Abstract
The clinicians usually prescribe antibiotics to reduce post-operative complications during third molar surgeries. However, in the absence of clear conclusions regarding the use of antibiotics in third molar surgeries, present systematic review was planned to assess the quality of systematic reviews evaluating the efficiency of antibiotics in reducing post-operative complications. The literature search was done in Cochrane Library, Cochrane Central Register of Controlled Trials (CENTRAL), PUBMED, EMBASE, and Google scholar. Systematic reviews published in English during the period from January 1990 to December 2019 were included. The maxillary and mandibular third molars indicated for extraction either because of infection, orthodontic or prophylactic reasons were included. From 526 screened studies, thirteen reviews were qualified for qualitative analysis. The qualities of the included reviews were evaluated using the AMSTAR 2 tool. The included reviews were also evaluated based on the number of authors, geographic region, impact factor of the published journal, year of publication, and the number of citations for each review. One high quality, eight moderate quality, three low quality, and one critically low-quality reviews were observed in the present review. No statistically significant difference was observed between the included reviews based upon the analysis of the number of authors, geographic region, impact factor of the published journal, year of publication, and the number of citations for each review. Considering the observations form the high and moderate-quality reviews, the present systematic review concludes that antibiotics effectively aid in reducing the post-operative complications and frequency of observation of dry socket.
Keywords: Antibiotics, Third molar, Impacted tooth, Dry socket, Post-operative complications, Alveolar osteitis
1. Introduction
Third molar extraction is one of the most common minor oral surgical procedures in routine clinical practice. Though the incidence of complications is rare during third molar extraction, infection of the extraction site and dry socket are the common post-operative problems encountered.1 Systemic antibiotics are routinely used to treat the fore mentioned complications after third molar extractions.2 However, the use of systemic antibiotics after third molar extractions is still a controversial topic.3 Prophylactic antibiotic therapy during third molar surgery in healthy individuals may trigger adverse drug reactions or develop antibiotic resistance which raises the controversy on the use of antibiotics after third molar surgery.4 Though the prescription of systemic antibiotics was not advised from the past evidence-based reports,3 they are being prescribed as a routine treatment protocol until today.5,6
In this evidence-based era, systematic reviews (SR) and meta-analysis (MA) provide the highest level of evidence with conclusive results for any subject. SRs employ strenuous methodology in searching and collecting information from different publication sources, and further summarizes the research question by critically appraising the included studies.7 MAs is the quantitative analysis of pooled data of similar studies addressing the same research question statistically.7 Eventually, SRs and MAs aid in developing evidence-based conclusions for the questions of the uncertainty of the literature.
The importance of SRs was identified and their publication in health care research has increased in recent times in all fields of medicine. However, SRs are subjected to multiple sources of bias, if proper methodological steps were not followed.8 An SR and MA of poor quality can mislead the practitioners and can affect the proper clinical care of the patients.9 Thus, assessing the methodological quality of SRs and MAs is quite important in health care research. The Overview Quality Assessment Questionnaire (OQAQ) was developed as a validating tool for measuring the quality of SRs.10 Further modifications were done in the assessment questions and a new Measurement Tool to Assess Systematic Reviews (AMSTAR) was developed, which consists of eleven questions for SR analysis of randomized controlled specifically.11 Recently, AMSTAR 2 was developed to evaluate the SRs of both randomized controlled trials and non-randomized controlled trials of health care research.12 Considering the lacunae in the use of prophylactic antibiotics during third molar surgery, systematic reviews evaluating the efficacy of antibiotics during third molar surgery were analyzed using AMSTAR 2 tool in the present review.
2. Methodology
The protocol for the present review was registered in OSF Registries (A scholarly repository built for creating and aggregating registrations of research) and the review protocol was under review process in the ‘International Prospective Register of Systematic Reviews' (PROSPERO) with a reference number “199090". The research question of the present review was “to evaluate the methodological quality of systematic reviews and meta-analysis conducted on the use of antibiotics to reduce postoperative complications (pain, fever, swelling, trismus, and surgical site or wound infection) during third molar surgeries.” (Table 1).
Table 1.
Explanation of the research question according to PICOS.
| S. No | Question | Explanation |
|---|---|---|
| 1 | Population (P) | Patients undergoing the third molar extraction |
| 2 | Intervention (I) | Antibiotic prophylaxis prescribed during the third molar extraction |
| 3 | Comparison (C) | Antibiotics verses placebo during third molar extraction |
| 4 | Outcomes (O) | Post-operative complications (pain, fever, swelling, trismus, and surgical site or wound infection), Dry socket, and Adverse Events |
| 5 | Study design (S) | Systematic reviews |
2.1. Types of studies, participants, and interventions
Systematic reviews published in English from January 1990 to December 2019 were included in the present review. The references of the included trials were checked further to find any relevant articles to the present research question. The studies satisfying the following inclusion criteria were included: The systematic review evaluating the use of antibiotics during third molar surgeries to reduce post-operative complications; Systematic reviews evaluating the prescription of antibiotics through oral or parenteral routes to reduce pain during third molar surgeries; Systematic reviews evaluating only randomized controlled trials to check the effectiveness of antibiotic use during third molar surgeries. The exclusion criteria for rejecting the studies in the systematic review were: The systematic reviews evaluating the use of antibiotics during the extraction of teeth other than third molars; Systematic reviews evaluating the prospective trials for evaluating the antibiotic use for third molar extraction; Literature or descriptive reviews.
2.2. Type of outcome measures
The primary outcome evaluated in the present review was the reduction of postoperative complications (pain, fever, swelling, trismus, and surgical site or wound infection) by using antibiotics during third molar surgeries. The secondary outcomes analyzed in the present review were the development of dry socket and development of adverse reactions after third molar extraction.
