Abstract
A worldwide survey of cercosporoid ascomycete species on hosts of the genus Diospyros (persimmon) with key to the species based on characters in vivo is provided. Special emphasis is placed on species of the genus Pseudocercospora, which are in part also phylogenetically analysed, using a multilocus approach. Species of the latter genus proved to be very diverse, with a remarkable degree of cryptic speciation. Seven new species are described (Pseudocercospora diospyri-japonicae, P. diospyriphila, P. ershadii, P. kakiicola, P. kobayashiana, and P. tesselata), and two new names are introduced [P. kakiigena (≡ Cylindrosporium kaki, non Pseudocercospora kaki), and Zasmidium diospyri-hispidae (≡ Passalora diospyri, non Zasmidium diospyri)]. Six taxa are lectotypified (Cercospora atra, C. diospyri, C. diospyri var. ferruginea, C. flexuosa, C. fuliginosa, C. kaki), and Pseudocercospora kaki is epitypified.
Keywords: Ascomycota, DNA phylogeny, epitypification, key, Mycosphaerellaceae, new taxa, persimmon trees, systematics
INTRODUCTION
Diospyros (Ebenaceae) is a large genus of deciduous and evergreen trees and shrubs, mainly distributed in tropical regions, currently comprising several hundred species [http://www.theplantlist.org/1.1/browse/A/Ebenaceae/Diospyros/], including various economically important widely used fruit and timber trees. Diospyros kaki (Japanese persimmon), one of the most widely cultivated members of this genus, is of enormous commercial relevance. It is cultivated throughout tropical-subtropical regions, but also in Europe, above all in Mediterranean countries, Israel, New Zealand, and the USA (California and Florida). Diospyros lotus (date plum) is another example for the economic importance of species of this genus, although less cultivated and less important compared to D. kaki. The fruits are used in raw and cooked form, the wood is utilized, and the tree is used as medical plant, e.g., in China (Lyle 2006, Hetzel & Jagel 2011). Despite the huge economic importance of several Diospyros species, phytopathogenic fungi, including cercosporoid leaf-inhabiting ascomycetes, infesting Diospyros spp. are less well examined. Ellis & Everhart (1887) described Cercospora kaki, the first ever cercosporoid species on Diospyros. Chupp (1954: 201–204) described six species on Diospyros spp. under Cercospora (C. diospyri, C. diospyri-erianthae, C. diospyri-morrisianae, C. flexuosa, C. fuliginosa, and C. kaki) and added a key to the species concerned. Hsieh & Goh (1990) published a detailed treatment of cercosporoid hyphomycetes in Taiwan, including two new species, viz., Pseudocercospora diospyricola and P. kaki. First phylogenetic analyses of sequences retrieved from Pseudocercospora spp. on Diospyros kaki and D. lotus (Crous et al. 2013, Bakhshi et al. 2014) raised doubts over the monophyly of Pseudocercospora kaki. Furthermore, the relation between Cercospora kaki, described from North America, and Pseudocercospora kaki, based on Asian type material, is still an open question, although both taxa are morphologically very close to each other. Numerous collections of Pseudocercospora on Diosyros kaki have recently been made in Brazil. The South American specimens are genetically and morphologically close to Asian samples assigned to P. kaki. In order to shed light on the taxonomy of Pseudocercospora on Diospyros spp., comprehensive molecular analyses of Asian and South Amerian strains have been performed. Furthermore, a descriptive survey of cercosporoid species described on Diospyros spp. is provided, in part based on results of previous still unpublished re-examinations of types and additional collections carried out by the senior author (UB).
MATERIALS AND METHODS
Isolates
Isolates included in this study were obtained from symptomatic leaves of diverse hosts, and identified as species of Pseudocercospora primarily based on the caespituli composed of conidiophores and pluriseptate conidia. In addition, several isolates were obtained from the culture collection of the herbarium of the Graduate School of Bioresources, Mie University (TSU-MUCC), Tsu, Japan; the culture collection of the Genebank, National Institute of Agrobiological Sciences (MAFF), Tsukuba, Japan; Westerdijk Fungal Biodiversity Institute (CBS culture collection), Utrecht, The Netherlands; the culture collection of Tabriz University (CCTU), Tabriz, Iran; the culture collection of the Iranian Research Institute of Plant Protection (IRAN), Tehran, Iran, and from the culture collection of Universidade de São Paulo, Brazil. Single conidial colonies were established from sporulating conidiomata on 2 % walter agar (WA), malt extract agar (MEA), potato-dextrose agar (PDA), and oatmeal agar (OA) (Nakashima et al. 2016, Crous et al. 2019), and incubated at 22 °C. The cultures included in this study are listed in Table 1.
Table 1.
Sources of fungal material and sequence database accession numbers.
| Species | Isolates1 | Source of Isolates | Country |
GenBank Accession Numbers |
|||
|---|---|---|---|---|---|---|---|
| actA | ITS | tef1 | rpb2 | ||||
| Pseudocercospora ershadii | CCTU1066 | Diospyros lotus | Iran | KM452842 | KM452865 | KM452887 | MN786460 |
| CCTU1191 | D. lotus | Iran | KM452843 | KM452866 | KM452888 | MN786461 | |
| CBS136114, CCTU 1206T | D. lotus | Iran | KM452844 | KM452867 | KM452889 | MN786459 | |
| IRAN 3428C | D. lotus | Iran | MN786451 | MN786443 | MN786447 | MN786455 | |
| IRAN 3429C | D. lotus | Iran | MN786452 | MN786444 | MN786448 | MN786456 | |
| IRAN 3430C | D. lotus | Iran | MN786453 | MN786445 | MN786449 | MN786457 | |
| IRAN 3431C | D. lotus | Iran | MN786454 | MN786446 | MN786450 | MN786458 | |
| P. fuliginosa | MAFF237710 | D. kaki | Japan | LC515779 | LC515776 | LC515789 | LC515799 |
| P. kaki | CC30 | D. kaki | Brazil | MK874007 | MK867670 | MK874044 | LC516699 |
| CC40 | D. kaki | Brazil | MK874016 | MK867679 | MK874053 | LC516700 | |
| CC43 | D. kaki | Brazil | MK874019 | MK867682 | MK874056 | LC516701 | |
| MAFF235880 | D. kaki | Japan | LC512005 | LC511999 | LC515781 | LC515792 | |
| MAFF237013 | D. kaki | Japan | LC512006 | LC512000 | LC515782 | LC515793 | |
| MAFF238214ET | D. kaki | Japan | LC512007 | LC512001 | LC515783 | LC515794 | |
| MUCC1063 | D. kaki | Japan | LC512008 | LC512002 | LC515784 | LC515795 | |
| MUCC903 | D. kaki | Japan | LC515777 | LC515773 | LC515785 | LC515796 | |
| P. kobayashiana | MAFF236999 | D. kaki | Japan | LC512004 | LC511998 | LC515780 | LC515791 |
| P. kakiicola | MAFF238238T | D. kaki | Japan | GU320431 | GU269729 | GU384442 | LC515786 |
| MAFF238215 | D. lotus | Japan | LC515778 | LC515774 | LC515787 | LC515797 | |
| MUCC941 | D. kaki | Japan | – | LC515775 | LC515788 | LC515798 | |
| P. diospyriphila | KACC47650 | D. kaki | Korea | GU512009 | LC512003 | LC515790 | – |
1CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CCTU: Culture Collection of Tabriz University, Tabriz, Iran; IRAN C: the culture collection of the Iranian Research Institute of Plant Protection, Tehran, Iran; MAFF: Genebank Project, NARO, Tsukuba, Ibaraki, Japan; MUCC: Culture collection, herbarium of Mie University (TSU), Mie, Japan. T, ET and NT indicate ex-type, ex-epitype and ex-neotype strains, respectively.
2ITS: internal transcribed spacers and intervening 5.8S nrDNA; actA: partial actin gene; rpb2: partial RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene.
DNA extraction, amplification (PCR), and phylogeny
Fungal mycelium of strains (Table 1) was harvested with a sterile scalpel and the genomic DNA was isolated using the Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA) or UltraClean Microbial DNA isolation kit (MoBio Laboratories, Inc., CA, USA), following the manufacturers’ protocols. Four partial nuclear genes were subjected to PCR amplification and sequencing: internal transcribed spacer regions and intervening 5.8S nrRNA gene (ITS) of the nrDNA operon, actin (actA), translation elongation factor 1-alpha (tef1), and DNA-directed RNA polymerase II second largest subunit gene (rpb2) using the primers listed in Table 2. The PCR amplifications were performed on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) or a BioRad T100 Thermal Cycler (Bio-Rad Laboratories, Inc., CA, US). The PCR mixtures consisted of 1 μL genomic DNA, 1× NH4 reaction buffer (Bioline, Luckenwalde, Germany), 2–2.5 mM MgCl2 (ITS and rpb2: 2.5 mM, actA: 2 mM and tef1: 2–5 mM), 20–80 μM of dNTPs (ITS and rpb2: 40 μM, actA 20 μM, and tef1: 20–80 μM), 5–5.5 % dimethyl sulfoxide (DMSO; tef1: 5.6 %), 0.2 μM of each primer and 0.5 U Taq DNA polymerase (Bioline) in a total volume of 12.5 μL. The PCR cycling conditions for ITS, actA, and tef1 were: initial denaturation (94 °C, 3 min); 40 cycles amplification (denaturation 94 °C for 30 s; annealing (Table 2); extension 72 °C for 45 s), and final extension (72 °C, 5 min). The PCR cycling conditions for rpb2 were: initial denaturation (94 °C, 3 min); 5 cycles amplification (denaturation 94 °C for 45 s, annealing 58 °C for 45 s, extension 72 °C for 2 min); 30 cycles amplification (denaturation 94 °C for 45 s, annealing 54 °C for 45 s, extension 72 °C for 2 min), and final extension (72 °C, 8 min). The resulting fragments were sequenced in both directions using the respective PCR primers and the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA). DNA sequencing amplicons were purified through Sephadex G-50 Superfine columns (Sigma-Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were analysed on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The DNA sequences generated were analysed and consensus sequences were computed using MEGA v. 7 (Kumar et al. 2016). All novel sequences generated in this study were deposited in GenBank (Table 1).
Table 2.
Details of primers used in this study for amplification and sequencing.
| Locus | Primer | Sequence (5′–3′) | Orientation | Annealing | Reference |
|---|---|---|---|---|---|
| ITS | V9G | TTA CGT CCC TGC CCT TTG TA | Forward | 48 °C for 30 s | de Hoog & Gerrits van den Ende (1998) |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | Reverse | White et al. (1990) | ||
| rpb2 | RPB2-5f2 | GGG GWG AYC AGA AGA AGG C | Forward | 60 °C for 45 s, 58 °C for 45 s, and 54 °C for 45 s | Sung et al. (2007) |
| fRPB2-5F | GAY GAY MGW GAT CAY TTY GG | Forward | Liu et al. (1999) | ||
| fRPB2-7cR | CCC ATR GCT TGT YYR CCC AT | Reverse | Liu et al. (1999) | ||
| actA | ACT-512F | ATG TGC AAG GCC GGT TTC GC | Forward | 48 °C for 30 s | Carbone & Kohn (1999) |
| ACT-783R | TAC GAG TCC TTC TGG CCC AT | Reverse | Carbone & Kohn (1999) | ||
| tef1 | EF1-728F | CAT CGA GAA GTT CGA GAA GG | Forward | 52 °C for 30 s | Carbone & Kohn (1999) |
| EF1-986R | TAC TTG AAG GAA CCC TTA CC | Reverse | Carbone & Kohn (1999) |
To analyze the phylogenetic relationships among Pseudocercospora isolates on Diospyros, maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI) analyses were conducted using a concatenated alignment. For the concatenated alignment composed of four loci, subsets of sequences from the alignments of Nakashima et al. (2016) were used as backbones. Loci were aligned with the online version of MAFFT v. 7 (Katoh et al. 2017) after which the alignments were manually checked and improved where necessary using MEGA v. 7 (Kumar et al. 2016).
Bayesian inference analyses were performed with BEAST v. 2.5 (Bouckaert et al. 2019). To estimate the posterior probabilities (PPs) of tree topologies, Metropolis-Coupled Markov Chain Monte Carlo searches (MCMCMC) were run for 20 M generations with trees sampled and saved every 1 000 generations with the evolutionary model set as HKY + G model. Maximum parsimony analyses were performed with PAUP v. 4.0b10 (Swofford 2003) using heuristic searches, each of which consisted of 100 random sequence additions and a tree-bisection-reconnection (TBR) algorithm for branch swapping. All the characters were unordered and unweighted, with alignment gaps treated as NEW STATE. Clade robustness of the obtained trees was assessed using 1 000 bootstrap (BS) replications (Felsenstein 1985). Tree scores, including tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI), were calculated. Maximum likelihood analyses were performed using RAxML v. 8.2 (Stamatakis 2014) with the GTR-GAMMA-I model used as the nucleotide substitution model for each locus including 500 bootstrap replicates.
All resulting trees were printed with FigTree v. 1.4.2 (Institute of Evolutionary Biology, University of Edinburgh, http://tree.bio.ed.ac.uk/software/figtree). The alignments and respective phylogenetic trees were deposited in TreeBASE, study number S25559.
Morphology
All fungal structures were examined by means of light microscopy, using an Olympus BX50 or Zeiss Axio imager A1 microscope, as far as new examinations and new description are concerned. Shear’s liquid or distilled water and lactic acid were used as mounting media, and aniline blue (cotton blue) was used to stain colourless structures. If possible, measurements of 30 conidia and other structures were made at a magnification of ×1 000, and the 95 % confidence intervals were determined (extreme values in parentheses). Cultures were studied on MEA. Colony colours were rated using the charts of Rayner (1970).
RESULTS
Phylogeny of Pseudocercospora spp. on Diospyros spp.
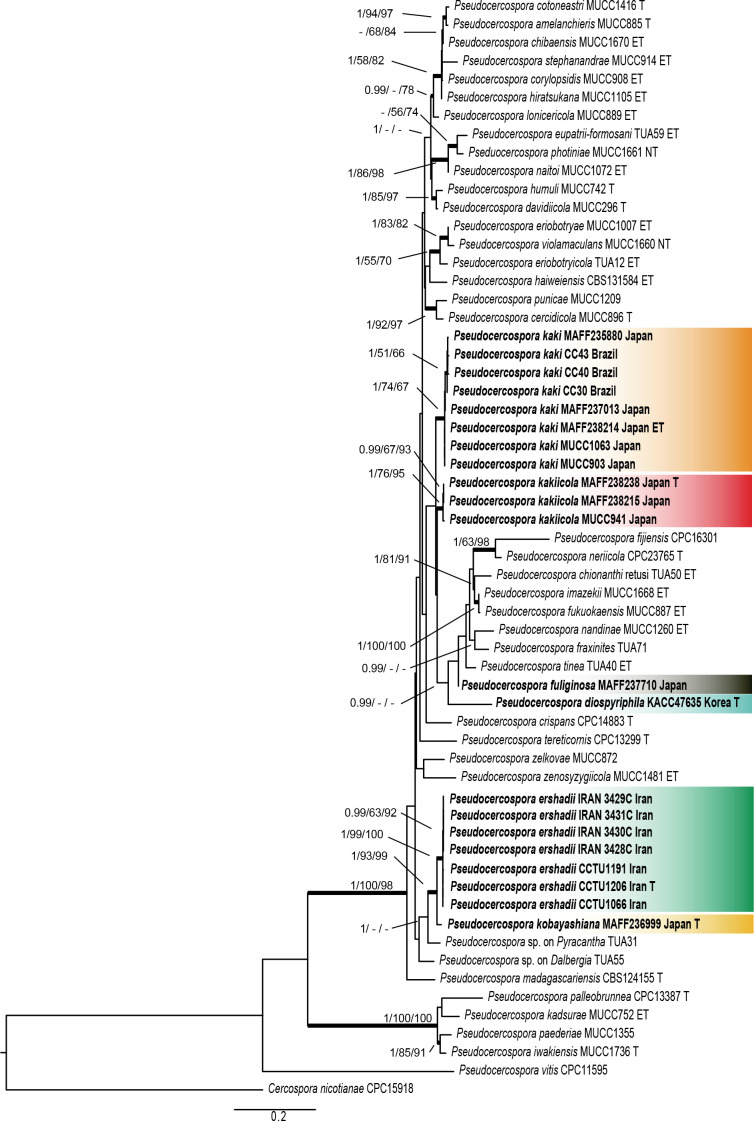
Combined ITS/actA/tef1/rpb2 phylogeny: The sequence matrix did not include all loci for all of the included isolates, mainly due to the fact that some loci failed to amplify for some isolates, even though several attempts were made to obtain a product suitable for sequencing. The alignment consisted of 59 OTU belonging to the genus Pseudocercospora. A strain of Cercospora nicotianae (CPC 15918) was used as outgroup taxon. The final alignment contained a total of 1 739 characters used for the phylogenetic analyses, including alignment gaps. A maximum-likelihood (ML) tree was generated (shown in Fig. 1). The robustness of nodes based on the bootstrap support values were indicated as the second value (ML-BS > 50 %). The Maximum Parsimony (MP) analyses generated 41 equally most parsimonious trees. A MP tree was selected from the equally most parsimonious trees based on the result of the Kishino-Hasegawa (KH) test (Kishino & Hasegawa 1989) in PAUP (Fig. 1; length = 1 986, CI = 0.494, RI = 0.699, RC = 0.345, HI = 0.506), and the bootstrap support values (MP-BS) were indicated on the Maximum Likelihood tree as the third value (Fig. 1; MP-BS > 50 % shown). From the analysed characters, 1 017 were constant, 276 were variable and parsimony-uninformative and 473 were parsimony-informative. The Bayesian analyses generated 20 001 trees. 15 001 trees were sampled after 25 % of the trees were discarded as burn-in based on the effective sample size (ESS) calculated by Tracer v. 1.7.1 software package (Rambaut et al. 2018). The posterior probability values (PP) were calculated from the 15 001 trees (Fig. 1; first value: PP > 0.95 shown).
Fig. 1.
Phylogenetic tree of Pseudocercospora species on Diospyros spp. generated from analysis of an actA, ITS, tef1, and rpb2 combined dataset. Parsimony (MP) and Maximum Likelihood (ML) bootstrap values > 50 % and Bayesian posterior probabilities (PP) > 0.95 are shown (PP/MP-BS/ML-BS). Isolates sequenced in this study are indicated in bold and oblique type. Status of reference isolates are indicated; T: ex-type, ET: ex-epitype, and NT: ex-neotype.
Most species of the genus Pseudocercospora are represented in the phylogenetic tree (Fig. 1) as they have been reported in previous studies [Crous et al. (2013), Nakashima et al. (2016), Videira et al. (2017)], supplemented by several phylogenetically hitherto not yet examined taxa, including the new species described in the present study.
Taxonomy
Taxonomic treatment and synopsis of cercosporoid ascomycetes on Diospyros spp.
