Abstract
Antarctica has hosted a wide range of ecosystems over the past 500-million years. Early in the Mesozoic, the Antarctic portion of southern Pangaea had a more habitable climate, but its position within the polar circle imposed extreme photoperiod seasonality on its resident flora and fauna. It remains unclear to what degree physiological adaptations underpinned the ability of tetrapods to establish the terrestrial communities captured in the fossil record. Here we use regular and stressful growth marks preserved in the dentine of ever-growing tusks of the Early Triassic mammalian predecessor, Lystrosaurus, to test for adaptations specific to this polar inhabitant. We find evidence of prolonged stress indicative of torpor when compared to tusk samples from non-polar populations of Lystrosaurus. These preliminary findings are to our knowledge the oldest instance of torpor yet reported in the fossil record and demonstrate unexpected physiological flexibility in Lystrosaurus that may have contributed its survivorship through the Permo-Triassic mass extinction.
Subject terms: Palaeontology, Evolution
Whitney and Sidor examine the growth marks on Lystrosaurus tusks from the Early Triassic, and demonstrate evidence of torpor in polar specimens. These preliminary findings give insight into physiological adaptations that could have aided in survival and recovery from the Permo-Triassic mass extinction.
Introduction
Antarctica is today the coldest and driest continent with extreme variation in light availability throughout the year, restricting vertebrate life to coastal regions and rendering most of the continent uninhabitable1. These modern environmental conditions are anomalous, however, considering the deep history of life in Antarctica when flora and fauna occupied large regions of the continent2. Although more habitable with a warmer climate than today, Antarctica remained at a high latitudinal position for much of the Phanerozoic3, subjecting its inhabitants to extreme photoperiod seasonality4–6. Extant vertebrates living in highly seasonally variable climates have evolved a variety of mechanisms to curb the effects of regular intervals of stress including daily torpor, hibernation, and brumation7. These adaptations are largely behavioral thus rendering them difficult to study directly in the fossil record. Importantly, however, these adaptations reflect underlying metabolic changes in response to resource limitations, and therefore should be recognizable in fossil hard tissues that preserve chronological records of physiology8–13.
Geological data from the Early Triassic document prolonged and unfavorable environments following the Permo-Triassic mass extinction (PTME)14. PTME survivors and newly evolved species would have had to adapt to high global temperatures, oceanic anoxia, and low nutrient availability that created unstable and highly variable environmental conditions15. Polar regions are thought to have shielded their inhabitants from the extremes of these conditions both during the PTME and subsequently in recovery. The Fremouw Formation of Antarctica provides some of the earliest records of terrestrial vertebrates of Early to Middle Triassic age (~250–230 million years old)16,17. Furthermore, the flora and fauna of the Fremouw Formation is taxonomically similar to those found in non-polar regions of southern Pangaea, especially the Karoo Basin of South Africa17–19, facilitating direct comparisons of polar and non-polar populations.
Here, we compare the frequency and patterns of growth marks in tusks of the Early Triassic non-mammalian synapsid Lystrosaurus, from polar Antarctica to those from the non-polar Karoo Basin of South Africa. Dentine, a major tissue of the vertebrate dentition, is deposited during times of regular incremental growth as well as times of arrested growth reflecting metabolic stress (Fig. 1). Compared to bone or enamel, dentine acts as a particularly sensitive recorder of daily-to-monthly physiological activity providing a robust chronology of both regular and stressed growth and has previously been used to assess responses to environmental change8–13,20. Despite this, the lack of sufficient experimental data on dentine deposition in extant tetrapods makes specifying the absolute amounts of time recorded by each growth mark (i.e. daily growth, yearly growth, etc.) difficult. Instead, we adopt an agnostic terminology reflective of growth patterns where fine-scale, regular marks denote baseline or routine growth and thicker more pronounced growth marks are referred to as stress marks.
Fig. 1. Measurements for stress and regular growth were recorded from the tusks of Lystrosaurus.