2.3. Information sources and search strategy
The electronic search for systematic reviews evaluating the efficacy of pre/post-operative antibiotics in reducing the postoperative complications after third molar surgeries were done in The Cochrane Library, The Cochrane Central Register of Controlled Trials (CENTRAL), PUBMED, EMBASE and Google scholar. The following search strategy was used for PUBMED: “Antibiotics” (MESH Term) AND Third molar (MESH Term); “Antibiotics” (MESH Term) AND Third molar surgery” (All fields); “Antibiotics” (MESH Term) AND Third molar impaction” (All fields); “Antibiotics” (MESH Term) AND Third molar (MESH Term) AND “Postoperative complications” (MESH Term); “Antibiotics” (MESH Term) AND Third molar (MESH Term) AND “Dry socket” (MESH Term).
2.4. Study collection and data extraction
Titles and Abstract of all the identified studies were read by two authors independently and the relevant articles were selected. Full-text articles of selected studies were obtained and read by two reviewers to check whether the included reviews met the inclusion criteria or not. Any disagreement regarding the selection and inclusion of the reviews was finalized by the decision of the third reviewer.
The data extraction from the included reviews was done by two authors individually and a structured data extraction form was developed to collect the relevant information from the selected reviews. The following data were extracted from the included reviews: Bibliographic data (Number of authors, country of the corresponding author, year of publication, number of citations for the included review and impact factor of the journal), number of randomized controlled trials of each included systematic review, outcomes assessed in each systematic review and mode of drug delivery in each systematic review.
2.5. Quality assessment – risk of bias
The included systematic reviews were analyzed using the AMSTAR (A MeaSurement Tool to Assess systematic Reviews) 2 tool published in 2017.12 The AMSTAR 2 contains a total of sixteen questions (7 critical and 9 non-critical) and the responses were recorded as ‘Yes’ or ‘Partial Yes’ or ‘No’ or ‘No Meta-analysis conducted’.12 Two review authors filled the sixteen questions for each included systematic review digitally using the link “https://amstar.ca/Amstar_Checklist.php” and the quality grade generated was recorded separately. Any disagreement regarding the scoring was again resolved with the third reviewer.
3. Data analysis
The characteristics of each included systematic review were summarized using the descriptive analysis. The analysis of response to each question in the AMSTAR 2 tool was tabulated and was graded according to the scores. The methodological quality of the included reviews was also analyzed based upon the number of authors for each review, geographic region, impact factor of the published journal, publication year, and number citations for each review using the One Way ANOVA test. The inter-observer rating was analyzed using Cohen's Kappa coefficient. SPSS Version 21 was used for the analysis and a P value of less than 0.05 was considered significant.
4. Results
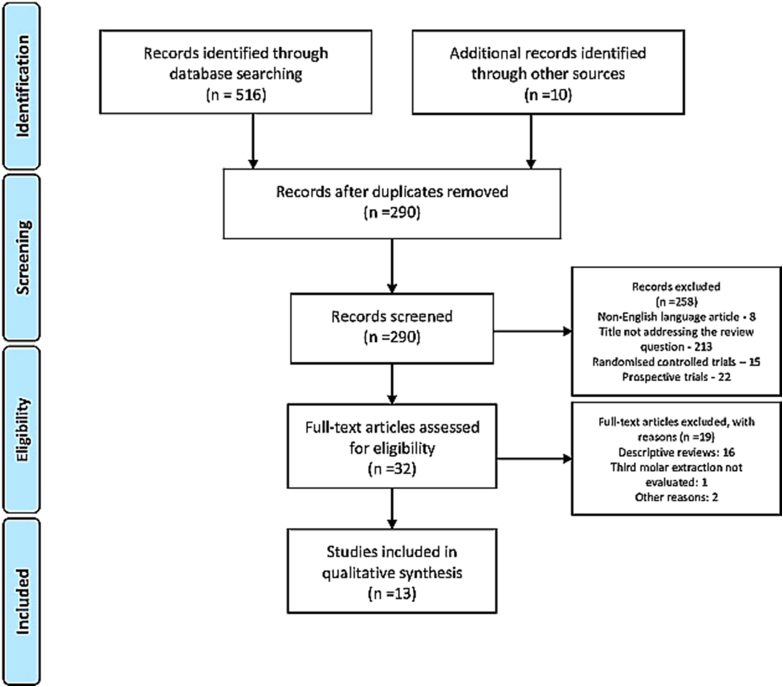
A total of 516 articles were searched form the databases and additional ten articles were found by hand searching. After removal of the duplicates, non-English language articles, articles other than systematic reviews, and articles not specific to the research question (494 articles) were excluded and 32 articles were assessed for full-text review. A total of 19 reviews were excluded (descriptive reviews and other reasons) and 13 articles13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 were included for qualitative analysis in the present review (Fig. 1). A total of 252 randomized controlled trials were analyzed form thirteen systematic reviews in the present review. Regarding the language restrictions in the included trials five reviews14, 15, 16, 22, 25 were completely restricted to the English language only, four reviews used English and other languages13,19,20,23 and four reviews17,18,21,24 were not restricted to the English language.
Fig. 1.
PRISMA flow diagram of the included trials.
