Abstract
Objectives
Compare the morphologic, laboratory, and clinical features of asymptomatic and symptomatic Castleman disease in the pediatric population.
Methods
We reviewed clinical records and histopathology of patients with Castleman disease from 2 pediatric institutions.
Results
Of 39 patients with pediatric Castleman disease, 37 had unicentric disease, all classified with the hyaline vascular variant of Castleman disease, 8 of which were clinically symptomatic. These 8 patients demonstrated abnormal laboratory findings, including microcytic anemia, elevated erythrocyte sedimentation rate and C-reactive protein, and hypoalbuminemia. In addition, histopathologic evaluation showed that the 8 symptomatic cases had more hyperplastic germinal centers, fewer atrophic or regressed germinal centers, fewer mantle zones containing multiple germinal centers, reduced “onion skinning” of mantle zones, and fewer “lollipop” formations compared with the asymptomatic cases.
Conclusions
This series of pediatric Castleman disease showed that lymph nodes from asymptomatic patients generally demonstrated the more classic hyaline vascular histology, whereas those with symptoms could lack or have only focal classic findings. As such, reactive lymph nodes with subtle Castleman-like features should prompt clinical correlation to ensure proper diagnosis.
Keywords: Castleman disease, Lymph node, Morphology, Pediatric, Symptomatic
Castleman disease (CD), previously called angiomatous lymphoid hamartoma and angiofollicular lymph node hyperplasia, is a lymphoproliferative disorder that is uncommon in the pediatric population. Clinically, it is divided into localized (unicentric CD [UCD]) and multicentric CD (MCD) disease. UCD is defined as a single lymph node or multiple lymph nodes within a single region; most patients present asymptomatically or with symptoms relating to mass effect. A small percentage of patients have inflammatory findings that usually disappear with complete surgical excision.1-7 MCD involves lymph nodes in 2 or more regions and commonly presents with systemic symptoms including fever, weight loss, and fatigue, with laboratory values identifying anemia, hypergammaglobulinemia, hypoalbuminemia, elevated erythrocyte sedimentation rate (ESR), elevated C-reactive protein (CRP), and elevated interleukin-6 (IL-6).1,2,4,5,8-10 MCD is further classified as HHV-8–associated disease and HHV-8–negative idiopathic MCD (iMCD); the former is further delineated as HIV-positive or HIV-negative disease.9-13 Diagnostic criteria for iMCD include both laboratory and clinical abnormalities, histologic features, and exclusion of other possible coexisting disorders that can mimic MCD.11 A clinically distinctive variant form of iMCD was recently described: TAFRO syndrome is defined as thrombocytopenia, anasarca, fever, reticulin fibrosis of the bone marrow, and organomegaly, although in some instances the R represents renal dysfunction.14-16
The gold standard for diagnosis is histologic examination of a lymph node.7 Pathologically, CD is divided into the hyaline vascular (HV), plasma cell (PC), and mixed subtypes.5,12,17,18 HV features account for the majority of unicentric cases, ranging from 74.4% to 92.5% in large series of pediatric and adult patients.1,10,17,19 The HV form of CD is characterized by numerous regressed follicles with increased hyaline deposition. “Onion skinning” of concentric mantle zone lymphocytes may be present around germinal centers. Mantle zones may encompass multiple germinal centers. The interfollicular areas are notable for small mature lymphocytes with increased vascularity. Many of the vessels are hyalinized and occasionally penetrate the mantle zones of regressed follicles, creating a “lollipop” appearance. The PC subtype of CD has pathology demonstrating reactive germinal centers with abundant interfollicular plasma cells. HHV-8–associated MCD demonstrates increased interfollicular vascularity, reactive germinal centers, and variable numbers of plasmablasts within the mantle zones that express HHV-8 by immunohistochemistry.9,13,20 In iMCD, an HV-like “hypervascular” histology is identified in 21% to 41% of cases, whereas PC/plasmacytic and mixed forms are present in 32% to 44% and 27% to 39% of cases, respectively.8,11 TAFRO usually demonstrates a mixed histology with atrophic germinal centers, increased vasculature, and variable numbers of plasma cells.14,15,21,22
Based on our experience in the pediatric population, we hypothesized that the histopathology of symptomatic and asymptomatic UCD differ, often with the symptomatic forms having “less characteristic” features of the disease, presenting a diagnostic challenge. Consequently, in the current study, we systematically addressed the histopathologic differences between symptomatic vs asymptomatic forms of pediatric CD. Further exploration of this pathology may help determine which patients may eventually need additional therapy if their symptoms do not fully resolve on excision.
Methods and Materials
With institutional review board approval and waiver of consent, pathology databases from our institutions were queried for lymph nodes with a diagnosis of CD or Castleman-like features from 1980 to 2018. All cases were reviewed, and only cases of CD were selected for this study. Medical records were reviewed for demographics, clinical features, laboratory values, treatment, and outcome. Age, sex, presentation signs and/or symptoms, physical findings, length of symptoms to diagnosis, and any underlying immunodeficiency were recorded. The location(s) of lymphadenopathy, size, and largest dimension were noted. Surgical excision vs biopsy, treatment, disease evolution, recovery, and length of follow-up were also documented. Laboratory values including hemoglobin, mean corpuscular volume (MCV), WBC count, platelet count, ESR, CRP, albumin, IgG level, and IL-6 levels were recorded.
Blinded to the presence or absence of symptoms or abnormal laboratory findings, both pathologists reviewed H&E-stained slides and any available immunohistochemical stained slides and categorized the cases as morphologic HV disease, mixed, or PC variant CD. The following morphologic features were scored in a binary fashion as present (1) or absent (0): hyperplastic germinal centers, cauliflower floret germinal center remnants, lollipop formations, arborizing mantle vascular proliferation, interfollicular histiocyte proliferation, interfollicular fibrosis, perinodal fibrosis, and atypical follicular dendritic cells. The following morphologic features were scored semiquantitatively as 0 to 3 (0 = absent, 0%; 1 = mild amounts, <10%; 2 = moderate amounts, 10%-30%; and 3 = present in abundance, ≥30%): atrophic/regressed germinal centers, multiple germinal centers per mantle zone, onion skinning, interfollicular vascular proliferation, and interfollicular plasma cell proliferation. The categories of atrophic or regressed germinal centers, lollipop formation, arborizing mantle zone vasculature, interfollicular vascular proliferation, interfollicular fibrosis, and perinodal fibrosis all included hyalinization. Statistics were performed using the Student t test for continuous variables and Fisher exact test for categorical variables (GraphPad Software).
