS-CoV-2 infection in adults (Wu and McGoogan, 2020). Children are less infected by the virus compared to other age groups, and most of them are asymptomatic or exhibit mild symptoms (Castagnoli et al., 2020, Dong et al., 2020, Xia et al., 2020).
Recently, pediatricians from Europe (Riphagen et al., 2020, Verdoni et al., 2020, Whittaker et al., 2020) and North America (Kaushik et al., 2020) placed a warning related to hospitalizations of critically ill children presenting with circulatory shock and a hyperinflammatory state, sharing features with other pediatric inflammatory conditions including Kawasaki disease (KD), toxic shock syndrome (TSS), bacterial sepsis with gastrointestinal symptoms, and macrophage activation syndrome (MAS). This experience prompted an alert and guidelines from the Royal College of Paediatrics and Child Health (United Kingdom) (RCPCH, 2020) on May 2020, referring to this entity as the “paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS)”. On May 14, the United States Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) named the entity ‘multisystem inflammatory syndrome in Children’ (MIS-C) and described new case definitions (CDC, 2020, WHO, 2020). Since then, several groups have reported series of similarly affected children in different regions, albeit not yet from Latin America (Cheung et al., 2020, Feldstein et al., 2020, Licciardi et al., 2020).
Chile is a high-income Latin American country (according to the current World Bank classification 2020) with a population of near 19 million inhabitants. As of July 4, 2020, a total of 330,183 COVID-19 cases had been reported, representing the sixth highest incidence rate worldwide and third highest incidence rate in South America (Coronavirus Resource Center, 2020), with a case fatality rate of 2.2%. Most cases (77%) have been reported in the Metropolitan Region (population of approximately eight million), where Santiago is situated, with 4.8% involving children younger than 15 years (Ministry of Health (MINSAL), Chile, 2020). Chilean children, similar to children in other regions, have had milder symptoms and have been less likely to be hospitalized when compared to adults, representing less than 5% of the total hospitalization rate (MINSAL, 2020). Total cases increased to over 4000 per day during May (May 20), and similar to other latitudes (Hennon et al., 2020, Verdoni et al., 2020, Whittaker et al., 2020), pediatricians began to report severe cases of a hyperinflammatory syndrome (MIS-C) in pediatric hospitals of Santiago 2–4 weeks after the peak of acute infections.
The aim of this study was to describe the epidemiological and clinical characteristics of hospitalized children who met the criteria for MIS-C in Santiago, Chile.
Patients and methods
Overall study design
This was an observational, retrospective and prospective study including children less than 15 years of age, with a diagnosis that met the MIS-C definition criteria (Centers for Disease Control and Prevention, USA) (CDC, 2020) from May 1 to June 24, 2020, in the three main public pediatric reference hospitals in Santiago, Chile: Hospital Roberto del Río (HRR), Hospital Exequiel González Cortés (HEGC), and Hospital Luis Calvo Mackenna (HLCM). HRR receives patients <15 years of age from the north area, HEGC from the south, and HLCM from the eastern area of Santiago (referral population of 160,746, 234,738, and 91,623 patients, respectively). The study was approved by the institutional review board of each institution and by the Ethics Committee for Clinical Investigation in Humans of the Faculty of Medicine, Universidad de Chile.
