Abstract
Context
The relationship between plasma fibroblast growth factor 21 (FGF21), insulin resistance, and steatohepatitis has not been systematically assessed.
Objective
To determine if higher plasma FGF21 is associated with worse steatohepatitis on liver biopsy in patients with nonalcoholic fatty liver disease (NAFLD).
Design and Setting
Cross-sectional study in a university hospital.
Patients Interventions and Main Outcome Measures
Patients with a body mass index >25 (n = 187) underwent: (i) euglycemic hyperinsulinemic clamp to assess tissue-specific insulin resistance (IR); (ii) liver magnetic resonance spectroscopy for intrahepatic triglyceride quantification, (iii) liver biopsy (if NAFLD present; n = 146); and (iv) fasting plasma FGF21 levels.
Methods and Results
Patients were divided into three groups: (i) No NAFLD (n = 41); (ii) No nonalcoholic steatohepatitis (NASH) (patients with isolated steatosis or borderline NASH; n = 52); and (iii) NASH (patients with definite NASH; n = 94). Groups were well-matched for age/sex, prevalence of type 2 diabetes mellitus, and hemoglobin A1c. During euglycemic hyperinsulinemic insulin clamp, insulin sensitivity in skeletal muscle and adipose tissue worsened from No NAFLD to NASH (both P < 0.001). Plasma FGF21 levels correlated inversely with insulin sensitivity in adipose tissue (r = −0.17, P = 0.006) and skeletal muscle (r = −0.23, P = 0.007), but not with liver insulin sensitivity. Plasma FGF21 was higher in patients with NASH (453 ± 262 pg/mL) when compared with the No NASH (341 ± 198 pg/mL, P = 0.03) or No NAFLD (325 ± 289 pg/mL, P = 0.02) groups. Plasma FGF21 increased with the severity of necroinflammation (P = 0.02), and most significantly with worse fibrosis (P < 0.001), but not with worsening steatosis (P = 0.60).
Conclusions
Plasma FGF21 correlates with severity of steatohepatitis, in particular of fibrosis, in patients with NASH. Measurement of FGF21 may help identify patients at the highest risk of disease progression.
We assessed the relationship between plasma FGF21 levels and steatohepatitis in patients with NAFLD. We found that higher FGF21 levels correlated with the severity of liver fibrosis.
Nonalcoholic fatty liver disease (NAFLD), a frequently overlooked complication of obesity, it is characterized by insulin resistance (IR) and hepatic triglyceride accumulation in the absence of other identifiable causes for hepatic steatosis (1). Its more severe form known as nonalcoholic steatohepatitis (NASH) is characterized by hepatocyte necrosis, inflammation, and often fibrosis (1). Although the mechanisms of progression from obesity to NAFLD and NASH are poorly understood, there are several lines of evidence to link fibroblast growth factor 21 (FGF21) to liver pathophysiology (2) and to suggest that effects in the liver of FGF21 lead to decreased fat accumulation and reduced inflammation and fibrosis (2).
In the past decade, FGF21 has emerged as a metabolic regulator that under certain stimuli (i.e., fasting, ketogenic diet, cold exposure) can increase energy expenditure (3), stimulate insulin sensitivity, and induce weight loss when administered as a pharmacological treatment (4). FGF21 is widely expressed in liver (3, 5) and adipose tissue (6) and can act on multiple target tissues in an endocrine or paracrine fashion (3). In the liver, FGF21 plays an important role in the regulation of hepatic glucose production and in regulation of fatty acid oxidation (3), possibly through activation of peroxisome proliferator-activated receptor-gamma coactivator-1α and enhancing mitochondrial function and biogenesis (2, 3), although the link remains controversial (7). Circulating FGF21 derives largely from the liver and correlates well with hepatic mRNA expression (2, 8). Li et al. (5) reported a correlation between liver mRNA expression of FGF21 concentration and intrahepatic triglyceride content (IHTG). Paradoxical to these beneficial metabolic effects of FGF21, FGF21 serum levels are elevated in insulin-resistant states such as obesity (9, 10), and are about twofold higher in NAFLD (5), suggesting that FGF21 action and signaling may be impaired in these conditions (2, 11) or that FGF21 levels may be increased compensatory to maintain insulin sensitivity (12).
As previously shown by work of Fisher et al. (11), Maratos-Flier (2, 3) and others (12, 13), in the context of obesity and IR, insulin levels rise compensatory and drive increased hepatic lipogenesis, which in turn triggers increase in liver-derived FGF21. Because FGF21 rises with obesity and IR, within the liver, resistance to FGF21 signaling develops, so that the maximum actions to drive fatty acid oxidation and reduce inflammation are not achieved (2). However, similar to IR, pharmacologic doses of FGF21 exogenously can compensate for this (2).
Administration of FGF21 analogs (LY2405319) (14) or pegylated FGF21 (BMS-986036) daily or weekly in overweight or obese subjects with type 2 diabetes (15, 16) results in increased adiponectin levels and improved fasting glucose, whole-body insulin sensitivity, and lipid profile (15, 16). Modest weight loss (of 1.8%) when compared from baseline was also reported in a short-term study (14).
In animal models of NAFLD, FGF21 showed anti-inflammatory and antifibrotic effects in the liver (2). Recently, treatment with the FGF21 analog (BMS-986036) was reported to decrease hepatic steatosis in patients with NASH (F1-F3) (16) and plasma level of pro-C3, a fibrosis marker (15). However, there is an incomplete understanding of the relationship between FGF21 levels and the severity of liver disease in NASH.
Therefore, the aim of this study was to determine the role of FGF21 in NAFLD and NASH as assessed by severity of liver histology and its relationship with gold-standard measures of IR in liver, muscle, and adipose tissue.
Materials and Methods
Subjects
Participants were recruited from the general population of San Antonio, TX, via newspaper advertisements and from the endocrinology and hepatology clinics at Audie L. Murphy Veterans Administration Medical Center, San Antonio, TX, and the University of Texas Health Science Center at San Antonio, TX. All patients had a screening 2-hour oral glucose tolerance test (OGTT) to diagnose or confirm a diagnosis of prediabetes or diabetes. Prediabetes was defined as impaired fasting glucose [5.6 to 6.9 mmol/L (100 to 125 mg/dL)], impaired glucose tolerance [7.8 to 11.1 mmol/L (140 to 199 mg/dL) on an OGTT], or a hemoglobin A1c (A1c) level of 5.7% to 6.4%. Diabetes was defined as fasting glucose ≥126 mg/dL and either a 2-hour glucose ≥200 mg/L on OGTT or A1c ≥6.5%. Patients were excluded from the study if they had a history of alcohol abuse (≥30 g/d in men and over ≥20 g/d in women) (17), liver disease other than NAFLD/NASH (i.e., hepatitis B/C, autoimmune hepatitis, hemochromatosis, Wilson disease, or other), type 1 diabetes mellitus, or if they exhibited clinically important renal, cardiac, pulmonary, or neurologic disease. Some of the patients analyzed here have been reported previously regarding elevation of plasma aminotransferases in NAFLD (18, 19) or participated in an earlier clinical trial (20). The study was approved by the University of Texas Health Science Center at San Antonio’s Institutional Review Board and written informed consent was obtained from each participant before participation.