Results
CD Classification
A total of 39 patients with a clinical-pathologic diagnosis of CD were included in this study. Chart review demonstrated that 37 of 39 patients (95%) had UCD, and the remaining 2 had MCD. All UCD patients had HV CD on pathologic exam. Of those 37 patients, 29 were completely asymptomatic, presenting with only an enlarging mass, usually peripherally in the neck, axilla, or chest wall, or with an incidentally identified mass. Two patients presented with symptoms related to mass effect, one with cough and shortness of breath due to a mediastinal mass and another with abdominal pain caused by obstruction by an enlarged lymph node in the mesentery near the ileocecal vale. However, the remaining 6 patients with HV UCD had more systemic symptoms. Both MCD patients had symptomatic disease. One patient with MCD pathologically demonstrated the plasmacytic variant, lacked HHV-8 staining, and was classified as iMCD. The other patient was originally diagnosed in 2002 and would meet the criteria for TAFRO syndrome,14 demonstrating all 5 symptoms (thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly), lack of hypergammaglobulinemia, diffuse but small volume lymphadenopathy, mixed HV and PC morphology, and hypercellular bone marrow with megakaryocyte hyperplasia; he also had thrombotic microangiopathy (TMA) leading to renal insufficiency and then renal failure. He was negative for HHV-8 by lytic and latent antibody serum testing.
Clinical Features of CD in Asymptomatic and Symptomatic Patients
For all patients, the male-to-female ratio was 0.625:1, with a median age of 12 years (range, 2 to 22 years). The single patient older than 18 years had been symptomatic for 3.5 years and was seen at a pediatric institution for evaluation. Table 1 demonstrates the clinical characteristics of the asymptomatic vs symptomatic cohorts, with additional categorization of UCD vs MCD in those with symptomatic disease. There was no significant difference in male-to-female ratio or ages between these groups. As demonstrated, 25 of 29 asymptomatic patients (86%) had primary disease in the neck, axilla, or chest wall, with only 3 patients demonstrating mediastinal disease and 1 patient having retroperitoneal disease. The unicentric symptomatic patients were more likely to have involvement of deeper anatomy including the small bowel mesentery, periportal region, and retroperitoneum, with only 1 case in the mediastinum. The patient with TAFRO had diffuse lymphadenopathy, albeit with only mildly enlarged lymph nodes (largest was 1.8 cm), whereas the patient with iMCD had mediastinal, pericardial, pulmonary hilar, right axillary, and supraclavicular lymphadenopathy. Only 1 patient with asymptomatic CD had a history of iatrogenic immunodeficiency due to chemotherapy, radiation, and stem cell transplant for neuroblastoma. Two patients with symptomatic CD had known immunodeficiencies, one with a common variable immune deficiency–like disorder with hypogammaglobulinemia and chronic Epstein-Barr virus infection and the other with CD4+ T cell lymphopenia. Patients with symptomatic UCD had significantly longer durations of disease to diagnosis compared with those with asymptomatic UCD or MCD (P = .010). The sizes of lymph nodes or masses were also larger in those with unicentric and multicentric symptomatic disease (P = .001 and P = .015, respectively). Most patients with unicentric disease underwent excision, although some cases had second procedures to obtain full excision.
Table 1.
Clinical Characteristics of Patients With Asymptomatic and Symptomatic Castleman Disease
| Asymptomatic UCD (n = 29) | Symptomatic | ||
|---|---|---|---|
| UCD (n = 8) | MCD (n = 2) | ||
| Sex, male:female | 11:18 | 2:6 | 2:0 |
| Age, y, median (range) | 11 (2-18) | 11.5 (6-22) | 13.5 (13-14) |
| Location of primary disease, n (%) | |||
| Neck/parotid | 20 (69) | 0 (0) | 1 (50) |
| Axilla | 3 (10) | 0 (0) | 1 (50) |
| Chest wall | 2 (7) | 0 (0) | 0 (0) |
| Mediastinum | 3 (10) | 1 (13) | 1 (50) |
| Small bowel mesentery | 0 (0) | 3 (38) | 0 (0) |
| Periportal | 0 (0) | 1 (13) | 0 (0) |
| Retroperitoneum | 1 (3) | 3 (38) | 0 (0) |
| Diffuse lymphadenopathy | 0 (0) | 0 (0) | 1 (50) |
| Length of signs/symptoms to diagnosis, mo, median (range) | 5.0 (0.13-36) | 25.5 (2-72)a | 7.5 (1-14) |
| Largest dimension of single lesion, cm, median (range) | 4.0 (2.2-7) | 5.0 (4.1-11)a | 8.15 (1.8-14.5)a |
| Complete surgical excision on first surgery, No. (%) | 23 (79) | 4/7 (57) | 0 (0) |
| Complete reexcision performed, No. (%) | 3/6 (50) | 1/3 (33) | 1/2 (50) |
| Clinical follow-up documented, No. (%) | 16 (55) | 6 (75) | 2 (100) |
| Length of follow-up, mo, median (range) | 5.5 (0.75-102) | 16 (8-84) | 12.5 (7-18) |
MCD, multicentric disease; UCD, unicentric disease; .
aSignificantly increased compared with asymptomatic patients.
Table 2 lists the symptoms and abnormal laboratory values in the patients with symptomatic CD compared with those without symptoms. The most common symptoms included fever, fatigue, weight loss, and gastrointestinal complaints. Those with MCD were more likely to have night sweats than those with asymptomatic and symptomatic UCD (P = .002 and P = .022, respectively). Laboratory values were more often abnormal in those with symptomatic disease. The most common abnormal laboratory finding was a microcytic anemia with decreased hemoglobin level (P < .001 asymptomatic vs all symptomatic) and MCV (P < .001 asymptomatic vs all symptomatic). Additional features more commonly identified in symptomatic patients included elevated ESR, (P < .001 asymptomatic vs all symptomatic), elevated CRP (P = .006 asymptomatic vs all symptomatic), and decreased albumin (P = .002 asymptomatic vs all symptomatic). Hypergammaglobulinemia was also identified in 3 symptomatic patients. The patient with TAFRO and multicentric disease demonstrated thrombocytopenia, normal immunoglobulin levels, and no elevation of IL-6.