Signed informed consent was obtained from the parents and informed assent from children ≥14 years old prior to inclusion in the study. The data collection was performed and managed by a researcher at each center who had previously been trained in data entry, using REDCap electronic data capture tools hosted at the Faculty of Medicine, Universidad de Chile (Harris et al., 2009, Harris et al., 2019). Variables recorded from admission to discharge were extracted from the clinical records assuring anonymity and confidentiality. These included (1) demographic characteristics: age, sex, nationality; (2) medical history of comorbidities, e.g. chronic heart or respiratory disease, primary or secondary immunosuppression, diabetes, hypertension, allergies, obesity, and cancer. Obesity was defined according to the WHO child growth standards, using the weight/age ratio for infants and with the body mass index (BMI) for children and teenagers (at or above the 95th percentile according to age and sex) (Whitlock et al., 2005). (3) Physical examination: symptoms/signs at admission, including vital signs, weight/height, fever, cough, runny nose, dyspnea, headache, sore throat, vomiting, diarrhea, and myalgia; (4) laboratory tests: blood cell counts, C-reactive protein (CRP), procalcitonin, liver function tests, serum creatinine, d-dimer, ferritin, blood cultures, and nasopharyngeal sample for viral testing. Viral testing included RT-PCR for SARS-CoV-2 and direct fluorescent antibody testing and/or molecular methods for respiratory viruses. Rapid serology tests to detect IgM/IgG antibodies against SARS-CoV-2 were performed to confirm the infection (OnSite COVID-19 IgG/IgM Rapid Test, CTK Biotech, Standard Q COVID-19 IgM/IgG Duo, SD Biosensor, COVID-19 IgG/IgM Rapid Test Cassette, Zhejiang Orient Gene Biotech). (5) Imaging, including chest X-ray, chest computed tomography scan, and echocardiographic examination; (6) clinical outcome variables: length of hospitalization, days of oxygen requirement, use of antimicrobials, use of corticosteroids, intravenous gamma globulin, acetylsalicylic acid, or anticoagulant treatment, admission to the pediatric intensive care unit (PICU), and mortality.
Case definition
The MIS-C definition provided by the US CDC was used in this study, which considers the following criteria: (1) an individual aged <21 years presenting with fever (temperature ≥38.0 °C for ≥24 h, or report of subjective fever lasting ≥24 h), (2) laboratory evidence of inflammation, including, but not limited to, one or more of the following: an elevated CRP, erythrocyte sedimentation rate (ESR), fibrinogen, procalcitonin, d-dimer, ferritin, lactic acid dehydrogenase (LDH), or interleukin 6 (IL-6), elevated neutrophils, reduced lymphocytes, and low albumin, (3) evidence of a clinically severe illness requiring hospitalization, with multisystem (two or more) organ involvement (cardiac, renal, respiratory, hematological, gastrointestinal, dermatological, or neurological), (4) a lack of an alternative plausible diagnoses, and (5) positivity for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test, or exposure to a suspected or confirmed COVID-19 case within the 4 weeks prior to the onset of symptoms.
Statistical analysis
Demographic and clinical characteristics were described using frequencies or percentages for categorical variables and measures of central trends and dispersion for continuous variables. Clinical characteristics and laboratory findings were compared according to the requirement for PICU admission. Comparisons were performed using the Chi–square test or Mann–Whitney rank sum test according to the data distribution. Statistical analyses were performed using R package and Stata 12 software (StataCorp, College Station, TX, USA), considering a p-value of <0.05 as statistically significant.
Results
Clinical variables at admission
A total of 220 pediatric patients with positive SARS-CoV-2 PCR were admitted to the hospitals included in this study between May 8 and June 24, 2020 (HRR = 92, HLCM = 62, HEGC = 66). During this period, 27 patients met the MIS-C definition (CDC, 2020): 16 were hospitalized in HRR, nine in HEGC, and two in HLCM (Table 1 ). According to the national weekly survey, which includes 25 Chilean hospitals and clinics, a total of 42 cases of MIS-C were reported up until June 28. Thus, the 27 cases included in this study represented 64% of the national cases (Torres, 2020). The median age of these patients was 6 years, and 14 of the 27 were male (52%). The nationality of the parents was Chilean in 85% of cases. Venezuelan (n = 2), Haitian (n = 1), and Peruvian (n = 1) parents were also registered. Overall, 26% had comorbidities including overweight or obesity (n = 4), asthma (n = 1), primary immunodeficiency, GATA 3 deficiency (n = 1), prematurity, and gestational age of 33 weeks (n = 1).
Table 1.
