Abstract
Pectinase as a biocatalyst play a significant role in food and textile industries. In this study, the pectinase was immobilized by encapsulation within polyacrylamide gel to enhance its catalytic properties and ensure the reusability for continuous industrial processes. 9.5% acrylamide and 0.5% N, N′- methylenebisacrylamide concentration gave high percentage of pectinase immobilization yield within gel. The catalytic properties of immobilized pectinase was determined with comparison of soluble pectinase. The immobilization of pectinase within polyacrylamide gel didn't effect catalytic properties of pectinase and both the free and immobilized pectinase showed maximum pectinolytic activity at 45 °C and pH 10. The Michaelis-Menten kinetic behavior of pectinase was slightly changed after immobilization and immobilized pectinase showed somewhat higher Km and lower Vmax value as compared to soluble pectinase. Polyacrylamide gel encapsulation enhanced the thermal stability of pectinase and encapsulated pectinase showed higher thermal stability against various temperature ranging from ranging from 30 °C to 50 °C as compared free pectinase. Furthermore, the surface topography of polyacrylamide gel was analyzed using scanning electron microscopy and it was observed that the surface topography of polyacrylamide gel was changed after encapsulation. The encapsulation of pectinase within polyacrylamide gel enhanced the possibility of reutilization of pectinase in various industries and pectinase retained more than 50% of its initial activity even after seven batch of reactions.
Keywords: Biotechnology; Encapsulation; Acrylamide; N, N′-methylenebisacrylamide; Pectinase; Characterization
Biotechnology, Encapsulation, acrylamide, N, N′-methylenebisacrylamide, pectinase, characterization.
1. Introduction
Pectinase is a generic term used for complex group of enzymes including polygalacturnase, pectin estrase and pectn lyase, and breakdown the pectin into various units. Pectinase have been used in various industrial processes such as fruit juice extraction, textile processing and bioscouring of cotton fibers, retting of plants fibers, waste water treatment, coffee and tea fermentation and oil extraction [1, 2, 3]. The commercialization and industrial utilization of enzymes depend on its stability against temperature, pH and other chemical additives used in industrial preparation. Protein engineering, chemical modification, adding additives and immobilization are usually used for modification of enzymes to meet the industrial needs. Immobilization is the method which not only increases the stability of enzyme but also ensures the reusability of costly enzyme for continuous industrial activity. Physical adsorption, entrapment, encapsulation or covalent bonding have been used for the immobilization of enzymes on various supports [4, 5, 6, 7]. The physical attachment of enzymes on support is done through ionic interactions between supports and enzymes but the binding is very weak and the enzyme can be detached from support under harsh ionic industrial conditions. The covalent bonding is much stronger as compare to physical adsorption and detachment of enzymes from supports are less possible when it is covalently attached but the chemical interaction may bring some structural modification in enzymes which may make the non-functional by irreversibly deactivating [8]. The entrapment or encapsulation is very simple method to physically retained the enzymes within polymeric network of natural and synthetic polymers and overcome limitation of enzyme detachment and irreversible deactivation issues.
Polyacrylamide gel is a polymer of cross-linked network of acrylamide and have enough porous structure to retained enzyme with exchange substrates and products. Furthermore, polyacrylamide is inert in nature and never react with protein as well as non-toxic and biocompatible. A robust enzyme containing polymer bead design synthesis requires the availability of optimum amount of acrylamide monomers, bis-acrylamide crosslinker, free radical ions and catalytically active enzyme. The encapsulation or entrapment of enzymes within polyacrylamide gel is done by initiating the polymerization of acrylamide and bis with the enzyme solutions using free radicals. The reactivity and stability of encapsulated enzymes are depended on porous structure of gel and higher pore size of gel may outflow the enzymes from the gel, and lower size may restrict the entery of substrate into the gel. The concentration acrylamide and crosslinker define the pore size of gel, so the optimiztaion of concentration ratio of acrylamide and crosslinker is necessary to trap the enzyme within gel and make the substrate and product exchangeable across the porous structure of gel [9, 10, 11, 12, 13, 14, 15, 16, 17].
