Highlights
-
•
Assessed relation of maternal prenatal distress to amygdalae and hippocampi volumes.
-
•
Newborn amygdalar volumes negatively related to maternal prenatal distress in males.
-
•
No association between maternal prenatal distress and newborn hippocampal volumes.
-
•
Prenatal maternal distress seems to affect newborn brain in a sex-dependent way.
Keywords: Prenatal anxiety, Prenatal depression, Newborn brain, Amygdala, Hippocampus, Sexual dimorphism
Abstract
Maternal psychological distress during pregnancy (PPD)1 has been associated with changes in offspring amygdalar and hippocampal volumes. Studies on child amygdalae suggest that sex moderates the vulnerability of fetal brains to prenatal stress. However, this has not yet been observed in these structures in newborns. Newborn studies are crucial, as they minimize the confounding influence of postnatal life.
We investigated the effects of maternal prenatal psychological symptoms on newborn amygdalar and hippocampal volumes and their interactions with newborn sex in 123 newborns aged 2–5 weeks (69 males, 54 females). Based on earlier studies, we anticipated small, but statistically significant effects of PPD on the volumes of these structures. Maternal psychological distress was measured at gestational weeks (GW)2 14, 24 and 34 using Symptom Checklist-90 (SCL-90, anxiety scale)3 and Edinburgh Postnatal Depression Scale (EPDS)4 questionnaires.
Newborn sex was found to moderate the relationship between maternal distress symptoms at GW 24 and the volumes of left and right amygdala. This relationship was negative and significant only in males. No significant main effect or sex-based moderation was found for hippocampal volumes.
This newborn study provides evidence for a sex-dependent influence of maternal psychiatric symptoms on amygdalar structural development. This association may be relevant to later psychopathology.
1. Introduction
Maternal prenatal anxiety and depressive symptoms – main features of maternal prenatal psychological distress (PPD) – are common among pregnant women (Andersson et al., 2006, Andersson et al., 2004, Matthey et al., 2004, Rubertsson et al., 2014). Studies increasingly associate maternal PPD with compromised offspring neurodevelopment, observed as behavioral and emotional disturbances, increased fearful temperament as well as delayed cognitive and motor development (Glover, 2014, Huizink et al., 2003, Sandman et al., 2012). PPD is additionally associated with alterations in offspring brain structures and function (Adamson et al., 2018, Buitelaar et al., 2003, Buss et al., 2011, El Marroun et al., 2016, Miguel et al., 2019, O'Donnell et al., 2014). The anatomical alterations are partly similar to those seen in psychopathologies of autism, depression, conduct disorder and post-traumatic stress disorder (PTSD), for instance involving changes in volume and connectivity of the limbic system (Frodl et al., 2003, Killion and Weyandt, 2020, Mosconi et al., 2009, Rogers and De Brito, 2016, Schumann et al., 2009, Valera et al., 2007).
Studies have started unpacking the links between PPD and offspring brain development. Evidence of a connection between PPD and altered cortical morphology is convincing in newborn and child studies (Buss et al., 2010, Lebel et al., 2016, Qiu et al., 2015b, Sandman et al., 2012, Sandman et al., 2015). However, the relationships between PPD and brain regions closely related to emotional, cognitive and memory functions – such as the amygdala and hippocampus (Qiu et al., 2017, Qiu et al., 2015a, Wen et al., 2017, Wu et al., 2020) – are less studied. Alterations in these structures may explain attentional deficits and other behavioural problems in offspring (Van den Bergh et al., 2018). The mechanisms that may link PPD and offspring neurodevelopment are nevertheless still mostly unknown. Suggested possible mechanisms include proinflammatory processes, increased levels of glucocorticoids, changes in placental function, and genetic factors (Davis et al., 2017, Graham et al., 2018, McEwen, 2010, Moisiadis and Matthews, 2014a, Qiu et al., 2017). Especially important seem to be changes in maternal concentrations of corticosteroids (cortisol), as these are critical to fetal programming (Amugongo and Hlusko, 2013, Davis et al., 2013, Moisiadis and Matthews, 2014a), partly via placental changes (Charil et al., 2010, Sandman et al., 1999). Fetal exposure to excess glucocorticoids has been proposed to alter hypothalamic–pituitaryadrenal (HPA) axis function, thus affecting the structural development of the cortex and subcortical regions (Moisiadis and Matthews, 2014a). However, contradictory findings of no association between PPD and maternal prenatal cortisol levels also exist (Davis et al., 2017, Deuschle et al., 2018, Petraglia et al., 2001). According to previous newborn/child MRI studies, an important time connecting PPD and the possible alterations in subcortical structures seems to be mid-pregnancy (Qiu et al., 2017, Qiu et al., 2013, Wen et al., 2017), although the possible impacts of other time points in pregnancy remain elusive.
Regarding subcortical structures, maternal prenatal depressive symptoms have been associated with higher cortisol levels and larger right amygdalar volumes in 4.5 and 7-year-old females (Wen et al., 2017), as well as more affective problems (Buss et al., 2012). Interestingly, greater pregnancy-related anxiety has been observed to associate with larger left amygdalar volumes in females, and with smaller left amygdalar volumes in 4-year-old males (Acosta et al., 2019). In newborns, a positive relationship between maternal depressive symptoms and larger right amygdalar volume has been observed, but only in a genetic population with a higher risk for major depressive disorder and with no reported sex differences (Qiu et al., 2017). However, Rifkin-Graboi et al. found no such connection in newborns with no specific genetic risk in neither sex (Rifkin-Graboi et al., 2013). Overall, some findings suggest the relationship between PPD and amygdalar volume to be sex-dependent (Acosta et al., 2019, Wen et al., 2017). The impact of PPD on hippocampal volumes measured at six months of age seems to be growth-restricting in both sexes, especially on the right side (Qiu et al., 2013), but opposite effects have also been observed in newborns with higher genetic risk for major depressive disorder (Qiu et al., 2017). Overall, only few studies have investigated associations between PPD and newborn amygdalar and hippocampal volumes. Instead, most studies have focused on other brain outcomes or older children, which increases the risk of postnatal factors affecting the results. In addition, even though comorbidity and overlap of depressive and anxiety symptoms are common, previous research has not considered the possible effect of PPD’s comorbidity, or additive effects of maternal depressive and anxiety symptoms, on offspring subcortical structures. One study found that in a population of 190 pregnant women with a psychiatric diagnosis, 20.5% had comorbid depressive and anxiety disorders (Andersson et al., 2006), which is a similar prevalence to that in non-pregnant populations (Andersson et al., 2006, Kendler et al., 2007, Mathew et al., 2011, Middeldorp et al., 1999). This is important, as maternal comorbid symptomatology might influence the developing fetus differently than one symptom dimension alone, for example through differential stress hormone activity (Evans et al., 2008). Further, as depressive and anxiety disorders are all arbitrary diagnoses based on the number, duration and severity/impact of symptoms, their aetiologies are overall unclear, and their subclinical manifestations may overlap one another to a greater extent.
To the best of our knowledge, no study using newborn brain volumes as outcomes has used a more robust construct of maternal PPD by combining depressive and anxiety symptoms into one measure. In this study, we explored: 1) the association of PPD and newborn amygdalar and hippocampal volumes, as well as 2) the possible interaction of this association with newborn sex. We measured PPD as a combination of anxiety and depressive symptoms, with which we aimed to have a more comprehensive measure of PPD. According to earlier studies, we expected to observe relationships mainly regarding mid-pregnancy symptoms but also investigated early and late pregnancy.
2. Methods
The study was conducted according to the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Hospital District of Southwest Finland (ETMK:31/180/2011).
2.1. Participants
Mothers involved in this study were drawn from the broader FinnBrain Neurodevelopment study (Karlsson et al., 2018). They were recruited at three healthcare locations in Southwest Finland during their first trimester ultrasound visits at gestational week (GW) 12. From this broader participant pool, 189 newborn-mother dyads were recruited into this study. They were recruited based on willingness to participate, availability of the newborn to have an MRI two to five weeks after birth, childbirth being after GW 31, birthweight more than 1500 g, and not having a previously diagnosed central nervous system (CNS) anomaly or abnormal findings in a previous MRI scan. After explaining the study’s purpose and protocol, written informed consent was obtained from the parent(s). Of these 189 newborn participants, 64 had motion artefacts in the MR images. Additionally, two mothers had missing prenatal distress questionnaires. Therefore 123 newborn-mother dyads were eligible for statistical analyses.
Participant descriptives are presented in Table 1. Background information was gathered by questionnaires and included monthly income, educational level, diagnosed medical conditions, CNS affecting medications, and substance use during pregnancy. For CNS affecting medications, only serotonin and norepinephrine reuptake inhibitors or benzodiazepines were reported. No substance use other than alcohol or tobacco were reported. Maternal education, medication, and substance use were included in a sensitivity analysis. Obstetric data were retrieved from the Finnish Medical Birth Register of the National Institute for Health and Welfare (www.thl.fi).
Table 1.
Descriptives.
| Variable | Whole sample (N = 123) | Males (N = 69) | Females (N = 54) | p |
|---|---|---|---|---|
| M ± SD (range) | ||||
| Age at birth (w) | 39.9 ± 1.1 (36.3–42.1) | 39.8 ± 1.1 (36.3–42.1) | 39.9 ± 1.2 (37.6–42) | 0.657 |
| Age at scan (days from due date) | 24.9 ± 7.4 (8–45) | 24.9 ± 6.9 (8–45) | 24.9 ± 8 (14–40) | 0.671 |
| Age at scan (days from birth) | 26.2 ± 7.7 (11–54) | 26.4 ± 7.9 (11–43) | 25.9 ± 7.4 (14–54) | 0.973 |
| Total age at scan (GW + days from birth) | 305.2 ± 7.4 (291–325) | 305.3 ± 7 (294–320) | 305.1 ± 7.8 (291–325) | 0.894 |
| Head circumference (cm) | 35 ± 1.3 (32.5–37.5) | 35.3 ± 1.3 (33–37.5) | 34.7 ± 1.3 (32.5–37) | 0.015* |
| Birth weight (g) | 3480.5 ± 431.8 (2530–4700) | 3564.8 ± 451.9 (2720–4700) | 3370.6 ± 380.7 (2530–4340) | 0.013* |
| Birth length (cm) | 50.5 ± 1.9 (44–56) | 50.8 ± 2 (46–56) | 50 ± 1.7 (44–53) | 0.020* |
| Maternal age at term (y) | 30.2 ± 4.4 (19.1–41.3) | 30.5 ± 4.6 (19.1–41.1) | 29.8 ± 4.3 (21.5–41.3) | 0.359 |
| Paternal age at term (y) | 31.9 ± 4.9 (20–47.5) | 31.4 ± 5.2 (20–47.5) | 32.4 ± 4.5 (24–43.4) | 0.368 |
| Maternal prepregnancy weight (kg) | 66.6 ± 12.6 (42–115) | 67.9 ± 13.3 (46–115) | 65 ± 11.5 (42–101) | 0.208 |
| Maternal BMI (kg/m2) | 24.1 ± 4 (17.5–38.4) | 24.5 ± 4.2 (18–38.4) | 23.7 ± 3.7 (17.5–35) | 0.329 |
| Frequencies | ||||
| APGAR 5 min < 5 | 1 | 1 | 0 | 0.383 |
| Respirator/CPR (yes) | 1/1 | 1/1 | 0 | 0.383 |
| Asphyxia (yes) | 14 | 6 | 8 | 0.289 |
| Gestational diabetes (GDM) (yes) | 15 | 8 | 7 | 0.811 |
| Prenatal alcohol consumption (yes) | 29 | 20 | 9 | 0.112 |
| Prenatal smoking (yes) | 9 | 5 | 4 | 0.950 |
| CNS affecting medication | 9 | 7 | 2 | 0.177 |
| Maternal education (low/middle/high) | 34/38/49 | 18/20/30 | 16/18/19 | 0.355 |
| Maternal ethnicity: Caucasian | 119 | 66 | 53 | |
| Parents married/living together | 119 | 61 | 48 |
In the right column p-values (of t-tests or chi-squared tests) for sex differences in the sample are listed; *Significant results (p < 0.05); GW = gestational week; CPR = cardiopulmonary resuscitation; CNS affecting medication: serotonin and norepinephrine reuptake inhibitors, benzodiazepines; maternal education: low = high school degree or lower, middle = vocational degree, high = master's degree or higher.