Demographic and clinical characteristics, and laboratory parameters of Chilean children with MIS-C.
| All patients | Ward unit | ICU | |
|---|---|---|---|
| n = 27 | n = 11 | n = 16 | |
| Demographic and clinical characteristics at admission, n (%) | |||
| Age in yearsa | 6 (0–14) | 6 (0–13) | 6.5 (0–14) |
| Male sex | 14 (52) | 5 (45) | 9 (56) |
| No comorbidities | 20 (74) | 8 (73) | 12 (75) |
| Days of symptoms at admissiona | 4 (2–9) | 5 (2–7) | 2 (2–9) |
| Fever | 27 (100) | 11 (100) | 16 (100) |
| Abdominal pain | 17 (63) | 7 (64) | 10 (62) |
| Diarrhea | 17 (63) | 6 (55) | 11 (69) |
| Vomiting | 13 (48) | 6 (55) | 7 (44) |
| Rash | 14 (52) | 5 (45) | 9 (56) |
| Conjunctival injection | 13 (48) | 6 (55) | 7 (44) |
| Oral mucosal changes | 11 (41) | 4 (36) | 7 (44) |
| Cough | 7 (26) | 3 (27) | 4 (25) |
| Peripheral extremity changes | 7 (26) | 3 (27) | 4 (25) |
| SARS-CoV-2 test results, n (%) | |||
| Positive nasopharyngeal RT-PCR | 14 (52) | 7 (64) | 7 (44) |
| Positive serology | 10 (77)b | 2 (100) | 8 (73) |
| History of COVID-19 (+) contact | 9 (33) | 5 (45) | 4 (25) |
| Laboratory tests at admission, median (IQR) | |||
| Hemoglobin (g/dl) | 11.5 (10.7–12.7) | 11.6 (10.3–12.7) | 11.4 (10.8–12.7) |
| WBC count (cells x 109/L) | 10.3 (7.5–15.1) | 10.5 (7.5–19.3) | 10.3 (7.4–13.6) |
| Absolute lymphocyte count (cells x 109/L) | 1.3 (0.65–1.74) | 1.5 (0.76–4.26) | 0.903 (0.57–1.65) |
| Platelets (x 109/L3) | 182 (114–240) | 202 (149–281) | 150 (81–224) |
| C-reactive protein (mg/l) | 169 (88–301) | 126 (56–202) | 184 (137–306) |
| D-dimer (μg/mL) | 1.77 (1.15–3.17) | 1.26 (0.62–2.16) | 2.35 (1.42–4) |
| Albumin (g/l)* | 3.1 (2.7–3.6) | 3.5 (3.3–3.9) | 2.9 (2.6–3.2) |
| Ferritin (ng/mL)c | 316 (111–542) | 221 (158–622) | 431 (111–542) |
COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range (p25-p75); MIS-C, multisystem inflammatory syndrome in children; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cell.
Statistically significant, p < 0.05.
Median (range).
Serology test performed on 13 patients (two from the basic ward unit and 11 from the pediatric ICU).
Ferritin at admission in 10 patients.
Fever was the first sign in 22 cases (82%), and the median length of symptoms before admission was 4 days (range 2–9 days). Overall, in addition to fever, which is included in the definition of MIS-C, the most common clinical presentations were gastrointestinal disorders (diarrhea (63%) and abdominal pain (63%)), followed by clinical features of KD, which were present in 18 patients. However, although 67% of patients presented at least one KD symptom, only four cases met the complete KD criteria. Of the 18 patients presenting KD clinical features, eight (44%) required vasoactive drugs, the same proportion as in patients with no KD signs or symptoms (4/9, 44%). A similar clinical presentation was observed for patients who required admission to the PICU when compared to those who stayed in the basic ward units.
Laboratory tests to confirm SARS-CoV-2 infection included RT-PCR at admission in all patients, and serology in 13 patients. Infection was confirmed in 22 patients (82%): 12 with positive RT-PCR, eight with positive serology, and two with positive RT-PCR and serology. Of the five patients with a negative SARS-CoV-2 result, four had been in close contact with confirmed COVID-19 cases and one with suspected cases (family). After clinical anamnesis, nine patients (33%) mentioned an epidemiological link with a COVID-19 patient.
At admission, laboratory parameters were compatible with acute inflammation in most children, with increased d-dimer (>1.0 μg/mL) and CRP (>100 mg/l) in 16/20 and 20/27 individuals, respectively. Also, lymphopenia (absolute lymphocyte count <1.5 cells/×109/l.l) was detected in 16/27 patients. As shown in Table 1, after comparisons between cases according to the requirement for PICU admission, significantly lower albumin was found in the PICU patients.
