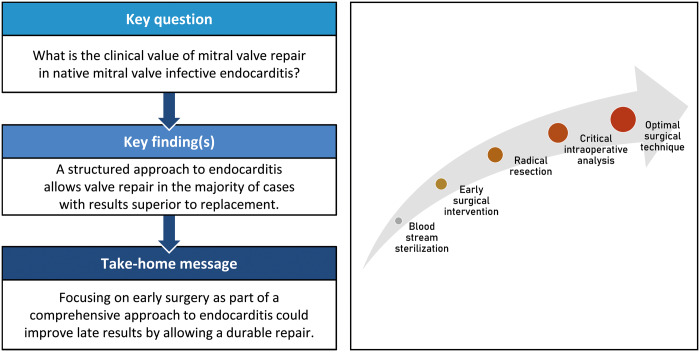
Abstract
OBJECTIVES
Mitral valve repair in native active infective endocarditis is technically challenging. The survival benefit over valve replacement is poorly established and possibly absent because of the high risk of repair failure and reoperation. In this study, we explore the results of our structured approach in these patients.
METHODS
Between January 2000 and January 2017, 149 patients underwent surgery for native mitral infective endocarditis. Among them, 97 (66%) patients underwent valve repair and 52 (34%) underwent valve replacement. Our structured approach consisted of early surgery, radical resection of infected tissue, liberal use of prosthetic materials and ‘patch’ repair techniques. A critical assessment of expected repair durability was made intraoperatively and repair was not performed if concerns of long-term durability existed. To study the effects of valve repair on overall survival, landmark analysis was performed.
RESULTS
In-hospital mortality was 15.4% (14 repair vs 9 replacement patients; P = 0.642). There were no residual infective endocarditis cases or early reoperations. On Cox proportional hazards analysis, valve replacement was not inferior to repair within 1-year post-surgery [hazard ratio (HR) 1.134, 95% confidence interval (CI) 0.504–2.540; P = 0.76]. Beyond 1 year post-surgery, replacement was associated with decreased survival (HR 2.534, 95% CI 1.002–6.406; P = 0.049). There were no differences in freedom from recurrent infective endocarditis (P = 0.47) and mitral valve reintervention (P = 0.52).
CONCLUSIONS
Active mitral valve endocarditis remains a complex disease with significant early and late morbidity and mortality. A structured approach allows valve repair in two-thirds of patients. Clinical results could be improved by focussing on early surgery, prior to extensive valve destruction, to enable durable repairs and improve late outcomes.
Keywords: Mitral valve repair, Mitral valve replacement, Infective endocarditis
INTRODUCTION
Dreyfus et al. [1] were amongst the first to show that mitral valve (MV) repair can be successfully performed even in patients with active native infective endocarditis. This allowed the benefits of valve repair, previously established in the surgical treatment of MV disease of other aetiologies, to be translated to this patient group as well. The expected clinical benefit of valve repair over replacement might, however, be less pronounced than in other types of MV disease due to higher repair complexity and concerns regarding long-term repair durability.
The benefits of MV repair over replacement in active infective endocarditis remain poorly established due to the general lack of properly sized and designed studies [2, 3]. While a number of studies suggested valve repair to be superior to a replacement, they did not consider the time-varying differences in hazard of time-related events as well as time-varying differences in the risk factors related to event occurrence. In previous studies, valve repair has largely been related to a survival benefit in the early postoperative phase. Thereafter, parallel survival curves are often seen [4–6] and studies have failed to explore these observations. Survival in the early postoperative period is largely affected by the wide scope of comorbidities and poor clinical condition that patients with active infective endocarditis usually present with. Patients undergoing valve replacement are usually poorer surgical candidates, providing a partial explanation for the previously observed superiority of valve repair. Moreover, infective endocarditis presents a complex disease, necessitating multimodality treatment and tailoring treatment details to patient and disease characteristics.
The aim of this study was to determine the effectiveness of our centre’s approach to native MV endocarditis; we aimed to critically evaluate our selection criteria for valve repair or replacement and performed a landmark analysis to evaluate the effect of treatment modality on patient survival in the early and late postoperative phase.
METHODS
Patient selection
Patients undergoing MV intervention between January 2000 and January 2017 were potential candidates to be included in the study. Inclusion criteria were active MV infective endocarditis and ≥18 years of age. Patients with a history of MV intervention were excluded. Active infective endocarditis was defined as surgery within 6 weeks after the initiation of antibiotic treatment and/or macroscopic evidence of valve endocarditis and/or positive cultures of valvular tissue obtained during surgical intervention. The final study cohort consisted of 149 patients of whom 97 (65.1%) underwent valve repair and 52 (34.9%) patients underwent valve replacement.