This survey comprises taxonomic treatments of cercosporoid species on Diospyros spp., including descriptions, illustrations, and additional data. The degree of examination of the particular species is rather different. Almost all species have been morphologically investigated, including re-examinations of type material. Type collections and other specimens of a few species were not available. Particular emphasis was placed on Pseudocercospora species on Diospyros spp., including cultures that have been used for description in vitro and phylogenetic analyses.
Cercospora diospyricola Munjal, Lall & Chona, Indian Phytopathol. 14(2): 181. [“1961”] 1962.
Literature: Kamal (2010: 41).
Illustration: Munjal et al. (1962: 180, fig. 1).
Description in vivo: Leaf spots subcircular to irregular, 2–12 mm diam, scattered, sometimes confluent, yellowish to brown. Caespituli amphigenous, mostly epiphyllous. Mycelium internal. Stromata slightly developed, small, consisting of pigmented swollen hyphal cells, subglobose, up to 38.5 μm diam, dark brown. Conidiophores in dense fascicles, arising from small stromatic hyphal aggregations, erect, somewhat geniculate, unbranched, about 40–90 × 4–6 μm, septate, olivaceous brown, darker in mass, conidiogenous loci conspicuous (thickened and darkened). Conidia solitary, obclavate(-cylindrical), straight to curved, tapering towards an obtuse tip, base obconically truncated, 15–77 × 4–6 μm, pluriseptate, hyaline, thin-walled, hila thickened and darkened.
Typus: India, Uttar Pradesh, Kathgodam, Nainital, on Diospyros sp., 23 Oct. 1959, J.N. Kapoor (HCIO 26864 – holotype).
Host range and distribution: On Diospyros sp., Asia (India: Uttar Pradesh).
Notes: This is a true species of Cercospora s. str., characterised by having thickened and darkened conidiogenous loci and conidial hila and colourless condia formed singly. Due to obclavate conidia, this species does not pertain to the Cercospora apii compex, which contains numerous plurivorous species. Type material was not available. The description is based on Munjal et al. (1962).
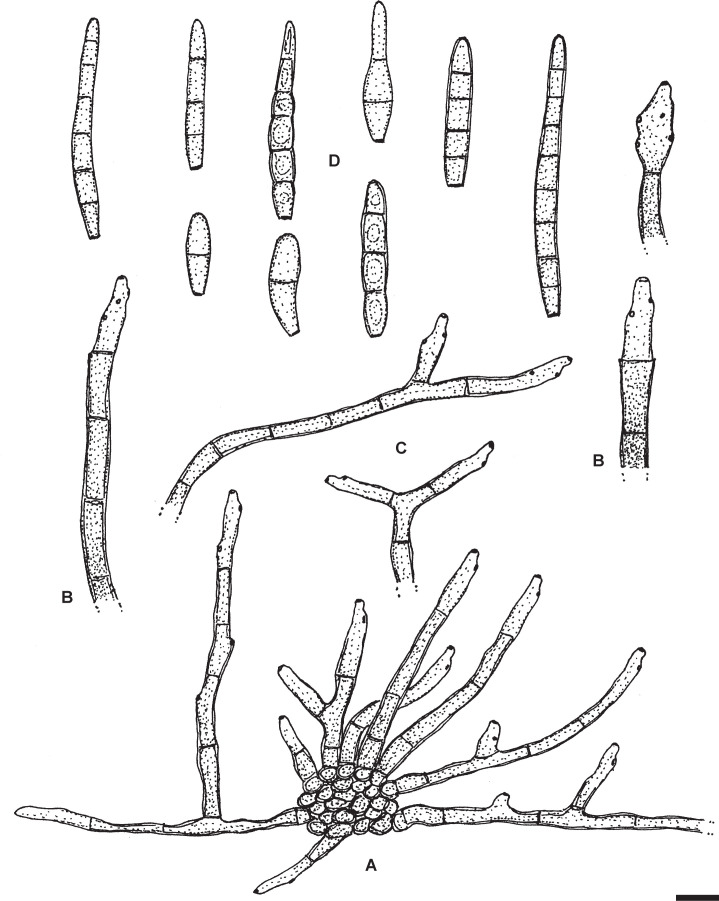
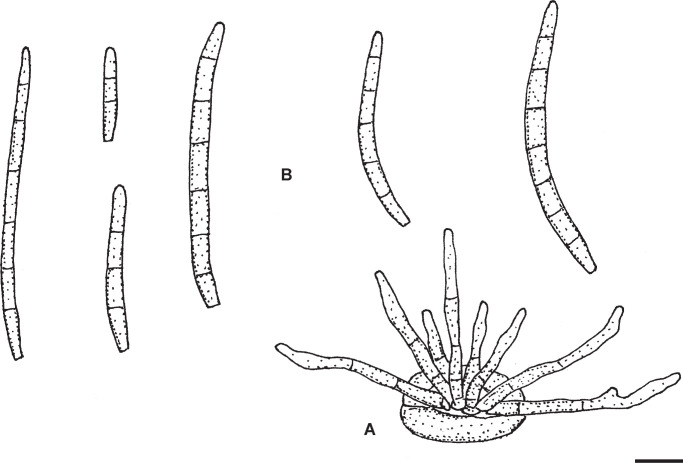
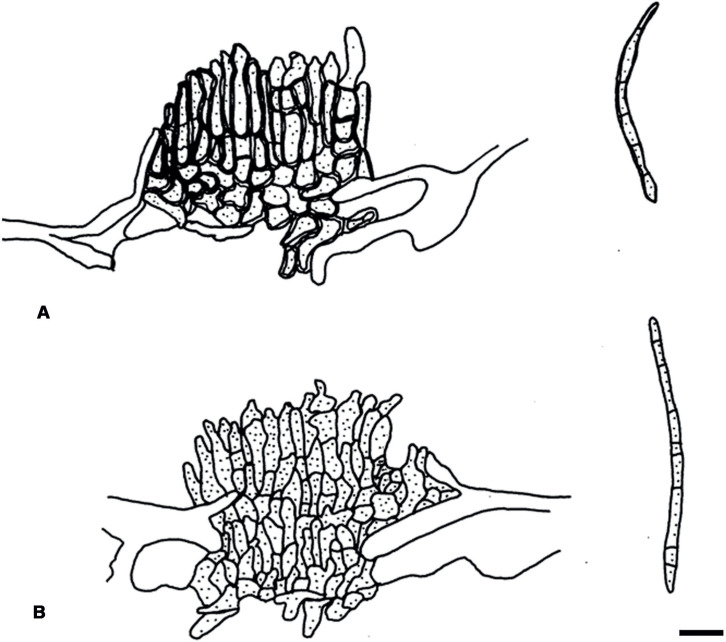
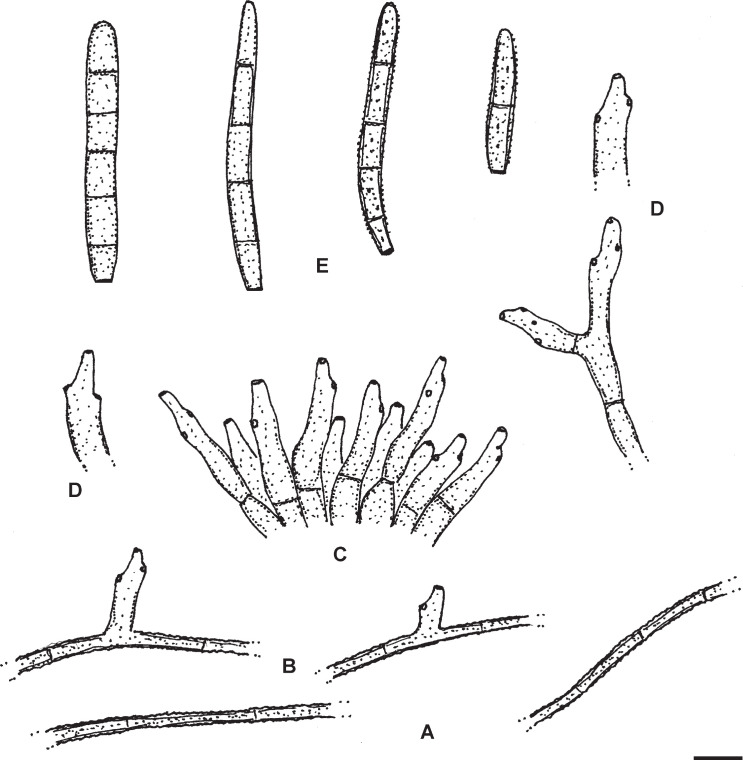
Passalora flexuosa (Tracy & Earle) U. Braun & Crous, in Crous & Braun, CBS Diversity Ser. (Utrecht) 1: 187. 2003. Fig. 2.
Fig. 2.
Passalora flexuosa (NY 937016 – lectotype). A. Fasciculate conidiophores and superficial hyphae with solitary conidiophores arising from a stromatic hyphal aggregation. B. Conidiophores. C. Superficial hyphae with solitary conidiophores. D. Conidia. Scale bar = 10 μm. U. Braun del.
Basionym: Cercospora flexuosa Tracy & Earle, Bull. Torrey Bot. Club 22: 178. 1895.
Synonyms: Mycovellosiella flexuosa (Tracy & Earle) U. Braun, Schlechtendalia 2: 1. 1999.
Cercospora diospyri var. ferruginosa G.F. Atk., J. Elisha Mitchell Sci. Soc. 8(2): 63. 1892 [type: USA, Alabama, Lee, Auburn, on Diospyros virginiana, 6 Oct. 1891 [B.M. Duggar, 2254] G.F. Atkinson (CUP-A-002254a#1(AL)) – lectotype, designated here, MBT390098; CUP-A-002254a#2(AL), CUP-039697 – isolectotypes].
Literature: Chupp (1954: 202).
Description in vivo: Leaf spots lacking or indefinite. Colonies hypophyllous, effuse, olivaceous or in sooty patches, sometimes not very distinct. Mycelium internal and external; superficial hyphae usually emerging through stomata, often from substomatal stromata, simple or branched, often similar to decumbent branched conidiophores, 1.5–4 μm wide, pale olivaceous, septate, thin-walled, smooth. Stromata relatively small, 10–30 μm diam, substomatal, brown, composed of swollen hyphal cells, 2–6 μm diam. Conidiophores in small to moderately large fascicles, loose to dense, arising from internal hyphae or substomatal stromata, erect to decumbent (decumbent threads often developing to superficial hyphae with lateral conidiophores), or solitary, arising from superficial hyphae, lateral or terminal, straight, subcylindrical to flexuous, geniculate-sinuous, unbranched to often branched, (10–)20–200 × 2.5–6 μm, continuous to pluriseptate, olivaceous, yellowish to medium dark brown throughout or paler towards the tip, wall thin to somewhat thickened, smooth; conidiogenous cells integrated, terminal, intercalary, occasionally pleurogenous, (5–)10–20(–30) μm long, proliferation sympodial, occasionally percurrent, conidiogenous loci conspicuous, 1–1.5 μm diam, slightly thickened and darkened. Conidia solitary, obclavate-cylindrical, straight to somewhat curved, 20–75 × 4–6 μm, (1–)3–8(–12)-septate, occasionally somewhat constricted at the septa, distance between septa 3–10 μm, pale olivaceous, olivaceous brown to medium dark brown, wall thin to somewhat thickened, sometimes with distinct inner lumen, imitating thick-walled conidia, smooth, apex obtuse, base rounded to short obconically truncated, hila 1–2 μm wide, slighty thickened and darkened.
Typus: USA, Mississippi, Jackson, Ocean Springs, on Diospyros virginiana, 10 Oct. 1889, F.S. Earle (NY 937016 – lectotype, designated here, MBT390099). Isolectotypes: BPI 436442, 436443; CHRB-F-002383; CUP 39826; FH 01012190, 01012191; MSC 0233701; NCU-F-0025379; NY 937017, 937018; RMS 8718; WIS-F-0012667. Topotype material (28 Aug. 1895): FLAS-F-23555; HBG; ILLS 117708; MICH 328319; MIN 955285; NY 03616823, 03616824; RMS 8718; UC 653439; WIS-F-0012677, 0012705; WSP 64924.
Host range and distribution: On Diospyros virginiana, North America (USA: Alabama, Florida, Georgia, Illinois, Michigan, Mississippi).
Notes: Based on the examination of topotype material, Braun (1999) assigned Cercospora flexuosa to the genus Mycovellosiella, and Braun and Crous, in Crous & Braun (2003), reallocated it to Passalora s. lat. Cercospora flexuosa is a mycovellosiella-like cercosporoid species characterised by forming superficial hyphae with solitary conidiophores in vivo and conspicuous (thickened, darkened) conidiogenous loci and conidial hila. However, phylogenetic examinations of the whole Passalora s. lat. complex revealed that this genus constitutes a polyphyletic complex composed of numerous genera without any clear and strict consistencies between morphological traits and phylogenetic units. Hence, phylogenetic analyses are necessary to assign species within this complex to certain genera. Since such data are not yet available for Cercospora flexuosa, we prefer to maintain this fungus in Passalora, at least for the interim until its phylogenetic generic affinity will be revealed.
In the original description of Cercospora diospyri var. ferruginosa, Atkinson (1892) cited in the protologue “D.M. Duggar, 2254” and “26 Oct. 1891”, but there are three syntype collections deposited in herb. Atkinson at CUP, all specimens without reference to “Duggar” and with the date 6 Oct. 1891.
Pseudocercospora diospyricola Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and Similar Fungi from Taiwan (Taipei): 106. 1990.
Literature: Guo & Hsieh (1995: 89–90), Guo et al. (1998: 105).
Illustrations: Hsieh & Goh (1990: 106, fig. 80), Guo & Hsieh (1995: 90, fig. 81), Guo et al. (1998: 106, fig. 85).
Description in vivo: Leaf spots amphigenous, angular, vein-limited, 1–2.5 mm diam, dark olivaceous brown, Mycelium internal and external; superficial hyphae with solitary conidiophores abundant, hypophyllous, emerging through stomata, arising from the base of conidiophore fascicles, unbranched or sparingly branched, 1–3 μm wide, very pale olivaceous to pale olivaceous brown, thin-walled, smooth. Stromata amphigenous, small to well-developed, larger on the upper side, lacking or smaller below, 10–45 μm diam, immersed or substomatal, olivaceous brown to brown. Conidiophores on the upper leaf surface in well-developed, larger fascicles, arising from stromata, loose to dense, below in smaller and usually loose fascicles, arising from internal hyphae or smaller stromata, through stomata, and solitary, arising from superficial hyphae, erect, straight, subcylindrical to conical when short, longer ones usually geniculate-sinuous, occasionally branched, 5–35 × 3–5 μm, 0–3-septate, sometimes somewhat constricted at the septa, very pale to medium olivaceous brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5–20 μm long, conidiogenous loci inconspicuous or discernable by being subdenticulate, but always unthickened and not darkened. Conidia solitary, obclavate-subcylindrical, straight to somewhat curved, apex subacute or subobtuse, base short obconically truncated, 15–50 × 2–3 μm, 1–8-septate, very pale olivaceous, hila 1–1.5 μm wide, neither thickened nor darkened.
Typus: Taiwan, Yuanlin, Changhwa Hsien, on Diospyros oldhamii, 23 Oct. 1985, T.K. Hsieh (NCHUPP-185 – holotype). Isotype: IMI 312974.
Host range and distribution: On Diospyros oldhamii, Asia (Taiwan).
Notes: This species is obviously confined to Diospyros oldhamii, a small tree species endemic in Japan (Ryukyu Islands) and Taiwan. It is well characterised by forming abundant superficial hyphae on the lower leaf side with numerous solitary conidiophores and larger, almost sporodochial conidiomata on the upper side, combined with pale, short, narrow conidia.
Pseudocercospora diospyri-erianthae Goh & W.H. Hsieh, Trans. Mycol. Soc. Republ. China 2(2): 90. 1987.
Synonym: Cercospora diospyri-erianthae Sawada, Rep. Gov. Res. Inst. Dept. Agric., Formosa 85: 103. 1943, nom. inval. (Art. 39.1).
Literature: Hsieh & Goh (1990: 107), Guo & Hsieh (1995: 90–91), Guo et al. (1998: 105–106).
Illustrations: Hsieh & Goh (1990: 108, fig. 81), Guo & Hsieh (1995: 91, fig. 82), Guo et al. (1998: 107, fig. 86).
Description in vivo: Leaf spots amphigenous, subcircular, 1.5–6 mm diam, reddish brown, margin black. Caespituli hypophyllous. Mycelium internal. Stromata well-developed, globose, 40–85 μm diam, dark brown. Conidiophores numerous, in dense fascicles, arising from stromata, divergent, cylindrical, uniform in pigmentation and width, straight to curved or sinuous, occasionally branched, 10–25 × 2–4 μm, 0–1-septate, subhyaline to pale olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores mostly reduced to conidiogenous cells, apex rounded to truncated, but conidiogenous loci always inconspicuous, neither thickened nor darkened. Conidia solitary, obclavate-cylindrical, apex subacute, base subtruncated to obconically truncated, straight to somewhat curved, 35–90 × 2.5–3.5 μm, 3–9-septate, subhyaline to yellowish olivaceous, thin-walled, smooth, hila 1–2 μm wide, unthickened, not darkened.
Typus: Taiwan, Keelung, on Diospyros eriantha, 24 May 1926, K. Sawada (NTU-PPE, herb. Sawada – holotype). Isotypes: BPI 435800, 435801.
Host range and distribution: On Diospyros eriantha, Asia (Taiwan).
Notes: This species is probably confined to Diospyros eriantha, an Asian tree or shrub distributed in China, Indonesia, Japan, Laos, Malaysia, Taiwan, and Vietnam. It is morphologically reminiscent of other Pseudocercospora species on Diospyros spp. characterised by having large stromata with numerous conidiophores, in combination with lacking superficial mycelium, like P. ershadii and P. kaki, but the conidia are long and narrow, 35–90 × 1.5–3.5 μm, and attenuated towards a more or less pointed tip. The record of Cercospora diospyri-erianthae on Diospyos kirkii (= D. latifolia), an African species, from China (Tai 1979) is doubtful.
