Highlights
-
•
Motor and pain-related intrinsic neural networks alter after spinal cord injury.
-
•
Alterations are inversely and simultaneously related to mobility and neuropathic pain.
-
•
Disabilities of mobility and neuropathic pain may be mutually influenced by supraspinal plasticity.
Keywords: Power spectral density, Resting-state functional magnetic resonance imaging, Mobility, Neuropathic pain, Spinal cord injury
Abstract
Background
The mechanisms by which mobility function and neuropathic pain are mutually influenced by supraspinal plasticity in motor- and pain-related brain networks following spinal cord injury (SCI) remains poorly understood.
Objective
To determine cortical and subcortical resting-state network alterations using power spectral density (PSD) analysis and investigate the relationships between these intrinsic alterations and mobility function and neuropathic pain following SCI.
Methods
A total of 41 patients with incomplete SCI and 33 healthy controls were included. The degree of mobility and balance function and severity of neuropathic pain and depressive mood were evaluated. The resting-state functional magnetic resonance imaging data of low-frequency fluctuations were analyzed based on PSD. Differences in PSD values between patients with SCI and controls were assessed using the two-sample t-test (false discovery rate-corrected P < 0.05). The relationship between PSD values and mobility function and pain intensity was assessed using Pearson’s correlation coefficient adjusted for the severity of depressive mood.
Results
Compared with healthy controls, lower PSD values in supplementary motor and medial prefrontal areas (the anterior cingulate cortex, ventral medial prefrontal cortex, and superior orbito-prefrontal cortex) were associated with greater pain severity and poorer postural balance and mobility (P < 0.05) in patients with SCI, whereas higher PSD values in the primary motor cortex, premotor cortex, thalamus, and periaqueductal gray were associated with greater pain severity and poorer postural balance and mobility (P < 0.05).
Conclusions
Cortical and subcortical plastic alterations in intrinsic motor- and pain-related networks were observed in patients with SCI and were simultaneously associated with neuropathic pain intensity and degree of mobility function.
1. Introduction
Spinal cord injury (SCI) interrupts efferent motor and afferent sensory connections between the cerebral cortex and spinal cord and leads to motor, sensory, and autonomic system dysfunctions (Dietz and Curt, 2006, North, 1999). Mobility disability and neuropathic pain frequently develop below the spinal lesion level as a secondary complication in patients with SCI, severely affecting the functional independence and quality of life (Siddall and Loeser, 2001, Siddall et al., 2003). Understanding the neural mechanism of plastic changes in the brain would help establish rehabilitation therapy strategies for patients with mobility disabilities and neuropathic pain following SCI.
Previous neuroimaging studies in patients with SCI have focused on plastic changes in sensorimotor cortices, which would be expected after a descending corticospinal tract disruption and sensory deafferentation following SCI (Cohen-Adad et al., 2011, Freund et al., 2011, Grabher et al., 2015, Jain et al., 2000, Lotze et al., 1999, Lundell et al., 2011, Mole et al., 2014, Nardone et al., 2013, Villiger et al., 2015, Wrigley et al., 2008). Atrophic changes and demyelination of the corticospinal tracts have been observed above the spinal level of the lesion (Cohen-Adad et al., 2011, Freund et al., 2011, Wrigley et al., 2008). Cortical reorganization of the primary motor cortex (M1) and primary somatosensory cortex (S1) has been shown to be associated with motor and sensory impairments (Grabher et al., 2015, Lotze et al., 1999, Lundell et al., 2011, Mole et al., 2014, Nardone et al., 2013). As a consequence of deafferentation, Wallerian degeneration occurs in the ascending sensory fiber tracts, inducing structural and functional changes in transsynaptic sensory relay nuclei of the brainstem following SCI (Jain et al., 2000, Villiger et al., 2015). Furthermore, structural and functional changes in sensorimotor cortices were observed in patients with SCI with neuropathic pain as compared with those without neuropathic pain (Grabher et al., 2015, Gustin et al., 2009, Jutzeler et al., 2016, Mole et al., 2014). However, functional changes in supraspinal circuits following SCI and possible associations between these functional alterations and disabling symptoms such as mobility dysfunction and neuropathic pain following SCI are not well defined. Especially, associations between functional alterations in brain motor regions and pain or between alterations in brain regions related to pain and disabling symptoms have not been frequently investigated.
Resting-state functional magnetic resonance imaging (rs-fMRI) shows spontaneous low-frequency fluctuations in blood oxygen level-dependent (BOLD) signals and is temporally correlated with distinct functional resting-state networks (Fox and Raichle, 2007, Fox et al., 2005). Power spectral density (PSD) analysis of rs-fMRI characterizes the frequency distribution of signal variance in a time series within a low-frequency range (0.01–0.1 Hz) (Duff et al., 2008, Zuo et al., 2010). Although the detailed physiological origin of PSD remains to be determined, PSD is thought to reflect regional intrinsic neuronal activity at resting-state and demonstrates a possible application in neurorehabilitation (Min et al., 2019). For SCI, PSD analyses of rs-fMRI have been used to study spontaneous brain activities in non-human primate SCI models (Rao et al., 2015, Rao et al., 2014). However, no study has investigated PSD alterations of the whole-brain networks in human SCI to date.
In the present study, the primary aim was to investigate possible neural reorganizations including intrinsic neural network alterations in resting-state brain networks in patients with SCI using PSD analysis. Furthermore, the secondary aim was to investigate associations between these neural alterations in the brain and functional disabilities such as mobility dysfunction, postural stability, and neuropathic pain in patients with SCI. In particular, we aimed to determine whether functional alterations in brain motor regions are associated with neuropathic pain or with mobility dysfunctions. This particular aim seems to have a significant implication in neurorehabilitation as it can identify fundamental mechanisms on whether sensorimotor improvements after SCI can boost improvements in neuropathic pain in patients with SCI.