Study methods
The local institutional ethics committee approved this study (number P16.003, date of approval 20 February 2017) and patient consent was obtained to allow data collection and anonymous data analysis. Preoperative, intraoperative and postoperative data were collected from our computerized patient database. Clinical follow-up data were obtained during routine postoperative visits and through telephonic interviews with patients. No patients were lost to follow-up with regards to survival. Median patient survival follow-up time was 5.7 years (interquartile range 3.1–9.2). Clinical follow-up was 97% complete with a median follow-up time of 5.5 years (interquartile range 2.6–9.2).
Perioperative and intraoperative management
Individually tailored patient treatment was based on a structured approach consisting of 5 cornerstones: bloodstream sterilization, early surgical intervention, radical resection of all infected tissue, critical intraoperative assessment of MV reparability and employment of optimal surgical repair and replacement techniques.
The diagnosis of infective endocarditis was based on the modified Duke criteria [7]. Empiric antibiotic therapy was promptly initiated after blood cultures were obtained. After the identification of the causative pathogen, antibiotic therapy was adjusted as needed. Provided that the patients were haemodynamically stable and without evidence of rapid disease progression or uncontrolled infection, a period of 48 h of systemic antibiotic therapy was deemed necessary to secure bloodstream sterilization.
The indication for surgery was based on the respective guidelines [8]. Additionally, surgical intervention was considered in the presence of severe valve regurgitation and low surgical risk, even in the absence of other indications. Typically, after an indication for surgery was established, surgical intervention was performed within a few days to prevent further destruction of infected valve tissue that would possibly prevent a durable MV repair.
All operations were performed by experienced MV surgeons. The surgical intervention consisted of radical resection of all macroscopically infected tissue, regardless of the effect this was to have on the subsequent possibility of valve repair. Once radical resection was performed, the intraoperative field was rinsed with a rifampicin solution. Hereafter, the MV was critically analysed for the possibility of a durable repair. The extensiveness of the infective process observed intraoperatively (e.g. presence of aortic root abscess) beyond the scope of the MV did not affect the decision to attempt valve repair. As a general rule of thumb, at least two-thirds of the free edge of the MV and one commissure needed to remain intact in order to attempt valve repair. We have previously described the technical details of valve repair in cases of infective endocarditis in our centre [9]. Our surgical strategy was based on the principles of MV repair proposed by Carpentier and consisted of preservation or restoration of normal leaflet motion, securing a large area of leaflet coaptation and stabilizing the MV annulus. Prosthetic ring annuloplasty was considered in all cases and performed in 86/97 (88.7%) patients who underwent valve repair. The presence of active infection was not considered a contraindication for prosthetic ring annuloplasty as the probability of residual infection was considered to be lower as a result of radical resection and perioperative systemic antibiotic therapy. On the other hand, annular stabilization was considered beneficial for repair durability; this presumption was based on the common presence of underlying MV disease, the involvement of the MV annulus in the infective process and/or patch leaflet repair that was expected to alter normal valve mechanics.
When a durable repair was deemed technically unfeasible, primary MV replacement was performed. Additionally, valve replacement was performed in case of an unsuccessful repair attempt (residual mitral regurgitation ≥grade 2+ on intraoperative echocardiography). In the case of valve replacement, chordal sparing techniques were employed to prevent postoperative deterioration of left ventricular function. Alternatively, in the presence of extensive destruction resulting in resection of both MV leaflets, implantation of neochordae was performed to restore the valvulo-ventricular continuity.
Oral anticoagulation with a target international normalized ratio of 2.0–3.0 (2.5–3.5 in case of mechanical MV replacement) was continued for 3 months after surgery in case of MV repair with concomitant prosthetic ring implantation or biological MV replacement and indefinitely in case of the mechanical aortic valve or MV replacement. In the presence of other indications, oral anticoagulation was continued as indicated.
End points
Study end points were defined according to the joint Society of Thoracic Surgeons, American Association of Thoracic Surgery and the European Association for Cardio-Thoracic Surgery guidelines [10]. Early mortality was defined as mortality within 30 days of intervention or during the index hospitalization. Secondary end points were the recurrence of infective endocarditis and freedom from MV reintervention.
Statistical analysis
Categorical data are displayed as counts and percentages. Continuous data are displayed as mean ± standard deviation in cases of normally distributed data or median with interquartile range in cases where the data did not adhere to a normal distribution. The normality of distribution was assessed with the Kolmogorov–Smirnoff test. Inter-group comparison of categorical variables was made using the χ2 test and Fisher’s exact test (when the expected value in any of the cells in the contingency table was <5). For continuous data, an independent two-tailed Student’s t-test or Mann–Whitney U-test was used if data were normally or non-normally distributed, respectively.