Study design
For this cross-sectional analysis, we recruited 187 subjects with a body mass index (BMI) >25 kg/m2. A complete medical history, physical examination, electrocardiogram, comprehensive metabolic and lipid panel, and A1c were performed in all patients at screening. In addition, we measured (i): fasting plasma FGF21 levels using ELISA (quantitative human FGF21 ELISA; R&D Systems, Minneapolis, MN) (ii); intrahepatic triglyceride content by magnetic resonance imaging (MRI) and spectroscopy (1H-MRS) (iii); body composition and total body fat by dual-energy X-ray absorptiometry (DXA, Hologic Inc., Waltham, MA) (iv); visceral adipose tissue by MRI; and (v) insulin sensitivity in the liver, skeletal muscle, and adipose tissue during fasting or euglycemic hyperinsulinemic clamp with 3-3H-glucose infusion (DuPont-NEN, Boston, MA). All patients underwent a two-step screening algorithm for NAFLD: they underwent liver 1H-MRS, followed by a liver biopsy if the hepatic triglyceride content was higher than 5.56% to assess for the presence of NASH (N = 146).
IHTG
For the measurement of hepatic fat content, localized proton nuclear MR spectra of the liver were acquired on three different areas of the liver (30 × 30 × 30 mm each) as previously described (21). Percentage of hepatic triglyceride content was calculated as the area under the curve of fat peak divided by area under the curve of fat plus water peak, multiplied by 100. Measurements were corrected for T1 and T2 relaxation. A liver fat content of ≥5.56% was considered diagnostic of NAFLD as previously defined (22).
Euglycemic hyperinsulinemic clamp
Patients were admitted to the research unit after a 12-hour overnight fast and insulin sensitivity was measured as previously reported by our group (20, 23, 24). In brief, a catheter was inserted into an antecubital vein with a three-tailed Y-connector for infusion of all test substances; a second catheter was inserted retrogradely into an ipsilateral wrist vein. The hand was kept in a heated box at 55°C for collection of arterialized blood sampling. A primed [25 μCi 3 (fasting glucose/100)] continuous (0.25 μCi/min) infusion of 3-3H glucose was initiated and continued until completion of the study. During the last 30 minutes of the basal equilibration period (150 to 180 minutes), plasma samples were taken every 5 to 10 minutes for determination of plasma glucose, insulin concentrations, free fatty acids (FFAs), and 3-3H glucose–specific activity. Subsequent to the basal equilibration period, insulin was administered as a primed continuous infusion at 10 mU/m2 per minute for 120 minutes to assess suppression of endogenous glucose production (EGP), followed by another 120 minutes at an infusion rate of 80 mU/m2 per minute to assess whole-body insulin-stimulated glucose disappearance (Rd). A variable infusion of 20% glucose was adjusted to maintain plasma glucose concentration at 90 to 100 mg/dL with a coefficient of variation <5%. Plasma samples were collected every 5 to 10 minutes for determination of plasma glucose, insulin, FFA concentrations, and 3-3H glucose–specific activity. Liver and adipose tissue IR were measured as percentage of suppression of EGP (∼90% coming from the liver) and FFA concentration (a reflection of suppression of lipolysis) respectively, during low-dose insulin infusion. Finally, skeletal muscle IR was measured by calculating Rd during the high-dose insulin infusion (25, 26).
Liver biopsy
An ultrasound-guided liver biopsy was performed in patients with NAFLD (>5.56% liver fat) by MRI and 1H-MRS. Biopsies were evaluated in a blinded fashion (i.e., with the pathologist unaware of the participants’ identity or clinical information). Histological characteristics for the diagnosis of NASH were determined on low- to medium-power microscopy based on the presence of at least grade 1 in each of the following three components (i): steatosis (5% to 33% of parenchyma is involved for grade 1, >33% to 66% for grade 2, and >66% for grade 3); (ii) lobular inflammation (<2 foci are present per ×200 field for grade 1, 2, to 4 foci for grade 2, and >4 foci for grade 3); and (iii) hepatocellular ballooning (few or many ballooning cells are present per high-power field for grade 1 or 2, respectively). NAFLD Activity Score (NAS) was determined as the sum of scores of steatosis (0 to 3), lobular inflammation (0 to 3), and hepatocellular ballooning (0 to 2) (27). Necroinflammation score was determined as the combined scores of lobular inflammation and hepatocellular ballooning (0 to 5).
Statistical analysis
Data were expressed as mean ± SD or mean ± SE for numeric variables or as a percentage for categorical variables. Categorical variables were compared performing χ2 or Fisher’s exact test. Comparisons among groups were performed with ANOVA (Bonferroni method for post hoc testing) or the Kruskal-Wallis test. Pearson or Spearman correlations were used for numerical variables according to their characteristics. A two-tailed P < 0.05 was considered to indicate statistical significance. For the linear and logistic regression analysis, β coefficient or odds ratios and the 95% CIs were estimated respectively. Analyses were performed with Stata V.11.1 (StataCorp LP, College Station, TX).
Results
Patients’ characteristics
We recruited 187 patients with BMI >25 kg/m2 and divided them into three groups based on IHTG content and liver histology results: patients without NAFLD (No NAFLD group), patients with NAFLD but without definite NASH (No NASH group), and patients with definite NASH (NASH group). Patients in the No NAFLD group were defined as having <5.56% liver fat on liver 1H-MRS (n = 41). These patients did not have a liver biopsy. Patients with >5.56% liver fat on 1H-MRS but without a diagnosis of definite NASH by standard histological criteria were labeled as No NASH (28) (n = 52). About 65% of participants in the No NASH group had isolated steatosis (steatosis alone or steatosis with minimal inflammation) on liver biopsy and 35% of patients had steatosis but did not meet criteria for definite NASH (i.e., missed one point for either steatosis, inflammation, or ballooning). The final group, NASH, included those patients with >5.56% liver fat on 1H-MRS and definite NASH by standard histological criteria, with or without fibrosis (17, 28) (n = 94).