2. Materials and methods
2.1. Participants
This cross-sectional study included patients 1) who were diagnosed with SCI more than 3 months after the injury; 2) with incomplete injury, defined as some degree of retained motor or sensory function below the site of injury, including sacral root segments (S4-5) according to the American Spinal Injury Association (ASIA) Impairment scale; 3) with at- or below-level SCI neuropathic pain according to the definition by the International Association for the Study of Pain (IASP); 4) who were ≥18 years old at the time of injury and were 18–80 years old at the time of assessment; and 5) who received inpatient rehabilitation and physical and occupational therapies 3 h daily for 60 days at the Department of Rehabilitation Medicine. The exclusion criteria were as follows: 1) history of concurrent traumatic hemorrhage or contusion on brain CT, 2) history of any neuropsychiatric disorder, 3) history of any central or peripheral nervous system disorder, or 4) history of alcohol and/or drug abuse. Written informed consent was obtained from all participants, and ethical approval was provided by the Institutional Review Board of Kyungpook National University Chilgok Hospital (No. 2018-12-004).
2.2. Resting-state fMRI acquisition
Rs-fMRI data were obtained using a 3T-MRI scanner (Discovery MR 750W, GE healthcare, Milwaukee, WI, USA). A 24-ch head coil was used for image acquisition. To obtain rs-fMRI data, participants performed no task and were instructed to close their eyes during scanning without falling asleep. For the resting-state imaging, 240 volumes were acquired with interleaved ascending order using T2*-weighted echo planar imaging pulse sequence with an echo time (TE) of 30 ms, repetition time (TR) of 2000 ms, flip angle (FA) of 90, field of view (FOV) of 23 cm, acquisition matrix of 64 × 64, slice thickness of 4 mm, and no gap. Structural brain images were obtained using 3D T1-weighted fast spoiled gradient echo sequence with a TR of 8.5 ms, TE of 3.2 ms, FA of 13, FOV of 25.6 cm, acquisition matrix of 256 × 256, and iso-voxel resolution of 1 mm.
2.3. Resting-state fMRI data preprocessing
The statistical parametric mapping software SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and MATLAB (The MathWorks, Inc., Natick, MA, USA) were used for fMRI data image preprocessing. Preprocessing of fMRI data images included slice timing, realignment (realigned and ART-based outlier identification was conducted using an outlier image: whether the composite movement from a preceding image exceeded 0.5 mm or the total mean intensity was >3 standard deviations from the mean image intensity), coregistration (the individual T1 structural image volume was coregistered to the mean echo planar image), segmentation of the structural image (simultaneously segmented into gray matter, white matter, and cerebrospinal fluid [CSF]), and normalization into the standard stereo-taxic coordinate space (Montreal Neurological Institute). Normalized images were spatially smoothed with a Gaussian kernel of full width at the half maximum of 8 mm. Rs-fMRI data were then prepared to analyze PSD of spontaneous low-frequency BOLD signal fluctuation. In order to identify and eliminate components such as physiological influences on fMRI data, we used a component-based noise correction method (CompCor) in the denoizing part of CONN toolbox (https://www.nitrc.org/projects/conn/). Using this method, 5 eigenvectors of the principal component decomposition were extracted from the white matter and CSF signals (derived via anatomical segmentation) using principal component analysis. The 5 components were then removed from gray matter signals through standard linear regression. Additionally, the confounding effects of 12 motion-related parameters (comprising 6 head motion parameters and first-order temporal derivatives) of realignment and regressors derived from ART-based outlier identification, were also removed (Whitfield-Gabrieli and Nieto-Castanon, 2012).
2.4. Analysis of fractional amplitude of low-frequency fluctuations
PSD analysis was performed on the preprocessed rs-fMRI data using a fractional amplitude of low-frequency fluctuation (fALFF) analysis. Maps with PSD values were computed using detrended fluctuation analysis. The signal was extracted from each voxel, and then temporally band-pass filtered (0.01–0.1 Hz) to reduce low-frequency drift and high-frequency noise. The PSD of the denoized low-frequency band signal, the frequency used in many rs-fMRI studies, was normalized using the following equation (Zuo et al., 2010):
To standardize power raw measures, Z-transform was performed for the PSD maps, which can improve the subsequent statistical analyses on the group level. Using the Z-score map, a one-sample t-test was performed within each group and a two-sample t-test was performed to evaluate the PSD difference between the two groups. Voxels on one-sample and two-sample t-tests were thresholded at a false discovery rate (FDR)-corrected P-value of <0.05 for multiple comparisons.
2.5. Statistical analysis
Regions of interest (ROIs) were defined at the peak of each cluster retrieved from the group difference analysis Z-score map and extracted mean Z-scores from ROIs (spheres of 5-mm radius). Pearson correlation analyses were used to determine correlations between clinical assessments and the Z-score of the PSD in the SCI group. All statistical analyses were performed using the SPSS software version 23 (SPSS, Inc, Armonk, NY, USA), using a significance level of P < 0.05.
2.6. Clinical assessments
Neurological and psychological symptoms in patients with SCI were assessed at the time of rs-fMRI acquisition. The injury severity and SCI grading, mobility and balance function, depressive mood severity, and neuropathic pain severity were evaluated.
The ASIA impairment scale consists of an anorectal examination, a dermatome-based sensory examination, and a myotome-based motor examination using an International Standards for Neurological Classification of SCI worksheet. Motor strength was graded using a 6-point scale (from 0 to 5) in the bilateral upper and lower extremities. The maximal motor score was 100 for all extremities. Sensory function was evaluated with 28 specific dermatomes using a 3-point scale (from 0 to 2) using light touch and pinprick stimulations. The maximal sensory score was 112 for the unilateral side (Kirshblum et al., 2011, Maynard et al., 1997). The neurologic level of injury (NLI) was defined as the most caudal functioning root level with intact sensation and grade ≥3 motor functions.
