Abstract
The field of digital health, and its meaning, has evolved rapidly over the last 20 years. For this article we followed the most recent definition provided by FDA in 2020. Emerging solutions offers tremendous potential to positively transform the healthcare sector. Despite the growing number of applications, however, the evolution of methodologies to perform timely, cost-effective and robust evaluations have not kept pace. It remains an industry-wide challenge to provide credible evidence, therefore, hindering wider adoption. Conventional methodologies, such as clinical trials, have seldom been applied and more pragmatic approaches are needed. In response, several academic centers such as researchers from the Institute of Global Health Innovation at Imperial College London have initiated a digital health clinical simulation test bed to explore new approaches for evidence gathering relevant to solution type and maturity. The aim of this article is to: (1) Review current research approaches and discuss their limitations; (2) Discuss challenges faced by different stakeholders in undertaking evaluations; and (3) Call for new approaches to facilitate the safe and responsible growth of the digital health sector.
Subject terms: Health policy, Translational research
Introduction
Digital health has evolved rapidly since the concept was first introduced in 2000 by Seth Frank1,2. The FDA considers digital health as a broad scope that includes categories such as mobile health, health information technology, wearable devices, telehealth and telemedicine, and personalized medicine3, a definition we follow in this article. Indeed, the numbers of digital health solutions are booming, for example, more than 300,000 health applications exist with more than 200 added daily4. Digital solutions can be grouped as follows, based on potential risk to patients5: (1) Solutions that improve system efficiency but with no measurable patient outcome benefit; (2) Mobile digital health, that inform or deliver basic monitoring, and encourage behavior change and self-management; (3) Clinical decision support (CDS), and prediction models, that guide treatment, deliver active monitoring, calculate and/or diagnose.
The evidence requirements of regulators are determined by a product’s intended use claims, as such, a large proportion of digital health solutions (e.g. administrative tools and wellness apps) fall outside of their jurisdiction. Therefore, a huge challenge for end users, such as patients and providers (e.g. healthcare professionals, hospital administrators), is how to determine a new solution’s credibility and compliance with standards. Furthermore, end users have different thresholds for acceptance of innovation and can be grouped into five archetypes: innovators, early adopters, early majority, late majority, and laggards6. In addition, aging adults, considered amongst the most digitally divided demographic group7, present unique challenges and dedicated efforts exist to develop strategies for implementation7–10. Conversely, challenges exist for healthcare innovators to best demonstrate solution impacts and to ensure compliance with standards, these include: unclear end-user expectations; uncertainty of evidence generation approaches; and, keeping up to date with the evolving compliance landscapes.
This article discusses the challenges for providing timely and robust evidence, to meet end-user expectations, in the context of digital health solutions. Specifically, we consider how the cadence of traditional research approaches are misaligned with the “fail fast, fail often” mantra espoused by technology start-ups. In addition, we introduce clinical simulation-based research as a potential opportunity to bridge the evidence gap.
A rapidly evolving guidance and regulatory landscape
Over the last 10 years a plethora of guidance has been developed for digital health innovators. In Table 1, we highlighted 10 of the key guidance (e.g., Continua Design Guidelines 2010, WHO monitoring and evaluating digital health solutions 2016, NICE evidence standards framework 2019; US FDA pre-certification program—a working model 2019, and FDA Proposed Regulatory Framework for modifications to Artificial intelligence/Machine learning-based Software as a Medical Device 2019). We ordered them by date first published and provided for each guidance a brief summary, applicable areas within digital health, releasing organization, and its main activities (Table 1). We observed that development of such documents follows a pattern: initial development by industry, optimization by non-government organizations, and finally refinement by government agencies. In addition, academic initiatives and institutions have produced critical thought leadership, often acting as counterbalance to industry proposals (Table 2; The digital health scorecard 2019). In Table 2, we highlighted five academic recommendations relevant to undertaking evidence generation studies for digital health solutions.
Table 1.
Selected guidance and discussion documents relevant to digital health solutions (not exhaustive).
| Document title | Document descriptions | Applicable areas within digital health | Date first published | Organization responsible | Main activities of releasing organization |
|---|---|---|---|---|---|
| Continua Design Guidelines (CDG)97 | Defining a framework of underlying standards and criteria that are required to ensure the interoperability of components used for applications monitoring personal health and wellness. | Connected devices and mobile applications (particular emphasis on interoperability and data standards) | 2010 | Personalized Connected Healthcare Alliance (PCHA) | PCHA is a membership-based Healthcare Information and Management Systems Society (HIMSS) Innovation Company. HIMSS is a global advisor and thought leader supporting the transformation of health through the application of information and technology. |
| FDA’s benefit-risk framework for medical devices11 | Providing a general framework to evaluate medical devices in both the benefits (7 dimensions, such as types, magnitude, likelihood of patients experiencing one or more benefits, etc.) and risks (7 dimensions, such as risk severity, likelihood of risk, false-positive or false-negative results, patient tolerance of risk, etc.) | All digital health solutions | 2016 | Food & Drug Administration (FDA) | The FDA is responsible for protecting and promoting public health throught the control and supervision, amongst other things, e.g., medical devices. |
| WHO monitoring and evaluating digital health interventions18 | Provides a general framework for the evaluation and validation of digital solutions along its product mature life-cycle | All digital health solutions | 2016 | World Health Organization (WHO) | International Public Health |
| Guidelines on the Qualification and Classification of Stand Alone Software Used in Healthcare within the Regulatory Framework of Medical Devices (MEDDEV 2.1/6)98 | Describing when software does and does not qualify as a medical or in vitro diagnostic device | Healthare software | 2016 | European Commission | Executive branch of the European Union |