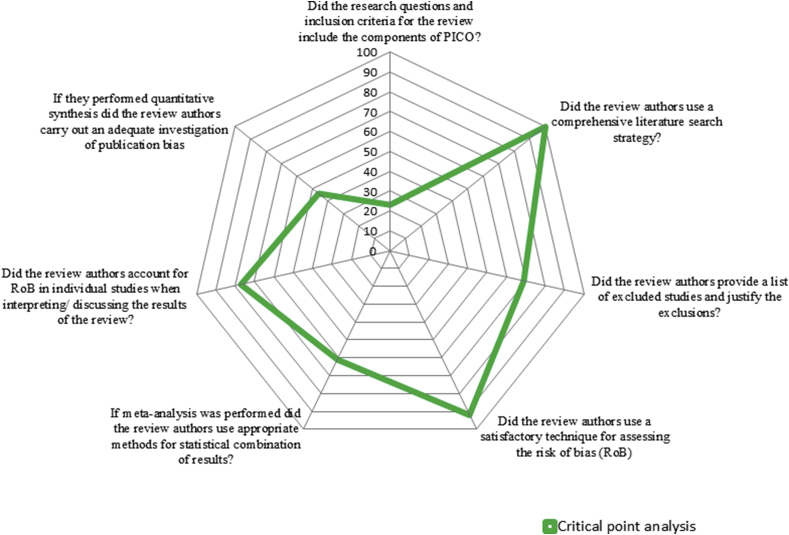
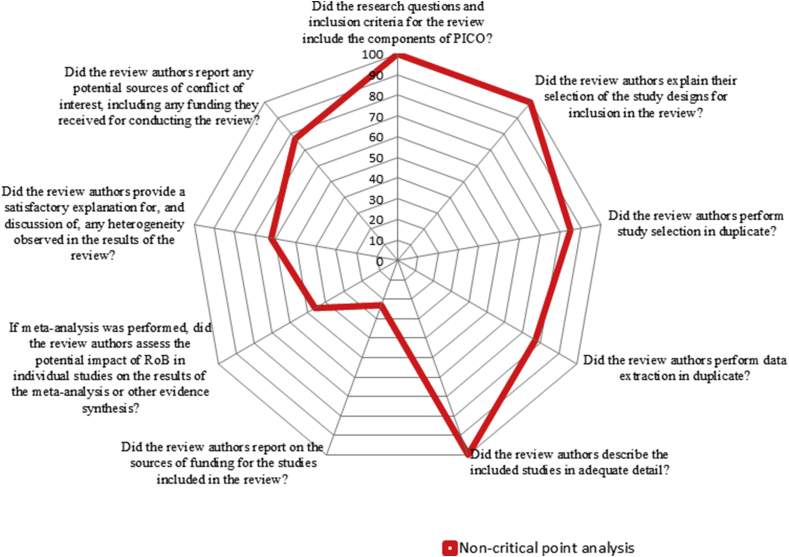
Postoperative complications (pain, fever, swelling, trismus, and surgical site or wound infection) as an outcome measure were assessed in twelve reviews,13, 14, 15, 16, 17, 18, 19, 20, 21, 22,24,25 dry sockets as a post-operative complication was assessed in six articles,17,18,20,21,23,24and evaluation of adverse events post-operatively as an outcome measure was assessed in five reviews.15,17, 18, 19,21Regarding the mode of drug delivery, the oral route of drug delivery was evaluated in five reviews,15, 16, 17,19,23whereas oral route along with the parenteral route was evaluated in eight reviews.13,14,18,20, 21, 22,24,25 Nine reviews15, 16, 17, 18, 19, 20, 21,24,25have done the meta-analysis for included randomized trials and four reviews13,14,22,23were only systematic reviews (Table 2). The percentage of responses to the AMSTAR 2 questions for all the included reviews were presented in Table 3. The first question (Did the research questions and inclusion criteria for the review include the components of Population (P), Intervention (I), Control (C) and Outcome (O) ?) and the third question (Did the review authors explain their selection of the study designs for inclusion in the review?) are answered positively in all the included reviews (Fig. 2, Fig. 3).
Table 2.
Study characteristics of the included systematic reviews and meta-analysis.
| S. No | Author | Year of publishing | Language restrictions | Number of RCTs included | Number of databases included | Outcomes assessed | Mode of drug delivery | Meta-analysis conducted |
|---|---|---|---|---|---|---|---|---|
| 1. | Blatt S et al.13 | 2019 | English or German | 80 | 2 | Surgical site infection | Intramuscular, intravenous, oral. | No |
| 2. | Cervino G. et al.14 | 2019 | Only English | 12 | 3 | Pain, swelling, fever, edema, reduced mouth opening, or postoperative surgical site infection | Oral Intra-muscular |
No |
| 3. | Gill AS et al.15 | 2018 | Only English | 4 | 6 | Post-operative infections Adverse events due to antibiotics |
Oral | Yes |
| 4. | Menon RK et al.16 | 2018 | English | 8 | 4 | Post-operative complications | Oral | Yes |
| 5. | Arteagoitia MI et al.17 | 2016 | No language restrictions | 10 | 10 | Incidence of dry socket and/or postoperative infections; Adverse events | Oral | Yes |
| 6. | Ramos E et al.18 | 2016 | No language restrictions | 21 | 10 | Prevention of dry socket and postoperative infection; Adverse events | Oral and parenteral routes | Yes |
| 7 | Isiordia-Espinoza MA et al.19 | 2015 | English or Spanish | 5 | 6 | Surgical wound infection and the adverse effects of amoxicillin | Oral | Yes |
| 8. | Marcussen KB et al.20 | 2015 | English, French, German, Danish, Swedish, Norwegian and Spanish | 10 | 3 | Surgical site infection and/or alveolar osteitis | per-orally, intravenously, intramuscularly or topically |
Yes |
| 9. | Lodi G et al.21 | 2012 | No language restrictions | 18 | 5 | Postoperative complications (Pain, swelling, fever, and trismus), Dry socket, and Local site of infection | Oral, Intra-muscular, Intra-venous | Yes |
| 10. | Oomens MAE et al.22 | 2012 | Only English | 23 | 3 | Post-operative complications | Intravenous and Oral | No |
| 11. | Hedström L et al.23 | 2007 | English, French, German, or any of the Nordic languages |
32 | 2 | Dry socket, alveolar osteitis, alveolitis sicca dolorosa, fibrinolytic alveolitis, and localized osteitis | Oral | No |
| 12. | Ren YF et al.24 | 2007 | No language restrictions | 20 | 3 | Alveolar osteitis and wound infection | Intramuscular, intravenous, oral. | Yes |
| 13 | Schwartz AB et al.25 | 2007 | Only English | 9 | 5 | Prevention of postoperative adverse outcomes and postoperative complications. | Oral and Intravenous | No |
Table 3.