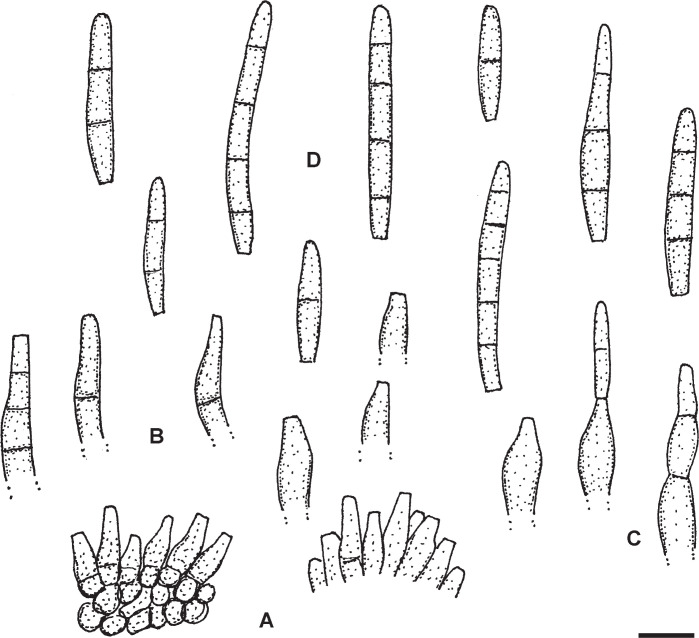
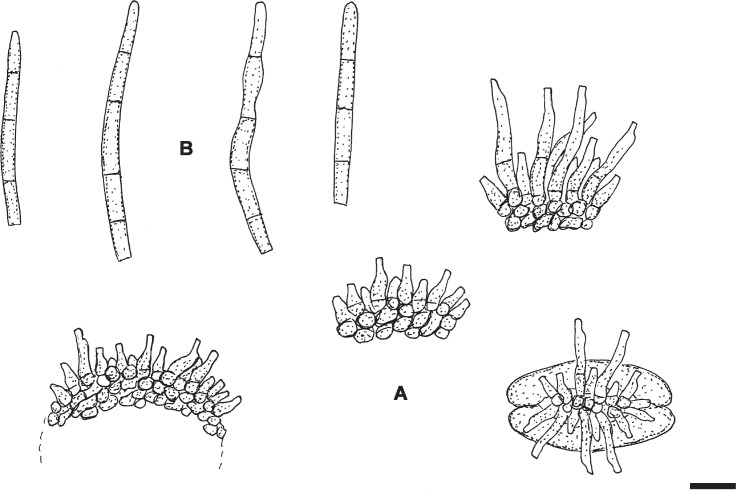
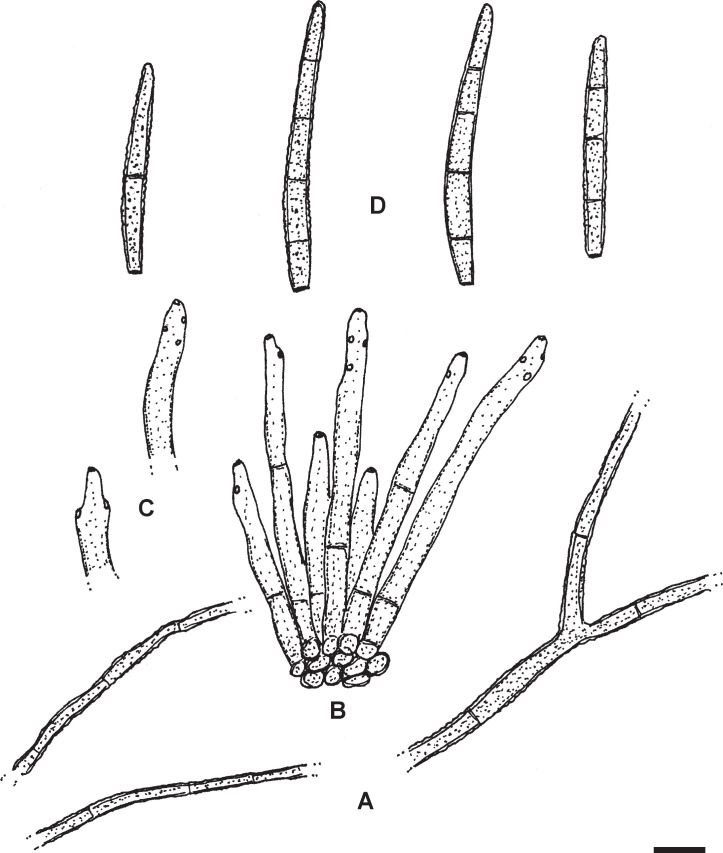
Pseudocercospora diospyri-japonicae U. Braun, sp. nov. MycoBank MB833835. Fig. 3.
Fig. 3.
Pseudocercospora diospyri-japonicae (BPI 1109707 – holotype). A. Conidiophore fascicles. B. Conidiophores. C. Conidiophores, in the middle with attached young conidium. D. Conidia. Scale bar = 10 μm. U. Braun del.
Etymology: Epithet derived from the host name, Diospyros japonica.
Diagnosis: Morphologically similar to Pseudocercospora kobayashiana, but conidia apically obtuse (rounded), i.e., not pointed, and 1–5-septate, conidiophores 0–3(–4)-septate, and conidiogenous loci and hila 2–3(–4) μm wide; formation of obvious annellations not observed.
Description in vivo: Leaf spots amphigenous, angular-irregular in shape, 2–12 mm diam, pale to medium brown, limited by darker slightly raised veins. Colonies amphigenous, punctiform, scattered to gregarious, dark brown. Mycelium internal. Stromata amphigenous, above all epiphyllous, immersed, 15–60 μm diam, subglobose, sometimes oblong or irregular, dark olivaceous brown. Conidiophores numerous, in dense fascicles, arising from stromata, erumpent, usually straight, subcylindrical to conical, somewhat attenuated towards the tip, ampulliform, sometimes flexuous-sinuous, but usually not geniculate, unbranched, 5–30 × 2–6 μm, with young still attached conidia even longer, 0–3(–4)-septate, subhyaline to medium olivaceous or olivaceous brown, wall smooth or almost so, thin-walled, later sometimes slightly thickened; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5–20 μm long, usually with a single terminal locus (unilocal, apex truncated), 2–3(–4) μm wide, neither thickened nor darkened, usually not percurrently proliferating or growth monopodial, but without conspicuous annellations. Conidia solitary, subcylindrical or somewhat attenuated towards the tip (subacicular) to almost obclavate, 10–55(–60) × 3–5 μm, 1–5-septate, subhyaline to olivaceous brown, thin-walled, smooth, apex obtuse, rounded, base truncated or short obconically truncated, hila 2–3 μm wide, neither thickened not darkened.
Typus: China, Beijing, Miaofengshan, on Diospyros japonica, 15 Oct 1959, X.J. Liu (BPI 1109707 – holotype). Isotype: HMAS 59051.
Host range and distribution: Only known from the type collection.
Notes: This species is morphologically reminiscent of Pseudocercospora kobayashiana, which differs, however, in having conidiogenous cells with percurrent proliferations and 4–14-septate conidia with pointed apex, as well as conidiogenous loci and hila 1.5–2.5 μm wide.
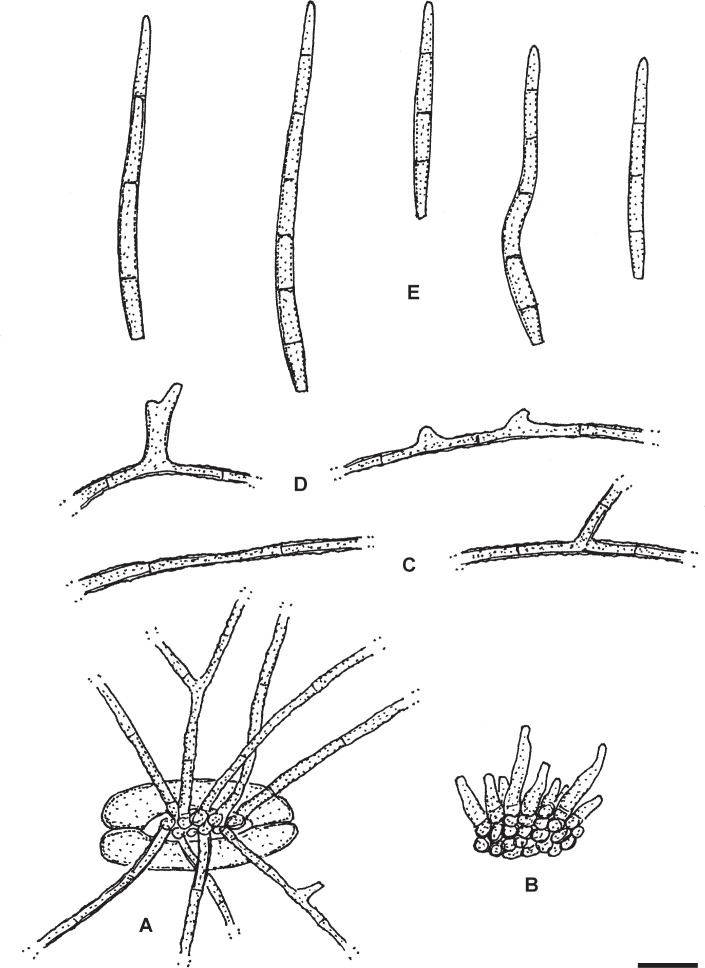
Pseudocercospora diospyri-lycioides Crous & U. Braun, Mycol. Res. 99(1): 34. 1995. Fig. 4.
Fig. 4.
Pseudocercospora diospyri-lycioides (PREM 51106 – holotype). A. Stromata and conidiophore fascicles. B. Conidiophores. C. Conidia. Scale bar = 10 μm. U. Braun del.
Illustration: Crous & Braun (1995: 34, fig. 6).
Description in vivo: Leaf spots amphigenous, circular to somewhat irregular, 1–5 mm diam, brown, margin dark brown. Caespituli amphigenous, punctiform, scattered, medium brown. Mycelium internal. Stromata immersed or substomatal, erumpent, 10–40 μm diam, olivaceous to olivaceous brown or yellowish brown. Conidiophores fasciculate, arising from stromata, forming sporodochioid conidiomata, 25–50 μm wide and 20–35 μm high, individual conidiophores erect, straight to curved, unbranched, subcylindrical, conical, ampulliform or somewhat geniculate-sinuous, 5–15 × 1–3 μm, usually reduced to conidiogenous cells (aseptate), sympodially proliferating, subhyaline, pale yellowish green or very pale olivaceous, thin-walled, conidiogenous loci inconspicuous, unthickened, not darkened, sometimes visible as truncated tips. Conidia solitary, narrowly subcylindrical to almost fusiform, straight to somewhat curved, 15–110 × 1–2 μm, 0–8-septate, very pale olivaceous, apex acute, subacute or rounded, base short obconically truncated or rounded, hila 0.5–1 μm wide, unthickened and not darkened.
Typus: South Africa, Gauteng Province, Pretoria, Roodeplaat, Experimental farm, Vegetable & Ornamental Research Institute, on Diospyros lycioides, Mar. 1988, E.J. van der Linde (PREM 51106 – holotype).
Host range and distribution: Only known from the type collection.
Pseudocercospora diospyri-morrisianae Goh & W.H. Hsieh, Trans. Mycol. Soc. Republ. China 2(2): 90. 1987.
Synonym: Cercospora diospyri-morrisianae Sawada, Rep. Gov. Res. Inst. Dept. Agric., Formosa 85: 103. 1943, nom. inval. (Art. 39.1).
Literature: Hsieh & Goh (1990: 108–109), Guo & Hsieh (1995: 91), Guo et al. (1998: 107–108).
Illustrations: Hsieh & Goh (1990: 109, fig. 82), Guo & Hsieh (1995: 92, fig. 83), Guo et al. (1998: 107, fig. 87).
Description in vivo: Leaf spots indistinct on the upper leaf surface, below visible as pale fuliginous angular spots, 3–7 mm diam. Colonies hypophyllous, sparingly effuse. Mycelium internal and external; superficial hyphae emerging through stomata. Stromata lacking. Conidiophores in small, loose fascicles, arising from internal, substomatal hyphae, emerging through stomata, and solitary, arising from superficial hyphae, erect, straight, subcylindrical or conical when short to curved or somewhat geniculate-sinuous, simple or branched, 5–50 × 2.5–4.5 μm, 0–2-septate, olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci inconspicuous, neither thickened nor darkened. Conidia solitary, narrowly obclavate, straight to somewhat curved, apex subacute or subobtuse, base short obconically truncated, 40–90 × 2–3 μm, 3–7-septate, subhyaline, thin-walled, smooth, hila 1–1.5 μm wide, unthickened, not darkened.
Typus: Taiwan, Yilan, on Diospyros morrisiana, 25 Jul. 1926, K. Sawada (NTU-PPE, herb. Sawada – holotype). Isotypes: BPI 435802, BPI 435803.
Host range and distribution: On Diospyros morrisiana, Asia (Taiwan).
Notes: This species is, as far as known, confined to Diospyros morrisiana, an Asian shrub and tree known from China, Japan, Taiwan, and Vietnam. It is well characterised by forming small conidiophore fascicles and solitary conidiophores arising from superficial hyphae, and long, narrowly obclavate, subhyaline conidia, 40–90 × 2–3 μm. Records of P. diospyros-morrisianae on Diospyros kaki and D. lotus are not conspecific and belong to another species (see Pseudocercospora diospyriphila).
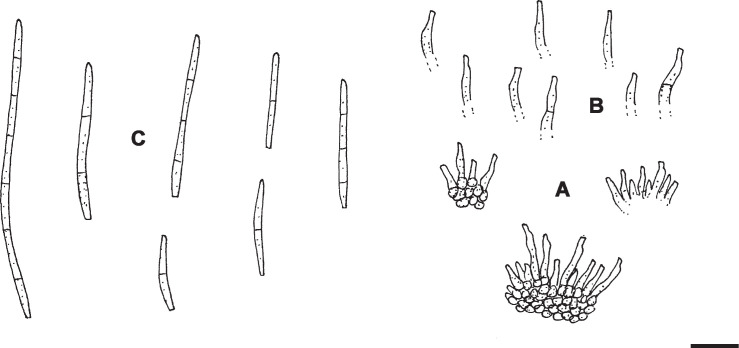
Pseudocercospora diospyriphila H.D. Shin & U. Braun, sp. nov. MycoBank MB833835. Fig. 5.
Fig. 5.
Pseudocercospora diospyriphila (KUS-F11042 – holotype). A. Conidiophore fascicle. B. Conidia. Scale bar = 10 μm. U. Braun del.
Etymology: Composed of Diospyros, the genus name of the host plant, and -philus (loving).
Literature: Shin & Braun (1993: 356), Shin (1995: 205), Kim & Shin (1998: 442), Shin & Kim (2001: 187–189).
Illustrations: Shin (1995: 199, fig. 1 g, 205, fig. 5 a, b), Shin & Kim (2001: 188, fig. 83).
Diagnosis: Similar to Pseudocercospora diospyri-morrisianae, but on Diospyros lotus, with amphigenous leaf spots, filiform-subcylindrical longer conidia, pale olivaceous brown, and wider, 15–85 × 3–4.5 μm.
Description in vivo: Leaf spots amphigenous, scattered, occasionally confluent, circular to angular, 1–10 mm diam, at first indistinct or formed as rather indistinct discolorations with indistinct margin, later reddish brown with blackish brown margin, finally forming angular olivaceous patches on the lower leaf surface. Caespituli amphigenous, mostly hypophyllous, forming pale olivaceous patches. Mycelium internal and external; external hyphae superficial, emerging through stomata, branched, 2–5 μm wide, septate, subhyaline to pale olivaceous, thin-walled, smooth, forming solitary conidiophores, lateral. Stromata lacking or almost so. Conidiophores in loose, small fascicles, 4–10, arising from internal hyphae or rudimentary substomatal stromatic hyphal aggregations, erect, almost straight to somewhat curved, rarely geniculate, somewhat narrowed towards rounded or conic tips, usually unbranched, 15–55 × 2.5–3.5 μm, 0–3-septate, olivaceous brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci inconspicuous or visible as truncated tips, but neither thickened nor darkened. Conidia solitary, subcylindrical, longer ones almost filiform, shorter ones may be obclavate, almost straight to curved, 15–85 × 3–4.5 μm, 2–11-septate, non or only slightly constricted at the septa, pale olivaceous brown, thin-walled, smooth, apex subacute or subobtuse, base obconically truncated, hila 1–2 μm wide, neither thickened nor darkened.
Description in vitro: Colonies on PDA reaching 30 mm diam after 28 days at 25 °C in the dark; surface folded, radially wrinkled, erumpent with scarce aerial mycelium and irregular lobate margins; surface grey olivaceous in the centre, dark grey in the outer region; reverse dark grey to nearly black, non-sporulating.
Typus: Korea, Kangnung, on Diospyros lotus (Ebenaceae), 9 Sep. 1991, H.D. Shin (KUS-F11042 – holotype).
Additional materials examined: Korea, Kangnung, on Diospyros lotus, 3 Oct. 1991, H.D. Shin (KUS-F11214); Kangnung, on D. lotus, 1 Sep. 1993, H.D. Shin (KUS-F12585); Kangnung, on D. lotus, 30 Oct. 1994, H.D. Shin (KUS-F13280); Kangnung, on D. lotus, 20 Oct. 1998, H.D. Shin (KUS-F12585); Seoul, on D. lotus, 20 Oct. 1998, H.D. Shin (KUS-F15515); Seoul, on D. lotus, 23 Oct. 1998, H.D. Shin (KUS-F15546); Ganghwa, on D. lotus, 28 Sep. 1999, H.D. Shin (KUS-F16854); Jeonju, on D. lotus, 23 Sep. 2000, H.D. Shin (KUS-F17589); Kangnung, on D. lotus, 17 Oct. 2000, H.D. Shin (KUS-F17900); Jinju, on D. lotus, 29 Oct. 2000, H.D. Shin (KUS-F17960); Jeju, on D. kaki, 1 Oct. 2013, H.D. Shin (KUS-F27662), culture – KACC47650.
Host range and distribution: On Dioscorea kaki and D. lotus, Asia (Korea).
Notes: Collections of this species were previously assigned to Pseudocercospora diospyri-morrisianae, described from Taiwan on Diospyros morrisiana, but Korean collections on Diospyros lotus are morphologically readily distinguishable (see diagnosis) and warrant a species of its own. This species has also been recorded on Diospyros kaki in Korea (Shin & Kim 2001). A culture obtained from this species on D. kaki has been used to generate sequences used for phylogenetic analyses, which confirmed it to represent a novel species.