Clinical and laboratory characteristics
In Table 1, we have summarized patients’ clinical characteristics, dividing them into three groups as outlined previously. The cohort included patients between 24 to 71 years age of; 80% were obese. Groups were well-matched for sex, A1c, and prevalence of type 2 diabetes mellitus (no important difference between groups). Patients without NAFLD had a lower BMI (32.1 ± 3.8 kg/m2) compared with the NASH group (34.8 ± 4.8 kg/m2), P < 0.05. As shown in Table 1, when compared with the No NAFLD group, visceral fat, liver fat, total cholesterol, triglycerides, liver enzymes, and fasting plasma insulin were higher in the NASH group (all P < 0.05). Additionally, subjects with NASH had higher liver enzymes and triglycerides when compared with the No NASH group (all P < 0.05) and lower high-density lipoprotein-cholesterol when compared with the No NAFLD (P < 0.05) group. Plasma cytokeratin-18 (CK-18) levels were significantly higher in those with NASH when compared with both the No NAFLD and No NASH groups. There were no substantial differences in A1c, fasting plasma glucose, adiponectin levels, or high-sensitivity C-reactive protein among the three groups (Table 1).
Table 1.
Clinical and Laboratory Characteristics of Subjects
| No NAFLD (N = 41) | No NASH (N = 52) | NASH (N = 94) | P (by ANOVA) | |
|---|---|---|---|---|
| Age, y | 55 ± 11 | 48 ± 10a | 50 ± 11a | 0.01 |
| Male, n (%) | 28 (68) | 38 (73) | 69 (73) | 0.82 |
| BMI, kg/m2 | 32.1 ± 3.8 | 33.8 ± 4.4 | 34.8 ± 4.8a | 0.005 |
| Total body fat by DXA, % of body weight) | 31 ± 7 | 33 ± 7 | 33 ± 7 | 0.56 |
| Visceral fat by MRI, cm2 | 139 ± 51 | 163 ± 43 | 187 ± 63a | <0.001 |
| Liver fat by 1H-MRS, % | 3 ± 1 | 15 ± 8a | 18 ± 9a | <0.001 |
| Patients with T2DM, n (%) | 21 (51) | 28 (54) | 51 (54) | 0.95 |
| Fasting glucose, mg/dL | 116 ± 25 | 118 ± 22 | 123 ± 27 | 0.26 |
| Plasma insulin, µU/mL | 8 ± 7 | 13 ± 10a | 16 ± 12a | <0.001 |
| A1C, % | 6.2 ± 1.1 | 6.3 ± 1.0 | 6.4 ± 1.0 | 0.54 |
| Cholesterol, mg/dL | 161 ± 35 | 171 ± 49 | 187 ± 45a | 0.006 |
| Triglycerides, mg/dL | 116 ± 52 | 148 ± 73 | 206 ± 144a,b | <0.001 |
| LDL, mg/dL | 95 ± 27 | 105 ± 44 | 110 ± 41 | 0.14 |
| HDL, mg/dL | 43 ± 12 | 38 ± 7a | 37 ± 8a | 0.001 |
| ALT, IU/L | 33 ± 27 | 47 ± 23 | 72 ± 39a,b | <0.001 |
| AST, IU/L | 29 ± 17 | 32 ± 12 | 51 ± 28a,b | <0.001 |
| Cytokeratin-18, U/L | 188 ± 186 | 228 ± 204 | 403 ± 293a,b | <0.001 |
| Adiponectin, μg/dL | 10.7 ± 6.0 | 7.8 ± 4.5 | 8.6 ± 6.5 | 0.07 |
| hsCRP, mg/L | 7.2 ± 8.6 | 6.6 ± 6.5 | 7.4 ± 7.2 | 0.98 |
Data are shown as mean ± SD for continuous variables and as number (%) for categorical variables. P values represent the comparison between all groups by ANOVA. Statistical significance was set at P < 0.05. Post hoc comparisons between two groups were performed adjusting for multiple comparisons by Bonferroni method.
Abbreviations: HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; T2DM, type 2 diabetes mellitus.
P < 0.05 compared with the No NAFLD group.
P < 0.05 compared with the No NASH group.
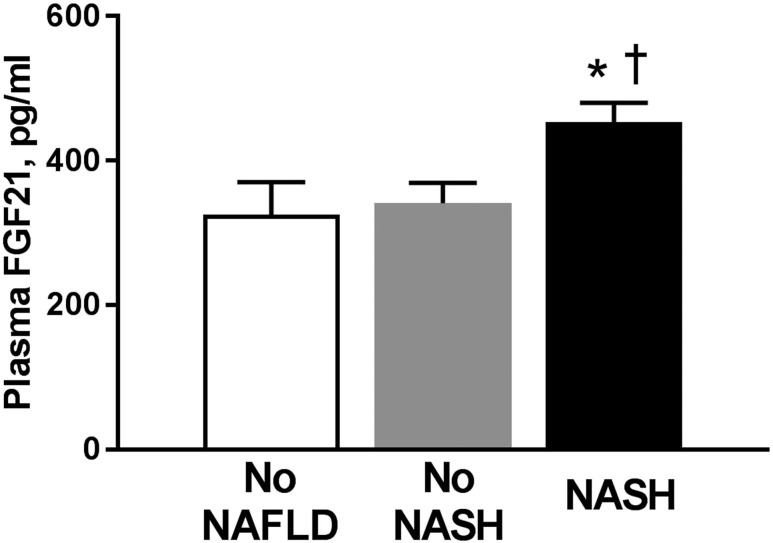
As seen in Fig. 1, plasma FGF21 levels were higher in patients with NASH compared with the No NASH or No NAFLD groups (453 ± 262 vs 341 ± 198 vs 325 ± 289 pg/mL, respectively; P = 0.03 and P = 0.02, respectively). The difference in plasma FGF21 levels was independent of the effect of obesity because the No NASH and NASH groups had comparable BMIs (33.8 ± 4.4 vs 34.8 ± 4.8, respectively), suggesting that obesity was not the main driver for the increased FGF21 levels observed in subjects with NASH. Of note, plasma FGF21 levels also correlated with visceral fat on MRI (Pearson correlation coefficient r = 0.26, P = 0.007), but not with total body fat as measured by DXA (r = 0.12, P = 0.13), confirming that factors beyond total adiposity play a role in the control of FGF21 metabolism.
Figure 1.
Plasma FGF21 levels in patients without NAFLD (No NAFLD group, n = 41), isolated steatosis or steatosis with minimal inflammation (No NASH group, n = 52), and NASH (n = 94). Data are shown as mean ± SE. *P < 0.05 when compared with the No NAFLD group. †P < 0.05 when compared with the No NASH group.