Newborns were generally scanned two to five weeks after birth, though twelve scans were performed after this age, and two before (Table 1). All newborns exceeded 2500 g at birth and all but one were born full-term [between GW 37 and 42; preterm at GW 36.3]. Newborns’ background information was gathered from register data; 15 newborns had mild asphyxia, one had cardiopulmonary resuscitation (CPR) and respirator treatment, and one individual scored four points in the five-minute Apgar. These birth complications were included in a sensitivity analysis as a single binary variable.
2.2. Maternal psychological distress questionnaire data
Maternal psychological distress during pregnancy was measured at GWs 14, 24 and 34 with two separate stress questionnaires: Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987) for depressive symptoms, and the anxiety subscale of Symptom Checklist 90 (SCL-90) (Deogratis et al., 1973) for anxiety. Although originally developed for screening postnatal depression, EPDS is also validated for use during pregnancy (Bergink et al., 2011, Bunevicius et al., 2009, Rubertsson et al., 2011, Tendais et al., 2014). The 10 items of EPDS cover the previous 7 days on a total score range from 0 to 30. While no consensus on the most optimal EPDS cut-point for prenatal depression exists (Alvarado-Esquivel, 2014, Gibson et al., 2009, Rubertsson et al., 2011), a score of 10 or more has been employed to indicate clinically meaningful symptoms of depression in pregnancy (Vázquez and Míguez, 2019). The anxiety subscale of SCL-90 is a standard tool for measuring anxiety (Bech et al., 2014, Deogratis et al., 1973) and widely used during the prenatal period (Adib-Rad et al., 2019, Kamel et al., 1999, Lin et al., 2017, Van den Heuvel et al., 2015). The SCL-90 anxiety subscale consists of 10 items, each on a 5-point scale of distress (0–4) with a total score range of 0–40. As for EPDS, no established cut-off score exists for the SCL-90 anxiety subscale, however, a score higher than 10 has been used to indicate relevant symptoms of anxiety (Karlsson et al., 2018, Korja et al., 2017). The number of scorings exceeding the selected cut-off points for probable clinical depression/anxiety are presented in Table 2. However, for this study, no clinical cut-off scores were set for depressive or anxiety symptoms as this would have excluded subjects with subclinical levels of these symptoms, which may be important in terms of PPD’s impact on fetal development. The number of respondents at each timepoint was 120 ± 3 out of 189 regardless of questionnaire type (Table 2). If questionnaire data was missing in one of the timepoints, data was imputed by the MissForest method (Stekhoven and Buhlmann, 2012). The number of missing data for EPDS and SCL-90 were 3/123 at GW 14, 1/123 at GW 24, and 6/123 at GW 34, meaning that a mother with missing data had neither of the two distress scores at that certain time point. SCL-90 and EPDS scores were combined to create an overall continuous distress score because this study sample was drawn from the general population and the frequency of subjects with clinical depression and anxiety was expectedly low. The scores were standardized (mean = 0, SD = 1) for each scale and then summed. This overall distress score was calculated for each gestational timepoint (GW 14: SCL1 + EPDS1, GW 24: SCL2 + EPDS2, GW 34: SCL3 + EPDS3).
Table 2.
Descriptive information on distress questionnaires.
| M ± SD (range) (N of imputed symptom scores) |
|||||
|---|---|---|---|---|---|
| Questionnaire | Whole sample (N = 123) | Males (N = 69) | Females (N = 54) | p | p (Q) |
| SCL score GW14 | 3.53 ± 4.50 (0–19) (3) | 3.40 ± 4.13 (0–16) | 3.68 ± 5.04 (0–19) | 0.558 | <0.001 (18.48) |
| SCL score GW24 | 4.56 ± 5.32 (0–28) (1) | 4.78 ± 5 (0–19) | 4.27 ± 5.73 (0–28) | 0.274 | |
| SCL score GW34 | 3.44 ± 4.12 (0–19) (6) | 3.67 ± 3.67 (0–12) | 3.15 ± 4.66 (0–19) | 0.101 | |
| EPDS score GW14 | 5.46 ± 5.15 (0–25) (3) | 5.40 ± 4.78 (0–21) | 5.54 ± 5.63 (0–25) | 0.788 | 0.635 (0.84) |
| EPDS score GW24 | 5.58 ± 5.35 (0–25) (1) | 5.66 ± 5.14 (0–23) | 5.48 ± 5.66 (0–25) | 0.645 | |
| EPDS score GW34 | 5.48 ± 4.95 (0–20) (6) | 5.66 ± 4.89 (0–17) | 5.25 ± 5.06 (0–20) | 0.574 | |
| Distress sum score | |||||
| SCL 1 + EPDS 1 | 1.28E-16 ± 1.92 (−1.84–6.43) (6) | −0.04 ± 1.76 (−1.84–6.43) | 0.05 ± 2.12 (−1.84–6.43) | 0.695 | 0.524 (1.29) |
| SCL 2 + EPDS 2 | 3.47E-18 ± 1.85 (−1.90–5.78) 6) | 0.06 ± 1.77 (−1.90–5.78) | −0.07 ± 1.96 (−1.90–5.78) | 0.403 | |
| SCL 3 + EPDS 3 | 5.38E-17 ± 1.84 (−1.94–6.71) (12) | 0.09 ± 1.67 (−1.94–6.71) | 0.12 ± 2.01 (−1.94–6.71) | 0.230 | |
| Frequencies (%) | |||||
| SCL 1 cut off score ≥ 10 | 16 (13) | 6 (8.7) | 10 (18.5) | ||
| SCL 2 cut off score ≥ 10 | 21 (17) | 12 (17.4) | 9 (16.7) | ||
| SCL 3 cut off score ≥ 10 | 12 (9.6) | 7 (10.1) | 5 (9.3) | ||
| EPDS 1 cut off score ≥ 10 | 16 (13) | 8 (11.6) | 8 (14.8) | ||
| EPDS 2 cut off score ≥ 10 | 26 (21.1) | 15 (21.7) | 11 (20.4) | ||
| EPDS cut off score ≥ 10 | 23 (18.7) | 13 (18.8) | 10 (18.5) | ||
Columns from right to left: p-values of Friedman test for differences between distress scores; p-values of Mann-Whitney U tests for sex differences in the sample. Significant results (p < 0.05) are in blue; GW, gestational week; SCL, Symptom Checklist −90 (range of total sum score 0–40); EPDS, Edinburgh Postnatal Depression Scale (range of total sum score 0–30).
2.3. MRI acquisition
The newborns were scanned without anesthesia with a Siemens Magnetom Verio 3 T scanner (Siemens Medical Solutions, Erlangen, Germany). The 40-minute imaging protocol included axial PD-T2-TSE (Dual-Echo Turbo Spin Echo) and sagittal 3D-T1 MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) sequences with isotropic 1.0 mm3 voxels and whole brain coverage. Repetition time (TR) time of 12 070 ms and effective Echo time (TE) of 13 ms and 102 ms were used in PD-T2 TSE sequence to produce both PD-weighted and T2-weighted images from the same acquisition. Total number of 1 mm thick slices was 128. TR of 1900 ms, TE of 3.26 ms, and inversion time (TI) of 900 ms were used for 3D-T1-MPRAGE sequence. Total number of slices was 176. A detailed description of the scanning protocol is provided in a previous publication by the same research team (Lehtola et al., 2019).
All brain images were assessed for incidental findings by a pediatric neuroradiologist (author RP). If found, parents were given a follow-up opportunity with a pediatric neurologist (author TL). Developmental status was age appropriate by the age of two years for all participants. The incidental findings (intracranial hemorrhages, N = 12, 6.9%) have been found to be common and clinically insignificant in previous studies (Kumpulainen et al., 2020, Rooks et al., 2008, Whitby et al., 2004). Intracranial hemorrhages were minor, situated far from the regions of interest and were deemed to be clinically insignificant by the pediatric neuroradiologist (author RP), and thus, these individuals were not excluded from the current study. All the brain images were checked visually by multiple researchers for motion artefacts, initially by three independent raters and the final segmentations were viewed by authors JDL and JJT. No specific rating scale was used but a binary classification.
2.4. Construction of an unbiased population-specific template
The measurements used in analysis were derived using fusion-based methods that rely on a labelled template. These methods depend on achieving good registrations between the subjects and the template. This is increasingly difficult to achieve the further the template is from the subjects in terms of similarity. Thus, a template was constructed based on the subjects in this study.
All 123 MRIs were used to construct a population-specific dual-contrast template. The T2 was linearly registered to the T1, and then the two together were linearly registered to the MNI 152 template (Fonov et al., 2011). The average scaling from the native MRIs to the MNI 152 template was then computed, and the inverse used to scale the MNI 152 template to the average size of the study population, which served as an initial target for construction of the population-specific template. The template construction procedure has been previously published (Fonov et al., 2011). It is an iterative procedure that, given a set of MRI volumes, builds a template which minimizes the mean squared intensity difference between the template and each subject’s MRI, and minimizes the magnitude of all deformations used to map the template to each subject’s MRI.
This method was applied to the T1 scans producing non-linear transformations from the template space to each scan, then these transformations were used to map the T1 scans to the template space, where they were averaged to create the T1 template; these transformations were also combined with the T2 to T1 transformations to map the T2 scans into the common space where they were averaged to create the T2 template.
2.5. Labelling the template
The structures of interest, the amygdalae and hippocampi, were manually labelled on the dual-contrast template. To ensure these labels were accurate, multiple variants of the template were produced, and each variant was manually labelled. Altogether 21 variants were produced, each a non-linear transformation of the template to overlay one of the subjects in the population. These variants represented well the morphological variation in the data and provided enough manual segmentations to be able to have confidence in the majority-vote final labels (see below).
The non-linear transformations derived from the template construction procedure were used to cluster the subjects into 21 groups where the anatomical within-group variability was smaller than the inter-group variability. As the basis for clustering, the Jacobian was computed for the non-linear transform mapping each subject to the template. The values in the Jacobian were then extracted as a vector for each voxel within the template brain mask. These Jacobian vectors were then clustered using an equal combination of cosine similarity and Euclidean distance with Ward’s clustering method (Ward, 1963), with the number of clusters chosen to be 21. Then, within each cluster, the sum-squared distance from each subject to each other subject was computed, and the subject with the minimum sum-squared distance was taken as the central-most subject of the cluster. The dual-contrast template constructed in the previous step was then warped to these 21 representative subjects, and provided for manual segmentation (without those doing the segmentation being aware that these were, in fact, 21 different versions of the same template) with standard procedures (Hashempour et al., 2019).
The 21 manual segmentations were then warped back to the standard template, and each voxel was assigned a label based on the majority vote across all 21 manual segmentations. This yielded the final labels for the amygdalae and hippocampi on the standard template.
2.6. Manual segmentation
Initially one template was segmented by the primary rater NH and senior rater JJT and externally reviewed by JDL. Then the other templates were segmented with standard procedures (Hashempour et al., 2019). The final segmentations were a consensus between the primary and senior raters and were used in the subsequent labelling step. A majority agreement for the final labels was obtained for 67.26% (left hippocampus), 65.99% (right hippocampus), 59.77% (left amygdala) and 56.49% (right amygdala) of the non-zero voxels. Generalized conformity indices (GCI) were also calculated to determine the inter-rater agreement in spatial overlap for the infant template (left hippocampus: CGI = 0.77, right hippocampus: CGI = 0.76, left amygdala: CGI = 0.72, right amygdala: CGI = 0.69). CGI scores of 0.7–1.0 are regarded as excellent (Kouwenhoven et al., 2009, Visser et al., 2019).