| IMDRF SaMD Key Definitions (N10), IMDRF SaMD Risk Categorization Framework (N12), IMDRF SaMD Quality Management Systems (N23), and IMDRF SaMD Clinical Evaluation (N41)99 | Documents developed by the International Medical Device Regulators Forum (IMDRF) as the basis for SaMD regulatory efforts in various countries | Software as a Medical Device (SaMD) | 2017 | International Medical Device Regulators Forum (IMDRF) | The International Medical Device Regulators Forum (IMDRF) was conceived in February 2011 as a forum to discuss future directions in medical device regulatory harmonization. It is a voluntary group of medical device regulators from around the world who have come together to build on the strong foundational work of the Global Harmonization Task Force on Medical Devices (GHTF), and to accelerate international medical device regulatory harmonization and convergence. |
| US FDA mobile medical apps guidance12 | Risk-based approach (not all mobile apps are subject to FDA regulation). The agency oversees most mobile apps that are intended to treat, diagnose, cure, mitigate, or prevent disease or other conditions as medical devices under federal statute. | Mobile medical applications | 2018 | Food & Drug Administration (FDA) | The FDA is responsible for protecting and promoting public health throught the control and supervision, amongst other things, e.g., medical devices. |
| Code of conduct for data-driven health and care technology13 | Provides 10 principles for developing and evaluating digital health solutions | All digital health solutions | 2019 | UK Department of Health & Social Care | Responsible for policy on health and social care matters in England & Wales |
| NICE evidence standards framework5 | Providing guidance on evidence generation for effectiveness standards (section A) and economic standards (section B). Guiding the user through identifying the functional classification of their product (three-tier based on risk to users). For products with higher tier functions, the gold standard for evaluating their effectiveness is a high-quality intervention study or randomized controlled trial (RCT). | Digital health solutions (excl. products with adaptive artificial intelligence algorithms) | 2019 | National Institute for Health and Care Excellence (NICE) | Executive non-departmental public body of the Department of Health in the United Kingdom which publishes guidelines, amongst other things, the use of health technologies within the National Health Service (NHS)/ |
| US FDA pre-certification program (a working model)14 | Provides a voluntary pathway for efficient oversight of software-based medical devices from manufacturers who have demonstrated a robust culture of quality and organizational excellence (CQOE) and are committed to monitoring real-world performance | Low risk digital health solutions | 2019 | Food & Drug Administration (FDA) | The FDA is responsible for protecting and promoting public health throught the control and supervision, amongst other things, e.g., medical devices. |
| Proposed Regulatory Framework for modifications to Artificial intelligence/Machine learning-based Software as a Medical Device (SaMD)100 | Proposed framework for modifications to AI/ML- based SaMD that is based on the internationally harmonized International Medical Device Regulators Forum (IMDRF) risk categorization principles, FDA’s benefit-risk framework, risk management principles in the software modifications guidance, and the organization-based TPLC approach as envisioned in the Digital Health Software Precertification (Pre-Cert) Program. It also leverages practices from current premarket programs, including the 510(k), De Novo, and PMA pathways | AI and ML algorithms-based solutions | 2019 (draft document released for feedback) | Food & Drug Administration (FDA) | The FDA is responsible for protecting and promoting public health throught the control and supervision, amongst other things, e.g., medical devices. |
Table 2.
Selected academic recommendations relevant to undertaking evidence generation studies for digital health solutions (not exhaustive).
| Tool/framework | Document descriptions | Applicable areas within digital health | Date first published |
|---|---|---|---|
| Quality in prognosis studies (QUIPS)101 | 6 factors to consider when evaluating validity and bias in studies of prognostic factors: participation, attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting | Prognosis models (incl., individualized predictive model) | 2006 |
| The Cochrane risk-of-bias tool for randomized trials (RoB2)102 | Set of domains of bias to guide the evaluation about features of a trial that are relevant to risk of bias based on answers to the signaling questions | Randomized studies (suitable for individually randomized, parallel-group trials) | 2008 (updated in 2011) |
| The risk of bias in nonrandomized studies of interventions (ROBINS-I)103 | Tool to assess risk of bias in non-randomized studies over 7 domains (e.g., missing data, participant selection, etc.) | Non-randomized studies | 2016 |
| PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies104 | Tool to assess the risk of bias and applicability of prediction model studies (20 questions). Informed by a Delphi procedure involving 38 experts and refined through piloting. It is not suitable for comparative studies. | Predictive models (incl., CDS algorithms) | 2019 |
| The digital health scorecard2 | Academic developed framework that proposes validation should include three aspects: (1) technical validation (e.g., how accurately does the solution measure what it claims?), (2) clinical validation (e.g., does the solution have any support for improving condition-specific outcomes?), (3) system validation (e.g., does the solution integrate into patients’ lives, provider workflows, and healthcare systems). | All digital health solutions | 2019 |
Until recently regulators relied upon modifications to existing medical device (software) regulations and innovators were encouraged to conform to development standards, as shown in Table 3, where we highlighted eight regulations and standards relevant to digital health solutions (e.g., IEC Medical device software, ISO Health informatics—requirements for an electronic health record architecture). However, the speed of development, diversity of interventions, and potential risks has finally prompted policy-makers to produce more targeted guidance on solution classification and evidence requirements5,11–14 (Tables 1 and 3). For example, one initiative, the FDA Pre-certification Program14, seeks to streamline the approval of Software as a Medical Device (SAMD), and proposes to assess both development organization and product capabilities. Notwithstanding, current guidance does not go far enough to enable innovators and end-users to know what evidence generation approaches are appropriate, and practical, for all classes of digital health solutions throughout the product lifecycle.
Table 3.
