Abstract
Cadmium is a ubiquitous, non-essential metal that has earned a spot on the World Health Organizations top 10 chemicals of major public health concern. The mechanisms of cadmium-induced adverse health outcomes, such as cardiovascular disease, renal toxicity and cancer, are well studied in adults. However, the implications for early life exposures to low-level cadmium leading to increased risk of developing diseases in adulthood remains elusive. Epidemiological investigation of the long term implications of cadmium-associated adverse birth outcomes are limited and studies do not extend into adulthood. This review will summarize the literature on the non-lethal, adverse health effects associated with prenatal and early life exposure to cadmium and the implications of these exposures in the development of diseases later in life. In addition, this review will highlight possible mechanisms responsible for these outcomes as well as address the inconsistencies in the literature. More recent studies have addressed sex as a biological variable, showing prenatal cadmium exposure elicits sex-specific outcomes that would otherwise be masked by pooling male and female data. Furthermore, researchers have begun to investigate the role of prenatal and early life cadmium exposures in the development of diet-induced diseases with evidence of altered essential metal homeostasis as a likely mechanism for cadmium-enhanced, diet-induced diseases. Although novel experimental models are beginning to be established to study the association between prenatal cadmium exposure and adverse health outcomes in adulthood, the studies are few, highlighting a major need for further investigation.
Keywords: Cadmium, prenatal exposure, fetal development, birth outcomes, Developmental Origins of Health and Disease (DOHaD) hypothesis, environmental contaminant
1. Introduction
Cadmium is a naturally occurring, non-essential metal that has been recognized as an occupational and environmental risk factor for decades (ATSDR, 2012; Tinkov et al., 2017). Ranked number 7 on the Agency for Toxic Substances and Disease Registry list of environmental chemical hazards (ATSDR, 2012), cadmium is one of the most common and detrimental metals present in our environment (Jacobo-Estrada et al., 2017). Over the last century, exposure to cadmium has dramatically increased (IPCS, 1992) due to its use in the production of batteries, pigments and plastics. Anthropogenic sources of cadmium include mining, burning of fossil fuels and incineration of household wastes, play a significant role in generating concentrated sources of cadmium and releasing it into the environment (Jarup and Akesson, 2009).
In adults, the association between chronic cadmium exposures and development of adverse health effects such as renal toxicity, cardiovascular disease and cancer is well documented (Jarup and Akesson, 2009; Nawrot et al., 2010). In children, pre- and postnatal exposure to cadmium is associated with reduced birthweight, impaired fetal growth, trace element deficiencies and congenital malformations (Al-Saleh et al., 2014; Hudson et al., 2019; Jin et al., 2016; Kippler et al., 2012; Taylor et al., 2016); however, little is known about the impact of these early life exposures on the development of diseases later in life.
The Developmental Origins of Health and Disease (DOHaD) hypothesis suggests that exposures to environmental stressors during sensitive stages of human development (in utero and early childhood) increases susceptibility to adverse health outcomes in adulthood (Barouku et al., 2012; Gluckman et al., 2010; Thayer et al., 2012). Due to the widespread production and use of metals, in parallel with the knowledge that metals can be passed from mothers to offspring via placenta and/or breast milk, concern has risen about prenatal exposure to metals and the long-term adverse health implications (Wang et al., 2014; Young et al., 2018). For some metals, such as arsenic, the connection between in utero exposures and increased risk of disease development in adulthood is evident in both human and animal studies (Young et al., 2018); however, for other metals, such as cadmium, this connection remains unclear.
Studies were considered for this review if they were published between 1970 and 2020 (last 50 years); however most of the studies discussed in this review were published after 2000 as the topic is new to the field and still emerging. Both Google Scholar and PubMed were searched using key terms (cadmium, prenatal exposure, early life exposure, fetal development, birth outcomes, Developmental Origins of Health and Disease (DOHaD) hypothesis, sex differences and sexual dimorphism). The most representative studies for each discussion point were then chosen for discussion in this review article.
2. Maternal Cadmium Exposure and Placental Toxicology
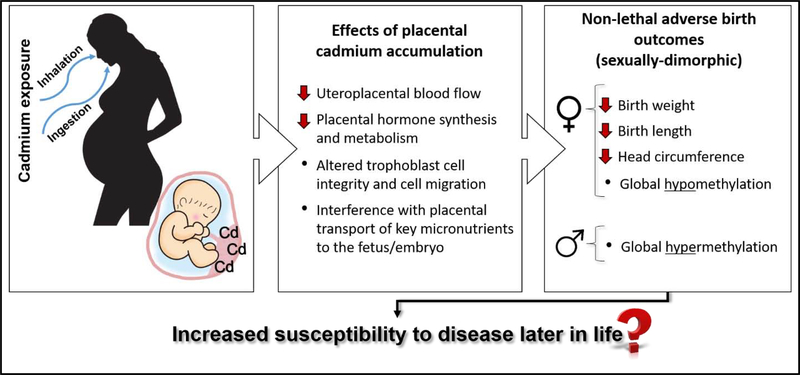
The main route of cadmium uptake in humans is by inhalation and ingestion, with 10–50% of inhaled cadmium being absorbed (dependent on particle size) and 5–10% of ingested cadmium being absorbed (dependent on an individuals’ essential metal load) (Friberg 1983; Nordberg et al., 2007; Röllin et al., 2015). Cadmium primarily accumulates in the kidneys and liver, with an estimated half-life of 6 to 38 years and 4–19 years, respectively (Jacobo-Estrada et al., 2017; Kjellstrom and Nordberg, 1978); however, cadmium also accumulates in the placenta with limited direct transfer to the fetus. (Kippler et al., 2010; Korpela et al., 1986; Osman et al., 2000). During pregnancy, the absorption of cadmium is enhanced as a result of physiological changes that occur to ensure nutritional needs of the mother and fetus are met during gestation (Astbury et al., 2015; Moya et al., 2014). For example, the divalent metal transporter 1 (DMT1) is abundantly expressed in the placenta throughout gestation. The major function of DMT1 is iron uptake and transfer; however DMTs also readily facilitate cellular uptake of other divalent cations, such as cadmium (Bressler et al., 2004; Chong et al 2005; Georgieff et al., 2000).