Percentage of responses to each question in AMSTAR 2 tool.
| S. No | Question | Yes n (%) | Partial Yes n (%) | No n (%) | Meta-analysis was not conducted n (%) |
|---|---|---|---|---|---|
| 1 | Did the research questions and inclusion criteria for the review include the components of PICO? | 13 (100) | – | – | – |
| 2 | Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? | 2 (15) | 1 (8) | 10 (77) | – |
| 3 | Did the review authors explain their selection of the study designs for inclusion in the review? | 13 (100) | – | – | – |
| 4 | Did the review authors use a comprehensive literature search strategy? | 11 (85) | 2 (15) | – | – |
| 5 | Did the review authors perform study selection in duplicate? | 11 (85) | – | 2 (15) | – |
| 6 | Did the review authors perform data extraction in duplicate? | 10 (77) | – | 3 (23) | – |
| 7 | Did the review authors provide a list of excluded studies and justify the exclusions? | 9 (69) | – | 4 (31) | – |
| 8 | Did the review authors describe the included studies in adequate detail? | 8 (62) | 5 (38) | – | – |
| 9 | Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? | 9 (69) | 3 (23) | 1 (8) | – |
| 10 | Did the review authors report on the sources of funding for the studies included in the review? | 3 (23) | – | 10 (77) | – |
| 11 | If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results? | 8 (62) | – | – | 5 (38) |
| 12 | If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | 6 (46) | 2 (15) | 5 (38) | – |
| 13 | Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? | 10 (77) | – | 3 (23) | – |
| 14 | Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | 8 (62) | – | 5 (38) | – |
| 15 | If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? | 6 (46) | – | 2 (15) | 5 (38) |
| 16 | Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | 10 (77) | – | 3 (23) | – |
Fig. 2.
Percentage scores of critical points.
Fig. 3.
Percentage scores of non-critical points.
The responses to AMSTAR 2 tool questions were scored by the two different reviewers and an inter-observer agreement was 0.92 which shows a high-quality correlation. The disagreement in the responses was resolved and finalized by the third reviewer. Overall grade for each study was calculated based upon response to the total sixteen questions of the AMSTAR 2 tool (Table 4). One high grade,21 eight moderate grades,13,15,17,18,22, 23, 24, 25 three low grade,16,19,20 and one critically low grade13 were observed from the included systematic reviews by AMSTAR 2 tool (Table 5). The name of the journals and their corresponding impact factors (IF) and their mean values are presented in Table 5. The overall confidence of the methodological quality of the included systematic reviews can be stated as ‘moderate’ as eight (62%)13,14,18,20, 21, 22,24,25 of the thirteen included reviews are rated as ‘moderate’ according to the AMSTAR 2 tool. The descriptive statistics of the primary and secondary outcomes for high and moderate-quality studies in the present review were presented in Table 6.
Table 4.
Assessment of each included systematic review using AMSTAR 2 tool.
| S. No | Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Overall Grade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Blatt S et al.13 | Yes | No | Yes | Partial Yes | No | No | No | Partial Yes | Yes | No | Meta-analysis was not conducted | Meta-analysis was not conducted | Yes | No | Meta-analysis was not conducted | Yes | Moderate |
| 2 | Cervino G. et al.14 | Yes | PY | Yes | Partial Yes | Yes | No | No | Partial Yes | Partial Yes | No | Meta-analysis was not conducted | Meta-analysis was not conducted | No | No | Meta-analysis was not conducted | Yes | Critically Low |
| 3 | Gill AS et al.15 | Yes | No | Yes | Partial Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Moderate |
| 4 | Menon RK et al.16 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Low |
| 5 | Arteagoitia MI et al.17 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 6 | Ramos E et al.18 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 7 | Isiordia-Espinoza MA et al.19 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | Low |
| 8 | Marcussen KB et al.20 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Partial Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Low |
| 9 | Lodi G et al.21 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| 10 | Oomens MAE et al.22 | Yes | No | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Meta-analysis was not conducted | Meta-analysis was not conducted | Yes | No | Meta-analysis was not conducted | No | Moderate |
| 11 | Hedström L et al.23 | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Partial Yes | No | Meta-analysis was not conducted | Meta-analysis was not conducted | Yes | No | Meta-analysis was not conducted | No | Moderate |
| 12 | Ren YF et al.24 | Yes | No | Yes | Yes | Yes | Yes | Yes | Partial Yes | Partial Yes | No | Yes | Yes | Yes | Yes | Yes | No | Moderate |
| 13 | Schwartz AB et al.25 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | No | Meta-analysis was not conducted | Meta-analysis was not conducted | Yes | No | Meta-analysis was not conducted | Yes | Moderate |
Table 5.
Description of included articles according to the quality GRADE (High quality to Very low quality).
| S. No | GRADE | Author details | Number of authors | Journal | Impact factor | Year of publishing |
|---|---|---|---|---|---|---|
| 1 | High | Lodi G et al.21 | 6 | The Cochrane Library | 6.24 | 2012 |
| 2 | Moderate | Blatt S et al.13 | 2 | Infection | 2.92 | 2019 |
| Gill AS et al.15 | 3 | Medicina | 1.42 | 2018 | ||
| Arteagoitia MI et al.17 | 5 | Medicina oral, patología oral y cirugía bucal | 1.07 | 2016 | ||
| Ramos E et al.18 | 5 | Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology |
1.49 | 2016 | ||
| Oomens MAE et al.22 | 2 | Oral Surg Oral Med Oral Pathol Oral Radiol | 1.49 | 2012 | ||
| Hedström L et al.23 | 2 | Oral Surg Oral Med Oral Pathol Oral Radiol Endod | 1.49 | 2007 | ||
| Ren YF et al.24 | 2 | Journal of oral and maxillofacial surgery | 1.33 | 2007 | ||
| Schwartz AB et al.25 | 2 | Journal of Dentistry | 3.45 | 2007 | ||
| 3 | Low | Menon RK et al.16 | 5 | International Journal of Oral and Maxillofacial Surgery | 1.52 | 2018 |
| Isiordia-Espinoza MA et al.19 | 4 | British Journal of Oral and Maxillofacial Surgery | 0.46 | 2015 | ||
| Marcussen KB et al.20 | 4 | Journal of Oral and Maxillofacial Surgery | 1.33 | 2015 | ||
| 4 | Very low | Cervino G et al.14 | 7 | Antibiotics | 2.92 | 2019 |
Table 6.