Selected regulations and standards relevant to digital health solutions (not exhaustive).
| Document title | Document descriptions | Applicable areas within digital health | Date first published | Organization responsible | Main activities of releasing organization |
|---|---|---|---|---|---|
| IEC 62304: Medical device software– software life cycle processes105 | Providing life cycle requirements for the development of medical software and software within medical devices. It is harmonized by the European Union (EU) and the United States (US) | Medical device software | 2006 | International Standards Organization (ISO) | International Electrotechnical Commission |
|
ISO 18308:2011 Health informatics— Requirements for an electronic health record architecture106 |
Defines the set of requirements for the architecture of a system that processes, manages and communicates electronic health record (EHR) information: an EHR architecture | IT applications in healthcare technology | 2011 | International Standards Organization (ISO) | The International Organization for Standardization is an international standard-setting body composed of representatives from various national standards organizations. ISO (35.240.80) pertains to IT Applications in Health Care Technology |
|
ISO/TR 12300:2014 Health informatics— Principles of mapping between terminological systems107 |
Provides guidance for organizations charged with creating or applying maps to meet their business needs. | IT applications in healthcare technology | 2014 | International Standards Organization (ISO) | As above |
|
ISO/HL7 10781:2015 [HL7] Health Informatics— HL7 Electronic Health Records-System Functional Model, Release 2 (EHR FM)108 |
Provides a reference list of functions that may be present in an Electronic Health Record System (EHR-S) | IT applications in healthcare technology | 2015 | International Standards Organization (ISO) | As above |
| US 21st Century Cures Act109 | Providing broad scope related to health, with one part covering expedited product development programs, including the regenerative medicine advanced therapy, and the breakthrough devices program (designed to speed the review of certain innovative medical devices) | All digital health solutions | 2016 | Passed by Congress and signed into law by the President | NA |
| Medical Device Regulation (MDR)— Regulation (EU) 2017/745110 | The entirety of the Regulation is applicable for SaMD products, however, classification Rule 17 specifically applies to software products | Software as a Medical Device (SaMD) (Rule 17) | 2017 | European Commission | Executive branch of the European Union |
|
ISO 25237:2017 Health informatics— Pseudonymization111 |
Contains principles and requirements for privacy protection using pseudonymization services for the protection of personal health information. | IT applications in healthcare technology | 2017 | International Standards Organization (ISO) | As above |
|
ISO/IEC CD 23053 Framework for Artificial Intelligence (AI) Systems Using Machine Learning (ML)112 |
Under development | IT applications in healthcare technology | Upcoming | International Standards Organization (ISO) | As above |
Traditional approaches to evaluation of digital health solutions
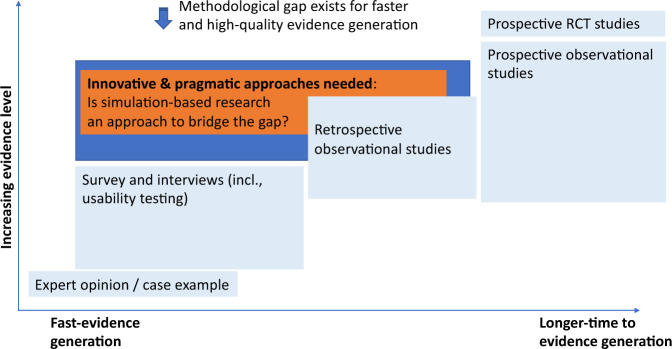
The most commonly recognized evidence for healthcare interventions is the randomized controlled clinical trial (RCT)15,16, yet, only a handful of products have been tested in this way as shown by recent systematic review17 and our searching results in Table 4, where we illustrated recent studies evaluating digital solutions and their methods (including study designs, study length, sample size, etc.). Indeed, a recent systematic review of publications between 1995 and 2016 identified just 24 RCTs for the high-risk CDS category17. In our opinion, this lack of studies indicates that these methods are no longer practicable, likely due to the speed of digital product development and iterative upgrading. In Fig. 1, we mapped existing approaches along two dimensions; strength of evidence and study duration, which demonstrated the current methodological gap to evidence needs and opportunity for more innovative and agile approaches. In this section we highlight a few of the more common methodologies, discuss strengths and limitations, and provide examples of their application (Table 4).
Table 4.
Recent studies utilizing various methodologies in evaluating digital health solutions (not exhaustive).
| Evaluation approach | Digital solution | Design/method details | Sample size | Number of sites involved during evaluation | Study length |
|---|---|---|---|---|---|
| Prospective: Randamized comparative design | Web-mediated follow-up algorithm113 | A web-mediated follow-up algorithm based on self-reported symptoms improved OS (18 vs. 12 months in the experimental and control arm, respectively) due to early relapse detection and better performance status at relapse | 120 patients | Single | 2 years (2014–2016) |
| Patient-reported outcomes collected via tablet36 | Patients randomly assigned to routine outpatient chemotherapy for advanced solid tumors with patient-reported outcomes vs. usual care with symptom monitoring at the discretion of clinicians | 766 patients, with 441 in the intervention cofthagenondition and 325 in the control condition (usual care) | Single | 4 years (2007–2011) | |
| Text messaging37 | Randomized trial of text messaging to reduce early discontinuation of aromatase inhibitor therapy in women with breast cancer | 338 patients on Text messaging (TM) condition, and 338 on non-TM | Multi | 3 years | |
| Digitally enabled care pathway for acute kidney injury management114 | Clinical outcome data were collected from adults with AKI on emergency admission before and after deployment at the intervention site and another not receiving the intervention (multi-site, pre- & post intervention design) | Implementation site: 767 patients in pre vs. 439 in post; Control site: 1016 in pre- and 422 in post | Multi | 2 years (2016–2017) | |
| WellDoc® mobile diabetes management tool38 | Patients with type 2 diabetes were recruited from three community physician practices and evenly randomized between intervention (cell phone-based software designed by endocrinologists, etc.) and control (One Touch Ultra™ BG meters) | 13 patients with type 2 diabetes in the intervention group vs. 13 in control | Multi | End-2-end study length not found in the paper | |
| Prospective: custer randomized design | Internet-delivered pain self-management program (WebMAP)115 | Protocol proposal for employing a stepped wedge design in which the WebMAP mobile intervention is sequentially implemented in 8 specialty pain clinics following a usual care period | 120 children to be recruited | Multi | NA |
