Abstract
Objectives
The natural history of human papillomavirus (HPV) infection has been studied extensively in young women; this study investigated HPV infection in adult women.
Methods
Data from 3817 women aged 24–45 years in a global trial of the 4-valent HPV (6/11/16/18) vaccine were used to calculate prevalence of anogenital infections containing 9-valent (9v) HPV vaccine types (6/11/16/18/31/33/45/52/58) and five non-vaccine types (35/39/51/56/59). Incidence of infections and persistent infections was estimated for 989 placebo recipients naive to all 14 HPV types at baseline. Age-adjusted hazard ratios were calculated for various sociodemographic factors.
Results
Prevalence of anogenital infection was highest in France at 29.2% (9vHPV types) and 21.7% (non-vaccine types) and lowest in the Philippines at 7.6% (9vHPV types) and 5.1% (non-vaccine types). Overall, HPV incidence (per 100 person-years) was 5.2 (9vHPV types) and 4.7 (non-vaccine types), and incidence of persistent infection was 2.7 (9vHPV types) and 2.1 (non-vaccine types). Factors associated with new HPV infections included younger age, younger age at first intercourse, being single, current use of tobacco, and higher number of past and recent sex partners.
Conclusions
Because mid-adult women acquire new HPV infections, administration of the 9vHPV vaccine could reduce HPV-related morbidity and mortality in this population.
Keywords: Human papillomavirus, Adult women, Persistent infection, Incidence, HPV vaccine, Risk factor
Highlights
-
•
Prevalence of HPV infection in mid-adult women varies by country for age.
-
•
Mid-adult women still acquire new HPV infections, including persistent infections.
-
•
Risk factors for acquiring new HPV infections are similar in mid-adult and young women.
Abbreviations
- 2vHPV
bivalent HPV vaccine
- 4vHPV
quadrivalent HPV vaccine
- 9vHPV
nonavalent HPV vaccine
- CIN
cervical intraepithelial neoplasia
- HPV
human papillomavirus
- IARC
International Agency for Research on Cancer
- PCR
polymerase chain reaction
1. Introduction
Human papillomavirus (HPV) is the commonest sexually transmitted viral infection worldwide [1]. HPV infection causes most cases of cervical (~100%), anal (~88%), and vaginal (~78%) cancers, and substantial proportions of penile (~51%), vulvar (≥25%), and oropharyngeal (30%–70%) cancers [[2], [3], [4], [5]]. HPV vaccination is an efficient strategy to reduce risk of HPV-related cancers by reducing the pool of circulating HPV, and therefore, lessening the occurrences of persistent HPV infection and high-grade cancer precursor lesions. There are currently three licensed HPV vaccines: bivalent (2vHPV; Cervarix®, GSK, Rixensart, Belgium), quadrivalent (4vHPV; Gardasil®, Merck & Co., Inc., Kenilworth, NJ, USA), and 9-valent (9vHPV; Gardasil 9, Merck & Co., Inc., Kenilworth, NJ, USA). The 4vHPV vaccine was first licensed in 2006 and the 2vHPV vaccine in 2009 in the United States. The 9vHPV vaccine was first licensed in the United States in 2014 for males (9–15 years old) and females (9–26 years old), and in 2015 for males (16–26 years old) [6]. In 2018, the 9vHPV vaccine was additionally approved in the United States for use in men and women 27 through 45 years old [7]. Consequently, in 2019 the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) updated its recommendations to include catch-up HPV vaccination for all persons through age 26 [8]. In addition, recognizing that some mid-adults who were not previously adequately vaccinated may benefit, the ACIP recommended shared clinical decision making for vaccination of adults 27–45 years old [8].
All three licensed HPV vaccines target HPV 16 and 18, which cause approximately 70% of cervical cancers worldwide. The 4vHPV and 9vHPV vaccines also target HPV 6 and 11, which cause approximately 90% of anogenital warts, as well as a rare respiratory condition (recurrent respiratory papillomatosis) [9,10]. The 9vHPV vaccine additionally protects against five other frequently detected high-risk HPV types (31/33/45/52/58) [11,12], thereby increasing coverage against HPV types that cause approximately 90% of cervical cancer cases worldwide [12,13]. Some high-risk types, such as 35/39/51/56/59, are not included in currently licenced HPV vaccines and are attributed to a smaller proportion of cervical cancers worldwide [11,14].
In addition to cancer protection, HPV vaccines also protect against low- and high-grade cervical intraepithelial neoplasia grades 1 through 3 (CIN1, CIN2, CIN3) caused by the vaccine types. CIN2 and CIN3 are potential precursors to cervical cancer and represent a substantial disease burden in young and adult women worldwide. For example, the CDC estimates that approximately 196,000 cases of CIN2, CIN3 and adenocarcinoma in situ (AIS) (collectively referred to as “CIN2+”) were diagnosed in 2016 in the United States [15]. Approximately 76% (150,000) of the CIN2+ cases were attributable to 9vHPV types and 97,000 of these cases were diagnosed in women aged 30 years or older [15].
Since licensure, the 9vHPV vaccine has been incorporated into standard immunization schedules in many countries, including Australia, Austria, Canada, Germany, and the United States [[16], [17], [18], [19]]. The 2vHPV and 4vHPV vaccines also continue to be used in many parts of the world.
HPV vaccination prior to sexual debut offers the greatest benefit for prevention of HPV infection and HPV-related cancers. HPV immunization programs therefore generally target adolescents (aged 11–13 years, varying by country). In addition, some countries have established multiple age-cohort (i.e., “catch-up”) vaccination programs for teens and young women who are older than the targeted adolescent age range. Observational studies using real-world evidence have demonstrated the impact of HPV vaccination on genital HPV infection, anogenital warts, and histologically-confirmed high-grade cervical intraepithelial neoplasia (CIN2+) in sexually active young women, particularly in settings of high vaccine coverage (>50% of population) and catch-up vaccination programs [20,21].