2.7. Labelling the subjects
Segmentation into left and right amygdalae and hippocampi was done for each subject using a label-fusion-based labeling technique based on Coupé et al. (Coupé et al., 2011) and further developed by Weier et al. (Weier et al., 2014) and Lewis et al. (Lewis et al., 2019). The approach uses a population-specific template library. In the current work, the library was constructed by clustering (similarly to the method described above) the deformation fields from the non-linear transforms produced during construction of the template and using the central-most subject of each cluster to construct the entries in the template library. Thus, the template library represented the range of deformations found in the population. The clustering was done as described above but using a dilated mask of the amygdalae and hippocampi to capture the anatomical context of the nonlinear registration in that region of the brain, and with the number of clusters now chosen as the square of the natural log of the number of subjects. The representative subject for each cluster was chosen as described above. This was done per hemisphere to accommodate hemispheric asymmetries.
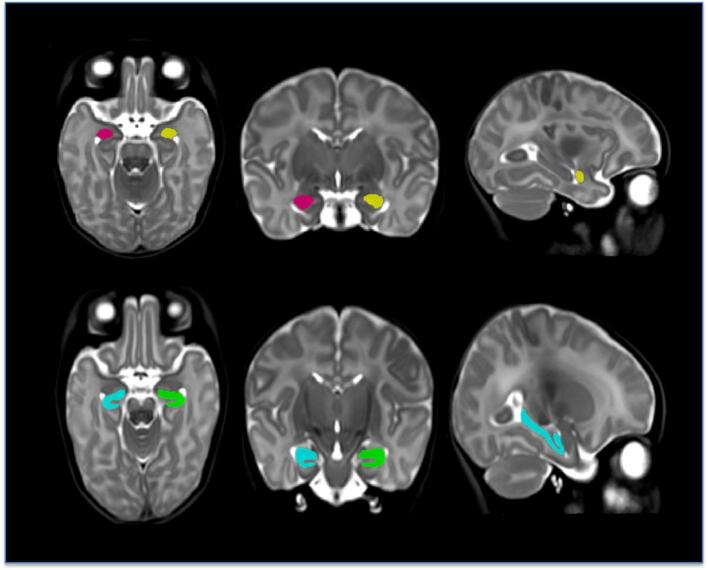
To create the library entry for a cluster, the non-linear transform for the central-most subject was used to warp the template together with the segmentation defined on it, and this pair was added to the template library. The template library was thus a set of warped copies of the template together with their correspondingly warped segmentations. Once the template library had been created, each subject in the population was non-linearly registered to the n closest templates in the library (here, n = 7), and the resulting transforms were used to warp their corresponding segmentations to the subject; the final labelling was then established via a per-voxel majority vote. This was also done separately for each hemisphere. An example of such a labelling is shown in Fig. 1. The volumes of each of the final labellings were then computed and scaled to native space based on the scaling factors in the subject’s linear transforms.
Fig. 1.
Segmentation example from a study subject. First row from left to right: the segmentation of the amygdala; axial, coronal and sagittal planes. Second row from left to right: the segmentation of the hippocampus; axial, coronal and sagittal planes.
Lastly, a brain mask was created on the template, and that template mask was then warped back to the native space of each subject and used to estimate intracranial volume (ICV).
2.8. Code availability
Please contact the corresponding author for code related questions.
2.9. Statistical analyses
IBM SPSS Statistics Version 23 for MAC was used for statistical analyses (Armonk, NY: IBM Corp.). Data normalities for descriptive variables, brain volumetric measures and distress scores were checked by visual inspection and by the Shapiro-Wilk test. If data was shown to be non-normally distributed, a non-parametric test was used. Distress score variables were not normally distributed, but as normality is not assumed for independent variables in regression, no adjustment was made. The normality of the residuals from the regression was checked using the Shapiro-Wilk test. Dependent variables used in the analyses were the relative (absolute volumes divided by ICV) volumes of the left and right amygdala and hippocampus (Table 3). Results for absolute amygdalar and hippocampal volumes were analyzed for consistency with these dependent variables but were not considered in the main analyses. Associations between the study variables (maternal and newborn health parameters, distress questionnaire scoring, volumetric measures) were investigated with zero-order Pearson correlations. Sex differences in mean volumetric measures, newborn age, head circumference, birthweight and length were analyzed with Independent samples T-test (Table 1, Table 3), sex differences in birth complications and maternal categorical variables with Chi-square Tests (Table 1) and in distress scores with Mann-Whitney U Test (Table 2). Differences between distress scores at different pregnancy time points were analyzed with Friedman test (Table 2).
Table 3.
Mean absolute and relative volumes of the amygdala and hippocampus in the whole group and in the subgroups of males and females.
| M ± SD (range) | ||||
|---|---|---|---|---|
| Volumetric measures (mm3) | Whole sample (N = 123) | Males (N = 69) | Females (N = 54) | p |
| ICV | 621 668 ± 47 015.3 (517 422–739 571) | 634 952.7 ± 45 996.2 (547 872–739 571) | 604 760.2 ± 43 041 (517 422–692 269) | <0.001* |
| Left amygdala | 267.7 ± 37.2 (188–358.4) | 274.3 ± 40.9 (188–358.4) | 259.1 ± 30.3 (203.9–356.1) | 0.024* |
| Right amygdala | 266.6 ± 39 (181.9–364.2) | 278.4 ± 39 (181.9–364.2) | 251.5 ± 33.7 (185.9–349.2) | <0.001* |
| Left hippocampus | 767.4 ± 114.6 (514.6–1082.6) | 793.6 ± 118.6 (514.6–1082.6) | 734 ± 100.9 (549.3–989.9) | 0.004* |
| Right hippocampus | 770.2 ± 107.8 (467.7–1020.9) | 788.6 ± 106.1 (560.7–1020.9) | 746.7 ± 106.3 (467.7–974.9) | 0.032* |
| Left amygdala /ICV | 4.3E-4 ± 5E-5 (3.1E-4–5.6E-4) | 4.3E-4 ± 5.2E-5 (3.1E-4–5.4E-4) | 4.3E-4 ± 4.7E-5 (3.3E-4–5.6E-4) | 0.943 |
| Right amygdala /ICV | 4.3E-4 ± 5.2E-5 (3.1E-4–5.6E-4) | 4.4E-4 ± 5.1E-5 (3.1E-4–5.3E-4) | 4.2E-4 ± 5.2E-5 (3.2E-4–5.6E-4) | 0.030* |
| Left hippocampus /ICV | 1.2E-3 ± 1.5E-4 (8.3E-4–1.6E-3) | 1.2E-3 ± 1.5E-4 (8.4E-4–1.6E-4) | 1.2E-3 ± 1.5E-4 (9.5E-4–1.6E-3) | 0.284 |
| Right hippocampus /ICV | 1.2E-3 ± 1.4E-4 (8.4E-4–1.6E-3) | 1.2E-3 ± 1.3E-4 (9E-4–1.5E-3) | 1.2E-3 ± 1.6E-4 (8E-4–1.6E-3) | 0.969 |
In the right column p-values of t-tests for sex differences in the sample are listed. *Significant values (p < 0.05); ICV = intracranial volume.
General linear modelling (GLM) was used to investigate the relationships between distress scores and volumetric measures of the amygdalae and hippocampi. GLM analyses were performed in two parts: 1) the main effect model and 2) the interaction model. The first model (Model A in Table 4A, Table 4B) explored the main effects of the distress scores on amygdalar and hippocampal volumes. The second model (Model B in Table 4A, Table 4B) explored the interaction between sex and distress scores by adding an interaction variable (sex*distress score). Both models were tested separately for both sides of the amygdala and hippocampus in each pregnancy time point (GW 14, 24 and 34, respectively). Thus, altogether 24 tests were performed. Covariates were added stepwise into the analyses: step 1) newborn age at scan (days counted from estimated due date) and newborn sex; step 2) maternal prenatal medication (CNS-affecting medication), substance use (alcohol or tobacco) and maternal education; step 3) gestational diabetes and birth complications (asphyxia, CRP, respirator treatment, 5 min Apgar score under five). Steps 2 and 3 were regarded as sensitivity analyses. Maternal gestational diabetes was included as it may impair fetal neurodevelopment (Torres-Espínola et al., 2018). Additional sensitivity analyses were conducted excluding the only preterm born, all newborns with birth complications and mothers with prenatal CNS affecting medication (N = 25, males N = 15). The main effect models for the whole sample were also performed separately in males and females as post-hoc analyses to further describe the results yielded in the interaction model.
Table 4A.
General linear model for infant brain volumetric measures and maternal prenatal distress scores in each trimester.
|
STEP 1 | ||||||
|---|---|---|---|---|---|---|
| Volumetric measures | Adj R2 | β | p | Partial η2 | CI | |
| LEFT AMYGDALA / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | −0.019 | −1.3E-6 | 0.586 | 0.002 | −6.0 E-6–3.4E-6 |
| EPDS 2 + SCL 2 | −0.009 | −3.0E-7 | 0.223 | 0.012 | −7.9E-6–1.9E-6 | |
| EPDS 3 + SCL 3 | −0.015 | −2.1E-6 | 0.401 | 0.006 | −7.0E-6–2.8E-6 | |
| MODEL B | sex * EPDS 1 + SCL1 | −0.016 | 5.5E-6 | 0.252 | 0.011 | −3.9E-6–1.5E-5 |
| sex * EPDS 2 + SCL 2 | 0.018 | 10.0E-6 | 0.042* | 0.034 | 3.5E-7 – 2.0E-5 | |
| sex * EPDS 3 + SCL 3 | −0.004 | 7.7E-6 | 0.126 | 0.020 | −2.2E-6 – 1.7E-5 | |
| RIGHT AMYGDALA / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | 0.019 | 1.8E-6 | 0.461 | 0.005 | −3.0E-6 – 6.6E-6 |
| EPDS 2 + SCL 2 | 0.016 | 1.2E-6 | 0.623 | 0.002 | −3.8E-6 – 6.3E-6 | |
| EPDS 3 + SCL 3 | 0.015 | 3.1E-7 | 0.905 | < 0.001 | −4.8E-6 – 5.4E-6 | |
| MODEL B | sex * EPDS 1 + SCL1 | 0.024 | 6.2E-6 | 0.203 | 0.014 | −3.4E-6 – 1.6E-6 |
| sex * EPDS 2 + SCL 2 | 0.048 | 1.1E-5 | 0.029* | 0.040 | 1.2E-6–2.1E-5 | |
| sex * EPDS 3 + SCL 3 | 0.035 | 9.6E-6 | 0.061 | 0.029 | −4.6E-7–1.0E-5 | |
| LEFT HIPPOCAMPUS / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | −0.007 | −5.1E-6 | 0.478 | 0.004 | −1.9E-5–9.2E-6 |
| EPDS 2 + SCL 2 | −0.011 | 6.2E-7 | 0.934 | <0.001 | −1.4E-5–1.6E-5 | |
| EPDS 3 + SCL 3 | −0.002 | 8.0E-6 | 0.291 | 0.009 | −6.9E-6–2.3E-5 | |
| MODEL B | sex * EPDS 1 + SCL1 | −0.013 | −8.0E-6 | 0.548 | 0.003 | −3.7E-5–2.1E-5 |
| sex * EPDS 2 + SCL 2 | −0.017 | −9.4E-6 | 0.536 | 0.003 | −3.9E-5–2.1E-5 | |
| sex * EPDS 3 + SCL 3 | −0.002 | −1.6E-5 | 0.306 | 0.009 | −4.6E-5–1.4E-5 | |
| RIGHT HIPPOCAMPUS / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | −0.013 | 2.1E-6 | 0.757 | 0.001 | −1.1E-5–1.5E-5 |
| EPDS 2 + SCL 2 | −0.012 | 3.9E-6 | 0.577 | 0.003 | −9.8E-6–1.8E-5 | |
| EPDS 3 + SCL 3 | −0.010 | 4.6E-6 | 0.506 | 0.004 | −9.1E-6–1.8E-5 | |
| MODEL B | sex * EPDS 1 + SCL1 | −0.018 | −9.1E-6 | 0.496 | 0.004 | −3.6E-5–1.7E-5 |
| sex * EPDS 2 + SCL 2 | −0.020 | −2.1E-6 | 0.881 | <0.001 | −3.0E-5–2.5E-5 | |
| sex * EPDS 3 + SCL 3 | −0.019 | −2.8E-6 | 0.840 | <0.001 | −3.1E-5–2.5E-5 | |
Models A and B were tested separately in each pregnancy time point. *Significant uncorrected results (p < 0.05); Step 1) adjusted for infant sex, infant age at scan counted from due date; ICV = intracranial volume; SCL, Symptom Checklist −90; EPDS, Edinburgh Postnatal Depression Scale.
