Abstract
We propose use of bispecific monoclonal antibody (mAb) complexes bound to erythrocytes to redress the lack of efficacy of anti‐amyloid beta mAbs in Alzheimer's disease treatment. Our paradigm leverages erythrocyte complement receptor 1 to promote rapid and quantitative removal of amyloid beta from the circulation, and its subsequent removal from the brain as well.
Keywords: Alzheimer's disease, amyloid beta, bispecific monoclonal antibodies, complement receptor 1, erythrocyte, human
Strategies focused on the utility of monoclonal antibodies (mAbs) to target amyloid beta (Aβ) in the prevention or treatment of Alzhemier's disease (AD) have unfortunately failed to meet clinical expectations although some evidence suggests that aducanumab may have a modest level of efficacy. 1 , 2 , 3 , 4 , 5 , 6 Anti‐Aβ mAbs have been designed to promote neutralization or phagocytosis of Aβ‐containing plaques, or to bind to soluble Aβ and to soluble Aβ oligomers (possibly the most neurotoxic forms of the peptide) and then mediate their neutralization and removal from the brain. However, infusion of large amounts of mAbs that bind to soluble Aβ substantially reduces its rate of elimination from the body by increasing its concentration in the bloodstream. Under these conditions it is cleared as an immunoglobulin G (IgG)‐Aβ immune complex with a half life of ≈ 30 days in contrast to a half‐life of 3 hours for the free peptide. 6 , 7 , 8 Only small amounts of infused IgG mAbs can penetrate the blood‐brain barrier; 1 , 6 , 9 therefore, it is not clear if these mAb infusions can actually lower Aβ levels in the brain, considering the markedly increased levels in the bloodstream.
We propose an alternative targeting approach to ensure rapid elimination of nascent Aβ–mAb immune complexes from the bloodstream (and we anticipate subsequently from other compartments). This strategy is based on intravenous infusion of bispecific, tetravalent mAb complexes to simultaneously capture soluble Aβ in the circulation and bind it to erythrocytes via complement receptor 1 (CR1; CD35). The complexes will then be cleared from the circulation due to the action of Fcγ receptors on resident macrophages in the liver and spleen 10 , 11 , 12 (Figure 1). As additional Aβ then diffuses across the blood‐brain barrier and enters the bloodstream, it will be handled by the same mechanism.
FIGURE 1.
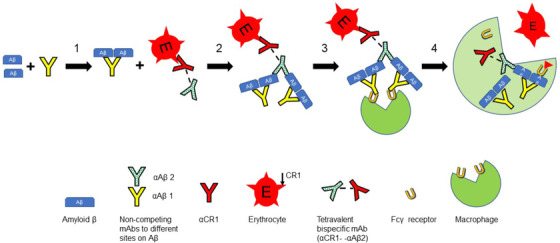
Schematic diagram of the alternative targeting strategy. Step 1, a non‐competing monoclonal antibody (mAb) binds to amyloid beta (Aβ) in the bloodstream. The initial concentration of Aβ is ∼ 0.07 nM [8, 20]. Step 2, the erythrocyte‐bound bispecific construct mediates binding of the Aβ‐mAb complexes to the erythrocyte. Under these conditions as many as four Aβ can be bound per complement receptor 1 (CR1). Step 3, the erythrocyte‐bound immune complex traffics to the liver and spleen where it will be engaged by Fcγ receptors on the macrophage. Step 4, the immune complex, along with CR1, is taken up and internalized by the macrophage but the erythrocyte returns to the circulation. Based on an average of 500 CR1 per erythrocyte, and 5 × 1012 erythrocytes per liter of blood, the concentration of erythrocyte‐associated CR1 is ≈ 4 nM. Therefore, the system has the potential to clear as much as 16 nM Aβ
To have optimal conditions to promote effective and rapid clearance of targeted, erythrocyte‐bound Aβ, a non‐competing anti‐Aβ IgG mAb that binds to a distinct site on soluble Aβ will also be infused; this allows for generation of larger immune complexes that will be readily recognized by macrophage Fcγ receptors and will be more efficiently cleared. Use of this IgG mAb will also lead to further amplification of binding of Aβ to erythrocyte CR1 (Figure 1) .