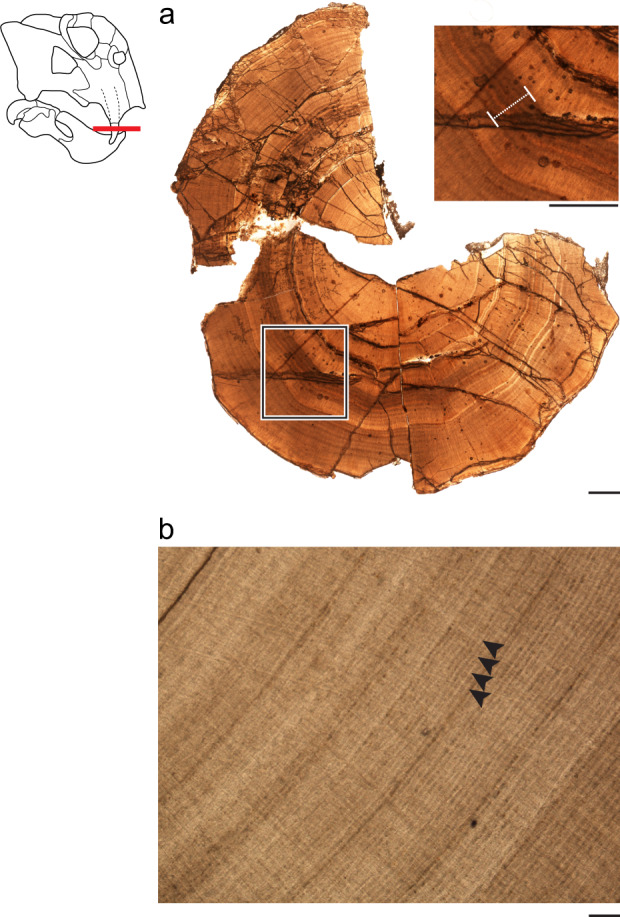
a A cross-section of Antarctic specimen UWBM 118025 with a “hibernation zone” highlighted at a higher magnification. Scale bars = 1000 μm. b Well-preserved regular incremental growth marks from the South African specimen UWBM 118028, lacking “hibernation zones”. Arrows denote individual lines with an average spacing of 16–20 μm. Scale bar = 100 μm.
The tusks of Lystrosaurus serve as particularly extensive markers of seasonality because they have been shown to be ever-growing21. Such tusks can therefore capture extended periods of time and control for the developmental stage of the tooth, reducing any bias toward rapid growth in newly erupted teeth. Furthermore, ever-growing dentitions such as incisors of modern rodents are known to preserve evidence of seasonal stress and hibernation8–11 as well as in fossil rodents12 and the tusks of mammoths13. Previous descriptions of a “hibernation zone” in these dentitions are recorded as episodes of shortened intervals between stress lines8,9,12 and as such, we accordingly employ these characteristics in testing for torpor in our Early Triassic sample.
We find evidence of prolonged and repeated metabolic stress events in the tusks of Antarctic Lystrosaurus that differ from the relatively steady growth observed in South African Lystrosaurus tusks. The patterns of stress found in polar Lystrosaurus are similar to previously described “hibernation zones” suggesting that Antarctic Lystrosaurus experienced seasonal torpor, likely similar to hibernation. This preliminary finding supports the growing body of evidence that Lystrosaurus was endothermic and highlights the role of polar regions in the recovery of terrestrial vertebrates from the PTME.
Results
Regular growth marks
We found that the spaces between regular, incremental growth marks in the tusks of Antarctic Lystrosaurus were not significantly different from those of South African Lystrosaurus (Fig. 2) indicating that although geographically separated by over 900 km in the Triassic, the baseline physiology and growth was generally similar in both populations and across individuals in our sample. These baseline indicators of physiology allow us to control for alternative sources of variation in growth patterns between the two populations (i.e. differences in growth rate due to ontogeny or species/individual variance). In modern ever-growing dentitions, these regular growth marks have been demonstrated to vary with factors such as age22, species of varying metabolisms23, and dentine deposition is sensitive to nutrient inputs24. Completely controlling for alternative sources of variation was particularly difficult given the small and fragmentary nature of the specimens that were available for destructive sampling, however, we consider these regular growth marks to be at least a general control for those sources of variation in growth that are unrelated to seasonality.