Table 4B.
General linear model for infant brain volumetric measures and maternal prenatal distress scores in each trimester.
|
STEP 2 | ||||||
|---|---|---|---|---|---|---|
| Volumetric measures | Adj R2 | β | p | Partial η2 | CI | |
| LEFT AMYGDALA / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | −0.027 | 2.2E-7 | 0.935 | −5.1E-6 | −5.1E-6–5.5E-6 |
| EPDS 2 + SCL 2 | −0.025 | −1.5E-6 | 0.606 | 0.002 | −7.1E-6–4.2E-6 | |
| EPDS 3 + SCL 3 | −0.017 | −8.0 E-7 | 0.767 | 0.001 | −6.2E-6–4.5E-6 | |
| MODEL B | sex * EPDS 1 + SCL1 | −0.025 | 6.0E-6 | 0.259 | 0.012 | −4.5E-6–1.7E-6 |
| sex * EPDS 2 + SCL 2 | 0.007 | 1.2E-5 | 0.035* | 0.040 | 8.7E-6–2.3E-6 | |
| sex * EPDS 3 + SCL 3 | −0.018 | 7.6E-6 | 0.165 | 0.018 | −3.2E-6–1.8E-5 | |
| RIGHT AMYGDALA / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | 0.037 | 3.3E-6 | 0.225 | 0.013 | −2.1E-6–8.6E-6 |
| EPDS 2 + SCL 2 | 0.040 | 3.9E-6 | 0.180 | 0.016 | −1.8E-6–9.6E-6 | |
| EPDS 3 + SCL 3 | 0.032 | 2.0E-6 | 0.480 | 0.005 | −3.5E-6–7.4E-6 | |
| MODEL B | sex * EPDS 1 + SCL1 | 0.040 | 6.3E-6 | 0.249 | 0.012 | −4.4E-6–1.7E-5 |
| sex * EPDS 2 + SCL 2 | 0.066 | 1.2E-5 | 0.048* | 0.035 | 9.0E-8–2.2E-5 | |
| sex * EPDS 3 + SCL 3 | 0.041 | 8.4E-6 | 0.129 | 0.021 | −2.5E-6–1.9E-5 | |
| LEFT HIPPOCAMPUS / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | <0.001 | −1.9E-6 | 0.813 | 0.001 | −1.8E-5–1.4E-5 |
| EPDS 2 + SCL 2 | 0.003 | 4.9E-6 | 0.580 | 0.003 | −1.2E-5–2.2E-5 | |
| EPDS 3 + SCL 3 | −0.007 | 1.1E-5 | 0.172 | 0.017 | −5.1E-8–2.8E-5 | |
| MODEL B | sex * EPDS 1 + SCL1 | 0.002 | −1.8E-5 | 0.271 | 0.011 | −5.0E-5–1.4E-5 |
| sex * EPDS 2 + SCL 2 | 0.009 | −2.2E-5 | 0.193 | 0.015 | −5.6E-5–1.2E-5 | |
| sex * EPDS 3 + SCL 3 | 0.029 | −2.6E-5 | 0.113 | 0.023 | −5.9E-5–6.4E-6 | |
| RIGHT HIPPOCAMPUS / ICV | ||||||
| MODEL A | EPDS 1 + SCL 1 | −0.009 | 5.1E-6 | 0.493 | 0.004 | −9.6E-6–2.0E-5 |
| EPDS 2 + SCL 2 | −0.004 | 7.7E-6 | 0.966 | 0.008 | −8.1E-6–2.3E-5 | |
| EPDS 3 + SCL 3 | −0.030 | 6.3E-6 | 0.415 | 0.006 | −8.9E-6–2.1E-5 | |
| MODEL B | sex * EPDS 1 + SCL1 | −0.006 | −1.7E-5 | 0.258 | 0.012 | −4.6E-5–1.3E-5 |
| sex * EPDS 2 + SCL 2 | −0.009 | −1.1E-5 | 0.495 | 0.004 | −4.2E-5–2.0E-5 | |
| sex * EPDS 3 + SCL 3 | −0.015 | −7.8E-5 | 0.612 | 0.002 | −3.8E-5–2.3E-5 | |
Models A and B were tested separately in each pregnancy time point. *Significant uncorrected (p < 0.05); Step 2) adjusted for infant sex, infant age at scan counted from due date, maternal education, maternal prenatal CNS affecting medication and substance use; ICV = intracranial volume; SCL, Symptom Checklist −90; EPDS, Edinburgh Postnatal Depression Scale.
P-values smaller than 0.05 were considered significant. Given the exploratory nature of the study, uncorrected values are reported.
3. Results
3.1. Description of the sample: distress scores, brain volumetric measures and control variables
Distress scores were highly intercorrelated (SCL1 + EPDS1 r = 0.832, p < 0.001; SCL2 + EPDS2 r = 0.700, p < 0.001; SCL3 + EPDS3 r = 0.664, p < 0.001). Cross-sectional SCL + EPDS or EPDS sum scores did not significantly differ between time points or newborn sexes (Table 2), however, SCL-90 scores at GW 24 were higher compared to GW 14 and 34. Newborn absolute left (r = 0.540, p < 0.001) and right (r = 0.548, p < 0.001) amygdalar volumes were positively correlated with ICV as were absolute left (r = 0.551, p < 0.001) and right (r = 0.595, p < 0.001) hippocampal volumes. Both absolute right amygdalar and hippocampal volumes correlated positively with newborn age at scan (ramygdala = 0.223, p < 0.012; rhippocampus = 0.303, p = 0.001) and newborn total age (ramygdala = 0.234, p < 0.009; rhippocampus = 0.295, p = 0.001), but significant correlations were not found for the left counterparts. Due to the correlations of volumetric measures with ICV and newborn age, brain volumes were adjusted to ICV and newborn age was added as a control variable. The absolute volumetric measures of the ICV, amygdalae and hippocampi were greater in males than females. In the relative volumes (to ICV), only that of the right amygdala was greater in males. (Table 3)
3.2. Maternal distress sum score and newborn sex as predictors of newborn amygdalar and hippocampal volumes
In the first part of the analysis (main effect model), no significant relationships were detected between SCL + EPDS sum scores of any time point and relative amygdalar or hippocampal volumes (Table 4A, Table 4B).
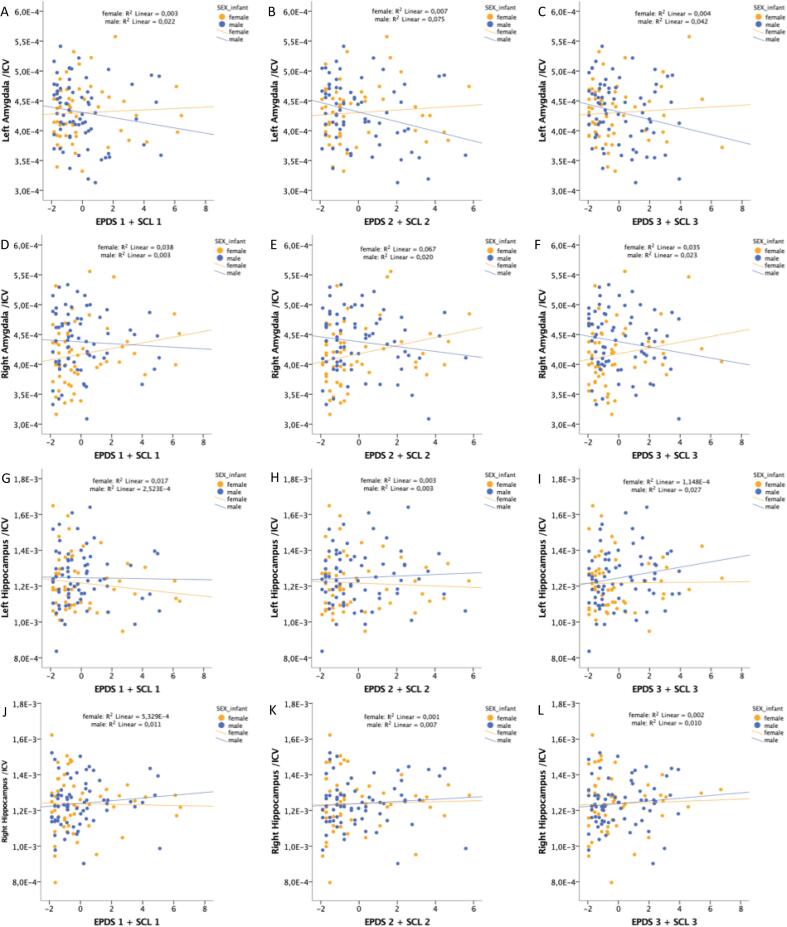
In the second part of the analysis (sex-interaction model), significant relationships were found between relative volumes of both the left and right amygdala and the interaction term of sex by SCL2 + EPDS2 (Table 4A, Fig. 2) showing a more negative association with amygdalar volumes in males than in females. This association persisted after adding control variables (Table 4B). In the sensitivity analysis for birth complications and gestational diabetes, the findings for the relative amygdalar volumes were no longer significant (left p = 0.057, B = 8.43E-6; right p = 0.099, B = 9.28E-6). Of note, the amygdala results of part one and two as well as of the sensitivity analyses, i.e. all the analyses, were similar when replacing newborn age at scan (counted from due date) with total age at scan (pregnancy + days after birth). No significant associations were found between SCL + EPDS sum scores and hippocampal volumes in the sex-interaction model (Table 4A, Table 4B, Fig. 2). As controlling for ICV can have complex effects on results (Mills et al., 2016), the models were also performed with uncorrected amygdalar and hippocampal volumes, which yielded similar results (Supplement, Tables 5A&B).
Fig. 2.
Scatter plots of the uncontrolled relationships between newborn brain volumetric measures and maternal prenatal distress scores. A) Relative left amygdalar volume and SCL1 + EPDS1. B) Relative left amygdalar volume and SCL2 + EPDS2. C) Relative left amygdalar volume and SCL3 + EPDS3. D) Relative right amygdalar volume and SCL1 + EPDS1. E) Relative right amygdalar volume and SCL2 + EPDS2. F) Relative right amygdalar volume and SCL3 + EPDS3. G) Relative left hippocampal volume and SCL1 + EPDS1. H) Relative left hippocampal volume and SCL2 + EPDS2. I) Relative left hippocampal volume and SCL2 + EPDS2. J) Relative right hippocampal volume and SCL1 + EPDS1. K) Relative right hippocampal volume and SCL2 + EPDS2. L) Relative right hippocampal volume and SCL3 + EPDS3.
To exclude the confounding effect of the one preterm born individual, birth complications and maternal CNS affecting medication, the main and interaction analyses were also performed without these 25 individuals (N = 15 males) and in the main effect model, there now was a significant relationship between SCL2 + EPDS2 and right amygdalar volume (B = 6.6E-6, p = 0.049). However, the result did not persist after controlling for other variables (Supplement, Tables 6A&B). In the sex-interaction model, left amygdalar volume was more negatively associated in males than in females with SCL1 + EPDS1 (B = 1.8E-5, p = 0.01) and SCL2 + EPDS2 (B = 1.9E-5, p = 0.006) and this finding remained significant after adding the control variables. The association with SCL3 + EPDS3 was not significant (B = 1.2E-5, p = 0.05) (Supplement, Tables 6A&B).
As a post hoc test for the whole sample, the main effect model analyses were performed separately in both males and females to test the significant sex interactions in amygdalar volumes and yielded a similar result: SCL2 + EPDS2 associated negatively with left amygdalar volume in males (p = 0.026, B = -1.08E-5), and the association persisted with all the control variables (Supplement, Table 7). In females, no significant results were observed, but a trend between SCL2 + EPDS2 and larger right amygdala volume was detected (p = 0.052, B = 7.84E-6) (Supplement, Table 7).
