Abstract
Background
Broad-spectrum antibiotics are commonly prescribed in critically ill patients. While it is commonly believed that only patients with impaired renal function need dose adjustment, augmented renal clearance (ARC) is a phenomenon that warrants dose adjustment as well. In critically ill patients ARC is often undetectable because it is associated with a normal or decreased serum creatinine concentration (SCr). This study’s objective was to assess pharmacists’ knowledge about ARC identification, risk factors, affected antimicrobials, and dosing of antibiotics in patients with ARC.
Methods
In January 2020, we carried out a cross-sectional study by sending out an online survey to the Saudi Pharmaceutical Society, Kuwait Pharmaceutical Association, and Oman Pharmaceutical Society. Due to the expected low response rate, we administered an electronic questionnaire to pharmacists attending Dubai International Pharmaceuticals and Technologies Conference and Exhibition 2020 (DUPHAT).
Results
Data were collected from 288 respondents. However, only 134 were included in the final analysis following the exclusion of incomplete responses, no experience working in in-patient settings, and respondents who chose “no” universal ARC definition. Those who chose “yes” or “I do not know” regarding the universal definition of ARC were asked about SCr status in ARC. Elevation in SCr was chosen by 67/134 (50%) compared to those who chose decreased or normal (48/134, 35.8%). Regarding risk factors, only 1/134 (0.7%) respondent selected all risk factors. Two/134 (1.4%) respondents chose all hydrophilic antibiotics that are likely to be affected by ARC. Concerning the appropriate dose and frequency of piperacillin-tazobactam and meropenem, they were selected by 60.4% and 30.5%, respectively.
Conclusion
Pharmacists’ knowledge about ARC was limited. Implementation of educational programs targeting hospital pharmacists, especially those practicing in critical care settings, and developing antimicrobial institutional guidelines are important.
Electronic supplementary material
The online version of this article (10.1007/s40121-020-00310-9) contains supplementary material, which is available to authorized users.
Keywords: Antibiotics, Augmented renal clearance, Beta-lactams, Critically ill patients, Pharmacist, Pharmacodynamic dosing, Pharmacokinetics/pharmacodynamics, Probability of target attainment
Key Summary Points
| Why carry out this study? |
| Sepsis and septic shock are commonly encountered in critically ill patients; hence, broad-spectrum antibiotics are commonly used in this population. |
| Aside from renal impairment and the need for dose adjustment, renal elimination enhancement is an important factor that can affect pharmacokinetic/pharmacodynamic indices. |
| Little is known about pharmacists’ knowledge about augmented renal clearance (ARC). |
| What was learned from the study? |
| Generally, pharmacists’ knowledge about ARC was poor. |
| This poor knowledge was evident as ARC identification based on definition, risk factors, and antibiotics that could be affected by this phenomenon was poor. |
| Educational programs targeting pharmacists taking care of critically ill patients are needed. |
Introduction
Broad-spectrum antibiotics are among the most commonly prescribed medications in general wards and intensive care units (ICU) [1–3]. In patients with septic shock, several factors can affect the outcomes, including initial administration of effective empiric antibiotics, timing of antibiotic administration, and the appropriate dosing of antibiotics [4–9]. Moreover, several factors can impact the antimicrobial pharmacokinetics in critically ill patients. For instance, the volume of distribution and renal clearance of hydrophilic antibiotics is usually increased, which may result in subtherapeutic tissue and plasma concentrations [10, 11]. There is a misconception that only patients with impaired renal function need a dose adjustment. Augmented renal clearance (ARC) is a phenomenon that warrants dose adjustment in critically ill patients [12]. Generally, ARC is defined as a creatinine clearance (CrCl) value > 130 ml/min [13, 14]. This enhancement in renal elimination can result in subtherapeutic concentrations and negatively impact the outcomes [13, 15–19]. There is limited knowledge on the mechanisms of ARC, and several mechanisms were hypothesized. ARC is a hyperdynamic response secondary to changes in vascular permeability, renal blood flow, and elevation in the body temperature. These changes can elevate the glomerular filtration rate, renal tubular secretion, and renal tubular reabsorption [15].