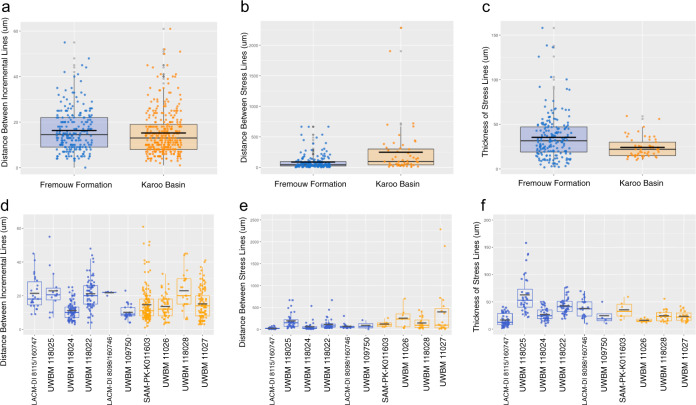
Fig. 2. Stress and regular incremental data for Antarctic (blue) and South African (orange) Lystrosaurus tusks.
a The mean distance between regular incremental growth marks was insignificantly different between the two populations (p = 0.06543; n = 550) suggesting similar baseline metabolic activity in Antarctic and South African Lystrosaurus populations. b The distance between growth marks was significantly greater in South African specimens (p = 7.43e-05; n = 234) indicating longer durations of uninterrupted growth. c The thickness of stress lines in Antarctic specimens was significantly greater than South African specimens (p = 1.45e-03; n = 246) indicating longer periods of inactive growth. d–f Measurements averaged in a–c separated by data collected for each specimen. Considerable variation was observed, but even with outliers removed, the average significant differences remained consistent.
Stress marks
While our sample contained individuals of similar baseline metabolic activity, indicators of metabolic stress were different between Antarctic and South African specimens. Quantitatively, both the duration of stress and the short intervals between stressful events suggest that Antarctic Lystrosaurus experienced relatively frequent and pronounced metabolic strain (Fig. 2). The amount of growth between stressful events was, on average, significantly greater in South African Lystrosaurus suggesting longer durations of regular growth without stressful interruptions for non-polar populations that inhabited the Karoo Basin. Furthermore, stressful events appear to have lasted for longer durations in Antarctic fossils as suggested by the significantly thicker lines observed in tusks from the Fremouw Formation. Antarctic tusks preserve stressed growth marks akin to previous descriptions of a “hibernation zone” where a series of thick stress marks are found close to one another while South African specimens typically display a single stress mark followed by regular growth that constitutes the majority of the growth record of this population (Fig. 3 and Supplementary Fig. 1).
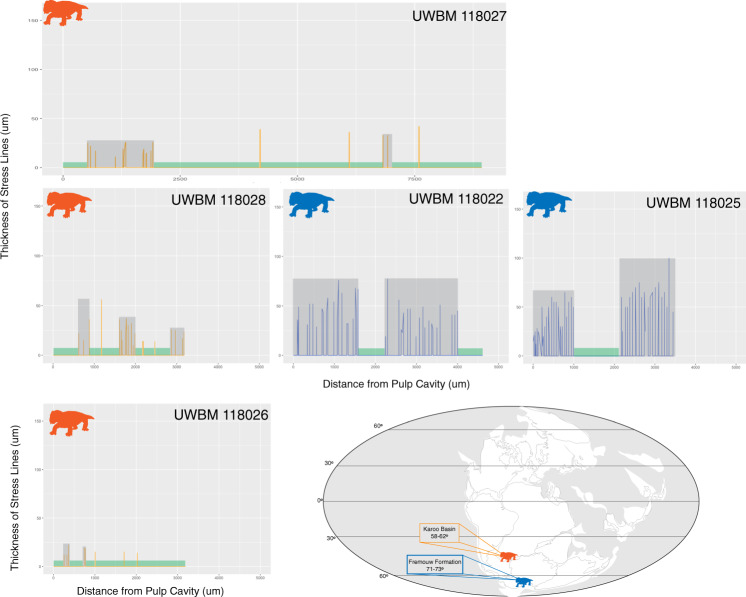
Fig. 3. Summary of stress mark data from a selection of South African (orange) and Antarctic (blue) specimens of Lystrosaurus.
These graphs represent transects collecting the thickness (μm) of stress lines moving from the pulp cavity (to left) to the outer edge of the tusks (to right). Green boxes highlight durations without visible stress lines and gray boxes highlight portions of the tusk with closely spaced stress lines suggesting a stressful interval. The height of the boxes represents the thickest stress line during such period. See Supplementary Fig. 1 for transects from entire sample.
Intrapopulation variation
Substantial variation within localities does exist. However, even when outliers are removed from our quantitative analyses, statistically significant differences in stress marker measures persist. In fact, this variation is an important consideration given that not every stress line necessarily represents torpor, even in the Antarctic populations (Fig. 4). Short periods of metabolic reduction may be caused by a variety of factors, but the unique patterns here observed in Antarctic tusks, where occurrences of closely spaced stress lines are present, are consistent with torpor at high latitudes.