4. Discussion
This study explored the associations of maternal PPD with newborn amygdalar and hippocampal volumes as well as the moderating effect of newborn sex. We used a combined score of depressive and anxiety symptoms to obtain a more comprehensive measurement tool for PPD. While there were no main effects, we found newborn sex to significantly moderate the relationship between mid-pregnancy PPD and newborn amygdalar volumes; the relationship being more negative in males than females. Moreover, in post hoc analyses conducted separately for the two sexes, a main effect of mid-pregnancy PPD associating negatively with left amygdalar volume was observed in males. Together, these findings suggest that newborn sex makes offspring differentially susceptible to PPD during fetal neurodevelopment. Our results support previous research showing that neurodevelopmental trajectories exhibit sex-specific vulnerability to prenatal stress (Acosta et al., 2019, Buss et al., 2012). We are the first to demonstrate a sex-moderated relationship between PPD and amygdalar volumes in newborns, providing new evidence that neurodevelopmental sex differences might occur early on. Additionally, we found the strongest associations between PPD and amygdalar volumes regarding mid-pregnancy PPD, which may be related to sensitive periods of brain development when the cytoarchitectonic foundations are forming (Arnold and Trojanowski, 1996, Nikolić and Kostović, 1986, Setzer and Ulfig, 1999, Ulfig et al., 2003, Ulfig et al., 1999).
We observed that more intense PPD is associated more negatively with amygdalar volumes of newborn males than females and this result was further confirmed in the post-hoc analyses done separately for both sexes. However, after controlling for gestational diabetes and birth complications, the observed sex-moderated relationship between PPD and the left amygdala no longer persisted. This may be due to the relatively high number of covariates in relation to the sample size. Another possibility is that birth complications and gestational diabetes in the context of maternal PPD may have currently unknown effects on brain development. This, however, would need further investigations in larger samples. In children, evidence that PPD differentially impacts male and female amygdalar volumes (Acosta et al., 2019, Wen et al., 2017) and behavioral outcomes (Sandman et al., 2013) has been reported. For instance, greater pregnancy related anxiety was associated with smaller left amygdalar volumes in 4-year-old males, and with more emotional symptoms, peer relationship problems, and overall child difficulties; whereas larger female left amygdalar volumes were associated with fewer of the described difficulties (Acosta et al., 2019). Placental functions are sexually dimorphic (e.g., distribution of placental glucocorticoid receptor subtypes; placental epigenetic mechanisms regulating gene expression as response to prenatal stress (Bock et al., 2015, St-Pierre et al., 2016)) and might be partially responsible for the observed sexually dimorphic association between PPD and amygdalar volumes. However, the literature on PPD having a stronger effect on the male amygdala is very limited. Further, the reasons for the observed amygdalar sex differences remain unclear but neuroendocrinological and genetic factors seem to be potential underlying mechanisms (Monk et al., 2019, Sandman et al., 2013).
It has been proposed that the sex difference in amygdalar volume after exposure to PPD might be the result of sexually diverse adaptation to higher cortisol concentrations, which in females leads to elevated stress reactivity and postnatal amygdalar enlargement, whereas in males the effects of higher cortisol without emotional arousal could present as smaller amygdalar volumes (Acosta et al., 2019). Thus, observing larger female amygdalar volumes might occur only later in development. In line with this, larger female right amygdalae have been related to exposure to prenatal maternal depressive symptoms in 4.5-year-olds (Wen et al., 2017) and higher maternal prenatal cortisol concentrations in 7-year-olds (Buss et al., 2012). This finding is interesting because amygdalar size partially mediated the effect of cortisol levels on the occurrence of affective problems in females (Buss et al., 2012). To the best of our knowledge though, the negative association between PPD and newborn male amygdalar volume has never been reported before. Moreover, that our main effect was insignificant points to the importance of considering sex; the opposing male and female trends may cancel each other out statistically in combined populations. Unfortunately, the reasons for the sex-dependent differences in amygdalar volumes related to PPD remain still unresolved in the literature. Rat studies on gene expression of various members of the corticotrophin-releasing hormone (CRH) family are an example of how offspring sex might moderate prenatal stress related genetic expression. These studies show heightened anxiety of prenatally stressed rats in a stressful environment, which is characterized by a higher expression of CRH mRNA and a lower expression of its receptor’s (CRHR2) mRNA in the female amygdala, and by a decrease in both CRH-binding protein (CRH-BP) and CRHR2 mRNA in the male amygdala. (Iwasaki-Sekino et al., 2009, Zohar and Weinstock, 2011). These findings are interesting, as reduced expression of CRH-BP in the amygdala has been observed in male, but not female, humans suffering from major depression, schizophrenia and bipolar disorder (Herringa et al., 2006).
The findings on PPD’s impact on hippocampal development are more modest compared to those regarding the amygdala. Our GLM analyses regarding hippocampal volumes did not find any significant main effects of PPD or moderating effect by newborn sex. However, a recent study observed smaller fetal hippocampal volumes in both sexes after exposure to maternal anxiety between GW 24 to 40 (Wu et al., 2020). Increased prenatal anxiety has also been connected to slower bilateral hippocampal growth during the first 6 months of life. However, in that study, after controlling for postnatal maternal anxiety, only the result for the right hippocampus persisted. (Qiu et al., 2013) Furthermore, a meta-analytic study observed that childhood maltreatment-related PTSD is associated with bilateral hippocampal volume reduction in adults but not in children (Woon and Hedges, 2008). This could imply that the altering effects of early stress on the hippocampus might not present while active growth is ongoing (Woon and Hedges, 2008). Conflictingly, in a human study, maternal depression was positively related to right hippocampal volumes in both newborn sexes at high genetic risk for major depressive disorder (MDD), in an Asian study population (Qiu et al., 2017). However, as with the amygdala, ethnicity-based differences were observed, with no association found between maternal depressive symptoms and newborn hippocampal volumes, in an equally high-risk American population (Qiu et al., 2017). Interestingly, early life stress has shown to induce microglial changes and to impair synaptic maturation, normal axonal growth and myelination in rat pups (Delpech et al., 2016, Wei et al., 2015), which might also explain the observed volume reductions in human studies. Taken together, the findings on the associations of prenatal stress and hippocampal volumes are somewhat contradictory. Nevertheless, they suggest that hippocampal size may be affected by early stress, but these changes are sensitive to postnatal growth, genetic influence and experiences. Regarding the moderating effect of newborn sex, only subtle trends for smaller male hippocampi have been observed in children with higher cortisol concentrations (Buss et al., 2012), although also negative findings exist (Qiu et al., 2013). Overall, studies on newborn hippocampal volumes are few, the results inconclusive, and further longitudinal studies are needed.
Amygdala and hippocampus together with many cortical regions are a part of a complex neuronal network dedicated to memory, spatial orientation and integration of emotional states with cognition and behavior (Catani et al., 2013). As a sign of accelerated neurodevelopment after exposure to PPD, previous studies have found increased functional and decreased white matter structural connectivity (Posner et al., 2016, Qiu et al., 2015a) and cortical thinning (Buss et al., 2010, Lebel et al., 2016, Marečková et al., 2019, Sandman et al., 2015) in regions of this network connected to the amygdala and hippocampus. Subjects exposed to PPD have also shown more impulsive or externalizing behavior and lower intelligence abilities (Davis et al., 2007, Sandman et al., 2015, Van den Bergh et al., 2005). Together, these findings suggest PPD might alter the limbic system so that susceptibility to psychopathology increases (Buss et al., 2011, Davis et al., 2007, O'Donnell et al., 2014, Sandman et al., 2015, Van den Bergh et al., 2005). Moreover, similar brain findings have been observed in many psychiatric disorders, such as MDD, autism and schizophrenia, where dysfunctions of the limbic system are implicated (Catani et al., 2013).
Our results suggest mid-pregnancy to be an important time at which PPD impacts amygdalar neurodevelopment. This aligns with previous research (Acosta et al., 2019, Qiu et al., 2017, Qiu et al., 2015a, Wen et al., 2017) and with the broader neurodevelopment literature, which highlights mid-pregnancy as a critical time of brain plasticity, consisting of cell growth, migration, and synaptogenesis in the amygdala (Arnold and Trojanowski, 1996, Kier et al., 1997, Nikolić and Kostović, 1986, Setzer and Ulfig, 1999, Ulfig et al., 2003, Ulfig et al., 1999). Regarding some of the mentioned studies, however, there might be an observational bias in the time of exposure to PPD, as distress was only measured in one timepoint (GW 25 or 26) (Qiu et al., 2017, Qiu et al., 2015a, Wen et al., 2017). Moreover, Acosta et al. observed that pregnancy related anxiety in the third trimester associated positively with larger amygdala volume in their whole sample, a finding driven by the female subsample (Acosta et al., 2019). In our sensitivity analysis, after excluding some of the individuals (see above), we also observed a positive relationship between PPD and the right amygdala in the whole sample, although in the second trimester. Additionally, in the same sensitivity analysis, PPD was associated more negatively with left amygdala volume in males than in females in early pregnancy. Thus, although the majority of the literature (Acosta et al., 2019, Buss et al., 2010, Lebel et al., 2016, Qiu et al., 2017, Qiu et al., 2013, Rifkin-Graboi et al., 2013, Sandman et al., 2015, Wen et al., 2017), including our study, has presented mid-pregnancy distress having the strongest associations with newborn/child neuroanatomical measures, neuromodulating exposure to distress during other trimesters cannot be excluded without directly examining differences between time points of exposure. In addition, the differential associations of the pregnancy time points with PPD might be related to the diversity in the temporal courses of depressive and anxiety symptoms during pregnancy. Korja et al. identified, in their sample of 3202 pregnant women, several trajectories for both depressive and anxiety symptoms, for instance, constantly low or high, low and increasing and high and decreasing trajectories (Korja et al., 2018). The exclusion of individuals may thus change the sample so that some symptom trajectory groups may be emphasized over others in different time points of pregnancy. Moreover, the reasons for the observed structural changes of the amygdala remain unclear. The deep subcortical structures have a greater growth trajectory in the second trimester compared to the third, and as mentioned, the left hemisphere demonstrates slightly larger volume earlier in pregnancy than the right (Andescavage et al., 2016). This time of greater growth could make the brain, especially the left side, more vulnerable to the effects of PPD (Qiu et al., 2015a). This aligns with our finding of hemispheric difference in the effect on amygdalar volume.
The mediation of maternal PPD to the fetus likely happens through several mechanisms (Rakers et al., 2017). As previously noted, maternal cortisol might induce differing sex-dependent adaptation mechanisms that can affect brain development (Acosta et al., 2019). Some evidence of cortisol’s effect in early pregnancy exists (Buss et al., 2012). However, previous reports have either found only weak associations (Mustonen et al., 2019) or failed to demonstrate any association between maternal PPD and cortisol measures (Davis et al., 2017, Deuschle et al., 2018, Petraglia et al., 2001). Researchers have speculated that the severity or type of PPD, the timepoint in gestation, HPA-axis activation or a combination of these factors might regulate whether PPD influences fetal cortisol exposure (Deuschle et al., 2018, Mustonen et al., 2019). Genetic influence also deserves consideration. One study that explored genome-wide SNP’s (single nucleotide polymorphisms) interactions with prenatal environments indicated that gene-environment interdependence best explains variation in amygdalar and hippocampal measures: right amygdalar volume in interaction with prenatal maternal depression, and hippocampal white matter asymmetry with prenatal maternal anxiety and household income (Ong et al., 2019). In an Asian population, maternal depressive symptoms were connected with larger right amygdalar volumes among newborns having a high genetic risk profile for depression, whereas, in contrast, the same positive connection was observed in a low risk group in an American population. The researchers suggested that differences in active allele types and frequencies may cause risk genes to induce opposing responses to environmental factors. (Qiu et al., 2017) Another potential mechanism is epigenetic variation (Moisiadis and Matthews, 2014b). Prenatal depression has been shown to influence offspring DNA methylation of glucocorticoid and mineralocorticoid receptor genes and also the methylation of other essential promoters in neurodevelopment, and some of these effects were detected only in males (Braithwaite et al., 2015). These findings offer a plausible link between PPD and sex differences in brain development and susceptibility to psychopathology. In addition, the individual function of the epigenome (including DNA methylation) reflects the interaction between child genotype and early environment, and thus, may result in region-specific structural changes in the brain (Chen et al., 2015). Overall, genetic moderation influences stress responses in perceiving stress (Wüst et al., 2000) and in HPA-activity (Ouellet-Morin et al., 2008), as well as susceptibility to psychiatric disorders (Rice et al., 2010). We could not discern the possible confounding effect of shared genetic susceptibility in our study population and therefore suggest this to be addressed in future studies.