Table 2.
Symptoms and Laboratory Values of Patients With Asymptomatic and Symptomatic Castleman Disease
| Asymptomatic UCD (n = 29) | Symptomatic | ||
|---|---|---|---|
| UCD (n = 8) | MCD (n = 2) | ||
| Symptoms | |||
| Fever | 0/29 (0) | 3/8 (38)a | 2/2 (100)a |
| Fatigue | 0/29 (0) | 2/8 (25)a | 2/2 (100)a |
| Failure to thrive/weight loss | 0/29 (0) | 3/8 (38)a | 1/2 (50) |
| Nausea/vomiting/abdominal pain | 0/29 (0) | 3/8 (38)a | 1/2 (50) |
| Gastrointestinal bleeding | 0/29 (0) | 1/8 (13) | 0/2 (0) |
| Joint pain and swelling | 0/29 (0) | 1/8 (13) | 0/2 (0) |
| Shortness of breath and/or cough | 0/29 (0) | 1/8 (13) | 0/2 (0) |
| Congestion | 0/29 (0) | 0/8 (0) | 1/2 (50) |
| Headache | 0/29 (0) | 0/8 (0) | 1/2 (50) |
| Night sweats | 0/29 (0) | 0/8 (0) | 2/2 (100)a |
| Splenomegaly | 0/29 (0) | 1/8 (13) | 1/2 (50) |
| Ascites | 0/29 (0) | 0/8 (0) | 1/2 (50) |
| Pleural effusion | 0/29 (0) | 0/8 (0) | 1/2 (50) |
| Pericardial effusion | 0/29 (0) | 1/8 (13) | 0/2 (0) |
| Renal failure | 0/29 (0) | 0/8 (0) | 1/2 (50) |
| Bone marrow myelofibrosis | 0/29 (0) | 0/8 (0) | 1/2 (50) |
| Paraneoplastic pemphigus | 0/29 (0) | 1/8 (13) | 0/2 (0) |
| Abnormal laboratory values | |||
| Decreased hemoglobin, No./total No. (%) | 0/15 (0) | 7/7 (100)a | 2/2 (100)a |
| Hemoglobin, g/dL, median (range) | 13.4 (10.8-14.5) | 8.4 (5.2-10.6)a | 8.7 (8.3-9.0)a |
| Decreased MCV, No./total No. (%) | 0/15 (0) | 5/6 (83)a | 1/2 (50) |
| MCV, fL, median (range) | 84.3 (78.1-94.0) | 64 (56.1-76.6)a | 75 (67-83)a |
| Leukocytosis, No./total No. (%) | 1/15 (7) | 0/6 (0) | 1/2 (50) |
| Leukopenia, No./total No. (%) | 0/15 (0) | 1/6 (17) | 0/2 (0) |
| WBC, x 103/μL, median (range) | 7.63 (4.5-10.99) | 6.79 (3.23-8.45) | 11.3 (9.8-12.7)a |
| Thrombocytosis, No./total No. (%) | 2/15 (13) | 4/7 (57) | 1/2 (50) |
| Thrombocytopenia, No./total No. (%) | 0/15 (0) | 1/7 (14) | 1/2 (50) |
| Platelets, x 103/μL, median (range) | 268.5 (204-574) | 485 (75-746) | 352 (83-621) |
| Elevated ESR | 4/8 (50) | 6/6 (100) | 2/2 (100) |
| ESR, mm/h, median (range) | 16.5 (1-37) | 96 (28 to >140)a | 116 (100-132)a |
| Elevated CRP, No./total No. (%) | 3/6 (50) | 5/6 (83) | 2/2 (100) |
| CRP, mg/dL, median (range) | 1.0 (0.1-4.8) | 11.37 (0.06-17.15)a | 25.6 (24.1-27)a |
| Hypoalbuminemia, No./total No. (%) | 0/5 (0) | 4/6 (67) | 2/2 (100) |
| Albumin, g/dL, median (range) | 4.4 (4.1-4.6) | 3.3 (2.0-4.1)a | 3.0 (2.7-3.2)a |
| Elevated IgG, No./total No. (%) | 0/5 (0) | 2/6 (33)b | 1/2 (50) |
| IgG, mg/dL, median (range) | 991 (892-1431) | 1,004 (655-13,744) | 2,605 (1,180-4,030) |
| Elevated IL-6, No./total No. (%) | 1/2 (50) | 1/2 (50) | 0/1 (0) |
| IL-6, pg/mL, median (range) | <10 (<5 to 15) | 41 (5-77) | <10 (<10) |
CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; MCD, multicentric disease; MCV, mean corpuscular volume; UCD, unicentric disease.
aSignificantly different compared with asymptomatic patients.
bTwo values determined >1 mo after excisional biopsy, and both remained elevated.
Of the 6 patients with symptomatic UCD and clinical follow-up, 2 had resolution of symptoms within 2 months of complete excision and another within 7.5 months of full excision. A patient with a mediastinal mass had a biopsy only, and her lesion remained stable with symptoms over the following 20 months. One 10-year-old girl with joint pain and swelling, fevers, abdominal pain, and anemia underwent an excisional biopsy with resolution of abdominal pain within 1 month; normalization of hemoglobin, ESR, and IgG within 20 months; normalization of CRP within 31 months; and normalization of MCV within 54 months. The most severely affected patient with UCD developed paraneoplastic pemphigus with oral and genital ulcers, skin eruptions, and fevers; this patient had localized nodal and retroperitoneal masses in the region of the right common iliac artery at the site of bifurcation of the external and internal iliac arteries. Although she underwent a staged full excision of disease and was treated with rituximab and daily intravenous immunoglobulin, she eventually succumbed to her disease 8 months after her diagnosis. Of the 2 patients with MCD, the patient with TAFRO underwent biopsy only, but after 3.5 months and treatment with high-dose methylprednisolone and 3 cycles of cyclophosphamide, his symptoms resolved. The patient with iMCD underwent a 2-step full excision; within 3 weeks of the second surgery, he had recovered from all symptoms.