Meta-analysis data of high and moderate quality articles for outcome assessed.
| S. No | Outcome assessed | Experimental group (Observations/Total number of extractions) | Control group (Observations/Total number of extractions) | Number needed to treat (NNT) | Relative Risk/Odds ration | 95% Confidence interval (Lower limit – Upper limit) | P value |
|---|---|---|---|---|---|---|---|
| 1 | Post-operative complicationsa | ||||||
| Gill AS15 | 10/379 | 14/332 | – | 0.74 | 0.34–1.65 | 0.47 | |
| Lodi G21 (Pain) | 46/390 | 36/285 | – | 0.60 | 0.32–1.11 | 0.10 | |
| Lodi G21 (Fever) | 9/458 | 14/258 | – | 0.34 | 0.06–1.99 | 0.23 | |
| Lodi G21 (Swelling) | 70/233 | 31/101 | – | 0.92 | 0.65–1.30 | 0.63 | |
| Lodi G21 (Trismus) | 18/119 | 10/56 | – | 0.84 | 0.42–1.71 | 0.64 | |
| Lodi G21 (Surgical site infection) | – | – | – | 0.29 | 0.16–0.50 | 0.0001 | |
| Ren YF24 (Surgical site infection) | 44/1110 | 78/1286 | 25 (15–73) | 1.79 | 1.19–2.68 | 0.263 | |
| 3 | Dry socketb | ||||||
| Arteagoitia MI17 | 27/1072 | 74/925 | 18 (13–29) | 0.35 | 0.21–0.57 | <0.0001 | |
| Ramos E18 | 79/1825 | 167/1479 | 14 (11–19) | 0.43 | 0.33–0.56 | <0.0001 | |
| Lodi G21 | – | – | – | 0.75 | 0.42–1.33 | 0.32 | |
| Ren YF24 | 84/1350 | 228/1582 | 13 (9–16) | 2.17 | 1.56–3.03 | 0.147 | |
| 4 | Adverse eventsc | ||||||
| Gill AS15 | 14/575 | 3/421 | – | 1.84 | 0.59–5.77 | 0.30 | |
| Arteagoitia MI17 | 136/741 | 86/596 | 26 | 1.18 | 0.65–2.14 | 0.56 | |
| Ramos E18 | – | – | 16 (11–32) | 1.28 | 0.86–1.88 | 0.21 | |
| Lodi G21 | 48/540 | 19/390 | – | 1.98 | 1.10–3.59 | 0.02 | |
In evaluating post-operative complications outcome, data from nine studies were not provided as meta-analysis was not done in three studies13,22,24; three were low quality studies16,19,20; One is very low-quality study14; and Meta-data was not provided in two studies.17,18
In evaluating dry socket outcome, data from two studies was not provided as one is a low quality study20 and meta-analysis was done in one study.23
In evaluating adverse events outcome, data from one study was not provided as it was a low quality study.19
5. Discussion
Third molar extraction is one of the most common minor oral surgical procedures carried out in the oral and maxillofacial surgery specialty.26 Third molars are indicated for extraction either because of the recurrent infection/gross decay in the tooth27 or as a preventive measure to reduce the resorption of adjacent tooth28 or as a prophylactic measure.29 Antibiotics are prescribed usually in all minor oral surgical procedures including third molar extractions to prevent post-operative complications even in healthy individuals.30,31 Absence of clear consensus regarding the prescription of antibiotics during third molar surgery, risk of development of antibiotic resistance and adverse drug reactions are major concerns in patients.32 Thus, it is important to form clear guidelines regarding the prescription of antibiotics during third molar surgery. Evidence-based conclusions can be made only through well-conducted systematic reviews and meta-analysis. However, with an increase in the publication of systematic reviews,33 it is again important to check the quality of published systematic reviews.34, 35, 36, 37, 38, 39
The original AMSTAR instrument was used and cited widely for reporting the methodological quality of systematic reviews.12 In addition to the appreciation, original AMSTAR was also critiqued extensively mainly regarding over-lapping appraisal items.12 In a perspective of increasing the applicability of AMSTAR as a critical appraisal instrument for systematic reviews in health care interventions, the expert group made revisions and made AMSTAR 2 tool.12 In AMSTAR 2, four domains were added in addition to the original AMSTAR instrument. Two of them are taken from the ROBINS-I (Risk Of Bias In non-randomized Studies of Interventions) tool (Elaboration of the PICO and use of the risk of bias in evidence synthesis),40 third is the possible causes and significance of heterogeneity and fourth is the justification of the study designs selected. Hence, with the improvement in the assessing quality of the AMSTAR 2 tool, it was used to assess the methodological quality of systematic reviews in the present review. The overall quality of the included systematic reviews was considered as ‘moderate’ as the moderate scores were observed in eight out of thirteen systematic reviews (62%). However, only one ‘High’ quality review was observed which was a Cochrane review.21 As moderate quality reviews were observed in the majority, results from high and moderate-quality reviews were only used for the preparation of evidence-based conclusions in the present review. The present review findings cannot be compared with any previous reports as no such analysis was done earlier, neither with AMSTAR 2 tool nor with the original AMSTAR tool.
Twelve reviews13, 14, 15, 16, 17, 18, 19, 20, 21, 22,24,25 analyzed the evaluation of post-operative complications after using the antibiotics in third molar surgery and out of which one was a high-quality review,21 seven were moderate quality,13,15,17,18,22,24,25 three were of low quality,16,19,20 and one was very low quality.14 Considering the observations form high and moderate-quality reviews it can be concluded that the antibiotics prevent the postoperative complications (pain, fever, swelling, and trismus) after third molar surgery. However, regarding surgical site or wound infection three reviews13,15,17 did not recommend the use of the pre/post-operative use of antibiotics in third molar surgeries.
One high quality,21 four moderate quality,17,18,23,24 and one low-quality review20 evaluated the efficacy of antibiotics in reducing the frequencies of dry socket or alveolar osteitis after third molar surgery. One high quality21 and three18,23,24 out of the four moderate reviews recommend the use of antibiotics to reduce the frequency of dry socket after third molar surgeries and one review17 claims that there is no enough evidence to show the reduction of dry socket incidence after pre/post-operative antibiotic usage in third molar surgeries. Considering the majority of the reviews favoring the use of antibiotics to reduce the dry socket after third molar surgeries, it can be concluded that antibiotics reduce the incidence of dry socket or alveolar osteitis.