Udy et al. conducted a multicenter prospective study to determine the prevalence of ARC. The authors found that 65.1% (182/281) of patients admitted to the ICUs had at least one occasion of ARC during the first 7 days of admission [16]; not surprisingly, the enhancement in renal elimination in ARC has been found to affect the clinical outcomes negatively [17]. The probability of pharmacodynamic target attainment at a particular minimum inhibitory concentration can be achieved by increasing the dose, changing the dosing frequency by extending the infusion, utilizing continuous infusion, or combining both [15].
ARC among critically ill patients is often undetectable because it is associated with a normal or reduced serum creatinine (SCr) concentration [13]. Identification of patients with ARC by estimating CrCl using Cockcroft-Gault or modification of diet in renal disease has not been validated in this patient population because of fluctuating renal function [20]. However, using measured CrCl through continuous urine collection at 2, 8, 12, and 24 h has been studied. Still, the optimal length of collection has not been established; 8–24 h is more convenient as it is not subjected to diurnal variation in the elimination of drugs [15]. Several risk factors predispose critically ill patients to ARC include, but are not limited to, young age, particularly ≤ 50 years, central nervous system infection, and extensive trauma upon admission [15].
ARC can be missed and may compromise antimicrobial therapy in critically ill patients. Little is known about pharmacists’ knowledge of ARC. This study’s objective was to assess pharmacists’ knowledge concerning ARC identification, risk factors, affected antimicrobials, and dosing of antibiotics in patients with ARC.
Methods
Design, Inclusion Criteria, and Study Sample
This was a cross-sectional, survey-based study conducted by administering an electronic questionnaire to pharmacists attending Dubai International Pharmaceuticals and Technologies Conference and Exhibition 2020 (DUPHAT). Participants were provided with a tablet device and self-administered the survey. Participants were approached during the scientific conference by a pharmacy student who was trained and prepared to collect data. Bearing in mind the nature of ARC, only licensed pharmacists were allowed to participate.
Initially, the link was shared via an online link with three scientific organizations: Saudi Pharmaceutical Society, Kuwait Pharmaceutical Association, and Oman Pharmaceutical Society. The online tool generated a confidential link sent to the societies mentioned earlier who distributed the link to their members using their email listservs. However, to avoid the expected low response rate with online surveys, the authors believed that a purposive sampling technique would be the best approach to overcome the possible low response rate and achieve the purpose of the study [21]. SurveyMonkey® is an online questionnaire generator that was utilized to design and distribute the questionnaire. The survey was designed to allow only one attempt per respondent. The survey was available between 16 January and 27 February 2020.
Contents of the Tool
The survey was not derived from any previously published questionnaire. The questionnaire consisted of five domains (Table 1). The first domain was a cover letter that provided general information about the purpose of the survey (supplementary file). The second domain consisted of demographic data (i.e., age, gender, year of initial pharmacy licensure, and “Are you a hospital pharmacist”).
Table 1.
Questionnaire
| First domain |
| Cover letter (supplementary file) |
| Second domain |
|
Age ……… Gender A. Male B. Female Year of initial pharmacy license ……… Are you a hospital pharmacist? A. Yes B. No |
| Third domain |
|
Do you have experience working as an in-patient pharmacist? A. Yes B. No |
| Fourth domain |
|
Is there a universal definition of ARC? A. Yes B. No C. I do not know |
| Fifth domain |
|
In patients with ARC, SCr is usually A. Elevated B. Decreased C. Normal D. I do not know What are the risk factors for ARC? Select all that apply A. Young age B. Traumatic brain injury C. Subarachnoid hemorrhage D. SOFA score ≥ 4 How to assess kidney function in patients with ARC? A. Cockroft-Gault equation B. Jelliffe C.Urine collection Antibiotics more likely to be affected by ARC; select all that apply A. Beta-lactams B. Vancomycin C. Linezolid D. Daptomycin To obtain probability target attainment of 85% or greater the following antibiotic should be administered as Piperacillin–tazobactam A. 4.5 g every 6 h (4-h infusion) B. 3.375 g every 6 h (3-h infusion) C. 3.375 g every 6 h (30-min infusion) To obtain probability target attainment of ≥ 85% the following antibiotic should be administered as Meropenem A. 2 g every 8 h (3-h infusion) B. 1 g every 8 h (30-min infusion) C. 500 mg every 6 h (30-min infusion) |
The third domain served to ask hospital pharmacists whether they had experience working as in-patient pharmacists. In case “no” was selected, respondents were directed to the end of the survey.
