Abstract
Purpose
Factor I (FI) is a serine protease regulator of the complement system. Genetic variants in CFI are associated with advanced age-related macular degeneration (AAMD). However, the clinical and functional impact of these variants is unknown. This study assessed the functional significance of rare CFI variants using a serum-based assay.
Methods
Carriers of rare variants with (n = 78) and without AAMD (n = 28), and noncarriers with (n = 49) and without AMD (n = 44) were evaluated. Function of FI was determined by measuring the proteolytic cleavage of C3b to iC3b, using the cofactor protein, Factor H.
Results
CFI variants were categorized into three groups based on antigenic and functional assessments. Type 1 variants (n = 18) in 35 patients with AAMD demonstrated low serum FI levels and a corresponding decrease in FI function. Type 2 variants (n = 6) in 7 individuals demonstrated normal serum FI antigenic levels but reduced degradation of C3b to iC3b. Type 3 variants (n = 15) in 64 individuals demonstrated normal antigenic levels and degradation of C3b to iC3b. However, iC3b generation was low when measured per unit of FI. Thus most rare CFI variants demonstrate either low antigenic levels (type 1) or normal levels but reduced function (types 2 or 3).
Conclusions
Results provide for the first time a comprehensive functional assessment in serum of CFI rare genetic variants and further establish FI's key role in the pathogenesis of AAMD.
Translational Relevance
Stratifying patients in the clinic with a rare CFI variant will facilitate screening and targeting patients most likely to benefit from complement therapies.
Keywords: age-related macular degeneration, complement, rare genetic variants, Factor I, functional analysis
Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness worldwide, and the number of people with AMD is projected to increase from 196 million in 2020 to 288 million in 2040.1 This slowly progressive, neurodegenerative disease of the retina usually manifests after 60 years of age and can markedly reduce quality of life. Loss of central vision can occur secondary to disruption and death of photoreceptor cells in the macula related to atrophy or neovascularization. Although diet, smoking, and other behavioral factors are associated with the risk of advanced AMD (AAMD),2 it has become increasingly evident that polymorphisms and rare variants in genes encoding complement system proteins also play a critical predisposing role.2,3
The complement system not only defends the host against microbes but also facilitates processing and clearance of damaged/altered cells and tissues. Complement is activated via three major cascades: the classical, lectin, and alternative pathways. Each is uniquely engaged but they share the common goal of promoting the inflammatory response and altering the membranes of infectious microbes. Because the complement system provides a rapidly activated as well as a potent surveillance and effector mechanism, strict control is required to avoid damage to self-tissue. Thus, inhibition of complement activation is mediated by both plasma and cell-bound regulators. For example, the serine protease complement factor I (FI), in conjunction with cofactor proteins such as factor H (FH) or membrane cofactor protein (MCP, CD46), modulates the complement cascade through proteolytic cleavage of C3b generated by each pathway. The prevailing hypothesis is that an overly exuberant inflammatory response, driven by an inadequately regulated complement cascade resulting primarily from genetic alterations, is a major player in the pathophysiology of AAMD.3–7 Thus, for a given degree of retinal injury, there is likely an excessive degree of complement activation, which is damaging to the retina. This undesirable response to injury may affect the retinal pigment epithelium, drusen formation, and/or the local vasculature leading to a predisposition and an acceleration of the disease process.
Genetic variants in CFI and CFH are associated with AAMD.6,8–13 These variants range from common polymorphisms, conferring relatively low to moderate risk, to rare variants with nearly complete penetrance and high risk. Also, the rare variants with high impact which occur in families lead to younger age of onset of AMD.9,11,13,14 The association between AMD and a common variant in CFI was first implicated in 2009 by Fagerness et al.12 In 2013, a targeted sequencing study of 681 genes and 2493 individuals led to the discovery of a burden of 59 rare CFI variants associated with AMD.6 Loss of function variants had the greatest impact on increasing risk of AMD.6 Studies have also noted a strong predisposition to AAMD in those with reduced serum FI levels and, in a few cases, a reduction in catalytic activity has been implicated.6,7 However, a majority of the variants in the CFI gene are still uncharacterized. To further investigate the pathophysiologic implications of harboring a rare variant in CFI, we interrogated the functional activity of this serine protease in serum samples using a serum- based assay (ARVO 2018, Baciu et al. IOVS 2018; 59(9):790 and ARVO 2020, Java et al, accepted abstract, IOVS 2020; 61(7):3651).
