Abstract
Aim and background
To assess the use of hypofractionated (HG-RT) versus normofractionated radiation therapy (NF-RT) in Breast Cancer in German speaking countries.
Materials and methods
Between July 2017 and August 2017, an email-based survey was sent to all 1408 physicians that are members of the German Society of Radiation Oncology (DEGRO). The survey was completed by 180 physicians including 10 private practice owners and 52 heads of departments. The majority (82.1%) of the participants had >15 years of experience in radiation therapy (RT).
Results
The majority (83.9%) of the heads of the departments agreed on using the normofractionated regimen of RT as standard treatment for breast cancer. Several physicians were skeptical about HF-RT with 6.5% of the heads refusing to use HF-RT. 40.3% of the departments had not seen the new German guidelines suggesting HF-RT as the standard treatment for all patients as positive or merely adopted a neutral position toward the guidelines (33.9%). The main points of criticism were increased side effects, an impaired toxicity profile and insufficient data. Most departments (46.8%) that perform HF-RT do so in an individual based manner.
Conclusions
HF-RT remains controversial in German speaking countries. Our data shows that NF-RT remains the predominant method of treatment. HF-RT is only used in a defined group of patients as most German physicians agree that particular patients, especially those at higher risk of RT late effects, may benefit from a less intense, extended fractionation schedule.
Abbreviations: DEGRO, German Society of Radiation Oncology; Gy, gray; HF-RT, hypofractionated radiotherapy; IORT, intraoperative radiation therapy; RT, radiation therapy; SIB, simultaneous integrated boost; SOP, standard operating procedure
Keywords: Breast cancer, Patterns of care, Hypofractionated radiotherapy, Normofractionated radiotherapy
1. Background
Breast cancer, with an incidence of 57,000 new cases a year in Germany is the most common cancer among women and the leading cause of cancer death in women worldwide. More than 18,000 breast cancer patients die every year even though the mortality rate continues to fall.1, 2 Radiation therapy (RT) is the main pillar of the interdisciplinary treatment of breast cancer. Due to modern treatment options in RT side effects could be reduced continuously. Furthermore many randomized clinical trials have shown that RT reduces local recurrences after breast-conserving surgery (BCS) and improves overall survival in a defined subset of patients.3
Over the years various RT concepts have been established, which are used depending on differing clinical factors. According to international standards, RT is mostly given continuously over a certain period. With dose and duration determining the treatment regimen RT concepts can be classified as:
-
-
Normofractionated radiotherapy (NF-RT): the standard method of treatment that uses lower single doses of 1.8 Gy/2 Gy over a longer period (28/25 fractions) resulting in a total dose of 50.4/50 Gy. In addition, a sequential/simultaneous boost is applied.
-
-
Hypofractionated radiotherapy (HF-RT): this method uses higher doses over a shorter time period (40.05–42.72 and 2.67 Gy). Usually used in patients who underwent breast conservation surgery, HF-RT is typically followed by a boost dose to the tumor bed with 5 times 2 Gy.
Obviously, HF-RT is associated with less fractions and thus with shorter treatment times. This is a key argument in some countries: In comparison to the United Kingdom or Canada the care capacity in Germany is higher and, thus, there are less resource limitations. Consequently, normofractionated treatment protocols can be used and are – for most radiotherapy departments – financially better rewarded than HF-regimes.
In 2017, there was a change in the German treatment recommendations: the new S3,4 and AGO guidelines,5 recommend HF-RT as the new standard of treatment for breast cancer. These recommendations are based on several prospective clinical trials.6, 7 There are, however, limitations concerning the underlying studies investigating HF-RT, such as the short length of follow up, particularly important for cardiovascular side effects. Older preclinical data has shown that HF-RT to the heart is likely to increase side effects.8 There is also little data concerning patients being treated with a combination of HF-RT and chemotherapy.