Histologic Features of CD in Asymptomatic and Symptomatic Patients
Table 3 lists the histologic lymph node features of the asymptomatic and symptomatic patients with CD. All asymptomatic and symptomatic cases of UCD were classified as the HV subtype. In general, biopsies from asymptomatic patients with CD were more likely to contain the “classic” features of HV CD including atrophic/regressed germinal centers; concentric lymphocyte rimming (onion skinning) of mantle zones; hyalinized blood vessels radially penetrating germinal centers as lollipops; and multiple regressed germinal centers per mantle zone. Image 1 demonstrates these histologic figures. Image 2 demonstrates other less common features seen in typical asymptomatic CD including arborizing mantle zone vasculature and cauliflower floret germinal center remnants. Perinodal fibrosis and interfollicular histiocytes were seen in both asymptomatic and symptomatic patients with CD. Biopsies from patients with symptomatic UCD more often demonstrated foci of hyperplastic germinal centers Image 3, whereas those with MCD contained more interfollicular plasma cells Image 4. Because the symptomatic UCD cases lacked significant plasma cell proliferation, they could not be classified as mixed or PC subtypes. Interfollicular fibrosis was more commonly, but not significantly, increased in symptomatic patients.
Table 3.
Histologic Features of Asymptomatic and Symptomatic Castleman Diseasea
| Asymptomatic UCD (n = 29) | Symptomatic | ||
|---|---|---|---|
| UCD (n = 8) | MCD (n = 2) | ||
| Pathologic diagnosis, No. (%) | |||
| Hyaline vascular | 29 (100) | 8 (100) | 0 (0) |
| Plasma cell variant | 0 (0) | 0 (0) | 1 (50)b |
| Mixed | 0 (0) | 0 (0) | 1 (50)b |
| Hyperplastic germinal centers present, No./total No. (%) | 6/29 (21) | 5/8 (63)b | 1/2 (50) |
| Atrophic/regressed germinal centers average score | 2.48 | 1.94c | 0.75c |
| Multiple germinal centers/mantle zone average score | 1.88 | 0.63c | 0c |
| Cauliflower florette germinal center remnants present, No./total No. (%) | 40/29 (14) | 0/8 (0) | 0/2 (0) |
| Lollipop formation present, No./total No. (%) | 25/29 (85) | 1/8 (13)c | 0/2 (0)c |
| Atypical follicular dendritic cells present, No./total No. (%) | 6/29 (21) | 1/8 (13) | 0/0 (0) |
| Mantle zone onion-skinning score | 1.88 | 0.88c | 1.00 |
| Arborizing mantle zone vasculature present, No./total No. (%) | 10/29 (34) | 1/8 (13) | 0/2 (0) |
| Interfollicular vascular proliferation score | 2.14 | 2.19 | 2.00 |
| Interfollicular plasma cell proliferation score | 0.09 | 0.25 | 2.50b |
| Interfollicular histiocyte proliferation present, No./total No. (%) | 7/29 (24) | 4/8 (50) | 0/0 (0) |
| Interfollicular fibrosis present, No./total No. (%) | 18/29 (62) | 7/8 (88) | 2/2 (100) |
| Perinodal fibrosis present, No./total No. (%) | 12/29 (41) | 3/8 (38) | 2/2 (100) |
MCD, multicentric disease; UCD, unicentric disease.
aAtrophic/regressed germinal centers, lollipop formation, arborizing mantle zone vasculature, interfollicular vascular proliferation, interfollicular fibrosis, and perinodal fibrosis all include hyalinization. Score represents semiquantitative score by pathologists (0 = absent; 1 = mild amounts; 2 = moderate amounts; and 3 = present in abundance).
bIncreased compared with asymptomatic patients.
cDecreased compared with asymptomatic patients.
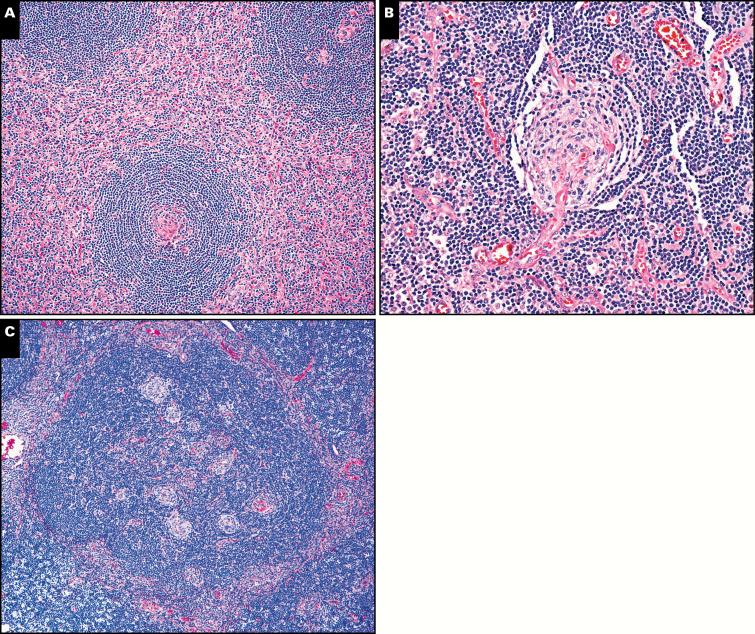
Image 1.
Castleman morphologic features more often present in asymptomatic cases. A, Atretic germinal center surrounded by mantle zone “onion skinning” is more often seen in asymptomatic cases of Castleman disease (×100). This case also demonstrates a high interfollicular vascular proliferation score. B, A vessel penetrates the mantle and germinal center forming a “lollipop,” a finding also more common in asymptomatic cases (×200). C, This enlarged mantle zone contains multiple atretic germinal centers, a finding more common in asymptomatic cases (×100).