Methods
Patients
Individuals with and without rare heterozygous CFI genetic variants were identified based on our previous studies.6,7 Briefly, targeted sequencing was performed in a cohort of 2828 individuals (1870 with and 958 without AAMD) in which 172 individuals were found to carry a rare allele (minor allele frequency <0.01) in CFI (Supplementary Fig. S1). A search for variants was further extended by conducting Sanger sequencing on samples from 838 individuals (396 with and 442 without AAMD) from which 59 additional individuals with CFI genetic variants were identified. Thus the number of individuals carrying a rare CFI variant was 231 (172 + 59) from an initial cohort of 3666 (6.3%). To explore the effects of rare CFI variants on FI antigenic levels and functional activity, we next identified individuals for whom serum samples were available. There were 106 carriers of rare variants with serum available including individuals with (n = 78) and without (n = 28) AAMD. The comparison cohort included 93 noncarriers of rare variants in CFI matched to carrier individuals by age at date of sample collection within two years, with AMD (n = 49) and without AMD (n = 44). Presence or absence and stage of maculopathy was graded using our five-stage Clinical Age-Related Maculopathy Staging system.15 Advanced AMD was defined as either geographic atrophy or neovascular disease, based on review of ocular records, fundus photography, optical coherence tomography, and autofluorescence images.
This research followed the tenets of the Declaration of Helsinki. All individuals in this study were participants in ongoing genetic and epidemiologic studies of macular degeneration approved by the institutional review board. Informed consent was obtained from each subject.
FI Antigenic Concentration
We obtained serum samples according to our standard protocol, which included drawing a fasting blood sample, refrigerated centrifugation within 10 to 30 minutes and storage at −140°C. Measurement of FI antigenic levels was conducted by the National Jewish Health Advanced Diagnostic Laboratory (Denver, CO, USA), using radial immunodiffusion (normal range 29.3– 58.5 µg/mL).16 C3 was quantitated by standard clinical laboratory nephelometric methodology (normal range 0.79–1.67 mg/mL).
FI Activity Assay
A previously described cofactor assay was modified in which FI converts substrate C3b to iC3b independent of the C3b and FH concentration in the serum sample (Supplementary Fig. S2).17 The effect of endogenous FH and C3b was minimized by diluting serum to 0.04% in PBS (phosphate-buffered saline) solution (Supplementary Fig. S3). FI activity was then determined after the addition of purified, exogenous C3b and FH (final concentration 1.9 µmol/L and 290 nmol/L, respectively; Complement Technology, Tyler, TX, USA) at 37°C for 20 minutes (Supplementary Fig. S4). At the end of the incubation period, the reaction was halted by chilling in an ice bath followed by a 1:500 dilution in enzyme-linked immunosorbent assay (ELISA) buffer (Quidel, San Diego, CA, USA).
Concentration of iC3b was determined by ELISA using MicroVue iC3b EIA (Quidel) per the manufacturer's instructions. Under these conditions, up to ∼ 40% of the C3b can be processed to iC3b. This assay was also performed in the absence of exogenous C3b and in the presence of patient serum alone. The iC3b was not detected in either of these conditions. Each patient sample was run twice with iC3b levels quantitated in duplicate. Data points represent the average of the two runs. Coefficient of variability of the iC3b assay within a plate was 4.2% ± 3.6% and between plates was 3.5% ± 2.6%.
The mean iC3b level was also used to quantitate the ratio iC3b/FI; i.e., the quantity of iC3b generated per unit (20 µg/mL) of FI. Data were collected using ELISA reader MR5 (Molecular Dynamics, Sunnyvale, CA, USA) and analyzed using Softmax Pro V6.3 (Molecular Probes, Invitrogen, OR, USA). Data were graphed and statistical analyses performed using Prism Graph v6.
Statistical Analysis
To compare the FI antigenic concentrations among individuals with and without rare variants, box plots were created with medians and quartiles [first quartile (Q1) and third quartile (Q3)]. The box length ranging from Q1 to Q3 is the interquartile range (IQR). To show possible outliers, the whiskers were shortened to a length of 1:5 times the box length, and points beyond that are outliers (Q3 + 1.5*IQR). Similarly, box plots were created for the iC3b generation and for the iC3b generation per each unit of FI. P values were computed to test whether FI antigenic levels and functional activity of carriers were different from those of noncarriers by fitting an analysis of variance model (ANOVA) and performing Tukey's “Honest Significant Difference” method for multiple test correction. In addition, P values were computed to test whether there were differences between the carriers of rare CFI variants and non-carriers, with respect to demographic (age, sex, race), behavioral (smoking status and body mass index [BMI]) and AAMD status. Fisher's exact test was performed for categorical data and a two-sample t-test was performed for the remaining continuous data (age and BMI). These box plots and P values were generated with R programming language (https://www.r-project.org/).