Clinical course and treatment
More than half of the children (59%) required admission to the PICU. In these cases, the disease was characterized by lower hemoglobin and albumin levels and platelet counts. Also, higher d-dimer levels were found in patients who required PICU admission (Table 2 ).
Table 2.
Laboratory findings, treatment throughout hospitalization, and outcomes of Chilean children with MIS-C.
| All patients | Ward unit | ICU | |
|---|---|---|---|
| n = 27 | n = 11 | n = 16 | |
| Laboratory resultsa, median (IQR) | |||
| Hemoglobin (g/dl)* | 9.4 (8.4–10.9) | 10.9 (9.8–12.5) | 8.7 (8.2–9.5) |
| WBC count (cells x 109/L) | 12.4 (5.8–19.8) | 12.4 (5.8–19.3) | 13.5 (5.7–20.3) |
| Absolute lymphocyte count (cells x 109/L) | 1.31 (0.64–1.83) | 1.66 (0.64–2.29) | 0.91 (0.59–1.63) |
| Platelets (x 109/L3)* | 153 (123–240) | 202 (150–341) | 130 (75–160) |
| C-reactive protein (mg/l) | 173 (127–275) | 132 (60–202) | 227 (135–301) |
| D-dimer (μg/mL)* | 3.61 (1.42–5.0) | 1.93 (0.62–2.16) | 4.08 (3.61–5.25) |
| Albumin (g/l)* | 2.3 (2.1–3.2) | 3.3 (2.9–3.6) | 2.2 (2–2.3) |
| Ferritin (ng/mL) | 309 (156–696) | 230 (156–298) | 542 (135–835) |
| Treatment, n (%) | |||
| Antibiotic treatment* | 24 (89) | 8 (73) | 16 (100) |
| Acetylsalicylic acid* | 17 (63) | 4 (36) | 13 (81) |
| Anticoagulation therapy (LMWH) | 18 (67) | 6 (55) | 12 (75) |
| Intravenous immunoglobulin* | 19 (70) | 5 (45) | 14 (87) |
| Systemic corticosteroids* | 17 (63) | 4 (36) | 13 (81) |
| Clinical outcome, n (%) | |||
| Days of hospitalizationb, * | 9 (6–13) | 6 (4–9) | 12 (11–17) |
| Oxygen* | 13 (48) | 1 (9) | 12 (75) |
| Invasive mechanical ventilation* | 12 (44) | 0 (0) | 12 (75) |
| Vasoactive drugs* | 12 (44) | 0 (0) | 12 (75) |
| Death | 0 (0) | 0 (0) | 0 (0) |
ICU, intensive care unit; IQR, interquartile range (p25-p75); LMWH, low molecular weight heparin; MIS-C, multisystem inflammatory syndrome in children; WBC, white blood cell.
Statistically significant, p < 0.05.
Includes the most abnormal laboratory test value.
Median (IQR).
Antimicrobials were prescribed for 73% of patients treated in the hospital ward and for 100% of patients treated in the PICU (Table 2). Anticoagulation therapy was completed in 18 patients, of whom 16 received a prophylaxis regimen and two received treatment doses. Specific anti-inflammatory treatment (including intravenous immunoglobulin (IGIV) and/or systemic corticosteroids) was given to 24 patients: 12 children received both (IVIG + corticoids), seven children only IGIV, and five completed only corticosteroid treatment. Of the 17 patients who received corticosteroids, 15 were treated with methylprednisolone (15/17), one with dexamethasone, and one received hydrocortisone. Additionally, a cytokine storm syndrome was suspected in two patients, and an IL-6 inhibitor (tocilizumab) was administered.
The median duration of hospitalization was 12 days for PICU patients and 6 days for children treated in the hospital ward; three of these patients remained hospitalized at the time of this report. Oxygen support was required in 13 patients (all but one in the PICU), and mechanical ventilation was required in 12 cases. In the latter, the duration of mechanical ventilation was a median of 5 days, ranging from 2 to 6 days. Also, 12 patients required inotropic support in the PICU. No deaths occurred in this group of patients (Table 2).