In which extent the two concepts are used for breast cancer patients in the daily clinical routine is still unknown. Thus, the aim of our survey was to conduct a patterns of care survey in German speaking countries. The available literature is discussed, especially with regard to the advantages and disadvantages of NF-RT and HF-RT techniques.
2. Materials and methods
Using the member directory of the German Society of Radiation Oncology (DEGRO) we sent out a survey regarding the use of NF-RT and HF-RT in patients with breast cancer. Ethics approval by our committee was not applicable for a pattern of care study involving online questionnaires sent to radiation oncologists. To generate the questionnaire, we collected all items relevant to the topic. We then formulated the questions and performed a test with radiation oncologists in our department. Thus, we checked whether questions were understandable, the answers were easy to choose from and whether important information was missing. After validation within this cohort the questionnaire was adapted and sent out to the whole test population.
Doctors of different ranks, who were all involved in treating breast cancer patients, were allowed to respond to the questions. Participants were from both genders and different age groups. The participants were given freedom to choose multiple options and to voice a different opinion from those listed in the predetermined responses they could choose from. The survey followed the ethics of survey research including maintenance of confidentiality, anonymity and giving the participants informed consent.
The questionnaire consisted of 15 questions, investigating the use of hypo- vs. normofractionated RT and the reasons behind preferring one or the other. The participants could choose from several predetermined responses and include individual answers in the free text option. This allowed the participants to give their personal and professional opinion. The responses of the participants were listed, according to their statement, as a reflection of an individual opinion or as the opinion of their respective departments.
The questionnaires returned were evaluated anonymously using the SPSS statistical program (IBM SPSS Statistics, Version 25, Armonk, USA).
3. Results
This research survey was conducted for 28 days from the 12th of July 2017 to the 8th of August 2017. All 1408 DEGRO members were contacted by email. The survey was completed by 180 physicians including 10 private practice owners and 52 head of departments. This amounts to 12% of the DEGRO members contacted. A total of 68.8% of all participants were 40–60 years old. The majority (82.1%) of the participants had >15 years of experience in the use of RT. Male participants (58.3%) dominated the survey.
The majority of the participants (81.7%) stated that their answers were consistent with their internal standard operating procedure. To avoid multiple answers from the same institution, that would distort the results of this survey, only the answers of the heads of the institutions and the private practice owners were analyzed for questions 1, 2, 3, 4 and 6. The answers given by the heads and owners are referred to as a general opinion of their respective departments.
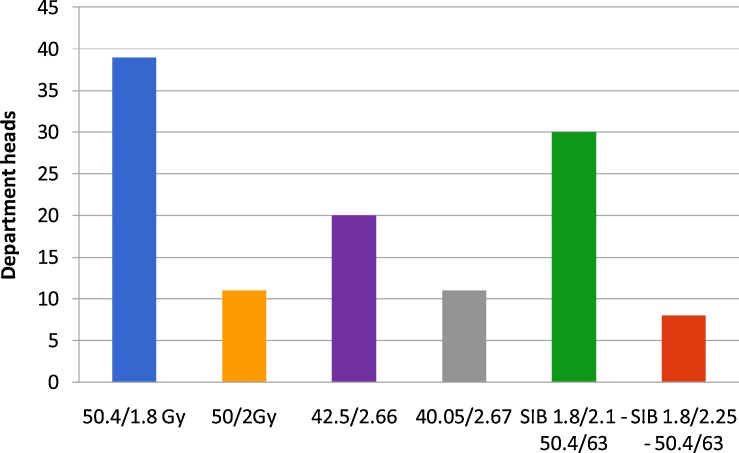
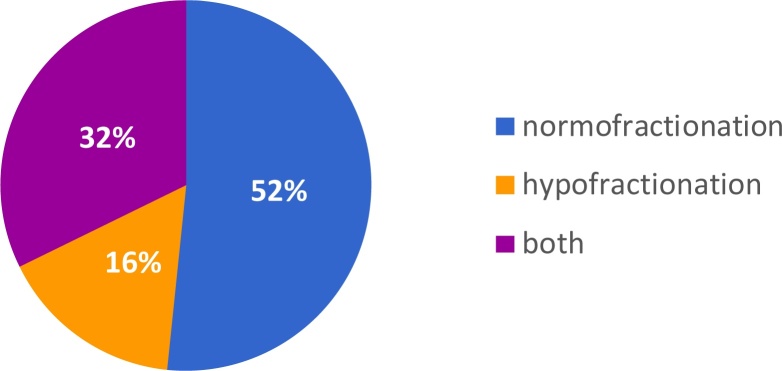
The majority (83.9%) of the departments agreed on using the normofractionated regimen of RT as one of the standard treatment options for node-negative breast cancer. 62.9% of the departments used and recommended a normofractionated RT with 50.4/50 and 1.8/2 Gy as default. 51.7% also used HF-RT (Fig. 1). A simultaneous boost (1.8 to 50.4 ± 2.1 to 58.8/2.25 to 63 Gy) is used in 59.7% of departments. When asked about the most used method in their department most heads chose normofractionation (52%). Hypofractionation was used the most in 16% of departments (Fig. 2).