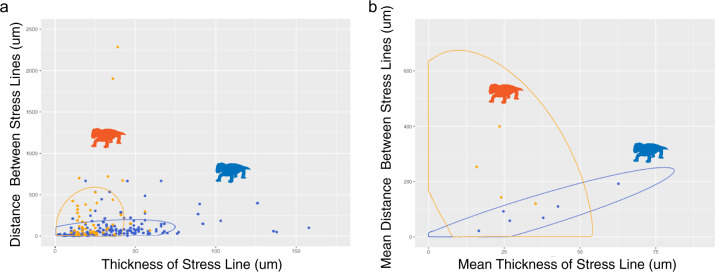
Fig. 4. Comparative measurements of stress lines in Antarctic (blue) and South African (orange) tusks.
There is substantial variability within each population, however, South African stress lines tend to be thinner and farther apart than Antarctic specimens which are more likely to occur closer together and more frequently. This is apparent as individual data points (a) and when specimens are assigned a mean value for these measurements (b).
Discussion
From this exploratory study, we find evidence of severe and prolonged periods of stress in Antarctic Lystrosaurus tusks that support the conclusion that polar populations adapted to their high-latitude environment by means of seasonal reduction in metabolic activity, otherwise referred to as torpor. The “hibernation zones” denoted here by temporarily reduced dentine deposition between stress lines are quantitatively (Fig. 2) and qualitatively (Fig. 3 and Supplementary Fig. 1) akin to heterothermic activity observed in modern endotherms. Heterothermic ectotherms reduce their metabolic activity from at least half of to nearly complete quiescent metabolic activity25. Heterothermic endotherms, on the other hand, can enter a state of torpor generally reducing metabolic activity by at most a third although most reduce by no more than 10% of normal activity7. Generally, ectothermic heterotherms are not able to reactivate metabolic activity during unfavorable environmental conditions and enter times of brumation whereas endothermic heterotherms, even hibernators, frequently will come out of metabolic dormancy either daily, weekly, or monthly26. The zones of stress observed here in Antarctic Lystrosaurus are marked by iterative reactivation of metabolic activity similar to those seen in torpor patterns of modern endotherms. This contributes additional support for a growing body of evidence that dicynodonts like Lystrosaurus were likely endothermic27–30.
These data also shed light on Antarctica’s role as a refugium during the PTME. Antarctic rocks have yielded Early Triassic tetrapod taxa that are missing from other contemporaneous, but otherwise much better sampled localities such as the Karoo Basin of South Africa. These discrepancies in otherwise very similar faunal assemblages17–19, have supported the hypothesis that the Antarctic portion of Pangaea was a high-latitude refugium from the global climatic events marked by the PTME31,32. Furthermore, geologic data suggest that polar regions were, in fact, the first to begin a prolonged recovery in the Early Triassic15. With its relatively temperate climate, Antarctica may have acted as a haven for terrestrial vertebrates through an extinction boundary and subsequent recovery.
Although more insulated from the effects of global climate change, tetrapods living in Antarctica during the Early Triassic would have had to adapt to extreme seasonality with long periods of limited light availability. A Permian tetrapod assemblage has yet to be recorded from Antarctica33, however, Lystrosaurus tusks from both the Permian and Triassic of the Karoo Basin do not record patterns of hibernation-like reductions in their metabolic activity (Fig. 3). Thus, these data suggest that upon expanding its geographic range to the Antarctic portion of southern Pangaea Lystrosaurus adapted with extended periods of reduced metabolic activity, although continued testing of this initial observation is required.
Lystrosaurus survivorship through the PTME and its subsequent abundance in the fossil record of the earliest Triassic34 was likely predicated on a flexible physiology that could modulate typically elevated metabolic activity according to the limiting resources of a fluctuating environment34,35. This agrees with Valentine’s36 suggestion that more stable environments tend to select for narrow niche partitioning among species, whereas those with unstable resources, such the Early Triassic15, tend to select for species with greater flexibility and generalization. Indeed, the near-global distribution of Lystrosaurus, with records known from China, Russia, India, Africa, and Antarctica, implies a remarkable ecological breadth for this lineage37. Furthermore, Triassic rocks from both South Africa and Antarctica preserve fossil evidence of tetrapod burrowing38–40, including for Lystrosaurus38. We suggest that a combination of a flexible physiology and burrowing served as exaptations to the acquisition of torpor for Antarctic populations of Lystrosaurus.