Echocardiographic evaluation
During the first day following admission, 26 patients were evaluated by echocardiography. Normal findings were reported in 18 patients, but myocardial dysfunction (e.g., left ventricular systolic function with an ejection fraction below 60%, diastolic dysfunction, or regional wall motion abnormalities) and/or coronary artery abnormalities (e.g., refringence or increased z-score) were detected in eight patients (31%) (Table 3 ). Consecutive echocardiography evaluations were performed in all patients, and abnormalities were detected in four additional cases. Thus, a total of 12 patients (12/26; 46%) presented pericardial effusion (3/26; 11%) and/or myocardial dysfunction (4/26; 15%) and/or coronary artery abnormalities (5/12; 19%): nine were patients who required PICU admission and three were in the milder disease group.
Table 3.
Echocardiography findings at admission and throughout hospitalization of 27 Chilean children with MIS-C.
| n (%) | |
|---|---|
| Echocardiography at admissiona | 26 (96) |
| Normal | 18 (69) |
| Myocardial dysfunction | 4 (15) |
| Coronary abnormalities | 3 (12) |
| Myocardial and coronary abnormalities | 1 (4) |
| Abnormal echocardiography throughout hospitalization | 12 (46) |
MIS-C, multisystem inflammatory syndrome in children.
Echocardiography during the first 24 h from admission.
Epidemiological characteristics of the SARS-CoV-2 outbreak and MIS-C case emergence
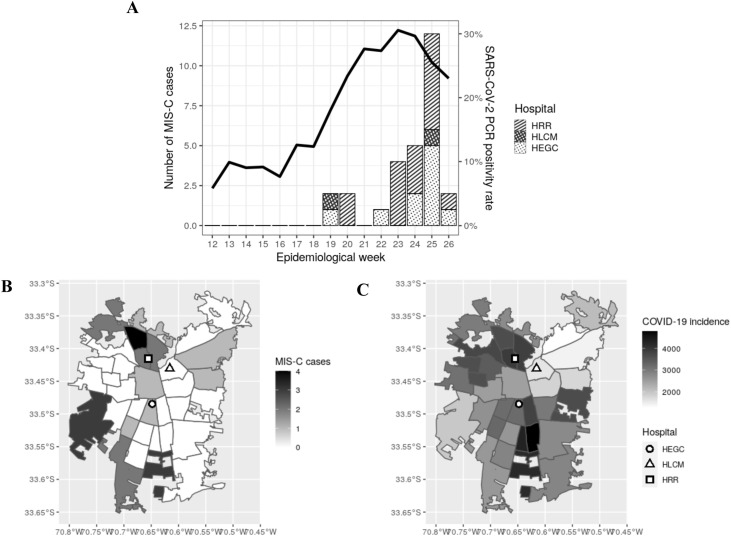
As shown in Figure 1 A, most of the cases (23/27) occurred after epidemiological week 22 (after May 30, 2020), with a similar increase slope to the rise in RT-PCR positivity rate that occurred 4 weeks before. Thus, the positivity of SARS-CoV-2 tests increased from 12% to nearly 30% between weeks 18 and 21 and remained higher than 25% for 4 weeks. Moreover, 25 of the 27 MIS-C cases were children from the northern and southern areas of Santiago, who were hospitalized in HRR and HEGC, respectively (Figure 1B). This was coincident with the higher cumulative case rates observed in the northern and southern areas of the Metropolitan Region, as shown in Figure 1C.
Figure 1.
XXX.
Discussion
We report one of the first series of patients from a Latin American country with MIS-C, of whom 59% required admission to a PICU, with no deaths. MIS-C cases occurred approximately 1 month after the peak of COVID-19, similar to reports in the northern hemisphere. In France (Belot et al., 2020), a series of 79 confirmed cases of MIS-C were reported 4–5 weeks after the peak of COVID-19 cases, as observed in Santiago, Chile. In addition, two main clusters were observed, one in the north and the other in the south of Santiago, areas with the most vulnerable populations. The first report of a series of cases of multisystemic inflammatory syndrome in Latin America came from Brazil (Prata-Barbosa et al., 2020). Of the 10 children, none died. They had a length of stay in the PICU of 5.5 days; only two of them were treated with corticosteroids and only one required invasive mechanical ventilation (Prata-Barbosa et al., 2020). Another study that included some Latin American countries (CAKE study) reported preliminary results in 17 children admitted to the PICU in Chile and Colombia (González-Dambrauskas et al., 2020).
