Abstract
Bronchopulmonary dysplasia (BPD) is a condition of neonatal chronic lung disease due to disruption or dysregulation of pulmonary development. However, the pathophysiology of BPD in the larger conducting airways is not yet fully understood. The objective of our study was to determine if the area of the central airways are altered in patients with a history of BPD. We hypothesized that compared to age- and sex-matched controls, BPD patients would have decreased area of the central conducting airways. Twenty-two BPD patients (n=10 male, n=12 female; median age=10 [range:1–49] yrs) and n=22 matched controls (n=10 male, n=12 female; median age=10 [range:1–48] yrs) who had undergone a chest computed tomography (CT) scan were retrospectively identified. Measurement and analysis was performed using software that reconstructs the airways into 3D. Measurements of airway area were conducted at three points based on anatomic bifurcations for each of the following structures: trachea, left main bronchus, left upper lobe, left lower lobe, right main bronchus, intermediate bronchus, and right upper lobe. The luminal area for each airway was calculated based on the averages of the three measures. Airway luminal area was not different between BPD patients and matched controls for any of the measured airways (p>0.05). Total lung volume detected in the CT scans was not different between BPD patients and matched controls (median [range]; 2775 [522–6215] vs 2969 [851–5612] cm3, p>0.05). Our results suggest the luminal areas of the large conducting airways in patients with BPD are not different from matched controls.
Keywords: bronchus, conducting airways, trachea
Introduction
Bronchopulmonary dysplasia (BPD) is a condition of chronic disease of the airways and lung parenchyma beginning in the neonatal period due to disruption or dysregulation of pulmonary development. BPD affects ~10,000–15,000 infants annually in the United States and ~17–75% of infants born <28 weeks globally [1, 2]. The notable risk factors for BPD are postnatal mechanical ventilation, supplemental oxygen, low birth weight, and increasingly preterm birth before 32 weeks [3, 4]. Patients with BPD have increased mortality, however existing treatments such as intratracheal surfactant and antenatal steroids have alleviated the burden for some infants.
BPD is associated with decreased alveolar septation, alveolar hypoplasia, altered proliferation and aging of alveolar epithelial cells, and abnormal disruption of pulmonary alveolar capillaries resulting in increased airway resistance [5]. Small airways pathology is extensive, as observations of infants with BPD revealed severe bronchiolitis, bronchiolar and alveolar fibrosis, and damage to the bronchial ciliary apparatus [6]. These issues do not resolve in adulthood [7]. However, the pathophysiology of the large conducting airways in BPD is not fully understood, despite their importance as the major determinant of airway resistance [8]. Previous studies have shown that infants with BPD have greater airway resistance [9]. The objective of our study was to determine if the luminal area of the central airways, as measured by computed tomography imaging (CT), are altered in patients with a history of BPD. We hypothesized that compared to age- and sex-matched controls, BPD patients would have decreased luminal area of the central conducting airways.
Methods
This retrospective study was approved by the Institutional Review Board at Mayo Clinic (IRB# 17–008537) and conformed to Declaration of Helsinki. Inclusion criteria: CT images and a history of admission to the neonatal ICU needing high fraction oxygenation. A total of n=43 subjects met the initial study criteria and a manual chart review was conducted by a physician to evaluate the history of BPD and apply exclusion criteria. BPD was defined as requiring oxygen at 28 days of life and/or 36 weeks postmenstrual age [10]. Exclusion criteria: airways could not be visualized due to poor-quality imaging, or having any severe complicating respiratory ailments (e.g. cystic fibrosis, bronchiectasis). Of the remaining 28 eligible patients, n=22 (10 males, 12 females) were analyzed because they had an available control matched for age, sex, height, and body mass index. Controls (10 males, 12 females) were obtained from previously published data [11, 12].
Measurement and analysis were performed by a single investigator using commercially available software (Aquarius, TeraRecon Inc.) that reconstructs airways in three dimensions. Luminal airway areas were measured at three points based on anatomic bifurcations for the following structures: trachea, left main bronchus, left upper lobe, left lower lobe, right main bronchus, bronchus intermedius, and right upper lobe. Airway luminal area was calculated as the average of the three measures obtained, and was height-controlled by dividing luminal area by height. The non-parametric Mann-Whitney U test was used to determine any difference between the groups. Significance was set at P<0.05 and values are presented as median±range.
Results
Controls and BPD patients did not differ by age (10 [1–49] vs. 10 [1–48] years), height (129 [66–184] vs. 139 [80–185] cm), or BMI (19.1 [14.6–39.8] vs. 16.7 [12.8–35.5] kg/m2, all p>0.05). Total lung volume (via CT scans) was not different between BPD patients and healthy controls (2775 [522–6215] vs 2969 [851–5612] cm3, p>0.05). Regardless of whether we adjusted airway size for height, airway luminal area of the large central airways was not different between BPD patients and healthy controls (Figure 1). Size of all airways in BPD patients were 112±4% of controls on average, but BPD patients did not consistently have larger airways than their respective controls. Airway size of individual BPD patients ranged from being 179% to 59% of their respective controls. To confirm that this represented normal biological variation, a healthy cohort of individuals [11,12] was analyzed to find that individuals’ airway size ranged from 36% to 166% of the cohort’s mean airway size.