One high quality,21 three moderate quality,15,17,18 and one low quality review19 evaluated the adverse effects after suing the antibiotics during third molar surgery. Both the high quality21 moderate quality reviews15,17,18 reported the presence of adverse effects after using the antibiotics during third molar surgery. However, moderate quality reviews showed no significant difference between the treatment and placebo groups, whereas the high quality review showed significant difference between the treatment and placebo groups. Though the presence of adverse effects was observed, conflicting results of significance levels in the moderate and high quality studies limit the authors from drawing evidence based conclusions.
Regarding the importance of prior protocol registration in the included trials, only three reviews (one high,21 moderate,23 and critically low-quality review14) have the prior protocol registration whereas ten reviews 13,15, 16, 17, 18, 19, 20,22,24,25 were not registered before starting the review. The present review findings are in accord with the past literature where Cochrane reviews are usually rated as high quality as they follow the rigorous methodology and have a pre-defined protocol for the review procedure.41 Similarly, one Cochrane review21 in the present review was rated as high quality which has the prior registered protocol. Though two reviews one moderate23 and one critically low quality14 are having the prior registered protocols, they failed to achieve the high-quality grade. The improvement in the quality review, with a prior registered protocol, was explained in the past literature as well, where it was reported that prior registered reviews are high quality when compared with the reviews without registration.42 Accordingly, the rest of the included reviews13,15, 16, 17, 18, 19, 20,22,24,25 in the present review failed to achieve the high-quality grade in the AMSTAR 2 analysis. So, protocol registration for the systematic reviews needs to be made mandatory to achieve a high-quality systematic review.
The risk of bias (RoB) analysis was analyzed in twelve reviews (92%) of all thirteen included reviews in the present analysis. The AMSTAR 2 tool only describes the methodological quality of the systematic reviews and their components but it does not describe the quality and impact of RoB of included trials on the results part of the current review. The results from the present review need to be implied carefully as the AMSTAR 2 tool does not assess the quality of primary studies (randomized controlled trials) of the included systematic reviews. Besides, only 46% of the included trials reported regarding the publication bias of all the included reviews. The deficiency in reporting publication bias in the included trials may overestimate the treatment effects and result in faulty evidence-based conclusions.43
5.1. Observations from the included studies
The observations from Blatt S et al.13 states that there was a good quality of evidence for not recommending the perioperative antibiotic therapy in healthy patients, and can be recommended in a higher risk of infection patients. The number needed to treat (NNT) was considerably high with a low prevalence of infection and a lack of serious complications in placebo groups. Gill AS et al.15 found that there is no enough evidence to support the routine use of antibiotics prophylactically in young patients as the risk of microbial resistance and allergic/toxic reactions outweigh its benefit. However, in situations of prolonged surgery with bone removal preemptive antibiotics were recommended. Atreagoitia MI et al.17 observed that the NNT was 40 for amoxicillin and 10 for amoxicillin plus clavulanate. Considering the low rate of serious infectious complications, NNT, risk of adverse reactions, and antibiotic resistance at a population level, the routine use of antibiotics was not recommended. Ramos E et al.18 found that 11–19 patients receive antibiotics to prevent 1 case of alveolar osteitis or infection and also reported that the prophylactic use of antibiotics reduces the risk of infection by 57%. Lodi G et al.21 observed that prophylactic antibiotics reduce the risk of infection by 70% with NNT of 12. Lodi et al.,21 also reported that the administration of antibiotics just before and/or after third molar extraction reduces the risk of infection, pain, and development of dry socket. However, their role in the prevention of fever, swelling, or problems with restricted mouth opening in patients who have had wisdom teeth removed had no evidence. Lodi et al.21 concluded that antibiotics given to healthy people to prevent infections may cause more harm than benefit to both the individual patients and the population as a whole. Oomens MA et al.22 found that numerous antibiotics were not successfully proven in third molar surgery and concluded that the current recommendation was not to prescribe antibiotics as prophylaxis unless clinical risk factors warrant so. Hedstorm L et al.23 observed that local treatment with tetracycline had strong evidence in the prevention of dry socket following third molar surgery. Ren YF et al.,24 observed that prescribing antibiotics during third molar surgeries reduces the risk of developing alveolar osteitis by 2.2 times and wound infection by 1.8 times. Ren YF et al.,24 recommended a single dose of penicillin 1 h before the third molar surgeries, and for patients with known risk factors like age, smoking, poor hygiene continue postoperatively for 2–5 days. Schwartz AB et al.,25 found that there is little evidence for the use of antibiotic prophylaxis in healthy patients undergoing third molar surgery.
5.2. Strengths, limitations, and future directions
The present systematic review generates evidence-based results regarding the recommendation of antibiotics in third molar surgeries. The present review also highlights some strength of the included trials such as reporting the review question and inclusion criteria, reporting regarding the study design of the included trials, literature search strategies, performance of the data selection and data extraction in duplicate, presentation of the list of included and excluded trials and reporting the conflict of interests of the included trials. Significantly, all included trials in the present review mentioned regarding the explanation of inclusion criteria according to the PICO statement and type of studies included in their systematic review.
The authors have observed an interesting feature regarding the assessment of grade quality of the included systematic reviews. The grade quality of the included reviews assessed using the digital form has resulted in one high-grade review, eight moderate quality reviews, three low-quality reviews, and one critically low-quality reviews. However, the quality grade of the included reviews differs if the analysis was done using the criteria given by Shea et al.12 If the quality was assessed using the criteria given by Shea et al. nine critically low quality, three low quality, and one high-quality review were observed. This, variation in the assessment of the quality of systematic reviews questions the confidence of the results observed in the present review.