Results
Individuals with and without rare heterozygous CFI genetic variants were identified based on our previous experiments (Supplementary Fig. S1).6,7 Demographic data for the carriers and non-carriers of CFI variants are shown in Supplementary Table S1. There were no significant differences between carriers and non-carriers of rare CFI variants regarding age, race, sex, smoking status or BMI. In our previous analyses, measurement of serum antigenic levels demonstrated that patients with AAMD carrying a rare CFI variant had lower mean FI compared with non-AAMD individuals (P < 0.001).6,7 However, although the effect of CFI genetic variants resulting in low FI levels in the pathogenesis of AAMD was apparent, the functional impact of the rare genetic variants which did not result in low antigenic levels of the protein remained unclear. Therefore we used a serum-based assay to more definitively ascertain the enzymatic activity of such variants and correlated these results with the antigenic levels. This key role of FI, known as cofactor activity, prevents C3b from engaging the feedback loop of the alternative pathway. In this assay, FI in the presence of a cofactor protein, FH cleaves the α′ chain of C3b to form iC3b (Supplementary Fig. S2). The effect of endogenous FH and C3b was eliminated by diluting serum to 0.04% in PBS (phosphate-buffered saline solution) (Supplementary Fig. S3). Generation of iC3b showed a linear correlation (r, 0.82) with FI antigenic levels (Supplementary Fig. S4).
Measurement of FI antigenic levels and quantification of the cofactor activity for each variant (iC3b generation and iC3b per unit of FI) using the serum-based assay allowed us to categorize the variants into three types (Table). Type 1 variants were defined as those that demonstrated low FI antigenic levels and low iC3b generation, but the iC3b generated per unit of FI (iC3b/FI) was normal when compared to controls (non-carriers of rare variants with and without AMD). Type 2 variants were defined as those that had normal FI antigenic levels but exhibited low iC3b generation and low iC3b/FI. Variants defined as Type 3 demonstrated normal FI levels as well as normal iC3b generation, but iC3b/FI was low.
Table.
Classification of the Three Complement Factor I (CFI) Rare Variant Types Based on Antigenic Levels and Functional Activity
| Variant Type | FI Antigen Level | iC3b Generation | iC3b Per 20 µg/mL FI |
|---|---|---|---|
| 1 | Low | Low | Normal |
| 2 | Normal | Low | Low |
| 3 | Normal | Normal | Low |
FI, Factor I; iC3b, A complement cleavage product derived from C3b by the combined action of Factor H and Factor I, known as cofactor activity.
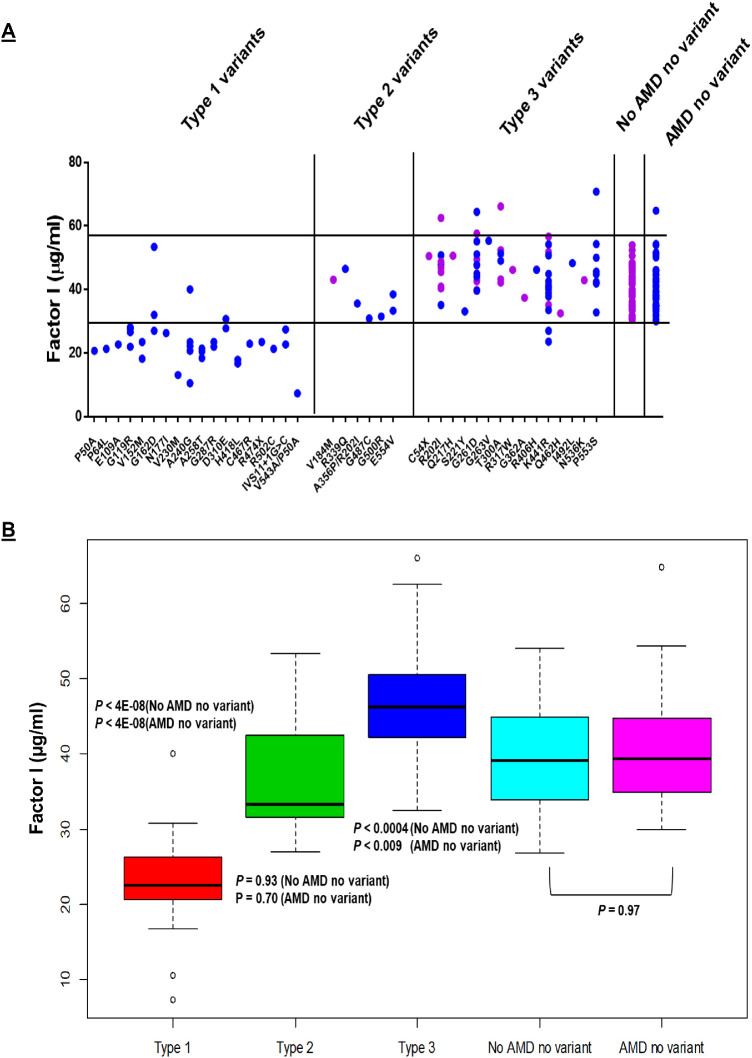