Effects of placental cadmium accumulation on the developing embryo include decreased uteroplacental blood flow, altered trophoblast cell integrity and cell migration, reduced synthesis and metabolism of placental hormones (Alvarez et al., 2011; Chertok et al., 1984; Lin et al., 1997; Stasenko et al., 2010). In addition, cadmium can interfere with placental transport of key micronutrients to the fetus/embryo, such as calcium and zinc (Lin et al., 1997; Wier et al., 1990). Wier et al (1990) showed impaired transfer of zinc from maternal to fetal circulation in human placentas perfused with 10 nmol/ml of cadmium. Mechanistically, cadmium accumulation in the placenta induces synthesis of the metal binding protein metallothionein (MT) and subsequent formation of cadmium-MT complexes in order to avoid transfer of cadmium to the fetus, although some cadmium crosses the placenta via DMTs. High concentrations of MT can reduce the amount of free, available zinc in the placenta through the formation of zinc-MT complexes, and reduce zinc transfer to the fetus (Kippler et al., 2010; Ronco et al., 2006). Since it is evident that placental cadmium accumulation can disrupt fetal growth and development (Cheng et al., 2017, Jacobo-Estrada et al., 2017) the relationship between prenatal cadmium exposure and birth outcomes has received growing interest.
3. Prenatal Cadmium Exposure and Non-lethal Birth Outcomes
Although several studies have reported associations between prenatal cadmium exposure and impaired fetal growth (low birth weight, small for gestation age and preterm birth) (Huang et al., 2017; Ikeh-Tawari et al., 2013; Lou et al., 2017; Salpietro et al 2002; Röllin et al., 2015; Sun et al., 2014; Wang et al., 2016; Zhang et al., 2018), the findings are inconsistent likely because they heavily rely on correlative measurements of cadmium at birth. For example, in a prospective pregnancy cohort study of 1027 women from Durham, North Carolina maternal blood samples collected at the time of delivery had a mean cadmium level of 0.46 μg/L (<0.08 – 2.52 μg/L) and infants born to women with blood cadmium levels in the highest tertile of exposure were more likely to have low birthweight and be small for gestational age (Salpietro et al., 2002). Similarly, a cross-sectional study of pregnant women in Saudi Arabia designed to evaluate the association between heavy metal exposure during pregnancy and adverse outcomes at birth showed cadmium in umbilical cord blood (median = 0.704 μg/L) taken at delivery was associated with low birth weight, reduced crown-heel length and were at greater risk of being small for gestational age (Al-Saleh et al., 2014).
In contrast, other studies have not observed associations between maternal cadmium levels and size at birth (Osman et al., 2000; Thomas et al., 2015); however these studies report cadmium levels much lower than the aforementioned studies. For example, a Canadian study of 1835 pregnant women found no association between average maternal blood concentrations from the first and third trimester (0.2 μg/L) and risk for small for gestational age (Thomas et al., 2015). Osman et al (2000) measured cadmium in the placenta, cord blood and maternal blood (median concentration: 5.17, 0.02 and 0.157 μg/L, respectively) at gestational week 36 in 106 Swedish women and found no association between cadmium and birth length, height or head circumference.
Furthermore, some studies have observed limited associations between birth outcomes and cadmium concentrations in umbilical cord blood, but not maternal blood, urine or placental tissue. For example, a study by Zhang et al (2004) of 44 pregnant women from a cadmium polluted area in the Hubei province of China found no association between maternal blood (0.80 to 25.20 μg/L) or placental cadmium (0.084 to 3.97 μg/g) levels and pregnancy outcomes (neonatal asphyxia or premature labor), neonatal birth weight or neonatal birth height. However, cord blood cadmium levels >0.40 μg/L were negatively associated with birth height (Zhang et al., 2004). In another agriculturally polluted area of China, the Jiangsu Province, a birth cohort study of 1073 mother-newborn pairs found no association between maternal urinary cadmium levels (median = 0.19 μg/L) and birth outcomes including birth weight, length, head circumference and ponderal index (a measure of leanness based on the relationship between height and mass) but did find cord blood cadmium levels (median = 0.40 μg/L) to be negatively associated with ponderal index in male newborns only (Guo et al., 2017). Overall, those studies that report associations between prenatal cadmium exposure and impaired fetal growth report the cadmium effects at levels of 0.40 ug/L and above. Thus, suggesting a potential threshold for cadmium-associated fetal growth impairment.