Fig. 1.
Regimens commonly used for adjuvant treatment of breast cancer in hospitals and private practices. The majority (83.9%) of the departments use the normofractionated regimen of RT as one of the standard treatment options for node-negative breast cancer. 62.9% of the departments used a normofractionated RT with 50.4/50 and 1.8/2 Gy as default. 51.7% also used HF-RT.
Fig. 2.
Standard treatment regimen in hospitals and private practices. Normofractionation was the most used method chosen by 52% of department heads. Hypofractionation was used most in 16% of departments.
Three of the 62 department heads selected interstitial brachytherapy as a method of administering the boost and 6 selected intraoperative radiation therapy.
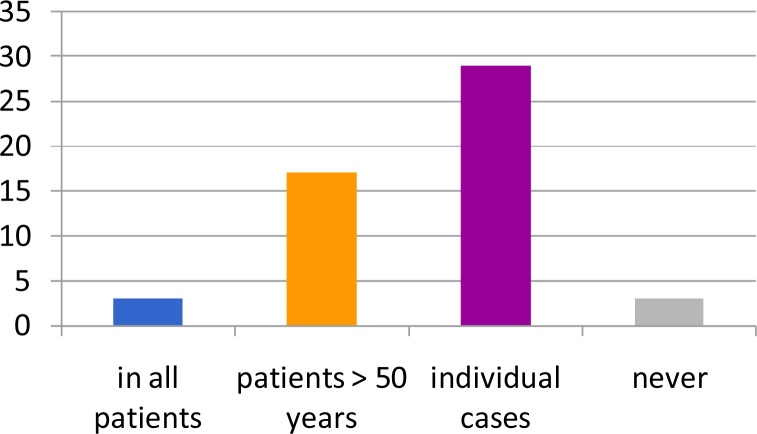
The majority (87.1%) of the departments used HF-RT. 4.8% applied it in all patients with node-negative breast cancer while most followed specific indications. Most departments that perform HF-RT do so in an individual based manner (46.8%) or in patients older than 50 years (27.4%). Several physicians were skeptical about HF-RT with 4.8% of the heads refusing to use HF-RT (Fig. 3).
Fig. 3.
Use of hypofractionated regimen departments: most departments that perform HF-RT do so in an individual manner (46.8%) or in patients older than 50 years (27.4%). About 4.8% of the heads never use HF-RT.
HF-RT can also be used to irradiate the lymphatic drainage pathways. The absolute majority (100%) of the heads, however, agreed on using normofractionated RT by default for this propose.
The survey also included a question about the new AGO guideline,5 that establishes HF-RT as the new standard treatment for breast cancer. 33.9% of the heads accepted this new guideline with a neutral point of view, while 21% saw it in a “positive” light. 40.3% perceived it as “negative”.