The children in the series presented herein had a relatively late consultation with the emergency department after the onset of MIS-C symptoms (median of 4 days), which reinforces the need to educate both parents and health teams on early diagnosis of the disease. The median duration of hospitalization was 9 days (interquartile range 6–13.5 days) and the most frequent clinical presentation was fever, abdominal pain, and features compatible with incomplete KD, similar to reports in Europe (Toubiana et al., 2020) and the United States (Feldstein et al., 2020). There were no deaths in our series, possibly due to the limited number of children and/or the high index of suspicion within the health team due to MIS-C alerts coming from the northern hemisphere. Likewise, in our cases, 59% of the children required admission to the PICU, slightly lower than reported in the USA (Feldstein et al., 2020) and France (Belot et al., 2020, Toubiana et al., 2020), which showed a range of 67%–81%, respectively. The median age of 6 years in our population is similar to other reports (Belot et al., 2020, Whittaker et al., 2020), as is the occurrence of gastrointestinal symptoms, such as diarrhea and abdominal pain, which have been most common, in addition to fever (Feldstein et al., 2020, Whittaker et al., 2020).
MIS-C is increasing during the winter months in Chile (MINSAL, 2020), and the possible role of co-circulation of other respiratory viruses (Kim et al., 2020) is unknown. To date, more than 60 cases of MIS-C have been reported in Chile, and several are ongoing, which may be reflecting an increase in episodes; incidence rates cannot yet be determined.
MIS-C is an emerging disease (Verdoni et al., 2020) of unknown long-term impact and sequelae, especially related to coronary and or neurological disorders. In addition, suitable biomarkers for better management and monitoring of the disease are currently unknown. d-dimer, troponin, and IL-6 in serum can increase in MIS-C as in other non-infectious and infectious diseases, and specific cut-off values for MIS-C are unknown. Early suspicion, especially in developing countries, is critical for prompt and timely care to achieve a favorable clinical outcome. In Latin America, ethnicity might be determining higher incidence rates of MIS-C. In the USA (Feldstein et al., 2020, Godfred et al., 2020), 31–40% of cases were reported to have occurred in children of Hispanic ethnicity, and in France (Belot et al., 2020), 11/21 (57%) MIS-C cases were found to have African ancestry. According to Belot et al., based on 108 children with confirmed, probable, and possible cases, MIS-C incidence rates would be less than two per 10,000 children. In the echocardiography study, a cardiac compromise was found in 12 patients, and 5/27 (18%) children presented coronary artery anomalies, slightly higher than the rate reported in the US series, which described these findings in 8–14% (Feldstein et al., 2020, Whittaker et al., 2020).
Most children in our series received IVIG and corticosteroids, with a relatively rapid positive response observed. Similar responses have been reported in France (universal use of IVIG and use of corticosteroids in 48%) and in the USA (use of IVIG in 77% and use of corticosteroids in 49%) (Feldstein et al., 2020, Toubiana et al., 2020). Only three of our patients (11%) received a second dose of IVIG and an increase in steroid dose. In other series, tocilizumab has been used (Kaushik et al., 2020, Nakra et al., 2020), while only two children in our series received this drug.
This study has limitations. We included roughly half of the cases reported to date in Chile, and several remain hospitalized (and thus lack a definite outcome) or are in the process of data collection. Furthermore, sequelae cannot be established, as long-term follow-up with pediatric, cardiac, and neurological evaluations are needed. Underreporting is possible, as reporting of MIS-C is not mandatory and there are no active or passive surveillance strategies. MIS-C can be seen in children and adolescents. In the present study, only children up to 14 years of age were included, since 15 years is the age limit for hospitalization in the three pediatric hospitals included in the study. Thus, cases that occurred in older adolescents were not considered. We mainly included an echocardiogram to assess cardiac involvement. Data on cardiac biomarkers such as troponin and B-type natriuretic peptide (BNP) were available for only a few children, as well as the study of possible arrhythmias. It would have been interesting to have a more extensive cardiology study. Serial assessment for the detection of SARS-CoV-2 and the immune response to SARS-CoV-2 (IgM, IgG) would have been desirable in all cases; however, this was not performed in all patients, although it was done at patient admission. Finally, with the number of cases in this series, we cannot propose treatment recommendations for MIS-C.