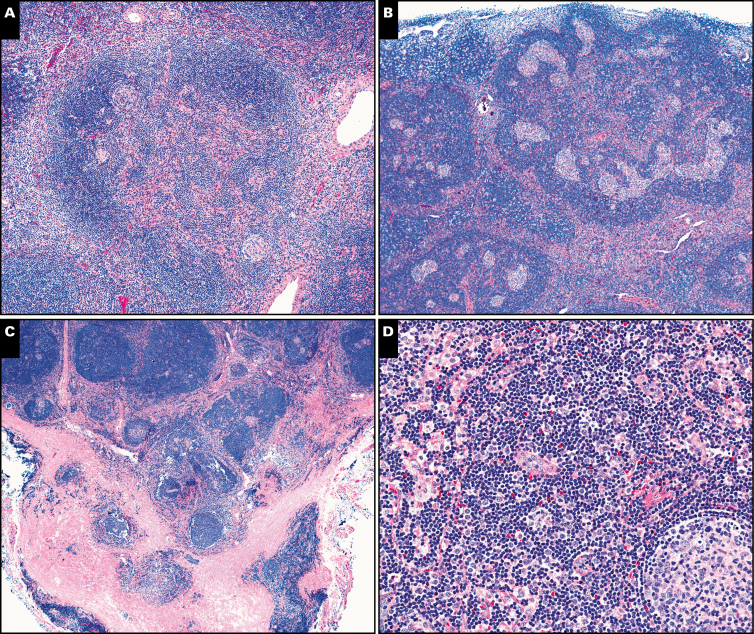
Image 2.
Less common morphologic features in asymptomatic and symptomatic Castleman disease. A, This expanded mantle zone contains small atretic germinal centers and an arborizing vascular proliferation (×100). B, The large right upper mantle zone contains multiple atretic germinal centers, forming a cauliflower floret pattern (×40). C, Perinodal fibrosis was prominent in this case (×20). D, Interfollicular histiocytes can be seen in this case (×200).
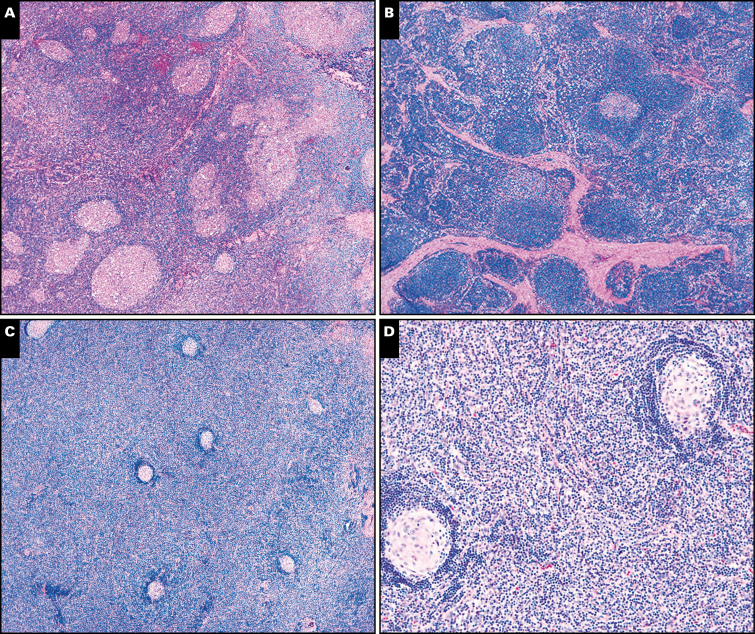
Image 3.
Castleman morphologic features more often present in unicentric symptomatic cases. A, This patient with unicentric Castleman disease (UCD) had fevers, fatigue, anemia, and slowed growth demonstrated hyperplastic germinal centers in his lymph node excision (×40). B, This symptomatic patient with UCD and fevers, joint pain and swelling, abdominal pain, and anemia had increased interfollicular fibrosis in her lymph node biopsy (×40). C and D, This patient with UCD died of paraneoplastic pemphigus. Histology demonstrated markedly expanded interfollicular regions comprising lymphocytes, numerous histiocytes, fibroblasts, plasma cells, and immunoblasts, with marked mantle zone regression; ×40 (C); ×20 (D). Flow cytometry did not identify any abnormal B- or T-cell proliferation. Immunohistochemical stains showed that most interfollicular lymphocytes were CD3-positive T cells, and the numerous histiocytes were highlighted by CD68 and CD163. Plasma cells were polytypic, and Epstein-Barr virus-encoded RNA in situ hybridization was negative.
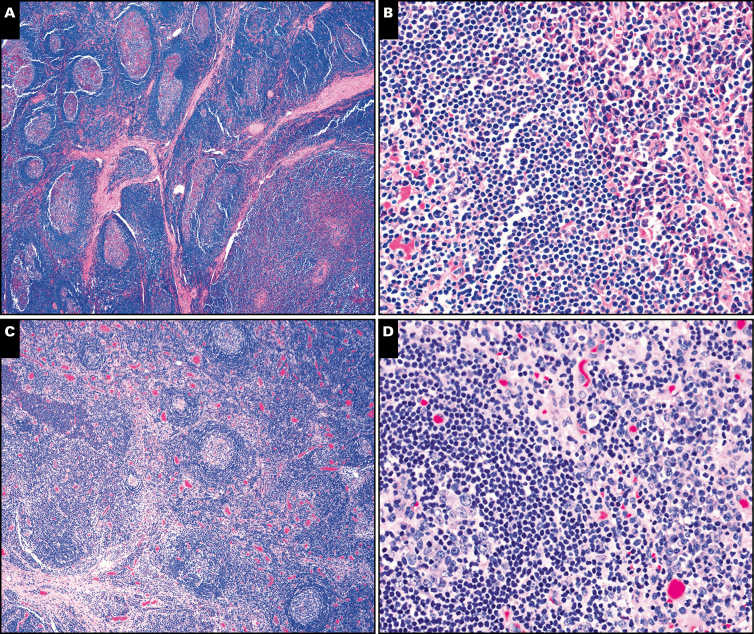
Image 4.