The fourth domain was to assess the respondents’ general knowledge about ARC. The question in the fourth domain was about the definition of ARC; a branch logic was created to customize the survey’s path. Respondents who chose “yes” or “I do not know” were directed to the fifth domain where they had to answer the status of SCr in such a condition, predisposing factors, how to diagnose, which antibiotics are more likely to be affected, and the dosing regimen of piperacillin-tazobactam and meropenem to obtain the probability target attainment of ≥ 85%. Since a universal definition of ARC is lacking, those who answered both results with “Yes” and “I do not know” were combined [15, 18, 22]. Additionally, provided that SCr in ARC could be normal or reduced, as many factors can affect SCr levels, answers to both choices were combined in the analysis [15, 17, 23].
Content Validity
Six pharmacists with considerable experience in pharmacy practice were invited to review the survey and provide face validity. None of the invited pharmacists were members of the aforementioned professional societies. The questionnaire was amended based on the comments or recommendations pointed by the invited pharmacists.
Ethical Approval
The Research Ethics Committee at King Faisal University approved this study (reference no. #KFU-REC/2019-12-06). The approval included the distribution of the survey to other countries (i.e., Oman, and Kuwait). Additionally, the study complied with the national guidelines of each country. No personal information was asked in the survey (i.e., name or contact number). Survey settings were modified to disable IP addresses and location identification. Additionally, written consent (i.e., in the form of a cover letter) was also provided. Respondents had to agree and give their consent to proceed to the survey.
Statistical Analysis
Analyses were carried out using descriptive statistics provided by SurveyMonkey®. Data were summarized as percentages (i.e., frequencies) for categorical data and means (± standard deviation [SD]) for continuous data.
Results
There were 288 responses; 35 participated via the online link and 253 by attending the scientific conference. However, nine respondents did not continue the survey resulting in a total of 279 responses. Additionally, because ARC is mainly encountered in critically ill patients, only pharmacists with experience working as in-patient pharmacists were considered in the analysis. Of the 279 respondents 155/279 (55.5%) had experience working in in-patient settings. The mean age was 34 years (8). There were 73/155 (47%) males and 82/155 (52.9%) females participating in this study. The majority of the respondents received their initial pharmacy license in 2012 and in 2009, 17/157 and 14/157, respectively (Table 2).
Table 2.
Demographics of the participants
| Parameter | |
|---|---|
| Age (years), mean (± SD) | 34 (8) |
| Gender, n (%) | |
| Male | 73 (47) |
| Female | 82 (52.9) |
| Initial pharmacy licensea | 2012 (1960–2018) |
aMode (range)
Pharmacists' Knowledge About ARC
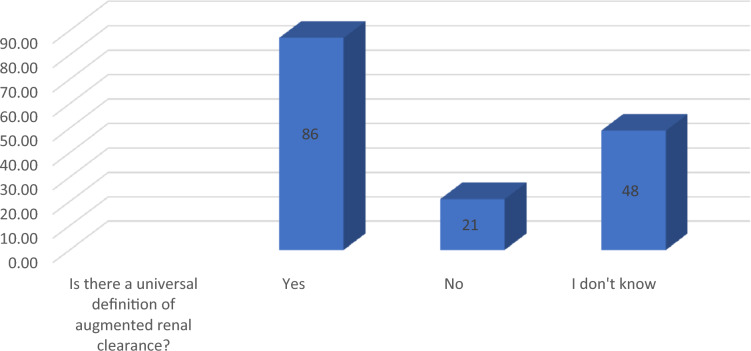
Eighty-six (86/155, 55.4%) of the respondents stated that there is a universal definition of ARC, and 48/155 (30.9%) stated that they do not know if there is an agreed-upon definition of ARC (Fig. 1). Those who chose no answer were directed to the end of the survey, resulting in a total of 134 (86%) involved in the final analysis (Fig. 1). Both those who said that there is a universal definition of ARC and who did not know if there is an accepted definition of ARC were asked about the status of SCr in ARC patients. Elevation in SCr was chosen by 67/134 (50%) (Table 3). Interestingly, only 48/134 (35.8%) stated that SCr decreases or is normal in ARC patients (Table 3). With respect to risk factors, only one respondent selected all risk factors (1/134, 0.7%) (Table 3). Urine collection was chosen as the recommended assessment method of kidney function by 86/134 (64%) in both groups combined (Table 3). Only two respondents (2/134, 1.4%) chose all hydrophilic antibiotics as those most likely to be affected by ARC (Table 3). Concerning the dosing of piperacillin-tazobactam and meropenem, the appropriate dose and frequency were chosen by 81/134 (60.4%) and 41/134 (30.5%), respectively.