Type 1 variants demonstrate reduced FI expression (P < 4E-08, compared to no AMD and no variant) (Fig. 1) and iC3b generation (P < 4E-08, compared to no AMD and no variant) (Fig. 2) with both measures falling within the lower quartile of controls. Consequently, these patients are haploinsufficient due to lack of protein being synthesized or secreted by the variant allele. This was further confirmed by the results indicating that per unit (20 µg/mL of FI) activity was not significantly different from controls (Fig. 3). Thus, if functional activity is expressed as iC3b generated per unit of FI, the measurement normalizes (P = 0.188, compared to no AMD and no variant), in keeping with expression of a functionally intact protein by the wild-type allele. Eighteen variants identified in 35 patients with AAMD belong to Type 1. Of note, one of five patients carrying the CFI variant A240G and two of three patients carrying the CFI variant G162D have normal FI antigenic concentrations (Fig. 1A). A limitation of this assay is that we are unable to quantitate the relative proportion of the mutant vs wild type protein. It has long been known that FI is an acute phase protein and that serum levels can vary substantially.18 The potential therefore exists in an individual with a Type 1 mutant allele for the wild type allele to be upregulated, normalizing the FI levels, and thereby masking the effect of the Type 1 variant. We have demonstrated by in vitro analysis that these mutations (G162D and A240G) are not secreted (not shown), and we can therefore surmise that all of the FI in both of these patients is the wild type protein. Furthermore, these variants have been reported previously in patients in whom the FI levels were low.6,7,19,20
Figure 1.
FI antigenic levels. (A) Individual values are shown as solid blue circles for patients with AAMD and purple circles for individuals without AAMD. Normal range (29.3–58.5 µg/mL) is represented by the black horizontal lines. The rare genetic variants are listed on the X-axis. The numbering system includes the signal peptide of 18 amino acids. (B) Box plots demonstrate that Type 1 variants have low FI antigenic levels (P < 4E-08, compared with no AMD and no variant; P < 4E-08, compared to AMD and no variant) whereas type 2 (P = 0.93, compared with no AMD and no variant; P = 0.70, compared with AMD and no variant) and type 3 variants have normal to high antigenic levels compared with controls (P < 0.0004, compared with no AMD and no variant; P < 0.009 compared to AMD and no variant). Controls (noncarriers of rare variants, with and without AMD), demonstrate normal antigenic levels of FI.
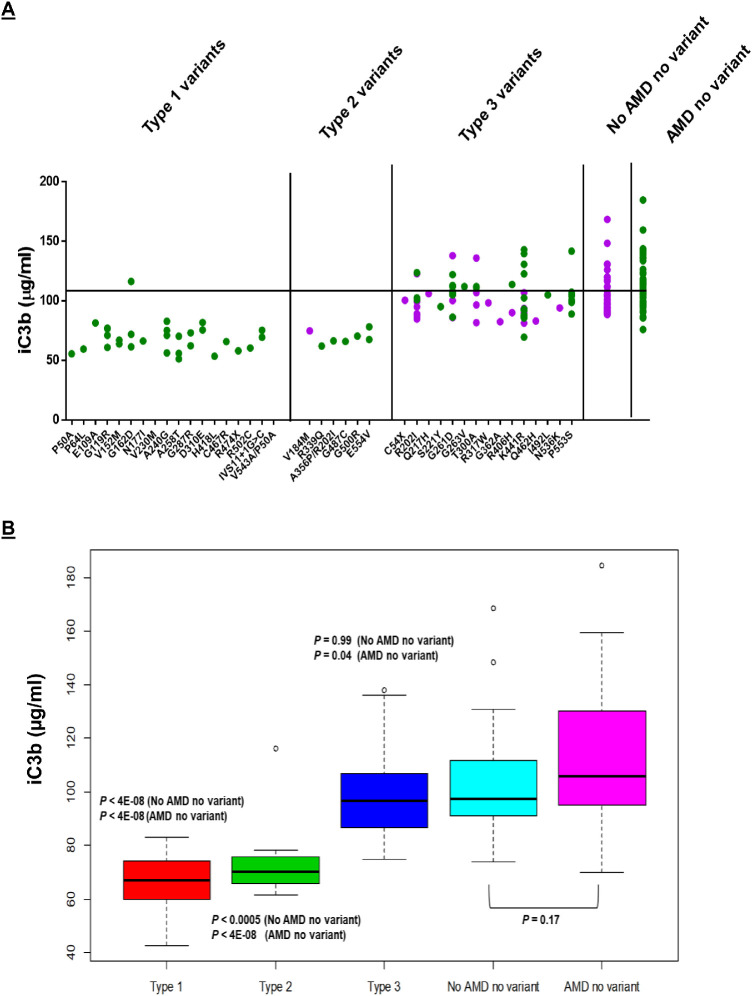
Figure 2.