This study was done to immobilize pectinase from B. lichenformis KIBGE-IB21 with polyacrylamide gel through encapsulation techniques. The concentration of monomers was optimized to encapsulate maximum enzymes with maximum reactivity and stability. The catalytic reactivity and stability of encapsulated pectinase against pH, temperature, and substrate concentration were determined with comparison of free pectinase.
2. Material and methods
2.1. Pectinase production
Pectinase was obtained through production of B. licheniformis KIBGE-IB21 was used for the production of pectinase [18]. Cell free filtrates were precipitated using ammonium sulfate (50%) and the precipitants were obtained through centrifugation of cell filtrate at 10,000 rpm for 20 min. The precipitants were then dissolved in buffer (Glycine–NaOH 50mM pH 10) and desalinated through membrane based dialysis against the same buffer for over night. The dailyzed protein solution were used for encapuslation of pectinase within polyacrylamide gel.
2.2. Encapsulation of pectinase
The encapsulation of pectinase within polyacrylamide gel was performed through polymerization of acrylamide and bis – acrylamide with partially purified pectinase solution by addition of ammonium per sulfate and TEMED (Tetramethylethylenediamine). Then the gel was cut into 2mm pieces and washed with buffer (Glycine-NaOH buffer 50mM, pH-10), and deionized water to clean the non-encapsulated enzymes from gel. The percent encapsulation yield of pectinase was calculated through following equation;
2.3. Determination of enzyme activity of encapsulated and soluble pectinase
The enzyme assay of both encapsulated and soluble pectinase was performed using pectin as substrate and the concentration of galacturonic acid produced by enzyme assay was determined by 3, 5-dinitrosalicylic acid method using D- (+)-galacturonic acid monohydrate as a standard [19]. One unit of enzyme was defined as the “amount of enzyme required to catalyze the formation of 1μmol of galacturonic acid per minute under standard assay conditions”.
2.4. Concentration effect of monomer and crosslinker on the encapsulation of pectinase
The effect of monomer and crosslinker concentration on the encapsulation of pectinase within polyacrylamide gel was investigated through utilization of various concentration of acrylamide and bis-acrylamide in encapsulation procedure.
2.5. Reaction time effect on the catalytic performance of polyacrylamide gel encapsulated pectinase
The incubation time effect on the catalytic reaction of enacapsulated pectinase was determined through performing the enzyme assay for different time intervals with comparison of soluble pectinase.
2.6. pH effect on the catalytic performance of polyacrylamide gel encapsulated pectinase
The pH effect on the catalytic performance of encapsulated of pectinase was analyzed through measuring the enzyme assay in various pH level (5.0–10.0) with different buffers (acetate buffer pH 5.0–6.0, phosphate buffer pH 7.0–8.0, glycine-NaOH buffer pH 9.0–12.0) in comparison to soluble pectinase.
2.7. Temperature effect on the catalytic performance of polyacrylamide gel encapsulated pectinase
The temperature effect on the catalytic performance of encapsulated pectinase was estimated through measuring the enzyme assay in different reaction temperature (30°C–60 °C) with comparison of soluble pectinase.
2.8. Kinetic parameters
The kinetic parameters including Km and Vmax value of encapsulated pectinase were calculated by performing the enzyme assay using various concentration of substrate ranging 1.0 mg/ml to 20 mg/ml with comparison of soluble enzymes.
2.9. Thermal stability of polyacrylamide gel encapsulated pectinase
Thermal stability of encapsulated pectinase against various temperatures was estimated by keeping the gel encapsulated enzyme in different temperatures ranging for different time intervals (24–120 h). After every 24 h, defined amount enzyme was taken to measure the residual activity.
2.10. Reusability of polyacrylamide gel encapsulated pectinase
The reusability of encapsulated pectinase was measured to reusing of same enzymes in various batch of reactions. The encapsulated pectinase polyacrylamide gel were washed with deionized water and buffer (glycine NaOH buffer of pH 10), and added fresh substrate for next catalytic reaction.
2.11. Surface morphologies of encapsulated polyacrylamide gel
The surface topologies of encapsulated pectinase polyacrylamide gel was scanned using scanning electron microscope (JSM 6380A Jeol, Japan) with reference to polyacrylamide gel without encapsulated pectinase [4, 20].