In the present review, four studies20,22, 23, 24 have specified lower third molar as specific inclusion criteria, rest of the studies have just mentioned third molar. It is presumed that it includes all third molars but mainly the lower third molars only. This is one lacunae of the present review as specific number of upper or lower third molars treated could not be defined. The other major pitfalls were observed in reporting the registration of study protocols and funding sources of the each included primary studies of concerned systematic reviews, and many studies do not have predefined criteria of infection.
6. Conclusion
Oral surgery is a clean-contaminated site, so antibiotic seems reasonable, but in young healthy patient's immune defenses are sufficient enough. The findings of the present review also demonstrate ‘moderate’ level confidence regarding the efficacy of antibiotics in the reduction of postoperative complications (pain, fever, swelling, trismus, and surgical site or wound infection) and episodes of dry socket during third molar surgery. Though adverse events were observed after prescribing antibiotics, clear consensus could not be framed to let us oppose the use antibiotics during third molar surgery. However low risk of associated serious infections, lack of clear recommendations proving the role of antibiotics in reducing surgical site/wound infections and risk of more long term and widespread harm of antibiotic resistance all oppose the regular use of antibiotics in third molar surgeries in healthy individuals. In authors view the role of anti-inflammatory drugs and better local measures should be preferred than systemic antibiotics in routine use. Systemic antibiotics should be reserved for medically compromised patients, cases suspected for poor bone remodeling, local infectious conditions warranting antibiotic supplement and cases in which extensive bone drilling is done. Thus antibiotics may not be recommended routinely but it is the responsibility of the surgeon to use his clinical experience and acumen and consider all potential factors before making the decision on use of antibiotics.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Nil.
Acknowledgments
None.
References
- 1.Cho H., Lynham A.J., Hsu E. Postoperative interventions to reduce inflammatory complications after third molar surgery: review of the current evidence. Aust Dent J. 2017;62:412–419. doi: 10.1111/adj.12526. [DOI] [PubMed] [Google Scholar]
- 2.Reiland M.D., Ettinger K.S., Lohse C.M., Viozzi C.F. Does administration of oral versus intravenous antibiotics for third molar removal have an effect on the incidence of alveolar osteitis or postoperative surgical site infections? J Oral Maxillofac Surg. 2017;75:1801–1808. doi: 10.1016/j.joms.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 3.Lang M.S., Gonzalez M.L., Dodson T.B. Do antibiotics decrease the risk of inflammatory complications after third molar removal in community practices? Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e5–12. doi: 10.1016/j.joms.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 4.Oberoi S.S., Dhingra C., Sharma G., Sardana D. Antibiotics in dental practice: how justified are we. Int Dent J. 2015;65:4–10. doi: 10.1111/idj.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braimah R.O., Ndukwe K.C., Owotade J.F., Aregbesola S.B. Impact of oral antibiotics on health-related quality of life after mandibular third molar surgery: an observational study. Niger J Clin Pract. 2017;20:1189–1194. doi: 10.4103/1119-3077.183235. [DOI] [PubMed] [Google Scholar]
- 6.Kreutzer K., Storck K., Weitz J. Current evidence regarding prophylactic antibiotics in head and neck and maxillofacial surgery. BioMed Res Int. 2014;2014:879437. doi: 10.1155/2014/879437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murad M.H., Asi N., Alsawas M., Alahdab F. New evidence pyramid. Evid Base Med. 2016;21:125‐127. doi: 10.1136/ebmed-2016-110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasiak J., Shen A.Y., Tan H.B. Methodological quality assessment of paper‐based systematic reviews published in oral health. Clin Oral Invest. 2016;20:399‐431. doi: 10.1007/s00784-015-1663-5. [DOI] [PubMed] [Google Scholar]
- 9.El-Rabbany M., Li S., Bui S., Muir J.M., Bhandari M., Azarpazhooh A. A quality analysis of systematic reviews in dentistry, Part 1: meta‐analyses of randomized controlled trials. J Evid Base Dent Pract. 2017;17:389‐398. doi: 10.1016/j.jebdp.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Pussegoda K., Turner L., Garritty C. Systematic review adherence to methodological or reporting quality. Syst Rev. 2017;6:131. doi: 10.1186/s13643-017-0527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea B.J., Grimshaw J.M., Wells G.A. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shea B.J., Reeves B.C., Wells G. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blatt S., Al-Nawas B. A systematic review of latest evidence for antibiotic prophylaxis and therapy in oral and maxillofacial surgery. Infection. 2019;47:519–555. doi: 10.1007/s15010-019-01303-8. [DOI] [PubMed] [Google Scholar]
- 14.Cervino G., Cicciù M., Biondi A. Antibiotic prophylaxis on third molar extraction: systematic review of recent data. Antibiotics. 2019;8:E53. doi: 10.3390/antibiotics8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh Gill A., Morrissey H., Rahman A. A systematic review and meta-analysis evaluating antibiotic prophylaxis in dental implants and extraction procedures. Medicina. 2018;54:E95. doi: 10.3390/medicina54060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon R.K., Gopinath D., Li K.Y., Leung Y.Y., Botelho M.G. Does the use of amoxicillin/amoxicillin-clavulanic acid in third molar surgery reduce the risk of postoperative infection? A systematic review with meta-analysis. Int J Oral Maxillofac Surg. 2019;48:263–273. doi: 10.1016/j.ijom.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Arteagoitia M.I., Barbier L., Santamaría J., Santamaría G., Ramos E. Efficacy of amoxicillin and amoxicillin/clavulanic acid in the prevention of infection and dry socket after third molar extraction. A systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2016;21:e494–504. doi: 10.4317/medoral.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos E., Santamaría J., Santamaría G., Barbier L., Arteagoitia I. Do systemic antibiotics prevent dry socket and infection after third molar extraction? A systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:403–425. doi: 10.1016/j.oooo.