Mobility and balance function were evaluated using functional ambulation categories (FAC) (Holden et al., 1986) and Berg balance scale (BBS) (Jung et al., 2006). FAC evaluates the ability of functional ambulation using a 6-point scale (from 0 to 5) (Holden et al., 1986). BBS is a gold standard for evaluating static and dynamic balance function as scored from 0 to 56 (Jung et al., 2006). Low FAC and BBS scores indicate poor motor function and reduced mobility.
The IASP defines neuropathic pain as spontaneous and/or evoked burning, stabbing, and shooting sensation caused by a central nervous system lesion or disease (Siddall et al., 1997). Depending on the NLI, neuropathic pain is classified as either at-level or below-level SCI neuropathic pain. At-level SCI pain is perceived in a segmental pattern anywhere within the dermatome of the NLI and/or within the three dermatomes below this level. Below-level SCI pain is perceived in more than three dermatomes below the dermatome of the NLI (Bryce et al., 2012, Siddall et al., 1997). Visual analog scale (VAS), scored from 0 to 100, was assessed as the average neuropathic pain intensity over the past 7 days. Higher VAS scores indicate more severe neuropathic pain.
The Beck Depression Inventory (BDI)-II was used to measure the severity of depressive mood (Hahn, 1982), comprising 21 items, with each answer being graded from 0 to 3, with higher BDI-II scores indicating greater severity (Hahn, 1982).
3. Results
3.1. Demographic and clinical characteristics of participants
A total of 41 patients with incomplete SCI (51.5 ± 13.7 years) and 33 healthy controls (49.5 ± 13.6 years) were enrolled in this study. Patients’ demographic data and clinical characteristics are presented in Table 1 and Supplementary Table S1. Differences in age and sex were not significant between healthy controls and patients with incomplete SCI (P = 0.53 and P = 0.81, respectively).
Table 1.
Demographic and clinical characteristics in patients with incomplete spinal cord injury.
| SCI (N = 41) | |
|---|---|
| Age, mean ± SD years | 51.5 ± 13.7 |
| Sex, n (%) | |
| Male | 28 (68.3%) |
| Female | 13 (31.7%) |
| Etiology of Injury, n (%) | |
| Traumatic | 25 (61.0%) |
| Non-traumatic | 16 (39.0%) |
| Time since injury, mean ± SD days | 408.8 ± 323.9 |
| ASIA impairment scale | |
| B/C/D, n (%) | 1 (2.4%)/10 (24.4%)/30 (73.1%) |
| Motor scores (Upper/Lower extrimity), mean ± SD | 35.0 ± 13.4/27.8 ± 11.2 |
| Sensory scores (Rt side/Lt side), mean ± SD | 75.4 ± 18.8/76.5 ± 19.7 |
| Level of injury (Cervical/Thoracic/Lumbar), n (%) | 27 (65.9%)/11 (26.8%)/3 (7.3%) |
| FAC | 2.5 ± 1.7 |
| BBS | 32.7 ± 19.2 |
| VAS | 33.1 ± 20.4 |
| BDI-II | 13.9 ± 10.3 |
ASIA, American Spinal Injury Associations; BBS, Berg balance scale; BDI-II, Beck Depression Inventory-II; FAC, Functional ambulation categories; VAS, visual analogue scale
3.2. PSD group analyses
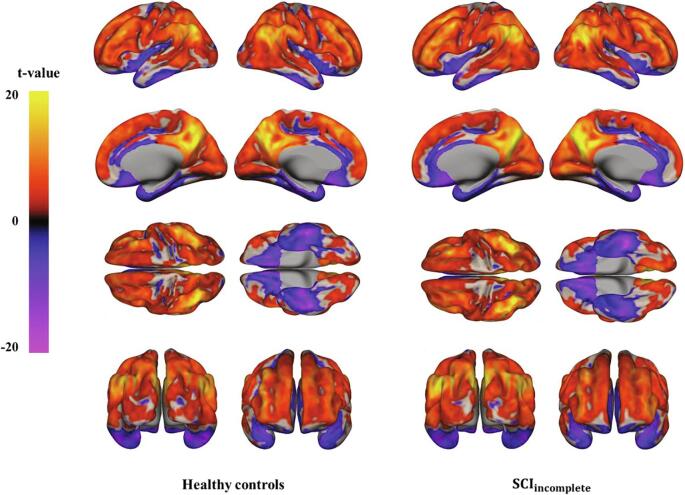
Z-scores of PSD values in each group were assessed using the one-sample t-test (Fig. 1, FDR-corrected P < 0.05). In patients with incomplete SCI, negative PSD values were observed in the bilateral orbito-frontal cortices, bilateral temporal cortices, medial prefrontal cortices, and cingulate cortex. Positive PSD values were observed in the bilateral frontal and parietal cortices. In healthy controls, negative PSD values were observed in the bilateral precentral gyri, bilateral postcentral gyri, bilateral orbito-frontal cortices, bilateral temporal cortices, cingulate cortex, and medial prefrontal cortices. Positive PSD values were observed in the bilateral frontal and parietal cortices in healthy controls (Fig. 1).
Fig. 1.
Power spectral density (PSD) map of a low-frequency band (0.01–0.1 Hz) in the resting brain of patients with spinal cord injury (SCI) (right) and healthy controls (left). The map was thresholded at an FDR-corrected P-value of <0.05 with a minimum cluster size of 10.
