Abstract
The COVID‐19 pandemic necessitated adaptations to standard operations and management of clinical studies, after lockdown measures put in place by several governments to reduce the spread of SARS‐COV‐2. In this paper, we describe our telehealth strategy developed for transitioning our dementia prevention clinical observational prospective study from face‐to‐face visits to virtual visits, to ensure the ongoing collection of longitudinal data. We share the lessons learned in terms of challenges experienced and solutions implemented to achieve successful administration of study assessments. Our methods will be useful for informing longitudinal observational or interventional studies that require a feasible model for remote data collection, in cognitively unimpaired adults.
Keywords: COVID‐19 pandemic, longitudinal studies, preclinical Alzheimer's disease, telehealth, virtual visit
1. INTRODUCTION
National governments and public health authorities around the world are acting to contain the COVID‐19 pandemic. Most countries have instituted lockdown guidelines requiring people to stay home and only travel for essential work, shopping, and exercise. Strategies of social distancing, self‐isolation, and shielding are recommended for the public, those who have symptoms or have been in contact with someone with symptoms of COVID‐19, and those who are over 70 years of age or have chronic underlying medical conditions. These measures are likely to be in place for several months and may be further extended if a second wave occurs. They have had a major impact on clinical research studies, in which participants normally attend a hospital or other clinical research facility for evaluations. In most countries, studies with older people (except for COVID‐19–related trials) are no longer possible at research/clinical sites, during a period of lockdown, and many have therefore been put on hold. On the other hand, the current pandemic has prompted several health authorities to relax regulations on the use of telehealth and telemedicine, thus facilitating their wider adoption in the clinical care of patients in the United States 1 and the rest of the world. 2
Since 2015, the Aging Epidemiology (AGE) research unit, part of the School of Public Health at Imperial College London, has been following up a large cohort of older community dwelling cognitively healthy (at baseline) volunteers in the CHARIOT PRO SubStudy (CPSS) with extensive cognitive, lifestyle, and clinical assessments (Udeh‐Momoh et al. under review). Participants were included in equal numbers above and below a positivity threshold of brain amyloid deposition (n∼409), based either on amyloid positron emission tomography (amyloid PET) or cerebrospinal fluid (CSF) measurements. The overall aim of the CPSS is to determine which cognitive tests and biomarkers, including amyloid status determination, are most sensitive as predictive tools for cognitive decline and dementia. Exploratory objectives are to evaluate a wide range of risk and protective factors and their markers. Persons (n = 2121) aged 60 to 85 years were screened to include those who met inclusion criteria and were cognitively healthy, based on cognitive, clinical, and neurological screening measures. Participants were required to be fluent in English and have adequate hearing and visual acuity to complete the required assessments.
Participants attend the research facility every 3 months for cognitive, medical, biometric, and self‐report questionnaire assessments, with annual bio‐sample collection and study partner informant assessments. Types and schedule of all study assessments are fully described in (Udeh‐Momoh et al. under review ).
As the COVID‐19 pandemic started to spread in the UK, it became evident that this older population cohort would not be permitted to travel to the site, nor would it be possible for research staff to visit their homes to conduct assessments. Thus, we devised a bespoke model using telehealth strategies to adapt the physical visits to a virtual format.
2. OPERATIONAL METHODS AND STRATEGY
A fast‐track review procedure, instigated by the UK Health Research Authority (HRA) in response to the COVID‐19 pandemic, expedited ethical approval for the transition from on‐site to remote assessments (3). To operationalize the transition, the clinical study delivery team was split into three parts: an administration and visit coordination team, a study delivery team, and a data management team. Here we describe strategies adopted to implement virtual visits (shown in Figure 1). We recount challenges faced in the set‐up and ongoing management of this mode of clinical study delivery and discuss lessons learned.
FIGURE 1.