| Telehealth programs41 | A Cluster-Randomized Program Evaluation in the Veterans Health Administration to evaluate the impact of availability of Telehealth Programs on Documented HIV Viral Suppression | Immediate telehealth availability (n = 925 patients in service areas of 13 primary care clinics offering telehealth) vs. availability 1 year later (n = 745 patients in 12 clinics) | Multi | 2015–2016 | |
| Digital medicine offering (DMO; medication taken with ingestible sensor) measuring medication ingestion adherence, physical activity, etc.116 | Participants with elevated systolic BP (SBP ≥ 140 mm Hg) and HbA1c (≥7%) failing antihypertensive (≥2 medications) and oral diabetes therapy were enrolled in this three-arm, 12-week, cluster-randomized study | 109 participants (12 sites) in total, within 80 participants (7 sites) in the DMO condition, and 29 participants (5 sites) in usual care | Multi | 12 weeks for the conduction stage; end-to-end study length not reported | |
| Prospective: Micro-randomization design | HeartSteps, an mHealth intervention that encourages regular walking via activity suggestions tailored to the individuals’ current context52 | A micro-randomized trial to evaluate the efficacy of HeartSteps’ activity suggestions to optimize the intervention; Contextually tailored suggestions could be delivered up to five times per day at user-selected times, for each participant on each day of the study, HeartSteps randomized whether to provide an activity suggestion, and, if so, whether to provide a walking or an antisedentary suggestion | 44 adults were recruited | Single | Recruitment took place from August 2015 to January 2016; study conduction took 6 weeks; in total it took 4 years from recruitmnt (2015) to publishment (2019) |
| Prospective: Pre–post intervention design | Clinical decision support system to aid computerized physician order entry of chemotherapy order (C-CO)117 | Computerized chemotherpay order (C-CO) versus paper based chemotherapy order (P-CO) in a 30-bed chemotherapy bay of a tertiary hospital. | 9279 chemotherapy orders from patients | Single | Not reported |
| Computerized provider order entry (CPOE)118 | CPOE system was implemented throughout the hospital, and impacts (e.g., active order number, medication-related patient safety events) were measured | 212 medication-related events | Single | Not reported | |
| Prospective: computational simulation | A deep-learning framework (Med3R) as clinical decision support119 | Evaluating a deep-learning framework (Med3R), which utilizes a human-like learning and reasoning process, its performance was measured against general human examinees and similar leading product (i.e., WatsonQA system) in mocked written test of National Medical Licensing Examination in China | Before officially taking National Medical Licence Examination for China (NMLEC) 7 practice tests were undertaken by Med3R to evaluate performance. In 2017 the Med3R system was officially entered as a “special examineee” and succesfully passed with a score of 78%. | Multi | Not reported |
| Direct order entry by physicians into computer-based medical information systems81 | A computer simulation model was developed to represent the process through which medical orders are entered into a digital hospital information system and estimate the impacts on process improvement, reduction of errors, and improved communication etc. | No participant was involved for real-time; instead, four weeks of patient data were extracted from the information system; 227 simulations of order entry were conducted | Single | Simulation itself took 16 h; end-to-end timeline not reported | |
| Prospective: Clinical simulation | A CDS tool that embedded clinical prediction rules into primary care workflow in EMR systems90 | Physicians interacting with video clips of standardized trained patient actors enacting the clinical scenarios (Pneumonia and Strep cases) | 8 (3 resident and 5 faculty providers) in the clinical simulation phase | Single | Not reported |
| Enhanced electronic health records system with features such as automatic sorting and decision support instructions91 | Physicians randomly assigned to baseline EHR or enhanced EHR; cognitive load for physicians and performMazurance were evaluated for each condition | 38 (20 in baseline, 18 in intervention) | Single | 9 months (2016 April–Dec) | |
| Medication management system known as “Patient Safety through Intelligent Procedure in Medication” (PSIP-DK)120 | Physicians randomly assigned to baseline (local standard mangement system) or the new system (PSIP-DK); participating doctors were asked to perform a ward round on the five patients; impact on medical safefy were evaluated based on semi-structural interviews | 15 (10 doctors and 5 patients) and 50 simulation runs—25 using PSIP-DK and 25 using standard system | Single | 6+ months | |
| CDSS with weight-loss prediction model121 | Physicians with varying experience levels were then recruited to evaluate 100 patients in an independent validation data set of head and neck cancer twice (i.e., a pre-post design) | 4 physicians evaluating 100 patient cases | Single | Not reported | |
| Voice assistants (recognition of commonly dispensed medications)122 | Voice recordings of 46 participants were played to voice assistants (e.g., Alexa, google assitant, siri) and compare the recognition accuracy rates | 46 participants (voice recorded as stimuli) | Single | 2+ months (All voice recordings were analyzed between mid-December 2018 and mid-January 2019—end-to-end study length not reported) | |
| Retrospective (incl. hybrid with prospective) | Watson for Oncology67 | Treatment recommendations made by WFO (638 breast cancers) and the tumor board were compared to evaluate the concordance in hospitals of India | 638 breast cancer patients | Not applicable/reported | 2 years (2016–2018) |
| Electronic symptom screening assessment scale (ESAS)123 | Retrospective chart reviews on cancer patient visits at a regional cancer centeremote and/or distributedr to examine whether patient visits with higher ESAS symptom scores are associated with higher rates of symptom documented in the chart and symptom-specific actions being taken | 912 visits were identified | Single | Study length not reported |
Fig. 1. Existing approaches for health digital solution evaluation, current methodological gap and emerging innovative pragmatic approaches to fill such gap.
Note, the position of each methodology is meant to be illustrative and reflecting general cases.