3. Results and discussion
3.1. Effect of monomer and crosslinker concentration on the encapsulation of pectinase
The concentration ratio of monomer and crosslinker manage the pore size of polyacrylamide gel and have significant impact on the immobilization yield of enzymes [1]. The percent immobilization yield of encapsulated pectinase was increased with the increase of acrylamide and N,N′- methylenebisacrylamide concentration and maximum yield (89%) was achieved at the concentration of acrylamide and N,N′- methylenebisacrylamide was maintained to 9.5 % and 0.5% of, respectively (Table 1). The relative activity of encapsulated pectinase was reduced when the concentration ratio of acrylamide and N,N′- methylenebisacrylamide was increased which might be due to the hindrance effect of polymer to allow the substrate to reach the active site of enzyme. The higher concentration of monomer and crosslinker reduced the pores size of polyacrylamide gel and higher molecule weight like pectin could not cross the pore to get in form enzymatic reaction. The concentration of 9.5% monomer and 0.5% cross linker were optimum to retained the maximum enzymatic activity of pectinase.
Table 1.
Effect of various concentration of acrylamide and N,N′- methylenebisacrylamide on the immobilization of pectinase from Bacillus licheniformis KIBGE-IB21.
| Acrylamide (%) | N,N′- methylenebisacrylamide | Immobilization yield (%) |
|---|---|---|
| 6.65 | 0.35 | 34 |
| 7.60 | 0.40 | 45 |
| 8.55 | 0.45 | 60 |
| 9.50 | 0.5 | 89 |
| 10.45 | 0.55 | 71 |
| 11.40 | 0.6 | 57 |
| 12.35 | 0.65 | 44 |
| 13.30 | 0.70 | 31 |
| 14.25 | 0.75 | 24 |
3.2. Reaction time effect on the relative activity of polyacrylamide encapsulated pectinase
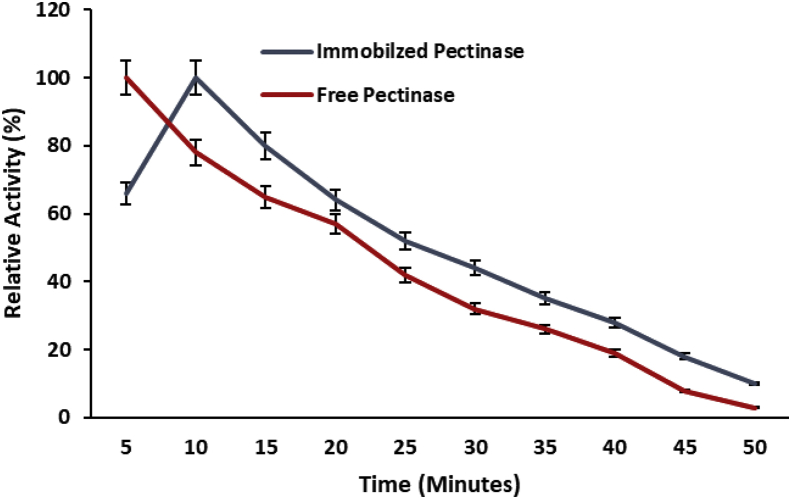
The time is important for enzymatic catalysis reaction because the enzyme need optimum time to convert maximum substrate into product. Therefore, the enzyme assay of encapsulated pectinase was performed in different time intervals with the comparison of soluble pectinase. The encapsulation within polyacrylamide changed the catalytic reaction incubation period of pectinase from 05 to minutes with reference to soluble enzyme (Figure 1). The porous structure of polyacryalamide gel may limit the movement of substrate from open aqueous environment to micro-environment of gel and substrate took some more time to collide with the active site of encapsulated pectinase as compared to soluble pectinase which directly interact with substrate.
Figure 1.