The iC3b generation. FI functional activity is ascertained by the quantity of iC3b generated as determined by ELISA. (A) Individual values are shown as solid green circles for AAMD and purple circles for those without AAMD. Black horizontal line represents mean iC3b value for controls. (B) Box plots demonstrate that both type 1 (P < 4E-08, compared with no AMD and no variant; P < 4E-08, compared with AMD and no variant) and type 2 variants (P < 0.0005, compared with no AMD and no variant; P < 4E-08, compared with AMD and no variant) have decreased iC3b generation compared to controls. Type 3 variants portray normal iC3b generation (P = 0.99, compared with no AMD and no variant; with P = 0.04, compared with AMD and no variant). Noncarriers (with and without AMD) also demonstrate normal iC3b generation.
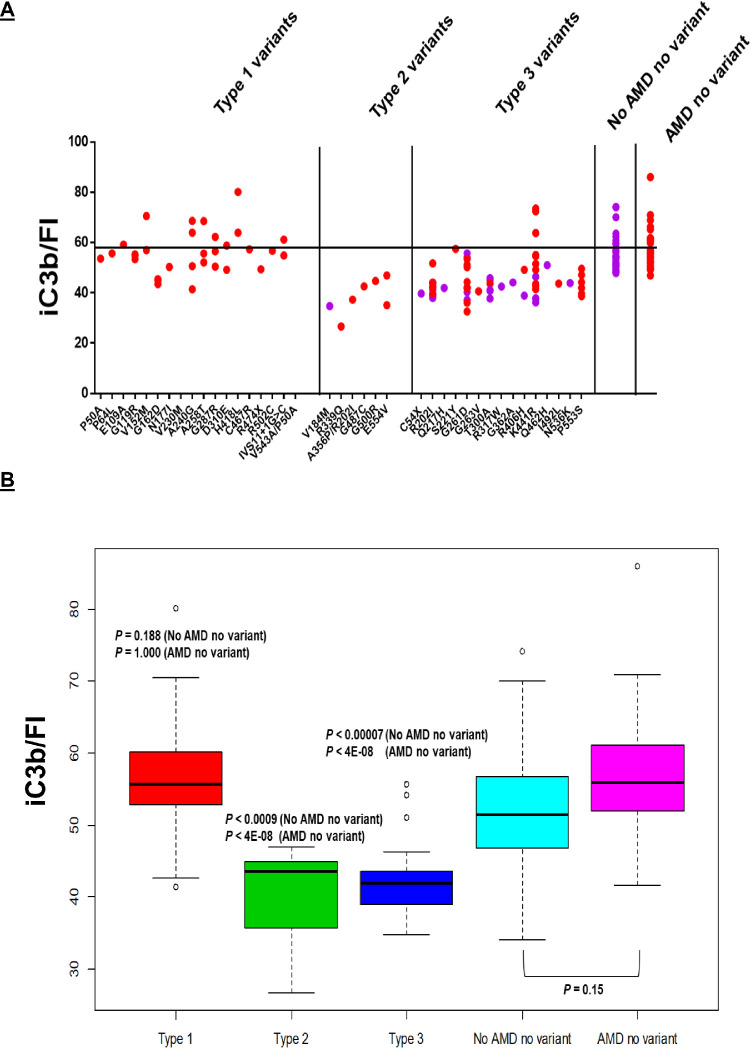
Figure 3.
The iC3b generation per antigenic unit (20 µg/mL) of FI. (A) Individual values are represented by solid red circles for patients with AAMD and purple circles for those without AAMD. Black horizontal line represents mean iC3b/FI for controls (noncarriers of rare variants with and without AMD). (B) Box plots demonstrate that Type 1 variants have a normal iC3b per unit of FI (P = 0.188, compared to no AMD and no variant; P = 1.00, compared with AMD and no variant). This is consistent with variants that result in expression by one allele (haploinsufficiency) of a functionally normal protein. Type 2 (P < 0.0009, compared to no AMD and no variant; P < 4E-08, compared to AMD and no variant) and type 3 variants (P < 0.00007, compared to no AMD and no variant; P < 4E-08, compared to AMD and no variant) demonstrate decreased iC3b per unit of FI since these variants result in a protein that is secreted normally but has decreased function. Noncarriers have normal iC3b generation/FI.
Type 2 variants show FI antigenic levels that are not different from controls (P = 0.93, compared with no AMD and no variant) but demonstrate significant decreases in degradation of C3b to iC3b (P < 0.0005, compared with no AMD and no variant) (Figs. 1 and 2). Relative to the results in Type 1, if expressed as iC3b generated per unit of FI, the level is decreased when compared to controls (P < 0.0009, compared with no AMD and no variant) (Fig. 3). This is in keeping with a rare variant in which the protein is secreted normally but has reduced functional activity (consistent with a haploinsufficiency phenotype). Six variants identified in seven individuals (six with and one without AAMD at the time of analysis) belong to this category.