The International Agency for Research on Cancer (IARC) highlighted the need for HPV studies in older women and their male counterparts, including cohort studies with repeated measures that assess sexual practices and immunity [22]. A focus on adult women is therefore relevant to individuals up to age 45 years who were never vaccinated against HPV, as well as those who remain susceptible to the 9vHPV types that are not covered in the 2vHPV or 4vHPV vaccines. Data on the natural history of HPV infection in older women have the potential to inform existing HPV prevention guidelines by shedding light on prevalence, incidence, and persistence of HPV infections among women who are typically not targeted for HPV immunization. In addition, pre-vaccine-era measures of the burden of HPV infection are important to provide context for interpreting real-world evidence of the impact of the vaccine [22]. For example, HPV prevalence generally declines with age, but in some parts of the world, a second peak of infection is observed in middle-aged women [23,24]. Factors such as these are important to understand when comparing disease burden in the vaccine era to that in the pre-vaccine era [23,[25], [26], [27], [28]].
Using data from a global 4vHPV vaccine clinical trial in adult women (aged 24–45 years), we addressed the following objectives: (1) determining country-specific prevalence of anogenital infection containing 14 of the most common HPV types; (2) calculating incidence of HPV anogenital infections and incidence of persistent infection among women in the placebo group who were HPV-naive at the start of the trial; and (3) detailing factors associated with incident HPV infection. Analyses focus on prevalence and incidence of 9vHPV vaccine types (6/11/16/18/31/33/45/52/58), 4vHPV vaccine types (6/11/16/18), the contribution to prevalence and incidence of five additional types covered in the 9vHPV vaccine (31/33/45/52/58), as well as five other high-risk HPV genotypes for which data are available (35/39/51/56/59).
2. Materials and methods
Data in this report are from a randomized, double-blind, placebo-controlled clinical trial of the safety, immunogenicity, and efficacy of 4vHPV vaccine in adult women aged 24 to 45 years (NCT00090220). Trial methods, inclusion and exclusion criteria, and participant characteristics have been described [29]. Briefly, between June 2004 and April 2005, 3819 women aged 24 to 45 years were randomly assigned to either 4vHPV (n = 1911) or placebo (n = 1908). Healthy, non-pregnant adult women with no history of anogenital warts, vulvar intraepithelial neoplasia, or vaginal intraepithelial neoplasia were enrolled from 38 study sites in seven countries (Colombia, France, Germany, Philippines, Spain, Thailand, United States).
Baseline characteristics in the trial population were generally similar between the vaccination and placebo arms of the trial and our analysis cohort. Approximately 14% of the cohort was from North America, 42% from Latin America, 13% from Europe, and 31% from the Asia/Pacific region. Mean age at baseline was 34 years, mean age at first sexual experience was 19 years, and 78% were married or in a permanent relationship. More than 90% reported no new sexual partners in the 6 months before enrollment, and the median number of lifetime sex partners was 2, with an interquartile range of 1 to 4 [29].
On day 1 and at months 7, 12, 18, 24, 30, 36, 42, and 48, an endo/ectocervical swab and a combined labial/vulvar/perianal swab (herein collectively referred to as an anogenital swab) were collected from all participants. A polymerase chain reaction (PCR)–based assay was used to test HPV DNA in the anogenital swab samples [30,31]. The assay included 14 HPV types: vaccine types 6, 11, 16, 18, 31, 33, 45, 52, and 58, and five additional HPV types not included in these vaccines (35, 39, 51, 56, and 59).
In the present analysis, prevalent, genotype-specific HPV infections were calculated from baseline data from all women (vaccinated and control group). The prevalence of type-specific infection was defined as the number of women who tested positive by PCR for a given HPV genotype in a day 1 anogenital swab, divided by the number of women who had a valid day 1 HPV test for that HPV type. For variables describing combinations of HPV types, a woman was considered infected if at least one HPV type in the combination was detected (e.g. HPV 31/33/45/52/58 means that a woman could have an infection with type 31 and/or 33 and/or 45 and/or 52 and/or 58). More than 99% of randomly assigned women had complete baseline data that were suitable for our prevalence analyses.
Analyses of incident and incident-persistent HPV infections were based on data from women who were randomly assigned to the placebo arm of the trial. Incident infection with a given HPV type was defined as a new detection (i.e. starting at month 6 or thereafter) of HPV by PCR in anogenital swabs collected among women who (1) had normal Pap test results at baseline; (2) were naive at day 1 to the 4vHPV vaccine types in serum (6, 11, 16, 18); and (3) were naive at day 1 to all 14 HPV types measured in swabs (6, 11, 16, 18, 31, 33, 45, 52, 58, 35, 39, 51, 56, 59). Incident persistent infections were defined as detection of a new HPV genital infection by PCR in anogenital swabs collected on at least two consecutive visits spaced 6 months apart (±4-week window). Age-adjusted Cox proportional hazard models were used to estimate the risk of incident infection associated with selected baseline characteristics.
3. Results
The prevalence of HPV infection varied with geographic region and by age of the trial participants (Table 1, Supplemental Tables 1 and 2). Prevalence of HPV infection with any of the 9vHPV vaccine types was 16.4% overall, ranging from 7.6% (Philippines) to 29.2% (France) among adult women. The global prevalence of any 9vHPV type was higher among women aged 24 to 34 (20.0%) than among women aged 34 to 45 (12.7%). The magnitude of age-related differences in infection prevalence also varied by country. For example, in Colombia, 9vHPV type prevalence was 24.3% and 14.7% among women ages 24 to 34 and 35 to 45 years, respectively, but in the Philippines, corresponding prevalence in the 2 age groups was nearly identical: 7.7% and 7.6%.
Table 1.