We show preliminary evidence that Lystrosaurus used torpor to respond to the seasonal stress incurred specifically in the polar regions of southern Pangaea during the Early Triassic. It was this ability to sustain activity in a variety of stressful environmental conditions that may have served as a critical adaptation in surviving and recovering from the largest mass extinction the earth has experienced to date. The Fremouw Formation preserves a diverse assemblage of tetrapod taxa17–19 with presumably an array of metabolic adaptations to the extremes of seasonal light availability. Continued testing of seasonal responses in Lystrosaurus, other non-mammalian synapsids, early reptiles, and the abundant temnospondyl fossils recovered from these localities can reveal how this polar ecosystem evolved despite unstable environmental conditions and eventually facilitated post-extinction recovery.
Methods
Overview
Following standard thin-sectioning protocol established by Lamm41, transverse and longitudinal sections of six Antarctic and four South African Lystrosaurus tusks were made and analyzed (Supplementary Table 1). Paleolatitude was estimated from tectonic plate reconstructions using paleomagnetic reference frame developed by Torsvik et al.42 and made available through the online calculator developed by van Hinsbergen et al.43.
Dentine growth mark terminology
Generally, there are three kinds of growth marks recognized in dentine: (1) daily increments that correspond to a circadian rhythm called lines of von Ebner, (2) 6–10 day increments called Andresen lines, and (3) periods of stress where dentine deposition essentially ceases creating particularly thick growth marks44,45. While these three marks are recognized in primates, especially humans, there is little investigation into the consistency of this hierarchy outside of mammals. This is in large part due to the range of reported distances between these growth marks. Dean44 reviewed the discrepancies in both reported short periods between lines of von Ebner (varying from 1.7 to 20 μm) and long periods between Andresen lines (varying from 4 to 20 μm) for which values overlap. Erickson46 was one of the few to experimentally test for daily lines of von Ebner in a non-mammalian vertebrate (Alligator mississipiensis). Erickson, however, only reported that lines of von Ebner were spaced <20 μm apart and did not discuss the variation of space between lines or whether or not there was a distinction between short- and long-term periods. Furthermore, that study was conducted on juvenile alligators that were rapidly growing and thus reported distances between growth marks may not be truly comparable to the adult primates previously studied or representative of adult dentine growth more generally. In this study, we follow Dean’s recognition of short-term and long-term period growth marks generally as regular incremental lines, assigning no specific amount of time, but rather that they represent routine or baseline dentine deposition (Fig. 1).
Statistics and reproducibility
An additional aim of this study was to develop a more quantitative and objective method for measuring and counting growth marks, especially in fossil material where visuals can be impaired by taphonomic alteration. Using NIS Nikon Elements software ROI Measurement Tool, we ran transects across live images of tusks that collected measurements on light intensity. The output of these transects records decreases and increases in light intensity that can be confirmed in the live image feed as growth marks and spaces between growth marks are recorded. Relative dips in light intensity are associated with regular and stress lines whereas relative increases in light intensity represent times of growth and tissue deposition. The space between regular increments (n = 550) as well as distance between (n = 234) and thickness of stress intervals (n = 246) were captured in these light transects. Mann–Whitney U tests were used to determine statistical significance in these nonparametric data sets (Fig. 2). Ellipses were constructed assuming a multivariate t-distribution at 95% confidence intervals (Fig. 4).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Funding for this project came from NSF PLR-1341304 (awarded to C.A.S.) and NSF DEB-1701383 (awarded to M.R.W.). Y. Tse, A. Huttenlocker, and K. Button assisted in specimen preparation. We thank Z. Skosan (Iziko: South African Museum) and M. Walsh (Natural History Museum of Los Angeles County) for access to specimens. We thank the 2017–2018 Shackleton Glacier field team (P. Braddock, P. Makovicky, J. McIntosh, A. Shinya, N. Smith, R. Smith, and C. Wooley) and W. Hammer for Antarctic field collections. Finally, logistical support for this project in Antarctica was provided by the U.S. National Science Foundation through the U.S. Antarctic Program and we thank the Shackleton deep field camp staff and pilots for supporting this research.