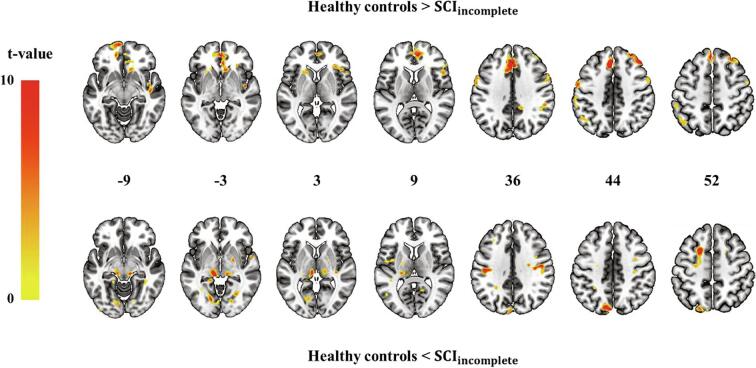
Differences in PSD values between patients with incomplete SCI and healthy controls are shown in Fig. 2 (FDR-corrected P < 0.05) and Table 2 (*P < 0.05 and **P < 0.01). Lower PSD values of the left superior orbito-prefrontal cortex, anterior cingulate cortex (ACC), right ventral medial prefrontal cortex (vmPFC), left medial prefrontal cortex, right middle frontal gyrus, and left supplementary motor area (SMA) were observed in patients with incomplete SCI as compared with the healthy controls. Higher PSD values of the left midbrain, left thalamus, right M1, and left premotor cortex (PMC) were observed in patients with incomplete SCI than in healthy controls.
Fig. 2.
Group differences in the power spectral density (PSD) of low-frequency fluctuations (0.01–0.1 Hz). The top map shows lower PSD values in patients with incomplete SCI than that in healthy controls. The bottom map shows higher PSD values in patients with SCI than that in healthy controls. The map was thresholded at an FDR-corrected P-value of <0.05 with a minimum cluster size of 10.
Table 2.
Group difference displayed in brain power spectral density of low frequency (0.01–0.1 Hz).
| Comparison | Region | Left/Right | Cluster size | Coordinates (mm) |
Peak T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| SCI < HC | Dorsal medial prefrontal cortex | L | 625 | −4 | 34 | 38 | 8.02 |
| Middle frontal gyrus | R | 340 | 38 | 34 | 46 | 6.33 | |
| Supplementary motor area | L | 81 | −2 | 12 | 64 | 5.28 | |
| Medial prefrontal cortex | L | 116 | −2 | 50 | 24 | 5.92 | |
| Anterior cingulate cortex | R | 157 | 5 | 34 | −4 | 5.91 | |
| Ventral medial prefrontal cortex | R | 94 | 4 | 48 | −6 | 6.21 | |
| Superior orbito-prefrontal cortex | L | 174 | −4 | 34 | 38 | 5.50 | |
| SCI > HC | Precentral gyrus | R | 184 | 50 | −10 | 32 | 6.09 |
| Premotor cortex | L | 104 | −20 | −4 | 58 | 5.31 | |
| Thalamus | L | 61 | −16 | −23 | 10 | 5.76 | |
| Midbrain | L | 136 | −4 | −32 | −7 | 6.53 | |
SCI, spinal cord injury; HC, healthy controls; P < 0.05, FDR corrected for multiple comparison and minimum cluster size of 10.
3.3. Correlation analyses between the mean Z-score of PSD and degree of motor function and neuropathic pain intensity
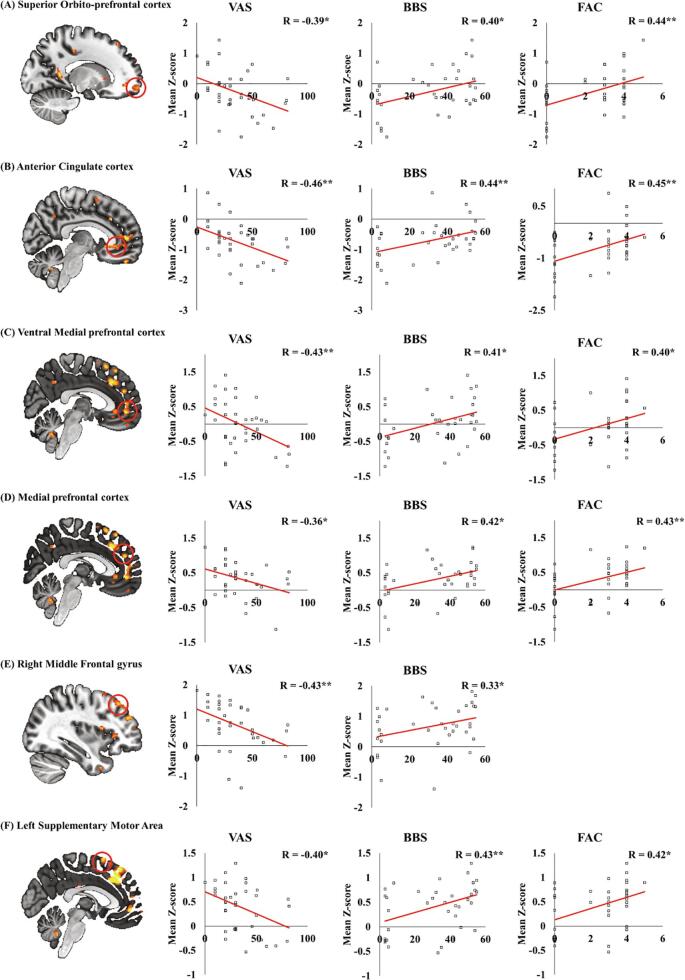
The mean Z-score values of PSD in group difference were associated with degree of mobility function and neuropathic pain intensity while adjusting for the degree of depressive mood (BDI score). In lower PSD values in patients with SCI compared with control, Z-scores of PSD in ACC, right vmPFC, left superior orbito-prefrontal cortex, left medial prefrontal cortex, right middle frontal gyrus, and left SMA were negatively correlated with VAS scores, but positively correlated with BBS and/or FAC scores (Fig. 3, *P < 0.05 and **P < 0.01).
Fig. 3.