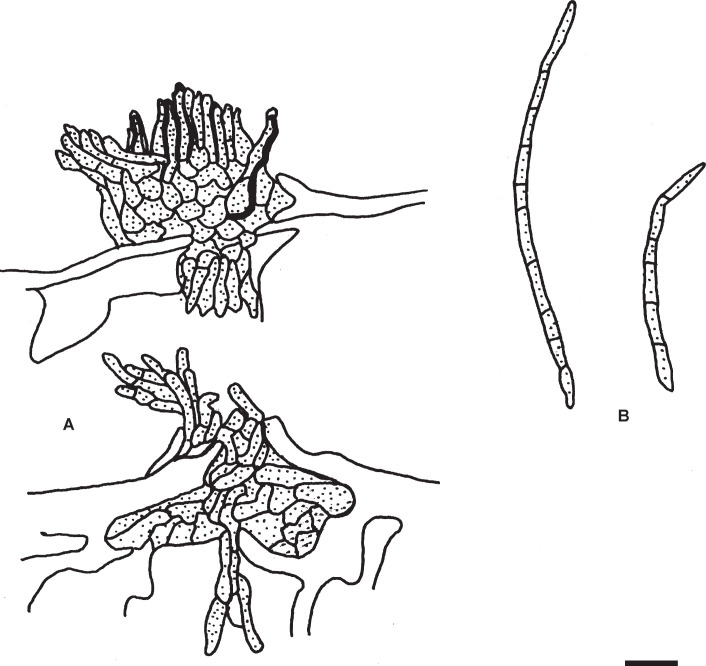
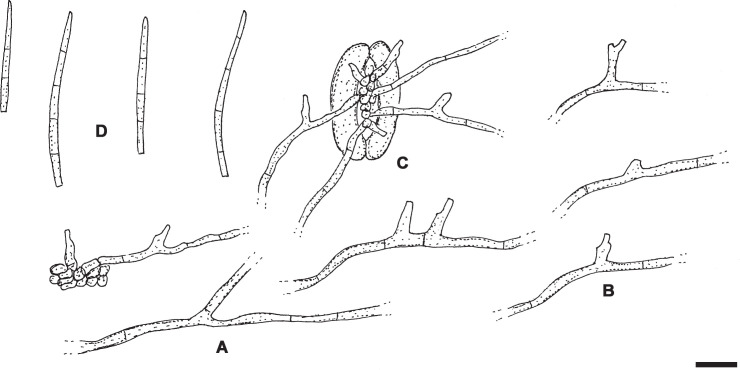
Pseudocercospora ershadii M. Bakhshi, U. Braun & Zare, sp. nov.MycoBank MB833836. Fig. 6.
Fig. 6.

Pseudocercospora ershadii (IRAN16456F – holotype). A, B. Symptoms on leaves. C–F. Conidiophore fascicles. G–K. Conidia. Scale bars = 10 μm.
Etymology: Named in honour of Djafar Ershad, Iranian mycologist, for his outstanding contribution to the knowledge of the fungi in Iran.
Literature: Bakhshi et al. (2014: 258, as Pseudocercospora sp. B).
Illustrations: Bakhshi et al. (2014: 259, fig. 9, as Pseudocercospora sp. B).
Diagnosis: Morphologically very similar to Pseudocercospora kaki, but confined to Diospyros lotus and genetically distinct, forming a separate clade distant from P. kaki; colonies amphigenous, sporodochial on the upper leaf surface, with large stromata, 20–70 μm diam, less conspicuous below, with smaller stromata and fasciculate conidiophores emerging through stomata, conidia mostly 25–65 × 1.5–4.5 μm, with narrow hila, 1–2 μm wide.
Description in vivo: Leaf spots amphigenous, distinct, scattered, subcircular to angular-irregular, 2–7 mm diam, brown, later centre pale brown, margin darker brown, slightly raised. Caespituli amphigenous, conspicuous on the upper leaf surface, punctiform by sporodochial conidiomata with large stromata, dark brown to blackish, scattered, less conspicuous below. Mycelium internal. Stromata well-developed and large on the upper side, 20–70 μm diam, immersed to erumpent, olivaceous brown to brown, composed of swollen hyphal cells, subcircular to slightly angular-irregular in outline, 2–6 μm diam, stromata almost lacking or smaller on the lower leaf side, 10–30 μm diam, substomatal. Conidiophores on the upper leaf surface in sporodochial conidiomata, numerous, dense, arising from well-developed stromata, on the lower side in smaller fascicles, arising from internal hyphae or smaller substomatal stromata, emerging through stomata or erumpent, erect, straight, subcylindrical or somewhat attenuated towards the tip to somewhat geniculate-sinuous, unbranched, apex rounded to subtruncated, (5–)10–25(–45) × 2–4.5 μm, pale olivaceous, olivaceous brown to medium brown, paler towards the tip, shorter ones sometimes subhyaline, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores often reduced to conidiogenous cells, 5–25 μm long, conidiogenous loci inconspicuous, sometimes distinctly truncated, 1–2.5 μm wide, but always unthickened and not darkened. Conidia solitary, obclavate-cylindrical, 25–65(–130) × 1.5–4.5 μm, (1–)3–8(–15)-septate, subhyaline to pale olivaceous or pale brownish, apex obtuse or subobtuse, base mostly obconically truncated, hila 1–2.5 μm wide, unthickened, not darkened.
Description in vitro: Colonies on MEA reaching 28 mm diam after 20 days at 25 °C in the dark; surface folded, erumpent with moderate aerial mycelium and irregular lobate margins; surface honey in the centre, dark brown in the outer region; reverse dark brown, non-sporulating.
Typus: Iran, Mazandaran Province, Ramsar, on Diospyros lotus (Ebenaceae), Oct. 2012, M. Bakhshi (IRAN 16456F – holotype); CCTU 1206 = CBS 136114 – ex-type culture.
Additional materials examined: Iran, Guilan Province, Shaft, Siahmazgi, 37°01′19.13″ N, 49°16′25.45″ E, 350 m alt., on D. lotus, 16 Aug. 2018, M. Bakhshi (IRAN 17594F; living culture IRAN 3431C); Guilan Province, Talesh, Kishunben, on D. lotus, Oct. 2012, M. Bakhshi (CCTU 1066); Golestan Province, Aliabad-e Katul, Kaboudval Waterfall, 36°52′ 31.7″ N, 54°53′ 13.7″, 332 m alt., on D. lotus, 4 Jul. 2017, M. Bakhshi (IRAN 17592F, IRAN 17591F; living cultures IRAN 3429C, IRAN 3428C); Mazandaran Province, Jannat Roudbar, Dalikhani forest, 36°49′10.49″ N, 50°40′05.06″ E, 660 m alt., on D. lotus, 14 Aug. 2018, M. Bakhshi (IRAN 17593F; living culture IRAN 343°C); Mazandaran Province, Ramsar, Kotra, on D. lotus, Oct. 2012, M. Bakhshi (CCTU 1191).
Host range and distribution: On Diospyros lotus, Asia (Iran).
Notes: This species is morphologically close to Pseudocercospora kaki, but superficial mycelium is always lacking. Pseudocercospora ershadii differs from P. kaki in forming abundant hypophyllous caespituli. Furthermore, the new species from Iran is genetically clearly distinct from P. kaki. An older specimen collected in Iran agrees well with the latter species: Iran, Sistan and Beluchestan, Goryā n, on Diospyros sp. (cf. lotus), Sep. 1940, E. Esfandiari, ex herb. Petrak (M-0291601, Fig. 7).
Fig. 7.
Pseudocercospora ershadii (M-0291601). A. Conidiophore fascicles. B. Conidia. Scale bar = 10 μm. U. Braun del.
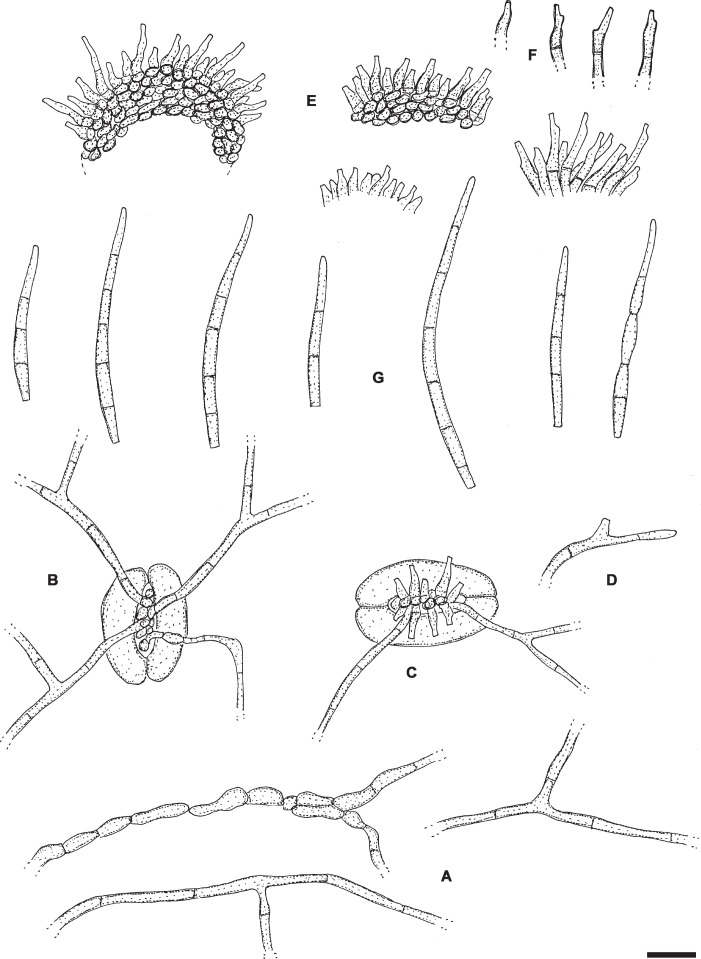
Pseudocercospora fuliginosa (Ellis & Kellerm.) W.X. Zhao & Y.L. Guo, Acta Mycol. Sin. 12(3): 195. 1993. Figs 8, 9.
Fig. 8.
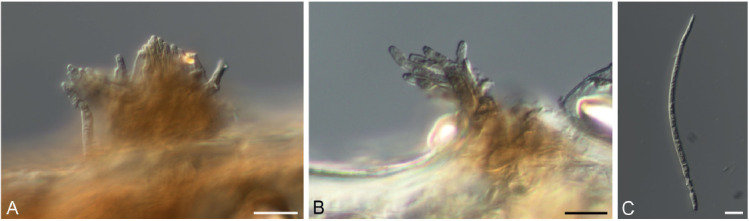
Pseudocercospora fuliginosa (CNS-219). A, B. Stromata with conidiophore fascicles. C. Conidia. Scale bars = 10 μm.
Fig. 9.
Pseudocercospora fuliginosa (CNS-219). A. Stromata with conidiophore fascicles. B. Conidia. Scale bar = 10 μm. C. Nakashima del.
Basionym: Cercospora fuliginosa Ellis & Kellerm. (“fuligniosa”), J. Mycol. 3(9): 103. 1887.
Synonyms: Passalora fuliginosa (Ellis & Kellerm.) Crous et al., Mycotaxon 64: 412. 1997.
Cercospora atra Ellis & Everh., J. Mycol. 4(1): 4. 1888 [type: USA, Delaware, New Castle, Faulkland, on Diospyros virginiana, 8 Aug. 1887, A. Commons 591 (NY 03615240 – lectotype, designated here, MycoBank MBT390100; NY 03615241, PH 811, PH 812 – isolectotypes)].
Pseudocercospora hunanensis Y.L. Guo, Mycosystema 35(1): 17. 2016 [type: China, Hunan Province, Zhangjiajie, on Diospyros kaki, 15 Sep. 1987, W.X. Zhao 179 (HMAS 62125 – holotype)].
Literature: Saccardo (1892: 648), Chupp (1954: 203), Katsuki (1965: 29), Guo & Hsieh (1995: 92), Guo et al. (1998: 108–109), Crous & Braun (2003: 191).
Illustrations: Guo & Hsieh (1995: 93, fig. 84), Guo et al. (1998:108, fig. 88), Guo (2016: 17, fig. 2).
Description in vivo: Leaf spot amphigenous, 0.5–8 mm diam, subcircular to mostly angular, vein-limited, sometimes confluent, brown, dark brown to blackish on the upper leaf surface, reddish brown below. Caespituli usually hypophyllous, scattered, punctiform, dark. Mycelium internal. Stromata lacking or almost so or globular, 10–50 μm diam, brown. Conidiophores in small to moderately large fascicles, usually 5–20, loose to dense and compact, arising from internal hyphae or stromata, erect, straight, subcylindrical to somewhat geniculate-sinuous, unbranched, 10–75 × 2.5–4 μm, 0–3-septate, pale olivaceous brown or brown, thin-walled, smooth, tips obtuse; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, about 10–25 μm long, conidigenous loci inconspicuous or subdenticulate, neither thickened nor darkened. Conidia solitary, obclavate to obclavate-cylindrical, straight to curved or somewhat sigmoid, 20–105 × 2.5–4 μm, 1–9-septate, pale olivaceous, thin-walled, smooth, apex obuse, subobtuse to subacute, base obconically truncated, hila about 2–2.5 μm wide, unthickened and not darkened.
Typus: USA, Kansas, Mound City, on Diospyros virginiana, Jul. 1887, W. A. Kellerman 1010 (NY 03616841 – lectotype, designated here, MycoBank MBT390101). Isolectotype: CHRB-F-0002285.
Additional material examined: Japan, Gunma, Shinto, Gunma Pref. Forest Exp. Stn., on Diospyros kaki, 10 Sep. 1997, T. Kobayashi & C. Nakashima (CNS-219); MAFF237710 = MUCC1271 – cultures.
Host range and distribution: On Diospyros chloroxylon [= D. tomentosa] (Asia: India: West Bengal), D. kaki (Asia: China, Japan; North America: USA: Alabama, Oklahoma, Oregon, Texas), D. lotus (Asia: Japan), D. nana (North America: USA: California), D. texana (North America: USA: Alabama, Florida, Georgia, Illinois, Mississippi, Texas), D. virginiana (North America: USA: Alabama, Delaware, Florida, Georgia, Illinois, Kansas, Mississippi, North Carolina, Ohio, Texas).
Notes: In the original description the name “C. fuligniosa” was used, which was undoubtedly a typographical error. Saccardo and Sydow (in Saccardo 1899) corrected the spelling to “fuliginosa”. Chupp (1954: 203) used the wrong spelling in the text, but listed C. fuliginosa in the register (p. 656). Furthermore, Chupp (l.c.) reduced the name Cercospora diospyri Viégas to synonymy with C. fuliginosa. Crous et al. (1997) re-examined type material of C. diospyri Viégas, but followed Chupp’s (l.c.) treatment of this name as synonym of C. fuliginosa and reallocated the latter name to Passalora. However, Chupp’s (l.c.) synonymy was incorrect. Therefore, Crous, Alfenas and Barreto (in Crous & Braun 2003) introduced the new name Passalora diospyri (now Zasmidium diospyri-hispidae) for Cercospora diospyri Viégas (non C. diospyri Thüm., 1878). Braun & Crous (2005: 409) discussed the nomenclature and taxonomy of the names involved and emphasised that C. fuliginosa represents a true species of the genus Pseudocercospora, confirmed by the re-examination of type material deposited at NY. Based on the erroneous reallocation of C. fuliginosa to Passalora by Crous et al. (1997), Guo (2016) introduced Pseudocercospora hunanensis for the collection on Diospyros kaki in China previously published as Pseudocercospora fuliginosa (Guo & Hsieh 1995, Guo et al. 1998). She undoubtedly overlooked the clarification of the latter name published by Braun & Crous (2005). Diospyros kaki is known as host of P. fuliginosa in North America. Diospyros virginiana is the type host of P. fuliginosa. Collections on D. kaki and D. virginiana are morphologically barely distinguishable and for the time being placed in a single species. Whether two species are involved can only be clarified on the basis of molecular sequence analyses including data retrieved from collections on D. virginiana as well as Asian and North America collections on D. kaki.
Chupp (1954) examined type material of Cercospora fuliginosa and C. atra and provided a detailed description. Ellis & Kellerman (1887) described much longer conidiophores, 100–150 μm, and short conidia, 35–40 μm.
An Asian record of C. fuliginosa on Diospyros lotus refers to Katsuki (1965) and Kobayashi (2007) but needs to be confirmed by re-examinations of the corresponding specimens or new collections (Katsuki, l.c., emphasised that he had never seen any Japanese specimens of this species), and the same applies to a record on the Asian species D. nana in California, which has not yet been re-examined. However, a Japanese specimen, including culture, collected on D. kaki has been examined and sequence data have been retrieved. The Japanese sequence confirmed the involvement of a species of its own distant from all other Pseudocercospora spp. on Diospyros, but sequence data obtained from P. fuliginosa on D. virginiana in the USA are still needed for comparison and confirmation.
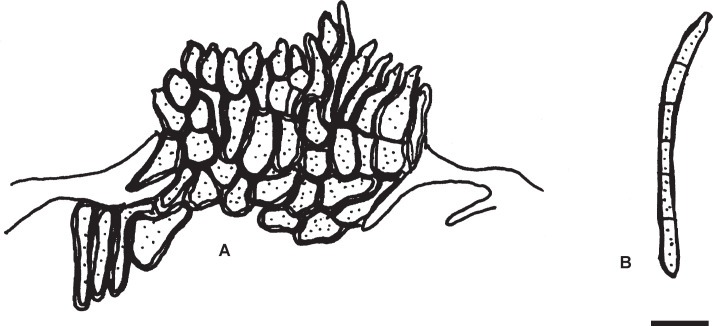
Pseudocercospora kaki Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and Similar Fungi from Taiwan (Taipei): 109. 1990. Figs 10–13.
Fig. 10.
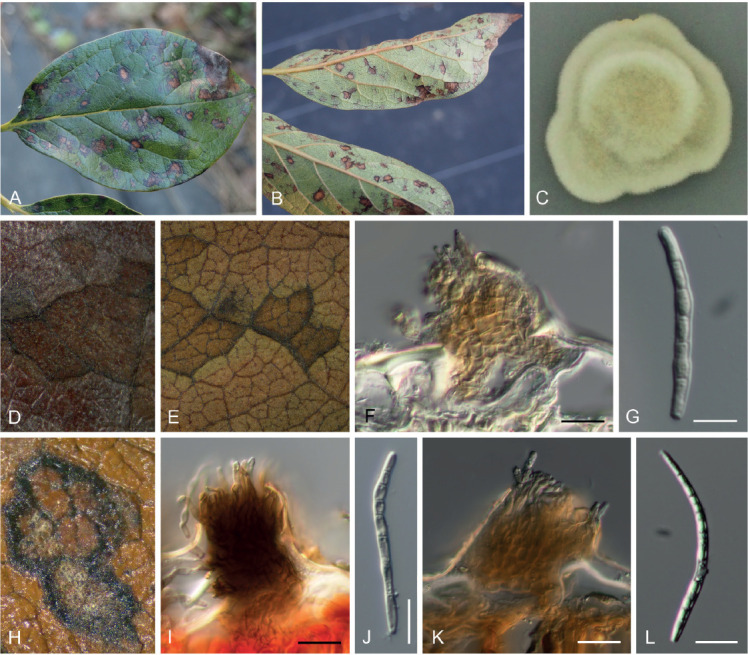
Pseudocercospora kaki, Japanese collections. A. Leaf symptoms on the upper surface. B. Leaf symptoms on the lower surface. C. Culture on PDA (MAFF238214 – ex-epitype culture). D. Magnified symptoms on the upper surface (MUMH-11899 – epitype). E. Magnified symptoms on the lower surface (MUMH-11899 – epitype). F. Stromata and conidiophores (MUMH-11899 – epitype). G. Conidium. H. Magnified symptom on D. kaki from Brazil (NIAES 127-1-8). I. Stroma and conidiophores (NIAES 127-1-8). J. Conidium (NIAES 127-1-8). K. Stroma (CNS-29). L. Conidium (CNS-29). Scale bars = 10 μm.
Fig. 13.