FGF21 levels in relationship to insulin sensitivity
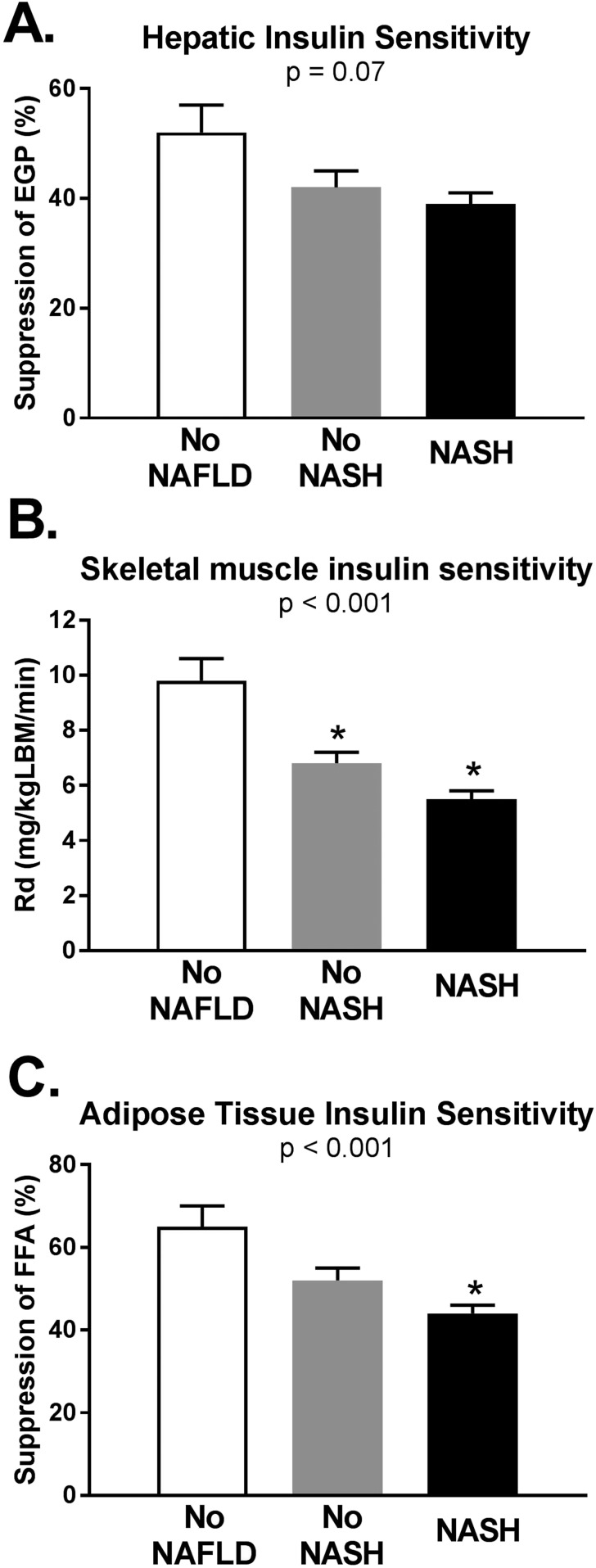
Next, we compared insulin sensitivity at the liver, skeletal muscle, and adipose tissue among the three groups (Fig. 2). Hepatic insulin sensitivity (measured as suppression of EGP by low-dose insulin infusion) did not differ significantly among the three groups (shown in Fig. 2A as mean ± SE): 52 ± 5 vs 42 ± 3 vs 39 ± 2% in the No NAFLD vs No NASH vs NASH groups, respectively; P = 0.07 by ANOVA. Muscle insulin sensitivity worsened from the No NAFLD to No NASH and NASH groups (Rd in Fig. 2B): 9.8 ± 0.84 vs 6.8 ± 0.44 vs 5.5 ± 0.28 mg/kg lean body mass/min, respectively; P < 0.001 by ANOVA. Adipose tissue insulin sensitivity (FFA suppression in Fig. 2C) was worse in the NASH vs No NASH or No NAFLD groups: 44 ± 2.1 vs 52 ± 2.9 and 65 ± 4.8%; P < 0.001 by ANOVA. Moreover, there were significant inverse linear correlations between plasma FGF21 levels and muscle (r = −0.23, P = 0.007) and adipose tissue insulin sensitivity (r = −0.17, P = 0.006).
Figure 2.
Tissue-specific measures on insulin sensitivity during the euglycemic insulin clamp studies in the three groups: No NAFLD (n = 41), No NASH (n = 52), and NASH (n = 94). (A) Hepatic insulin sensitivity (i.e., suppression of EGP by low-dose insulin infusion) was not significantly different among the three groups. (B) Skeletal muscle (Rd) and (C) adipose tissue (suppression of plasma FFA by low-dose insulin infusion) insulin sensitivity worsened from the No NAFLD to NASH group. Higher values indicate greater insulin sensitivity. Data are shown as mean ± SE. LBM, lean body mass. *P < 0.05 when compared with the No NAFLD group.