Overall, our finding on the sex-by-maternal PPD interaction and altered newborn volumetric brain measures implies the existence of a sex-dependent susceptibility of the brain to maternal anxiety and depressive symptoms. Consistent with this, Lebel at al. found a sex-by-depressive symptom interaction in the middle temporal cortex: female children exposed to maternal PPD had a thinner cortex than males, while a more negative correlation between postpartum EPDS scores and diffusivity was observed in males (Lebel et al., 2016). This finding further highlights sexual dimorphism in brain structures, beyond the dimorphism seen in normative brain development (Bakker, 2018, Blakemore et al., 2010, Lehtola et al., 2019, Lenroot et al., 2007). Evidently, large individual variation exists in responses to stress based upon genetic background (Rice et al., 2010), as well as intrinsic and extrinsic environmental background (McEwen, 2010), which is further modified by hormonal influences, including changes in HPA-axis activity and sex hormone levels. In addition to these factors, sex-specific neurodevelopment may change the individual response to adversity and increase the vulnerability to psychiatric disorders. (McEwen, 2010, Swaab et al., 2005) This theory might partly explain the pronounced sex biases in the incidences of autism, ADHD and affective problems (Bale and Epperson, 2015, Davis and Pfaff, 2014). Because of the complex relationship between PPD and brain development, future research should aim to collect multimodal longitudinal MRI data from subjects including subjective stress questionnaire data and objective biological markers of stress, while also working to understand how different genotypes and epigenetic changes affect brain structure and function, and later psychopathology. This information could enable prevention of deviant developmental paths, such as by implementing supportive measures to families with mothers suffering from anxiety and/or depressive symptoms.
4.1. Strengths and limitations
This study has several strengths. The young age of the newborn participants minimized the possible effects of postnatal events on brain development. This has been a noted limitation in several previous studies. In addition, it was important to include both amygdalar and hippocampal volumes, as these structures are closely related. The sample size is relatively large although it did not enable reliable trajectory models of the maternal distress symptoms over the pregnancy (Korja et al., 2017). Moreover, very few newborn studies have yet reported these volumes. Finally, simultaneously measuring maternal anxiety and depressive symptoms, and then creating one parameter from them may better simulate the overall distress a pregnant mother experiences, as these symptoms very often coincide (Andersson et al., 2006, Kendler et al., 2007, Mathew et al., 2011, Middeldorp et al., 1999). The study was also subject to certain limitations. First, measurements of prenatal distress were based on self-report questionnaires. However, the questionnaires we used are validated and widely accepted. Second, and although we had several time points for distress measurements, the newborn brains were scanned only once, thus impeding the generation of causality between PPD and postnatal newborn brain growth. Further, with this design, common shared genetic factors possibly rendering mother and offspring both more susceptible to volumetric brain changes or psychiatric symptomatology could not be taken into account. Evidence shows, after all, that vulnerability to later psychopathology might be restricted to certain populations with certain risk genes (Chen et al., 2015, Qiu et al., 2017). However, according to twin studies, genetics do not account for more than e.g. 38% of the incidence in depression, which highlights the importance of continuing to explore the role of different exposures, also very early ones (Kendler et al., 2006).
5. Conclusions
Overall, this exploratory study offers insight into how sex may play an important role in the susceptibility of limbic brain structures exposed to early stress. However, considering the complex relationship between the early environment and brain development, further research should focus on exploring how genotype modifies this relationship, in longitudinal study protocols and with multimodal techniques.
CRediT authorship contribution statement
Satu J. Lehtola: Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. Jetro J. Tuulari: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - review & editing. Noora M. Scheinin: Investigation, Writing - review & editing, Supervision, Funding acquisition. Linnea Karlsson: Conceptualization, Resources, Project administration, Funding acquisition. Riitta Parkkola: Investigation, Resources, Writing - review & editing. Harri Merisaari: Methodology, Data curation. John D. Lewis: Methodology, Validation, Formal analysis, Writing - original draft. Vladimir S. Fonov: Methodology, Writing - original draft. D. Louis Collins: Resources. Alan Evans: Resources. Jani Saunavaara: Methodology, Writing - review & editing. Niloofar Hashempour: Methodology, Visualization. Tuire Lähdesmäki: Methodology, Investigation, Writing - review & editing. Henriette Acosta: Writing - review & editing, Supervision. Hasse Karlsson: Conceptualization, Resources, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank our radiographer Krisse Kuvaja for performing the imaging, the FinnBrain staff and the participating families.
Funding
This work was supported by the Academy of Finland (264363, 253270, 134950, 308176); the Azrieli Neurodevelopmental Research Program in partnership with the Brain Canada Multi-Investigator Research Initiative (ANRP-MIRI13-3388). This work was further supported by individual grants to authors through Maire Taponen Foundation; Juho Vainio Foundation; Finnish State Grants for Clinical Research (ERVA); Emil Aaltonen Foundation; Alfred Kordellin Foundation; Sigrid Jusélius Foundation; Signe and Ane Gyllenberg Foundation; NARSAD Brain and Behavior Research Foundation YI Grant 1956; Foundation for Paediatric Research; Jane and Aatos Erkko Foundation; the Canadian Institutes of Health Research; the Natural Sciences and Engineering Research Council of Canada. The research also benefited from computational resources provided by Compute Canada (www.computecanada.ca) and Calcul Quebec (www.calculquebec.ca).
Footnotes
prenatal psychosocial distress
gestational weeks
Symptom Checklist-90
Edinburgh Postnatal Depression Scale
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102380.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Acosta, H., Tuulari, J.J., Scheinin, N.M., Hashempour, N., Rajasilta, O., Lavonius, T.I., Pelto, J., Saunavaara, V., Parkkola, R., Lähdesmäki, T., Karlsson, L., Karlsson, H., 2019. Maternal Pregnancy-Related Anxiety Is Associated With Sexually Dimorphic Alterations in Amygdala Volume in 4-Year-Old Children. Front. Behav. Neurosci. 13, 175. https://doi.org/10.3389/fnbeh.2019.00175. [DOI] [PMC free article] [PubMed]
- Adamson B., Letourneau N., Lebel C. Prenatal maternal anxiety and children's brain structure and function: a systematic review of neuroimaging studies. J. Affect. Disord. 2018;241:117–126. doi: 10.1016/j.jad.2018.08.029. [DOI] [PubMed] [Google Scholar]
- Adib-Rad H., Basirat Z., Faramarzi M., Mostafazadeh A., Bijani A. Psychological distress in women with recurrent spontaneous abortion: a case-control study. tjod. 2019;16(3):151–157. doi: 10.4274/tjod.galenos.2019.88899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel, Validation of the Edinburgh postpartum depression scale in a population of adult pregnant women in Mexico. J. Clin. Med. Res. 2014;6:374–378. doi: 10.14740/jocmr1883w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amugongo S.K., Hlusko L.J. Impact of maternal prenatal stress on growth of the offspring. Aging Dis. 2013;5:1–16. doi: 10.14336/AD.2014.05001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L., Sundström-Poromaa I., Wulff M., Aström M., Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: a population-based study. Am. J. Epidemiol. 2004;159:872–881. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- Andersson Liselott, Sundström-Poromaa Inger, Wulff Marianne, Åström Monica, Bixo Marie. Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstet. Gynecol. Scand. 2006;85(8):937–944. doi: 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- Andescavage N.N., du Plessis A., McCarter R., Serag A., Evangelou I., Vezina G., Robertson R., Limperopoulos C. Complex trajectories of brain development in the healthy human fetus. Cereb. Cortex. 2016;27:5274–5283. doi: 10.1093/cercor/bhw306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.E., Trojanowski J.Q. Human fetal hippocampal development: I. Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J. Comp. Neurol. 1996;367:274–292. doi: 10.1002/(SICI)1096-9861(19960401)367:2<274::AID-CNE9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bakker J. Springer; Berlin, Heidelberg: 2018. The Sexual Differentiation of the Human Brain: Role of Sex Hormones Versus Sex Chromosomes; pp. 45–67. [DOI] [PubMed] [Google Scholar]
- Bale Tracy L, Epperson C Neill. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18(10):1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech P., Bille J., Møller S.B., Hellström L.C., Østergaard S.D. Psychometric validation of the Hopkins Symptom Checklist (SCL-90) subscales for depression, anxiety, and interpersonal sensitivity. J. Affect. Disord. 2014;160:98–103. doi: 10.1016/j.jad.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Bergink Veerle, Kooistra Libbe, Lambregtse-van den Berg Mijke P., Wijnen Henny, Bunevicius Robertas, van Baar Anneloes, Pop Victor. Validation of the Edinburgh depression scale during pregnancy. J. Psychosom. Res. 2011;70(4):385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock Joerg, Wainstock Tamar, Braun Katharina, Segal Menahem. Stress in utero: prenatal programming of brain plasticity and cognition. Biol. Psychiatry. 2015;78(5):315–326. doi: 10.1016/j.biopsych.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Braithwaite E.C., Kundakovic M., Ramchandani P.G., Murphy S.E., Champagne F.A. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics. 2015;10(5):408–417. doi: 10.1080/15592294.2015.1039221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitelaar J., Huizink A., Mulder E., de Medina P., Visser G. Prenatal stress and cognitive development and temperament in infants. Neurobiol. Aging. 2003;24(Suppl 1):S53–S60. doi: 10.1016/s0197-4580(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Bunevicius Adomas, Kusminskas Laima, Pop Victor J., Pedersen Cort A., Bunevicius Robertas. Screening for antenatal depression with the Edinburgh Depression Scale. J. Psychosomatic Obstetrics Gynecol. 2009;30(4):238–243. doi: 10.3109/01674820903230708. [DOI] [PubMed] [Google Scholar]
- Buss Claudia, Davis Elysia Poggi, Muftuler L. Tugan, Head Kevin, Sandman Curt A. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35(1):141–153. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Hobel C.J., Sandman C.A. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14(6):665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. 2012;109(20):E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani Marco, Dell’Acqua Flavio, Thiebaut de Schotten Michel. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013;37(8):1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Charil Arnaud, Laplante David P., Vaillancourt Cathy, King Suzanne. Prenatal stress and brain development. Brain Res. Rev. 2010;65(1):56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Chen Li, Pan Hong, Tuan Ta Anh, Teh Ai Ling, MacIsaac Julia L., Mah Sarah M., McEwen Lisa M., Li Yue, Chen Helen, Broekman Birit F.P., Buschdorf Jan Paul, Chong Yap Seng, Kwek Kenneth, Saw Seang Mei, Gluckman Peter D., Fortier Marielle V., Rifkin-Graboi Anne, Kobor Michael S., Qiu Anqi, Meaney Michael J., Holbrook Joanna D. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism influences the association of the methylome with maternal anxiety and neonatal brain volumes. Dev. Psychopathol. 2015;27(1):137–150. doi: 10.1017/S0954579414001357. [DOI] [PubMed] [Google Scholar]