Variability in cadmium exposure, analytical methods and timing of fetal cadmium exposure and specimen collection likely all play a role in these inconsistent epidemiological findings. To begin to address the inconsistent findings in the literature Cheng et al (2017) assessed trimester-specific effects of prenatal cadmium exposure on birth weight, birth length and ponderal index using creatinine adjusted urinary cadmium levels which are thought to be more reflective of whole-body burden than blood samples which are more reflective of recent, transient exposures (Cheng et al., 2017; Hays et al., 2008). More specifically, urinary cadmium levels were collected during each trimester in 282 pregnant women from the Wuhan Women and Children Medical Center in China (Cheng et al., 2017). Urinary creatinine, which is excreted at a relatively contestant rate in the urine, was measured and used to control for variations in spot urine sample dilutions. The creatinine adjusted urinary cadmium levels during the first, second and third trimesters were 0.51, 0.59 and 0.61 μg cadmium/g creatinine, respectively. Birth size in males was not associated with maternal cadmium levels in any trimester. In contrast, female birth size was inversely associated with high maternal cadmium levels in the first trimester only, suggesting that the critical window of susceptibility to cadmium-associated adverse effects on birth size can occur during earlier periods of pregnancy and can be sex-specific. The sex-dependent outcome of this study is consistent with other studies investigating in utero cadmium exposure (Kippler et al., 2012; Röllin et al., 2015; Vahter et al., 2007) and provides insight into the variability surrounding the literature.
4. Sex-Specific Effects
The contradictory results in the effects of prenatal cadmium exposure on birth outcomes is likely to be due to studies pooling male and female data, which masks the sex-specific effects, a phenomenon observed with the pharmacokinetics and toxicity studies of other metals (Vahter et al., 2007). Thus, the experimental study design has shifted to account for sex as a biological variable in order to more accurately and informatively address research questions. For example, Romano et al (2016) showed a negative association between maternal urinary cadmium levels (0.31 μg/g creatinine) and female offspring birth length, but a positive association with male offspring birth length in a subset of 396 women form a prospective cohort study in Seattle, Washington. In another study using whole blood samples collected in the first trimester from 4191 pregnant women enrolled in the Avon Longitudinal Study for Parents and Children, Taylor et al (2016) found an adverse association between maternal blood cadmium levels (mean, 0.56 μg/L) and birthweight, head circumference and crown-heel length in females, but not males. Similarly, maternal urinary cadmium (median, 0.63 μg/L with 75% < 1 μg/L) collected during early gestation (on average gestation week 8) from 1616 women in rural Bangladesh was negatively associated with birth weight and head circumference in female, but not male neonates (Kippler et al., 2012).
The “moderate” cadmium levels found in maternal blood and urine [defined by the Human Biomonitoring Commission of the German Federal Environment Agency as anything below the 1 μg/L reference level (Schulz et al., 2007)], in the aforementioned studies are similar to those reported in other developed countries (Taylor et al., 2014). Interestingly, the sex-specific associations between maternal cadmium levels and adverse birth outcomes continue to be evident at even lower cadmium concentrations. Specifically, Röllin et al (2015) found a negative association between lower birth weight in female, but not male neonates, and maternal blood cadmium levels (mean: 0.25 μg/L) half that of previously reported studies correlating cadmium with sex-specific adverse outcomes (Kippler et al., 2012; Röllin et al., 2015; Taylor et al., 2016).
Of the three epigenetic mechanisms involved in regulating gene expression (DNA methylation, histone modifications and gene silencing mediated by non-coding RNAs), cadmium research has focused exclusively on DNA methylation (Paul and Bhattacharje, 2016; Vilahur et al., 2015). Sex-specific effects have been reported in DNA methylation patterns associated with prenatal cadmium exposure in both epidemiological and animal studies, providing a possible mechanism by which birth size is correlated to maternal cadmium exposure (Castillo et al., 2012; Kippler et al., 2013; Mohanty et al., 2015). In a study of 24 maternal-infant pairs, Mohanty et al (2015) found sex-specific associations between placental genome-wide DNA methylation and placental cadmium levels. In females, differentially methylated sites were near transcriptional start sites for cell damage response genes whereas in males the sites were close to transcriptional start sites involved in organ development, cell differentiation and angiogenesis (Mohanty et al., 2015). More specifically, in female infants cadmium was associated with hypomethylation of cell damage genes SIAH3, HS3ST4, and TP53G1 where as in male infants cadmium was associated with hypomethylation of MECOM, and hypermethylation of SALL1. Whether these genes were upregulated or downregulated by the observed altered methylation patterns was not addressed in this study.
In another study, Kippler et al (2013) found altered DNA methylation patterns in mononuclear cells from cord blood were associated with maternal blood cadmium levels taken at gestational week 14 (mean of 1.3 μg/kg) and the associations were sex-specific. Of the top 500 CpG sites, 96% of the sites showed positive correlations between cadmium exposure and cord blood methylation in males where as in females only 29% were positively correlated, with most associations being inverse. Cadmium exposure was associated with more global hypermethylation in males and hypomethylation in females. The top 6 genes with differential DNA methylation associated with cadmium exposure were hypomethylated in females and hypermethylated in males. In females, methylation changes were reported to be associated with genes involved in morphology and mineralization of bone as well as organ development. In males, methylation changes were associated with genes related to cell death (Kippler et al., 2013).
These studies provide clear evidence that prenatal cadmium exposure alters DNA methylation patterns in a sex-specific manner with cadmium-related hypomethylation more prominent in females and hypermethylation more prominent in males. Further studies are needed to elucidate the consequences of these altered DNA methylation patterns on child health and development. In addition, the cadmium-induced epigenetic modifications during fetal development may cause persistent alternations in gene expression that increase the risk for long-term adverse health effects, a phenomenon observed with prenatal exposure to other metals, such as arsenic (Vilahur et al., 2015; Wang et al., 2012; Young et al., 2018). In addition, the altered DNA methylation patterns observed in the placenta and newborns of cadmium exposed mothers may, at least in part, explain the sex-specific differences adverse birth outcomes, as sex-specific differences in methylation patterns are generated early after fertilization (Gabory et al., 2013).