Baseline prevalence of anogenital HPV infection in 3817 women aged 24 to 45 years, by country.
| HPV type | Total |
Colombia |
France |
Germany |
Philippines |
Spain |
Thailand |
United States |
|---|---|---|---|---|---|---|---|---|
| N = 3817 |
N = 1610 |
N = 106 |
N = 310 |
N = 400 |
N = 65 |
N = 782 |
N = 544 |
|
| % | % | % | % | % | % | % | % | |
| 6 | 1.9 | 1.8 | 6.7 | 4.6 | 0.8 | 3.1 | 0.3 | 2.6 |
| 11 | 0.2 | 0.3 | 0.0 | 0.7 | 0.0 | 0.0 | 0.4 | 0.0 |
| 16 | 4.5 | 4.9 | 9.6 | 7.8 | 1.8 | 4.8 | 1.8 | 6.4 |
| 18 | 2.1 | 2.2 | 1.9 | 3.3 | 0.8 | 1.6 | 0.6 | 4.3 |
| 31 | 2.8 | 3.2 | 8.7 | 5.6 | 0.8 | 7.9 | 0.8 | 2.6 |
| 33 | 0.7 | 0.8 | 1.9 | 1.0 | 0.0 | 0.0 | 0.8 | 0.7 |
| 35 | 1.3 | 2.0 | 1.0 | 0.3 | 0.3 | 1.6 | 0.1 | 2.4 |
| 39 | 3.0 | 3.4 | 2.9 | 2.6 | 0.8 | 1.6 | 2.3 | 4.8 |
| 45 | 1.7 | 1.9 | 1.0 | 2.3 | 1.3 | 4.8 | 0.4 | 3.0 |
| 51 | 3.8 | 4.1 | 9.6 | 5.9 | 1.8 | 4.8 | 1.8 | 4.5 |
| 52 | 4.5 | 5.1 | 9.6 | 3.6 | 2.6 | 3.2 | 3.9 | 5.0 |
| 56 | 6.4 | 8.0 | 8.7 | 10.5 | 2.0 | 14.3 | 1.4 | 8.2 |
| 58 | 2.7 | 4.3 | 1.9 | 2.3 | 0.8 | 0.0 | 1.2 | 2.0 |
| 59 | 2.2 | 2.5 | 5.8 | 3.3 | 1.3 | 0.0 | 1.0 | 2.8 |
| 6/11 | 2.1 | 2.1 | 6.7 | 4.6 | 0.8 | 3.1 | 0.6 | 2.6 |
| 16/18 | 6.2 | 6.8 | 11.5 | 10.1 | 2.0 | 6.5 | 2.3 | 9.9 |
| 6/11/16/18 | 7.9 | 8.6 | 15.4 | 13.7 | 2.8 | 8.1 | 3.0 | 12.0 |
| 31/33/45/52/58 | 10.7 | 13.1 | 19.0 | 12.4 | 5.1 | 11.5 | 6.1 | 12.0 |
| 35/39/51/56/59 | 14.0 | 16.9 | 21.7 | 17.7 | 5.1 | 19.0 | 5.7 | 19.5 |
| 6/11/16/18/31/33/45/52/58 | 16.4 | 19.3 | 29.2 | 20.9 | 7.6 | 15.0 | 8.6 | 20.5 |
“HPV”: human papillomavirus.
Prevalence of HPV 6/11 was 2.1% overall, ranging from 0.6% in Thai women to 6.7% in French women. Likewise, prevalence of HPV 16/18 was 6.2% overall, ranging from 2.0% in the Philippines to 11.5% in France. Although HPV 16/18 prevalence was typically higher in women aged 24 to 34 years at enrollment, infections were also observed in women aged 35 to 45. For example, HPV 16/18 prevalence was 9.5%, 7.8%, 6.7%, and 6.3% among women aged 35 to 45 in France, Germany, United States, and Spain, respectively. Prevalence of infection with the other five HPV types targeted by the 9vHPV vaccine (31/33/45/52/58) was 10.7% overall, with similar geographic differences as observed for other HPV types. The prevalence of non-vaccine HPV types (35/39/51/56/59) was 14.0%, also with patterns similar to the 9vHPV types, except in Spain, where the older women (aged 35–45 years) had higher prevalence than the younger women (aged 24–34 years).
Table 2 summarizes relationships between baseline factors and the risk of incident (new) 9vHPV anogenital infection among women in the placebo group who were HPV naive at baseline. Relative to women aged 24 to 29 years, older women had lower risk of HPV infection, with women aged 40 to 45 years having approximately 45% to 48% lower risk of infection. Similarly, as age at first intercourse increased, incidence of high-risk infections decreased. Women who were not in their first marriage at the time of enrollment had significantly elevated risk of incident HPV infection, as did women who tested positive for chlamydia or gonorrhea at baseline. Infection risk also increased with the number of lifetime sex partners and the number of new sex partners in the 6-month period before enrollment. Relative to current smokers, former and never smokers had lower risk of incident infection.
Table 2.
Association between selected baseline characteristics and incident anogenital HPV infection in women aged 24 to 45 years.