Effect of reaction time on the activity of polyacrylamide gel encapsulated pectinase with comparison of free pectinase. Symbols (means ± S.E., n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
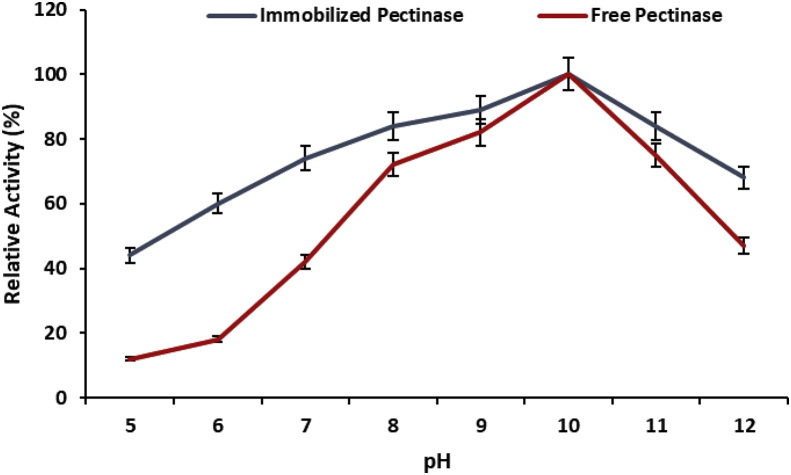
3.3. pH effect on the relative activity of encapsulated pectinase
The ionic strength causes significant variation on the relative activity of enzyme because the molecular association of substrate and enzyme need a ionic solution which support to bring the structural orientation of both molecules to react one another. The relative activity of both the encapsulated and soluble pectinase have significantly changed when the relative activity of these enzymes were analyzed in different pH solution ranging from pH-5 to pH-12 (Figure 2). The optimum pH of pectinase was not changed after encapsulation within polyacrylamide and encapsulated pectinase higher relative activity at pH 10 similar to soluble pectinase. The encapsulation broadened the relative activity of pectinase and encapsulated pectinase maintained higher relative activity in wide range of acidic and basic pH. The enhancement of relative activity of pectinase against different pH after encapsulation might be due to pH change between microenvironment of polymer and reaction mixture of solution [21].
Figure 2.
Effect of pH on the activity of polyacrylamide gel encapsulated pectinase with comparison of free pectinase. Symbols (means ± S.E., n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
3.4. Temperature effect on the relative activity of encapsulated pectinase
The relative activity of encapsulated pectinase was measured against different temperature ranging from 30 °C to 60 °C. The polyacrylamide gel encapsulation didn't change the relative activity of enzyme and encapsulated pectinase performed higher activity at 45 °C similar to free pectinase (Figure 3). Whereas, the relative activity of pectinase against various temperatures was become higher after and encapsulated pectinase mantained highest relative activities in various temperature as compared soluble pectinase. The reduction of relative activity of soluble pectinase at higher temperature is because of the conformational changes of pectinase at higher temperature. The encapsulated pectinase faced less conformational changes from temperature due to protective sheet of polyacrylamide gel and retained greater relative activity at higher temperature as compared to soluble pectinase which was exposed to temperature directly [21].
Figure 3.
Effect of different temperatures on the activity of polyacrylamide gel encapsulated pectinase with comparison of free pectinase. Symbols (means ± S.E., n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
3.5. km and Vmax values of encapsulated pectinase
The kinetic parameters (Km and Vmax value) pectinase of encapsulated pectinase was measured using lineweaver-Burk plot to investigate that how the encapsulation effect the Km and Vmax values enzyme. The Km and Vmax values of free provide information about the affinity between substrate and enzymes, and these value determine the efficacy of supporting material and method used for the immobilization [22]. The encapsulation of polyacrylamide slightly changed the kinetic parameters of pectinase and the encapsulated pectinase nearly gave close Km and Vmax values with the comparison of pectinase (Figure 4). It indicates that the polyacrylamide gel encapsulation didn't affect the affinity of enzyme and substrate due to having less steric obstruction effect on enzyme substrate collusion, and flexibility of enzyme and substrate were maintained for catalytic reaction [23].
Figure 4.
A. Michaelis–Menten and reciprocal Lineweaver–Burk plots of polyacrylamide gel entrapped pectinase. B. Michaelis-Menten and Lineweaver-Burk plot of pectinase.