Survival, freedom from reintervention and recurrence of infective endocarditis rates were calculated and displayed using the Kaplan–Meier method. The log-rank test was used to compare the survival distributions of the 2 groups. Cox proportional hazards models at different time points were fit to identify the risk factors for mortality as a function of time after the intervention. Specifically, for each of the following periods: intervention to follow-up closure, intervention to 1 year (early phase) and 1 year after the intervention to follow-up closure (late phase), we developed a Cox proportional hazards model to determine the risk factors related to the event occurrence. The cut-off of 1 year was based on the clinical assumption that, in this complex patient group, the preoperative and perioperative factors influence the possibility of event occurrence (mortality) primarily during this period while the influence of treatment modality (repair or replacement) is expected to affect event occurrence primarily in the later phase. This was supported by graphical analysis of the Kaplan–Meier curves where the estimated hazard of event occurrence stabilized after 1 year after surgery for both groups. For each model, a univariable analysis was initially performed. Variables demonstrating a P-value <0.20 were included in the multivariable model with a backward selection method. Treatment modality (repair or replacement) was forced into the model. Variables included in the univariable and multivariable analyses can be found in Supplementary Material, Tables S1 and S2. Statistical analyses were performed with SPSS statistical software package (Version 23.0. Armonk, NY, USA; IBM Corp.).
RESULTS
Baseline characteristics
The baseline characteristics of the whole study population are presented in Table 1. There was a significantly higher proportion of male patients in the repair group. In general, the prevalence of extracardiac comorbidities was comparable with the exception of diabetes mellitus that was less often present in the repair group. Microorganisms from the Streptococcus group were the most common causative microorganism identified and a considerable proportion of patients from both groups presented with a history of clinically manifested peripheral embolism. Several MV specific characteristics differed significantly between the two groups with underlying MV disease and annular involvement more frequently seen amongst replacement patients.
Table 1:
Baseline patient characteristics
| Mitral valve repair (n = 97) | Mitral valve replacement (n = 52) | P-value | |
|---|---|---|---|
| Age (years) | 57 ± 13 | 61 ± 13 | 0.005 |
| Male gender | 20 (20.6) | 22 (42.3) | 0.005 |
| Hypertension | 35 (36.1) | 20 (38.4) | 0.774 |
| Renal impairment | |||
| CC <50 mmol/min | 46 (47.4) | 28 (53.8) | 0.495 |
| Preoperative dialysis | 4 (4.1) | 6 (11.5) | 0.098 |
| Chronic lung disease | 8 (8.2) | 6 (11.5) | 0.512 |
| Diabetes mellitus | 8 (7.9) | 13 (25.0) | 0.005 |
| Symptomatic mitral regurgitation | 34 (35.1) | 16 (30.8) | 0.598 |
| Atrial fibrillation | 18 (18.6) | 5 (9.6) | 0.150 |
| Impaired left ventricular function | 25 (25.8) | 8 (15.4) | 0.145 |
| Previous cardiac surgery | 24 (24.7) | 6 (11.5) | 0.055 |
| Peripheral embolism | 23 (23.7) | 17 (32.7) | 0.238 |
| Causative micro-organism | 0.453 | ||
| Streptococcus spp. | 52 (53.6) | 27 (51.9) | |
| Staphylococcus aureus | 14 (14.4) | 11 (21.2) | |
| Staphylococcus spp. (other) | 5 (5.2) | 1 (1.9) | |
| Enterococcus faecalis | 10 (10.3) | 8 (15.4) | |
| Other or culture-negative | 16 (16.5) | 5 (9.6) | |
| Underlying mitral valve disease | 24 (24.7) | 27 (51.9) | 0.001 |
| Annular infection | 6 (6.2) | 12 (23.1) | 0.003 |
| Annular calcification | 6 (6.2) | 9 (17.3) | 0.035 |
| Concomitant surgery | |||
| Aortic valve surgerya | 46 (47.4) | 23 (44.2) | 0.710 |
| Tricuspid valve surgery | 19 (19.6) | 12 (23.1) | 0.617 |
| CABG | 12 (12.4) | 4 (7.7) | 0.379 |
| Aortic cross clamp time (min) | 193 ± 99 | 208 ± 123 | 0.537 |
| Cardiopulmonary bypass time (min) | 245 ± 134 | 246 ± 110 | 0.935 |
| Primary indications for surgery | 0.100 | ||
| Heart failure | 8 (8.2) | 8 (15.4) | |
| Uncontrolled infection | 29 (29.8) | 11 (22.2) | |
| Prevention of embolism | 20 (20.6) | 19 (36.5) | |
| Severe mitral regurgitation | 35 (36.1) | 12 (23.1) | |
| Severe aortic regurgitation | 5 (5.2) | 2 (3.8) | |
Data are presented as n (%) or means ± standard deviations.