Author contributions
Study design was developed by M.R.W. and C.A.S.; M.R.W. collected and analyzed histological data and both M.R.W. and C.A.S. contributed to the writing of this manuscript.
Data availability
Thin sections of UWBM specimens (118022, 118024, 118025, 118026, 118027, 118028, and 109750) are housed at the University of Washington Burke Museum, thin sections and additional material of LACM-DI 8115/180747 and LACM-DI 8098/160746 are housed at the Natural History Museum of Los Angeles County and thin sections and additional fossil material of SAM-PK-K011603 is housed at the Iziko: South African Museum. Histological images that support this study have been deposited in MorphoBank with the project number 3650 at http://morphobank.org/permalink/?P3650.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42003-020-01207-6.
References
- 1.Convey P, et al. Antarctic terrestrial life - challenging the history of the frozen continent? Biol. Rev. 2008;83:103–117. doi: 10.1111/j.1469-185X.2008.00034.x. [DOI] [PubMed] [Google Scholar]
- 2.Taylor, T. N. & Taylor, E. L. Antarctic Paleobiology: Its Role In The Reconstruction Of Gondwana (Springer Verlag, New York, 1990).
- 3.Scotese CR, Boucot AJ, McKerrow WS. Gondwanan palaeogeography and palaeoclimatology. J. Afr. Earth Sci. 1999;28:99–114. doi: 10.1016/S0899-5362(98)00084-0. [DOI] [Google Scholar]
- 4.Taylor EL, Taylor TN, Cuneo NR. The present is not the key to the past: a polar forest from the permian of antarctica. Science. 1992;257:1675–1677. doi: 10.1126/science.257.5077.1675. [DOI] [PubMed] [Google Scholar]
- 5.Taylor, E. L. & Taylor, T. N. Tree rings and paleoclimate from the Triassic of Antarctica. The Nonmarine Triassic (eds Lucas, S. G. & Morales, M.), New Mexico Museum of Natural History History and Science Bulletin 3: 453–455 (1993).
- 6.Decombeix AL, Serbet R, Taylor EL. Under pressure? Epicormic shoots and traumatic growth zones in high-latitude Triassic trees from East Antarctica. Ann. Bot. 2018;121:681–689. doi: 10.1093/aob/mcx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiser F, Ruf T. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol. Zool. 1995;68:935–966. doi: 10.1086/physzool.68.6.30163788. [DOI] [Google Scholar]
- 8.Rinaldi C, Cole TM. Environmental seasonality and incremental growth rates of beaver (Castor canadensis) incisors: Implications for palaeobiology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;206:289–301. doi: 10.1016/j.palaeo.2004.01.008. [DOI] [Google Scholar]
- 9.Goodwin HT, Michener GR, Gonzalez D, Rinaldi CE. Hibernation is recorded in lower incisors of recent and fossil ground squirrels (Spermophilus) J. Mammal. 2005;86:323–332. doi: 10.1644/04-MAMM-A-034R.1. [DOI] [Google Scholar]
- 10.Batavia M, Nguyen G, Zucker I. The effects of day length, hibernation, and ambient temperature on incisor dentin in the Turkish hamster (Mesocricetus brandti) J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2013;183:557–566. doi: 10.1007/s00360-012-0729-9. [DOI] [PubMed] [Google Scholar]
- 11.Klevezal GA, Anufriev AI. Variations of increments and “hibernation zone” on incisor surface in marmots (Genus Marmota) Biol. Bull. 2014;41:601–615. doi: 10.1134/S1062359014070024. [DOI] [Google Scholar]
- 12.Trunova YE. Hibernation mark in incisors of fossil rodents. Dokl. Biol. Sci. 2001;377:163–165. doi: 10.1023/A:1019282312994. [DOI] [Google Scholar]
- 13.Fisher, D. C., Fox, D. L. & Agenbroad, L. D. Tusk growth rate and season of death of Mammuthus columbi from Hot Springs, South Dakota, USA. Adavances in Mammoth Research (Proceedings of the Second International Mammoth Conference, Rotterdam, May 16-20 1999) (eds Reumer, J. W. F., De Vos, J. & Mol, D.) DEINSEA 9: 117–133 (2003).