After the release of the new AGO guidelines 29.4% of the participants changed their treatment concepts. 43.9% adapted to these new guidelines in a case-based manner. 31.1% did not change their treatment regimens.
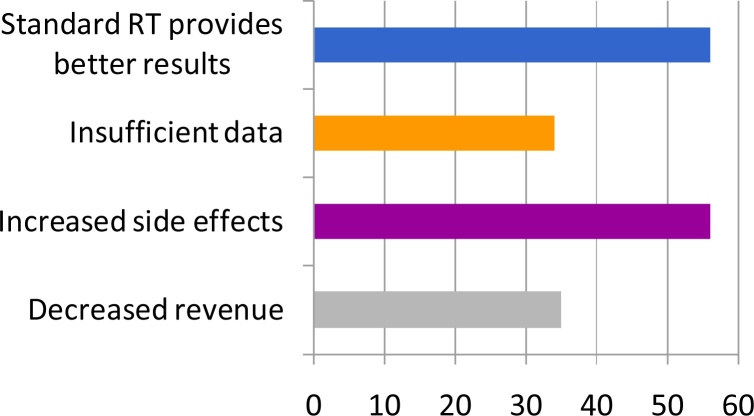
When asked for the reasoning for not adhering to the new guidelines, 33% voiced concerns about increased side effects. 32.4% believe that normofractionated RT is a better concept. 19.9% put financing as their reason. 19.3% indicated that the underlying data was insufficient (Fig. 4).
Fig. 4.
Reasons for not adhering with the new guidelines. 32.4% believe that normofractionated RT is the better concept. 33% voiced concerns about increased side effects. 19.3% indicated that the underlying data was insufficient. 19.9% put financing as their reason.
When asked about advantages of the HF-RT, 99.4% mentioned the shortened treatment time. 18.3% agreed on an improved toxicity profile. Some participants also saw an advantage in improved cosmetic outcome (11.7%) and a better tumor control (7.8%).
The main disadvantage the participants indicated was a worse financial compensation (57.8%). 22.8% see HF-RT with an impaired toxicity profile. 21.1% believe in an inferior cosmetic outcome.
4. Discussion
The results of our survey show that although the new guidelines of the S3,4 and AGO,5 recommend the use of HF-RT for breast cancer, the acceptance and use of HF-RT is not as common as might have been anticipated. There are several reasons most of which include the fear of any long-term toxicity as well as the lack of pressure in terms of limited resources.
The new AGO guideline,5 is based on several studies showing the advantages of HF-RT.
Asked about their view of this new guideline (AGO),5 54.9% of the heads voiced a neutral or positive opinion. 29.4% of the participants changed their treatment concepts accordingly, with 43.9% adapting to these new guidelines in a case-based manner.
Although the majority of departments still use NF-RT as default for treating breast cancer, HF-RT is used by 51.7% of the departments. While only a small percentage used it with all patients most departments treat patients using HF-RT in an individual manner. These findings align with Prades et al. reporting HF-RT for only 29% of eligible patients in Catalonia (Spain).9 A study conducted in New South Wales (Australia) identified a wide variability in the use of HF-RT.10
When asked about advantages of the HF-RT 99.4% mentioned the shortened treatment time. HF-RT is associated with a reduction in the length of treatment by 2–3 weeks compared to conventional schedules that require 6–7 weeks. A reduction of up to 35% in treatment time makes HF-RT schedules attractive for both patients and health care providers and would have a significant impact on daily RT practice, as it would accelerate patient turnover and save health care resources.11 The recently published 5-year efficacy of the FAST-Forward trail even suggests that radiation treatment of only 5 fractions may be considered as an alternative.12
18.3% of the participants of our survey agreed on an improved toxicity profile. Ciammella et al. found that hypofractionation seemed to be effective while showing acceptable side effects.13 Similar studies support this assumption.14, 15
Some participants also reported an advantage in improved cosmetic outcome (11.7%). Studies have suggested that cosmetic results are better and that patient satisfaction is improved.16, 17, 13 According to Haviland et al. some normal tissue effects were less common in the 40 Gy group than in the 50 Gy group (breast shrinkage, telangiectasia, and breast edema).7 Most patients, therefore, seem to gain from HF-RT regimens with a potential for fewer adverse effects.18
Some participants also mentioned a better tumor control. The Start B Trial showed that there were significantly fewer distant relapses up to 10 years in the 40 Gy group, which contributed to the significantly higher rates of disease-free survival and overall survival in the 40 Gy group compared with the 50 Gy group.7 These findings were confirmed by Livi et al. and Ortholan et al.14, 17