Castillo et al (2012) designed an animal study to investigate the impact of maternal cadmium exposure (50 ppm in drinking water) during pregnancy on altered fetal methylation patterns in the liver, focusing on altered glucocorticoid metabolism, a phenomenon linked to increased risk of cardiometabolic disorders in adulthood (Ronco et al., 2009; Seckl, 2004). Prenatal cadmium exposure resulted in increased expression of fetal DNA methyltransferase 3a (an enzyme involved in de novo methylation in embryonic development) in males and decreased expression in females. This sex-specific outcome correlated with hypomethylation of the hepatic glucocorticoid receptor in female fetuses and hypermethylation in male fetuses (Castillo et al., 2012). Although informative, it remains unclear if these altered methylation patterns remain throughout life or result in long-term health effects. Relative to cadmium exposure, hypomethylation of repetitive sequences has been reported in adult women suggesting implications for long term health effects such as hormone-related cancers (Hossain et al., 2012).
However, few epidemiological studies have followed cohorts of in utero cadmium exposed children past birth, and of those that do, follow-up ends before puberty (most ending before age 10). The adverse effect of cadmium exposure on fetal growth, including weight and height appear to remain until at least 5 years of age and are more apparent among females (Gardner et al., 2013). Kippler et al (2013) identified several CpG sites associated with cadmium exposure in newborns that persisted in 4.5-year old children. In children 9 years of age, Malin Igra et al (2019) correlated early life cadmium exposure with increases in bone-related biomarkers associated with osteotoxicity. Recently, Moynihan et al (2019) conducted a study that emphasized the sex-dependent effects of prenatal exposure to cadmium, observing a negative association between exposure to cadmium in utero and both peripheral and abdominal adiposity in females, but not males (median age 10).
Although it is well documented that prenatal exposure to cadmium is associated with adverse effects on child health and development, the implication for long term health remain elusive (Fig. 1) Few studies have considered the possibility that prenatal cadmium exposure may reprogram an individual to be more susceptible to pathologies later in life including cancer, type 2 diabetes and cardiovascular diseases, a concept known as the DOHaD hypothesis (Heindel et al., 2017; Vilahur et al., 2015).
Fig 1.
Summary of the effects of placental cadmium accumulation and the non-lethal adverse birth outcomes that may lead to increased susceptibility of disease development in adulthood.
5. Evidence for Increased Risk of Developing Adult Diseases
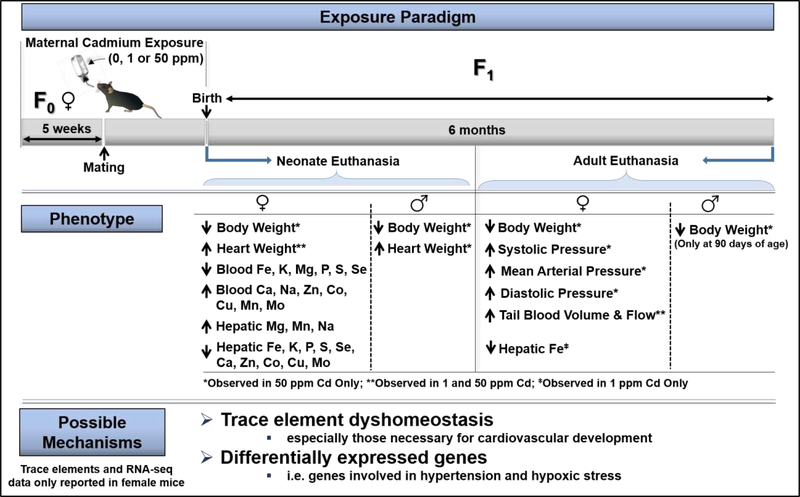
Epidemiologically, the link between early-life cadmium exposures and increased risk for adult diseases remains to be explored. Experimentally, researchers have begun to develop models to study the impact of early life cadmium exposure on disease development later in life. For instance, Hudson et al (2019) investigated the impact of maternal cadmium exposure on cardiovascular changes in offspring at birth or after 6 months of age (Fig. 2). Maternal cadmium exposure was associated with a hypertensive phenotype in adult female mice, but not in male mice; however, there were no differences in markers of circulating reactive oxygen species or essential trace element levels between control and exposed adult female mice. Thus, the authors focused on neonate tissues to identify possible early-life, cadmium-induce molecular changes (via RNA-seq) that may be sufficient to program adulthood hypertension (Hudson et al., 2019). RNA-seq was performed on the hearts of female neonates only as the females showed evidence of hypertension while the males did not. In association with maternal cadmium exposure, RNA-seq identified differentially expressed genes enriched for functions in cardiovascular disease, hypertension, enlarged hearts, hypoxic stress, cellular energy. Overall, the results of this study suggests maternal cadmium exposure causes changes during critical windows of development (perturbed trace element homeostasis and gene expression) that increases risk of hypertension in adulthood (Hudson et al., 2019). However, empirical evidence is still needed to strengthen these observations both in animal models and prospective birth cohort studies in humans.
Fig 2.
Summary of the findings in “Maternal cadmium exposure in the mouse leads to increased heart weight at birth and programs risk to hypertension in adulthood” (Hudson et al., 2019). The illustration was created by the authors based on the published work by Hudson et al (2009). NOTE: results summarized in this figure are from BxC mice only. Hudson et al (2019) used two different mating strategies creating B×C (B mother × C father) and C×B (C mother × B father) offspring (B = C57BL/6J and C = CAST/EiJ) to facilitate a study not yet published. Transcriptomics, essential trace metal levels and circulating reactive oxygen species levels were only determined in BxC offspring. In addition, these three endpoints were only measured in female BxC mice as they showed evidence of hypertension while the male mice did not.