- Coupé Pierrick, Manjón José V., Fonov Vladimir, Pruessner Jens, Robles Montserrat, Collins D. Louis. Patch-based segmentation using expert priors: application to hippocampus and ventricle segmentation. NeuroImage. 2011;54(2):940–954. doi: 10.1016/j.neuroimage.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression: development of the 10-item edinburgh postnatal depression scale. Br. J. Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davis Elysia Poggi, Glynn Laura M., Schetter Christine Dunkel, Hobel Calvin, Chicz-Demet Aleksandra, Sandman Curt A. Prenatal exposure to maternal depression and cortisol influences infant temperament. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis Elysia Poggi, Sandman Curt A., Buss Claudia, Wing Deborah A., Head Kevin. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol. Psychiatry. 2013;74(9):647–655. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Elysia Poggi, Head Kevin, Buss Claudia, Sandman Curt A. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology. 2017;75:56–63. doi: 10.1016/j.psyneuen.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Elysia Poggi, Pfaff Donald. Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25. doi: 10.1016/j.psyneuen.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech Jean-Christophe, Wei Lan, Hao Jin, Yu Xiaoqing, Madore Charlotte, Butovsky Oleg, Kaffman Arie. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun. 2016;57:79–93. doi: 10.1016/j.bbi.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deogratis L.R., Lipman R.S., Covi L. SCL-90: an outpatient psychiatric rating scale-preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- Deuschle Michael, Hendlmeier Ferdinand, Witt Stephanie, Rietschel Marcella, Gilles Maria, Sánchez-Guijo Alberto, Fañanas Lourdes, Hentze Sabine, Wudy Stefan A., Hellweg Rainer. Cortisol, cortisone, and BDNF in amniotic fluid in the second trimester of pregnancy: effect of early life and current maternal stress and socioeconomic status. Dev. Psychopathol. 2018;30(3):971–980. doi: 10.1017/S0954579418000147. [DOI] [PubMed] [Google Scholar]
- El Marroun H., Tiemeier H., Muetzel R.L., Thijssen S., van der Knaap N.J.F., Jaddoe V.W.V., Fernández G., Verhulst F.C., White T.J.H. Prenatal exposure to maternal and paternal depressive symptoms and brain morphology: a population-based prospective neuroimaging study in young children. Depress. Anxiety. 2016;33:658–666. doi: 10.1002/da.22524. [DOI] [PubMed] [Google Scholar]
- Evans Lynn M., Myers Michael M., Monk Catherine. Pregnant women’s cortisol is elevated with anxiety and depression — but only when comorbid. Arch Womens Ment Health. 2008;11(3):239–248. doi: 10.1007/s00737-008-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov Vladimir, Evans Alan C., Botteron Kelly, Almli C. Robert, McKinstry Robert C., Collins D. Louis. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl Thomas, Meisenzahl Eva Maria, Zetzsche Thomas, Born Christine, Jäger Markus, Groll Constanze, Bottlender Ronald, Leinsinger Gerda, Möller Hans-Jürgen. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol. Psychiatry. 2003;53(4):338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Gibson, J., McKenzie-Mcharg, K., Shakespeare, J., Price, J., Gray, R., 2009. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr. Scand. https://doi.org/10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed]
- Glover Vivette. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Practice Res. Clinical Obstetrics Gynaecol. 2014;28(1):25–35. doi: 10.1016/j.bpobgyn.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Graham Alice M., Rasmussen Jerod M., Rudolph Marc D., Heim Christine M., Gilmore John H., Styner Martin, Potkin Steven G., Entringer Sonja, Wadhwa Pathik D., Fair Damien A., Buss Claudia. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry. 2018;83(2):109–119. doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashempour N., Tuulari J.J., Merisaari H., Lidauer K., Luukkonen I., Saunavaara J., Parkkola R., Lähdesmäki T., Lehtola S.J., Keskinen M., Lewis J.D., Scheinin N.M., Karlsson L., Karlsson H. A novel approach for manual segmentation of the amygdala and hippocampus in neonate MRI. Front. Neurosci. 2019;13:1025. doi: 10.3389/fnins.2019.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa Ryan J, Roseboom Patrick H, Kalin Ned H. Decreased amygdala CRF-binding protein mRNA in post-mortem tissue from male but not female bipolar and schizophrenic subjects. Neuropsychopharmacol. 2006;31(8):1822–1831. doi: 10.1038/sj.npp.1301038. [DOI] [PubMed] [Google Scholar]
- Huizink Anja C., Robles de Medina Pascale G., Mulder Eduard J.H., Visser Gerard H.A., Buitelaar Jan K. Stress during pregnancy is associated with developmental outcome in infancy. J. Child Psychol. & Psychiat. 2003;44(6):810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino Azusa, Mano-Otagiri Asuka, Ohata Hisayuki, Yamauchi Naoko, Shibasaki Tamotsu. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kamel, H.S., Ahmed, H.N., Eissa, M.A., Abol-Oyoun, A.-S.M., 1999. Psychological and Obstetrical Responses of Mothers Following Antenatal Fetal Sex Identification. J. Obstet. Gynaecol. Res. 25, 43–50. https://doi.org/10.1111/j.1447-0756.1999.tb01121.x. [DOI] [PubMed]
- Karlsson, L., Tolvanen, M., Scheinin, N.M., Uusitupa, H.-M., Korja, R., Ekholm, E., Tuulari, J.J., Pajulo, M., Huotilainen, M., Paunio, T., Karlsson, H., FinnBrain Birth Cohort Study Group, 2018. Cohort Profile: The FinnBrain Birth Cohort Study (FinnBrain). Int. J. Epidemiol. 47, 15-16j. https://doi.org/10.1093/ije/dyx173. [DOI] [PubMed]
- Kendler Kenneth S., Gardner Charles O., Gatz Margaret, Pedersen Nancy L. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol. Med. 2007;37(03):453. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- Kendler Kenneth S., Gatz Margaret, Gardner Charles O., Pedersen Nancy L. A Swedish national twin study of lifetime major depression. AJP. 2006;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kier E.L., Kim J.H., Fulbright R.K., Bronen R.A. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am. J. Neuroradiol. 1997 [PMC free article] [PubMed] [Google Scholar]
- Killion Bryana E., Weyandt Lisa L. Brain structure in childhood maltreatment-related PTSD across the lifespan: a systematic review. Appl. Neuropsychol.: Child. 2020;9(1):68–82. doi: 10.1080/21622965.2018.1515076. [DOI] [PubMed] [Google Scholar]
- Korja Riikka, Nolvi Saara, Grant Kerry Ann, McMahon Cathy. The relations between maternal prenatal anxiety or stress and child’s early negative reactivity or self-regulation: a systematic review. Child Psychiatry Hum. Dev. 2017;48(6):851–869. doi: 10.1007/s10578-017-0709-0. [DOI] [PubMed] [Google Scholar]
- Korja, R., Nolvi, S., Kataja, E.L., Scheinin, N., Junttila, N., Lahtinen, H., Saarni, S., Karlsson, L., Karlsson, H., 2018. The courses of maternal and paternal depressive and anxiety symptoms during the prenatal period in the finnbrain birth cohort study. PLoS One 13. https://doi.org/10.1371/journal.pone.0207856. [DOI] [PMC free article] [PubMed]
- Kouwenhoven Erik, Giezen Marina, Struikmans Henk. Measuring the similarity of target volume delineations independent of the number of observers. Phys. Med. Biol. 2009;54(9):2863–2873. doi: 10.1088/0031-9155/54/9/018. [DOI] [PubMed] [Google Scholar]
- Kumpulainen V., Lehtola S.J., Tuulari J.J., Silver E., Copeland A., Korja R., Karlsson H., Karlsson L., Merisaari H., Parkkola R., Saunavaara J., Lähdesmäki T., Scheinin N.M. Prevalence and risk factors of incidental findings in brain MRIs of healthy neonates—the finnbrain birth cohort study. Front. Neurol. 2020;10 doi: 10.3389/fneur.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel Catherine, Walton Matthew, Letourneau Nicole, Giesbrecht Gerald F., Kaplan Bonnie J., Dewey Deborah. Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol. Psychiatry. 2016;80(11):859–868. doi: 10.1016/j.biopsych.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Lehtola S.J., Tuulari J.J., Karlsson L., Parkkola R., Merisaari H., Saunavaara J., Lähdesmäki T., Scheinin N.M., Karlsson H. Associations of age and sex with brain volumes and asymmetry in 2–5-week-old infants. Brain Struct. Funct. 2019;224(1):501–513. doi: 10.1007/s00429-018-1787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis John D., Fonov Vladimir S., Collins D. Louis, Evans Alan C., Tohka Jussi. Cortical and subcortical T1 white/gray contrast, chronological age, and cognitive performance. NeuroImage. 2019;196:276–288. doi: 10.1016/j.neuroimage.2019.04.022. [DOI] [PubMed] [Google Scholar]
- Lin Yanfen, Hu Wenjing, Xu Jian, Luo Zhongcheng, Ye Xiaofang, Yan Chonghuai, Liu Zhiwei, Tong Shilu. Association between temperature and maternal stress during pregnancy. Environ. Res. 2017;158:421–430. doi: 10.1016/j.envres.2017.06.034. [DOI] [PubMed] [Google Scholar]
- Marečková, K., Klasnja, A., Bencurova, P., Andrýsková, L., Brázdil, M., Paus, T., 2019. Prenatal Stress, Mood, and Gray Matter Volume in Young Adulthood. Cereb. Cortex 29, 1244–1250. https://doi.org/10.1093/cercor/bhy030. [DOI] [PMC free article] [PubMed]
- Mathew A.R., Pettit J.W., Lewinsohn P.M., Seeley J.R., Roberts R.E. Co-morbidity between major depressive disorder and anxiety disorders: shared etiology or direct causation? Psychol. Med. 2011;41(10):2023–2034. doi: 10.1017/S0033291711000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthey S., Phillips J., White T., Glossop P., Hopper U., Panasetis P., Petridis A., Larkin M., Barnett B. Routine psychosocial assessment of women in the antenatal period: frequency of risk factors and implications for clinical services. Arch. Womens Ment. Health. 2004;7(4):223–229. doi: 10.1007/s00737-004-0064-6. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann. N. Y. Acad. Sci. 2010;1204:38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp C.M., Cath D.C., Van Dyck R., Boomsma D.I. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol. Med. 1999;35(5):611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- Miguel Patrícia M, Pereira Lenir O, Silveira Patrícia P, Meaney Michael J. Early environmental influences on the development of children's brain structure and function. Dev. Med. Child Neurol. 2019;61(10):1127–1133. doi: 10.1111/dmcn.14182. [DOI] [PubMed] [Google Scholar]
- Mills Kathryn L., Goddings Anne-Lise, Herting Megan M., Meuwese Rosa, Blakemore Sarah-Jayne, Crone Eveline A., Dahl Ronald E., Güroğlu Berna, Raznahan Armin, Sowell Elizabeth R., Tamnes Christian K. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. NeuroImage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisiadis Vasilis G., Matthews Stephen G. Glucocorticoids and fetal programming part 1: outcomes. Nat. Rev. Endocrinol. 2014;10(7):391–402. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- Moisiadis Vasilis G., Matthews Stephen G. Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol. 2014;10(7):403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- Monk Catherine, Lugo-Candelas Claudia, Trumpff Caroline. Prenatal developmental origins of future psychopathology: mechanisms and pathways. Annu. Rev. Clin. Psychol. 2019;15(1):317–344. doi: 10.1146/annurev-clinpsy-050718-095539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi Matthew W., Cody-Hazlett Heather, Poe Michele D., Gerig Guido, Gimpel-Smith Rachel, Piven Joseph. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with Autism. Arch. Gen. Psychiatry. 2009;66(5):509. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen Paula, Karlsson Linnea, Kataja Eeva-Leena, Scheinin Noora M., Kortesluoma Susanna, Coimbra Bárbara, Rodrigues Ana João, Sousa Nuno, Karlsson Hasse. Maternal prenatal hair cortisol is associated with prenatal depressive symptom trajectories. Psychoneuroendocrinology. 2019;109:104383. doi: 10.1016/j.psyneuen.2019.104383. [DOI] [PubMed] [Google Scholar]