Although these studies show the advantages of HF-RT. our survey has shown that the majority of departments (83.9%) still use normofractionated RT for patients with breast cancer. 40.3% of the heads perceived the new AGO guideline,5 as negative and 31.1% did not change their treatment regimens. Several physicians were skeptical about HF-RT as a concept with 6.5% of the heads refusing to use HF-RT altogether. 32.4% believe that normofractionated RT is a better concept, although HF-RT has been proven to be safe by large randomized trials and derived metaanalyses.19, 20, 21
When asked for their reasoning for not adhering to the new guidelines, 33% voiced concerns about increased side effects. However, in the START A and B trials, the rate of confirmed ischemic heart disease in patients with left-sided breast cancer was not different between short and longer fractionation, although a follow-up of 10 years was early for this end point.7
Of all, 22.8% fear that HF-RT can be associated with an impaired toxicity profile. As mentioned above, studies have shown a low rate of severe toxicity.13 However, very long-term data are still missing. HF-RT may even reduce acute radiation toxicity for breast cancer treatment when compared to normofractionated RT.22 In contrast, older studies focusing on RT have shown that HF-RT might increase cardiac side effects; moreover, when focusing on tumor biology, especially high-risk histologies do not benefit from hypofractionation, and might be undertreated with the hypofractionated regimens since total dose according to EQD2 calculations is slightly lower than the established standard doses.
From all participants, 21.1% believe in an inferior cosmetic outcome. This is in contrast to the published data that have reported an improved cosmetic outcome: Taher et al. found no statistically significant difference between the two treatment groups as regards acute skin reactions and cosmetic appearance.23 However, Cimella et al. showed an increase of late subcutaneous and skin effects in patients who had received an additional boost.13
1/5 of the participants indicated financing as their reason for not following the new AGO guidelines.5 This was underlined by their answer that reduced financial compensation (57.8%) was the main disadvantage of HF-RT since especially private practice centers are reimbursed per fraction.
Regarding the relevance of the data, 19.3% of all respondents indicated that the underlying data was insufficient. START A/B and the Canadian trial reported equivalent results (regarding local control, survival, and post-radiation effects) between the standard fractionation schedule and a hypofractionated scheme for women with node-negative early breast cancer.24, 7 However, women who received adjuvant chemotherapy were not included. The START trials mentioned above can be criticized for having a relatively limited follow up time. There is also little data concerning patients treated with a combination of HF-RT and chemotherapy. The shortcomings of the above-mentioned studies (follow up time, inclusion criteria, etc.) should be addressed in long-term follow-ups. A limitation of this study was the low response rate and the high representation of the heads of departments over consultants. Further studies of the present situation are warranted to confirm these observations. Another limitation is the missing patient perspective benefiting from shorter treatment times. The recently published FAST-Forward trail suggests that even 5-fraction schedules may be considered.12
5. Conclusions
Our patterns of care survey showed that, although the new AGO guidelines recommend the use of HF-RT in breast cancer patients, this method is not the standard concept of radiation oncologists in German speaking countries. Although hypofractionation is more convenient for patients and less costly, it remains controversial. Our data shows that NF-RT remains the predominant method of treatment. HF-RT is only used in a defined group of patients as most German physicians agree that particular patients, especially those at higher risk of RT late effects, may benefit from an extended fractionation schedule.
Declarations
Availability of data and materials: The data supporting the conclusions of this article are included within the article.