Pseudocercospora kaki (NY 937048 – lectotype of Cercospora kaki). A. Superficial hyphae. B. Superficial hyphae emerging through a stoma. C. Superficial hyphae and conidiophores emerging through a stoma. D. Solitary conidiophore arising from a superficial hypha. E. Conidiophore fascicles. F. Conidiophores. G. Conidia. Scale bar = 10 μm. U. Braun del.
Synonym: Cercospora kaki Ellis & Everh., J. Mycol. 3: 17. 1887, non Pseudocercospora kaki Goh & W.H. Hsieh, 1990 [type: USA, Louisiana, Lafayette Parish, Lafayette, on Diospyros kaki, 21 Sep. 1886, A.B. Langlois, 722 (NY 937048 – lectotype, designated here, MycoBank MBT390102; BPI 437530 – isolectotype); topotype material – Ellis & Everh., North Am. Fungi 1758 (e.g. BPI 437526) and NY 838243 (Nov. 1886)].
Literature: Chupp (1954: 203–204), Katsuki (1965: 29–30), Guo & Hsieh (1995: 92), Guo et al. (1998: 109–110).
Illustrations: Hsieh & Goh (1990: 110, fig. 83), Guo et al. (1998: 109, fig. 89).
Description in vivo: Leaf spots amphigenous, at first visible as minute dark specks, later subcircular to angular-irregular, 1–10 mm diam, sometimes confluent and larger, to 20 mm diam, medium brown to dark or blackish brown, with narrow darker margin, later centre sometimes becoming paler, greyish brown to greyish white, often vein-limited. Caespituli in some collections exclusively or predominantly epiphyllous, punctiform, pustulate, dark, scattered to aggregated, in other collections amphigenous, conspicuous on the upper side, punctiform by sporodochial conidiomata, blackish, but less conspicuous below and more effuse and paler. Mycelium either exclusively internal or internal and external, superficial hyphal confined to the lower leaf surface; hyphae emerging through stomata, branched, 1–4 μm wide, septate, not or sometimes constricted at the septa, subhyaline to pale olivaceous or olivaceous brown, thin-walled, smooth, without or with solitary conidiophores, superficial hyphae sometimes with swollen hyphal cells, 2–5 μm diam. Stromata well-developed, on the upper side large, 20–100 μm diam, immersed, lacking or almost so or smaller below, 10–40 μm diam, usually substomatal, globose or somewhat irregularly shaped, occasionally oblong, medium to dark brown or olivaceous brown, hyphal cells 2–6 μm diam. Conidiophores on the upper side very numerous in dense fascicles, arising from large stromata, forming sporodiochial conidiomata, conidiophores on the lower side in smaller fascicles, loose to dense, arising from internal hyphae or smaller substomatal stromata, erumpent or emerging through stomata, erect, subcylindrical or slightly attenuated towards the obtuse or sometimes truncated tip, unbranched, straight to curved or somewhat sinuous, not or only slightly geniculate, 5–30(–40) × 1.5–5 μm, 0–1-septate, subhyaline to pale olivaceous or olivaceous brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores often reduced to conidiogenous cells, 5–20 μm long, proliferation sympodial, rarely percurrent, conidiogenous loci inconspicuous, 1.5–2 μm diam when truncated, unthickened, not darkened. Conidia solitary, narrowly obclavate-cylindrical, subacicular, straight to somewhat curved or sigmoid, apex obtuse to subacute, base truncated to usually short obconically truncated, rarely rounded, (15–)25–80(–100) × 2–4 μm, 1–9-septate, distance between septa 5–15 μm, rarely somewhat constricted at the septa, subyhaline to pale olivaceous or very pale brownish, thin-walled, smooth, hila neither thickened nor darkened, (1–)1.5–2(–2.5) μm wide.
Description in vitro: Colonies on PDA reaching 25–30 mm diam after 28 d at 25 °C in the dark; surface lanate, undulate margins; surface citrine green to greyish yellow-green; reverse dark gray to dull green, non-sporulating.
Typus: Taiwan, Taipei, on Diospyros kaki, 8 Oct. 1928, K. Sawada (NTU-PPE, herb. Sawada – holotype). Isotype: HMAS 04923. Epitype: Japan, Toyama, Kureha, on Diospyros kaki, 25 Sep. 1998, T. Kobayashi, E. Imaizumi, & R. Hori (MUMH11899, designated here, MycoBank MBT390103). Ex-epitype culture: MAFF238214 = MUCC903 and 1320.
Additional materials examined: (1) Sequenced herbarium samples with exclusively internal mycelium in vivo – Brazil, São Paulo, Porto Feliz, on Diospyros kaki, 16 Nov. 2017, R.F. Alves (strain CC30); São Paulo, Mogi das Cruzes, on D. kaki, 14 May 2018, R.F. Alves (strain CC40); São Paulo, Guararema, on D. kaki, 14 May 2018, R.F. Alves (strain CC43). Japan, Chiba, Togane, on D. kaki, 1 Oct. 1993, T. Kobayashi (TFM:FPH 7411; culture – MUCC1213 = MAFF237013); Chiba, Matsudo, on D. kaki, Oct. 1991, T. Kobayashi (culture – MUCC1189 = MAFF235880). Japan, Nagano, Kasakami, on D. kaki, 1 Oct, 1995, K. Kishi (MUMH11900; culture – MUCC1063); Chiba, Togane, on D. kaki, 1 Oct. 1993, T. Kobayashi (culture – MUCC1213 = MAFF237013); Chiba, Matsudo, on D. kaki, Oct. 1991, T. Kobayashi (TFM:FPH 7411; culture – MUCC1189 = MAFF235880); Nagano, Kawakami, on D. kaki, 1 Oct. 1995, K. Kishi (MUMH11900; culture – MUCC1063).
(2) Collections (herbarium material) with exclusively internal mycelium in vivo – Brazil, São Paulo, Mogi das Cruzes, on D. kaki, 10 April 1974, T. Hino (NIAES 127-1-8). China, Kwangsi, Yanso, on Diospyros kaki, 10 Sep. 1938, S.H. Ou 336 (BPI 437539). Taiwan, Taipei, on D. kaki, 8 Nov. 1933, W. Yamamoto (BPI 4375235, 437540, paratype material).
(3) Collections (herbarium material) with internal and external mycelium and solitary conidiophores arising from superficial hyphae in vivo – Bermuda, Agricultural Station, on D. kaki, Dec. 1921, H.S. Whetzel (BPI 437532). Brazil, Est. São Paulo, Cantareira, Sec. Fitopatologia, on Diospyros kaki, 23 Mar. 1940, S.C. Silva (CUP 29352); state of São Paulo, Piracicaba, on Diospyros kaki, 15 May 2019, M.B. Sposito (HAL 3320 F). China, Nanking, Kiangsu, on D. kaki, 28 Sep. 1928, R.H. Porter [ex herb. Univ. Nanking 850] (BPI 437538); Guangdong, Guangzhou, on Diospyros sp. (cf. kaki), 3 Sep. 1961, Q.M. Ma & X.J. Liu 1162, ex HMAS 59063 (BPI 1109709). Japan, Tottori, on D. kaki, 27 Sep. 1921, T. Fukushi (BPI 437536); Yokohama, Tozuka, Nakata, on D. kaki, 24 Aug. 1950, T. Miyakawa (CUP 40099); Japan (intercepted at San Pedro), on D. kaki, 1 Nov. 1967, M.E. Stroope (BPI 437527); (?) Tokyo, on D. kaki, 1911, S. Suzuki (CUP-S-0277, material very sparse). USA, Louisiana, Baton Rouge, on D. kaki, 5 Aug. 1908, W.A. Orton (BPI 437531); Texas, Gonzales, on D. kaki, 10 Sep. 1909, F.D. Heald & F.A, Wolf 2651 (BPI 437529); Texas, Hallettsville, on D. kaki, 13 Sep. 1909, F.D. Heald & F.A, Wolf 2778 (BPI 437528); Texas, San Antonio, on D. kaki, Nov. 1921, G.T. Ratliffe (BPI 437533); Alabama, on D. kaki, Oct. 1923, H.L. Dozier (BPI 437537).
Host range and distribution: On Diospyros kaki, Asia (China, Japan, Taiwan), North America (USA: Alabama, California, Florida, Louisiana, Texas), Bermuda, South America (Brazil).
Notes: Hsieh & Goh (1990) supposed that Pseudocercospora kaki and Cercospora kaki might be synonymous. Type material of the two species is, indeed, morphologically very similar, but C. kaki, based on North America type material, differs in forming abundant hypophyllous mycelium with solitary conidiophores, which are lacking in type material of P. kaki. However, several examined Chinese and Japanese herbarium specimens on Diospyros kaki agree well with type material of C. kaki in having superficial hyphae, sometimes with solitary conidiophores, suggesting that the previous assumption of Hsieh & Goh (1990) might be correct. Numerous specimens of Pseudocercospora on D. kaki, recently collected in Brazil, examined, cultivated and sequenced, turned out to be genetically conforming with P. kaki. All examined specimens from Brazil are characterised by forming superficial hyphae with solitary conidiophores in vivo, supporting that this species comprises collections with and without superficial mycelium. There are specimens without any trace of superficial hyphae, only with superficial hyphae (without solitary conidiophores), as well as with abundant superficial hyphae and solitary conidiophores. It is currently unknown which external influences are responsible for the formation or absence of superficial hyphae in vivo.
Cercospora kaki is reduced to synonymy with Pseudocerco-spora kaki, at least tentatively until suitable North American samples and cultures will be available for epitypification and phylogenetic confirmation. Japanese persimmon was introduced in Florida in the 1800s (Boning 2006), probably from Japan or other Asian countries, likely together with Pseudocercospora.
Chinese collections on Diospyros lotus assigned to P. kaki (Guo & Hsieh 1995) need to be re-examined and verified. They might rather pertain to P. kakiicola or P. ershadii. Older North American records of Cercospora kaki on Diospyros texana (Anonymous 1960) probably refer to Pseudocercospora fuliginosa. Vasudeva (1963) published a brief description of “C. kaki” on Diospyros melanoxylon from India, but this fungus belongs to another species (see Pseudocercospora tessellata). Kobayashi (2007) listed “C. kaki” on Diospyros japonica from Japan. Material on this host should be re-examined and its identity has to be proven. It is possible that the Japanese record on D. japonica rather pertains to P. diospyri-japonicae. A collection of “C. kaki” on Diospyros sandwicensis from Hawaii (BPI 607947 B) has been re-examined. It contains ascomata of Mycosphaerella sp., but a cercosporoid fungus has not been found. Diospyros kaki is infested by several Pseudocercospora species. Therefore, records of C. kaki on D. kaki from areas outside Asia and North America also need confirmation, e.g., from Australia (Simmonds 1966), and South Africa (Gorter 1977). Pirnia et al. (2012) listed Pseudocercospora kaki on Diospyros kaki and D. lotus from Iran, but without any description and illustration. Guo & Liu (1991) reported P. kaki on Diospyros “kaki var. japonica” from China. “Var. japonica” is an unpublished name which probably refers to Diospyros japonica. The Chinese record may refer to P. diospyri-japonicae. Other reports of C. kaki on various hosts have to be excluded or they are doubtful, e.g., Indian records on Diospyros tomentosa and D. virginiana (Kamal 2010) are unclear, doubtful, and require re-examinations.
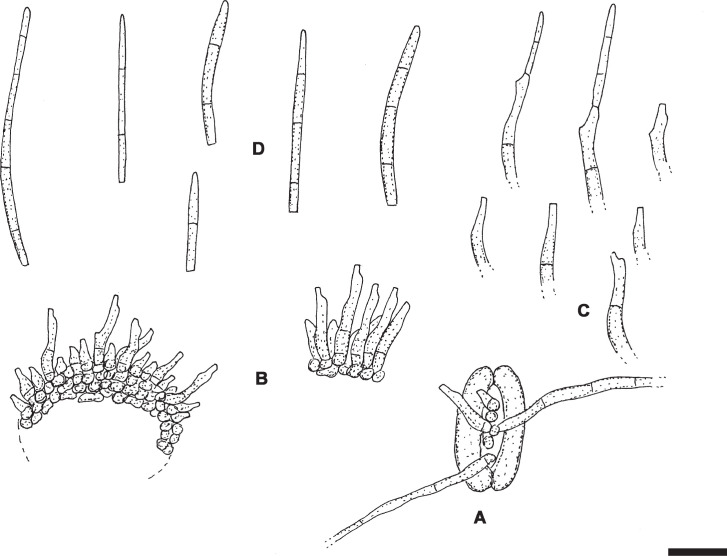
Pseudocercospora kakiicola C. Nakash., sp. nov. MycoBank MB833838. Figs 14, 15.
Fig. 14.
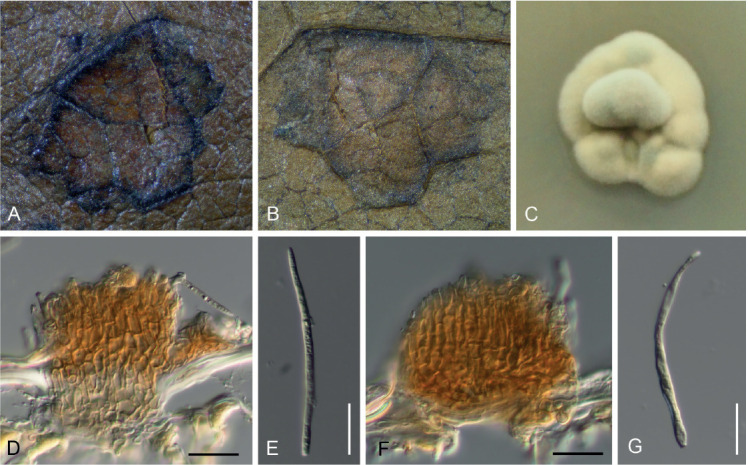
Pseudocercospora kakiicola. A. Magnified symptoms on the upper surface (MUMH-11898 – holotype). B. Magnified symptoms and sign on the lower surface (MUMH-11898 – holotype). C. Culture on PDA (MAFF238238 – ex-type culture). D. Stroma and conidiophores (MUMH-11898 – holotype). E. Conidium (MUMH-11898 – holotype). F. Stroma and conidiophores (MUMH-11901). G. Conidium (MUMH-11901). Scale bars = 10 μm.
Fig. 15.
Pseudocercospora kakiicola. Stromata with conidiophore fascicles and conidia. A. ex MUMH11901. B. ex MUMH11898. Scale bar = 10 μm. C. Nakashima del.
Etymology: Name composed of the epithet of the host plant, kaki, and -icola (from incola = dweller).
Diagnosis: Morphologically close to collections of Pseudocercospora kaki but conidiogenous cells sympodially and percurrently proliferating, conidia somewhat wider, 2.5–5 μm (vs. 2–4 μm), with numerous septa, 5–15 (vs. 1–9), and phylogenetically clearly distinct.
Description in vivo: Leaf spots amphigenous, at first visible as minute dark specks, later angular to angular-irregular, medium brown to dark or blackish brown on upper leaf surface, darker on lower surface, with blackish border, vein-limited, 1–10 mm diam, often confluent and larger, to 20 mm diam, medium brown to dark or blackish brown with narrow darker margin, resulting in reticular leaf spots with irregular blackish border. Caespituli amphigenous, dense, with abundant well-formed conidiophores and conidia. Mycelia internal and external; hyphae simple or branched, colourless to pale brown, smooth, 2–3 μm wide, septate. Stromata amphigenous, mainly epiphyllous, intraepidermal or in the stomatal cavity, erumpent or emerging through stomata, small, composed of few brown cells to well-developed, pale brown to brown, 20–100 μm diam. Conidiophores densely fasiciculate, arising from stromata, but also solitary, arising from external hyphae, very pale brown to brown, simple, straight to slightly curved, or geniculate at the apex, smooth, thin-walled, irregular in width, 0–1(–3)-septate, 5–25 × 2–5 μm; conidiogenous cells integrated, terminal, or conidiophores reduced to conidiogenous cells, proliferating sympodially or percurrently, unilocal to multilocal, short conically truncated at the apex, loci neither thickened nor darkened, 2–2.5 μm diam. Conidia solitary, formation holoblastic, obclavate, occasionally acicular, straight to curved, 22–80 × 2–5 μm, 4–15-septate, hyaline to very pale brown, smooth, straight to slightly curved, thin-walled, base truncated, apex acute, basal hilum 2–2.5 μm diam, neither thickened nor darkened.
Description in vitro: Colonies on PDA reaching 25–30 mm diam after 28 d at 25 °C in the dark; surface folded, radially wrinkled, lanate, and undulate to irregular lobate margins; surface grey, yellow-green, mottled; reverse dark grey to dull green, non-sporulating.
Typus: Japan, Chiba, Kimitsu, Nakaseiwa, on Diospyros kaki, 18 Sep. 1998, S. Uematsu & C. Nakashima (CNS464 – holotype). Isotype: MUMH11898. Ex-type culture: MAFF238238 = MUCC900.
Additional materials examined: Japan, Toyama, Kureha, on Diospyros lotus, 25 Sep. 1998, T. Kobayashi, E. Imaizumi & R. Hori (MUMH11901; culture – MAFF238215 = MUCC1091); Mie, Tsu, on Diospyros kaki, 2007, I. Araki (culture – MUCC941).
Host range and distribution: On Diospyros kaki and D. lotus, Asia (Japan).
Notes: Pseudocercospora kakiicola is morphologically very close to collections of P. kaki with superficial hyphae and solitary conidiophores, but it is genetically clearly distinct from the latter species (see Fig. 1). Without sequence data, the discrimination of the two species is difficult, although there are slight morphological differences. Pseudocercospora kaki has somewhat narrower conidia (2–4 μm), with fewer septa (1–9).
Pseudocercospora kakiigena U. Braun, nom. nov., MycoBank MB833839. Fig. 16.
Fig. 16.
Pseudocercospora kakiigena (S-F42111 – holotype). A. Branched hypha. B. Superficial hyphae with solitary conidiophores. C. Conidiophores and hyphae emerging through a stoma. D. Conidia. Scale bar = 10 μm. U. Braun del.
Basionym: Cylindrosporium kaki Syd. & P. Syd., Ann. Mycol. 11: 116. 1913, non Pseudocercospopra kaki Goh & W.H. Hsieh, 1990.
Etymology: Named after the epithet of the host species, Diospyros kaki, + -genus (borne or produced in a certain place).