| Baseline characteristics | Age-adjusted hazard ratio (95% CI) for incident HPV DNA infection |
||
|---|---|---|---|
| HPV 16/18 | HPV 31/33/45/52/58, not 16/18 | HPV 16/18/31/33/45/52/58 | |
| Age, years | |||
| 24–29 (n = 250) | 1.00 | 1.00 | 1.00 |
| 30–34 (n = 223) | 0.93 (0.53–1.63) | 0.72 (0.41–1.24) | 0.83 (0.55–1.26) |
| 35–39 (n = 231) | 0.39 (0.19–0.81) | 0.64 (0.37–1.11) | 0.58 (0.37–0.91) |
| 40–45 (n = 285) | 0.56 (0.30–1.02) | 0.52 (0.30–0.91) | 0.55 (0.36–0.85) |
| Marital status | |||
| Married, first marriage (n = 512) | 1.00 | 1.00 | 1.00 |
| All others (n = 477) | 1.80 (1.12–2.91) | 1.43 (0.94–2.18) | 1.48 (1.07–2.05) |
| Regiona | |||
| North America (n = 101) | 1.00 | 1.00 | 1.00 |
| Europe (n = 119) | 1.17 (0.42–3.29) | 0.46 (0.19–1.12) | 0.60 (0.30–1.18) |
| Latin America (n = 387) | 2.39 (1.02–5.60) | 1.18 (0.64–2.16) | 1.43 (0.87–2.34) |
| Asia (n = 382) | 0.61 (0.23, 1.62) | 0.36 (0.18, 0.74) | 0.42 (0.23–0.74) |
| Race/Ethnicity | |||
| White (n = 189) | 1.00 | 1.00 | 1.00 |
| Black (n = 16) | N/A (N/A) | 3.23 (1.08–9.67) | 1.80 (0.63–5.13) |
| Hispanic (n = 398) | 1.93 (1.06–3.52) | 1.86 (1.06–3.25) | 1.87 (1.22–2.86) |
| Asia/Pacific (n = 386) | 0.54 (0.26–1.14) | 0.62 (0.32–1.20) | 0.60 (0.36–0.99) |
| Smoking status | |||
| Current (n = 129) | 1.00 | 1.00 | 1.00 |
| Former (n = 63) | 0.69 (0.22–2.11) | 0.20 (0.05–0.84) | 0.33 (0.13–0.85) |
| Never (n = 797) | 0.74 (0.40–1.34) | 0.53 (0.33–0.87) | 0.61 (0.41–0.90) |
| Age at first intercourse, years | |||
| ≤17 (n = 332) | 1.00 | 1.00 | 1.00 |
| 18-19 (n = 251) | 0.98 (0.58–1.66) | 0.86 (0.53–1.37) | 0.88 (0.61–1.28) |
| 20-22 (n = 206) | 0.68 (0.36–1.26) | 0.54 (0.30–0.98) | 0.63 (0.41–0.98) |
| ≥23 (n = 199) | 0.21 (0.08–0.61) | 0.27 (0.12–0.61) | 0.26 (0.14–0.49) |
| Lifetime number of sex partners | |||
| 1 (n = 515) | 1.00 | 1.00 | 1.00 |
| 2–3 (n = 286) | 1.58 (0.93–2.69) | 2.23 (1.34–3.71) | 1.85 (1.27–2.71) |
| ≥4 (n = 186) | 1.90 (1.08–3.34) | 3.52 (2.12–5.85) | 2.66 (1.80–3.92) |
| Number of new sex partners in last 6 months | |||
| 0 (n = 936) | 1.00 | 1.00 | 1.00 |
| 1 (n = 47) | 1.73 (0.79–3.79) | 1.96 (0.98–3.94) | 2.05 (1.20–3.51) |
| 2–3 (n = 2) | 6.37 (0.88–46.29) | 6.22 (0.85–45.23) | 3.47 (0.48–25.02) |
| ≥4 (n = 1) | N/A (N/A) | N/A (N/A) | N/A (N/A) |
| Number of prior pregnancies | |||
| 0 (n = 162) | 1.00 | 1.00 | 1.00 |
| 1–2 (n = 441) | 0.83 (0.45–1.52) | 1.49 (0.80–2.78) | 1.10 (0.70–1.73) |
| 3–4 (n = 300) | 0.83 (0.41–1.68) | 1.71 (0.87–3.38) | 1.22 (0.74–2.02) |
| 5+ (n = 86) | 0.96 (0.36–2.55) | 1.02 (0.35–2.94) | 0.95 (0.45–2.01) |
| Chlamydia/gonorrhea positive | |||
| No (n = 956) | 1.00 | 1.00 | 1.00 |
| Yes (n = 33) | 2.09 (0.84–5.18) | 2.95 (1.43–6.10) | 2.19 (1.15–4.16) |
“CI”: confidence interval; “HPV”: human papillomavirus; “N/A”: not applicable.
North America includes United States; Europe includes France, Germany, and Spain; Latin America includes Colombia; and Asia includes Philippines and Thailand.
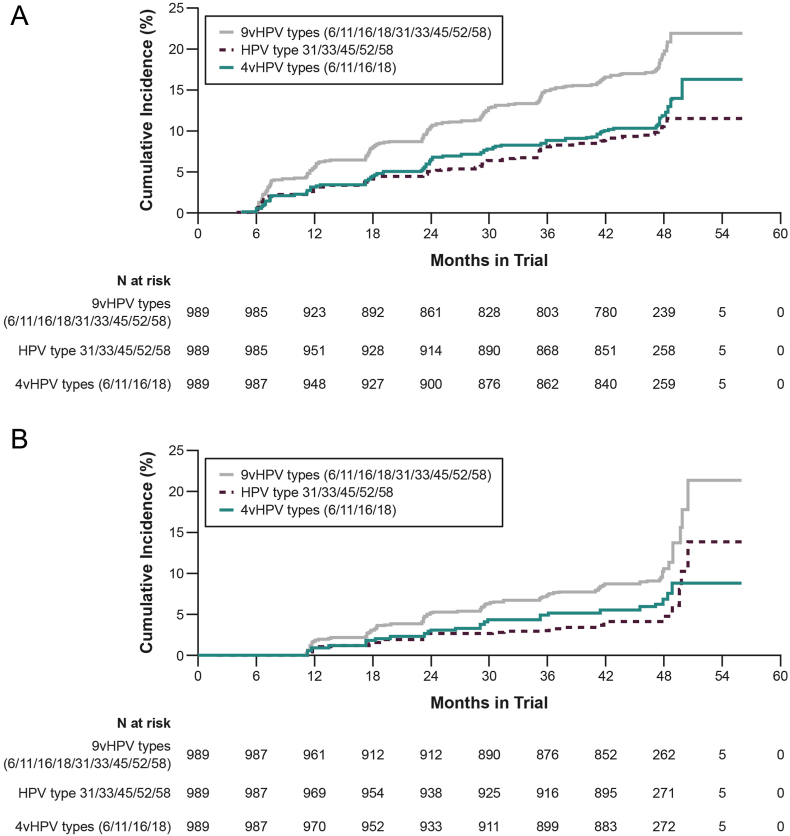
Among the HPV-naive placebo recipients, incidence (per 100 person-years) of anogenital infection was 5.22 (95% confidence interval [CI], 4.51–6.02) for any 9vHPV type, 3.07 (95% CI, 2.54–3.69) for any 4vHPV type, 2.72 (95% CI, 2.22–3.30) for HPV 31/33/45/52/58, and 4.72 (95% CI, 4.04–5.47) for non-vaccine types 35/39/51/56/59 (Table 3). Approximately half the incident infections persisted 6 or more months as follows: 54% (9vHPV types), 56% (4vHPV types), 46% (types 31/33/45/52/58), and 46% (non-vaccine types 35/39/51/56/59). Incidence was higher in younger women (aged 24–34 years) than in older women (aged 35–45 years) (Supplemental Table 3). For example, 9vHPV type incident infection rates (per 100 person-years) were 6.96 (95% CI, 5.76–8.32) in women aged 24–34 and 3.75 (95% CI, 2.94–4.72) in women aged 35–45. The 48-month cumulative incidence of anogenital infection was 19.4% (9vHPV types), 11.8% (4vHPV types) and 10.5% (HPV 31/33/45/52/58) (Fig. 1a), and for persistent infections, the cumulative incidence was 10.2% (9vHPV types), 6.8% (4vHPV types), and 4.4% (HPV 31/33/45/52/58) (Fig. 1b).