Aortic root replacement in 42 patients, aortic valve replacement in 22 patients and aortic valve repair in 5 patients.
CABG: coronary artery bypass surgery; CC: creatinine clearance.
Perioperative mortality and morbidity
Intraoperative details and perioperative morbidity and mortality data can be found in Supplementary Material, Table S1 and Table 2, respectively. Early mortality was 15.4% (23 patients) without significant differences between both groups [14.4% (14/97) for the repair group and 17.3% (9/52) for the replacement group, P = 0.64]. There were 5 (3.3%) intraoperative deaths, 2 (2.1%) in the repair group and 3 (5.7%) in the replacement group (P = 0.34). Ventricular failure was the cause of death in 1 repair and 3 replacement patients and uncontrollable haemorrhage was the cause of death in the remaining repair patient. Postoperatively, the most common cause of death was multi-organ failure followed by ventricular failure. No cases of residual infective endocarditis were observed. The incidence of prolonged mechanical ventilation and renal failure was relatively high but without significant differences between groups.
Table 2:
Postoperative morbidity and mortality
| Mitral valve repair (n = 97) | Mitral valve replacement (n = 52) | P-value | |
|---|---|---|---|
| Intraoperative mortality | 2 (2.1) | 3 (5.7) | 0.343 |
| Ventricular failure | 1 (1.0) | 3 (5.7) | |
| Haemorrhage | 1 (1.0) | 0 (0) | |
| In-hospital mortality (excluding intraoperative mortality) | 12 (12.4) | 6 (11.5) | 0.947 |
| Multi-organ failure | 6 (6.1) | 1 (1.9) | |
| Ventricular failure | 3 (3.1) | 2 (3.8) | |
| Myocardial infarction | 0 (0) | 1 (1.9) | |
| Rhythm abnormality | 1 (1.0) | 0 (0) | |
| Disseminated intravascular coagulation | 1 (1.0) | 0 (0) | |
| Cardiac tamponade | 0 (0) | 1 (1.9) | |
| Sudden death | 1 (1.0) | 0 (0) | |
| Intensive care unit stay (days) | 2 (1–5) | 2 (1–5) | 0.804 |
| Mechanical circulatory support | 9 (9.3) | 3 (5.8) | 0.543 |
| Prolonged mechanical ventilation (>48 h) | 23 (23.7) | 9 (17.3) | 0.527 |
| Re-exploration | 15 (15.5) | 6 (11.5) | 0.803 |
| Stroke | 2 (2.1) | 2 (3.8) | 0.605 |
| Renal failure | 16 (16.5) | 10 (19.2) | 0.649 |
| Pacemaker implantation | 8 (8.2) | 3 (5.8) | 0.749 |
Data are presented as n (%) or medians (IQR).
CABG: coronary artery bypass surgery; CC: creatinine clearance; IQR: interquartile range.
Overall survival
For the whole study population (all 149 patients), 10-year survival rates of 66.5% [95% confidence interval (CI) 55.5–77.5] and 36.9% (95% CI 11.2–62.6) were seen in the repair and replacement groups, respectively (P = 0.052; Figure 1). Cox proportional hazards regression analysis revealed patient age, preoperative dialysis, chronic lung disease and preoperative new-onset atrioventricular block as risk factors associated with mortality (Supplementary Material, Tables S2 and S3). In this analysis, MV replacement was not identified as a statistically significant risk factor for mortality [hazard ratio (HR) 1.430, 95% CI 0.760–2.690; P = 0.27].
Figure 1:
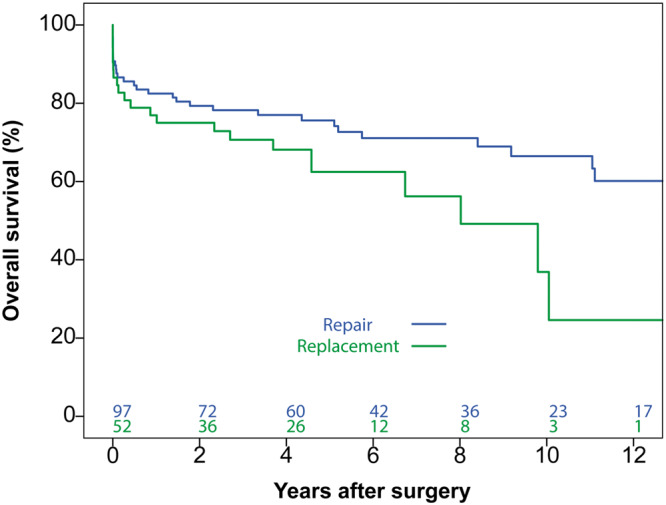
Overall survival for all patients for the whole study period. No statistically significant difference was observed between repair and replacement groups (P = 0.052).