Figure 1.
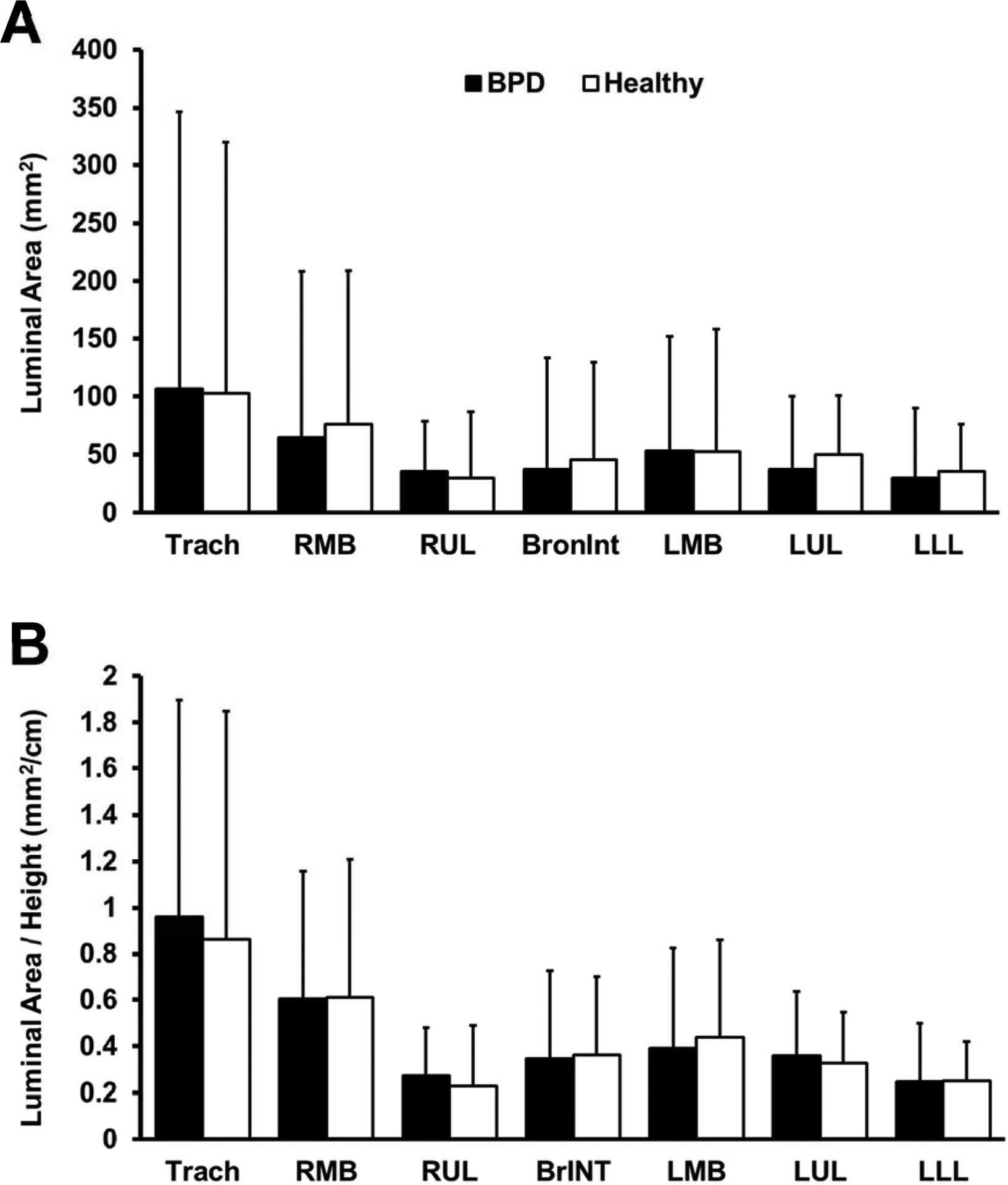
Airway luminal areas for BPD patients and their matched healthy controls, with values presented as absolute airway size (A) and airway size relative to patient height (B). Trach, trachea; RMB, right main bronchus; RUL, right upper lobe; BrINT, bronchus intermedius; LMB, left main bronchus; LUL, left upper lobe; LLL, left lower lobe.
Discussion
The major finding from our study is that the luminal area of the large airways is not different in BPD patients compared to controls. Thus, we suggest that the large airways are not part of the structural pathology associated with BPD.