6. Potential Role of Cadmium-Diet Interactions
Of growing interest is the role of diet-environment interaction in the initiation, progression and development of human metabolic diseases. Dietary factors, such as excessive fat intake, contribute to the formation of adult metabolic pathologies including type 2 diabetes, non-alcoholic fatty liver disease and cardiovascular disease, all of which increase susceptibility to a second hit which may promote progression to more severe pathologies (De Long and Holloway, 2017). Animal studies have been published investigating the relationship between diet and low level cadmium exposure, showing that the combination of high fat or high cholesterol diets with cadmium exposure results in increased risk of heart failure and altered bone quality (Türkcan et al., 2015; Zhang et al., 2020); however these studies do not consider the role of prenatal or early-life exposures as a potential “second hit” influencing susceptibility to chronic metabolic disease in adulthood.
Liang et al (2019) used a model of early life (in utero through 10 weeks post weaning) exposure to environmentally relevant doses of cadmium (0.5 and 5 ppm in drinking water) combined with post-weaning high fat diet (HFD), to investigate the combined effects on mouse cardiac remodeling. A second study, using the same cadmium exposure model, investigated whole-life exposure (in utero through 24 weeks post-weaning) on metal distribution in the blood, heart, kidney and liver in HFD-fed adult mice (Young et al., 2019). Based on these two studies using same animal model, the following key features can be obtained (Table 1).
Table 1.
Key results from two studies using the same animal model of early life exposure to cadmium combined with post-weaning HFD (Liang et al. 2019; Young et al. 2019)
| • Over time, cadmium exposure alone reduced body weight gain in female, but not male mice. |
| • Cadmium exposure combined with HFD reduced body weight gain in male mice only. |
| • Females accumulate more cadmium in liver and kidney compared to males. |
| • HFD increased cadmium levels in liver, kidney and heart tissue in both sexes. |
| • The combination of cadmium with HFD significantly altered essential metal levels in the blood, liver and kidney, but not the heart. |
| • The combined exposure induced cardiac hypertrophy and fibrosis in female mice only. |
| • Cardiac hypertrophy and fibrosis in female mice was independent of oxidative stress. |
These findings suggest that cadmium interacts with HFD to alter essential metal homeostasis in the liver and kidney, a phenomenon that may contribute to the underlying mechanism responsible for the development of obesity-associated pathologies in multiple organs. Additionally, the data suggests the mechanisms for cadmium-enhanced, diet-induced heart hypertrophy in female mice is independent of both oxidative stress and metal dyshomeostasis (Liang et al., 2019; Young et al., 2019). Although possibly more reflective of true environmental exposures where the offspring continues to be exposed to the same toxicant as the parents (either through food, water or air), these studies do not separate in utero and post-natal exposure and therefore conclusions on prenatal exposure alone cannot be deduced from these results.
7. Conclusions
It is evident that although the placenta acts as a significant barrier to cadmium during fetal development, there are several indirect effects implicated in the non-lethal, adverse birth outcomes related to in utero cadmium exposure. The implication of these outcomes in the development of diseases later in life remains elusive with little to no epidemiological investigation. Recently, however, the ability of cadmium to alter DNA methylation patterns and disrupt essential metal homeostasis has highlighted the need to investigate the effects of early-life cadmium exposure and increased susceptibility to disease in adulthood. Experimental models have just begun to address this very large knowledge gap and provide novel insights into mechanism by which prenatal cadmium exposure may increase the risk of adverse health complications later in life.
Future studies must continue to address sex as a risk factor as it has become clear that cadmium elicits sex-specific outcomes that are otherwise masked in mixed-sex studies (those that do not separate males and females). In addition, future studies should consider the implications of prenatal, low-dose cadmium exposure in the development of not only metabolic syndrome and cardiovascular disease, but also neurobehavioral disorders and cancers. Furthermore, it remains unclear if the increased risk of disease development seen in the first generation of offspring prenatally exposed to low-dose cadmium can be generationally transmitted. To address this important question, systemic mechanistic studies investigating epigenetic modifications and generational transition of diseases must be implemented. Subsequently, epidemiologic studies should include the collection of trimester specific biosamples and information, not just at birth, to address the gaps and variability in the data.
Future studies should also address how prenatal exposure to cadmium in combination with other metals impacts disease initiation and progression as cadmium is found in combination with many other metals, such as zinc, lead and copper in the environmental. Additionally, many of the hazardous waste sites, including Superfund sites, around the United States are contaminated with mixtures of metals and present with ideal metal mixture exposure scenarios for future studies.