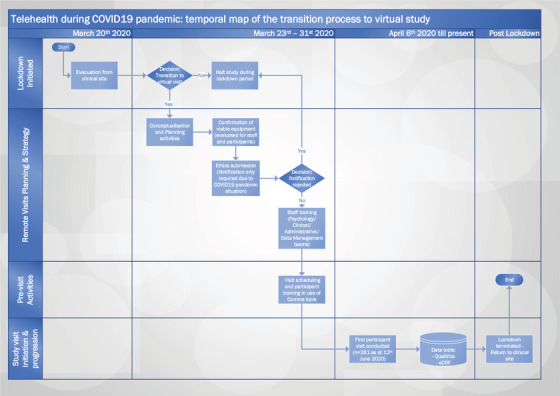
Telehealth during COVID‐19 Pandemic: Temporal Map of the transition process from physical to virtual study visits. eCRF – electronic case report forms. Swimlane map depicts the transition process from physical to virtual visit post clinical site closure in response to lockdown during the COVID‐19 Pandemic. Schematic depicts the timeline of the Go/No‐go decisions for adaptation and implementation of the clinical study protocol for remote administration. Post the site evacuation on 20th March 2020, strategy for remote visits was devised and implemented within 2 weeks, with first virtual visit undertaken by 6th April 2020. As at 12th June 2020, up to 261 participant visits (approximately 24 visits per week) were conducted remotely. Remote visits are planned to continue until the clinical site re‐opens post lock‐down.
2.1. Management and coordination of study activities
Successful and seamless implementation of the transition to virtual visits required the coherent working of all parties. Guidance documents and training were provided to the delivery teams. A study coordinator managed a shared online calendar for scheduling remote visits, allocating raters, as well as rescheduling missed or cancelled appointments, and convened daily meetings to identify and resolve or escalate operational challenges. For the first few weeks of the study, weekly meetings were scheduled to recap lessons learned and resolve more complex issues. The study sponsor provided additional operational support at bi‐monthly team meetings.
HIGHLIGHTS
Remote visits ensure ongoing data collection and participant retention in lockdown.
Standardized paper‐and‐pencil tests may be valid/adaptable for online assessment.
Seniors may need assistance using digital devices for web‐based assessments.
Opportunities needed for exploring novel remote technologies in dementia research.
Bridging gaps between novel and traditional tools may accelerate telehealth practice.
RESEARCH IN CONTEXT
Systematic review: A systematic literature review confirmed that applications of telehealth and telemedicine are expanding through remote clinical assessments and that adoption of such methodologies has significantly increased during the current COVID‐19 pandemic. Prospective dementia and aging research may benefit considerably, if remote assessments can be successfully applied in this field.
Interpretation: Our adaptation to COVID‐19 lockdown has demonstrated that rapid transition to virtual format is achievable within longitudinal cognitive aging research. We provide a blueprint of our adaptations, and describe challenges encountered and mitigating strategies.
Future directions: Continued data collection via remote platforms will help ensure that advances in dementia research are not stalled during crises such as this pandemic. Insights into how to adapt, mobilize, and motivate researchers and study participants under conditions of social isolation can be shared with the wider research community to escalate future research and trial design to a new level encompassing novel digital technology.
2.2. Remote visit operational schedule
2.2.1. Pre‐visit activities
Development of standard operating procedure (SOP), staff training, and ethics
SOPs were rapidly put in place, providing details on the specific roles and accountabilities of the different study team members and adapted mechanisms for the distinct trial activities. Specific training for the remote administration of the study assessments was provided to all staff. All suggestions from team members were debated, with the most viable added to the operations manual and implemented.
Some secondary cognitive assessments used in the study could not be administered remotely due to insurmountable obstacles (eg, the requirement for test raters to annotate the participants’ errors immediately in real time, and computerized tests that required bespoke equipment and software only suitable for onsite use). However, the primary cognitive measures used in the study, namely Preclinical Alzheimer's Cognitive Composite (PACC) and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), presented no such obstacles. For cognitively healthy participants, performance on remotely administered RBANS via videocall has been reported to be comparable to face‐to‐face (F2F) administration. 3
Self‐reported questionnaires/patient reported outcomes (PROs) were added to a secure data survey tool, designed to prevent missing information, which enabled remote completion of the questionnaires. For any questionnaire that required a special license and could not be readily uploaded into a survey format (eg, Qualtrics), assistance was sought from the questionnaire owner to facilitate remote provision to study participants.