We further classify a group of Type 3 variants that demonstrate normal to high normal FI antigenic levels (P < 0.0004, compared to no AMD and no variant) and apparently normal degradation of C3b to iC3b (P = 0.99, compared to no AMD and no variant) (Figs. 1 and 2). However, iC3b generation per unit of FI is low (P < 0.00007, compared to no AMD and no variant) (Fig. 3). Fifteen variants identified in 64 individuals belong to this group. The normalization of iC3b is likely associated with the modest increase in FI levels. Whether the elevated FI levels reflect an increase in the secretion of the WT allele (because of the acute phase nature of FI) or a prolonged half-life of the variant allele remains to be determined. Variants belonging to Type 3 are dysfunctional based on the low iC3b per unit of FI, but likely of lower penetrance compared to those belonging to Types 1 and 2. Of note, the most frequent variant in this group, K441R, identified in 16 individuals, demonstrates wide variability in the FI antigenic levels. This is possibly due to the ratio of WT and variant allele expression as explained above. To more definitively delineate the functional activity of this variant, we plan to produce this variant recombinantly. Another variant, R202I, is categorized as Type 3 but when present as a double mutant with A356P/R202I, it demonstrates a Type 2 defect. We believe this may likely be due to the combined deleterious effect of both variants. This result and its interpretation will also need to be validated using recombinant proteins.
As shown in Figures 1 to 3, noncarriers of rare CFI variants, with and without AAMD, have normal (one exception with a high value) FI antigenic levels (29.3–58.5 µg/mL), iC3b generation (108 ± 19.9 µg/mL), and iC3b/unit FI (56.6 ± 7.3). Also, there was no significant difference in FI antigenic levels (P = 0.92) or function (P = 0.17) in noncarriers regardless of disease status (with or without AAMD).
Discussion
Rare genetic variants in CFI have been associated with AAMD.6,7 However, a majority of the variants have not been functionally characterized and many are classified based on in-silico analyses as benign, likely benign, or variants of uncertain significance. This inability to ascertain the function of genetic variants impairs our understanding of the disease pathogenesis and is an impediment to personalized management of AAMD. In this study, we have implemented a high throughput serum-based functional assay, similar to that described by Lashkari et al.,17 to more definitively determine the significance of FI variants. As outlined below, our results produced several key findings.
First, a large majority (78 of 106) of individuals who are haploinsufficient for FI develop AAMD. Haploinsufficiency presents as either a quantitative defect (low antigenic and thus reduced functional activity of FI) with Type 1 variants or normal antigenic levels but low functional activity (Types 2 and 3). A variant belonging to Type 3 produces a protein that is less dysfunctional than those generated by genetic variants in Type 2, but it is not equivalent in regulatory activity to wild-type. Therefore it would also be expected that a moderate deficiency state would have a lower penetrance. The main point is that Type 3 variants have a functional deficiency, i.e., being less defective than Type 2 variants but not equivalent to wild-type. Importantly, FI-mediated complement regulatory activity is normal in most individuals without a rare variant (with or without AMD).
Second, remarkably, 42% of the rare genetic variants in this AAMD cohort have been observed in patients with atypical hemolytic uremic syndrome (aHUS),21–25 a thrombotic microangiopathy in which rare mutations in CFI are causative in 5% to 15% of patients. This association of variants causing two apparently disparate diseases is striking. Atypical hemolytic uremic syndrome features acute endothelial injury that often arises in about 50% of the time in early childhood, whereas AMD features biological debris above and below the retinal pigment epithelium and is a disease manifested primarily in older adults. An emerging theme over the past decade, particularly based on insights gained from genetic studies, is that modulation of complement inhibitory activity predisposes to acute thrombomicroangiopathies such as aHUS, as well as to dysfunctional debris handling causing a chronic disease such as AMD. Gaining insights into the functional capabilities of these variants may help to delineate patients who are at risk of developing an acute kidney disease early in life versus those who are at risk of manifesting another chronic debilitating condition at an older age. Type 1 variants P50A, P64L, G119R, V152M, G162D, N177I, A240G, A258T, G287R and R474X,17,19–22 Type 2 variant E554V,26,27 and Type 3 variants G261D, R317W, R406H, K441R and P553S have all been reported in aHUS.20,21,28–30 Further analyses of those variants that overlap versus those that are distinctly observed in one or the other disease could be informative.