Surveys and interviews
In the early stages of development innovators seek to establish product usability, feasibility, and efficacy18. Surveys and/or interviews are often employed, which are low-cost, efficient, scalable tools to collect attitudes, user experience, and suitability insights. Commonly used methods include usability testing, user-center design, net promoter score survey (e.g. to rate likelihood to recommend a product), online surveys, and log-file data analyses (e.g. to evaluate how users interact with the digital solution)19. Such approaches have been used to explore user views on the usefulness of digital storytelling20, to assess a web-based network for MS patients21, and to collect attitudes towards digital treatment for depression22. Despite being common, few efforts are turned into peer-reviewed publications19, likely because the main purpose was to generate insights for internal use (e.g. product development) or external customer communication (e.g. case studies, presentations), and can be challenging to pass the peer-review for such work due to its relatively lower evidence strength19,23.
A key approach for digital solution development is usability testing which has been widely utilized to examine whether specified users can achieve intended use effectively and efficiently24–26. Typically, an intended user completes tasks and is observed for where they encounter problems. This can be exploratory, to identify new features or functionalities, or comparative testing A vs. B27,28. Studies are conducted by UX researchers, who synthesize results and translate to actions (e.g. product improvements). Data collected can be qualitative (e.g. observations of problems) and/or quantitative (e.g. task time, task success rates). Evidence strength depends upon study design, for example, task-based and controlled studies that collect quantitative data and can be replicated in other settings/sites, generate stronger evidence, whilst surveys and self-reported behaviors provide weaker evidence, as suggested by UX practitioners29. Controversy exists regarding the appropriate number of participants. Whilst there is no “single correct number”, for formative testing 5 participants is common (“the magic number 5”), compared with 20 participants for summative tests, which offer a tighter confidence interval30.
Prospective studies
Prospective RCTs are the most accepted method for evaluating healthcare interventions31. For end-users, not considered “early adopters”, such studies are critical to justify adoption decisions. The randomization unit can be individuals, groups (“clusters”), or even specific solution components32. Choice of the study designs heavily depends on the digital solution and objectives of the evaluation.
Individual-randomization trials (IRTs) are well-suited for digital solutions targeting an individual user, such as patient-level randomization (e.g. symptom self-monitoring33) or clinician-level randomization (e.g. digital pathology algorithms for pathologists34). This is traditionally the most commonly used experimental design in healthcare research (e.g., clinical trials for the development of drugs and diagnostic tests)35, however for digital health solutions, we found few studies employed strict individual randomized designs (Table 4; e.g., refs. 36–38). One reason is that individual randomization is not always possible or appropriate as in the examples provided below.
Cluster-randomization trials (CRTs), by contrast, are better suited for digital solutions supporting group efforts (e.g. solutions supporting tumor board meetings39), and this approach has been increasingly adopted by public health researchers40–42. CRTs are often used in situations when contamination may occur; for example, where individuals in the same cluster have been randomized to different intervention groups, or for logistic, feasibility or ethical reasons43. Attractive features include: increased administrative efficiency; decreased risk of experimental contamination (e.g. where control group individuals adopt the intervention)43; and, enhancement of subject compliance44. In addition, CRTs allow both direct and indirect effects of an intervention to be evaluated—a particular advantage when both effects are hypothesized to be important, e.g., in vaccine field trials45. Disadvantages include: reduced statistical efficiency relative to IRTs46; overmatching; and, subsampling bias47,48. Analysis commonly employs multi-level modeling49,50.
Micro-randomization trials (MRTs) are helpful when researchers want to determine empirically the efficacy of a specific component (e.g., which component of an intervention should be delivered, and whether it had the intended effect)32. MRT involves randomly assigning an intervention option at each time point that the component could be delivered (e.g., see examples in the ref. 51 on p. 5 and ref. 52)51,52, and can be particularly powerful in the early stages of product development51. MRTs generate longitudinal data with repeated measures of participants’ behaviors, context, and psychosocial factors, and can be analyzed by methods, such as multilevel models and generalized estimating equation51,53,54.
The most commonly used method for evaluating digital health solutions, however, is the pre–post design, as demonstrated by a previous systematic review17 and supported by our own searches (Table 4). A standard approach of pre–post design involves: pre-phase, which provides control data; “washout” period55 (i.e., with no interventions implemented with a time gap up to several months), to allow familiarization and to limit bias related to implementation39,56; post-phase to collect data on solution effectiveness. Existing studies are often undertaken at a single site (vs. multi-site), which is typically more practical and affordable. Typically, this design requires a longer duration, making it difficult to evaluate continuous solution upgrades (i.e. new features and/or bug fixes), which are often observed in digital health products. In addition, it is not optimal for testing medium-term or longer-term clinical outcomes, because it is difficult to determine independent effects when patients may appear in both pre-phase and post-phase. Data analysis generally employs methods, such as analysis of variance (ANOVA) and analysis of covariance (ANCOVA) or non-parametric tests (depending on the underlying distributions)57.
Relatively few multi-site studies have been conducted17 (we also listed some examples in Table 4), nevertheless, a variety of designs have been attempted in this context including: pre–post58, cross-sectional with non-equivalent control59, cross-sectional with internal control60, and randomized controlled trial61. For multi-site RCTs, some sites are assigned as controls and the rest as the experimental condition. For this approach, control and experimental sites should be matched along key characteristics (e.g., workflow, patient characteristics), which can be difficult to achieve. The main advantage is reduction in study duration. Disadvantages include: higher set-up efforts; increased cost; and, challenges to identify matched sites. Various tests are employed such as t-test, non-parametric tests, or other advanced techniques (depending on the underlying distributions)62.