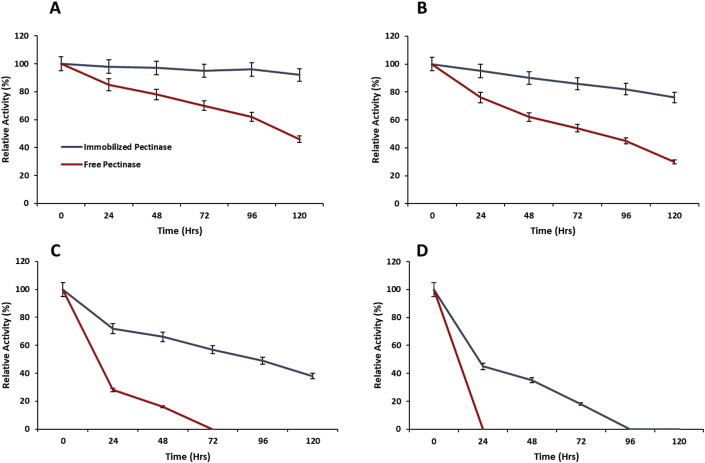
3.6. Thermal stability of encapsulated pectinase
The stability of biocatalysts against temperature is very important to tolerate the harsh environment conditions and the immobilization usually increased the stability of enzyme against temperature. The encapsulation of pectinase within polyacrylamide gel enhanced its catalytic activity against various temperatures and encapsulated pectinase showed higher relative activity as compared to soluble pectinase (Figure 5). The rate of thermal deactivation of encapsulated pectinase within polyacrylamide gel was minor than the soluble pectinase at different temperatures. The encapsulated pectinase maintained 95% and 80% relative activity at 30 °C and 40 °C after 120 h, respectively. The soluble pectinase lost its complete relative activity at 50 °C after 72 h, whereas the encapsulated pectinase demonstrated more than 50% of its original activity at same environment. The polyacrylamide gel increased the rigidity of pectinase, so as compared to soluble pectinase the three-dimensional structure of pectinase within polyacrylamide was less affected at higher temperatures [24].
Figure 5.
Thermal stability of polyacrylamide gel encapsulated pectinase at 30 °C (A); 40 °C (B); 50 °C (C) and 60 °C (D) with the comparison of free pectinase. Symbols (means ± S.E., n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
3.7. Reusabilty of polyacrylamide encapsulated pectinase
The recycling efficacy of immobilized enzymes play very imperative role for its cost effective applications in various industrial preparation. The reusability of polyacrylamide encapsulated pectinase was analyzed by measuring the activity of defined amount of encapsulated pectinase in batch reaction (Figure 6). The encapsulated pectinase exhibited effective recycling capability and maintained 90% of its catalytic activity after reusing three times. The decreased of enzymatic activity is due to leaching out of enzyme from the encapsulated polyacrylamide gel tablets by washing of these tablets after each cycle. It is been concluded that the polyacrylamide gel encapsulated pectinase could be re-utilize for number of catalytic reactions and capable to manage multiple industrial preparation continuously.
Figure 6.
Recycling efficiency of polyacrylamide gel encapsulated pectinase in batch reactions. Symbols (means ± S.E., n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
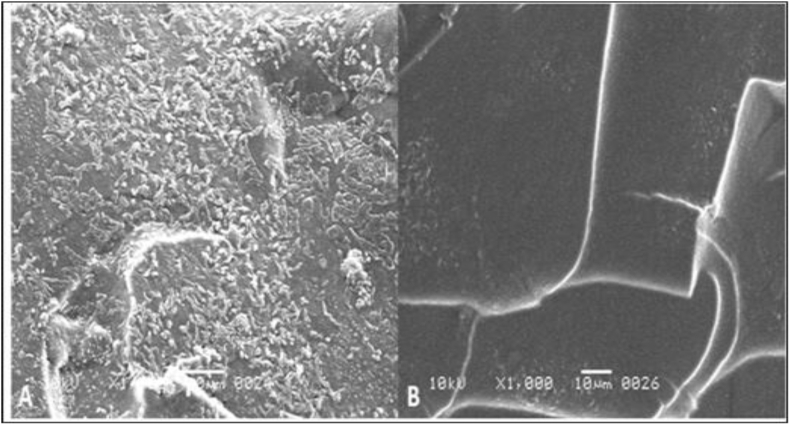
3.8. Scanning electron microscopy
The scanning electron microscopy was performed for the evaluation of topographical changes on polyacrylamide gel as well as to observe the enzymes particles on gel. The surface topography of polyacrylamide gel was changed after the encapsulation of pectinase and the surface of gel with encapsulated pectinase appeared rough due to entrapment of enzymes particles within/on the gel as compared to normal gel which appeared smooth (Figure 7). The surface topographical changes on polyacrylamide gel confirming the encapsulation of different molecules of enzyme on to the porous structure of polyacrylamide gel.