Early phase survival
Of the whole study population, 29 patients had died within one year after surgery (17 repair and 12 replacement patients; Figure 2). Six patients died after discharge, 2 of unknown causes and 1 due to progressive heart failure in the repair group and 2 of unknown causes and 1 due to natural causes in the replacement group. In the early phase, patient age, preoperative dialysis, impaired left ventricular function and preoperative new-onset atrioventricular block were identified as risk factors associated with mortality (Supplementary Material, Tables S2 and S3). MV replacement was not identified as a significant risk factor for mortality in this phase (HR 1.134, 95% CI 0.504–2.540; P = 0.76).
Figure 2:
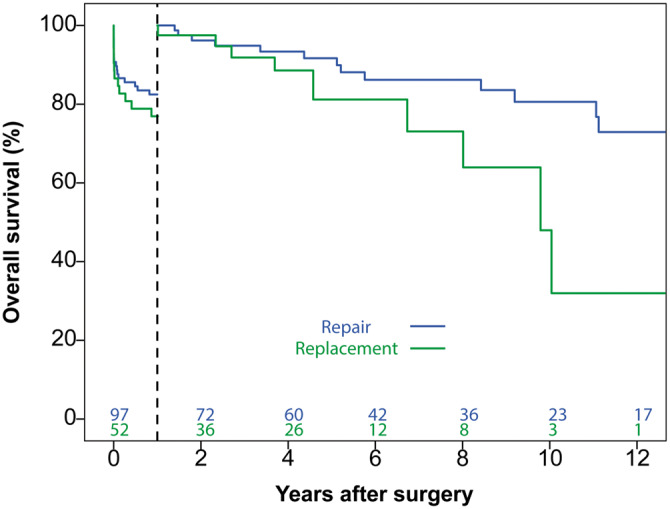
Overall survival in the early and late phase after surgery. No difference in overall survival was observed in the early phase (within 1 year after surgery). A significant difference in overall survival, favouring mitral valve repair, was observed in the late phase (beyond 1 year after surgery).
Late phase survival
One year after surgery, 120 patients (80 repair, 40 replacement) were still alive and available for the late phase analysis (Figure 2). The baseline characteristics of this patient population can be found in Supplementary Material, Table S4. The differences in patient characteristics between both groups resembled the differences observed in the whole patient population with more male patients and a lower prevalence of diabetes mellitus in the repair group. Interestingly, only 4/10 patients on preoperative dialysis were still alive 1 year after the operation. A total of 23 patients died in the late phase follow-up (13 repair and 10 replacement patients), of which 2 patients in the replacement died due to complications from reoperation, 6 due to unknown causes (2 repair vs 4 replacement), 2 due to terminal heart failure, 1 due to an intracranial haemorrhage (after previous mechanical MV replacement) and 12 due to non-cardiac, non-valve related causes.
In the late phase (Supplementary Material, Tables S2 and S3), MV replacement was the only statistically significant risk factor for mortality (HR 2.534, 95% CI 1.002–6.406; P = 0.049).
Recurrence and freedom from reintervention
Six patients developed recurrent infective endocarditis (5 in the repair group and 1 in the replacement group) without any significant differences between groups (P = 0.47; Figure 3). None of the recurrences occurred within one year of the initial operation. The diagnosis was established by the modified Duke criteria and no patients required reoperation due to recurrent MV endocarditis.
Figure 3:
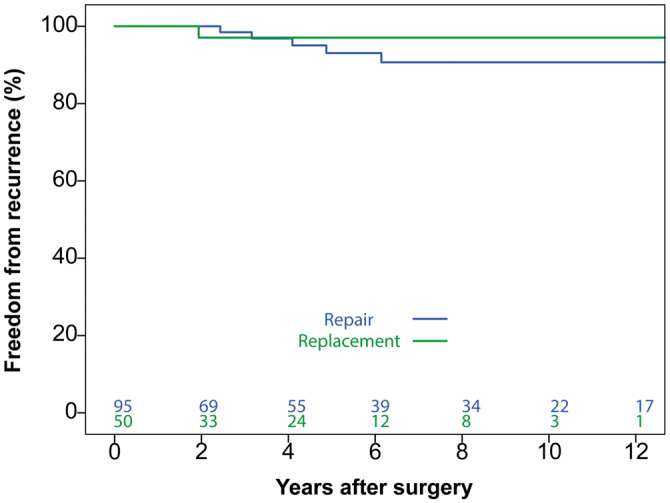
Freedom from recurrence of infective endocarditis. No significant difference was observed between repair and replacement groups (P = 0.47).