Retrospective studies
Retrospective studies can be employed to analyze pre-existing data, such as patient charts or electronic medical records. Types of retrospective studies include case series, cohort, or case-control studies. They are typically quicker, cheaper, and easier63 than prospective studies because data are already collected, and are commonly used to generate hypotheses for further investigation by prospective studies. The disadvantages are, that they are subject to biases and confounding factors, such as patient information loss or distortion during data collection64, risk factors present but not captured, normal growth or maturation influence, attrition bias (e.g. patients with unfavorable outcome(s) less likely to attend follow-up)63,65, and selection bias due to non-random assignment of participants65,66. Such biases threaten internal validity, therefore, retrospective studies are considered (particularly by the academic groups) inferior as compared to RCTs63–66. It remains as an open question whether this is still the case for digital health solutions, particularly for the ones of lower-risk class.
To date, few publications have evaluated digital solutions with retrospective data, likely due to limited use of digital solutions in clinical practice, and challenges for data access (e.g. GDPR). Nevertheless, one such study from India investigated concordance between the treatment recommendations of an artificial intelligence (AI) algorithm compared with actual tumor board recommendations67 (Table 4). Strictly speaking this study was a hybrid of retrospective (treatment recommendations from Tumor Board 2014–2016) and prospective (treatment recommendations from AI algorithm in 2016). A key limitation of the study was that breast cancer treatment knowledge was not constant for the two conditions, because of the evolving clinical practice standards. Additional, prospective studies would be required to examine impacts on clinical outcomes, efficiency, and mental fatigue of clinicians.
Systematic reviews
Systematic reviews have a key role in evidence-based medicine and the development of clinical guidelines68–70. Reviews on a specific solution can provide stronger evidence for its impacts, but require a sufficient number of individual evaluation studies. A possible limitation for such work in digital health is that included studies would need to be matched to the same mechanism of intervention, disease area, and measurable outcome.
Systematic reviews of prediction models are a new and evolving area and are increasingly undertaken to systematically identify, appraise, and summarize evidence on the performance of prediction models71–73. Frameworks and tools exist to facilitate this including: prediction model risk of bias assessment tool (PROBAST), quality in prognosis studies (QUIPS), revised Cochrane randomized comparative design (ROB), risk of bias in nonrandomized studies of interventions (ROBINS-I). Details provided in Table 2.
Economic evaluation
Demonstration of positive economic benefits are critical for the majority of end-users to justify solution adoption. In addition, such data is important for other critical actors (e.g. Payers, Government agencies, Professional Societies) to endorse the need for change. The World Health Organization (WHO) guidelines provide a good overview of options for economic evaluation (Table 4.8 in WHO guideline18) including: cost-effectiveness analysis, cost–benefit analysis, cost-consequence analysis, cost-minimization analysis, etc. However, for all of the aforementioned methods, tracking usage and performance data of users compared to non-users, is required.
The critical evidence gaps for digital health solutions
In general, approaches for evidence generation at early stages of product development deliver weaker evidence. Although, such efforts may be enough to support internal needs, and can convince “early adopters”, they are insufficient to satisfy the “majority” of a solution’s potential beneficiaries. These groups require, and expect, more robust, traditional evidence approaches. Currently, and in our opinion, there is a gap between quick, lower-cost approaches applied at the early stages of product development and higher-cost approaches needed to convince the majority of stakeholders.
The challenge of the traditional approach for digital health innovators
It is our opinion that traditional methods to develop more robust evidence are incongruent with the agile approach taken in software development (e.g., mismatch between the length of RCTs and the typical development and update cycle of software). As such, traditional approaches present fundamental limitations for researchers to create evidence for digital health solutions. In fact, evaluation of digital health solutions has been identified as requiring improvement, and has been cited as a major obstacle for wider adoption74–76. The paradox at the heart of this problem is that, “without evidence healthcare providers would not adopt a solution; without solution adoption it is very difficult to generate evidence to convince healthcare providers”.
Digital solution evaluation requires collective efforts from multiple parties, such as health authorities, healthcare providers (incl., academic medical centers), and manufacturers such as small and medium-sized enterprises (SMEs), multinational corporation (MNCs). Whilst they face shared difficulties with the current approaches for evidence generation (e.g. significant time and cost), they also have circumstance-specific challenges.
SMEs—Limited resources to undertake clinical studies
SMEs typically prioritize and allocate their research and development budget to product development. Anecdotal evidence suggests that close relationships between innovator and adopter are a critical driver of initial adoption decisions. Wider implementation requires robust evidence of benefit, yet this is difficult to prioritize given the many challenges for establishing new ventures. In addition, well designed and executed studies require skilled researchers, often via collaboration with academia, adding further complexity. Moreover, it has been estimated that the timescale for submitting a research proposal and receiving ethical approval for a pilot or trial study can take as long as 3 years19. As demonstrated in a recent report19, the biggest obstacle for providing evidence of effectiveness reported by companies, is the cost and timeframe for evaluation.
MNCs—Out of date evidence not an investment priority
Larger corporations have more resources to develop evidence but are equally limited by time. For internal budget allocation, it can be difficult to provide rationale for investments into expensive and time-consuming clinical studies for early-stage solutions when such products are constantly evolving. Given it typically takes 2–3 years to conduct a study, evidence published today may reflect a product that has been updated and refined multiple times. Furthermore, for many companies’ investments in sales and manufacturing, for example, are more tangible with more predictable return on investment than those in clinical studies.
The same challenges (as SMEs) exist around navigating the complex infrastructure of the healthcare system, dealing with the cultural resistance to digital solutions, and identifying appropriate principle investigators for the evaluation studies. Despite the long-existing collaborations between large health abd life science companies and principal investigators in, for example clinical trials for drug development, this group of researchers may not necessarily be willing to conduct studies to evaluate digital solutions, as they require different settings, capabilities and also deliver different scientific output—benefits on the operational level impacting cost and indirectly patient outcome versus a drug that can improve patient outcome directly.