Acknowledgements
The authors are in part supported by the following funding agencies: JLY, National Institutes of Health grant T32-ES011564; LC, American Diabetes Association grant 1-18-IBS-02.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Saleh I, Shinwari N, Mashhour A, Rabah A, 2014. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int. J. Hyg. Environ. Health. 217 (2–3), 205–218. 10.1016/j.ijheh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Alvarez MM, Chakraborty C, 2011. Cadmium inhibits motility factor-dependent migration of human trophoblast cells. Toxicol. In Vitro. 25 (8), 1926–1933. 10.1016/j.tiv.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Astbury S, Mostyn A, Symonds ME, Bell MC, 2015. Nutrient availability, the microbiome, and intestinal transport during pregnancy. Appl. Physiol. Nutr. Metab. 40 (11), 1100–1106. 10.1139/apnm-2015-0117. [DOI] [PubMed] [Google Scholar]
- ATSDR, 2012. Toxicological Profile for Cadmium. U.S. Department of Health and Human Services. Public Health Agency for Toxic Substances and Disease Registry. [PubMed] [Google Scholar]
- ATSDR, 2017. Substance Priority List. U.S. Department of Health and Human Services. Public Health Agency for Toxic Substances and Disease Registry. [Google Scholar]
- Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ, 2012. Developmental origins of noncommunicable disease: implications for research and public health. Environ. Health. 11 (42), 1–9. 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D, 2004. Divalent metal transporter 1 in lead and cadmium transport. Ann. N.Y. Acad. Sci. 1012, 142–152. 10.1196/annals.1306.011. [DOI] [PubMed] [Google Scholar]
- Castillo P, Ibáñez F, Guajardo A, Llanos MN, Ronco AM, 2012. Impact of cadmium exposure during pregnancy on hepatic glucocorticoid receptor methylation and expression in rat fetus. PLoS One. 7 (9), e44139 10.1371/journal.pone.0044139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhang N, Zheng T, Hu J, Zhou A, Bassig BA, Xia W, Savitz DA, Buka S, Xiong C, Braun JM, Zhang Y, Zhou Y, Pan X, Wu C, Wang Y, Qian Z, Yang A, Romano ME, Shi K, Xu S, Li Y, 2017. Critical Windows of Prenatal Exposure to Cadmium and Size at Birth. Int. J. Environ. Res. Public Health. 14 (1), 58 10.3390/ijerph14010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertok RJ, Kullgren B, Burbank D, 1984. The effects of CdCl2 on the maternal-to-fetal clearance of 67Cu and placental blood flow. Proc. Soc. Exp. Biol. Med. 176 (2), 138–142 10.3181/00379727-176-41853I. [DOI] [PubMed] [Google Scholar]
- Chong WS, Kwan PC, Chan LY, 2005. Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues. Hum. Reprod. 20 (12), 3532–3538. 10.1093/humrep/dei246. [DOI] [PubMed] [Google Scholar]
- De Long NE, Holloway AC, 2017. Early-life chemical exposures and risk of metabolic syndrome. Diabetes Metab. Syndr. Obes. 10, 101–109. 10.2147/DMSO.S95296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg L, 1983. Cadmium. Annu. Rev. Public. Health. 4, 367–373. 10.1146/annurev.pu.04.050183.002055. [DOI] [PubMed] [Google Scholar]
- Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C, 2013. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 4 (1), 5 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R, Kippler M, Tofail F, Bottai M, Hamadani J, Grander M, Nermell B, Palm B, Rasmussen KM, Vahter M, 2013. Environmental Exposure to Metals and Children’s Growth to Age 5 Years: A Prospective Cohort Study. Am. J. Epidemiol. 177 (12), 1356–1367. 10.1093/aje/kws437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff MK, Wobken JK, Welle J, 2000. Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta. 21, 799–804. 10.1053/plac.2000.0566. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Mitchell MD, 2010. Developmental origins of health and disease: reducing the burden of chronic disease in the next generation. Genome Med. 2 (2), 14 10.1186/gm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wu C, Qi X, Jiang S, Liu Q, Zhang J, Cao Y, Chang X, Zhou Z, 2017. Adverse associations between maternal and neonatal cadmium exposure and birth outcomes. Sci. Total Environ. 575, 81–587. 10.1016/j.scitotenv.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Hays SM, Nordberg M, Yager JW, Aylward LL, 2008. Biomonitoring Equivalents (BE) dossier for cadmium (Cd) (CASNo.7440–43-9). Regul. Toxicol. Pharmacol 51 (3Suppl.), S49–S56. 10.1016/j.yrtph.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Skalla LA, Joubert BR, Dilworth CH, Gray KA, 2017. Review of developmental origins of health and disease publications in environmental epidemiology. Reprod. Toxicol. 68, 34–48. 10.1016/j.reprotox.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Hossain MB, Vahter M, Concha G, Broberg K, 2012. Low- Level Environmental Cadmium Exposure is Associated with DNA Hypomethylation in Argentinean Women. Environ. Health Perspect. 120 (6), 879–884. 10.1289/ehp.1104600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Li H, Zhang B, Zheng T, Li Y, Zhou A, Du X, Pan X, Yang J, Wu C, Jiang M, Peng Y, Huang Z, Xia W, Xu S, 2017. Prenatal cadmium exposure and preterm low birth weight in China. J. Expo. Sci. Environ. Epidemiol. 27 (5), 491–496. 10.1038/jes.2016.41. [DOI] [PubMed] [Google Scholar]
- Hudson KM, Belcher SM, Cowley M, 2019. Maternal cadmium exposure in the mouse leads to increased heart weight at birth and programs susceptibility to hypertension in adulthood. Sci. Rep. 9 (1), 3553 10.1038/s41598-019-49807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeh-Tawari EP, Anetor JI, Charles-Davies MA, 2013. Cadmium level in pregnancy, influence on neonatal birth weight and possible amelioration by some essential trace elements. Toxicol. Int. 20 (1), 108–112. 10.4103/0971-6580.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCS, 1992. Cadmium - Environmental aspects. Geneva, World Health Organization, International Programme on Chemical Safety. Environmental Health Criteria; 135; http://www.inchem.org/documents/ehc/ehc/ehc135.htm (accessed 30 April 2020). [Google Scholar]