The 10-year freedom from MV reoperation rates were 88.3% (95% CI 80.7–95.9) and 61.1% (95% CI 23.9–98.3) for repair and replacement groups, respectively (P = 0.52; Figure 4). Amongst the repair group, 8 patients underwent MV reintervention (7 due to severe regurgitation and 1 due to MV stenosis). Causes of valve regurgitation were patch failure (tear/perforation) in 5 patients, patch calcification in 1 patient and commissural leaflet stiffness causing coaptation failure in 1 patient. The cause of the MV stenosis was attributed to extensive pannus formation. Amongst the MV replacement group, 5 patients underwent reintervention. The indication for reintervention was structural valve degeneration of the previously implanted bioprosthetic valve in all cases.
Figure 4:
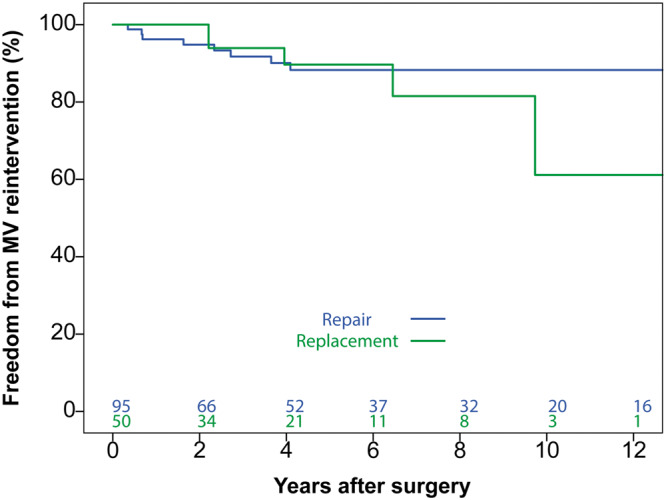
Freedom from mitral valve reintervention. No significant difference was observed between repair and replacement groups (P = 0.52).
DISCUSSION
Our results demonstrate that a structured approach to active native MV infective endocarditis will allow valve repair to be performed in about two-thirds of patients. Moreover, despite the liberal use of prosthetic materials and extensive use of patch techniques to reconstruct valvular and non-valvular cardiac structures, no residual infections occurred. The survival benefit of MV repair was not evident when both groups were compared during the entire follow-up period. However, when a landmark analysis was performed, improved patient survival in the late phase (beyond 1 year after surgery) was seen in patients who underwent valve repair.
Our study demonstrated that treatment modality significantly impacts clinical results. However, we believe that the optimization of perioperative care and the implementation of optimal surgical techniques, including stringent resection of all infected tissue, remain the true foundations of the treatment of infective endocarditis. Preoperative decision-making, in particular the timing of surgery and expansion of the indications for surgical intervention, played a crucial role in the intraoperative possibility of a durable repair to be performed. The timing of surgery is a matter of debate as the diagnosis of infective endocarditis is often delayed and preceded by a period of unspecific symptoms and undetected valve destruction; even early surgery will thus include a time period in which the disease is left untreated. Once an indication for surgery is established, operative correction should, in our opinion, promptly be performed. Respective guidelines advise emergency surgery to be performed only in case of refractory pulmonary oedema or cardiogenic shock [8]. For other indications, the timing of surgery is less clear and it is recommended that surgical intervention should be performed within a few days. This seems somehow contradictory to the results of a randomized trial by Kang [11] who demonstrated that, in case of left-sided infective endocarditis, early surgery (within 48 h) was effective at reducing disease-related morbidity when compared to the timing of surgery according to the guidelines (absolute risk reduction of 21%). Moreover, peripheral embolization is known to most commonly occur within the first days after the diagnosis of infective endocarditis, before the effect of systemic antibiotic therapy is achieved [12]. In the presence of an indication for surgery (heart failure, uncontrolled infection or prevention of embolism), the benefit of delaying surgery seems questionable and carries a considerable risk of haemodynamic deterioration, disease progression and peripheral embolization. We adhere to our policy of systemic antibiotic therapy for a period of 48 h to secure bloodstream sterilization and prevent the occurrence of residual endocarditis, when clinically feasible.