In conclusion, we describe the first series (n = 27) of children with MIS-C in a Latin American country, occurring during the autumn–winter season several weeks after the peak of SARS-CoV-2 cases. Episodes tended to occur within geographic clusters in the urban area of Santiago. Most children (60%) required PICU admission, and all had a favorable clinical outcome. During this pandemic, it will be relevant to emphasize to health teams the need for early diagnosis and timely management of MIS-C.
Funding source
The authors declare no funding source for this study.
Ethical approval
This study was approved by the institutional review boards of each institution and by the Ethics Committee for Clinical Investigation in Humans of the Faculty of Medicine, Universidad de Chile.
Conflict of interest
None declared.
Acknowledgements
We thank all of the “COVID-19 in pediatric patients” group, who have been alert to MIS-C cases in the participating hospitals: Dr Natalia Conca, Dr Cecilia Castillo, Dr Sofia Canals, Dr Karin Osorio, Dr Pietro Pietroboni, Dr Yenis Labraña, Dr M. Luisa Espinoza, and Dr Valentina Gutierrez. We are also grateful to Dr Miguel O’Ryan for his insightful advice.
References
- Torres J.P. (2020). No Title [Internet]. Informe COVID-19 Pediatría. Available from: https://virusresp.xyz/covid/.
- Belot A., Antona D., Renolleau S., Javouhey E., Hentgen V., Angoulvant F. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22):1–6. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020:2. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- CDC . 2020. No Title [Internet]. Health Alert Network (HAN): Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19)https://emergency.cdc.gov/han/2020/han00432.asp Available from: [Google Scholar]
- Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020:16–18. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Resource Center J.H.U. 2020. No Title [Internet]. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. Available from: https://coronavirus.jhu.edu/map.html. [Google Scholar]
- Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. http://www.ncbi.nlm.nih.gov/pubmed/32179660 [DOI] [Google Scholar]
- Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020:1–13. doi: 10.1056/NEJMoa2021680. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32598831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfred S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Dambrauskas S., Vásquez-Hoyos P., Camporesi A., Díaz-Rubio F., Piñeres-Olave B.E., Fernández-Sarmiento J. Pediatric critical care and COVID-19. Pediatrics. 2020;146(3) doi: 10.1542/peds.2020-1766. [DOI] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., Neal L.O. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019:1–24. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennon T.R., Penque M.D., Abdul-Aziz R., Alibrahim O.S., McGreevy M.B., Prout A.J. COVID-19 associated Multisystem Inflammatory Syndrome in Children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol. 2020:57. doi: 10.1016/j.ppedcard.2020.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J Pediatr. 2020:2–7. doi: 10.1016/j.jpeds.2020.06.045. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi F., Pruccoli G., Denina M., Parodi E., Taglietto M., Rosati S. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020 doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- MINSAL D de E. 2020. Informe Epidemiologico N°31 Enfermedad por SARS-CoV-2 (COVID-19) CHILE 05-07-2020. [Google Scholar]
- Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata-Barbosa A., Lima-Setta F., Santos G.R. Dos, Lanziotti V.S., de Castro R.E.V., de Souza D.C. Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J Pediatr (Rio J) 2020 doi: 10.1016/j.jped.2020.07.002. S0021-7557(20)30192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCPCH . 2020. No Title [Internet]. RCPCH Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated With COVID-19. Available from: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19. [Google Scholar]
- Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;6736(20):2019–2020. doi: 10.1016/S0140-6736(20)31094-1. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32493739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock E.P., Williams S.B., Gold R., Smith P.R., Shipman S.A. Screening and interventions for childhood overweight: a summary of evidence for the US Preventive Services Task Force. Pediatrics. 2005;116(1) doi: 10.1542/peds.2005-0242. [DOI] [PubMed] [Google Scholar]
- Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. No Title [Internet]. World Health Organization Scientific Brief: Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19. Available from: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xia W., Shao J., Guo Y., Peng X., Li Z., Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol [Internet] 2020;(February):1–6. doi: 10.1002/ppul.24718. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]