Ascertainment of technical capacity for delivery staff and study participants
Delivery staff were provided with suitable equipment for video teleconferencing enabling them to work from home. The team contacted all participants to discuss the new initiative, answer questions, seek verbal consent for undertaking the virtual visit, and evaluate whether their home computer equipment was suitable for study requirements. Remote visits were scheduled for consenting participants. Staff provided step‐by‐step guidance on how to download use the required software for video teleconferencing (TC). Table 1 shows the breakdown of participant response and feedback in relation to change to study visit mode due to COVID‐19 and technical capability.
TABLE 1.
Breakdown of participant response and feedback in relation to study visit mode changes due to COVID‐19*
| Participants scheduled to undertake remote visit (n = 324) | In ≈90% of actively participating volunteers, the general response was willingness to try out the new mode of conducting study visits, ie, remote study assessments. |
| Participants who declined remote visit due to unwillingness to use/learn/download technology/lost to contact (n = 22) | Of the remaining 10% who would not undertake the remote visits, majority of these participants advised that they may consider assessments conducted over the telephone or via email or post. Some participants revealed a lack of conducive environment at home for the study assessments. Others were not willing to undertake the remote visits due to health impediments and yet others were simply not willing to use the technological systems required for videocalls. |
| Participants without suitable equipment (n = 26) | These participants though willing to undertake the remote visits did not have suitable equipment for videocalls (ie, with audio/ visual capabilities—webcam/mic/computer/internet access). At the same time, these participants expressed willingness to try out the remote assessments by phone as well as e‐mail or postal questionnaires. |
Notes: Significant proportion of the actively participating cohort were willing and able to undertake study assessments remotely. Reasons for lack of participation in the remote visits ranged from non‐availability of suitable equipment for video teleconferencing/ communication tools to general unwillingness to learn and use any new technology.
Information valid as at June 12, 2020.
Prior to the study visit, participant readiness and technical capacity for the visit were confirmed.
Development of data management protocol for study assessments and storage of electronic medical records (EMRs)
A secure, encrypted, password‐protected folder was set up to enable electronic storage of all participant information, with clearly labeled sections for ease of access to relevant documents. Electronic versions of all study record forms including source worksheets were prepared. The study team received specific training on how to upload data into the secure folder during or immediately after visits, with revision training of information governance policies per GDPR (General Data Protection Regulation) and GCP (Google Could Platform).
2.2.2. Remote visit activities
Administration of trial assessments
Cognitive assessments: As far as possible, the process for administering assessments face‐to‐face was replicated remotely. Forms for participants to write or draw on were mailed out before the visit, marked “Do not open until instructed.” At the visit, participants were asked to ensure that they would be free of distractions during the assessment. After checking that the participant could see and hear the rater well, had received the mailed forms, and had sufficient desk space and a pen, testing commenced. Verbal stimuli were presented by computer audio or phone. Visual stimuli were displayed via webcam or PowerPoint. Once completed, participants displayed their drawings and written responses to the webcam and these were captured as screenshots by the raters. Raters completed paper record forms and retained these as original source documents. Electronic copies were saved in the participant e‐folder with the screenshots.
Clinical evaluations: The clinical evaluations, typically undertaken F2F, were performed over the phone. Assessments included administration of the Clinical Dementia Rating (CDR) scale 4 as well as recording of study events (serious and non‐serious), including COVID‐19–related signs and symptoms. These evaluations were performed by fully trained clinicians. As the study is observational, participants were given standard National Health Service guidance and advised to contact their medical provider for clinical follow‐up where this was indicated. For a subgroup of participants without suitable equipment for video TCs for cognitive testing, clinical evaluations were thus still possible.