Consent for publication: Not applicable.
Ethics approval and consent to participate: Not applicable.
Authors’ contributions
Study Design: MM, SEC. Data Collection: MM, SEC. Statistical Analysis: MM, MND, SEC. Data Interpretation: MM, CS, DH, MND, SEC. Manuscript Preparation MM, DH, SEC. Literature Search MM, CS, DH, MND, SEC. Funds Collection SEC. All authors read and approved the final manuscript.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgments
Not applicable.
References
- 1.Katalinic A., Pritzkuleit R., Waldmann A. Recent Trends in Breast Cancer Incidence and Mortality in Germany. Breast Care (Basel) 2009;4(2):75–80. doi: 10.1159/000211526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuire A., Brown J.A., Malone C., McLaughlin R., Kerin M.J. Effects of age on the detection and management of breast cancer. Cancers (Basel) 2015;7(2):908–929. doi: 10.3390/cancers7020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebctcg P., McGale C., Taylor C. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onkologie L. S3-Leitlinie Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Deutsche Krebsgesellschaft. 2017;4.0 [Google Scholar]
- 5.Onkologie A.G.k. 2017. Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. ago-online.de. [Google Scholar]
- 6.Overgaard M., Hansen P.S., Overgaard J. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy, Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 7.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 8.Lauk S., Ruth S., Trott K.R. The effects of dose-fractionation on radiation-induced heart disease in rats. Radiother Oncol. 1987;8(4):363–367. doi: 10.1016/s0167-8140(87)80187-1. [DOI] [PubMed] [Google Scholar]
- 9.Prades J., Algara M., Espinas J.A. Understanding variations in the use of hypofractionated radiotherapy and its specific indications for breast cancer: a mixed-methods study. Radiother Oncol. 2017;123(1):22–28. doi: 10.1016/j.radonc.2017.01.014. [DOI] [PubMed] [Google Scholar]; Background and purpose: Radiation oncology guidelines favour hypofractionated whole-breast radiotherapy (HWBRT) over more conventional schemes in the conservative treatment of breast cancer, but its adoption still varies in clinical practice. This study assessed the patterns of HWBRT adoption in Catalonia (Spain). Material and methods: We used a mixed-methods approach based on an explanatory sequential design, first collecting and analysing quantitative data on HWBRT use (>2.5 Gy per fraction) in 11 public radiotherapy centres (2005–2015) and then performing 25 semi-structured interviews with all department heads and reference radiation oncologist/s. Results: Of the 34,859 patients fulfiling the study criteria over the study period, just 12% were hypofractionated, reaching a percentage of 29% in 2015 (p < 0.001). Our analysis showed a narrowing age gap between patients receiving conventional fractionation and hypofractionation in centres leading adoption. However, there were important differences in clinicians’ interpretation of evidence (e.g. regarding the perceived risk of long-term toxicity) and selection of patients for specific indications, both within and between departments. Conclusions: Differences observed in the rate of adoption of HWBRT could not be tackled only using a rational, evidence-based approach. Factors related to the management of radiotherapy departments play a major role in the diffusion of therapeutic strategies.