Figure 7.
SEM images of polyacrylamide gel after (A) and before (B) encapsulation of pectinase.
4. Conclusion
Pectinase is the enzyme which involved in the degradation of pectin into D-galacturonic acid and has several industrial applications. Encapsulation of pectinase using polyacryalmide gel is practically and economically feasible technique to increase the stability of pectinase and making it reusable for continuous industrial processes. The concentration ratio of 9.5% and 0.5% of acrylamide and N,N′- methylenebisacrylamide gave high percent yield of immobilization. The immobilization did not alter the optimum temperature and pH of pectinase for enzyme activity, but the optimum temperature and pH of immobilized enzyme were broaden and showed greater level of relative activity as compared to free enzyme. The affinity of substrate to the enzyme did not change so much but the maximum reaction rate was apparently decreased after immobilization. Polyacrylamide gel encapsulation enhanced the stability of pectinase against different temperature and encapsulated pectinase maintained higher residual activity at different temperature as compared with free enzyme. The encapsulated pectinase managed its more than 80% relative activity after three times performance of catalytic reactions. The appearance of rough surface with different entrapped particles of enzymes in SEM image confirmed the encapsulation of pectinase within/on the polyacrylamide gel. The encapsulation of pectinase within polyacrylamide gel is seem to be promising method to meet the industrial needs of pectinase utilization.
Declarations
Author contribution statement
Haneef Ur Rehman: Conceived and designed the experiments; Analyzed and interpreted the data.
Muhammad Asif Nawaz, Sidra Pervez: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Muhsin Jamal, Mohammad Attaullah: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Afsheen Aman, Shah Ali Ul Qader: Conceived and designed the experiments.
Funding statement
This work was supported by the Karachi Institute of Biotechnology and Genetic Engineering (KIBGE), University of Karachi.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kaur G., Kumar S., Satyanarayana T. Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Bioresour. Technol. 2004;94:239–243. doi: 10.1016/j.biortech.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Hoondal G.S., Tiwari R.P., Tiwari R., Dahiya N., Beg Q.K. Microbial alkaline pectinases and their applications: a review. Appl. Microbiol. Biotechnol. 2002;59:409–418. doi: 10.1007/s00253-002-1061-1. [DOI] [PubMed] [Google Scholar]
- 3.Jayani R.S., Saxena S., Gupta R. Microbial pectinolytic enzymes: a review. Process Biochem. 2005;40:2931–2944. [Google Scholar]
- 4.Rehman H.U., Aman A., Silipo A., Qader S.A.U., Molinaro A., Ansari A. Degradation of complex carbohydrate polymer: immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem. 2013;139:1081–1086. doi: 10.1016/j.foodchem.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 5.Lei Z.L., Ren N., Li Y.L., Li N., Mu B. Fe3O4/SiO2-g-PSStNa polymer nanocomposites microspheres (PNCMs) from a surface-initiated atom transfer radical polymerization (SI-ATRP) approach for pectinase immobilization. J. Agric. Food Chem. 2009;57:1544–1549. doi: 10.1021/jf802913m. [DOI] [PubMed] [Google Scholar]
- 6.Li T., Li S., Wang N., Tain L. Immobilization and stabilization of pectinase by multipoint attachment onto an activated agar-gel support. Food Chem. 2008;109:703–708. doi: 10.1016/j.foodchem.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Lei Z., Bi S. Preparation and properties of immobilized pectinase onto the amphiphilic PS-b-PAA diblock copolymers. J. Biotechnol. 2007;128:112–119. doi: 10.1016/j.jbiotec.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Sheldon R.A. Enzyme immobilization: the quest for optimum performance. Advance Synthetic Catalyst. 2007;349:1289–1307. [Google Scholar]