Self‐reported study outcomes: Participants completed self‐reported questionnaires electronically. All participants were emailed a unique link to the questionnaires in Qualtrics and advised to complete them after the cognitive assessments and before the end of the day. Submission of the questionnaires was ascertained on each day and responses were entered into our secure electronic data capture system.
Operational considerations: Delivery team leaders were appointed on a daily rota to coordinate the visit activities and manage the schedule. This individual was responsible for ensuring all delivery team members required to conduct visit activities were available (finding alternatives for staff who were indisposed), and checking that each component of every study visit was completed. The team leader was contactable by participants and dealt with queries where possible, or escalated to the relevant staff member, and followed‐up to ensure resolution.
2.2.3. Post‐visit activities
Data validation and management
Data quality assurance measures: Senior psychologists regularly check completed assessment forms for quality and standardization in administration and scoring across the rater team. To enable this to be done remotely, a method of editing/quality checking e‐copies of source documents was implemented.
Data entry: Dedicated staff entered or downloaded all data from the remote assessments into the electronic database as per the usual process for the longitudinal data entry. The source documents will be examined and monitored by the sponsor on‐site monitoring team as per usual practice.
3. DISCUSSION
3.1. Operational challenges and mitigating strategies
Participants welcomed the initiative, with 90% of them willing to undergo assessments by videocall and 70% of visits successfully accomplished in the first few weeks. Although they all reported possession of suitable equipment at home, this information could not be relied upon in practice, as technical obstacles became evident when pre‐visit videocalls were attempted. In some cases, the problem was insurmountable (eg, no web camera). In other cases, staff were able to advise the participant, by phone, on how to enable the video link. These technical advisory phone calls initially took up to 2 hours but were ultimately reduced to approximately 30 minutes, once solutions for recurrent issues were identified. Issues included difficulty with download of software required for video teleconferencing, and was emphasised by device incompatibility, and/ or technical inabilities. Indeed in the first month of conducting the remote visits, 17% of the virtual visits could not proceed and had to be re‐scheduled due to technical issues and non‐compatible/‐viable equipment for video calls.
The virtual visits required suitable video quality for administration of cognitive assessments. Permission to use electronic copies of the stimulus materials made it possible to display clear‐quality images on the participant's screen via PowerPoint to minimize reliance on the quality of the webcam video display. Participants’ written and drawn responses were successfully viewed via the video link and saved as screenshots. Audio communication was successfully achieved either via computer or by telephone. Significant distractions were generally absent and, if present, could often be addressed by the rater. Assessment of orientation to date and time required consideration (as this information appears routinely on computer screens) and was addressed by asking the participant to close their eyes before orientation questions were posed. Remote quality checking and monitoring of paper record forms were made possible by uploading digitally captured record forms although, where possible, the physical transfer of forms by courier proved more efficient. All participants were able to complete self‐report questionnaires on their home computing devices, with some expressing a preference for this medium.
Early indicators suggest that the initiative is helping to maintain participant engagement and ensure retention. Initial feedback was positive, as most participants who have completed the assessments found the process more convenient than having to travel to the study site, and appreciated the opportunity to take part in a constructive activity during COVID‐19 lockdown restrictions.
3.2. Strengths and limitations
We did not change the format of any assessments other than the remote interface. Furthermore, the usual visit schedule and process was followed and coordinated by the study coordinator and daily delivery team lead. Success of the remote visit was facilitated by regular feedback sessions with staff and ongoing training. We were thus able to maintain the longitudinal assessments, thereby contributing to research during self‐isolation and providing a sense of purpose/involvement for both staff and participants.