Table 3.
Anogenital HPV incident infection rate and incident-persistent infection rate in women aged 24 to 45 years.
| HPV type | HPV incident infection rate |
HPV incident-persistent infection rate |
||||
|---|---|---|---|---|---|---|
| N positive | Person-years | Incidence per 100 person-years (95% CI) | N positive | Person-years | Incident-persistent infection per 100 person-years (95% CI) | |
| 6 | 38 | 3765.6 | 1.01 (0.72–1.38) | 15 | 3802.9 | 0.39 (0.22–0.65) |
| 11 | 7 | 3809.9 | 0.18 (0.07–0.38) | 2 | 3817.5 | 0.05 (0.01–0.19) |
| 16 | 57 | 3702.7 | 1.54 (1.17–1.99) | 37 | 3755.5 | 0.99 (0.69–1.36) |
| 18 | 23 | 3773.1 | 0.61 (0.39–0.91) | 12 | 3797.6 | 0.32 (0.16–0.55) |
| 31 | 18 | 3792.2 | 0.47 (0.28–0.75) | 11 | 3804.7 | 0.29 (0.14–0.52) |
| 33 | 12 | 3798.5 | 0.32 (0.16–0.55) | 3 | 3814.6 | 0.08 (0.02–0.23) |
| 35 | 18 | 3789.9 | 0.47 (0.28–0.75) | 6 | 3810.9 | 0.16 (0.06–0.34) |
| 39 | 35 | 3753.6 | 0.93 (0.65–1.29) | 16 | 3789.7 | 0.42 (0.24–0.68) |
| 45 | 20 | 3787.2 | 0.53 (0.32–0.81) | 9 | 3808.0 | 0.24 (0.11–0.45) |
| 51 | 59 | 3719.4 | 1.59 (1.21–2.04) | 27 | 3777.0 | 0.71 (0.47–1.04) |
| 52 | 35 | 3755.3 | 0.93 (0.65–1.29) | 17 | 3790.6 | 0.45 (0.26–0.72) |
| 56 | 83 | 3679.9 | 2.26 (1.80–2.79) | 31 | 3763.3 | 0.82 (0.56–1.17) |
| 58 | 28 | 3769.2 | 0.74 (0.49–1.07) | 9 | 3802.2 | 0.24 (0.11–0.45) |
| 59 | 25 | 3780.2 | 0.66 (0.43–0.97) | 12 | 3800.0 | 0.32 (0.16–0.55) |
| 6/11/16/18 | 111 | 3611.4 | 3.07 (2.54–3.69) | 62 | 3720.4 | 1.67 (1.28–2.13) |
| 31/33/45/52/58 | 99 | 3636.5 | 2.72 (2.22–3.30) | 45 | 3744.4 | 1.20 (0.88–1.60) |
| 35/39/51/56/59 | 166 | 3517.1 | 4.72 (4.04–5.47) | 77 | 3687.0 | 2.09 (1.65–2.60) |
| 6/11/16/18/31/33/45/52/58 | 181 | 3464.7 | 5.22 (4.51–6.02) | 98 | 3656.8 | 2.68 (2.18–3.26) |
| 16/18/31/33/45/52/58/35/39/51/56/59 | 266 | 3287.2 | 8.09 (7.18–9.08) | 142 | 3571.4 | 3.98 (3.36–4.67) |
| 31/33/45/52/58 no 6/11/16/18 no 35/39/51/56/59 | 70 | 3680.8 | 1.90 (1.49–2.40) | 33 | 3760.6 | 0.88 (0.60–1.23) |
| 6/11/16/18 no 31/33/45/52/58 no 35/39/51/56/59 | 87 | 3653.1 | 2.38 (1.91–2.93) | 47 | 3742.6 | 1.26 (0.92–1.67) |
| 35/39/51/56/59 no 31/33/45/52/58 no 6/11/16/18 | 137 | 3577.2 | 3.83 (3.22–4.51) | 59 | 3723.3 | 1.58 (1.21–2.04) |
“CI”: confidence interval; “HPV”: human papillomavirus.
Fig. 1.
48-month cumulative incidence Kaplan–Meier curves among women aged 24 to 45 years for (A) incident infection and (B) incident-persistent infection.
“4vHPV”: quadrivalent human papillomavirus; “9vHPV”: 9-valent human papillomavirus.
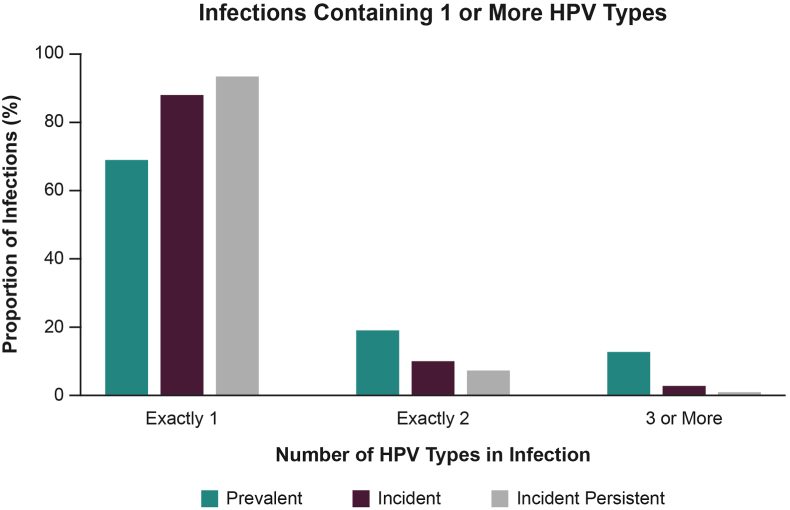
Only a single HPV type was found in most infections, irrespective of whether infections were prevalent at baseline or newly acquired during the trial (Fig. 2; Supplemental Table 4). Approximately 69% of infections that were prevalent at baseline contained a single HPV type, whereas an even greater proportion of single HPV-type infections were found in incident (88%) and incident-persistent (approximately 93%) infections.