Description in vivo: Leaf spots amphigenous, subcircular, elliptical to angular-irregular, 1–5 mm diam, occasionally oblong, sometimes confluent, forming larger patches, pale to greyish brown. Caespituli hypophyllous, more or less effuse, greyish white. Mycelium internal and external; superficial hyphae emerging through stomata, branched, septate, 1.5–3.5 μm wide, thin-walled, almost hyaline to pale olivaceous, smooth. Stromata lacking or only with small substomatal hyphal aggregations, 10–20 μm diam, olivaceous brown. Conidiophores solitary, arising from superficial hyphae, lateral, rarely terminal, occasionally single conidiophores or small, loose conidiophores emerging through stomata, arising from internal hyphae or from small substomatal stromatic hyphal aggregations, subcylindrical, conical, slightly geniculate-sinuous, often with subdenticulate shoulders caused by sympodial proliferation, 3–20 × 2–4 μm, 0–1-septate, subhyaline or pale, faintly greenish or olivaceous, thin-walled, smooth; conidiophores mostly reduced to conidiogenous cells, conidiogenous loci inconspicuous or visible as subdenticulate shoulders, but always unthickened and not darkened. Conidia solitary, obclavate-subcylindrical, subacicular, short, 15–50 × 1.5–3 μm, 2–8-septate, subhyaline, pale greenish, thin-walled, smooth, apex acute to subobtuse, base truncated to short obconically truncated, 1–2 μm wide, hila unthickened, not darkened.
Typus: Japan, Tōhoku region, Aomori Prefecture, Mutsu, Ishi, on Diospyros kaki, 1 Sep. 1911, M. Miura (S-F42111 – holotype).
Host range and distribution: On Diospyros kaki, Asia (Japan).
Notes: Braun (1993) examined type material of Cylindrosporium kaki, and, owing to similar morphological characters, reduced this species to synonym with Pseudocercospora diospyri-morrisianae. However, C. kaki on Diospyros kaki is clearly distinct from the latter species in forming obvious epiphyllous leaf spots, greyish white fungal colonies, abundant superficial hyphae with solitary conidiophores, lacking or sparingly developed conidiophore fascicles, subhyaline or very pale, greenish or pale olivaceous, and much shorter conidia, 15–50 × 1.5–3 μm (vs. 40–90 × 2–3 μm). There is an additional collection deposited as BPI 400794 (on Diospyros kaki, from Japan, intercepted at San Francisco, California).
Pseudocercospora kobayashiana C. Nakash., sp. nov. MycoBank MB833840. Figs 17, 18.
Fig. 17.
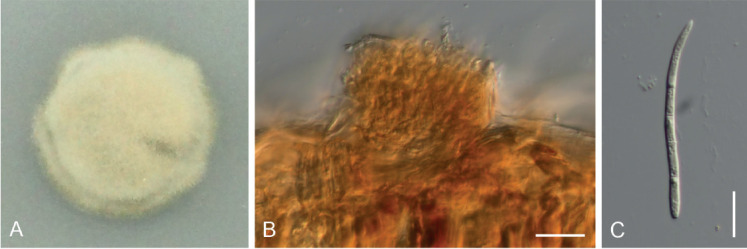
Pseudocercospora kobayashiana. A. Culture on PDA (MAFF236999 – ex-type culture). B. Stroma and conidiophores (CNS-993 – holotype). C. Conidium (CNS-993 – holotype). Scale bars = 10 μm.
Fig. 18.
Pseudocercospora kobayashiana. A. Stroma with conidiophore fascicle. B. Conidium. Scale bar = 10 μm. C. Nakashima del.
Etymology: Epithet dedicated to Takao Kobayashi, Japanese phytopathologist.
Diagnosis: Differs from all other Pseudocercospora species on Diospyros kaki in having very short brownish conidiophores, 8–25 × 2–5 μm, conidiogenous cells unilocal, percurrently proliferating (cercostigmina-like), and conidia with acute apex.
Description in vivo: Caespituli amphigenous, mainly epigenous, leaves densely colonised, with abundant conidiophores and conidia. Mycelium internal and external; superficial hyphae on the lower leaf surface, branched, hyaline to pale brown, smooth, 2–2.5 μm wide, septate. Stromata small, composed of few brown cells, to well-developed, amphigenous, mainly epigenous, epidermal, erumpent, stomatal, pale brown to brown, 30–70 μm diam. Conidiophores densely fasiciculate, short, emerging from the upper part of stromata, or emerging from hyphal cells, simple, subcylindrical, straight to slightly curved, 8–25 × 2–5 μm, 0–1-septate, pale brown, thin-walled; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, short conically truncated or rounded at the apex, with a single terminal locus, proliferating percurrently, loci unthickened, not darkened, 1.5–2.5 μm diam. Conidia solitary, acicular to obclavate, straight to flexuous, 22–75 × 2–5 μm, 4–14-septate, hyaline to very pale olivaceous, smooth, thin-walled, acute at the apex, short obconically truncated at the base, hila unthickened, not darkened, 1.5–2.5 μm wide.
Description in vitro: Colonies on PDA reaching 20 mm diam after 28 days at 25 °C in the dark; surface lanate, and slightly undulate to rounded margins; surface greenish glaucous to yellow-green; reverse dark grey to dull green, non-sporulating.
Typus: Japan, Kagoshima, Amami-Ohshima Is., Setouchi, Katsuura, on Diospyros kaki, 11 Nov. 1993, T. Kobayashi (CNS993 – holotype). Ex-holotype culture: MAFF236999 = MUCC1210.
Additional material examined: Japan, on D. kaki, Oct. 1991, T. Kobayashi (culture: MAFF235880).
Host range and distribution: On Diospyros kaki, Asia (Japan).
Notes: Sequences retrieved from cultures of this species were already included in a phylogenetic analysis (Crous et al. 2013) and turned out to form a separate lineage distant from P. kaki. Pseudocercospora kobayashiana is characterised by having short, percurrently proliferating conidiophores, i.e., this species is cercostigmina-like and differs in this respect from all other Pseudocercospora species on Diospyros.
Pseudocercospora montanae C.D. Sharma et al., J. Indian Bot. Soc. 75: 39. 1996.
Illustration: Sharma et al. (1996: 39, fig. 2).
Description in vivo: Leaf spots hypophyllous, irregularly scattered all over the leaf surface, mostly as very small spots, sometimes confluent, vein-limited, greyish black. Colonies hypophyllous, effuse, black. Mycelium immersed; hyphae branched, septate, light olivaceous, 1.5–4 μm wide, smooth. Stromata lacking or almost so, only composed of aggregations of a few swollen hyphal cells. Conidiophores fasciculate, in small, dense to larger, loose fascicles, arising from internal hyphae or small substomatal hyphal aggregations, erect, straight, subcylindrical to geniculate-sinuous, unbranched, 14.5–50 × 2.5–6.5 μm, 0–2-septate, pale to mid olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, proliferation symposial, with a single to several conidiogenous loci, unthickened, not darkened. Conidia formed singly, straight, sometimes curved, subcylindrical-obclavate, apex subacute to obtuse, base short obconically truncated, 17–125 × 1.5–5.5 μm, 2–13-septate, light olivaceous, thin-walled, smooth, hila unthickened, not darkened.
Typus: India, Madhya Pradesh, Amarkantak (S.F.D.), Shahdol circle, on Diospyros montana, Oct. 1992, C.D. Sharma, no. 5 (IMI 355871 – holotype).
Host range and distribution: Only known from the type collection.
Notes: Pseudocercospora montanae is morphologically very close to and barely distinguishable from P. fuliginosa. The conidiophores are somewhat shorter, to 50 μm, and wider, to 6.5 μm, and the conidia are somewhat longer, up to 125 μm, and wider, up to 5.5 μm. Otherwise, P. montanae agrees well with P. fuliginosa and is possibly synonymous. On the other hand, molecular sequence analyses based on North American collections of P. fuliginosa and Asian specimens of this species as well as P. montanae are not yet available, except for a single sequence retrieved from a Japanese sample. Therefore, a final taxonomic treatment of Asian and North American cercosporoid fungi resembling P. fuliginosa is not yet possible. P. montanae is, at least tentavively, maintained as a distinct species.
Pseudocercospora tessellata U. Braun, sp. nov. MycoBank MB833841. Fig. 19.
Fig. 19.
Pseudocercospora tessellata (CUP37217 – holotype). A. Superficial hyphae and conidiophores emerging through a stoma. B. Conidiophore fascicles. C. Conidiophores. D. Conidia. Scale bar = 10 μm. U. Braun del.
Etymology: tessellatus = tessellated, referring to the characters of the leaf spots.
Literature: Thirumalachar & Chupp (1948: 356, as “Cercospora kaki”), Vasudeva (1963: 128–129, as “Cercospora kaki”), Kamal (2010: 186, as “Cercospora kaki”).
Diagnosis: Similar to Pseudocercospora kakiicola, but on Diospyros melanoxylon, with tessellated lesions, smaller epiphyllous stromata, 10–50 μm diam, lacking hypophyllous stromata, sparingly developed hypophyllous mycelium, and (0–)1–4-septate conidia.
Description in vivo: Leaf spots amphigenous, scattered, angular-irregular, 0.5–5 mm diam, occasionally confluent, on the upper leaf surface at first brown, but soon greyish white or whitish, with distinct dark brown to almost blackish brown border or surrounded by dark somewhat raised veins, entire spots characteristically tessellated (composed of two to several whitish, dark-bordered spotlets), below dingy brown, greyish brown, with darker border or vein-limited. Caespituli amphigenous, on the upper leaf surface punctiform, dark brown to blackish, scattered, below inconspicuous. Mycelium internal and external; external hyphae superficial, emerging through stomata, confined to the lower leaf surface, sparingly developed, unbranched or sparingly branched, 1–3 μm wide, septate, colourless or very pale, thin-walled, smooth or almost so (solitary conidiophores arising from superficial hyphae not observed). Stromata epiphyllous, immersed to slightly erumpent, subglobose to irregularly shaped, 10–50 μm diam, medium to dark brown or olivaceous brown, cells 2–5 μm diam, hypophyllous stromata lacking. Conidiophores in loose to usually dense fascicles, numerous, arising from epiphyllous stromata, on the lower leaf surface occasionally with a single or few very short conidiophores emerging through stomata, erect, subcylindrical-conical, almost straight to curved or somewhat sinuous, rarely geniculate, usually somewhat narrowed towards rounded or conically truncated tips, unbranched, 5–20(–30) × 1.5–4 μm, 0–1(–2)-septate, hyaline, subhyaline or very pale olivaceous or olivaceous brown, thin-walled, smooth to somewhat rough-walled; conidiophores mostly reduced to conidiogenous cells, occasionally conidiogenous cells integrated, terminal, conidiogenous loci inconspicuous or visible as truncated tips, 1.5–2 μm wide, but neither thickened nor darkened. Conidia solitary, narrowly cylindrical to obclavate-cylindrical, straight to curved, occasionally slightly sigmoid, 15–60 × 2–4 μm, (0–)1–4-septate, septation not very conspicuous, hyaline, subhyaline or very pale, thin-walled, smooth or almost so, apex subacute or obtuse, base truncated or usually short obconically truncated, hila 1–2 μm wide, neither thickened nor darkened.
Typus: India, Karnataka, Bangalore, Yashavantapur, on Diospyros melanoxylon, 10 Feb. 1945, M.J. Thirumalachar (CUP 37217 – holotype).
Host range and distribution: On Diospyros melanoxylon, Asia (India: Karnataka, Madhya Pradesh).
Notes: Pseudocercospora tessellata is well characterised by forming tessellated leaf spots. This type of lesions differs from leaf spots caused by all other Pseudocercospora species on Diospyros spp. Pseudocercospora kaki and P. kakiicola on Diospyros spp. are similar but differ in having larger epiphyllous stromata, 30–100 μm diam, well-developed hypophyllous stromata (10–35 μm diam), and (1–)2–9(–15)-septate conidia.
Thirumalachar & Chupp (1948) reported a collection on D. melanoxylon from Bangalore, Nandi Hills, 15 Mar. 1945 under the name Cercospora kaki. This is not the collection deposited at CUP (see holotype data). Vasudeva (1963) cited two collections on Diospyros melanoxylon from Karnataka, one from Nandi Hills, agreeing with the report in Thirumalachar & Chupp (1948), and one from Bangalore, Mysore, 15 Aug. 1945. He described “fruiting hypophyllous” (the source of his description is unclear). Kamal (2010) listed “Pseudocercospora kaki” on Diospyros melanoxylon from Karnataka (Nandi Hills) and Madhya Pradesh (Amboli).
Zasmidium diospyri (Thüm.) U. Braun, Schlechtendalia 20: 101. 2010. Fig. 20.
Fig. 20.
Zasmidium diospyri (HAL, s.n., Thüm., Mycoth. Univ. 1273 – lectotype). A. Superficial hyphae. B. Superficial hyphae with solitary conidiophores. C. Conidiophore fascicle. D. Conidiophores. E. Conidia. Scale bar = 10 μm. U. Braun del.
Basionym: Cercospora diospyri Thüm., Mycoth. Univ., Cent. 13, no. 1273. 1878.
Synonyms: Helminthosporium diospyri Thüm., Rev. Mycol. (Toulouse) 1: 60. 1879.
Cercospora diospyri (Thüm.) Cooke, Grevillea 12(61): 31. 1883, nom. illeg. (Art. 52.1 and 53.1).
Sirosporium diospyri (Thüm.) Deighton, More Dematiaceous Hyphomycetes: 301. 1976.
Stenella diospyri (Thüm.) U. Braun, Mycotaxon 55: 239. 1995.
Literature: Ellis (1976: 301), Crous & Braun (2003: 163).
Illustration: Ellis (1976: 300, fig. 225D).
Description in vivo: Leaf spots lacking or diffuse discolorations. Caespituli hypophyllous, punctiform, scattered, loose to dense, often spread over the whole lower surface. Mycelium internal and external; superficial hyphae 1.5–4.5 μm wide, septate, sparingly branched, subhyaline to pale olivaceous, almost smooth to verruculose, thin-walled. Stromata small to well-developed, substomatal or immersed, 10–50 μm diam, sometimes confluent or oblong, up to 80 μm, medium to medium dark brown, cells circular to angular-irregular in outline. Conidiophores in small to mostly rather large fascicles, loose to moderately dense, arising from stromata, through stomata, erect, straight to curved or geniculate-sinuous, usually unbranched (rarely branched), 10–70 × 3–7 μm, 0–3-septate, pale to medium brown or olivaceous brown, thin-walled, smooth or almost so; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 10–30 μm long, proliferation sympodial, conidiogenous loci conspicuous, 1–1.5(–2) μm wide, sometimes subdenticulate, slightly thickened and darkened. Conidia solitary, obclavate-cylindrical, mostly straight, occasionally slightly curved, apex obtuse, base short obconically truncated, sometimes long obconically truncated, 30–80 × 4–7 μm, 1–7-septate, not or only occasionally slightly constricted at the septa, pale olivaceous, olivaceous brown or pale to medium brownish, thin-walled, smooth or almost so to faintly rough, hila 1–2 μm wide, slightly thickened and darkened.
Typus: USA, South Carolina, Aiken, on Diospyros virginiana, Thüm., Mycoth. Univ. 1273 (HAL, s.n. – lectotype, designated here, MycoBank MBT390104). Isolectotypes: Thüm., Mycoth. Univ. 1273, e.g., BPI 435791, FH 01012173, ILL 76928, LE, NEB 40164, NY 937000, 3616368, PUL-F1066.
Host range and distribution: On Diospyros virginiana, North America (USA: Florida, Illinois, Louisiana, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, Washington), South America (Brazil).
Notes: Records of this species from Brazil on Diospyros sp. (Mendes et al. 1998) and Mexico on D. kaki (Alvarez 1976) are doubtful and unconfirmed. A record on Diospyros hispida from Brazil (Pollack 1987) refers probably to Zasmidium diospyri-hispidae.
The complicated nomenclature of this species was discussed by Crous & Braun (2003), but it must be supplemented. Cercospora diospyri Thüm., the oldest name for this species, was based on Thüm., Mycoth. Univ. 1273, referring to Ravenel no. 2196. Helmintosporium diospyri Thüm. was published with reference to Ravenel 2196 as type, but Cercospora diospyri was not cited as basionym, i.e., Cercospora diospyri and Helminthosporium diospyri are homotypic synonyms, both based on Ravenel 2196. Cooke cited the name Cercospora diospyri Cooke, in Ravenel, Fungi Amer. Exs. 588 (referring to Ravenel 2659), but without any description or diagnosis), and without reference to Cercospora diospyri Thüm. Cooke (1883) published this name as “Cercospora diospyri (Thüm.) Cooke” referring to Ravenel, Fungi Amer. Exs. 588 (Ravenel 2659). A basionym was not cited, but the citation of “(Thüm.)” is undoubted an indirect reference to Helminthosporium diospyri, since a reference to Cercospora diospyri would not make any sense. Cooke’s name is, in any case, an illegitimate younger homonym, a superfluous name, and homotypic with C. diospyri.
Zasmidium diospyrigenum (Chaudhary et al.) Kamal, Cercosporoid Fungi of India (Dehra Dun): 241. 2010.
Basionym: Stenella diospyrigena Chaudhary et al., Journal of Basic and Applied Mycology 1(1): 27. 2002.
Description in vivo (based on the original description): Leaf spots amphigenous, irregularly shaped, spreading over the entire surface, black on the lower leaf surface, greyish above. Colonies hypophyllous, effuse. Mycelium internal and external; hyphae branched, septate, finely verruculose, light yellow, 2.5 μm wide. Stromata poorly developed, superficial. Conidiophores solitary, arising from superficial hyphae, lateral and terminal, almost cylindrical, straight to flexuous, unbranched, 9.5–44 × 3–4.5 μm, 1–3-septate, light brown, smooth; conidiogenous cells integrated, terminal and intercalary, with 1–3 conspicuous conidiogenous loci, proliferation sympodial. Conidia solitary, obclavate-cylindrical, straight to curved, apex subacute to rounded, base obconically truncated, 17.5–116 × 3.5–4.5 μm, (0–)1–7-septate, pale to mid olivaceous, verruculose.
Typus: India, Uttar Pradesh, Kusumi forest, on Diospyros sp., March 2000, S. Chaudhary (HCIO 43693 – holotype). Isotype: GPU.
Host range and distribution: Only known from the type collection.
Zasmidium diospyri-hispidae U. Braun, nom. nov., MycoBank MB833842. Fig. 21.
Fig. 21.
Zasmidium diospyri-hispidae (CUP39849 – isotype). A. Superficial hyphae. B. Conidiophore fascicle. C. Conidiophore. D. Conidia. Scale bar = 10 μm. U. Braun del.
Basionym: Passalora diospyri Crous et al., CBS Diversity Ser. (Utrecht) 1: 163. 2003, non Zasmidium diospyri (Thüm.) U. Braun, 2010.
Synonym: Cercospora diospyri Viégas, Bol. Soc. Brasil. Agron. 8: 23. 1945 (nom. illeg., Art. 53.1), non Cercospora diospyri Thüm., 1878.
Misapplied name: Passalora fuliginosa (Ellis & Kellerm.) Crous et al., Mycotaxon 64: 412. 1997.
Description: Crous et al. (1997: 412).
Illustration: Crous et al. (1997: 413, fig. 5).