- 10.Delaney G.P., Gandhidasan S., Walton R., Terlich F., Baker D., Currow D. The pattern of use of hypofractionated radiation therapy for early-stage breast cancer in New South Wales, Australia, 2008 to 2012. Int J Radiat Oncol Biol Phys. 2016;96(2):266–272. doi: 10.1016/j.ijrobp.2016.05.016. [DOI] [PubMed] [Google Scholar]; Purpose: Increasing phase 3 evidence has been published about the safety and efficacy of hypofractionated radiation therapy, in comparison with standard fractionation, in early-stage, node-negative breast cancer. However, uptake of hypofractionation has not been universal. The aim of this study was to investigate the hypofractionation regimen variations in practice across public radiation oncology facilities in New South Wales (NSW). Methods and materials: Patients with early breast cancer registered in the NSW Clinical Cancer Registry who received radiation therapy for early-stage breast cancer in a publicly funded radiation therapy department between 2008 and 2012 were identified. Data extracted and analyzed included dose and fractionation type, patient age at first fraction, address (for geocoding), year of diagnosis, year of treatment, laterality, and department of treatment. A logistic regression model was used to identify factors associated with fractionation type. Results: Of the 5880 patients fulfilling the study criteria, 3209 patients (55%) received standard fractionation and 2671 patients (45%) received hypofractionation. Overall, the use of hypofractionation increased from 37% in 2008 to 48% in 2012 (range, 7%–94% across departments). Treatment facility and the radiation oncologist prescribing the treatment were the strongest independent predictors of hypofractionation. Weaker associations were also found for age, tumor site laterality, year of treatment, and distance to facility. Conclusions: Hypofractionated regimens of whole breast radiation therapy have been variably administered in the adjuvant setting in NSW despite the publication of long-term trial results and consensus guidelines. Some factors that predict the use of hypofractionation are not based on guideline recommendations, including lower rates of left-sided treatment and increasing distance from a treatment facility.
- 11.Plataniotis G. Hypofractionated radiotherapy in the treatment of early breast cancer. World J Radiol. 2010;2(6):197–202. doi: 10.4329/wjr.v2.i6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray Brunt A., Haviland J.S., Wheatley D.A. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciammella P., Podgornii A., Galeandro M. Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: predictive clinical and dosimetric factors. Radiat Oncol. 2014;9:97. doi: 10.1186/1748-717X-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortholan C., Hannoun-Levi J.M., Ferrero J.M., Largillier R., Courdi A. Long-term results of adjuvant hypofractionated radiotherapy for breast cancer in elderly patients. Int J Radiat Oncol Biol Phys. 2005;61(1):154–162. doi: 10.1016/j.ijrobp.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 15.Plataniotis G.A., Theofanopoulou M.A., Sotiriadou K., Kyrgias G. Hypofractionated radiotherapy for breast cancer patients treated by breast-conserving surgery: short-term morbidity and preliminary results. Breast Cancer. 2010;17(1):42–47. doi: 10.1007/s12282-009-0102-3. [DOI] [PubMed] [Google Scholar]
- 16.Benson J.R., Jatoi I. Highlights of the San Antonio Breast Cancer Symposium 2015: part 1. Future Oncol. 2016;12(7):893–896. doi: 10.2217/fon-2016-0043. [DOI] [PubMed] [Google Scholar]
- 17.Livi L., Stefanacci M., Scoccianti S. Adjuvant hypofractionated radiation therapy for breast cancer after conserving surgery. Clin Oncol (R Coll Radiol) 2007;19(2):120–124. doi: 10.1016/j.clon.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Hopwood P., Haviland J.S., Sumo G. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol. 2010;11(3):231–240. doi: 10.1016/S1470-2045(09)70382-1. [DOI] [PubMed] [Google Scholar]
- 19.Group S.T., Bentzen S.M., Agrawal R.K. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holloway C.L., Panet-Raymond V., Olivotto I. Hypofractionation should be the new ‘standard’ for radiation therapy after breast conserving surgery. Breast. 2010;19(3):163–167. doi: 10.1016/j.breast.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Whelan T.J., Pignol J.P., Levine M.N. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 22.Kim K.S., Shin K.H., Choi N., Lee S.W. Hypofractionated whole breast irradiation: new standard in early breast cancer after breast-conserving surgery. Radiat Oncol J. 2016;34(2):81–87. doi: 10.3857/roj.2016.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taher A.N., El-Baradie M.M., Essa H., Zaki O., Ezzat S. Hypofractionation versus conventional fractionation radiotherapy after conservative treatment of breast cancer: early skin reactions and cosmetic results. J Egypt Natl Canc Inst. 2004;16(3):178–187. [PubMed] [Google Scholar]
- 24.Whelan T., MacKenzie R., Julian J. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94(15):1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]