Academic institutions—focus on research output not widespread adoption
A growing number of academic centers have created digital health research programs to develop and evaluate digital health solutions. However, such research units generally favor traditional research methodologies because of the increased likelihood of high-impact publication. As such, the timeliness of studies is largely immaterial, therefore, potentially valuable solutions may be delayed and/or are never implemented at scale. Obtaining sufficient research funding can also be a challenge.
Evolving pragmatic approaches for evidence generation
In our opinion, large differences exist between the evidence required for initial adopters (e.g., surveys and interviews, case studies), and that required for the majority (prospective RCT studies). Other research areas, such as drug development, have demonstrated that pragmatic approaches can be adopted to control cost at early stages (pragmatic clinical trials, basket of baskets, umbrella trials, etc.77–79). The “gold standard” RCT remains but for later-stage final assessment.
The concept of “simulation” is not new and is the methodological foundation for human behavior experimental research (e.g. neuroscience and experimental psychology). The assumption is that people behave similar to real-life if key components of the scenarios are extracted and fidelity maintained. Various approaches for simulation could be applied to evaluate digital solutions, such as, computational, system, and clinical simulation.
Computational simulation for software evaluation involves two steps: verification and validation80. Verification checks if a system was built according to specification, and validation checks that a system meets user expectations. The most common application of computational has been for verification. Typically, this involves simulated outcomes based on synthesized or real cases, before involving users/clinicians. Recent efforts have extended its use to non-regulated and on-market products (e.g., Google Alexa; Table 4). This approach is more applicable for products where the outputs can be evaluated for individual users, and not for clinical management tools where a group of users are targeted (e.g. multidisciplinary tumor boards).
System simulation adopts a system engineering view and methodology to model the effect of an intervention on a healthcare system (e.g. multi-site hospital network) without disrupting the real health care setting81. It has gained some traction (ASCO QCS Keynote topic by Joe Simone, literatures82,83), however, to date we are not aware of the use of system simulation to evaluate a digital health solution, perhaps because of the significant complexity to establish models that represent a healthcare system.
Clinical simulation was traditionally developed and used in training medical residents, and it was further developed as an approach to test systems and digital solutions with representative users doing representative tasks, in representative settings/environments84. In our opinion, can be complementary to many of the traditional approaches reviewed above that require the use of a digital solution in real clinical practice, and could bridge the evidence needs between those of “early adopters” and the “majority”. Clinical simulation provides a good balance between the strength of evidence (e.g., “near-live” clinical scenarios), whilst remaining cost-effective and timely for fast version updates (Fig. 1). Previous work demonstrated, the total cost for such a simulation was as little as 2750 USD, including set-up, subject and personnel cost85. A recent cost-effective analysis suggested that introducing simulation into a product development lifecycle could lead to cost savings of 37–79%86. Other advantages include: scalability19, flexibility in design of studies (e.g. different scenarios, various types of participants), feasibility in being implemented as remote and/or distributed87, and ability to collect behavioral and/or cognitive metrics. Sophisticated approaches and equipment can be employed, such as eye-tracker analysis or measurement of EEG, which would not be possible in real clinical practice. Furthermore, clinical simulation may also be helpful in facilitating patient engagement and/or Patient and Public Involvement and Engagement (PPIE), an initiative aiming to involving patients and/or representatives from relevant public bodies in the research88.
Clinical simulation has been increasingly used in evaluating digital health solutions, including five studies in Table 4, and a further twenty studies from ITX-lab evaluating clinical information systems89. For example, in one study90 primary care physicians interacted with videoclips of professional patient actors providing standardized responses to clinical scenarios and utilized a CDS tool of clinical prediction rules via an EMR system. In another recently published study91, cognitive load and performance of physicians was evaluated for different conditions by randomly assigning participants to baseline EHR (control) or enhanced EHR (simulated environment with features such as automatic sorting and decision support instructions). Moreover, a recent interview study of 10+ companies reported that they found this approach feasible for evidence generation for their own digital solution19.
Several academic centers have established clinical simulation test environments, including: The School of Health Information Science (University of Victoria); The Department of Development and Planning (Aalborg University); The IT Experimentarium (ITX) lab (Danish Institute for Medical Simulation)84; and, The Institute of Global Health Innovation (IGHI) (Imperial Colleague London)92. Indeed, researchers from IGHI have established a simulation test bed specifically to explore application to test digital health solutions. Initial work evaluated the impact of a digital solution on the conduction of cancer multidisciplinary team (MDT) meetings. 56 healthcare professionals (e.g. pulmonologist, oncologists, radiologists, clinical nurse specialists, and thoracic surgeons), who were regular participants at lung cancer tumor boards, were recruited to take 10 simulated MDT sessions. High-fidelity mock patient cases were developed by the study team and clinical experts93. Participants discussed up to 10 patient cases, using a standard UK approach to conduct MDTs (paper handout and PACS system) in the control condition, compared with the NAVIFY Tumor Board solution. A manuscript detailing the learnings and results from this pioneer work is under development.
Whilst clinical simulation offers opportunities to prospectively test a digital solution quickly, safely and cost-effectively prior to implementation, there are a few limitations in its use. First, high-fidelity is a prerequisite for generating valid and effective evidence. Therefore, researchers should take efforts to create scenarios representing real clinical practice, recruit the most representative end-users as participants, and provide comprehensive trainings of the digital solutions to the participants before their simulation sessions. Second, while the regulatory space evolves fast, we think clinical simulation results itself alone probably are not adequate for approval application from Health authorities, particularly for higher-risk group of digital solutions that would need to be approved as SaMD. Nevertheless, in these cases, clinical simulations can help to provide initial insights for product development, reduce safety risk for patients, and guide the design of large-scale real clinical studies. Third, for digital solutions that are already adopted in clinical practice, leveraging real-word data (RWD) is probably more suitable. RWD studies could be systematically employed to undertake near real-time evaluation during pilot implementation and post-market monitoring. Indeed, studies utilizing real-world data (RWD) have been encouraged to support regulatory decision making (e.g. The 21st Century Cures Act; Table 3); have been used for clinical evidence generation (e.g. diagnostic and treatment patterns)94–96; and can demonstrate solution utility (e.g. meta-data associated with solution features and functionalities).