Correlation between the mean Z-score of power spectral density (PSD), showing lower PSD values in patients with SCI than that in controls, degree of motor function, and neuropathic pain. The mean Z-score of PSD in the left superior orbito-prefrontal cortex (A), anterior cingulate cortex (B), right ventral medial prefrontal cortex (C), left medial prefrontal cortex (D), right middle frontal gyrus (E), and left supplementary motor area (F) were correlated with VAS, BBS, and/or FAC scores (*P < 0.05, **P < 0.001).
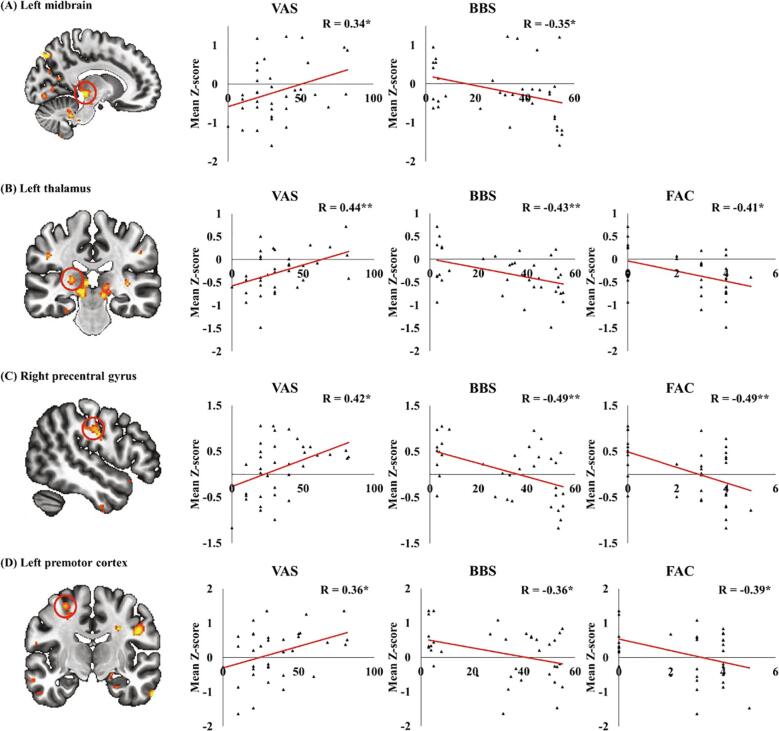
In higher values of PSD in patients with SCI compared with controls, the Z-score of PSD in the left periaqueductal gray (PAG), left thalamus, right M1, and left PMC were positively correlated with VAS scores, but negatively with BBS and/or FAC scores (Fig. 4, *P < 0.05 and **P < 0.01).
Fig. 4.
Correlation between the mean Z-score of power spectral density (PSD), showing higher values of PSD in patients with SCI than that in controls, degree of motor function, and neuropathic pain. The mean Z-score of PSD in the left midbrain (A), left thalamus (B), right precentral gyrus (C), and left premotor cortex (D) were correlated with VAS, BBS, and/or FAC scores (*P < 0.05, **P < 0.001).
4. Discussion
Our findings represent cortical and subcortical PSD alterations in brain areas related to motor function and pain; these alterations have been associated with disabilities and neuropathic pain following SCI. To our knowledge, this is the first study to reveal that alterations in intrinsic spontaneous BOLD oscillations are possibly associated with degree of mobility function and intensity of neuropathic pain in patients with incomplete SCI. Compared with healthy controls, patients with incomplete SCI showed low PSD values in motor-related areas (SMA) and pain-related medial prefrontal areas (ACC, vmPFC, and superior orbito-frontal cortex), whereas patients showed high PSD values in motor-related areas (M1 and PMC) and pain-related areas (thalamus and PAG). In patients with SCI, reduced mobility and poor balance functions were associated with low PSD values in SMA and medial prefrontal areas and high PSD values in M1, PMC, thalamus, and PAG. Conversely, severe neuropathic pain is related to low PSD values in SMA, ACC, vmPFC, and orbito-frontal cortex and high PSD values in M1, PMC, thalamus, and PAG. That is, both motor disabilities and neuropathic pain intensity were associated with PSD alterations not only in motor-related areas but also in pain-related areas. Simultaneously, neuropathic pain intensity was also associated with PSD alterations in motor- and pain-related areas.
Although the functional role of PSD in resting-state BOLD signal has not been well established, several studies have suggested that PSD can be used as a measure of regional intrinsic neuronal activity at the resting-state. The resting-state PSD measures the total power of a given BOLD time course data within a low frequency (<0.1 Hz). PSD analysis of low-frequency fluctuation has been used to examine local spontaneous neural activities and applied to various neurological and psychological diseases (Hoptman et al., 2010, Liu et al., 2014, Min et al., 2019, Park et al., 2018).
Although the severity of motor disability would be not directly related to pain intensity, our study demonstrated the mutual relationship between motor- and pain-related networks and their behaviors in patients with SCI complaining of neuropathic pain. Vlaeyen and Linton (Vlaeyen and Linton, 2000) proposed a fear-avoidance model that assumes the relationship between pain and avoidance behavior mediated by fear. Pain avoidance behavior results in long-term functional disabilities that may lead to motor neuroaxis changes such as in the motor unit, spinal cord, and brain (Hutchinson, 2017, Zale et al., 2013). Indeed, Simons et al. (Simons et al., 2014) reported changes of functional connectivity between the amygdala and motor-/pain-related areas in patients with complex regional pain syndrome. However, our study enrolled patients with SCI with neuropathic pain that interrupted the structural and/or functional sensorimotor connections between the brain and spinal cord. As a disruption between brain and spinal cord may alter supraspinal neural activity in our study, our clinical level of motor disability would be not associated with pain intensity.