Fig. 1.
Definition of ARC*. Respondents who answered “no” were directed to the end of the survey page
Table 3.
Fifth domain questions
| Frequency n (%)b | |
|---|---|
| In patients with ARC, SCr is usuallya | |
| Elevated | 67 (50) |
| Decreased | 25 (18.6) |
| Normal | 23 (17) |
| Decreased and normal combined | 48 (35) |
| I do not know | 19 (14) |
| What are the risk factors for ARC; select all that apply | |
| One risk factor chosen | 109 (81) |
| Two risk factors chosen | 19 (14) |
| Three risk factors chosen | 5 (3.7) |
| Four risk factors chosen | 1 (0.7) |
| How to assess kidney function in patients with ARC? | |
| Cockroft-Gault equation | 37 (27.6) |
| Urine collection | 86 (64) |
| Jelliffe | 11 (8.2) |
| Antibiotics most likely to be affected by ARC; select all that apply | |
| All hydrophilic antibiotics | 2 (1.4) |
| All hydrophilic antibiotics and linezolid | 2 (1.4) |
| Beta-lactam antibiotics and linezolid | 4 (2.9) |
| Beta-lactam antibiotics and vancomycin | 11 (8.2) |
| Beta-lactam antibiotics, vancomycin, and linezolid | 4 (2.9) |
| Vancomycin, linezolid, and daptomycin | 9 (6.7) |
| Vancomycin and linezolid | 9 (6.7) |
| Vancomycin and daptomycin | 1 (0.7) |
| Linezolid and daptomycin | 1 (0.7) |
| Beta-lactam antibiotics and daptomycin | 0 |
| Linezolid alone | 12 (8.9) |
| Daptomycin alone | 4 (2.9) |
| Vancomycin alone | 37 (27.6) |
| Beta-lactam antibiotics alone | 38 (28.3) |
| To obtain probability target attainment ≥ 85% the following antibiotic should be administered as: piperacillin-tazobactam | |
| 4.5 g every 6 h (4-h infusion) | 81 (60.4) |
| 3.375 g every 6 h (3-h infusion) | 36 (26.8) |
| 3.375 g every 6 h (30-min infusion) | 17 (12.6) |
| To obtain probability target attainment ≥ 85% the following antibiotic should be administered as: meropenem | |
| 2 g every 8 h (3-h infusion) | 41 (30.5) |
| 1 g every 8 h (30-min infusion) | 68 (50.7) |
| 500 mg every 6 h (30-min infusion) | 25 (18.6) |
aCombining both “Yes” and “I do not know” answers of the fourth domain
bBased on total of 134 (86 in the “Yes” and 48 in the “I do not know”)
Discussion
This present cross-sectional study was conducted to assess pharmacists’ knowledge about ARC. To the best of our knowledge, this is the first study to determine pharmacists’ knowledge concerning ARC. Generally, knowledge about ARC was high (86%) among pharmacists with an in-patient service experience. However, this general knowledge was discordant regarding ARC identification based on SCr assessment and risk factors. The majority of the respondents (50%) chose elevated SCr as the status of SCr in ARC patients.
Additionally, only one respondent (0.7%) selected all risk factors that could lead to ARC, which in turn could result in suboptimal antibiotic concentrations. Surprisingly, the majority (64%) chose urine collection as the assessment method of choice. This general and recent understanding indicates that formulas and equations are inadequate in critically ill patients [24]. Again, the high general knowledge was contradicted by the very low (1.4%) selection of all hydrophilic antibiotics that could possibly be affected by ARC.