BPD is characterized by disrupted pulmonary development, but the pathology is focused in the small airways, alveoli, and the surrounding vasculature and smooth muscle. Pulmonary hypertension and impairments of gas exchange result from deficiencies in alveolar and capillary development, and pulmonary resistance is further increased by small airway conditions such as bronchiolitis and fibrosis [5, 6]. Pulmonary function tests in infants with BPD demonstrate severe obstruction of the lower airways, with no recovery in lung capacity or maximal expiratory flow at 25% FVC in patients who had been mechanically ventilated for >10 months [13].
By contrast, central airways largely determine total airway resistance but do not seem to play a role in BPD pathology [8]. The limiting factors in patients with BPD are likely the impaired gas exchange. The increased airway resistance in BPD patients may be explained by reduced alveolarization and small airway obliteration [5, 6]. The unaffected central airways are likely due to the embryonic development timeline, as major airways are fully developed by 7 weeks [14]. Thus, even severe preterm birth (<29 weeks) and postnatal mechanical ventilation and supplemental oxygen are too late to impact large airway development. However, alveolarization begins at 36 weeks and is highly susceptible to disruption via premature birth and postnatal interventions.
A limitation is the range of ages of BPD patients which likely resulted in different treatments along with a lack of severity measures. However, we also split the BPD patients into the youngest (>20 years) and older groups and both had similar airway morphology as their respective controls.
In conclusion, this study is the first to directly quantify central airway luminal area in patients with BPD. Our results suggest the luminal areas of the large conducting airways in patients with BPD are not different from matched controls.
The pathophysiology of large airways in Bronchopulmonary dysplasia (BPD) is unclear
CT derived measures of central airways luminal area in BPD patients
Large conducting airways in patients with BPD are not different from controls
Funding information:
National Institutes of Health Grants R35-HL139854 (MJJ), and Natural Sciences and Engineering Research Council of Canada RGPIN-2019-04615 (PBD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, Watterberg KL, Saha S, Das A, Higgins RD. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics 2010; 126: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siffel C, Kistler KD, Lewis JFM, Sarda SP. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Mat Fetal Neonat Med; 2019; 0: 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res Part A: Clin Mol Teratol 2014; 100: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voynow JA. “New” bronchopulmonary dysplasia and chronic lung disease. Paediatr Respir Rev 2017; 24: 17–18. [DOI] [PubMed] [Google Scholar]
- 5.Mižíková I, Morty RE. The Extracellular Matrix in Bronchopulmonary Dysplasia: Target and Source. Front Med (Lausanne) 2015; 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonikos DS, Bensch KG, Northway WH, Edwards DK. Bronchopulmonary dysplasia: The pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Human Path 1976; 7: 643–666. [DOI] [PubMed] [Google Scholar]
- 7.Liu N, Cummings OW, Lagstein A, Hage CA, Chan KM, Zhang C. Lung Transplantation for Bronchopulmonary Dysplasia in Adults: A Clinical and Pathologic Study of 3 Cases. Am. J. Surg. Pathol 2020;. [DOI] [PubMed] [Google Scholar]
- 8.Ochs M, O’Brodovich H. 6 - The Structural and Physiologic Basis of Respiratory Disease In: Wilmott RW, Deterding R, Li A, Ratjen F, Sly P, Zar HJ, Bush A, editors. Kendig’s Disorders of the Respiratory Tract in Children (Ninth Edition) Philadelphia: Content Repository Only!; 2019. [cited 2020 Jan 21]. p. 63–100.e2Available from: [Google Scholar]
- 9.Udomittipong K, Sly PD, Patterson HJ, Gangell CL, Stick SM, Hall GL. Forced oscillations in the clinical setting in young children with neonatal lung disease. Eur Resp J. 2008; 31: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 10.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: predictions from oxygen requirements in the neonatal period. Pediatrics. 1988; 82(4) 527–532. [PubMed] [Google Scholar]
- 11.Ripoll JG, Guo W, Andersen KJ, Baker SE, Wiggins CC, Shepherd JRA, Carter RE, Welch BT, Joyner MJ, Dominelli PB. Sex differences in paediatric airway anatomy. Exp Physiol 2020; 105(4): 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominelli PB, Ripoll JG, Cross TJ, Baker SE, Wiggins CC, Welch BT, Joyner MJ. Sex differences in large conducting airway anatomy. J. Appl. Physiol 2018; 125: 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallory GB, Chaney H, Mutich RL, Motoyama EK. Longitudinal changes in lung function during the first three years of premature infants with moderate to severe bronchopulmonary dysplasia. Ped Pulmon 1991; 11: 8–14. [DOI] [PubMed] [Google Scholar]
- 14.Schittny JC. Development of the lung. Cell Tissue Res 2017; 367: 427–444 [DOI] [PMC free article] [PubMed] [Google Scholar]