Patients with incomplete SCI showed lower PSD values in SMA and medial prefrontal areas such as ACC, vmPFC, and orbito-frontal cortex. In addition, PSD values of these areas were positively correlated with mobility and balance function scores and negatively with pain intensity scores. These findings suggest that increased spontaneous activity of PSD in SMA and medial prefrontal areas is associated with improved mobility and balance function and neuropathic pain relief in patients with incomplete SCI. SMA activity plays a role in the control of postural stabilization and is functionally activated dependent on motor impairment following stroke and SCI (Hou et al., 2016, Penfield and Welch, 1951, Ward et al., 2003). In line with these previous studies, the degree of mobility and balance function increased in proportion to increased spontaneous activity of SMA in this study.
Although SMA is traditionally thought to be a motor area, this study revealed that spontaneous activity changes of SMA are associated with pain intensity. Previous studies have reported that decreased or increased activation of SMA is associated with pain processing during allodynic stimuli in patients with neuropathic pain (Becerra et al., 2006, Moisset and Bouhassira, 2007, Peyron et al., 2004). However, these studies did not clarify the relationship between the degree of activation in SMA and pain intensity. Our findings suggest that increased spontaneous neural activity of SMA plays an additional role in pain processing in patients with SCI with neuropathic pain. Furthermore, this study found that increased PSD values in medial prefrontal areas (ACC, vmPFC, and orbito-frontal cortex) associated with higher-order emotional function during pain processing (Gusnard et al., 2001, Vogt, 2005) were related to reduced pain intensity and improved mobility and balance function. These findings are in accordance with those of previous studies, which reported hypometabolism in medial prefrontal areas regardless of pain intensity in patients with SCI with neuropathic pain (Wrigley et al., 2008, Yoon et al., 2013). Additionally, our study demonstrated that the decreased activity in medial prefrontal areas was associated with increased pain intensity. In consideration with previous evidence, our results suggest that hypoactivities in SMA and medial prefrontal regions are involved in impaired mobility and neuropathic pain following SCI.
In patients with incomplete SCI, increased PSD values of the M1, PMC, thalamus, and PAG were positively associated with pain intensity and negatively with the degree of mobility function. Higher PSD values of the M1, PMC, thalamus, and PAG were associated with greater pain severity and poorer postural balance and mobility. This is consistent with the finding of a previous study by Lundell et al. (2011), who showed that increased activation of the ipsilateral M1 and bilateral PMC during an ankle dorsiflexion is associated with severe impaired motor function in patients with incomplete SCI. In addition, previous studies have reported the relationship between pain intensity and structural and functional changes in motor-related areas in patients with SCI with neuropathic pain (Gustin et al., 2009, Jutzeler et al., 2016, Peyron et al., 2007). In line with these previous studies, our findings seem to suggest that PMC and M1 hyperactivity after SCI influences pain intensity as modulating spontaneous neural activity in pain-related brain areas. Furthermore, the thalamus acts as a relay between the spinal nociceptive input and pain-processing networks (Moisset and Bouhassira, 2007, Schmidt-Wilcke, 2008). Our findings are consistent with that of a previous study, which reported that increased spontaneous firing rate in the posterior nucleus of the thalamus is associated with increased levels of pain stimuli in rodent models of SCI with central pain (Masri et al., 2009). Consequently, our results suggest that spontaneous hyperactivity of the PMC, M1, and thalamus after SCI is associated with severe neuropathic pain and poor mobility function.
Furthermore, increased PSD values of PAG in patients with SCI were strongly associated with severe neuropathic pain and reduced mobility and balance function. The nucleus of PAG, located around the cerebral aqueduct within the tegmentum of the midbrain, has a major role to play in emotional motor systems (Benarroch, 2012, Mantyh, 1983a). The PAG receives afferents from emotional systems including the medial prefrontal cortex, anterior cingulate cortex, amygdala, and hypothalamus. In addition, PAG outputs project to fronto-limbic cortices, which are associated with emotional controls and brainstem nuclei that are related to motor, sensory, and autonomic function (Benarroch, 2012, Krout and Loewy, 2000, Mantyh, 1983b). Because PAG is strongly implicated in emotions, the severity of depression was adjusted as a covariate variable in the correlation analysis to ensure that the observed associations between the mean Z-score PSD values and degree of mobility function and neuropathic pain intensity were not due to the depressive factor. In addition, PAG is a critical component of the pain modulatory system, which is associated with endogenous inhibition of pain and reticular formation system with gross movement and postural stability (Benarroch, 2012, Brownstone and Chopek, 2018). Taken together, our findings on PAG suggest that functional PSD alterations in PAG modulate spontaneous activities of supraspinal neural networks including motor- and pain-related circuits following SCI.
The main findings of the current study were that increased PSD activity in motor-related areas was associated not only with mobility dysfunction but also with pain and that decreased PSD activity in pain-related areas was associated not only with pain but also with mobility dysfunction. In neurorehabilitation settings, these findings seem to suggest important implications in functional motor recovery and neuropathic pain management. For example, neurorehabilitation strategy to reduce increased spontaneous neural activity in motor-related areas based on physical exercise may effectively improve the mobility and neuropathic pain relief following SCI. Moreover, physical exercise combined with pharmacologic treatment, which could further decrease spontaneous activity in pain-related areas, would be an effective neurorehabilitation strategy to improve mobility and relieve pain simultaneously.
This study has some limitations. We enrolled patients with heterogeneous interval since injury and had taken medication to relieve neuropathic pain in various periods at the point of assessment. In addition, participants were allowed to take medications on the rs-fMRI scan day. When analyzing the relationship between alterations of brain regions and clinical data, we could not adjust the type, dose, and duration of medication because of incomplete medical records. Furthermore, results from correlation analyses should also be considered as a limitation as it did not pass the FDR-corrected p-value of 0.05 when performed in the primary analyses (Benjamini and Hochberg, 1995). Finally, another limitation is the lack of physiological recordings during rs-fMRI data acquisition. This limitation is very important owing to possibly changed physiological pulsations in patients with high/cervical spinal cord injuries affecting their central influences on the autonomous nervous system. Therefore, we performed the group comparisons of intermediate results from CompCor denoising to evaluate the amount explained variance of noise in each of the groups and potential differences in the power spectra of the noise components. The results of the group comparisons showed that there were no statistical differences between groups in the power spectra density of the noise components (Supplementary Fig. S1).