Description in vivo: Leaf spots amphigenous, subcircular to angular-irregular, 2–7 mm diam, dark brown with indistinct margin above, medium brown with raised dark brown margin below. Caespituli hypophyllous, punctiform, scattered, dark brown. Mycelium internal and external; internal hyphae branched, 2–3 μm wide, light brown, thin-walled, smooth; external hyphae emerging throught stomata or from stromata, branched, septate, thin-walled, 1.5–3.5 μm wide, distinctly verruculose, subhyaline to pale olivaceous. Stromata immersed or substomatal, 10–50 μm diam, brown. Conidiophores in well-developed fascicles, divergent to dense, arising from stromata, erect, subcylindrical, straight to slightly curved, usually not geniculate-sinuous, unbranched, 30–110 × 3–5 μm, 0–5-septate, medium to medium dark brown or olivaceous brown, almost smooth to finely verruculose; conidiogenous cells integrated, terminal or intercalary, 20–40 μm long, light to medium brown, subcylindrical to somewhat attenuated or occasionally slightly wider towards truncated or rounded tips, with a single to usually several conspicuous conidiogenous loci, 1.5–2 μm diam, slightly thickened and darkened-refractive. Conidia solitary, obclavate-subcylindrical, straight to somewhat curved, guttulate, apex obtuse, base obconically truncated, 25–70 × 3–6 μm, 1–3(–4)-septate, subhyaline, olivaceous to pale brown, wall thin, finely but distinctly verruculose, hilum 1.5–2 μm wide, slightly thickened and darkened-refractive.
Typus: Brazil, Minas Gerais, Belo Horizonte, Jardim Botanico, on leaves of Diospyros hispida, 30 Jan. 1943, A.P. Viégas (IACM 4134 – holotype). Isotype: CUP 39849.
Host range and distribution: On Diospyros hispida, South America (Brazil).
Notes: The isotype material of this species, deposited at CUP, has recenty been re-examined. In the original description, the conidiophores and conidia of Passalora diospyri were described to be verruculose, as was confirmed by morphological analyses of the isotype material. However, verruculose conidia are not consistent with the concept and characeristics of Passalora s. lat., but they are more characteristic for Zasmidium. Species of the latter genus are usually characterised by forming verruculose superficial hyphae in vivo. Such hyphae have been found in the isotype material, although not mentioned and undoubtedly overlooked in the original description of Passalora diospyri. Zasmidium diospyri-hispidae is morphologically very close to the North American Z. diospyri on Diospyros virginiana, but besides the differences in the host range and geographical distribution, there are in addition some morphological differences. The lesions caused by the two species are quite distinct from each each other, the conidiophores of Z. diospyri-hispidae are longer, up to 110 μm, and fainly verruculose, solitary conidiophores arising from superficial hyphae are not developed, and the conidia are distinctly verruculose. These differences support the decision to maintain Zasmidium diospyri-hispidae as a distinct species.
There is another zasmidium-like hyphomycete on Diospyros hispida in Brazil. A collection deposited in Chupp’s herbarium at CUP has been examined (Brazil, Est. São Paulo, Mogy-Mirim, Cerrado, 12 Aug. 1943, A.P. Viégas & A.S. Lima, CUP 40101, Fig. 22): Leaf spots amphigenous, 2–10 mm diam, angular-irregular, at first dingy greenish, later brown to blackish brown, margin indefinite or darker, below often vein-limited. Caespituli amphigenous, punctiform, dark brown to blackish, scattered. Mycelium internal and external, superficial hyphae sparingly branched, 1–5 μm wide, subhyaline to pale olivaceous brown, septate, thin-walled, verruculose. Stromata amphigenous, immersed or substomatal, 20–60 μm diam, subglobose, brown, cells 2–6 μm diam. Conidiophores in dense fascicles, arising from stromata, or solitary, arsing from superficial hyphae, 2–30 × 2.5–4 μm, short and peg-like to subcylindrical or somewhat geniculate-sinuous, unbranched, aseptate or sparingly septate, subhyaline to olivaceous brown, thin-walled, smooth or almost so; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci inconspicuous or visible as truncated tips or shoulders, but neither distinctly thickened nor darkened-refractive. Conidia solitary, narrowly obclavate-cylindrical, straight to curved, occasionaly sigmoid, apex obtuse to subacute, 20–90 × 1.5–4 μm, 2–8-septate, subhyaline to pale olivaceous or olivaceous brown, verruculose, base obconically truncated, hila 1.5–2.5 μm wide, but not distinctly thickened and darkened. This fungus represents an undescribed species. However, the generic affinity of this species is unclear. The general morphology is strongly in favour of Zasmidium, but inconspicuous conidogenous loci are in conflict with the concept of ther latter genus. Cultures and results of phylogenetic examinations are necessary to confirm the generic affinity this fungus.
Fig. 22.
Zasmidium-like hyphomycete on Diospyros hispida from Brazil (CUP40101). A. Superficial hyphae emerging through a stoma. B. Conidiophore fascicle. C. Superficial hyphae. D. Solitary conidiophores arising from superficial hyphae. E. Conidia. Scale bar = 10 μm. U. Braun del.
Cercosporoid fungi of unclear generic affinity
Cercospora kakiivora Hara (as “kakivora”), J. Pl. Protect. [Byochu-gai zasshi], Tokyo, 16: 18. 1929.
Literature: Katsuki (1965: 30).
Description in vivo (based on the original Japanese description and Katsuki, l.c.): Leaf spots amphigenous, ellipsoid, oblong, angular-irregular, 2–10 mm diam, sometimes confluent, yellowish brown, brown, dark brown to reddish, margin indistinct or with dark marginal line, rarely with yellow or white halo. Caespituli amphigenous, mainly epiphyllous. Stromata immersed, globose, epidermal, erumpent, 30–80 μm diam. Conidiophores in dense fascicles, 10–20, arising from stromata, cylindrical, straight to curved or flexuous, usually unbranched, 55–105 × 5–6.5 μm, 2–7-septate, dark brown, paler towards the tip, wall thickened. Conidia vermiform or obclavate, widest part below, elongated and beak-like in the upper part, 100–170 × 5–6.6 μm, 9–17-septate (on average 11-septate), dark brown.
Typus: Japan, Prov. Shizuoka, Ichinomiya-mura, Shuchi-gun, on Diospyros kaki, Oct. 1927, K. Hara (probably not preserved).
Notes: Katsuki (1965) mentioned that he had examined type material of C. kakivora and cited “KOU” as corresponding herbarium. However, the latter acronym does not exist, and the type material could not be traced. Details of the conidiogenesis and of the structure of the conidiogenous loci and conidial hila are unknown. Therefore, the generic affinity of C. kakiivora remains quite unclear. It is even unknown whether the conidiogenesis is holoblastic or possibly tretic. Owing to the conidial shape and size, it is also possible that this species might rather be exosporioid, helmintosporioid or sporidesmioid.
Scolecostigmina diospyri Kamal (as “diospyrosis”), Cercosporoid Fungi of India (Dehra Dun), 259. 2010, nom. inval. (Art. 41.5).
Basionym: Stigmina diospyri G.P. Agarwal & N.D. Sharma (as “diospyrosis”), J. Indian Bot. Soc. 53(1-2): 81. 1974, nom. illeg. (Art. 53.1), non B.V. Patil & Thirum., 1968.
Illustration: Sharma & Agarwal (1974: 81, fig. 6).
Description in vivo: On living leaves. Conidiomata sporodochial, hypophyllous, punctiform, up to 400 μm diam. Mycelium internal; hyphae branched, septate, constricted at the septa, pale brown, 2.5–6.5 μm wide. Stromata immersed and superficial, 100–140 μm diam, brown to dark brown. Conidiophores arising from stromata, erect, straight to curved, cylindrical, 2–18 × 4–7.5 μm, 0–2 times percurrently proliferating, pale brown to brown. Conidia solitary, ellipsoid, cylindrical, obclavate-cylindrical, mostly rostrate, straight to curved, 16.5–92 × 6.5–11.5 μm, 1–14 times transversely septate and 0–3 times longitudinally septate, with constrictions at the septa, brown to dark brown, paler towards the apex, 3.3–5.5 μm wide at the base.
Notes: Kamal (2010) assigned this species to Scolecostigmina, but he failed to validate Stigmina diospyri G.P. Agarwal & N.D. Sharma (non B.V. Patil & Thirum.). Stigmina diospyri and Scolecostigmina diospyri were erroneously published as “diospyrosis” and the later name as “comb. nov.” (see Art. 58.1), but Kamal (2010) omitted to cite the correct page of the bibliographic reference since a page spread (“76–82”) was given. The gross morphology of this species is scolecostigmina-like (Braun et al. 1999), but abundant perpendicular septa are unusual, some conidia are even almost alternarioid. Details of the conidiogenesis and conidiogenous loci are not described. The evaluation of the true generic affinity of this species requires the re-examination of type material and phylogenetic analyses. Based on the phylogenetic placement of its type species, Stigmina has been reduced to synonymy with Pseudocercospora (Crous et al. 2013). The type species of Scolecostigmina clustered distant from the Pseudocercospora clade, suggesting that Scolecostigmina represents a genus of its own. On the other hand, other species previously assigned to the latter genus turned out to be true members of Pseudocercospora.
Stigmina diospyri B.V. Patil & Thirum., Sydowia 20(1–6): 38. 1968.
Original description: Leaf spots 3 mm wide, dark brown, fruiting bodies hypophyllous. Conidial fructifications in the substomatal space. Conidiophores cylindrical, pale brown, 10–22 × 4–8 μm, straight or bent and towards the apex, they end in circular ridges which indicate where the dispersal of conidia has taken place by successive proliferations of the conidiophores. Conidia develop singly as blown out ends of conidiophores, fusoid to clavate with rounded apex and base tapering gradually to a flat scar, 2–9-septate, dark brown, wall smooth about 1 μm thick, measuring 27–65 × 7–9 μm.
Typus: India, Bhilwadi (Nasik), on living leaves of Diospyros malabarica (= D. embryopteris), 28 Nov. 1960, B.V. Patil (probably not preserved).
Notes: This species was described without any illustration. It is unknown whether the type material is preserved, and, if this is the case, in which herbarium. A reassessment of the generic affiliation of this species is currently not possible and requires the examination of type material or other specimens, cultures and results of phylogenetic analyses. The phylogeny and taxonomy of the generic complex around Pseudocercospora, Scolecostigmina and Stigmina is intricate and has previously often been confused (see notes under Scolecostigmina diospyri).
Key to cercosporoid species on Diospyros spp. in vivo
1. Conidiogenous cells with thickened and darkened conidiogenous loci; conidia formed singly, obclavate, hyaline, 15–77 × 4–6 μm, basal hilum thickened and darkened; on Diospyros sp., India ...................................................... Cercospora disospyricola
1*Conidiogenous loci either inconspicuous, neither thickened nor darkened, or conidia pigmented ...........................................2
2. Conidiophores dark brown, 55–105 × 5–6.5 μm; conidia dark brown, 100–170 × 5–6.6 μm; on Diospyros kaki, Japan ....................................................................................................................................................................Cercospora kakiivora [generic affinity unknown]
2* Conidiophores and conidia much shorter and paler, not dark brown ........................................................................................ 3
3. Conidiogenous cells with conspicuous conidiogenous loci, thickened and darkened, superficial hyphae developed (Passalora and Zasmidium) ......................................................................................................................................................................... 4
3* Conidiogenous loci inconspicuous, neither thickened nor darkened or when subconspicuous by being subdenticulate, loci always unthickened and not darkened (Pseudocercospora) ....................................................................................................... 7
4. Superficial hyphae smooth; conidiophores (10–)20–200 × 3.5–6 μm, longer conidiophores pluriseptate; conidia solitary, obclavate-cylindrical, 20–75 × 4–6 μm, (1–)3–8(–12)-septate; on Diospyros virginiana, North America ........................... Passalora flexuosa
4* Superficial hyphae verruculose (Zasmidium) ............................................................................................................................. 5
5. Conidiophores solitary, arising from superficial hyphae, conidiophore fascicles lacking; conidia 17.5–116 × 3.5–4.5 μm, on Diospyros sp., India ........................................................................................................................ Zasmidium diospyrigenum
5* Well-developed fascicles of long conidiophores arising from stromata developed; conidia 25–80 × 3–7 μm, 1–7-septate ........... 6
6. Leaf spots lacking or only with diffuse discolorations; conidiophores smooth or almost so; solitary conidiophores arising from superficial hyphae developed; conidia 1–7-septate, smooth or almost so to delicately verruculose; on Diospyros virginiana, North America ............................................................................................................................................... Zasmidium diospyri
6* Distinct leaf spots developed; conidiophores delicately verruculose; solitary conidiophores arising from superficial hyphae not developed; conidia 1–3(–4)-septate, verruculose; on Diospyros hispida, Brazil ............................. Zasmidium diospyri-hispidae
7(3*) Conidiomata composed of small to moderately large stromata, 10–40 μm diam, and densely arranged, short conidiogenous cells, 5–15 × 2–3 μm, giving rise to long, very narrow, pale olivaceous, cylindrical-filiform conidia, 15–110 × 1–2 μm; on Diospyros lycioides, South Africa ......................................................................................... Pseudocercospora diospyri-lycioides
7* Stromata either much larger, up to about 80 μm diam, or stromata lacking or almost so; and/or conidia obclavate-cylindrical and wider, on average > 2 μm .................................................................................................................................................... 8
8. Caespituli hypophyllous, conidiophores fasciculate, emerging through stomata, stromata lacking or only forming small substomatal aggregations of a few swollen hyphal cells; mycelium internal; superficial hyphae lacking to well-developed, with lateral short conidiophores ........................................................................................................................................................ 9
8* Conidiophores in larger fascicles or sporodochial conidiomata, arising from larger stromata, about 20–85 μm diam, mainly epiphyllous or amphigenous; superficial hyphae either lacking or developed ......................................................................... 13
9. Caespituli greyish white; superficial mycelium well-developed; superficial hyphae with solitary conidiophores abundant; fasciculate conidiophores lacking or only with a few, loosely arranged conidiophores emerging through stomata; conidiophores short, 3–20 × 2–4 μm, very pale, subhyaline or very pale olivaceous; conidia short and narrow, acicular to obclavate, 15–50 × 1.5–3 μm, hyaline to pale greenish; on Diospyros kaki, Japan ......................................................... Pseudocercospora kakiigena
9* Conidiophores in well-developed fascicles; superficial mycelium lacking or sparingly developed, only with few superficial hyphae emerging through stomata; conidiophores longer, either 5–50 μm long or even up to 105 μm long, 2.5–6.5 μm wide, olivaceous, pale olivaceous brown to brown ............................................................................................................................10
10. Superficial hyphae lacking; conidia obclavate-cylindrical, pale olivaceous ............................................................................... 11
10* Superficial hyphae with solitary conidiophores developed; conidia either filiform-subcylindrical and pale olivaceous brown or subhyaline ................................................................................................................................................................................ 12
11. Conidiophores in well-developed, dense fascicles, 5–20, 10–75 μm long; conidia obclavate to obclavate-cylindrical, pale olivaceous, 20–105 × 2.5–4 μm; on Diospyros spp., Asia and North America ................................ Pseudocercospora fuliginosa
11* Conidiophores in smaller fascicles, to 50 μm long, but 6.5 μm wide; conidia to 125 μm long and 5.5. μm wide; on Diospyros montana, India ......................................................................................................................................................... P. montanae
12. Leaf spots lacking on the upper leaf surface; conidia subcylindrical-obclavate colourless or almost so, narrow, 40–90 × 2–3(–4) μm; on Diospyros morrisiana, Asia ................................................................................ Pseudocercospora diospyri-morrisianae
12* Leaf spots amphigenous; longer conidia filiform-subcylindrical and pale olivaceous brown, wider, 15–85 × 3–4.5 μm; on Diospyros lotus, Korea ................................................................................................................ Pseudocercospora diospyriphila
13(8*) On the upper leaf surface with large, often sporodochial conidiomata composed of numerous densely arranged conidiophores arising from large stromata, on the lower side with smaller conidiophore fascicles emerging through stomata and superficial hyphae with solitary conidiophores ......................................................................................................................................... 14
13* Superficial hyphae not developed; colonies either confined to the upper leaf surface, with large, well-developed stromata or, when amphigenous, on the lower side with smaller fascicles and smaller stromata ............................................................... 17
14. Stromata small to moderately developed, to 45 μm diam on the upper leaf surface; superficial hyphae with solitary conidiophores well-developed, abundant; conidia relatively short and narrow, 15–50 × 2–3 μm, colourless; on Diospyros oldhamii, Asia ..................................................................................................................................................... Pseudocercospora diospyricola
14* Stromata well-developed, larger, 10–80 μm diam; superficial hyphae sparingly developed to abundant, but solitary conidiophores lacking or only less developed; conidia hyaline, subhyaline to pale olivaceous or olivaceous brown; on Diospyros kaki, D. lotus or D. melanoxylon ............................................................................................................................................... 15
15. Forming tessellated lesions; epiphyllous stromata 10–50 μm diam, hypophyllous stromata lacking; hypophyllous mycelium sparingly developed; conidia (0–)1–4-septate; on D. melanoxylon, India ....................................... Pseudocercospora tessellata
15* Lesions not tessellated; stromata amphigenous, hypophyllous stromata 10–35 μm diam, epiphyllous stromata larger, 30–100 μm diam; hypophyllous mycelium abundant; conidia 1–15-septate; on Diospyros kaki and D. lotus, Asia, North and South America .................................................................................................................................................................................... 16
16. Conidiogenous cells sympodially proliferaring; conidia 2–4 μm wide, with 1–9 septa, subhyaline to pale olivaceous or very pale brownish; on Diospyros kaki, common in Asia, above all China and Japan, also in North America (USA) and South America (Brazil) ...................................................................................................................................................... Pseudocercospora kaki
16* Conidiogenous cells sympodially and percurrently proliferaring; conidia to 5 μm wide, often very pale, hyaline or subhyaline, with numerous septa, 4–15; on Diospyros kaki and D. lotus, only known from Japan...................................................................................................................................................... Pseudocercospora kakiicola
17(13*) Sporodochial conidiomata with large stromata mainly hypophyllous; conidia long and narrow, 35–90 × 1.5–3.5 μm, attenuated towards a more or less pointed tip; on Diospyros eriantha, Asia..................... Pseudocercospora diospyri-erianthae
17* Sporodochial conidiomata with large stromata confined to the upper leaf surface or, when amphigenous, lower side with smaller stromata and fasciculate conidiophores; on Diospyros kaki and D. lotus .......................................18
18. Conidiophores usually unilocal (apex rounded to truncated), determinate or percurrently proliferating; on Diospyros japonica or D. kaki.................................................................................................................................................................................. 19
18* Conidiophores uni- to multilocal, conidiogenous cells determinate to sympodially proliferating, at least sometimes geniculate; on Diospyros kaki or D. loti ...................................................................................................................................................... 20
19. Conidiophores 0–1-septate, conidiogenous cells percurrently proliferating; conidia apically pointed, 22–75 × 2–5 μm, 4–14-septate; conidiogenous loci and hila 1.5–2.5 μm wide; on Diospyros kaki ...................... Pseudocercospora kobayashiana
19* Conidiophores 0–3(–4)-septate, conidiogenous cells unilocal, determinate, percurrent proliferations not evident; conidia apically obtuse, rounded, 10–55 × 3–5 μm, 1–5-septate; conidiogenous loci and hila 2–3(–4) μm wide; on Diospyros japonica ........................................................................................................Pseudocercospora diospyri-japonicae
20(18*) Sporodochial conidiomata epiphyllous, rarely hypophyllous; conidia obclavate-cylindrical, 20–80 × 2–4(–5) μm, hila 1.5–2.5 μm wide; on Diospyros kaki, Asia .............................................................................................................. Pseudocercospora kaki
20* Fructification amphigenous, on the upper leaf surface with well-developed, large stromata, often sporodochial, stromata 20–70 μm diam, hypophyllous colonies less conspicuous, with smaller stromata and fascicuate conidiophores emerging through stomata, conidia 25–65(–130) × 1.5–4.5 μm, hila 1–2 μm wide; on Diospyros lotus, Iran ................ Pseudocercospora ershadii
DISCUSSION
A survey of cercosporoid ascomycetes (Mycosphaerellaceae) on host species of the genus Diospyros is given, including a key to the species involved. Previously published data, unpublished results based on examined type collections and other specimens, and outcomes of additional recently performed examinations of collections deposited in several herbaria, including BPI and CUP (herb. Chupp) have been the basis for the descriptions of cercosporoid fungi on Diospyros spp. in the present study. Passalora flexuosa is only tentatively retained in Passalora. The genus Passalora s. lat. has recently been split into several genera (Videira et al. 2017). Phylogenetic results are necessary to resolve the correct generic affinity of the latter species. Three cercosporoid species on Diospyros pertain to the genus Zasmidium, including Z. diospyri-hispidae (≡ Passalora diospyri, non Zasmidium diospyri). The re-examination of isotype material deposited at CUP revealed that the characteristics of this species, described from Brazil on Diospyros hispida, rather correspond with the circumscription of Zasmidium. However, the main focus of the present examinations of cercosporoid fungi on Diospyros was on species of the genus Pseudocercospora, above all with regard to the Cercospora kaki – Pseudocercospora kaki complex. First results of molecular sequence analyses (Crous et al. 2013, Bakhshi et al. 2014) suggested a high degree of genetic heterogeneity of this complex. But numerous crucial issues were still unresolved. Are Cercospora kaki and Pseudocercospora kaki conspecific, as usually assumed, or rather different species? What about Pseudocercospora on Diospyros kaki in Brazil? And what about Pseudocercospora collections on various other hosts, including Diospyros japonica, D. lotus and D. melanoxylon. In order to answer these questions, types of all species involved and additional specimens have been re-examined and all available cultures have been used as basis for phylogenetic examinations and analyses.