- Jacobo-Estrada T, Santoyo-Sánchez M, Thévenod F, Barbier O, 2017. Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models. Int. J. Mol. Sci. 18 (7), 1590 10.3390/ijms18071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarup L, Akesson A, 2009. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 238, 201–208. 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Jin X, Tian X, Liu Z, Hu H, Li X, Deng Y, Li N, Zhu J, 2016. Maternal exposure to arsenic and cadmium and the risk of congenital heart defects in offspring. Reprod. Toxicol. 59, 109–116. 10.1016/j.reprotox.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Johnston JE, Valentiner E, Maxson P, Miranda ML, Fry RC, 2014. Maternal cadmium levels during pregnancy associated with lower birth weight in infants in a North Carolina cohort. PLoS One. 9 (10), e109661 10.1371/journal.pone.0109661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippler M, Hoque AM, Raqib R, Ohrvik H, Ekstrom EC, Vahter M, 2010. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol. Lett. 192 (2), 162–168. 10.1016/j.toxlet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Kippler M, Tofail F, Gardner R, Rahman A, Hamadani JD, Bottai M, Vahter M, 2012. Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Enviro. Health. Perspectives. 120 (2), 284–289. 10.1289/ehp.1103711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippler M, Engström K, Mlakar SJ, Bottai M, Ahmed S, Hossain MB, Raqib R, Vahter M, Broberg K, 2013. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics. 8 (5), 494–503. 10.4161/epi.24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellstrom T, Nordberg GF, 1978. A kinetic model of cadmium metabolism in the human being. Environ. Res. 16 (103), 248–269. 10.1016/0013-9351(78)90160-3. [DOI] [PubMed] [Google Scholar]
- Korpela H, Loueniva R, Yrjanheikki E, Kauppila A, 1986. Lead and cadmium concentrations in maternal and umbilical cord blood, amniotic fluid, placenta, and amniotic membranes. Am. J. Obstet. Gynecol. 155 (5), 1086–1089. 10.1016/0002-9378(86)90356-X. [DOI] [PubMed] [Google Scholar]
- Liang Y, Young JL, Kong M, Tong Y, Qian Y, Freedman JH, Cai L, 2019. Gender Differences in Cardiac Remodeling Induced by a High-Fat Diet and Lifelong, Low-Dose Cadmium Exposure. Chem. Res. Toxicol. 32 (6), 1070–1081. 10.1021/acs.chemrestox.8b00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Fitzpatrick JW, Iannotti CA, Martin DS, Mariani BD, Tuan RA, 1997. Effects of cadmium on trophoblast calcium transport. Placenta. 18 (4), 341–356. 10.1016/S0143-4004(97)80069-0. [DOI] [PubMed] [Google Scholar]
- Luo Y, McCullough LE, Tzeng J-Y, Darrah T, Vengosh A, Maguire RL, Maity A, Samuel-Hodge C, Murphy SK, Mendez MA, Hoyo C, 2017. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Publ. Health. 17 (1), 354 10.1186/s12889-017-4225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin Igra A, Vahter M, Raqib R, Kippler M, 2019. Early-Life Cadmium Exposure and Bone-Related Biomarkers: A Longitudinal Study in Children. Environ. Health Perspect. 127 (3), 37003 10.1289/EHP3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty AF, Farin FM, Bammler TK, MacDonald JW, Afsharinejad Z, Burbacher TM, Siscovick DS, Williams MA, Enquobahrie DA, 2015. Infant sex-specific placental cadmium and DNA methylation associations. Environ. Res. 138, 74–81. 10.1016/j.envres.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya J, Phillips L, Sanford J, Wooton M, Gregg A, Schuda L, 2014. A review of physiological and behavioral changes during pregnancy and lactation: potential exposure factors and data gaps. J. Expo. Sci. Environ. Epidemiol. 24 (5), 449–458. 10.1038/jes.2013.92. [DOI] [PubMed] [Google Scholar]
- Moynihan M, Telléz-Rojo MM, Colacino J, Jones A, Song P, Cantoral A, Mercado-García A, Peterson KE, 2019. Prenatal Cadmium Exposure Is Negatively Associated With Adiposity in Girls Not Boys During Adolescence. Front. Public Health. 7, 61 10.3389/fpubh.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, Ruttens A, Smeets K, Clijsters H, Vangronsveld J, 2010. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals. 23 (5), 769–782. 10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Nogawa K, Nordberg M, Friberg L, 2007. Cadmium, in: Nordberg GF, Fowler BF, Nordberg M, Friberg L. (Eds), Handbook of the Toxicology of metals 3rd ed Amsterdam, The Netherlands: Elsevier, pp. 445–486. [Google Scholar]
- Osman K, Åkesson A, Berglund M, Bremme K, Schutz A, Ask K, Vahter M, 2000. Toxic and essential elements in placentas of Swedish women. Clin. Biochem. 33 (2), 131–138. 10.1016/S0009-9120(00)00052-7. [DOI] [PubMed] [Google Scholar]
- Paul S, Bhattacharjee P, 2016. Epigenetics and arsenic toxicity, in. States JC (Ed), Arsenic: exposure sources, health risks and mechanisms of toxicity. Wiley, Hoboken (NJ), pp. 81–109. [Google Scholar]