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Isiordia-Espinoza M.A., Aragon-Martinez O.H., Martínez-Morales J.F., Zapata-Morales J.R. Risk of wound infection and safety profile of amoxicillin in healthy patients which required third molar surgery: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. 2015;53:796–804. doi: 10.1016/j.bjoms.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Marcussen K.B., Laulund A.S., Jørgensen H.L., Pinholt E.M. A systematic review on the effect of single dose pre-operative antibiotics at surgical osteotomy extraction of lower third molars. J Oral Maxillofac Surg. 2016;74:693–703. doi: 10.1016/j.joms.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Lodi G., Figini L., Sardella A., Carrassi A., Del Fabbro M., Furness S. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst Rev. 2012 Nov 14;11:CD003811. doi: 10.1002/14651858.CD003811.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Oomens M.A., Forouzanfar T. Antibiotic prophylaxis in third molar surgery: a review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e5–12. doi: 10.1016/j.oooo.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Hedström L., Sjögren P. Effect estimates and methodological quality of randomized controlled trials about prevention of alveolar osteitis following tooth extraction: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:8–15. doi: 10.1016/j.tripleo.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Ren Y.F., Malmstrom H.S. Effectiveness of antibiotic prophylaxis in third molar surgery: a meta-analysis of randomized controlled clinical trials. J Oral Maxillofac Surg. 2007;65:1909–1921. doi: 10.1016/j.joms.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz A.B., Larson E.L. Antibiotic prophylaxis and postoperative complications after tooth extraction and implant placement: a review of the literature. J Dent. 2007;35:881–888. doi: 10.1016/j.jdent.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Rafetto L.K. Managing impacted third molars. Oral Maxillofac Surg Clin. 2015;27:363–371. doi: 10.1016/j.coms.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Dodson T.B., Susarla S.M. Impacted wisdom teeth. Clin Evid. 2014;9:1302. [PMC free article] [PubMed] [Google Scholar]
- 28.Oenning A.C., Freire A.R., Rossi A.C. Resorptive potential of impacted mandibular third molars: 3D simulation by finite element analysis. Clin Oral Invest. 2018;22:3195–3203. doi: 10.1007/s00784-018-2403-4. [DOI] [PubMed] [Google Scholar]
- 29.Shoshani-Dror D., Shilo D., Ginini J.G., Emodi O., Rachmiel A. Controversy regarding the need for prophylactic removal of impacted third molars: an overview. Quintessence Int. 2018;49:653–662. doi: 10.3290/j.qi.a40784. [DOI] [PubMed] [Google Scholar]
- 30.Xue P., Wang J., Wu B., Ma Y., Wu F., Hou R. Efficacy of antibiotic prophylaxis on postoperative inflammatory complications in Chinese patients having impacted mandibular third molars removed: a split-mouth, double-blind, self-controlled, clinical trial. Br J Oral Maxillofac Surg. 2015;53:416–420. doi: 10.1016/j.bjoms.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Rabi A., Maheshwari R., Srinivasan B., Warad L.P., Suvarna C.C., Tank K.S. Effectiveness of antimicrobial therapy after extraction of impacted mandibular third molar: a randomized clinical trial. J Contemp Dent Pract. 2018;19:81–85. doi: 10.5005/jp-journals-10024-2215. [DOI] [PubMed] [Google Scholar]
- 32.Aragon-Martinez O.H., Isiordia-Espinoza M.A., Tejeda Nava F.J., Aranda Romo S. Dental care professionals should avoid the administration of amoxicillin in healthy patients during third molar surgery: is antibiotic resistence the only problem? J Oral Maxillofac Surg. 2016;74:1512–1513. doi: 10.1016/j.joms.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Aromataris E., Fernandez R., Godfrey C.M., Holly C., Khalil H., Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Base Healthc. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 34.Madera Anaya M., Franco J.V.A., Ballesteros M., Solà I., Urrútia Cuchí G., Bonfill Cosp X. Evidence mapping and quality assessment of systematic reviews on therapeutic interventions for oral cancer. Canc Manag Res. 2018;11:117–130. doi: 10.2147/CMAR.S186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saletta J.M., Garcia J.J., Caramês J.M.M., Schliephake H., da Silva Marques D.N. Quality assessment of systematic reviews on vertical bone regeneration. Int J Oral Maxillofac Surg. 2019;48:364–372. doi: 10.1016/j.ijom.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Jayaraman J., Nagendrababu V., Pulikkotil S.J., Innes N.P. Critical appraisal of methodological quality of systematic reviews and meta-analysis in paediatric dentistry journals. Int J Paediatr Dent. 2018;28:548–560. doi: 10.1111/ipd.12414. [DOI] [PubMed] [Google Scholar]
- 37.Nagendrababu V., Pulikkotil S.J., Sultan O.S., Jayaraman J., Peters O.A. Methodological and reporting quality of systematic reviews and meta-analyses in endodontics. J Endod. 2018;44:903–913. doi: 10.1016/j.joen.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Pulikkotil S.J., Jayaraman J., Nagendrababu V. Quality of abstract of systematic reviews and meta-analyses in paediatric dentistry journals. Eur Arch Paediatr Dent. 2019;20:383–391. doi: 10.1007/s40368-019-00432-w. [DOI] [PubMed] [Google Scholar]
- 39.El-Rabbany M., Li S., Bui S., Muir J.M., Bhandari M., Azarpazhooh A. A quality analysis of systematic reviews in dentistry, Part 1: meta-analyses of randomized controlled trials. J Evid Base Dent Pract. 2017;17:389–398. doi: 10.1016/j.jebdp.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Sterne J.A., Hernán M.A., Reeves B.C. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almeida M.O., Yamato T.P., Parreira P.D.C.S., Costa L.O.P., Kamper S., Saragiotto B.T. Overall confidence in the results of systematic reviews on exercise therapy for chronic low back pain: a cross-sectional analysis using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) 2 tool. Braz J Phys Ther. 2020;24:103–117. doi: 10.1016/j.bjpt.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sideri S., Papageorgiou S.N., Eliades T. Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J Clin Epidemiol. 2018;100:103–110. doi: 10.1016/j.jclinepi.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Dwan K., Gamble C., Williamson P.R., Kirkham J.J. Reporting Bias Group. Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PloS One. 2013;8 doi: 10.1371/journal.pone.0066844. [DOI] [PMC free article] [PubMed] [Google Scholar]