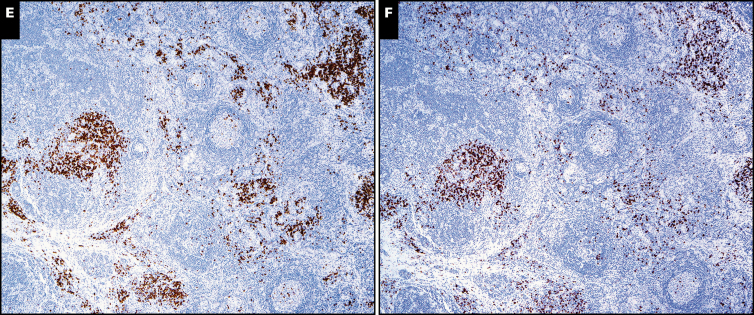
Castleman morphologic features in multicentric cases. A and B, The symptomatic patient with idiopathic multicentric Castleman disease demonstrated the plasma cell (PC) variant by morphology (×20) with reactive germinal centers (A) and numerous interfollicular plasma cells (B, right side of photomicrograph; ×200). C and D, The patient with TAFRO (thrombocytopenia, anasarca, fever, reticulin fibrosis of the bone marrow, and organomegaly) had lymph node morphology demonstrating mildly atrophic germinal centers (C; ×40) with moderate mantle zone “onion skinning” and abundant interfollicular plasma cells (D, right side of photomicrograph; ×200). The κ (E) and λ (F) stains show polytypic plasma cells in the lymph node biopsy from the patient with TAFRO (both at ×40).
Discussion
Comparison of the histologic features of asymptomatic vs symptomatic CD demonstrates that those biopsies or excisions from patients with symptomatic disease have less classic features of HV CD, which typically include atrophic germinal centers, multiple germinal centers per mantle zone, lollipop-penetrating vasculature into germinal centers, concentric onion-skinning mantle zone lymphocytes surrounding germinal centers, and an interfollicular vascular proliferation. In this study, the 8 lymph nodes from patients with UCD had fewer classic features, except for the interfollicular vascular proliferation, compared with our cohort of 29 asymptomatic UCD patients. In fact, many of these patients with UCD not only lacked regressed germinal centers but instead had hyperplastic germinal centers. In some respects, despite being classified as the HV variant and being unicentric, they histologically had slightly more mixed features and some similarities to the 2 iMCD patients in this series, but most lacked significant plasma cell proliferation. In addition, these symptomatic patients with UCD also had some symptoms and abnormal laboratory values (hemoglobin, MCV, ESR, CRP, albumin) similar to those with iMCD, although those with iMCD had greater degrees of most of these laboratory abnormalities. However, 5 of 6 symptomatic patients with UCD with follow-up had symptom resolution with excision alone, quite typical of HV UCD.
All 8 of the symptomatic UCD patients in our cohort had the main site of lymph node disease located internally compared with 86% of the patients with asymptomatic UCD, for whom disease was located peripherally. Their peripheral location may have led to earlier medical attention than those with symptomatic UCD. This time difference is supported by the increased length of time from mass detection or symptoms to diagnosis in those with symptomatic vs asymptomatic UCD. This difference may also have provided more time for lymph nodes to further enlarge, pathologically alter from the more typical features of HV CD, and produce more cytokines to lead to symptoms.
Symptoms in UCD are often associated with mass effect, such as mediastinal masses leading to cough and shortness of breath and mesenteric or peritoneal masses leading to abdominal pain.2 Only 2 of the 8 symptomatic patients with UCD in our series had symptoms that could be directly attributed to mass effect, with the remaining 6 demonstrating more systemic symptoms including fever, fatigue, and weight loss. The percentage of UCD patients with systemic symptoms (6/37, 16%) in our cohort was greater than in some adult cohorts1,9,10 but less than in the pediatric cohort of Sopfe et al,4 in which 8 of 18 (44%) UCD patients had systemic symptoms, and at least half of those were classified as HV CD. Similar to the cohort reviewed by Parez et al,2 those with systemic symptoms more frequently demonstrated internal or visceral mass locations such as mesenteric and retroperitoneal lymph nodes. However, our cohort is the first to separately analyze laboratory data of asymptomatic and symptomatic UCD. Our investigation identified increased laboratory abnormalities in UCD with symptoms, including microcytic anemia, elevated ESR and CRP, and hypoalbuminemia. These findings are not surprising because fever, weight loss, and other systemic symptoms usually correlate with increased inflammatory markers. It is unclear if the symptomatic patients in the cohort of Sopfe et al also correlated to those patients with more abnormal laboratory values.4
Although it has been shown that IL-6 is produced by lymph nodes involved in CD23,24 and that IL-6 production can increase systemic symptoms, not all cases of CD are associated with increased IL-6. Of the 5 patients in the current series with measured IL-6 levels, only 2 were increased. Similarly, only 33% of the tested patients in another pediatric series had increased IL-6.4 These lower percentages may have been related to the lower numbers of MCD cases in pediatrics, as one larger scale study encompassing all ages of only iMCD cases identified 57 of 63 patients as having elevated IL-6.11 Therefore, other cytokines and inflammatory markers must lead to increased symptoms in these IL-6 normal cases. This is supported by the finding that approximately 25% of cases treated with siltuximab, an IL-6 inhibitor, had no therapeutic response.10 Other cytokines have been implicated in CD including IL-1 (with therapeutic response with Anakinra treatment)25,26 and vascular endothelial growth factor.27 However, most of these cases studied have PC CD, whereas most cases of UCD are HV subtype. As such, more studies are warranted to determine what inflammatory cytokines may be differentially expressed in UCD vs MCD and in UCD with and without symptoms. It is possible that these increased inflammatory markers either directly or indirectly lead to altered lymph node morphology.
Genetics may also have a role in the difference between asymptomatic and symptomatic UCD. Recent studies have identified genetic mutations in FAS28and PDGFRB29 in a subset of UCD patients. An additional study identified copy number variants in HIST1H genes, PTPN6, ETS1, and TGFBR2 in cases of UCD.30 Stone et al31 identified an IL-6 receptor polymorphism present with increased frequency in iMCD compared with a healthy control population, and these patients had higher levels of the soluble IL-6 receptor, potentially leading to increased IL-6 activity. As such, underlying genetics may lead to increased susceptibility to develop symptomatic disease.