Third, as anticipated, the functional characterization of CFI variants facilitated more accurate interpretations compared to the in-silico predictions. As an example, E109A, N177I, R317W, R406H, K441R and G500R are predicted to be “benign” or “likely benign” by in-silico models, but based on our current data, as well as prior structure-function analyses of these variants using recombinant proteins,24,25 they are deleterious.
As noted, a further and in some cases a critical approach, to facilitate understanding of FI variants is to produce the variants recombinantly, and then to analyze their function, including (a) utilizing multiple cofactor proteins (C4 binding protein, membrane cofactor protein, complement receptor 1 as well as Factor H), (b) carrying out assessments on the cell membrane as well as in the fluid phase, and (c) monitoring cleavage of both the alternative and classical/lectin pathway fragments (C3b and C4b, respectively). We are now taking this overall approach for variants belonging to Type 3 to rigorously assess their functional repertoire as well as for other outliers and putative discrepancies.28,30
Measuring serum antigenic levels and functional activity of FI provides a quick, simple, practical and low-cost screening tool in the clinical laboratory. We recommend that patients with and without AAMD who have a rare variant in CFI should undergo an assessment of serum FI antigenic levels. If the antigenic level is low, as illustrated here and in the literature,7 the patient is appropriately classified as carrying a defective Factor I. If the antigenic level is normal, then a functional assessment for FI, such as the serum-based assay reported here, should be performed. If the assay shows low function, the patient has reduced cofactor activity and is therefore predisposed to AAMD.
Overall, these results provide compelling functional evidence for a key role of impaired FI-mediated regulatory activity of the complement system's alternative pathway in the pathogenesis of AAMD. Results also have direct clinical relevance. Identification of individuals with AAMD in the clinic who have a rare CFI variant, and stratifying them according to this assay into Type 1, 2 or 3 CFI mutations, will facilitate screening and targeting patients most likely to benefit from complement pathway related therapies. Such an assay therefore facilitates a personalized treatment strategy for AAMD as currently underway in some clinical trials. Results may also be relevant for complement-mediated renal diseases.
In conclusion, AMD is a chronic disease in which the therapeutic goals are to slow the rate of progression or, preferably, halt the retinal damage. In AAMD, approximately 10% of patients carry a rare genetic variant in FH or FI, the two plasma regulators that inhibit complement activation at the key C3 step. In a clinical trial using a complement-based therapeutic, the investigators would preferentially study such patients. To ascertain them, we would recommend a workup consisting of a genetic screen for a rare variant. If identified, then an antigenic level would be obtained. In approximately 30% of cases for FH and 50% for FI, a patient carrying a rare variant will be haploinsufficient with a low antigenic level. If level is in the normal range, a functional assay (such as on FI as performed in this study) would next be obtained. This personalized medicine approach is being used for clinical trials (https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-003712-39/GB; https://clinicaltrials.gov/ct2/show/NCT04246866?term = Gemini&cond = AMD&draw = 2&rank = 1) and, assuming an effective anti-complement therapeutic agent is identified, will be required to target AMD patients who are most likely to benefit from a complement therapeutic agent. This approach is already being accomplished in aHUS29 and should be undertaken in AAMD, and other diseases in which a patient carries a rare variant. In view of the drug costs and duration of therapy likely to be necessary to treat AAMD, these analyses will eventually be required.
Supplementary Material
Acknowledgments
The authors thanks Iris Lee (Washington University School of Medicine, St. Louis) for her helpful suggestions and Madonna Bogacki for editorial assistance.
Supported by NIH R01-EY011309, R01-EY028602, American Macular Degeneration Foundation, Northampton, MA; The Macular Degeneration Center of Excellence, University of Massachusetts Medical School, Department of Ophthalmology and Visual Sciences, Worcester, MA (JS); Wellcome Trust, The MRC, Kidney Research UK and the NIHR Biomedical Research Centre at Newcastle upon Tyne Hospitals, NHS Foundation Trust (DK); NIH R01-EY028602 (JA & AJ), NIH 2R01 GM099111 (JA); Barnes Jewish Hospital Foundation Fund, Division of Nephrology, Washington University School of Medicine in St. Louis (AJ).