This paradigm is derived from Nelson's immune adherence reaction, 11 , 13 which was additionally developed by Cornacoff et al., 14 who demonstrated that C3b‐opsonized IgG immune complexes bound to erythrocyte CR1 are cleared from the circulation. Application of this concept to clearance of Aβ was proposed by Rogers et al. in 2006, who reported that Aβ can activate complement and bind to erythrocyte CR1, thereby allowing for its peripheral clearance. 15 More recently the groups of Rogers and Tenner have reported in vitro investigations and a non‐human primate model which demonstrate that in the presence of a specific mAb, immune complexes containing Aβ can activate complement, capture C3b, and bind more effectively to erythrocytes and then be cleared from the circulation. 12 , 16 Under these conditions not all immune‐complexed Aβ binds to erythrocytes because high affinity multivalent binding of C3b‐opsonized immune complexes to erythrocyte CR1 requires capture of substantial amounts of C3b by the immune complexes. 10 In the configuration proposed here, Aβ‐IgG immune complexes will be quantitatively bound to erythrocyte CR1 via the high affinity anti‐CR1 mAb which serves as a surrogate for multiple copies of C3b. The construct should also enhance binding of Aβ to the erythrocytes of AD patients that may have lower densities of CR1. 12
The unique properties of erythrocyte CR1 strongly suggest that the Aβ immune complex will be rapidly cleared to the liver and spleen and destroyed. This mechanism has been amply documented in the clearance of other CR1‐associated erythrocyte‐bound bispecific mAb/immune complexes. Notably, under these conditions, the erythrocytes will not be phagocytosed or lysed, although CR1 will be removed during the clearance reaction. 10 , 17 The paradigm we describe may not allow for removal and/or solubilization of Aβ plaques in the brain, but has the potential to prevent Aβ deposition in the brain by substantially reducing the steady‐state concentration of Aβ in the bloodstream and other compartments as well.
There are questions that will need to be addressed with respect to timing and dosing, including optimizing construction of the tetravalent bispecific mAb complexes. Based on pharmacokinetics studies reported by Siemers et al., 7 infusion of small amounts (0.5 to 1 mg/kg) of the anti‐Aβ IgG mAb should generate circulating mAb‐Aβ immune complexes that will increase and reach a concentration of about 12 to 15 nM after 10 days. Next, the bispecific reagent would be infused intravenously to approximately saturate circulating levels of erythrocyte CR1 (16 nM). The immune complexes would then be bound to erythrocytes and rapidly cleared from the bloodstream. We anticipate that during the clearance process >90% of erythrocyte‐bound Aβ will be transferred to resident macrophages, and that during this process CR1 will be stripped from the erythrocytes. Newly formed erythrocytes have the highest levels of CR1, and therefore periodic therapeutic infusions would need to be properly spaced so as to allow time for restoration of CR1 as mediated by entry of new erythrocytes into the bloodstream. 18 In principle the cycle could be repeated every 21 days and would have the net effect of continuing to promote movement of soluble Aβ (and its soluble oligomers) out of the brain and ultimately to the liver and spleen for destruction. Use of relatively low doses of the anti‐Aβ mAb may allow for more convenient subcutaneous infusion, and should also reduce the potential of adverse side effects such as amyloid‐related imaging abnormalities. 2
The feasibility of this approach can first be investigated based on in vitro experiments with human erythrocytes and acceptor macrophages. The protocols required to quantitate binding of Aβ to human erythrocytes, mediated by the bispecific reagents, can be developed based on previous publications. 10 , 11 , 16 Similarly, transfer of the erythrocyte‐bound Aβ‐IgG immune complexes to acceptor macrophages can also be investigated. If the results of the in vitro experiments outlined here align with our predictions, a next step would be to extend the work to murine (transgenic for human CR1) or non‐human primate models 1 , 3 , 10 , 17 , 19 for pre‐clinical testing. Multiple investigations and trials have demonstrated that, although several anti‐Aβ mAbs are safe, they lack efficacy even when used at rather high doses. 1 , 2 We suggest that redirecting these mAbs in the new paradigm provides a reasonable and testable path forward for the prevention and/or treatment of AD.
CONFLICTS OF INTEREST
Ronald P. Taylor and Margaret A. Lindorfer have developed anti‐CR1 mAbs that have been licensed through the UVA Licensing and Ventures Group.