5. Conclusion
PSD alterations in the motor- and pain-related cortical and subcortical brain areas were found in patients with incomplete SCI. These PSD alterations were inversely and simultaneously associated with disabling symptoms such as mobility dysfunction, poor postural balance, and neuropathic pain. Therefore, mobility disabilities and neuropathic pain may be mutually influenced by supraspinal plasticity in motor- and pain-related brain networks after SCI.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2019R1C1C101017412).
CRediT authorship contribution statement
Tae-Du Jung: Conceptualization, Project administration, Writing - review & editing. Eunhee Park: Conceptualization, Investigation, Resources, Data curation, Writing - original draft. Hyunsil Cha: Formal analysis, Investigation, Methodology, Resources, Software, Visualization. Eunji Kim: Methodology, Resources, Software, Visualization. Yu-Sun Min: Supervision, Validation. Ae Ryoung Kim: Supervision, Validation. Hui Joong Lee: Supervision, Validation. Yongmin Chang: Conceptualization, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102342.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Becerra L., Morris S., Bazes S., Gostic R., Sherman S., Gostic J., Pendse G., Moulton E., Scrivani S., Keith D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J. Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. Periaqueductal gray: an interface for behavioral control. Neurology. 2012;78:210–217. doi: 10.1212/WNL.0b013e31823fcdee. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- Brownstone R.M., Chopek J.W. Reticulospinal systems for tuning motor commands. Front. Neural Circuits. 2018;12:30. doi: 10.3389/fncir.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce T.N., Biering-Sorensen F., Finnerup N.B., Cardenas D.D., Defrin R., Lundeberg T., Norrbrink C., Richards J.S., Siddall P., Stripling T., Treede R.D., Waxman S.G., Widerstrom-Noga E., Yezierski R.P., Dijkers M. International spinal cord injury pain classification: part I. Background and description. March 6–7, 2009. Spinal Cord. 2012;50:413–417. doi: 10.1038/sc.2011.156. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J., El Mendili M., Lehéricy S., Pradat P.-F., Blancho S., Rossignol S., Benali H. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55:1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. [DOI] [PubMed] [Google Scholar]
- Dietz V., Curt A. Neurological aspects of spinal-cord repair: promises and challenges. Lancet Neurol. 2006;5:688–694. doi: 10.1016/S1474-4422(06)70522-1. [DOI] [PubMed] [Google Scholar]
- Duff E.P., Johnston L.A., Xiong J., Fox P.T., Mareels I., Egan G.F. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum. Brain Mapp. 2008;29:778–790. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P., Weiskopf N., Ward N.S., Hutton C., Gall A., Ciccarelli O., Craggs M., Friston K., Thompson A.J. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134:1610–1622. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabher P., Callaghan M.F., Ashburner J., Weiskopf N., Thompson A.J., Curt A., Freund P. Tracking sensory system atrophy and outcome prediction in spinal cord injury. Ann. Neurol. 2015;78:751–761. doi: 10.1002/ana.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin S., Wrigley P., Siddall P., Henderson L. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb. Cortex. 2009;20:1409–1419. doi: 10.1093/cercor/bhp205. [DOI] [PubMed] [Google Scholar]
- Hahn H. A standardization study of Beck Depression Inventory in Korea. J. Korean Neuropsychiatr. Assoc. 1982;25:487–502. [Google Scholar]
- Holden M.K., Gill K.M., Magliozzi M.R. Gait assessment for neurologically impaired patients: standards for outcome assessment. Phys. Ther. 1986;66:1530–1539. doi: 10.1093/ptj/66.10.1530. [DOI] [PubMed] [Google Scholar]
- Hoptman M.J., Zuo X.-N., Butler P.D., Javitt D.C., D'Angelo D., Mauro C.J., Milham M.P. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr. Res. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Xiang Z., Yan R., Zhao M., Wu Y., Zhong J., Guo L., Li H., Wang J., Wu J. Motor recovery at 6 months after admission is related to structural and functional reorganization of the spine and brain in patients with spinal cord injury. Hum. Brain Mapp. 2016;37:2195–2209. doi: 10.1002/hbm.23163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. McGraw-Hill Education/Austrália; 2017. Brukner & Khan’s Clinical Sports Medicine: Injuries. [Google Scholar]
- Jain N., Florence S.L., Qi H.-X., Kaas J.H. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc. Natl. Acad. Sci. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.Y., Park J.H., Shim J.J., Kim M.J., Hwang M.R., Kim S.H. Reliability Test of Korean Version of Berg Balance Scale. J. Korean Acad. Rehab. Med. 2006;30:611–618. [Google Scholar]
- Jutzeler C.R., Huber E., Callaghan M.F., Luechinger R., Curt A., Kramer J.L., Freund P. Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. 2016;6:18534. doi: 10.1038/srep18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshblum S.C., Waring W., Biering-Sorensen F., Burns S.P., Johansen M., Schmidt-Read M., Donovan W., Graves D.E., Jha A., Jones L. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 2011;34:547–554. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krout K.E., Loewy A.D. Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 2000;424:111–141. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Liu C., Li C., Yin X., Yang J., Zhou D., Gui L., Wang J. Abnormal intrinsic brain activity patterns in patients with subcortical ischemic vascular dementia. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0087880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M., Laubis-Herrmann U., Topka H., Erb M., Grodd W. Reorganization in the primary motor cortex after spinal cord injury – A functional Magnetic Resonance (fMRI) study. Restor. Neurol. Neurosci. 1999;14:183–187. [PubMed] [Google Scholar]