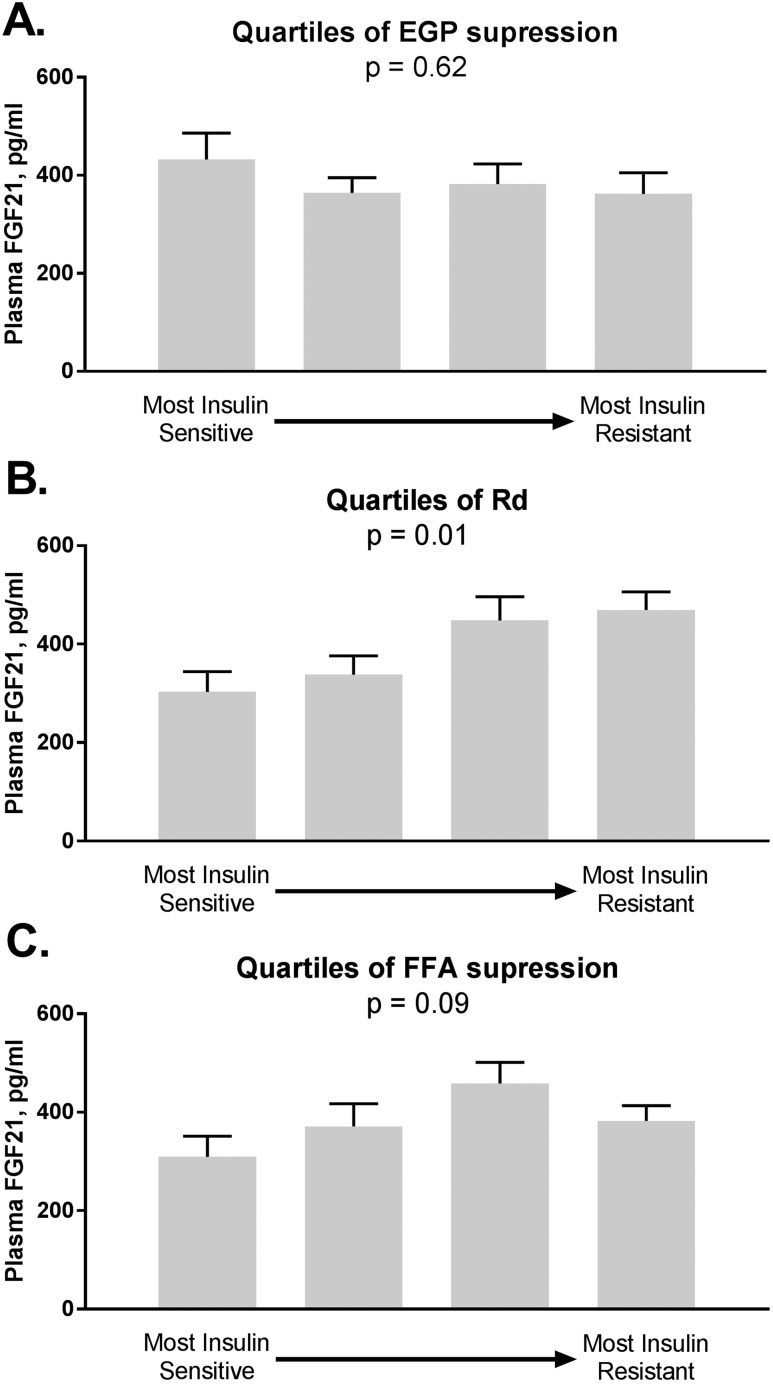
To further dissect the role of IR in relation to plasma FGF21 concentration, we analyzed FGF21 levels in relation to quartiles of hepatic, muscle, and adipose tissue insulin sensitivity (Fig. 3). We found no difference in levels of FGF21 by quartiles of EGP suppression, from most insulin sensitive to most insulin resistant (Fig. 3A, P = 0.620). In contrast, plasma FGF21 levels were lower in subjects with better muscle insulin sensitivity (Rd) (Fig. 3B, P = 0.01) and of borderline significance for adipose tissue insulin sensitivity, as assessed by suppression of FFA during low-dose insulin infusion (Fig. 3C, P = 0.09). These findings suggest that higher plasma FGF21 in NASH could be at least partially explained by changes in peripheral IR.
Figure 3.
Plasma FGF21 level in relationship to quartiles of insulin sensitivity: from most insulin sensitive to most insulin resistant. (A) FGF21 levels by quartiles of liver insulin sensitivity measured as suppression of EGP by low-dose insulin infusion. (B) FGF21 levels by quartiles of skeletal muscle insulin sensitivity shown as Rd. (C) FGF21 levels by quartiles of adipose tissue insulin sensitivity measured as suppression of FFA by low-dose insulin infusion. Data are shown as mean ± SE. N = 187. P values represent the comparisons among the four quartiles by ANOVA.
FGF21 levels in relationship to steatohepatitis
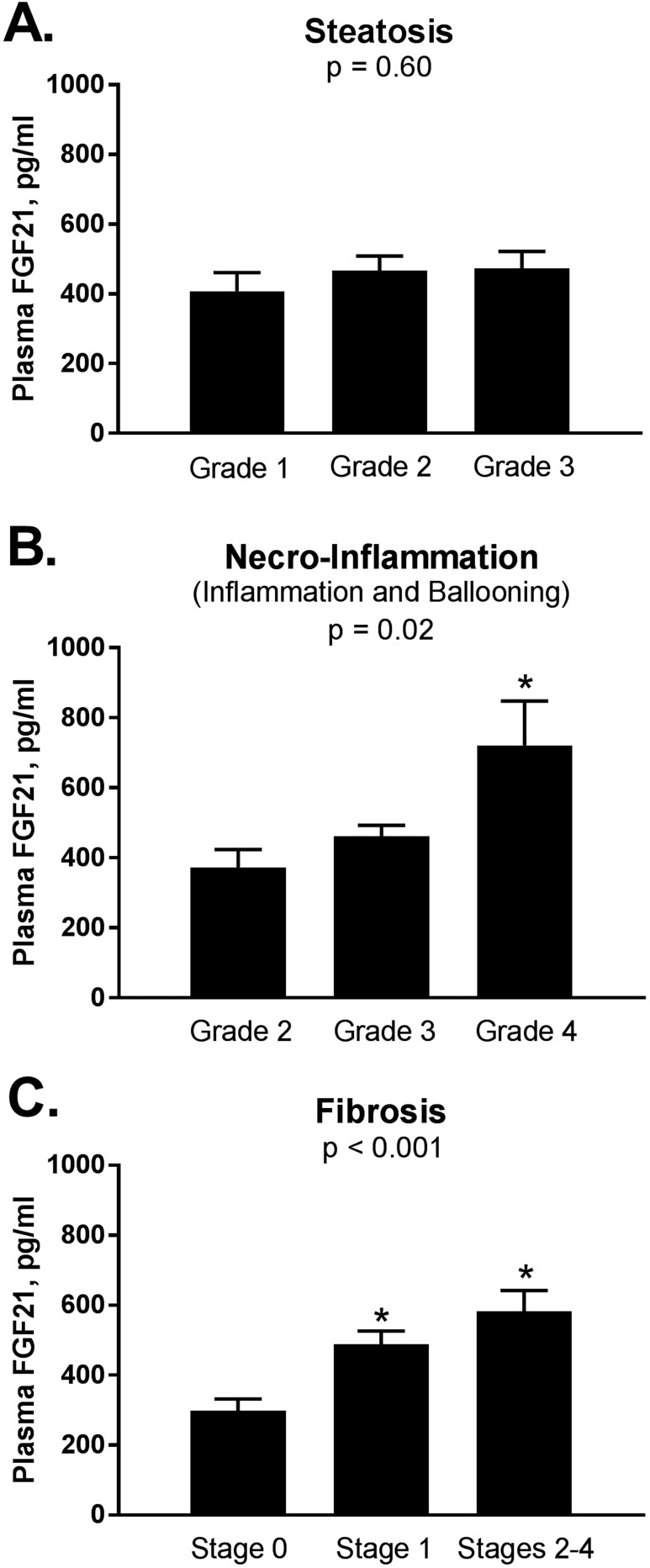
To further assess the relationship between FGF21 and steatohepatitis, we examined plasma FGF21 concentration in relationship with each parameter of liver histology in patients with definite NASH (n = 94, NASH group). We found that FGF21 levels were not significantly changed with worsening grades of steatosis (Fig. 4A): 408 ± 264 vs 467 ± 255 vs 474 ± 274 pg/mL for steatosis grades 1, 2, and 3, respectively; P = 0.60. In addition, plasma FGF21 levels did not correlate with liver fat content by 1H-MRS (r = 0.15, P = 0.06) or percentage of steatosis as reported on liver histology.