Several limitations should be considered in implementing our transition to remote visits strategy. The first is of a possible selection bias: those who have agreed to take part and are able to learn and use the required technology may represent a biased higher functioning subsample. We will be able to evaluate if this is the case, given the availability of information on cognitive status of participants from prior assessments. Furthermore, study participants were not assessment‐naive, as they had already undergone on‐site assessments multiple times and were, thus, already familiar with the test procedures. Therefore, this study cannot fully inform on‐line trial designs where remote visits would be required from study start. Furthermore, it was not possible to administer the full protocol remotely: sample collection, a key element of physical visits for our longitudinal study, was not possible during the lockdown period and some cognitive assessments could not be adapted for remote administration. The full protocol will be resumed once on‐site visits are possible.
3.3. Implications for wider research and future considerations
This initiative has provided information on the viability and feasibility of administering remote study visits with older adults using their own available equipment, including rates of uptake and successful completion of assessments, as well as lessons learned.
It also provides an opportunity to evaluate the validity of traditional paper‐and‐pencil cognitive tests, under non‐standard remote administration conditions. Previous studies of remote administration of cognitive assessments have reported good correlations with results from F2F administration, though with potential variability according to age and type of assessment. 5 , 6 , 7 After the successful set‐up and implementation of the virtual visits, we plan to compare results of all assessments obtained through the remote visits with those obtained at last pre‐lockdown physical visit and the first post‐lockdown (typically within a 3‐month period). By evaluating which elements of the assessments retain validity under remote conditions and which are compromised, we hope to report findings to inform the choice of assessments in future studies.
Remote assessments may provide a useful means of testing large numbers of study participants for longitudinal, observational studies as well as for screening for inclusion and follow‐up in large‐scale prevention/intervention trials, 8 with high acceptance rate in seniors (90% in our study; 88.6% in Takeda et al. 8 ). A pilot study on remote assessment revealed that once older adults had coped with a first remote assessment in digital format the dropout was minimal. 9 While this remote method is suitable for a cognitively healthy elderly population conversant with the internet and digital devices, it is likely that, for cognitively impaired participants, difficulties in coping with technology may be accentuated over time, due to progressive memory and executive function loss. Participants may become frustrated and anxious, and some may require assistance. At the same time, there is much ongoing research and development of apps and wearable devices that could track patterns of behavior in individuals with mild cognitive impairment or dementia. These methods may help to bridge the gap between remote assessment for cognitively healthy and impaired participants of clinical research studies.
The potential for virtual clinical trials to improve trial efficiency, increase protocol flexibility, and foster volunteer participation and retention is an ongoing debate that has wide implications for future clinical trial operations, even as telemedicine strategies for dementia have been developing over the last 25 years (reviewed in Vilalta‐Franch et al. 10 ). Virtual clinical studies in other fields have reported several logistical benefits, such as cost efficiency, increased diversity of participants enrolled ,and rapid enrolment. 11 , 12 , 13 , 14
Further work is also needed to explore the validity and reliability of remote versus F2F assessment of cognitive function and clinical status, typically using validated paper‐and pencil tests (P&P), and for use in clinical populations. This could additionally be aided by digital health tools and devices, and the development of novel technologies in this field.
4. SUMMARY
The methods we have tried for the remote administration of a longitudinal brain aging research study showed that clinical research may continue without long‐term disruption, when a national or international crisis, such as the current pandemic, requires self‐ or social isolation. Transitioning physical to remote visits allows for uninterrupted longitudinal data collection and for participants to maintain their involvement in clinical research, when other avenues or outlets for usual activities are restricted. Similar studies have reported the validity of cognitive assessments even under non‐standard conditions. Implementing such strategies could reduce restrictions and impediments to future studies thereby enabling wider participation in research, for instance recruitment of participants who might not otherwise be able to take part and/ or retention.
For large‐scale clinical trials and longitudinal studies, our model of remote visits may result in reduced visit costs, compared to on‐site visits. This decentralized trial approach, in which entire clinical visits are conducted virtually, may merit further consideration for adoption in future study designs, as part of longitudinal observational and interventional trials.