Fig. 2.
Number of HPV types in anogenital infection among women ages 24 to 45 who had at least 1 of 12 measured high-risk HPV types.
“HPV”: human papillomavirus.
4. Discussion
Using data from a large clinical trial, this analysis shows that women aged 24 to 45 years (an age group rarely targeted for HPV immunization) are at risk for acquiring new HPV infections, including infections with HPV types targeted by the 9vHPV vaccine. In addition, these infections can persist for 6 or more months. Among women in the placebo group who were HPV-naive at baseline, new 9vHPV-type infections and incident-persistent infections were identified in 19.4% and 10.2% of the women, respectively, during the 48-month trial. Because some persistent infections can lead to CIN2+ and invasive cervical cancer, these findings suggest that infections acquired during adult years can be an important contributor to the cervical disease burden in adult women.
Cervical screening can detect CIN2+ and treatment of CIN2+ can prevent progression to invasive cervical cancer. In the United States as of 2016, approximately 81% of women 21–65 years old up to date with guidelines-recommended cervical screening [32]. Based on incidence rates of CIN2+ established from cervical screening in the United States, the CDC estimates that approximately 196,000 CIN2+ cases were diagnosed in the United States in 2016, of which 97,000 were attributable to 9vHPV types and were diagnosed in women aged 30 years or older [15,33]. The proportion of these CIN2+ cases that was caused by HPV infections acquired prior to age 27 is not known. However, rates of progression from infection to high-grade lesions are similar in young and adult women, and most infections clear or progress within 1 to 3 years [34,35], suggesting that infections acquired during the adult years can lead to high-grade lesions.
Our findings of the prevalence of HPV 6/11/16/18 at baseline are consistent with age-specific prevalence data from a meta-analysis that showed considerable levels of infection with high-risk HPV types among women aged 25 years and older [23]. The meta-analysis also noted second peaks among women aged ≥45 years in Central and South America, southern Asia, and southern Europe. Although our trial also included women from these regions, prevalence data are available only through age 45, raising the question of whether a wider age range would have revealed second peaks similar to those observed in other studies. With respect to baseline infection with the five additional HPV types targeted by the 9vHPV vaccine (31/33/42/52/58), the prevalence of these infections in adult women was equal to, or slightly greater than, the prevalence of 4vHPV in all study regions.
Our data also showed that the prevalence of infection with non-vaccine HPV types 35/39/51/56/59 was higher than the prevalence of 4vHPV types and types 31/33/45/52/58. Previous reports have shown that the prevalence of HPV types 35/39/51/56/59 among women aged 15 to 26 years was 19%, similar to the 17% we observed in women aged 24 to 34 years [36]. However, although these non-vaccine types contribute substantially to the burden of infection, they are not as prevalent in CIN2, CIN3, AIS, and invasive cervical cancer [36,37]. In fact, less than 7% of cervical cancers worldwide are attributed to HPV types 35/39/51/56/59 [37].
Our findings suggest that factors associated with HPV infection in mid-adult women are similar to those in younger women, such as younger age, younger age at first intercourse, being single, current use of tobacco, and higher number of past and recent sex partners [[38], [39], [40]]. Many of the point estimates relating sexual behavior characteristics to incident HPV were not statistically significant, likely owing to the small numbers of incident cases in some strata. Nonetheless, the magnitude of the point estimates associated with these characteristics were often large, a finding that suggests sexual behaviors continue to confer risk of HPV infections among women beyond the age range that is typically targeted in HPV immunization programs.
Several methodologic considerations warrant attention. Women in this trial were enrolled from primary care settings; more than 50% of the cohort had only one lifetime sex partner and more than 80% had three or fewer lifetime sex partners. The cohort is therefore not representative of women with a higher number of lifetime and recent sexual partners and less access to health care, groups that would be expected to have an even higher prevalence and incidence of HPV infection. As such, the data presented here may be biased to a lower prevalence and rate of new incident and persistent anogenital HPV infections among mid-adult-aged women. Although the trial cohort was followed for 48 months, analyses of persistent infections were based on a subset of data from generally HPV-naive women in the placebo arm of the trial who developed incident infections. The limited number of at-risk women in these analyses resulted in a relatively small number of persistent infections. In addition, the HPV-naive group was likely at lower risk than women who had evidence of a current or prior HPV infection at baseline.
This report also has several notable strengths. It addresses IARC's call for data on the natural history of HPV infection among older women, including data on persistent infection [22]. Results are drawn from a large, “average-risk” cohort of geographically diverse women. The size and global nature of the clinical trial from which data are drawn offer new insight, not only on regional differences in prevalence of high-risk HPV types, but also on how these infections are differentially distributed between women aged 24 to 34 years and those aged 35 to 45 years. The trial included repeated measures of HPV infection every 6 months over a period of 4 years, providing a robust dataset for this analysis. These data also provide insight on infection prevalence and incidence of five HPV types that are not currently included in any licensed vaccine. Although these non-vaccine HPV types had prevalence and incidence estimates similar to or higher than those of vaccine types, their combined relative contribution to high-grade cervical lesions and cervical cancers is considerably lower than the seven high-risk types targeted by the 9vHPV vaccine [12,36,41].