We believe that the timing of surgery has an important effect on the durability of repair. As recently demonstrated by Perotta et al. [4], non-radical resection of infected tissue will result in a considerable risk of residual infection. Radical resection has to be performed in all patients without considering its impact on the subsequent possibility of valve repair. In the presence of extensive valve destruction, valve replacement will be inevitable. Early surgery will prevent ongoing tissue destruction and enable durable repair to be performed. The repair rate in our study was relatively high but clearly lower than the repair rate of 80.7%, previously reported by de Kerchove et al. [13] in a comparable group of patients. We were reluctant to use very complex repair techniques (e.g. partial MV replacement with a homograft) due to the possible negative effect this may have on repair durability [14]. Early surgical intervention will enable a less complex and more durable valve repair and will hopefully help improve the outcomes of surgical intervention for MV infective endocarditis. In the past, concerns regarding potential colonization of newly implanted prosthetic materials in the setting of active infection have been raised [15]. However, the preventive methods used in our experience, such as systemic antibiotic therapy for a period of 48 h, radical resection and rinsing the operative field with rifampicin, were sufficient in preventing prosthetic material infection, as shown by the absence of such cases in our patient population. Radical tissue resection and unpredictable extent of native MV destruction often necessitate complex surgical techniques with long cardiopulmonary bypass times and surgery should best be reserved for experienced surgeons in all cases. As the incidence of infective endocarditis is low, centralization of care seems feasible as well as reasonable.
In a recent multicentre study, including 1970 patients undergoing surgery for active native MV infective endocarditis, Toyoda et al. [5] suggested that valve repair [performed in 367/1970 (19%) patients] was associated with better survival when compared to valve replacement. Interestingly, Kaplan–Meier analysis demonstrated that the survival benefit was largely based on the early postoperative period (within 1 year after surgery). Thereafter, the survival curves of the repair and replacement groups ran approximately parallel to one another. A comparable trend can be observed in a number of other studies on this topic [4–6]. To date, studies have failed to explore these observations and did not take into account the time-related differences in the hazards of event occurrence (mortality) as well as the differences in the risk factors related to mortality at different time periods.
Unlike previous studies, we failed to observe a survival benefit of valve repair in the early postoperative period. We speculate that this is related to the fact that, in our experience, the decision to perform valve repair was based predominantly on the extensiveness of the infective process with patient comorbidities playing only a minor role. The survival benefit of valve repair in the early postoperative period is complex to understand as the clinical benefits of repair (e.g. lower risk of recurrent infective endocarditis, thromboembolic and bleeding complications) are predominantly expected to appear in the late postoperative phase. In the case of valve repair with prosthetic annuloplasty, oral anticoagulation is indicated for a period of 3 months. Therefore, early complications related to oral anticoagulation use are, at least in our experience where annuloplasty was performed in almost 90% of repair patients, expected to affect the postoperative course after valve repair as well.
Interestingly, the freedom from recurrent infective endocarditis was lower in the valve replacement group, a difference that failed to reach statistical significance. This contradicts the observations from previous reports [4, 5]. However, the diagnosis was based on the modified Duke criteria that are not always accurate at diagnosing prosthetic valve endocarditis [16]. In all cases, a conservative approach was chosen and all patients recovered. On the other hand, the freedom from recurrent infective endocarditis in the valve replacement group was lower than previously reported [4, 5]. The high rates of recurrent endocarditis after valve replacement in previous studies might have been related to residual infection. Should an optimal surgical strategy be implemented, the freedom from recurrent endocarditis should not be different than the freedom observed after valve replacement for degenerative MV disease [17].
Limitations
Our study is retrospective in nature and subjected to flaws inherent to this type of study design. Importantly, the extensiveness of disease differed between patient groups and patients undergoing valve replacement demonstrated more extensive valve destruction. Moreover, other inter-group differences in patient characteristics could have affected the results observed. Appropriate statistical analyses were conducted to compensate for the absence of randomization and inter-group differences. Randomization in this patient group is most likely not feasible due to the low incidence of disease and high variety of disease presentations. We believe that the results observed confirm the known clinical benefits of MV repair over MV replacement, previously demonstrated for other types of MV disease, additionally supporting the validity of our results.
CONCLUSION
Infective endocarditis of the native MV is associated with high mortality and morbidity rates. Valve repair provides comparable early results and improved late survival when compared to replacement. The concept of a structured approach to MV infective endocarditis enables the identification of patients suitable for a durable repair. Clinical results could be improved by focussing on early surgery, prior to extensive valve destruction, to enable durable repairs and help further improve late outcomes.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.