This pediatric series of CD is the largest reported to date in the United States. As reported previously, most cases of pediatric CD are UCD with HV pathology, representing 75% in the series of Sopfe et al4 (78% of which were HV) and 87% in the series with literature review by Parez et al2 (of which 54% were HV subtype). The lower percentage of HV in the series with literature review by Parez et al may be caused by reporting bias of PC cases in the literature. Our cohort was composed of 95% UCD, all of which demonstrated HV morphology. The 37 UCD patients in this series also slightly differed from the literature by including a substantial percentage of cases involving the neck (69% of symptomatic UCD and 54% of all UCD). Most prior series, although predominantly in adults, have reported high incidence of cases in the mediastinum, up to 86%,17 with cases in the head and neck representing up to 29%1 in other series. A large pediatric series reported that the most common site of disease was the head and neck, affecting 44% of the UCD patients,4 and another literature review of pediatric neck CD identified substantially more of the HV subtype in its cohort (28 of 29 patients).3 As such, pediatric cases may be enriched in head and neck locations and HV morphology. Also similar to Sopfe and colleagues’ pediatric cohort,4 no cases of HHV8- or HIV-associated disease were identified in our institutions.
The patient with UCD and paraneoplastic pemphigus deserves special mention because this patient was the only one who died of this disease, and these biopsies prompted our investigation. Paraneoplastic pemphigus occurs in 1.3%-7.0% of CD1,9,19—even up to 32% in 1 series from China32—and is most commonly associated with HV UCD,1,6,9,32,33 although some series demonstrate rare cases of mixed-type UCD9,33 or mixed-type MCD.32 In fact, CD is the most common cause of paraneoplastic pemphigus in the pediatric population.34 Despite a full excision, rituximab, and daily intravenous immunoglobulin, our patient died, similar to published cases.33,34 Histologically, this patient demonstrated a predominance of atrophic germinal centers, regressed mantle zones, and a marked interfollicular expansion of small lymphocytes, histiocytes, plasma cells, fibroblasts, and small vessels. Onion-skinning mantle zones were not prominent. Image 3C and Image 3D demonstrate this atypical histology. This interfollicular expansion was the most dramatic of all symptomatic UCD cases, and it was the only case with mantle zone regression. It is unclear if the specific dysregulated humoral and cellular autoimmunity factors that lead to paraneoplastic pemphigus35 also directly affect the lymph node architecture or initiate a cascade of factors that lead to the altered architecture.
Both patients with iMCD demonstrated systemic symptoms and increased abnormal laboratory values, similar to prior literature.1,8,10,36 The lower percentage of iMCD cases in children compared with adults has been reported previously, with only 11% of iMCD cases occurring in those younger than 19 years in 1 review,8 and only 25% of iMCD in the pediatric CD cohort of Sopfe et al.4 In our series, the patient with iMCD had plasmacytic features, whereas the patient with TAFRO demonstrated mixed features of CD. Our patient with TAFRO also warrants further discussion because this disease is quite rare in children, with a reported median age of 50-57 years,14,15 and this patient, at age 13, could quite possibly represent the youngest reported in the literature. His disease was consistent with typical TAFRO, having small but diffuse adenopathy with the largest focus measuring only 1.8 cm. This small size markedly decreased the median MCD lymph node size because the other patient had multiple lymph nodes ranging from 3 to 14.5 cm. The patient with TAFRO also demonstrated renal failure due to TMA, a rare but only recently reported finding in pediatric CD.37 Cousin et al37 reported 4 adolescents, all older than our patient, with renal failure in MCD caused by TMA; it is unclear from their report if these teenagers met the complete criteria for TAFRO; although they all had more than 3 TAFRO symptoms, lack of hypergammaglobulinemia and small volume lymphadenopathy were not reported.
This study has limitations. In this series of 39 pediatric patients with CD, only 8 were classified as symptomatic UCD. A larger number of cases would have led to stronger findings. In addition, the histologic features were only graded subjectively in a binary or semiquantitative fashion; the review by 2 board-certified hematopathologists was performed to help decrease any variability. This study was also limited in that it was retrospective, and not all patients had laboratory values analyzed, again, possibly decreasing the sensitivity of our analyses. An additional analysis that compared all UCD patients with any abnormal laboratory values with those with normal laboratory values would have been educational but was not feasible given the number of unknown laboratory values in some patients. Clinical information was also incomplete for some cases that were submitted to the 2 institutions for consultation. Furthermore, follow-up was documented in only 24 of 39 patients (62%), with variable lengths of time, not allowing long follow-up analysis for relapse.
Conclusions
In this large pediatric series of CD, patients with typical features of HV UCD tended to have more peripheral disease and lack many abnormal inflammatory signs and symptoms. However, those patients with HV UCD who did have symptoms demonstrated less classic histology and more reactive findings, including reactive germinal center formation. As such, pathologists are urged to carefully correlate with the clinical presentation when lymph nodes show reactive findings with subtle Castleman-like features to make sure a diagnosis of symptomatic CD is not missed.
Acknowledgments:
This work is supported by Seattle Children’s Mark Alan Bomgardner Endowment (K.M.C.) and. R24 DK099808 (M.D.F.). We thank the dedicated clinicians who took care of these patients. We also thank the Histology Laboratory in the Boston Children’s Hospital Department of Pathology for performing the immunohistochemical stains in a subset of cases.