- 9.Baker J.P., Blanch H.W., Prausnitz J.M. Swelling properties of acrylamide-based ampholytic hydrogels - comparison of experiment with theory. Polymers. 1995;36:1061–1069. [Google Scholar]
- 10.Nottelmann H., Kulicke W.M. Preparation, characterization, and rheological behavior of water-swellable polymer networks. ACS (Am. Chem. Soc.) Symp. Ser. 1991;462:62–87. (chapter 4) [Google Scholar]
- 11.Pizarro C., González-Sáiz J. M., Sánchez-Jiménez J. J. Optimisation of polyacrylamide gel composition as support for enzyme immobilisation by covalent linking. J. Chem. Technol. Biotechnol. 2000;75:1040–1046. [Google Scholar]
- 12.Nawaz M.A., Aman A., Rehman H.U., Bibi Z., Ansari A., Islam Z., Qader S.A.U. Polyacrylamide gel-entrapped maltase: an excellent design of using maltase in continuous industrial processes. Appl. Biochem. Biotechnol. 2016;179:383–397. doi: 10.1007/s12010-016-2001-3. [DOI] [PubMed] [Google Scholar]
- 13.Stellwagen N.C. Electrophoresis of DNA in agarose gels, polyacrylamide gels and in free solution. Electrophoresis. 2009;30:S188–S195. doi: 10.1002/elps.200900052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asadi S., Tabani H., Nojavan S. Application of polyacrylamide gel as a new membrane in electromembrane extraction for the quantification of basic drugs in breast milk and wastewater samples. J. Pharmaceut. Biomed. Anal. 2018;151:178–185. doi: 10.1016/j.jpba.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Pizarro C., Fernández-Torroba M.A., Benito C., González-Sáiz J.M. Optimization by experimental design of polyacrylamide gel composition as support for enzyme immobilization by entrapment. Biotechnol. Bioeng. 1997;53:497–506. doi: 10.1002/(SICI)1097-0290(19970305)53:5<497::AID-BIT7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Rehman H.U., Qader S.A.U., Aman A. Polygalacturonase: production of pectin depolymersing enzyme from Bacillus licheniformis KIBGE IB-21. Carbohydr. Polym. 2012;90:387–391. doi: 10.1016/j.carbpol.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 17.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- 18.Palmer T. fourth ed. Prentice Hall/Ellis Horwood; New York, USA: 1995. Understanding Enzymes; pp. 356–365. [Google Scholar]
- 19.Arıca M.Y., Bayramoǧlu G. Reversible immobilization of tyrosinase onto polyethyleneimine-grafted and Cu (II) chelated poly (HEMA-co-GMA) reactive membranes. J. Mol. Catal. B Enzym. 2004;27:255–265. [Google Scholar]
- 20.Heitmann T., Wenzig E., Mersmann A. Characterization of three different potato starches and kinetics of their enzymatic hydrolysis by an α-amylase. Enzym. Microb. Technol. 1997;20:259–267. [Google Scholar]
- 21.Bayramoglu G., Altintas B., Arica M.Y. Cross-linking of horseradish peroxidase adsorbed on polycationic films: utilization for direct dye degradation. Bioproc. Biosyst. Eng. 2012;35:1355–1365. doi: 10.1007/s00449-012-0724-2. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y., Shi L., Huang Y., Gao J., Zhang X., Zhou L. Preparation of robust biocatalyst based on cross-linked enzyme aggregates entrapped in three-dimensionally ordered macroporous silica. ACS Appl. Mater. Interfaces. 2014;6:2622–2628. doi: 10.1021/am405104b. [DOI] [PubMed] [Google Scholar]
- 23.Qader S.A.U., Aman A., Azhar A. Continuous production of dextran from immobilized cells of Leuconostoc mesenteroides KIBGE HA1 using acrylamide as a support. Indian J. Microbiol. 2011;51:279–282. doi: 10.1007/s12088-011-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Saiz J.M., Pizarro C. Polyacrylamide gels as support for enzyme immobilization by entrapment. Effect of polyelectrolyte carrier, pH and temperature on enzyme action and kinetics parameters. Eur. Polym. J. 2001;37:435–444. [Google Scholar]