Figure 4.
Plasma FGF21 level in relationship to severity of liver histology in patients with NASH (n = 94). (A) FGF21 levels by severity of liver steatosis. Grade 1 represents between 5% and 33% of liver fat; grade 2 = 34% to 66%; and grade 3 ≥66%. (B) FGF21 levels by severity of necroinflammation, a combined lobular inflammation, and hepatocellular ballooning scores (grades 2 through 4). (C) FGF21 by severity of liver fibrosis, stages 0 through 4. Data are shown as mean ± SE. *P < 0.05 by Bonferroni post hoc analysis compared with first column. NASH was defined based on presence of at least grade 1 for each of the following three components: steatosis, lobular inflammation, and hepatocellular ballooning.
In contrast, as observed in Fig. 4B, plasma FGF21 levels were higher with greater necroinflammation scores (i.e., the sum of ballooning and inflammation scores): 373 ± 247 vs 462 ± 255 vs 721 ± 285 pg/mL in grade 2 vs 3 and 4, respectively; P = 0.02. Even more striking results were evident with the presence of moderate fibrosis (Fig. 4C): 298 ± 180 vs 488 ± 255 vs 583 ± 279 pg/mL when comparing stage 0 (no fibrosis) vs stage 1 (mild fibrosis) vs stages 2 through 4 (moderate and advanced), respectively; P < 0.001. When the same analysis was performed in the entire NAFLD cohort (all patients with a liver biopsy, n = 146; data not shown), results were similar: plasma FGF21 levels were not different across steatosis grades (P = 0.07), and again were higher with worsening liver inflammation (P = 0.02), hepatocyte ballooning (P = 0.04), and, hepatic fibrosis (P < 0.001). Furthermore, in the entire NAFLD cohort, plasma FGF21 levels were higher in patients with a NAS score ≥6 vs NAS score <2 (538 ± 290 vs 355 ± 199 pg/mL respectively; P for trend = 0.009).
To further elucidate if histology, and in particular fibrosis, is an independent predictor of higher circulating plasma FGF21 levels in NASH, we performed a multiple linear regression analysis. In this model, the plasma FGF21 concentration was strongly predicted by liver fibrosis stage with a β coefficient of 83 ± 20 (CI, 44 to 121; P < 0.001).
Furthermore, fibrosis remained an independent predictor of plasma FGF21 levels (β coefficient, 84 ± 25; CI, 34 to 134; P = 0.001) after adjusting for plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), fasting glucose, insulin, A1c, and tissue-specific parameters of IR including Rd. Of note, plasma CK-18 levels, considered a marker of hepatocyte apoptosis and fibrosis (29), showed a modest but significant correlation with plasma FGF21 (r = 0.28, P < 0.001).
Discussion
To best of our knowledge, our study is one of the largest studies to assess plasma FGF21 levels in a cohort of well-characterized patients, including in-depth measures of insulin sensitivity and liver histology and to provide strong evidence of the close association between plasma FGF21 and the severity of liver disease in NAFLD and NASH. In our cohort, elevated FGF21 concentration in NASH when compared with no NAFLD or isolated steatosis alone, was best explained by increased liver fibrosis on histology (higher levels with worse fibrosis). In contrast to previous reports (5, 10), in our study FGF21 levels were not impacted by the degree of steatosis. Our findings are similar to those reported recently in bariatric surgery patients, where neither basal or fructose stimulated FGF21 levels correlated with liver fat content on 1H-MRS or on liver biopsy (30) and maybe related to lack of a lean control group.
Previous study that reported close association of FGF21 with steatosis had only a small cohort of subjects with liver biopsy. For example, Li et al. (5) show that FGF21 mRNA liver expression was 14-fold higher in advanced steatosis (grade 2 to 3) (n = 4) and fourfold higher in grade 1 steatosis (n = 5) compared with no steatosis (n = 8). Of note, none of these subjects had NASH. In the same 17 subjects, logarithmic transformation of plasma FGF21 correlated with IHTG content. Dushay et al. (31) reported elevated FGF21 in NASH (n = 9) when compared with controls (n = 6), but lower when compared with simple steatosis (n = 6); however, there is no mention of presence or absence of fibrosis in this small NASH cohort. In a prospective epidemiological study, Wu et al. (32) report higher FGF21 levels at baseline in patients who developed NAFLD after 3 years of follow-up vs those who did not, suggesting a role for FGF21 in predicting development of simple steatosis. Somehow contradictorily, they also report both elevated liver FGF21 by immunohistochemistry in both groups of patients with simple steatosis or NASH (32), but no difference in FGF21 plasma levels in subjects (n = 69) with presumed simple steatosis (defined as NAFLD on ultrasound and ALT level <40 U/L) vs subjects with “suspected” NASH (n = 24, NAFLD patients with ALT level ≥40 U/L). Some of these reported differences, when compared with our study, may be related to lack of data from liver biopsy results and the way NAFLD and NASH was diagnosed (based on ultrasonography, which is less accurate than MRS and liver enzymes) with likely substantial overlap in between the two groups. Portillo-Sanchez et al. (18) have shown before that NASH can be present even when liver enzymes are normal. Finally, Yan et al.(33) reports FGF21 levels by quartiles of hepatic fat content by 1H-MRS in 136 subjects with NAFLD and show that serum FGF21 increased from quartiles 1 to 3 but decreased in quartile 4 (with the highest liver fat content >44.6%): 194.12 ± 126.96, 219.65 ± 141.74, 326.44 ± 149.47, and 258.75 ± 124.69 pg/mL, suggesting a lack of relationship between FGF21 levels and advanced steatosis.