Similarly, the selection of beta-lactam antibiotics and vancomycin together was relatively low (8.2%). This is extremely important as this combination is commonly administered in hospitalized and, more specifically, critically ill patients [2, 25, 26]. Although the appropriate dose and frequency of administration of piperacillin-tazobactam were chosen by the majority of respondents (60.4%), this could be due to the lack of implementation of the extended dosing interval of beta-lactam antibiotics and particularly piperacillin-tazobactam in some countries [27]. Unlike piperacillin-tazobactam, the appropriate dose and frequency of administration of meropenem were chosen by only 30.5%. Our findings agree with a previously published study conducted to determine ICU physicians’ attitudes regarding antibiotic dosing adjustments in patients with ARC. Only 15% of the respondents would modify the dose of beta-lactam antibiotics and vancomycin [28].
Several studies evaluated the effect of suboptimal antibiotic concentrations resulting from ARC and clinical outcomes [17–19], Claus et al. conducted a prospective observational study in a mixed ICU cohort. Therapeutic failure was higher in those with ARC 18 (27.3%) versus 8 (12.9%) [17]. Similarly, Carrie et al. conducted a prospective observational study to describe the relationship between ARC and the subsequent subtherapeutic beta-lactam antibiotic concentrations. A threshold CrCl of ≥ 170 ml/min was associated with beta-lactam antibiotic underdosing and more therapeutic failures [18]. Contrarily, other studies did not find an association between ARC-induced beta-lactam antibiotics renal elimination enhancement and mortality, which could be explained by the low prevalence of ARC in the population studied and the use of combination therapy, which could obscure the effect of ARC on clinical outcomes [13, 16, 29, 30].
This study has several limitations. First, the small number of participating pharmacists in this survey limits the generalization of our results to non-Gulf countries in the Middle East. The expected low response rate with online surveys triggered the authors who believed that the purposive sampling technique by attending a scientific conference would target the population of interest. Additionally, to avoid selection bias, only those with in-patient experience were allowed to participate. Second, no formal sample size calculation was carried out during the planning of this study, as none of the aforementioned scientific organizations were able to provide the authors with a specific number of registered licensed pharmacists. Their email listservs were not powered to categorize their members based on licensure (i.e., pharmacists vs. pharmacy technicians vs. pharmacy students). Third, a specific CrCl threshold was not included in the questionnaire to avoid respondent’s confusion given that studies used different thresholds [15, 18]. Finally, it was almost impossible to compare respondents’ knowledge based on the country of initial licensure as the response rate from the scientific organizations was low.
Conclusion
Pharmacists’ knowledge about ARC was limited. This gap in knowledge was clear when respondents were asked about risk factors, SCr status, and antibiotics most likely to be affected by ARC. Bearing in mind the importance of appropriate initial antibiotics and the importance of probability of target attainment in patients with septic shock, developing educational programs that target in-patient pharmacists and particularly those taking care of critically ill patients is vital to optimize antibiotic dosing in this population. Additionally, developing antimicrobial institutional guidelines will help unify dosing and administration practices.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Abdulaziz S. Almulhim, Batool A. Al-Dahneen, and Yazed S. Alsowaida have nothing to disclose.
Compliance with Ethics Guidelines
The Research Ethics Committee at King Faisal University approved this study (reference no. #KFU-REC/2019-12-06). The approval included the distribution of the survey to other countries (i.e., Oman and Kuwait). Additionally, the study complied with the national guidelines of each country. No personal information was asked in the survey (i.e., name or contact number). Survey settings were modified to disable IP addresses and location identification. Additionally, written consent (i.e., in the form of a cover letter) was also provided. Respondents have to agree and give their consent to proceed to the survey.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12465866.