We live in an era of digital phenotyping, big data analytics, and advancing new technologies. These opportunities have yet to be fully exploited within dementia research. Many promising technical innovations will take time to develop and validate and, in the meantime, steps can be taken immediately to capitalize on the technology which is already available and has become increasingly familiar as a result of the COVID‐19 pandemic. A unified effort among funders, industry, and the research community to support the development and validation of tools to bridge the gap between digital and the traditional P&P assessment tools would accelerate the incorporation of telehealth practices into dementia research.
TRIAL REGISTRATION
The CHARIOT:PRO SubStudy is registered with clinicaltrials.gov (NCT02114372). Notices of Protocol modifications will be made available through this trial registry.
ETHICS INFORMATION
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. To ensure the quality and integrity of research, CHARIOT:PRO SubStudy is conducted in accordance with GCP Guidelines, GPPs issued by ISPE, applicable national guidelines, and to the Declaration of Helsinki 2013, as modified by the 52nd World Medical Assembly (WMA), Edinburgh, Scotland, 2000, and clarified by the WMA General Assembly, Washington 2002 and Tokyo 2004. The study has received approval from the National Research Ethics Service (NRES) Committee London Central (reference 15/LO/0711 [IRAS 140764]), as well as independent ethics review by committee from the local site. Formal informed consent was taken using an informed consent form (ICF) from both participant and study partners before participation in the study. Consent for publication has been granted by the study sponsor clinical lead at Janssen R&D.
AUTHOR CONTRIBUTIONS
All authors contributed to initiating and designing the study, drafting and critically reviewing the manuscript. The study was supervised by LM. All authors approved this manuscript for publication.
CONFLICTS OF INTEREST
Prof Lefkos T. Middleton has received research funding for his Imperial team from Janssen, Takeda, AstraZeneca, Novartis, and UCB Pharmaceuticals; and does not hold any agreement with any of the funders in relation to patents, products in development relevant to this study, or marketed products. He has had consultancy agreements with Takeda, Eli Lilly, Astra Zeneca, Novartis, and Takeda. He is National (UK) Coordinator for the TOMMORROW, Amaranth and Generation I&II Clinical Trials and the Janssen Chariot PRO studies.
FUNDING INFORMATION
This work was supported by Janssen Pharmaceuticals Research & Development, LLC.
AVAILABILITY OF DATA
Data used in preparation of this article were obtained from the CHARIOT:PRO SubStudy database at Imperial College London. The primary goal of CHARIOT:PRO SubStudy is to identify and validate determinants of AD, alongside cognitive, functional and biological changes in older adults with or without detectable evidence of AD pathology at baseline. The Principal Investigator of CHARIOT:PRO SubStudy is Lefkos T Middleton, MD (email: l.middleton@imperial.ac.uk).
ACKNOWLEDGMENTS
The authors wish to thank the participants who continue to volunteer their time and commitment toward the CHARIOT:PRO SubStudy. We thank the staff at the Imperial College London site who facilitated execution of the transition to virtual visits, particularly Jenny Crispin, Heather McLellan, Dinithi Perera, Inthushaa Indrakumar, Stefan McGill‐Summers, Neil Beckford, Naia Headland‐Vanni, and Kristina Lakey (project coordination and administrative team); Parthenia Giannakopoulou and Banke Adeleke (data management team); Dimitra Kafetsouli and Seemulamoodi Yellappa Chowdary (clinical team); Jamie Ford, Isaac Walsh, Katrina Cosby, Claire Devlin‐Chisnall, Snehal Pandya, and Lesley Williamson (psychology team). We especially thank the sponsor for funding this work.
Udeh‐Momoh CT, de Jager‐Loots CA, Price G, Middleton LT. Transition from physical to virtual visit format for a longitudinal brain aging study, in response to the Covid‐19 pandemic. Operationalizing adaptive methods and challenges. Alzheimer's Dement. 2020;6:e12055 10.1002/trc2.12055
REFERENCES
- 1. AMA . CARES Act: AMA COVID‐19 pandemic telehealth fact sheet. 2020. [cited 2020 Accessed May 6, 2020]; Available from: https://www.ama-assn.org/delivering-care/public-health/cares-act-ama-covid-19-pandemic-telehealth-fact-sheet.