Our findings that plasma FGF21 levels are inversely correlated with peripheral insulin sensitivity are consistent with previous reports (34); however, the increased FGF21 levels with severity of necroinflammation and fibrosis could suggest an important link between impaired FGF21 signaling (11, 12) and progression of liver disease in NASH. In our NAFLD cohort, plasma FGF21 levels were higher in patients with higher NAS scores and correlated with CK-18 levels, a marker of hepatocyte apoptosis and fibrosis (29), similar to previous reports in the literature (34). Whether elevated FGF21 in NASH is a cause-and-effect relationship between plasma FGF21 and worse liver histology, or there are other confounding factors, such as adiposity or IR, playing a role, remains a matter of debate. In our study, NASH and the No NASH groups were equally obese; therefore, obesity was not a confounder. Consistent with previous reports by our group (19), adipose tissue (shown as suppression of FFA during the insulin clamp) and Rd deteriorated progressively from the No NAFLD to NASH (both P < 0.001) groups. The three groups were equally insulin resistant at the level of the liver, as shown by similar changes in suppression EGP; this could most likely be explained by the extreme sensitivity of the liver to developing IR in the setting of lipotoxicity, with even small increases in IHTG being associated with hepatic IR (19, 35). As previously shown by Bril et al. (19), small increases in liver fat >2% are already associated with decreased hepatic insulin sensitivity. Patients in the current study were overweight and obese and with a liver fat content that was already ∼3% to 4% in the No NAFLD group, which is already within a range associated with IR, and even higher in the groups with steatosis or NASH. Although FGF21 is known to be a strong stimulus for adiponectin secretion and improves glucose/lipid metabolism, patients with obesity have FGF21 resistance (2, 11, 12). This could explain our findings of higher plasma FGF21 levels in the face of worsening muscle and adipose tissue insulin sensitivity, the key players in NASH. This is similar to what happens in patients with IR in the setting of type 2 diabetes, where insulin still works as a treatment and has been shown to decrease liver fat (36). Despite FGF21 resistance in obesity, treatment with FGF21 analogs may still hold promise.
The studies looking at FGF21 analogs for treatment in humans are limited by small sample size and short duration of study. However, all show consistent effects on improving fasting triglycerides (14–16), fasting glucose, and whole body insulin sensitivity likely related to dose-dependent increase in adiponectin levels (15, 16, 37). In these short-term studies (4 weeks) modest weight loss of ∼1.8% was observed when compared from baseline in one study (14), and up to 5% to 6% with higher dose of a longer acting FGF21 analog, PF-05231023, in subjects with type 2 diabetes (37). Finally, Sanyal et al. (16) evaluated changes in liver fat by MRI-measured proton density fat fraction after administration of two doses (10 mg daily and 20 mg weekly) of pegylated FGF21 (BMS-986036) vs placebo (randomized 1:1:1) in 74 patients with NASH (38% of which had diabetes). After 16 weeks, liver fat decreased by 6.8% with the 10 mg/d dose and by 5.2% with the 20 mg/wk dose vs 1.3% in placebo (both P < 0.01). Plasma PRO-C3 (N-terminal type III collagen propeptide), a fibrosis marker, also decreased with FGF21 treatment (15, 16), suggesting that pharmacological dosing of FGF21 works.
The most prominent finding in our study, showing elevated FGF21 levels in steatohepatitis and in particular driven by worsened liver fibrosis, could be explained as adaptive or compensatory possibly triggered by the increased mitochondrial stress and inflammation. A number of observations support a role for FGF21 as a protective pathway in liver disease, and specifically, steatohepatitis. FGF21 is the main acute hormonal response to fructose ingestion, which is known to acutely induce ATP depletion in liver cells (38), possibly through increase in reactive oxygen species and hepatic mitochondrial stress, and in the long term to induce triglyceride accumulation (39).
FGF21 knockout mice demonstrate impaired ability to mobilize and use lipids and develop hepatic steatosis with ketogenic diet, suggesting a role of FGF21 in lipid oxidation (40). Up-regulation of FGF21 may also counteract endoplasmic reticulum stress-induced hepatic steatosis and protect against the NAFLD-induced lipotoxicity (41, 42). A long-acting FGF21, PsTag600-FGF21, reversed hepatic steatosis by enhanced fatty acid activation and mitochondrial β oxidation in the liver of choline-deficient, high-fat diet-induced NASH model in mice (43). Interestingly, compensatory protective increase in FGF21 is also observed in Tylenol-induced hepatotoxicity (44) and in hepatocellular cancer, where delays initiation of chemically induced liver carcinogenesis (45).
There are a few limitations to our study. Although higher levels of FGF21 were associated with an increase in fibrosis on histology, a direct cause-and-effect relationship cannot be proven from a cross-sectional analysis. In addition, because NASH is well known to be associated with IR, this could be a confounder as it relates to liver histology. However, the association remained substantial even after adjusting for insulin sensitivity across the groups.
In conclusion, increasing plasma FGF21 concentration was associated with the severity of IR (muscle > adipose tissue). More clinically relevant for the management of patients with NAFLD, higher plasma FGF21 levels correlated with the severity of NASH, with levels being highest in advanced fibrosis. These findings suggest that FGF21 could be a biomarker of NASH and that FGF21 resistance may play a role in the pathogenesis of NASH. The clinical implication is that measuring plasma FGF21 may help in identifying patients at the highest risk of disease progression.
Acknowledgments
Financial Support: K.C. received research support from the National Institutes of Health as principal investigator, Burroughs Wellcome Fund, and American Diabetes Association.
Author Contributions: D.B., F.B., and K.C. participated in data collection and writing and editing of this manuscript. F.B. performed the statistical analyses. S.K. performed the laboratory measurements. All authors read and approved the final manuscript submitted.
Disclosure Summary: K.C. is a consultant for BMS, Coherus, Deuterex, Eli Lilly and Company, Janssen Research & Development, LLC, Pfizer, Poxel, Novo Nordisk, Allergan, AstraZeneca, Cirius, Inventiva, Merck, Novartis, and Zydus and has received research support from Cirius, Inventiva, Janssen, Novartis, Novo Nordisk, Allergan and Zydus.