References
- 1.Curcio D, Alí A, Duarte A, Defilippi Pauta A, Ibáñez-Guzmán C, Chung Sang M, et al. Prescription of antibiotics in intensive care units in Latin America: an observational study. J Chemother. 2009;21(5):527–534. doi: 10.1179/joc.2009.21.5.527. [DOI] [PubMed] [Google Scholar]
- 2.Curcio D, Group LAAUiICU Antibiotic prescriptions in critically-ill patients: a Latin American experience. Ann Med Health Sci Res. 2013;3(2):220. doi: 10.4103/2141-9248.113666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almulhim AS, Alamer A. The prevalence of resistant Gram-negative bacteraemia among hospitalized patients in Tucson, Arizona over a 12-month period; a retrospective single center study. J Int Med Res. 2020;48(1):0300060519829987. doi: 10.1177/0300060519829987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. Predictors of mortality in patients with infections due to multi-drug resistant gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66(5):401–414. doi: 10.1016/j.jinf.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115(7):529–535. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 7.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122(1):262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 10.Pea F, Viale P, Furlanut M. Antimicrobial therapy in critically ill patients. Clin Pharmacokinet. 2005;44(10):1009–1034. doi: 10.2165/00003088-200544100-00002. [DOI] [PubMed] [Google Scholar]
- 11.Boucher BA, Wood GC, Swanson JM. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22(2):255–271. doi: 10.1016/j.ccc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance. Clin Pharmacokinet. 2010;49(1):1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney BC, Ungerer JP, et al. Subtherapeutic initial β-lactam concentrations in select critically ill patients. Chest. 2012;142(1):30–39. doi: 10.1378/chest.11-1671. [DOI] [PubMed] [Google Scholar]
- 14.Udy AA, Putt M, Boots RJ, Lipman J. ARC-augmented renal clearance. Curr Pharm Biotechnol. 2011;12(12):2020–2029. doi: 10.2174/138920111798808446. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs AL, Shea KM, Roberts KM, Daley MJ. Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacother J Hum Pharmacol Drug Ther. 2015;35(11):1063–1075. doi: 10.1002/phar.1653. [DOI] [PubMed] [Google Scholar]
- 16.Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42(3):520–527. doi: 10.1097/CCM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 17.Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28(5):695–700. doi: 10.1016/j.jcrc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Carrie C, Petit L, d’Houdain N, Sauvage N, Cottenceau V, Lafitte M, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018;51(3):443–449. doi: 10.1016/j.ijantimicag.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Carrie C, Bentejac M, Cottenceau V, Masson F, Petit L, Cochard J-F, et al. Association between augmented renal clearance and clinical failure of antibiotic treatment in brain-injured patients with ventilator-acquired pneumonia: a preliminary study. Anaesth Crit Care Pain Med. 2018;37(1):35–41. doi: 10.1016/j.accpm.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol. 2013;42(4):1012–1014. doi: 10.1093/ije/dys223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39(3):346–354. doi: 10.1002/phar.2231. [DOI] [PubMed] [Google Scholar]
- 23.Hoste EA, Damen J, Vanholder RC, Lameire NH, Delanghe JR, Van den Hauwe K, et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2005;20(4):747–753. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- 24.Sunder S, Jayaraman R, Mahapatra HS, Sathi S, Ramanan V, Kanchi P, et al. Estimation of renal function in the intensive care unit: the covert concepts brought to light. J Intensive Care. 2014;2(1):31. doi: 10.1186/2052-0492-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almulhim AS, Alotaibi FM. Comparison of broad-spectrum antibiotics and narrow-spectrum antibiotics in the treatment of lower extremity cellulitis. Int J Health Sci (Qassim). 2018;12(6):3–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Malacarne P, Rossi C, Bertolini G. Antibiotic usage in intensive care units: a pharmaco-epidemiological multicentre study. J Antimicrob Chemother. 2004;54(1):221–224. doi: 10.1093/jac/dkh299. [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Health. National antimicrobial therapy guidelines for community and hospital acquired infections in adults 2018. https://www.moh.gov.sa/en/CCC/healthp/regulations/Documents/National%20Antimicrobial%20%20Guidelines.pdf. Accessed 27 Apr 2020.
- 28.Dunning J, Roberts J. Assessment of renal function in dosing antibiotics in septic patients: a survey of current practice within critical care units in England: 22. Anaesthesia. 2015;22:70. [Google Scholar]
- 29.Kawano Y, Maruyama J, Hokama R, Koie M, Nagashima R, Hoshino K, et al. Outcomes in patients with infections and augmented renal clearance: a multicenter retrospective study. PLoS One. 2018;13(12):e0208742. doi: 10.1371/journal.pone.0208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udy AA, Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, et al. Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int J Antimicrob Agents. 2017;49(5):624–630. doi: 10.1016/j.ijantimicag.2016.12.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.