Disclosures: A. Java, Alexion Pharmaceuticals (C), Gemini Therapeutics (C), Novartis Pharmaceuticals (C); P. Baciu, Allergan (E); R. Widjajahakim, None; Y.J. Sung, None; J. Yang, Allergan (E); D. Kavanagh, Gyroscope Therapeutics (S), Alexion Pharmaceuticals (C), Sarepta (C), Actelion (C), Novartis (C), Apellis (C); J. Atkinson, Compliment Corporation (I), Kypha (I,C), Gemini Therapeutics (I), Q32BIO INC - formerly AdMiRx (I,C), Celldex Therapeutics (C), Clinical Pharmacy Services (C), Achillion Pharmaceuticals (C), BioMarin Pharmaceutical (C), Annexon Biosciences (C); J. Seddon, Laboratoires THEA (C), Gemini Therapeutics, Inc (F)
References
- 1. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. [DOI] [PubMed] [Google Scholar]
- 2. Seddon JM. Macular degeneration epidemiology: nature-nurture, lifestyle factors, genetic risk, and gene-environment interactions - The Weisenfeld Award Lecture. Invest Ophthalmol Vis Sci. 2017; 58: 6513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014; 61: 118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geerlings MJ, de Jong EK, den Hollander AI. The complement system in age-related macular degeneration: A review of rare genetic variants and implications for personalized treatment. Mol Immunol. 2017; 84: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liszewski MK, Java A, Schramm EC, Atkinson JP. Complement dysregulation and disease: insights from contemporary genetics. Annu Rev Pathol. 2017; 12: 25–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seddon JM, Yu Y, Miller EC, Reynolds R, Tan PL, Gowrisankar S, et al.. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013; 45: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kavanagh D, Yu Y, Schramm EC, et al.. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum Factor I levels. Hum Mol Genet. 2015; 24: 3861–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Triebwasser MP, Roberson ED, Yu Y, et al.. Rare variants in the functional domains of complement Factor H are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu Y, Triebwasser MP, Wong EK, et al.. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum Mol Genet. 2014; 23: 5283–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donoso LA, Vrabec T, Kuivaniemi H. The role of complement Factor H in age-related macular degeneration: a review. Surv Ophthalmol. 2010; 55: 227–246. [DOI] [PubMed] [Google Scholar]
- 11. Wagner EK, Raychaudhuri S, Villalonga MB, et al.. Mapping rare, deleterious mutations in Factor H: Association with early onset, drusen burden, and lower antigenic levels in familial AMD. Sci Rep. 2016; 6: 31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement Factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009; 17: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raychaudhuri S, Iartchouk O, Chin K, et al.. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011; 43: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrara D, Seddon JM.. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthalmol. 2015; 133: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006; 113: 260–266. [DOI] [PubMed] [Google Scholar]
- 16. Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009; 50: 5818–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lashkari K, Teague G, Chen H, et al.. A monoclonal antibody targeting amyloid beta (Abeta) restores complement Factor I bioactivity: potential implications in age-related macular degeneration and Alzheimer's disease. PLoS One. 2018; 13: e0195751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okroj M, Blom AM. Factor I. In: Barnum SR, Schien T, editors. The Complement Factsbook. 2nd ed Cambridge: Academic Press; 2018. p. 147–54. [Google Scholar]
- 19. Le Quintrec M, Lionet A, Kamar N, et al.. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant. 2008; 8: 1694–1701. [DOI] [PubMed] [Google Scholar]
- 20. Nilsson SC, Kalchishkova N, Trouw LA, Fremeaux-Bacchi V, Villoutreix BO, Blom AM. Mutations in complement Factor I as found in atypical hemolytic uremic syndrome lead to either altered secretion or altered function of Factor I. Eur J Immunol. 2010; 40: 172–85. [DOI] [PubMed] [Google Scholar]
- 21. Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat. 2010; 31: E1445–E1460. [DOI] [PubMed] [Google Scholar]
- 22. Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al.. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013; 8: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Westra D, Volokhina E, van der Heijden E, et al.. Genetic disorders in complement (regulating) genes in patients with atypical haemolytic uraemic syndrome (aHUS). Nephrol Dial Transplant. 2010; 25: 2195–2202. [DOI] [PubMed] [Google Scholar]
- 24. Bresin E, Rurali E, Caprioli J, et al.. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013; 24: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caprioli J, Noris M, Brioschi S, et al.. Genetics of HUS: the impact of MCP, CFH and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006; 108: 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sullivan M, Erlic Z, Hoffmann MM, et al.. Epidemiological approach to identifying genetic predispositions for atypical hemolytic uremic syndrome. Ann Hum Genet. 2010; 74: 17–26. [DOI] [PubMed] [Google Scholar]
- 27. Noris M, Caprioli J, Bresin E, et al.. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010; 5: 1844–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nilsson SC, Karpman D, Vaziri-Sani F, et al.. A mutation in Factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of Factor I in complement regulation. Mol Immunol. 2007; 44: 1835–1844. [DOI] [PubMed] [Google Scholar]
- 29. Java A, Pozzi N, Love-Gregory LD, et al.. A multimodality approach to assessing Factor I genetic variants in atypical hemolytic uremic syndrome. Kidney Int Rep. 2019; 4: 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kavanagh D, Richards A, Noris M, et al.. Characterization of mutations in complement Factor I (CFI) associated with hemolytic uremic syndrome. Mol Immunol. 2008; 45: 95–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.