In the present phylogenetic tree (Fig. 1), most of the included taxa exhibit that the particular Pseudocercospora spp. on hosts of different plant genera cluster separately, which is in accordance with previous studies (Crous et al. 2013, Nakashima et al. 2016, Videira et al. 2017) and indicate a sufficient resolution on species level. Pseudocercospora species on Diospyros spp. were found to be spread over a large portion in one of two well-supported major clades [PP/MP BS/ ML BS: 1/100/98] of the genus Pseudocercospora. In the present study, six species of this genus, P. diospyriphila, P. ershadii sp. nov., P. fuliginosa, P. kaki, P. kakiicola sp. nov., and P. kobayashiana sp. nov., have been phylogenetically examined using multi-locus analyses. Out of these, P. ershadii and P. kobayashian, and also P. diospyriphila and P. kakiicola, seem to have diverged from a common ancestor species on Diospyros, similar to a speciation pattern discovered in species on hosts belonging to the Amygdaloideae (Spiraeoideae) of Rosaceae (Nakashima et al. 2016). Moreover, P. kaki, including several strains, was verified in geographically distant areas, viz., Japan and Brazil. The genus Diospyros comprises widespread deciduous tree species in temperate to tropical areas. Although D. kaki, known as persimmon, is the most widely cultivated species of the genus Diospyros in China, Korea, Japan, and Brazil (FAOSTAT, http://www.fao.org/faostat/), its natural habitat remains largely unknown (Barwick 2004). Japanese Persimmon was introduced to Brazil in the 1910s through Japanese immigration (Mita 2010). Based on the historical background, it can be assumed that the occurrence of P. kaki in Brazil and the present Brazilian strains go back to this introduction from Japan, leading to its establishment in Brazil. However, more detailed studies are needed to examine gene flow between populations on the different continents. On the other hand, it might be suggested that the origin of P. fuliginosa could be attributed to particular host jumps.
The clarification of the phylogenetic/taxonomic relationships between Cercospora kaki, based on North American type material on Diospyros kaki, and Pseudocercospora kaki, described on the basis of Asian type material on Japanese persimmon, has not yet been possible due to lacking cultures and sequence data retrieved from North American collections, preventing phylogenetic analyses of North American strains and an epitypification of C. kaki, or to put it another way, the identity of C. kaki remains unresolved, i.e., this species, which is very probably conspecific with Pseudocercospora kaki, can currently only tentatively be reduced to synonymy with the latter species.
In conclusion, results of morphological re-examinations and first data of molecular analyses clearly revealed a high degree of diversity within the Pseudocercospora kaki complex, which is composed of several cryptic species that are described in the present revision of cercosporoid fungi on Diospyros. Several Pseudocercospora species occur on Diospyros kaki, including P. kaki and P. kakiicola, and on other species of the genus Diospyros, e.g., D. japonica and D. melanoxylon, which turned out to be hosts of other still undescribed Pseudocercospora species. The present study just represents a first step towards a more comprehensive revision of Pseudocercosora species on Diospyros. The distribution of the taxa involved is only incompletely known. The number of cultures and sequence data is still fragmentary. We need additional examinations of cercosporoid fungi from all areas worldwide associated with persimmon cultivation and also from wild Diospyros spp., above all in Asia, from China and Japan to Iran.
Fig. 11.
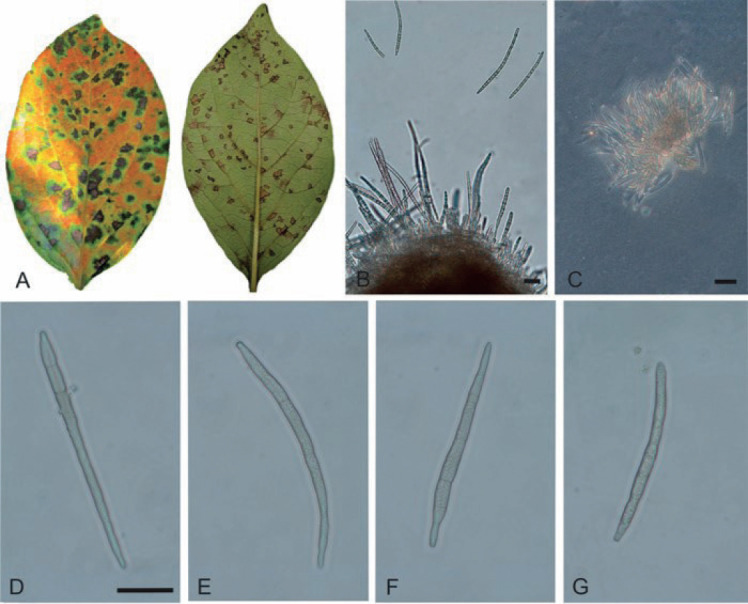
Pseudocercospora kaki, Brazilian collection (HAL 3320 F). A. Leaf symptoms. B, C. Conidiophore fascicles. D–G. Conidia. Scale bars = 10 μm.
Fig. 12.
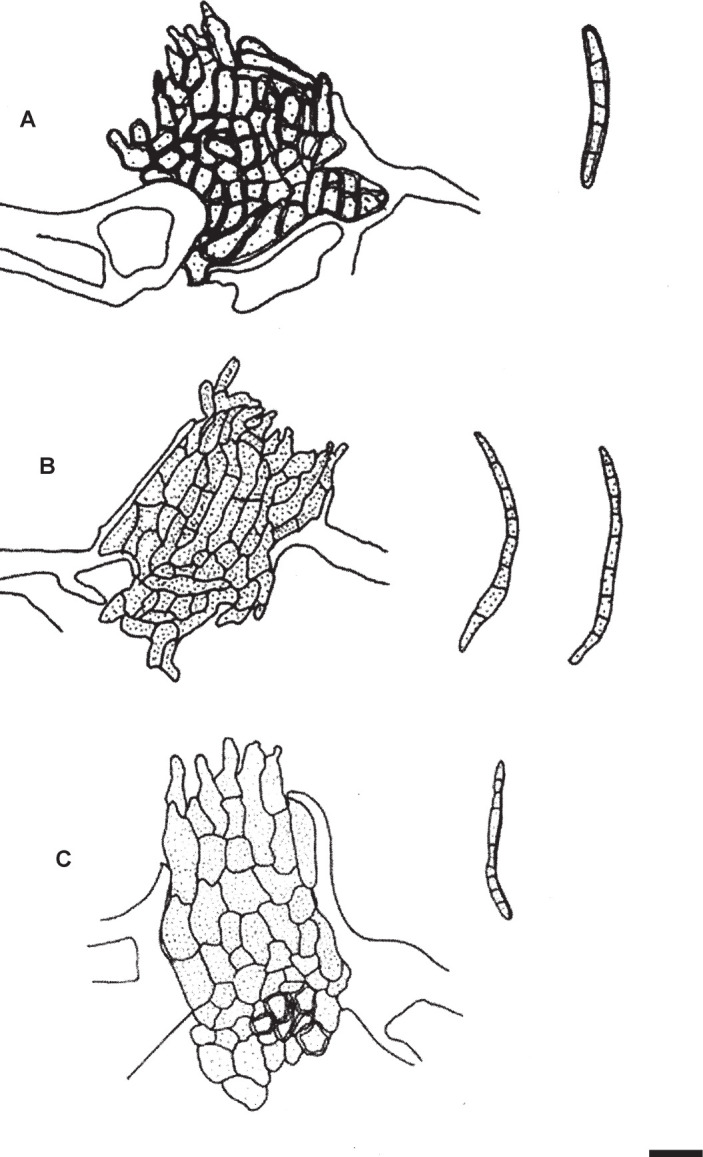
Pseudocercospora kaki. Stromata with fasciculate conidiophores. A. ex MUMH11900. B. ex MUMH11901. C. ex NIAES127-1-8. Scale bar = 10 μm. C. Nakashima del.
ACKNOWLEDGEMENTS
We are obliged to the curators of the herbaria BPI, CNS, CUP, K, MAFF, NIAES, NY, and S for the possibility to examine specimens in their keeping and to Rajesh Kumar (Pune, India) for his kind help in tracing the original description of Stenella diospyrigena. This study was partially supported by the Institute for Fermentation, Osaka, Japan, and a JSPS KAKENHI grant (no. 17K07837) to CN. Furthermore, the Iranian co-authors acknowledge the Research Deputy of the Iranian Research Institute of Plant Protection, Agricultural Research, Education and Extension Organization (AREEO) for financial support.
REFERENCES
- Alvarez MG. (1976). Primer catalogo de enfermedades de plantas Mexicanas. Fitofilo 71: 1–169. [Google Scholar]
- Anonymous (1960). Index of Plant Diseases in the United States. U.S.D.A. Agriculture Handbook 165: 1–531. [Google Scholar]
- Atkinson GF. (1892). Some Cercosporae from Alabama. Journal of the Elisha Mitchell Scientific Society 8(2): 33–67. [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-Ahari A, et al. , (2014). Multi-gene analysis of Pseudocercospora spp. from Iran. Phytotaxa 184(5): 245–264. [Google Scholar]
- Barwick M. (2004). Tropical and Subtropical Trees: A Worldwide Encyclopaedic Guide. Thames & Hudson Ltd; London. [Google Scholar]
- Boning CR. (2006). Florida’s Best Fruiting Plants. Pinapple Press, Sarasota. [Google Scholar]
- Bouckaert R, Vaughan TG, Barido-Sottani J, et al (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15(4): e1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U. (1993). Taxonomic notes on some species of the Cercospora complex (III). Mycotaxon 48: 275–298. [Google Scholar]
- Braun U. (1999). Taxonomic notes on some species of the Cercospora complex (V). Schlechtendalia 2: 1–28. [Google Scholar]
- Braun U, Crous PW. (2005). Additions and corrections to names published in Cercospora and Passalora. Mycotaxon 92: 395–416. [Google Scholar]
- Braun U, Mouchacca J, McKenzie EHC. (1999). Cercosporoid hyphomycetes from New Caledonia and some other South Pacific islands. New Zealand Journal of Botany. 37: 297–327. [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Chupp C. (1954). A monograph of the fungus genus Cercospora. Published by the author, Ithaca, New York. [Google Scholar]
- Cooke MC. (1883). New American Fungi. Grevillea 12(61): 22–33. [Google Scholar]
- Crous PW, Braun U. (2003). Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1–569. [Google Scholar]
- Crous PW, Alfenas AC, Barreto RW. (1997). Cercosporoid fungi from Brazil. 1. Mycotaxon 64: 405–430. [Google Scholar]
- Crous PW, Braun U, Hunter GC, et al. , (2013). Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. , (2019). Fungal Biodiversity. Westerdijk Laboratory Manual Series 1: 1–425. [Google Scholar]
- De Hoog GS, Gerrits van den Ende AHG. (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Ellis MB. (1976). More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, United Kingdom. [Google Scholar]
- Ellis JB, Everhart BM. (1887). Additions to Cercospora, Gloeosporium and Cylindrosporium. Journal of Mycology 3(2): 13–22. [Google Scholar]
- Ellis JB, Kellerman WA. (1887). New Kansas Fungi. Journal of Mycology 3(9): 102–105. [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Gorter GJMA. (1977). Index of plant pathogens and the diseases they cause in cultivated plants in South Africa. Department of Agricultural Technical Services, Plant Protection Research Institute Science Bulletin 392: 1–177. [Google Scholar]
- Guo YL. (2016). Three species of cercosporoid fungi from China. Mycosystema 35(1): 16–19. [Google Scholar]
- Guo Y-L, Hsieh W-H. (1995). The genus Pseudocercospora in China. International Academic Publishers, Beijing. [Google Scholar]
- Guo YL, Liu XJ. (1991). Studies on the genus Pseudocercospora in China V. Mycosystema 4: 99–118. [Google Scholar]
- Guo YL, Liu XJ, Hsieh WH. (1998). Pseudocercospora. [Flora Fungorum Sinicorum vol. 9.] Science Press, Beijing. [Google Scholar]
- Hetzel I, Jagel A. (2011). Diospyri kaki – Kaki, Kakipflaume (Ebenaceae). Jahrbuch des Bochumer Botanischen Vereins 2: 194–198. [Google Scholar]
- Hsieh WH, Goh TK. (1990). Cercospora and similar fungi from Taiwan. Maw Chang Book. Co., Taipei. [Google Scholar]
- Kamal (2010). Cercosporoid Fungi of India. Dehra Dun: Bishen Singh Mahendra Pal Singh. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2017). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S. (1965) Cercosporae of Japan. Transactions of the Mycological Society of Japan, Extra Issue 1: 1–100. [Google Scholar]
- Kim JD, Shin HD. (1998). Taxonomic studies on Cercospora and allied genera in Korea (IV). The Korean Journal of Mycology 26: 437–449. [Google Scholar]
- Kishino H, Hasegawa H. (1989). Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. Journal of Molecular Evolution 29: 170–179. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. (2007). Index of fungi inhabiting woody plants in Japan. Host, Distribution and Literature. Zenkoku-Noson-Kyoiku Kyokai Publishing Co., Ltd. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lyle S. (2006). Ultimate fruit & nuts. A comprehensive guide to the cultivation, uses and health benefits of over 300 food-producing plants. Frances Lincoln Ltd., London. [Google Scholar]
- Mendes MAS, da Silva VL, Dianese JC, et al (1998). Fungos em Plants no Brasil. Embrapa-SPI/Embrapa-Cenargen, Brasilia. [Google Scholar]
- Mita C. (2010). Foi um fruto da jornada global : a mudanca dos vegetais cultivados no Brasil e os imigrantes japoneses. Journal of Latin American Studies 39: 51–60. [Google Scholar]
- Munjal BL, Lall G, Chona BL. (1962 [“1961”]). Some Cercospora species from India – VI. Indian Phytopathology 14: 179–190. [Google Scholar]
- Nakashima C, Motohashi K, Chen CY, et al. , (2016). Species diversity of Pseudocercospora from far east Asia. Mycological Progress 15: 1093–1117. [Google Scholar]
- Pirnia M, Zare R, Zamanizadeh HR, et al. , (2012). New records of cercosporoid hyphomycetes from Iran. Mycotaxon 120: 157–169. [Google Scholar]
- Pollack FG. (1987). An annotated compilation of Cercospora names. Mycologia Memoir 12: 1–212. [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, et al. , (2018). Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology. syy032. doi:10.1093/sysbio/syy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. (1970). A Mycological Colour Chart. Kew, Surrey: Commonwealth Mycological Institute & British Myclogical Socienty. [Google Scholar]
- Saccardo PA. (1892). Sylloge fungorum omnium hucusque cognitum, Vol. 10 (Supplementum universal, Pars 2). Patavii. [Google Scholar]
- Saccardo PA. (1899). Sylloge fungorum omnium hucusque cognitum, Vol. 14 (Supplementum universal, Pars 4). Patavii. [Google Scholar]
- Sharma ND, Agarwal GP. (1974). Fungi causing plant diseases at Jabalpur (M.P.) – XVI. Journal of the Indian Botanical Society 53: 76–82. [Google Scholar]
- Sharma CD, Gadpandey KK, Rai AN, et al (1996). Some additions to the new hyphomycetous taxa from Indian sub-continent. Journal of the Indian Botanical Society 75: 37–40. [Google Scholar]
- Shin HD. (1995). New fungal diseases of economic resource plants in Korea (III). Korean Journal of Plant Pathology 11: 197–209. [Google Scholar]
- Shin HD, Braun U. (1993). Notes on Korean Cercosporae and Allied Genera (1). Mycotaxon 49: 351–362. [Google Scholar]
- Shin HD, Kim JD. (2001). Cercospora and allied genera from Korea. Plant Pathogens of Korea 7: 1–302. [Google Scholar]
- Simmonds JH. (1966). Host index of plant diseases in Queensland. Queensland Department of Primary Industries, Brisbane. [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, et al. , (2007). A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- Swofford DL. (2003). PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Tai FL. (1979). Sylloge Fungorum Sinicorum. Science Press, Academia Sinica, Peking. [Google Scholar]
- Thirumalachar MJ, Chupp C. (1948). Notes on some Cercosporae of India. Mycologia 40: 352–362. [Google Scholar]
- Vasudeva RS. (1963). Indian Cercosporae. New Delhi, Indian Council of Agricultural Research. [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. , (2017). Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Bruns T, Lee S, et al. , (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocol: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, et al., , eds). Academic Press, San Diego, USA: 315–322. [Google Scholar]