- Röllin HB, Kootbodien T, Channa K, Odland JØ, 2015. Prenatal Exposure to Cadmium, Placental Permeability and Birth Outcomes in Coastal Populations of South Africa. PLoS One. 10 (11), e0142455 10.1371/journal.pone.0142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Enquobahrie DA, Simpson C, Checkoway H, Williams MA, 2016. Maternal body burden of cadmium and offspring size at birth. Environ. Res. 147, 461–468. 10.1016/j.envres.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco AM, Garrido F, Llanos MN, 2006. Smoking specifically induces metallothionein-2 isoform in human placenta at term. Toxicology. 233 (1–2), 46–53. 10.1016/j.tox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Ronco AM, Urrutia M, Montenegro M, Llanos MN, 2009. Cadmium exposure during pregnancy reduces birth weight and increases maternal and foetal glucocorticoids. Toxicol. Lett. 188, 186–219. 10.1016/j.toxlet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Salpietro CD, Gangemi S, Minciullo PL, Briuglia S, Merlino MV, Stelitano A, Cristani M, Trombetta D, Saija A, 2002. Cadmium concentration in maternal and cord blood and infant birth weight: a study on healthy non-smoking women. J. Perinat. Med. 30 (5), 395–399. 10.1515/JPM.2002.061. [DOI] [PubMed] [Google Scholar]
- Schulz C, Angerer J, Ewers U, Kolossa-Gehring M, 2007. The German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health. 210 (3–4), 373–382. 10.1016/j.ijheh.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Seckl JR, 2004. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151 (Suppl 3), U49–U62. 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Stasenko S, Bradford EM, Piasek M, Henson MC, Varnai VM, Jurasovic J, Kusec V, 2010. Metals in human placenta: Focus on the effects of cadmium on steroid hormones and leptin. J. Appl. Toxicol. 30 (3), 242–253. 10.1002/jat.1490. [DOI] [PubMed] [Google Scholar]
- Sun H, Chen W, Wang D, Jin Y, Chen X, Xu Y, 2014. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere. 108, 33–39. 10.1016/j.chemosphere.2014.02.080. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Golding J, Emond AM, 2014. Lead, cadmium and mercury levels in pregnancy: the need for international consensus on levels of concern. Dev. Orig. Health Dis. 5 (1), 6–30. 10.1017/S2040174413000500. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Golding J, Emond AM, 2016. Moderate prenatal cadmium exposure and adverse birth outcomes: A role for sex-specific differences? Paediatr. Perinat. Epidemiol. 30 (6), 603–611. 10.1111/ppe.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA, 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ. Health Perspect. 120 (6), 779–789. 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W, 2015. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: the MIREC study. Environ. Res. 140, 430–439. 10.1016/j.envres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Tinkov AA, Filippini T, Ajsuvakova OP, Aaseth J, Gluhcheva YG, Ivanova JM, Bkorklund G, Skalnaya MG, Gatiatulina ER, Popova EV, Nemereshina ON, Vinceti M, Skalny AV, 2017. The role of cadmium in obesity and diabetes. Sci. Total. Environ. 601–602, 741–755. 10.1016/j.scitotenv.2017.05.224. [DOI] [PubMed] [Google Scholar]
- Türkcan A, Scharinger B, Grabmann G, Keppler BK, Laufer G, Bernhard D, Messner B, 2015. Combination of cadmium and high cholesterol levels as a risk factor for heart fibrosis. Toxicol. Sci. 145 (2), 360–371. 10.1093/toxsci/kfv057. [DOI] [PubMed] [Google Scholar]
- Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M, 2007. Gender differences in the disposition and toxicity of metals. Environ. Res. 104 (1), 85–95. 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vilahur N, Vahter M, Broberg K, 2015. The Epigenetic Effects of Prenatal Cadmium Exposure. Curr. Environ. Health. Rep. 2 (2), 195–203. 10.1007/s40572-015-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li Y, Shao C, Tan Y, Cai L, 2012. Cadmium and Its Epigenetic Effects. Curr. Med. Chem. 19 (16), 2611–2620. 10.2174/092986712800492913. [DOI] [PubMed] [Google Scholar]
- Wang G, Chen Z, Bartell T, Wang X, 2014. Early life origins of metabolic syndrome: the role of environmental toxicants. Curr. Environ. Health Rep. 1 (1), 78–89. 10.1007/s40572-013-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu L, Hu Y-F, Hao JH, Chen YH, Su PY, Fu L, Yu Z, Zhang GB, Wang L, Tao FB, Xu DX, 2016. Maternal serum cadmium level during pregnancy and its association with small for gestational age infants: a population-based birth cohort study. Sci. Rep. 6, 22631 10.1038/srep22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier PJ, Miller RK, Maulik D, DiSant’Agnese PA, 1990. Toxicity of cadmium in the perfused human placenta. Toxicol. Appl. Pharmacol. 105 (1), 156–171. 10.1016/0041-008X(90)90367-4. [DOI] [PubMed] [Google Scholar]
- Young JL, Cai L, States JC, 2018. Impact of prenatal arsenic exposure on chronic adult diseases. Systems biology in reproductive medicine. Syst. Biol. Reprod. Med. 64 (6), 469–483. 10.1080/19396368.2018.1480076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Yan X, Xu J, Yin X, Zhang X, Arteel GE, Barnes GN, States JC, Watson WH, Kong M, Cai L, Freedman JH, 2019. Cadmium and High-Fat Diet Disrupt Renal, Cardiac and Hepatic Essential Metals. Sci. Rep. 9 (1), 14675 10.1038/s41598-019-50771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li X, Sheng X, Wang S, Li B, Tao S, Zhang Z, 2020. Effects of Combined Exposure to Cadmium and High-Fat Diet on Bone Quality in Male Mice. Biol. Trace. Elem. Res.193 (2), 434–444. 10.1007/s12011-019-01713-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu X, Chen A, Davuljigari CB, Zheng X, Kim SS, Dietrich KN, Ho SM, Reponen T, Huo X, 2018. Maternal urinary cadmium levels during pregnancy associated with risk of sex-dependent birth outcomes from an e-waste pollution site in China. Reprod. Toxicol. 75, 49–55. 10.1016/j.reprotox.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Zhao YC, Wang JX, Zhu HD, Liu QF, Fan YG, Wang NF, Zhao JH, Liu HS, Ou-Yang L, Liu AP, Fan TQ, 2004. Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: a study on healthy pregnant women in China. J. Environ. Sci. Health Part A. 39 (9), 2507–2515. 10.1081/ESE-200026331. [DOI] [PubMed] [Google Scholar]