References
- 1. Haap M, Wiefels J, Horger M, et al. . Clinical, laboratory and imaging findings in Castleman’s disease—the subtype decides. Blood Rev. 2018;32:225-234. [DOI] [PubMed] [Google Scholar]
- 2. Parez N, Bader-Meunier B, Roy CC, et al. . Paediatric Castleman disease: report of seven cases and review of the literature. Eur J Pediatr. 1999;158:631-637. [DOI] [PubMed] [Google Scholar]
- 3. Rabinowitz MR, Levi J, Conard K, et al. . Castleman disease in the pediatric neck: a literature review. Otolaryngol Head Neck Surg. 2013;148:1028-1036. [DOI] [PubMed] [Google Scholar]
- 4. Sopfe J, Endres A, Campbell K, et al. . Castleman disease in pediatrics: insights on presentation, treatment, and outcomes from a two-site retrospective cohort study. Pediatr Blood Cancer. 2019;66:e27613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szalat R, Munshi NC. Diagnosis of Castleman disease. Hematol Oncol Clin North Am. 2018;32:53-64. [DOI] [PubMed] [Google Scholar]
- 6. Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255:677-684. [DOI] [PubMed] [Google Scholar]
- 7. Wong RSM. Unicentric Castleman disease. Hematol Oncol Clin North Am. 2018;32:65-73. [DOI] [PubMed] [Google Scholar]
- 8. Liu AY, Nabel CS, Finkelman BS, et al. . Idiopathic multicentric Castleman’s disease: a systematic literature review. Lancet Haematol. 2016;3:e163-e175. [DOI] [PubMed] [Google Scholar]
- 9. Oksenhendler E, Boutboul D, Fajgenbaum D, et al. . The full spectrum of Castleman disease: 273 patients studied over 20 years. Br J Haematol. 2018;180:206-216. [DOI] [PubMed] [Google Scholar]
- 10. Yu L, Tu M, Cortes J, et al. . Clinical and pathological characteristics of HIV- and HHV-8-negative Castleman disease. Blood. 2017;129:1658-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fajgenbaum DC, Uldrick TS, Bagg A, et al. . International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129:1646-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123:2924-2933. [DOI] [PubMed] [Google Scholar]
- 13. Wang HW, Pittaluga S, Jaffe ES. Multicentric Castleman disease: where are we now? Semin Diagn Pathol. 2016;33:294-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwaki N, Fajgenbaum DC, Nabel CS, et al. . Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91:220-226. [DOI] [PubMed] [Google Scholar]
- 15. Kawabata H, Takai K, Kojima M, et al. . Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop. 2013;53:57-61. [DOI] [PubMed] [Google Scholar]
- 16. Takai K, Nikkuni K, Shibuya H, et al. . Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly [in Japanese]. Rinsho Ketsueki. 2010;51:320-325. [PubMed] [Google Scholar]
- 17. Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29:670-683. [DOI] [PubMed] [Google Scholar]
- 18. Wu D, Lim MS, Jaffe ES. Pathology of Castleman disease. Hematol Oncol Clin North Am. 2018;32:37-52. [DOI] [PubMed] [Google Scholar]
- 19. Talat N, Schulte KM. Castleman’s disease: systematic analysis of 416 patients from the literature. Oncologist. 2011;16:1316-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soumerai JD, Sohani AR, Abramson JS. Diagnosis and management of Castleman disease. Cancer Control. 2014;21:266-278. [DOI] [PubMed] [Google Scholar]
- 21. Igawa T, Sato Y. TAFRO syndrome. Hematol Oncol Clin North Am. 2018;32:107-118. [DOI] [PubMed] [Google Scholar]
- 22. Kurose N, Futatsuya C, Mizutani KI, et al. . The clinicopathological comparison among nodal cases of idiopathic multicentric Castleman disease with and without TAFRO syndrome. Hum Pathol. 2018;77:130-138. [DOI] [PubMed] [Google Scholar]
- 23. Yoshizaki K, Matsuda T, Nishimoto N, et al. . Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74:1360-1367. [PubMed] [Google Scholar]
- 24. Yoshizaki K, Murayama S, Ito H, et al. . The role of interleukin-6 in Castleman disease. Hematol Oncol Clin North Am. 2018;32:23-36. [DOI] [PubMed] [Google Scholar]
- 25. El-Osta H, Janku F, Kurzrock R. Successful treatment of Castleman’s disease with interleukin-1 receptor antagonist (Anakinra). Mol Cancer Ther. 2010;9:1485-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galeotti C, Tran TA, Franchi-Abella S, et al. . IL-1RA agonist (anakinra) in the treatment of multifocal castleman disease: case report. J Pediatr Hematol Oncol. 2008;30:920-924. [DOI] [PubMed] [Google Scholar]
- 27. Nishi J, Arimura K, Utsunomiya A, et al. . Expression of vascular endothelial growth factor in sera and lymph nodes of the plasma cell type of Castleman’s disease. Br J Haematol. 1999;104:482-485. [DOI] [PubMed] [Google Scholar]
- 28. Baker TS, Gambino KJ, Schriefer L, et al. . A novel FAS mutation with variable expressivity in a family with unicentric and idiopathic multicentric Castleman disease. Blood Adv. 2018;2:2959-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Z, Lan X, Li C, et al. . Recurrent PDGFRB mutations in unicentric Castleman disease. Leukemia. 2019;33:1035-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagy A, Bhaduri A, Shahmarvand N, et al. . Next-generation sequencing of idiopathic multicentric and unicentric Castleman disease and follicular dendritic cell sarcomas. Blood Adv. 2018;2:481-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stone K, Woods E, Szmania SM, et al. . Interleukin-6 receptor polymorphism is prevalent in HIV-negative Castleman disease and is associated with increased soluble interleukin-6 receptor levels. PLoS One. 2013;8:e54610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong Y, Wang M, Nong L, et al. . Clinical and laboratory characterization of 114 cases of Castleman disease patients from a single centre: paraneoplastic pemphigus is an unfavourable prognostic factor. Br J Haematol. 2015;169:834-842. [DOI] [PubMed] [Google Scholar]
- 33. Han SP, Fu LS, Chen LJ. Masked pemphigus among pediatric patients with Castleman’s disease. Int J Rheum Dis. 2019;22:121-131. [DOI] [PubMed] [Google Scholar]
- 34. Mimouni D, Anhalt GJ, Lazarova Z, et al. . Paraneoplastic pemphigus in children and adolescents. Br J Dermatol. 2002;147:725-732. [DOI] [PubMed] [Google Scholar]
- 35. Nguyen VT, Ndoye A, Bassler KD, et al. . Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol. 2001;137:193-206. [PubMed] [Google Scholar]
- 36. Smir BN, Greiner TC, Weisenburger DD. Multicentric angiofollicular lymph node hyperplasia in children: a clinicopathologic study of eight patients. Mod Pathol. 1996;9:1135-1142. [PubMed] [Google Scholar]
- 37. Cousin E, Flodrops H, Boyer O, et al. . Renal failure in pediatric Castleman disease: four French cases with thrombotic microangiopathy. Pediatr Blood Cancer. 2018;65:e27045. [DOI] [PubMed] [Google Scholar]