- 14.Hallam A. Why was there a delayed radiation after the end-Palaeozoic extinctions? Hist. Biol. 1991;5:257–262. doi: 10.1080/10292389109380405. [DOI] [Google Scholar]
- 15.Kidder DL, Worsley TR. Causes and consequences of extreme Permo-Triassic warming to globally equable climate and relation to the Permo-Triassic extinction and recovery. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;203:207–237. doi: 10.1016/S0031-0182(03)00667-9. [DOI] [Google Scholar]
- 16.Collinson JW, Hammer WR, Askin RA, Elliot DH. Permian-Triassic boundary in the central Transantarctic Mountains, Antarctica. GSA Bull. 2006;118:747–763. doi: 10.1130/B25739.1. [DOI] [Google Scholar]
- 17.Kitching JW, Collinson JW, Elliot DH, Colbert EH. Lystrosaurus Zone (Triassic) Fauna from Antarctica. Science. 1972;175:524–527. doi: 10.1126/science.175.4021.524. [DOI] [PubMed] [Google Scholar]
- 18.Sidor CA, Damiani R, Hammer WR. A new Triassic temnospondyl from Antarctica and a review of Fremouw Formation biostratigraphy. J. Vertebrate Paleontol. 2008;28:656–663. doi: 10.1671/0272-4634(2008)28[656:ANTTFA]2.0.CO;2. [DOI] [Google Scholar]
- 19.Peecook, B. R., Smith, R. M. H. & Sidor, C. A. (2019). A novel archosauromorph from Antarctica and an updated review of a high-latitude vertebrate assemblage in the wake of the end-Permian mass extinction. J. Vertebr. Paleontol. https://doi.org/10.1080/02724634.2018.1536664 (2019).
- 20.Thackery JF. Growth increments in the teeth of Diictodon (Therapsida) Koedoe. 1991;34:7–11. [Google Scholar]
- 21.Whitney MR, Tse Y, Sidor CA. Histological evidence of trauma in tusks of southern African dicynodonts. Palaeontol. Afr. 2019;53:75–80. [Google Scholar]
- 22.Klevezal GA, Shchepotkin DV. Incisor growth rate in rodents and the record of the entire annual cycle in the incisors of Marmota baibacina centralis. Biol. Bull. 2012;39:684–691. doi: 10.1134/S1062359012080079. [DOI] [Google Scholar]
- 23.Schour I, Hoffman MM. The rate of apposition of enamel and dentine in man and other mammals. J. Dent. Res. 1939;15:161–175. doi: 10.1177/00220345390180020601. [DOI] [Google Scholar]
- 24.Tjäderhane L, Hietala E-L, Svanberg M, Larmas M. Morphological analysis of dentine formation in young rat molars during the recovery phase with calcium along or combined with xylitol following a low-calcium dietary regimen. Arch. Oral Biol. 1995;40:707–711. doi: 10.1016/0003-9969(95)00036-O. [DOI] [PubMed] [Google Scholar]
- 25.Ultsch GR. Ecology and physiology of hibernation and overwintering among freshwater fishes, turtles and snakes. Biol. Rev. Camb. Philos. Soc. 1989;64:435–516. doi: 10.1111/j.1469-185X.1989.tb00683.x. [DOI] [Google Scholar]
- 26.Geiser F. Hibernation. Curr. Biol. 2013;23:188–193. doi: 10.1016/j.cub.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 27.Botha-Brink J, Angielczyk KD. Do extraordinarily high growth rates in Permo-Triassic dicynodonts (Therapsida, Anomodontia) explain their success before and after the end-Permian extinction? Zool. J. Linn. Soc. 2010;160:341–365. doi: 10.1111/j.1096-3642.2009.00601.x. [DOI] [Google Scholar]
- 28.Laaß M, et al. New insights into the respiration and metabolic physiology of Lystrosaurus. Acta Zool. 2011;92:363–371. doi: 10.1111/j.1463-6395.2010.00467.x. [DOI] [Google Scholar]
- 29.Rey K, et al. Oxygen isotopes suggest elevated thermometabolism within multiple Permo-Triassic therapsid clades. eLife. 2017;6:1–25. doi: 10.7554/eLife.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraure-Brac MG, Cubo J. Were the synapsids primitively endotherms? A palaeohistological approach using phylogenetic eigenvector maps. Philos. Trans. R. Soc. B. 2020;375:20190138. doi: 10.1098/rstb.2019.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erwin DH, Bowring SA, Yugan J. End-Permian mass extinctions: a review. Spec. Pap. Geol. Soc. Am. 2002;356:363–383. [Google Scholar]
- 32.Fröbisch J, Angielczyk KD, Sidor CA. The Triassic dicynodont Kombuisia (Synapsida, Anomodontia) from Antarctica, a refuge from the terrestrial Permian-Triassic mass extinction. Naturwissenschaften. 2010;97:187–196. doi: 10.1007/s00114-009-0626-6. [DOI] [PubMed] [Google Scholar]
- 33.Miller, M. F., Sidor C. A. & Isbell J. L. Permian Vertebrates In Antarctica? Probably Not. Gondwana 12: Geological And Biological Heritage Of Gondwana, Abstracts. 257 (Academia National de Ciencias, Cordoba, Argentina, 2005).