Taylor RP, Lindorfer MA, Atkinson JP. Clearance of amyloid‐beta with bispecific antibody constructs bound to erythrocytes. Alzheimer's Dement. 2020;6:e12067 10.1002/trc2.12067
REFERENCES
- 1. Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid‐β‐targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73‐88. [DOI] [PubMed] [Google Scholar]
- 2. Van Dyck CH. Anti‐amyloid‐β monoclonal antibodies for Alzheimer's disease: pitfalls and promises. Biol Psychiatry. 2018;83:311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walsh DM, Selkoe DJ. Amyloid β‐protein and beyond: the path forward in Alzheimer's disease. Curr Op Neurobiol. 2020;61:116‐124. [DOI] [PubMed] [Google Scholar]
- 4. Tolar M, Abushakra S, Sabbagh M. The path forward in Alzheimer's disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 2019). 1–8. 10.1016/j.jalz.2019.09.075. [DOI] [PubMed] [Google Scholar]
- 5. Howard R, Liu KY. Questions EMERGE as biogen claims aducanumab turnaround. Nat Rev Neurol. 2020;16:63‐64. [DOI] [PubMed] [Google Scholar]
- 6. Levites Y, Smithson LA, Price RW, et al. Insights into the mechanisms of action of anti‐Aβ antibodies in Alzheimer's disease mouse models. FASEB J. 2006;20:E2002‐E2014. [DOI] [PubMed] [Google Scholar]
- 7. Siemers ER, Friedrich S, Dean RA, et al. Safety and changes in plasma and cerebrospinal fluid amyloid β after a single administration of an amyloid β monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharm. 2010;33:67‐73. [DOI] [PubMed] [Google Scholar]
- 8. Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to CNS amyloidosis. Alzheimers Dement. 2017;13:841‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid‐β peptide across the blood‐brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2009;8:16‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindorfer MA, Hahn CS, Foley PL, Taylor RP. Heteropolymer‐mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol Rev. 2001;183:10‐24. [DOI] [PubMed] [Google Scholar]
- 11. Krych‐Goldberg M, Atkinson JP. Structure‐function relationships of complement receptor type 1. Immunol Rev. 2001;180:112‐122. [DOI] [PubMed] [Google Scholar]
- 12. Tenner AJ. Complement‐mediated events in Alzheimer's disease: mechanisms and potential therapeutic targets. J Immunol. 2020;204:306‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nelson RA. The immune‐adherence phenomenon. A hypothetical role of erythrocytes in defense against bacteria and viruses. Proc Royal Soc Med. 1955;49:55‐58. [PMC free article] [PubMed] [Google Scholar]
- 14. Cornacoff JB, Hebert LA, Smead WL, VanAman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte‐immune complex‐clearing mechanism. J Clin Invest. 1983;71:236‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rogers J, Li R, Mastroeni D, et al. Peripheral clearance of amyloid β peptide by complement C3‐dependent adherence to erythrocytes. Neurobiol Aging. 2006;27:1733‐1739. [DOI] [PubMed] [Google Scholar]
- 16. Crane A, Brubaker WD, Johansson JU, et al. Peripheral complement interactions with amyloid β peptide in Alzheimer's disease: 2. Relationship to amyloid β immunotherapy. Alzheimers Dement. 2018;14:243‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor RP, Ferguson PJ, Martin EN, et al. Immune complexes bound to the primate erythrocyte complement receptor (CR1) via anti‐CR1 mAbs are cleared simultaneously with loss of CR1 in a concerted reaction in a rhesus monkey model. Clin Immunol Immunopathol. 1997;82:49‐59. [DOI] [PubMed] [Google Scholar]
- 18. Craig ML, Reinagel ML, Martin EN, Schlimgen R, Nardin A, Taylor RP. Infusion of bispecific monoclonal antibody complexes into monkeys provides immunologic protection against later challenge with a model pathogen. Clin Immunol. 1999;92:170‐180. [DOI] [PubMed] [Google Scholar]
- 19. de Oliveira RB, Wang JP, Ram S, Gazzinelli RT, Finberg RW, Golenbock DT. Increased survival in B‐cell‐deficient mice during experimental cerebral malaria suggests a role for circulating immune complexes. Mbio. 2014;5:00949‐00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts KF, Elbert DL, Kasten TP, et al. Amyloid‐β efflux from the CNS into plasma. Ann Neurol. 2014;76:837‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]