- 2. Wang CJ, Car J, Zuckerman BS. The power of telehealth has been unleashed. Pediatr Clin North Am. 2020;67(4):xvii‐xviii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galusha‐Glasscock JM, Horton DK, Weiner MF, Cullum CM. Video teleconference administration of the repeatable battery for the assessment of neuropsychological status. Arch Clin Neuropsychol. 2016;31(1):8‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566‐572. [DOI] [PubMed] [Google Scholar]
- 5. Randoplh C, Williams J, Hannesdottir K, Eurevecko E, Tariot P, Farlow M, Galvin J Langois C, Hunt C, Olsson T, Poole M, Weber C, Boehm P, Cohen E, Garzio L, & Alexander R, et al., P1‐177 Telephone administration of the CDR: excellent agreement with face‐to‐face administration. Alzheimer’s & Dementia. 2014. 10, P364–P364. 10.1016/j.jalz.2014.05.415. [DOI] [Google Scholar]
- 6. Dauphinot V, Boublay N, Moutet C, Achi S, Bathsavanis A, Krolak‐Salmon P. Comparison of instrumental activities of daily living assessment by face‐to‐face or telephone interviews: a randomized, crossover study. Alzheimers Res Ther. 2020;12(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta‐analysis. Neuropsychol Rev. 2017;27(2):174‐186. [DOI] [PubMed] [Google Scholar]
- 8. Takeda C, Guyonnet S, Ousset PJ, Soto M, Vellas V. Toulouse Alzheimer's clinical research center recovery after the COVID‐19 crisis: telemedicine an innovative solution for clinical research during the coronavirus pandemic. J Prev Alzheimer's Dis. 2020. 10.14283/jpad.2020.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sano M, Egelko S, Ferris S, et al. Pilot study to show the feasibility of a multicenter trial of home‐based assessment of people over 75 years old. Alzheimer Dis Assoc Disord. 2010;24(3):256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vilalta‐Franch J, Garre‐Olmo J, López‐Pousa S, Coll‐De Tuero G, Monserrat‐Vila S. [Telemedicine and dementia: a need for the 21st century]. Rev Neurol. 2007;44(9):556‐561. [PubMed] [Google Scholar]
- 11. Bedlack RS, Wicks P, Vaughan T, et al. Lunasin does not slow ALS progression: results of an open‐label, single‐center, hybrid‐virtual 12‐month trial. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(3‐4):285‐293. [DOI] [PubMed] [Google Scholar]
- 12. Wicks P, Vaughan TE, Massagli MP, Heywood J. Accelerated clinical discovery using self‐reported patient data collected online and a patient‐matching algorithm. Nat Biotechnol. 2011;29(5):411‐414. [DOI] [PubMed] [Google Scholar]
- 13. Dorsey ER, Wagner JD, Bull MT, et al. Feasibility of virtual research visits in fox trial finder. J Parkinsons Dis. 2015;5(3):505‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orri M, Lipset CH, Jacobs BP, Costello AJ, Cummings SR. Web‐based trial to evaluate the efficacy and safety of tolterodine ER 4 mg in participants with overactive bladder: rEMOTE trial. Contemp Clin Trials. 2014;38(2):190‐197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in preparation of this article were obtained from the CHARIOT:PRO SubStudy database at Imperial College London. The primary goal of CHARIOT:PRO SubStudy is to identify and validate determinants of AD, alongside cognitive, functional and biological changes in older adults with or without detectable evidence of AD pathology at baseline. The Principal Investigator of CHARIOT:PRO SubStudy is Lefkos T Middleton, MD (email: l.middleton@imperial.ac.uk).