- Lundell H., Christensen M.S., Barthelemy D., Willerslev-Olsen M., Biering-Sorensen F., Nielsen J.B. Cerebral activation is correlated to regional atrophy of the spinal cord and functional motor disability in spinal cord injured individuals. Neuroimage. 2011;54:1254–1261. doi: 10.1016/j.neuroimage.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Mantyh P.W. Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections. J. Neurophysiol. 1983;49:567–581. doi: 10.1152/jn.1983.49.3.567. [DOI] [PubMed] [Google Scholar]
- Mantyh P.W. Connections of midbrain periaqueductal gray in the monkey. II. Descending efferent projections. J. Neurophysiol. 1983;49:582–594. doi: 10.1152/jn.1983.49.3.582. [DOI] [PubMed] [Google Scholar]
- Masri R., Quiton R.L., Lucas J.M., Murray P.D., Thompson S.M., Keller A. Zona incerta: a role in central pain. J. Neurophysiol. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard F.M., Jr, Bracken M.B., Creasey G., Ditunno J.F., Jr, Donovan W.H., Ducker T.B., Garber S.L., Marino R.J., Stover S.L., Tator C.H. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;35:266. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Min Y.-S., Park J.W., Jang K.E., Lee H.J., Lee J., Lee Y.-S., Jung T.-D., Chang Y. Power spectral density analysis of long-term motor recovery in patients with subacute stroke. Neurorehabil. Neural Repair. 2019;33:38–46. doi: 10.1177/1545968318818900. [DOI] [PubMed] [Google Scholar]
- Moisset X., Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37:S80–S88. doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Mole T.B., MacIver K., Sluming V., Ridgway G.R., Nurmikko T.J. Specific brain morphometric changes in spinal cord injury with and without neuropathic pain. NeuroImage: Clinical. 2014;5:28–35. doi: 10.1016/j.nicl.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R., Holler Y., Brigo F., Seidl M., Christova M., Bergmann J., Golaszewski S., Trinka E. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res. 2013;1504:58–73. doi: 10.1016/j.brainres.2012.12.034. [DOI] [PubMed] [Google Scholar]
- North N. The psychological effects of spinal cord injury: a review. Spinal Cord. 1999;37:671. doi: 10.1038/sj.sc.3100913. [DOI] [PubMed] [Google Scholar]
- Park J.-S., Seo J., Cha H., Song H.-J., Lee S.-H., Jang K.E., Lee H.J., Park J., Lee H.-W., Chang Y. Altered power spectral density in the resting-state sensorimotor network in patients with myotonic dystrophy type 1. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-19217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W., Welch K. The supplementary motor area of the cerebral cortex: a clinical and experimental study. AMA Arch. Neurol. Psychiatry. 1951;66:289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- Peyron R., Schneider F., Faillenot I., Convers P., Barral F.-G., Garcia-Larrea L., Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Peyron R., Faillenot I., Mertens P., Laurent B., Garcia-Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage. 2007;34:310–321. doi: 10.1016/j.neuroimage.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Rao J.-S., Ma M., Zhao C., Zhang A.-F., Yang Z.-Y., Liu Z., Li X.-G. Fractional amplitude of low-frequency fluctuation changes in monkeys with spinal cord injury: a resting-state fMRI study. Magn. Reson. Imaging. 2014;32:482–486. doi: 10.1016/j.mri.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Rao J.-S., Ma M., Zhao C., Liu Z., Yang Z.-Y., Li X.-G. Alteration of brain regional homogeneity of monkeys with spinal cord injury: a longitudinal resting-state functional magnetic resonance imaging study. Magn. Reson. Imaging. 2015;33:1156–1162. doi: 10.1016/j.mri.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T. Variations in brain volume and regional morphology associated with chronic pain. Curr. Rheumatol. Rep. 2008;10:467–474. doi: 10.1007/s11926-008-0077-7. [DOI] [PubMed] [Google Scholar]
- Siddall P., Loeser J. Pain following spinal cord injury. Spinal Cord. 2001;39:63. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Siddall P.J., Taylor D.A., Cousins M.J. Classification of pain following spinal cord injury. Spinal Cord. 1997;35:69–75. doi: 10.1038/sj.sc.3100365. [DOI] [PubMed] [Google Scholar]
- Siddall P.J., McClelland J.M., Rutkowski S.B., Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Simons L., Pielech M., Erpelding N., Linnman C., Moulton E., Sava S., Lebel A., Serrano P., Sethna N., Berde C. The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. PAIN®. 2014;155:1727–1742. doi: 10.1016/j.pain.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger M., Grabher P., Hepp-Reymond M.-C., Kiper D., Curt A., Bolliger M., Hotz-Boendermaker S., Kollias S., Eng K., Freund P. Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: a longitudinal pilot study. Front. Hum. Neurosci. 2015;9:254. doi: 10.3389/fnhum.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeyen J.W., Linton S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N., Brown M., Thompson A., Frackowiak R. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wrigley P.J., Gustin S.M., Macey P.M., Nash P.G., Gandevia S.C., Macefield V.G., Siddall P., Henderson L.A. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb. Cortex. 2008;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- Yoon E.J., Kim Y.K., Shin H.I., Lee Y., Kim S.E. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 2013;1540:64–73. doi: 10.1016/j.brainres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Zale E.L., Lange K.L., Fields S.A., Ditre J.W. The relation between pain-related fear and disability: a meta-analysis. J. Pain. 2013;14:1019–1030. doi: 10.1016/j.jpain.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Di Martino A., Kelly C., Shehzad Z.E., Gee D.G., Klein D.F., Castellanos F.X., Biswal B.B., Milham M.P. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.