Emerging real-world evidence demonstrates the benefit of HPV vaccination strategies that target multiple age cohorts and achieve high vaccine uptake [21]. In locations where vaccination strategies have included catch-up cohorts, such as older teens and young women, declines in the prevalence of vaccine-type HPV infection, anogenital warts, and CIN2+ lesions have been observed [21]. This real-world evidence is based on more than 8 years of follow-up in vaccinated women in developed countries. As it takes decades for cervical cancer to develop, the population-level impact of HPV vaccination on CIN2+ is the most proximal outcome to cervical cancer, and is recognized as a valid proxy for vaccine effectiveness against cervical cancer by regulatory agencies worldwide [7,[42], [43], [44]]. However, vaccine impact and effectiveness data are not yet available for women who were vaccinated during their adult years. Given the favorable efficacy and immunobridging data from the 4vHPV and 9vHPV vaccine clinical programs, real-world impact and effectiveness in adult women can be expected as vaccine uptake in this age group increases and when sufficient follow up time is available.
5. Conclusions
Our findings suggest that women who are older than the age typically targeted by HPV vaccination programs are at risk for incident and incident-persistent HPV anogenital infections, depending on sexual behavior. Although most new HPV infections clear, some persist and can lead to cervical precancer and cancer if left untreated. In addition, most infections in this low-risk group of women are single-type HPV infections, suggesting that a sizable proportion of adult women remain susceptible to infections with other HPV types to which they may not have yet been exposed. Our data highlight the need for primary prevention of HPV infection in at-risk adult women. This information can inform development or modification of HPV immunization programs and guidelines in a manner that is tailored to the needs of adult women, for whom few such guidelines currently exist.
CRediT authorship contribution statement
Daron G. Ferris: Writing - review & editing. Darron R. Brown: Writing - review & editing. Anna R. Giuliano: Writing - review & editing. Evan Myers: Conceptualization, Formal analysis, Writing - review & editing. Elmar A. Joura: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Suzanne M. Garland: Conceptualization, Writing - review & editing. Susanne K. Kjaer: Writing - review & editing. Gonzalo Perez: Investigation, Writing - review & editing. Alfred Saah: Conceptualization, Methodology, Investigation, Supervision, Writing - review & editing. Alain Luxembourg: Writing - review & editing. Christine Velicer: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of competing interest
D.G.F. reports grant support from Merck & Co., Inc. (Kenilworth, NJ) through his institution and personal fees for consultancy and advisory boards for Merck & Co., Inc.; D.R.B. reports grant support from Merck & Co., Inc. and a portion of funds from a confidential agreement with his institution and Merck & Co., Inc. as income; A.R.G. reports grant support from and is an advisory board member for Merck & Co., Inc; E.M. reports personal fees from Merck & Co., Inc.; E.A.J. reports grant support and personal fees from Merck & Co., Inc.; S.M.G. reports grant support and personal fees from and is a global advisory board member for Merck & Co., Inc.; S.K.K. reports grant support and personal fees from Merck & Co., Inc.; A.S., A.L., and C.V. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and hold shares in Merck & Co., Inc., Kenilworth, NJ, USA. G.P. previously was an employee of and is currently a paid consultant for Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, for the development of this manuscript.
Acknowledgements
The authors would like to thank Brady Dubin, Kathy Harkins, Se Li, Mary Ann Rutkowski, Weifeng Xu, and Xingshu Zhu for their logistical and statistical programming support. Medical writing assistance was provided by ApotheCom (New York, NY, USA) and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2020.100202.
Contributor Information
Daron G. Ferris, Email: dferris@augusta.edu.
Darron R. Brown, Email: darbrow@iupui.edu.
Anna R. Giuliano, Email: Anna.Giuliano@moffitt.org.
Evan Myers, Email: evan.myers@duke.edu.
Elmar A. Joura, Email: elmar.joura@gmail.com.
Suzanne M. Garland, Email: Suzanne.Garland@thewomens.org.au.
Susanne K. Kjaer, Email: susanne@cancer.dk.
Gonzalo Perez, Email: gonzalopamaya@gmail.com.
Alfred Saah, Email: alfred_saah@merck.com.
Alain Luxembourg, Email: alain_luxembourg@merck.com.
Christine Velicer, Email: christine_velicer@merck.com.
Funding sources
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. This study was designed, managed, and analyzed jointly by authors employed by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. and external authors who were not paid for their work. Medical writing assistance was provided by ApotheCom (New York, NY, USA) and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization Human papillomavirus (HPV) and cervical cancer [fact sheet] January 24, 2019. https://www.who.int/en/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer Available from:
- 2.Plummer M., de Martel C., Vignat J., Ferlay J., Bray F., Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob. Health. 2016;4:e609–616. doi: 10.1016/s2214-109x(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 3.de Sanjose S., Alemany L., Ordi J., Tous S., Alejo M., Bigby S.M. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur. J. Cancer. 2013;49:3450–3461. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Ndiaye C., Mena M., Alemany L., Arbyn M., Castellsague X., Laporte L. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/s1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 5.Mehanna H., Beech T., Nicholson T., El-Hariry I., McConkey C., Paleri V. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 6.Luxembourg A., Moeller E. 9-Valent human papillomavirus vaccine: a review of the clinical development program. Expert Rev. Vaccines. 2017;16:1119–1139. doi: 10.1080/14760584.2017.1383158. [DOI] [PubMed] [Google Scholar]
- 7.Gardasil 9 [prescribing information] Merck Sharp & Dohme Corp.; Whitehouse Station, NJ: October 2018. [Google Scholar]
- 8.Meites E., Szilagyi P.G., Chesson H.W., Unger E.R., Romero J.R., Markowitz L.E. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019;68:698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garland S.M., Steben M., Sings H.L., James M., Lu S., Railkar R. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 10.Novakovic D., Cheng A.T.L., Zurynski Y., Booy R., Walker P.J., Berkowitz R. A prospective study of the incidence of juvenile onset recurrent respiratory papillomatosis after implementation of a National HPV Vaccination Program. J. Infect. Dis. 2018;217:208–212. doi: 10.1093/infdis/jix498. [DOI] [PubMed] [Google Scholar]