Author contributions
Rufin J. Defauw: Conceptualization; Data curation; Writing—original draft. Anton Tomsic: Conceptualization; Data curation; Methodology; Writing—original draft. Thomas J. van Brakel: Writing—review & editing. Nina Ajmone Marsan: Writing—review & editing. Robert J.M. Klautz: Conceptualization; Supervision; Writing—review & editing. Meindert Palmen: Conceptualization; Supervision; Writing—review & editing.
Supplementary Material
Abbreviations
- CI
Confidence interval
- HR
Hazard ratio
- MV
Mitral valve
Presented at the 2019 Annual Meeting of the Heart Valve Society, Sitges, Spain, 11–13 April 2019.
REFERENCES
- 1. Dreyfus G, Serraf A, Jebara VA, Deloche A, Chauvaud S, Couetil JP. et al. Valve repair in acute endocarditis. Ann Thorac Surg 1990;49:706–11; discussion 12–3. [DOI] [PubMed] [Google Scholar]
- 2. Feringa HH, Shaw LJ, Poldermans D, Hoeks S, van der Wall EE, Dion RA. et al. Mitral valve repair and replacement in endocarditis: a systematic review of literature. Ann Thorac Surg 2007;83:564–70. [DOI] [PubMed] [Google Scholar]
- 3. Liu JZ, Li XF, Miao Q, Zhang CJ.. Surgical treatment of active native mitral infective endocarditis: a meta-analysis of current evidence. J Chin Med Assoc 2018;81:147–54. [DOI] [PubMed] [Google Scholar]
- 4. Perrotta S, Frojd V, Lepore V, Schersten H, Jeppsson A, Svensson G.. Surgical treatment for isolated mitral valve endocarditis: a 16-year single-centre experience. Eur J Cardiothorac Surg 2018;53:576–81. [DOI] [PubMed] [Google Scholar]
- 5. Toyoda N, Itagaki S, Egorova NN, Tannous H, Anyanwu AC, El-Eshmawi A. et al. Real-world outcomes of surgery for native mitral valve endocarditis. J Thorac Cardiovasc Surg 2017;154:1906–12.e9. [DOI] [PubMed] [Google Scholar]
- 6. Solari S, De Kerchove L, Tamer S, Aphram G, Baert J, Borsellino S. et al. Active infective mitral valve endocarditis: is a repair-oriented surgery safe and durable? Eur J Cardiothorac Surg 2019;55:256–62. [DOI] [PubMed] [Google Scholar]
- 7. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T. et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 8. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F. et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–128. [DOI] [PubMed] [Google Scholar]
- 9. Tomsic A, Versteegh MIM, Ajmone Marsan N, van Brakel TJ, Klautz RJM, Palmen M.. Early and late results of surgical treatment for isolated active native mitral valve infective endocarditis. Interact CardioVasc Thorac Surg 2018;26:610–16. [DOI] [PubMed] [Google Scholar]
- 10. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL. et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490–5. [DOI] [PubMed] [Google Scholar]
- 11. Kang DH. Timing of surgery in infective endocarditis. Heart 2015;101:1786–91. [DOI] [PubMed] [Google Scholar]
- 12. Dickerman SA, Abrutyn E, Barsic B, Bouza E, Cecchi E, Moreno A. et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J 2007;154:1086–94. [DOI] [PubMed] [Google Scholar]
- 13. de Kerchove L, Vanoverschelde JL, Poncelet A, Glineur D, Rubay J, Zech F. et al. Reconstructive surgery in active mitral valve endocarditis: feasibility, safety and durability. Eur J Cardiothorac Surg 2007;31:592–9. [DOI] [PubMed] [Google Scholar]
- 14. Zegdi R, Khabbaz Z, Flecher E, Latremouille C, Noghin M, Chauvaud S. et al. Management of commissural lesions in native mitral valve endocarditis: long-term results of valve repair and partial homograft replacement. J Heart Valve Dis 2006;15:356–9. [PubMed] [Google Scholar]
- 15. Renzulli A, Carozza A, Romano G, De Feo M, Della Corte A, Gregorio R. et al. Recurrent infective endocarditis: a multivariate analysis of 21 years of experience. Ann Thorac Surg 2001;72:39–43. [DOI] [PubMed] [Google Scholar]
- 16. Tanis W, Scholtens A, Habets J, van den Brink RB, van Herwerden LA, Chamuleau SA. et al. CT angiography and 18F-FDG-PET fusion imaging for prosthetic heart valve endocarditis. JACC Cardiovasc Imaging 2013;6:1008–13. [DOI] [PubMed] [Google Scholar]
- 17. Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF. et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation 2017;135:410–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.