- 34.Botha-Brink J, Smith RMH. Lystrosaurus species composition across the Permo-Triassic Boundary in the Karoo Basin of South Africa. Lethaia. 2007;40:125–137. doi: 10.1111/j.1502-3931.2007.00011.x. [DOI] [Google Scholar]
- 35.Botha-Brink J, Codron D, Huttenlocker AK, Angielczyk KD, Ruta M. Breeding young as a survival strategy during Earth’s greatest mass extinction. Sci. Rep. 2016;6:24053. doi: 10.1038/srep24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valentine JW. Resource supply and species diversity patterns. Lethaia. 1971;4:51–61. doi: 10.1111/j.1502-3931.1971.tb01278.x. [DOI] [Google Scholar]
- 37.Colbert EH. The distribution of Lystrosaurus in Pangaea and its implications. Geobios mémoire spécial. 1982;6:375–383. doi: 10.1016/S0016-6995(82)80126-5. [DOI] [Google Scholar]
- 38.Botha-Brink J. Burrowing in Lystrosaurus: preadaptation to a postextinction environment? J. Vertebr. Paleontol. 2017;37:e1365080. doi: 10.1080/02724634.2017.1365080. [DOI] [Google Scholar]
- 39.Miller MF, Hasiotis ST, Babcock LE, Isbell JL, Collinson JW. Tetrapod and large burrows of uncertain origin in Triassic high paleolatitude floodplain deposits, Antarctica. PALAIOS. 2001;16:218–232. doi: 10.1669/0883-1351(2001)016<0218:TALBOU>2.0.CO;2. [DOI] [Google Scholar]
- 40.Sidor CA, Miller MF, Isbell JL. Tetrapod burrows from the Triassic of Antarctica. J. Vertebr. Paleontol. 2008;28:277–284. doi: 10.1671/0272-4634(2008)28[277:TBFTTO]2.0.CO;2. [DOI] [Google Scholar]
- 41.Lamm, E.-T. Bone Histology of Fossil Tetrapods (eds K. Padian & E.-T. Lamm) 55–160 (University of California Press, 2013).
- 42.Torsvik TH, et al. Phanerozoic polar wander, palaeogeography and dynamics. Earth Sci. Rev. 2012;114:325–368. doi: 10.1016/j.earscirev.2012.06.007. [DOI] [Google Scholar]
- 43.van Hinsbergen DJJ, et al. A paleolatitude calculator for paleoclimate studies. PLoS ONE. 2015;10:e0126946. doi: 10.1371/journal.pone.0126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dean MC. Comparative observations on the spacing of short-period (von Ebner’s) lines in dentine. Arch. Oral. Biol. 1998;43:1009–1021. doi: 10.1016/S0003-9969(98)00069-7. [DOI] [PubMed] [Google Scholar]
- 45.Dean, M. C. Development, Function and Evolution of Teeth (eds M. F. Teaford, M. M. Smith, & M. W. J. Ferguson) 119–132 (Cambridge University Press, 2000).
- 46.Erickson GM. Daily deposition of dentine in juvenile Alligator and assessment of tooth replacement rates using incremental line counts. J. Morphol. 1996;228:189–194. doi: 10.1002/(SICI)1097-4687(199605)228:2<189::AID-JMOR7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Thin sections of UWBM specimens (118022, 118024, 118025, 118026, 118027, 118028, and 109750) are housed at the University of Washington Burke Museum, thin sections and additional material of LACM-DI 8115/180747 and LACM-DI 8098/160746 are housed at the Natural History Museum of Los Angeles County and thin sections and additional fossil material of SAM-PK-K011603 is housed at the Iziko: South African Museum. Histological images that support this study have been deposited in MorphoBank with the project number 3650 at http://morphobank.org/permalink/?P3650.