- 11.de Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Sanjose S., Serrano B., Tous S., Alejo M., Lloveras B., Quiros B. Burden of Human Papillomavirus (HPV)-related Cancers Attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectrum. 2018;2:pky045. doi: 10.1093/jncics/pky045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano B., de Sanjose S., Tous S., Quiros B., Munoz N., Bosch X. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur. J. Cancer. 2015;51:1732–1741. doi: 10.1016/j.ejca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Joura E.A., Pils S. Vaccines against human papillomavirus infections: protection against cancer, genital warts or both? Clin. Microbiol. Infect. 2016;22(Suppl 5):S125–S127. doi: 10.1016/j.cmi.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 15.McClung N.M., Gargano J.W., Park I.U., Whitney E., Abdullah N., Ehlers S. Estimated number of cases of high-grade cervical lesions diagnosed among women—United States, 2008 and 2016. MMWR Morb. Mortal. Wkly. Rep. 2019;68:337–343. doi: 10.15585/mmwr.mm6815a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Immunization schedules: table 1. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States. 2019. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html#note-hpv Updated February 5, 2019. Available from:
- 17.Australian Government Department of Health Clinical update: National Immunisation Program (NIP) childhood schedule changes from 1 July 2018. July 2018. https://www.health.gov.au/news/clinical-update-national-immunisation-program-nip-childhood-schedule-changes-from-1-july-2018 Available from:
- 18.Koch Institut Robert. Vaccination recommendations by STIKO. 2019. https://www.rki.de/EN/Content/infections/Vaccination/recommandations/recommendations_node.html Available from:
- 19.Sozialministerium Impfplan österreich [in German] 2019. https://www.sozialministerium.at/site/Gesundheit/Krankheiten_und_Impfen/Impfen/Oesterreichischer_Impfplan_2019 Available from:
- 20.Garland S.M., Kjaer S.K., Munoz N., Block S.L., Brown D.R., DiNubile M.J. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin. Infect. Dis. 2016;63:519–527. doi: 10.1093/cid/ciw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drolet M., Benard E., Perez N., Brisson M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394:497–509. doi: 10.1016/s0140-6736(19)30298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . Vol. 90. 2007. IARC monographs on the evaluation of carcinogenic risks to humans.https://www.ncbi.nlm.nih.gov/books/NBK321760/ Available from: [Google Scholar]
- 23.Bruni L., Diaz M., Castellsague X., Ferrer E., Bosch F.X., de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010;202:1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 24.Zhao F.H., Lewkowitz A.K., Hu S.Y., Chen F., Li L.Y., Zhang Q.M. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int. J. Cancer. 2012;131:2929–2938. doi: 10.1002/ijc.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith J.S., Melendy A., Rana R.K., Pimenta J.M. Age-specific prevalence of infection with human papillomavirus in females: a global review. J. Adolesc. Health. 2008;43:S5.E1–S5.E62. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Lazcano-Ponce E., Herrero R., Munoz N., Cruz A., Shah K.V., Alonso P. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int. J. Cancer. 2001;91:412–420. doi: 10.1002/1097-0215(20010201)91:3<412::AID-IJC1071>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.Herrero R., Castle P.E., Schiffman M., Bratti M.C., Hildesheim A., Morales J. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J. Infect. Dis. 2005;191:1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 28.Trottier H., Franco E.L. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–S15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 29.Munoz N., Manalastas R., Jr., Pitisuttithum P., Tresukosol D., Monsonego J., Ault K. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 30.Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Ault K.A., Giuliano A.R. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 31.Mao C., Koutsky L.A., Ault K.A., Wheeler C.M., Brown D.R., Wiley D.J. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet. Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 32.Watson M., Soman A., Flagg E.W., Unger E., Deapen D., Chen V.W. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009–2012. Prev. Med. 2017;103:60–65. doi: 10.1016/j.ypmed.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson M., Benard V., King J., Crawford A., Saraiya M. National assessment of HPV and Pap tests: changes in cervical cancer screening, national health interview survey. Prev. Med. 2017;100:243–247. doi: 10.1016/j.ypmed.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaisamrarn U., Castellsague X., Garland S.M., Naud P., Palmroth J., Del Rosario-Raymundo M.R. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PloS One. 2013;8 doi: 10.1371/journal.pone.0079260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skinner S.R., Wheeler C.M., Romanowski B., Castellsague X., Lazcano-Ponce E., Del Rosario-Raymundo M.R. Progression of HPV infection to detectable cervical lesions or clearance in adult women: analysis of the control arm of the VIVIANE study. Int. J. Cancer. 2016;138:2428–2438. doi: 10.1002/ijc.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joura E.A., Ault K.A., Bosch F.X., Brown D., Cuzick J., Ferris D. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol. Biomarkers Prev. 2014;23:1997–2008. doi: 10.1158/1055-9965.Epi-14-0410. [DOI] [PubMed] [Google Scholar]
- 37.de Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 38.Franco E.L., Villa L.L., Sobrinho J.P., Prado J.M., Rousseau M.C., Desy M. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J. Infect. Dis. 1999;180:1415–1423. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 39.Ho G.Y., Bierman R., Beardsley L., Chang C.J., Burk R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998;338:423–428. doi: 10.1056/nejm199802123380703. [DOI] [PubMed] [Google Scholar]
- 40.Sellors J.W., Karwalajtys T.L., Kaczorowski J., Mahony J.B., Lytwyn A., Chong S. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168:421–425. [PMC free article] [PubMed] [Google Scholar]
- 41.Saraiya M., Unger E.R., Thompson T.D., Lynch C.F., Hernandez B.Y., Lyu C.W. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J. Natl. Cancer Inst. 2015;107:djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagliusi S.R., Teresa Aguado M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine. 2004;23:569–578. doi: 10.1016/j.vaccine.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 43.Statement on human papillomavirus vaccine. An advisory committee statement (ACS) Can. Commun. Dis. Rep. 2007;33:1–31. [PubMed] [Google Scholar]
- 44.European Center for Disease Prevention and Control Guidance for the introdution of HPV vacines in EU countries, Published January 2008. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0801_GUI_Introduction_of_HPV_Vaccines_in_EU.pdf Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.