Glossary
Abbreviations:
- A1C
hemoglobin A1c
- ALT
alanine aminotransferase
- AST
aspartate aminotransferases
- BMI
body mass index
- CK-18
cytokeratin-18
- DXA
dual-energy X-ray absorptiometry
- EGP
endogenous glucose production
- FFA
free fatty acid
- FGF21
fibroblast growth factor 21
- 1H-MRS
proton magnetic resonance spectroscopy
- IHTG
intrahepatic triglyceride
- IR
insulin resistance
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- OGTT
oral glucose tolerance test
- Rd
rate of glucose disappearance
References
- 1. Barb D, Portillo-Sanchez P, Cusi K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1183–1195. [DOI] [PubMed] [Google Scholar]
- 2. Maratos-Flier E. Fatty liver and FGF21 physiology. Exp Cell Res. 2017;360(1):2–5. [DOI] [PubMed] [Google Scholar]
- 3. Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–241. [DOI] [PubMed] [Google Scholar]
- 4. Kharitonenkov A, Adams AC. Inventing new medicines: the FGF21 story. Mol Metab. 2013;3(3):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53(5):934–940. [DOI] [PubMed] [Google Scholar]
- 6. Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104(18):7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63(12):4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang F, Yu L, Lin X, Cheng P, He L, Li X, Lu X, Tan Y, Yang H, Cai L, Zhang C. Minireview: roles of fibroblast growth factors 19 and 21 in metabolic regulation and chronic diseases. Mol Endocrinol. 2015;29(10):1400–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. J Clin Endocrinol Metab. 2012;97(6):2143–2150. [DOI] [PubMed] [Google Scholar]
- 11. Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Markan KR. Defining “FGF21 resistance” during obesity: controversy, criteria and unresolved questions. F1000 Res. 2018;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markan KR, Naber MC, Small SM, Peltekian L, Kessler RL, Potthoff MJ. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Mol Metab. 2017;6(6):602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18(3):333–340. [DOI] [PubMed] [Google Scholar]
- 15. Charles ED, Neuschwander-Tetri BA, Pablo Frias J, Kundu S, Luo Y, Tirucherai GS, Christian R. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity (Silver Spring). 2019;27(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanyal AC, Charles ED, Neuschwander-Tetri B, Loomba R, Harrison S, Abdelmalek M, Lawitz EF, Halegoua-DeMarzio D, Kundu S, Noveiello S, Luo Y, Christian R. BMS-986036 (pegylated FGF21) in patients with non-alcoholic steatohepatitis: a phase 2 study. J Hepatol. 2017;66(1):S89–S90. [Google Scholar]
- 17. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. [DOI] [PubMed] [Google Scholar]
- 18. Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J, Cusi K. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100(6):2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, Weber MH, Budd JT, Lupi ME, Cusi K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–1144. [DOI] [PubMed] [Google Scholar]
- 20. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–315. [DOI] [PubMed] [Google Scholar]
- 21. Bril F, Ortiz-Lopez C, Lomonaco R, Orsak B, Freckleton M, Chintapalli K, Hardies J, Lai S, Solano F, Tio F, Cusi K. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35(9):2139–2146. [DOI] [PubMed] [Google Scholar]
- 22. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–E468. [DOI] [PubMed] [Google Scholar]
- 23. Bril F, Cusi K. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis. Ann Intern Med. 2017;166(3):230. [DOI] [PubMed] [Google Scholar]
- 24. Maximos M, Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Biernacki D, Suman A, Weber M, Cusi K. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology. 2015;61(1):153–160. [DOI] [PubMed] [Google Scholar]
- 25. Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E, Defronzo RA. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496–506. [DOI] [PubMed] [Google Scholar]
- 26. Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F, Hecht J, Cusi K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(6):2178–2187. [DOI] [PubMed] [Google Scholar]
- 27. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 28. Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ter Horst KW, Gilijamse PW, Demirkiran A, van Wagensveld BA, Ackermans MT, Verheij J, Romijn JA, Nieuwdorp M, Maratos-Flier E, Herman MA, Serlie MJ. The FGF21 response to fructose predicts metabolic health and persists after bariatric surgery in obese humans. Mol Metab. 2017;6(11):1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu G, Li H, Fang Q, Zhang J, Zhang M, Zhang L, Wu L, Hou X, Lu J, Bao Y, Jia W. Complementary role of fibroblast growth factor 21 and cytokeratin 18 in monitoring the different stages of nonalcoholic fatty liver disease. Sci Rep. 2017;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan H, Xia M, Chang X, Xu Q, Bian H, Zeng M, Rao S, Yao X, Tu Y, Jia W, Gao X. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 2011;6(9):e24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giannini C, Feldstein AE, Santoro N, Kim G, Kursawe R, Pierpont B, Caprio S. Circulating levels of FGF-21 in obese youth: associations with liver fat content and markers of liver damage. J Clin Endocrinol Metab. 2013;98(7):2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bril F, Cusi K. Liver fat accumulation as a barometer of insulin responsiveness again points to adipose tissue as the culprit. Hepatology. 2017;66(1):296–297. [DOI] [PubMed] [Google Scholar]
- 36. Cusi K, Sanyal AJ, Zhang S, Hoogwerf BJ, Chang AM, Jacober SJ, Bue-Valleskey JM, Higdon AN, Bastyr EJ III, Haupt A, Hartman ML. Different effects of basal insulin peglispro and insulin glargine on liver enzymes and liver fat content in patients with type 1 and type 2 diabetes. Diabetes Obes Metab. 2016;18(Suppl 2):50–58. [DOI] [PubMed] [Google Scholar]
- 37. Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, Brenner MB, Trimmer JK, Gropp KE, Chabot JR, Erion DM, Rolph TP, Goodwin B, Calle RA. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016;23(3):427–440. [DOI] [PubMed] [Google Scholar]
- 38. Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Lipkin EW, Balasubramanyam A, Bantle JP, Johnson RJ, Diehl AM, Clark JM; Fatty Liver Subgroup of Look AHEAD Research Group. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology. 2012;56(3):952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cioffi F, Senese R, Lasala P, Ziello A, Mazzoli A, Crescenzo R, Liverini G, Lanni A, Goglia F, Iossa S. Fructose-rich diet affects mitochondrial DNA damage and repair in rats. Nutrients. 2017;9(4):E323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150(11):4931–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rusli F, Deelen J, Andriyani E, Boekschoten MV, Lute C, van den Akker EB, Müller M, Beekman M, Steegenga WT. Fibroblast growth factor 21 reflects liver fat accumulation and dysregulation of signalling pathways in the liver of C57BL/6J mice. Sci Rep. 2016;6(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang S, Yan C, Fang QC, Shao ML, Zhang YL, Liu Y, Deng YP, Shan B, Liu JQ, Li HT, Yang L, Zhou J, Dai Z, Liu Y, Jia WP. Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. J Biol Chem. 2014;289(43):29751–29765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bao L, Yin J, Gao W, Wang Q, Yao W, Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. 2018;175(16):3379–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology. 2014;60(3):977–989. [DOI] [PubMed] [Google Scholar]
- 45. Huang X, Yu C, Jin C, Yang C, Xie R, Cao D, Wang F, McKeehan WL. Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Mol Carcinog. 2006;45(12):934–942. [DOI] [PubMed] [Google Scholar]