- Nikolić Ida, Kostović Ivica. Development of the lateral amygdaloid nucleus in the human fetus: transient presence of discrete cytoarchitectonic units. Anat. Embryol. 1986;174(3):355–360. doi: 10.1007/BF00698785. [DOI] [PubMed] [Google Scholar]
- O'Donnell Kieran J., Glover Vivette, Barker Edward D., O'Connor Thomas G. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev. Psychopathol. 2014;26(2):393–403. doi: 10.1017/S0954579414000029. [DOI] [PubMed] [Google Scholar]
- Ong Mei‐Lyn, Tuan Ta A., Poh Joann, Teh Ai L., Chen Li, Pan Hong, MacIsaac Julia L., Kobor Michael S., Chong Yap S., Kwek Kenneth, Saw Seang M., Godfrey Keith M., Gluckman Peter D., Fortier Marielle V., Karnani Neerja, Meaney Michael J., Qiu Anqi, Holbrook Joanna D. Neonatal amygdalae and hippocampi are influenced by genotype and prenatal environment, and reflected in the neonatal DNA methylome. Genes, Brain Behavior. 2019;18(7) doi: 10.1111/gbb.v18.710.1111/gbb.12576. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I., Boivin M., Dionne G., Lupien S.J., Arseneault L., Arsenault L., Barr R.G., Pérusse D., Tremblay R.E. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch. Gen. Psychiatry. 2008;65:211–218. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- Petraglia F., Hatch M.C., Lapinski R., Stomati M., Reis F.M., Cobellis L., Berkowitz G.S. Lack of Effect of Psychosocial Stress on Maternal Corticotropin-Releasing Factor and Catecholamine Levels at 28 Weeks' Gestation. J. Soc. Gynecol. Investig. 2001;8(2):83–88. [PubMed] [Google Scholar]
- Posner, J., Cha, J., Roy, A.K., Peterson, B.S., Bansal, R., Gustafsson, H.C., Raffanello, E., Gingrich, J., Monk, C., 2016. Alterations in amygdala–prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry 6, e935–e935. https://doi.org/10.1038/tp.2016.146. [DOI] [PMC free article] [PubMed]
- Qiu, A., Anh, T.T., Li, Y., Chen, H., Rifkin-Graboi, A., Broekman, B.F.P., Kwek, K., Saw, S.-M., Chong, Y.-S., Gluckman, P.D., Fortier, M.V, Meaney, M.J., 2015a. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl. Psychiatry 5, e508–e508. https://doi.org/10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed]
- Qiu, A., Rifkin-Graboi, A., Chen, H., Chong, Y.-S., Kwek, K., Gluckman, P.D., Fortier, M.V, Meaney, M.J., 2013. Maternal anxiety and infants’ hippocampal development: timing matters. Transl. Psychiatry 3, e306–e306. https://doi.org/10.1038/tp.2013.79. [DOI] [PMC free article] [PubMed]
- Qiu, A., Shen, M., Buss, C., Chong, Y.-S., Kwek, K., Saw, S.-M., Gluckman, P.D., Wadhwa, P.D., Entringer, S., Styner, M., Karnani, N., Heim, C.M., O’Donnell, K.J., Holbrook, J.D., Fortier, M.V, Meaney, M.J., the GUSTO study group, the G. study, 2017. Effects of Antenatal Maternal Depressive Symptoms and Socio-Economic Status on Neonatal Brain Development are Modulated by Genetic Risk. Cereb. Cortex 27, 3080–3092. https://doi.org/10.1093/cercor/bhx065. [DOI] [PMC free article] [PubMed]
- Qiu Anqi, Tuan Ta Anh, Ong Mei Lyn, Li Yue, Chen Helen, Rifkin-Graboi Anne, Broekman Birit F.P., Kwek Kenneth, Saw Seang-Mei, Chong Yap-Seng, Gluckman Peter D., Fortier Marielle V., Holbrook Joanna Dawn, Meaney Michael J. COMT haplotypes modulate associations of antenatal maternal anxiety and neonatal cortical morphology. AJP. 2015;172(2):163–172. doi: 10.1176/appi.ajp.2014.14030313. [DOI] [PubMed] [Google Scholar]
- Rakers, F., Rupprecht, S., Dreiling, M., Bergmeier, C., Witte, O.W., Schwab, M., 2017. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2017.02.019. [DOI] [PubMed]
- Lenroot Rhoshel K., Gogtay Nitin, Greenstein Deanna K., Wells Elizabeth Molloy, Wallace Gregory L., Clasen Liv S., Blumenthal Jonathan D., Lerch Jason, Zijdenbos Alex P., Evans Alan C., Thompson Paul M., Giedd Jay N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F., Harold G.T., Boivin J., van den Bree M., Hay D.F., Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol. Med. 2010;40(2):335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi Anne, Bai Jordan, Chen Helen, Hameed Waseem Bak’r, Sim Lit Wee, Tint Mya Thway, Leutscher-Broekman Birit, Chong Yap-Seng, Gluckman Peter D., Fortier Marielle V., Meaney Michael J., Qiu Anqi. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol. Psychiatry. 2013;74(11):837–844. doi: 10.1016/j.biopsych.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Rogers Jack C., De Brito Stéphane A. Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiatry. 2016;73(1):64. doi: 10.1001/jamapsychiatry.2015.2423. [DOI] [PubMed] [Google Scholar]
- Rooks V.J., Eaton J.P., Ruess L., Petermann G.W., Keck-Wherley J., Pedersen R.C. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am. J. Neuroradiol. 2008;29(6):1082–1089. doi: 10.3174/ajnr.A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubertsson Christine, Börjesson Karin, Berglund Anna, Josefsson Ann, Sydsjö Gunilla. The Swedish validation of Edinburgh Postnatal Depression Scale (EPDS) during pregnancy. Nord. J. Psychiatry. 2011;65(6):414–418. doi: 10.3109/08039488.2011.590606. [DOI] [PubMed] [Google Scholar]
- Rubertsson C., Hellström J., Cross M., Sydsjö G. Anxiety in early pregnancy: prevalence and contributing factors. Arch. Womens Ment. Health. 2014;17(3):221–228. doi: 10.1007/s00737-013-0409-0. [DOI] [PubMed] [Google Scholar]
- Sandman Curt A., Davis Elysia P., Buss Claudia, Glynn Laura M. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95(1):8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman Curt A., Buss Claudia, Head Kevin, Davis Elysia Poggi. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol. Psychiatry. 2015;77(4):324–334. doi: 10.1016/j.biopsych.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman Curt A, Glynn Laura M, Davis Elysia Poggi. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013;75(4):327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman Curt A., Wadhwa Pathik, Glynn Laura, Chicz-Demet Aleksandra, Porto Manuel, Garite Thomas J. Corticotrophin-releasing hormone and fetal responses in human pregnancy. Annals NY Acad. Sci. 1999;897:66–75. doi: 10.1111/j.1749-6632.1999.tb07879.x. [DOI] [PubMed] [Google Scholar]
- Schumann Cynthia Mills, Barnes Cynthia Carter, Lord Catherine, Courchesne Eric. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol. Psychiatry. 2009;66(10):942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer M., Ulfig N. Differential expression of calbindin and calretinin in the human fetal amygdala. Microsc. Res. Tech. 1999;46:1–17. doi: 10.1002/(SICI)1097-0029(19990701)46:1<1::AID-JEMT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- St-Pierre, J., Laurent, L., King, S., Vaillancourt, C., 2016. Effects of prenatal maternal stress on serotonin and fetal development. Placenta 48, S66–S71. https://doi.org/10.1016/j.placenta.2015.11.013. [DOI] [PubMed]
- Stekhoven D.J., Buhlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- Swaab Dick F., Bao Ai-Min, Lucassen Paul J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Tendais I., Costa R., Conde A., Figueiredo B. Screening for depression and anxiety disorders from pregnancy to postpartum with the EPDS and STAI. Span. J. Psychol. 2014;17:E7. doi: 10.1017/sjp.2014.7. [DOI] [PubMed] [Google Scholar]
- Torres-Espínola, F.J., Berglund, S.K., García, S., Pérez-García, M., Catena, A., Rueda, R., Sáez, J.A., Campoy, C., team, for the P., 2018. Visual evoked potentials in offspring born to mothers with overweight, obesity and gestational diabetes. PLoS One 13, e0203754. https://doi.org/10.1371/journal.pone.0203754. [DOI] [PMC free article] [PubMed]
- Ulfig, N., Setzer, M., Bohl, J., 2003. Ontogeny of the human amygdala. Ann. N. Y. Acad. Sci. 985, 22–33. https://doi.org/10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed]
- Ulfig N., Setzer M., Bohl J. Distribution of GAP-43-immunoreactive structures in the human fetal amygdala. Eur. J. Histochem. 1999;43:19–28. [PubMed] [Google Scholar]
- Valera Eve M., Faraone Stephen V., Murray Kate E., Seidman Larry J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Van den Bergh Bea R.H., Mennes Maarten, Oosterlaan Jaap, Stevens Veerle, Stiers Peter, Marcoen Alfons, Lagae Lieven. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci. Biobehav. Rev. 2005;29(2):259–269. doi: 10.1016/j.neubiorev.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Van den Bergh Bea R.H., Dahnke Robert, Mennes Maarten. Prenatal stress and the developing brain: Risks for neurodevelopmental disorders. Dev Psychopathol. 2018;30(3):743–762. doi: 10.1017/S0954579418000342. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel, M.I., Donkers, F.C.L., Winkler, I., Otte, R.A., Van den Bergh, B.R.H., 2014. Maternal mindfulness and anxiety during pregnancy affect infants’ neural responses to sounds. Soc. Cogn. Affect. Neurosci. 453–460. https://doi.org/10.1093/scan/nsu075. [DOI] [PMC free article] [PubMed]
- Vázquez M. Belén, Míguez M. Carmen. Validation of the Edinburgh postnatal depression scale as a screening tool for depression in Spanish pregnant women. J. Affect. Disord. 2019;246:515–521. doi: 10.1016/j.jad.2018.12.075. [DOI] [PubMed] [Google Scholar]
- Visser M., Müller D.M.J., van Duijn R.J.M., Smits M., Verburg N., Hendriks E.J., Nabuurs R.J.A., Bot J.C.J., Eijgelaar R.S., Witte M., van Herk M.B., Barkhof F., de Witt Hamer P.C., de Munck J.C. Inter-rater agreement in glioma segmentations on longitudinal MRI. NeuroImage: Clinical. 2019;22:101727. doi: 10.1016/j.nicl.2019.101727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward Joe H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963;58(301):236–244. [Google Scholar]
- Wei Lan, Hao Jin, Lacher Richard K., Abbott Thomas, Chung Lisa, Colangelo Christopher M., Kaffman Arie. Early-life stress perturbs key cellular programs in the developing mouse hippocampus. Dev. Neurosci. 2015;37(6):476–488. doi: 10.1159/000430861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier Katrin, Fonov Vladimir, Lavoie Karyne, Doyon Julien, Collins D. Louis. Rapid automatic segmentation of the human cerebellum and its lobules (RASCAL)-Implementation and application of the patch-based label-fusion technique with a template library to segment the human cerebellum: automatic segmentation of the cerebellum. Hum. Brain Mapp. 2014;35(10):5026–5039. doi: 10.1002/hbm.22529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, D.J., Poh, J.S., Ni, S.N., Chong, Y.-S., Chen, H., Kwek, K., Shek, L.P., Gluckman, P.D., Fortier, M.V, Meaney, M.J., Qiu, A., 2017. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl. Psychiatry 7, e1103–e1103. https://doi.org/10.1038/tp.2017.74. [DOI] [PMC free article] [PubMed]
- Whitby E.H., Griffiths P.D., Rutter S., Smith M.F., Sprigg A., Ohadike P., Davies N.P., Rigby A.S., Paley M.N. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. The Lancet. 2004;363(9412):846–851. doi: 10.1016/S0140-6736(04)15730-9. [DOI] [PubMed] [Google Scholar]
- Woon Fu L., Hedges Dawson W. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18(8):729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Wu Yao, Lu Yuan-Chiao, Jacobs Marni, Pradhan Subechhya, Kapse Kushal, Zhao Li, Niforatos-Andescavage Nickie, Vezina Gilbert, du Plessis Adré J., Limperopoulos Catherine. Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Netw Open. 2020;3(1):e1919940. doi: 10.1001/jamanetworkopen.2019.19940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst Stefan, Federenko Ilona, Hellhammer Dirk H, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Zohar, I., Weinstock, M., 2011. Differential Effect of Prenatal Stress on the Expression of Cortiocotrophin-Releasing Hormone and its Receptors in the Hypothalamus and Amygdala in Male and Female Rats. J. Neuroendocrinol. 23, 320–328. https://doi.org/10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.