Finally, we believe clinical simulation can be employed in combination with traditional study designs, e.g., individual-randomization, cluster-level randomization, and micro-randomization to examine different types of digital solutions. For example, clinical simulation-based study with micro-randomization design can be a powerful and pragmatic approach to evaluate the digital solutions with multiple components at early stage of the product development.
Conclusion
Innovators face significant challenges to overcome the “no evidence, no implementation—no implementation, no evidence” paradox in digital health. We believe that innovative approaches, such as simulation-based research, can enable the generation of higher-quality, lower-cost, and more timely evidence. By considering such methods, end-users will encourage developers to undertake research activities, rather than be intimidated by the complexity, cost, and duration of traditional approaches.
Acknowledgements
We acknowledge Nate Carrington from Roche for his valuable input on the regulations related to digital health solutions in the US and European markets.
Author contributions
All authors (C.H.G., H.A., S.G., G.F., C.G., M.P.) contributed to the conception of the work, analysis and interpretation of the data, drafting the work and revising critically, final approval of the completed version, and accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
All data supporting the findings of this study are available within the paper.
Competing interests
C.H.G. and M.P. are currently employees of Roche Diagnostics; H.A., S.G., G.F. and C.G. are employees of Imperial College London. H.A., S.G., G.F., C.G. receive infrastructure support provided by the NIHR Imperial Biomedical Research Center (BRC) at Imperial College London; S.G. is a co-founder of Psyma Ltd, a mobile app that allows clients to access psychological therapy through a live video call; M.P. is a co-founder of Open Medical Ltd, a cloud-based patient management platform.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frank SR. Digital health care—the convergence of health care and the Internet. J. Ambul. Care Manag. 2000;23:8–17. doi: 10.1097/00004479-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mathews SC, et al. Digital health: a path to validation. NPJ digital medicine. 2019;2:1–9. doi: 10.1038/s41746-019-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA. https://www.fda.gov/medical-devices/digital-health (2020).
- 4.IQVIA. IQVIA Institute for Human Data Science Study: Impact of Digital Health Grows as Innovation, Evidence and Adoption of Mobile Health Apps Accelerate. https://www.iqvia.com/newsroom/2017/11/impact-of-digital-health-grows-as-innovation-evidence-and-adoption-of-mobile-health-apps-accelerate/ (2017).
- 5.NICE. https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies (2019).
- 6.Rogers, E. M. Diffusion of innovations. Simon and Schuster (2010).
- 7.Ball C, et al. The physical–digital divide: exploring the social gap between digital natives and physical natives. J. Appl. Gerontol. 2019;38:1167–1184. doi: 10.1177/0733464817732518. [DOI] [PubMed] [Google Scholar]
- 8.Francis, J. et al. Aging in the digital age: conceptualizing technology adoption and digital inequalities. In Ageing and digital technology, 35–49 (Springer, Singapore, 2019).
- 9.Peek S, et al. What it takes to successfully implement technology for aging in place: focus groups with stakeholders. J. Med. Internet Res. 2016;18:e98. doi: 10.2196/jmir.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y-H, et al. Bridging the digital divide in older adults: a study from an initiative to inform older adults about new technologies. Clin. Interv. Aging. 2015;10:193–201. doi: 10.2147/CIA.S72399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. https://www.fda.gov/media/98657/download.
- 12.Shuren et al. FDA regulation of mobile medical apps. JAMA, 320, 337–338 (2018). [DOI] [PubMed]
- 13.https://www.gov.uk/government/publications/code-of-conduct-for-data-driven-health-and-care-technology/initial-code-of-conduct-for-data-driven-health-and-care-technology.
- 14.FDA. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program.
- 15.Chung KC, et al. Introducing evidence-based medicine to plastic and reconstructive surgery. Plast. Reconstr. Surg. 2009;123:1385. doi: 10.1097/PRS.0b013e31819f25ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song JW, et al. Observational studies: cohort and case-control studies. Plast. Reconstr. Surg. 2010;126:2234. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawloski PA, et al. A systematic review of clinical decision support systems for clinical oncology practice. J. Natl. Compr. Canc. Netw. 2019;17:331–338. doi: 10.6004/jnccn.2018.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://www.who.int/reproductivehealth/publications/mhealth/digital-health-interventions/en/. WHO (2016).
- 19.Ghafur, S. et al. A simulation test bed: the solution to the obstacles of evaluating the effectiveness of digital health interventions (in preparation).
- 20.Cumming GP, et al. Web-based survey on the effect of digital storytelling on empowering women to seek help for urogenital atrophy. Menopause Int. 2010;16:51–55. doi: 10.1258/mi.2010.010004. [DOI] [PubMed] [Google Scholar]
- 21.Lavorgna L, et al. Health-care disparities stemming from sexual orientation of Italian patients with Multiple Sclerosis: a cross-sectional web-based study. Mult. Scler. Relat. Disord. 2017;13:28–32. doi: 10.1016/j.msard.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Topooco N, et al. Attitudes towards digital treatment for depression: a European stakeholder survey. Internet Interv. 2017;8:1–9. doi: 10.1016/j.invent.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J. Clin. Nurs. 2003;12:77–84. doi: 10.1046/j.1365-2702.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 24.Schneiderheinze, H. et al. Development and usability analysis of a multimedia eConsent solution. Stud. Health Technol. Inform. In GMDS, 297–303 (2019). [DOI] [PubMed]
- 25.Hardy A, et al. How inclusive, user-centered design research can improve psychological therapies for psychosis: development of SlowMo. JMIR Ment. Health. 2018;5:e11222. doi: 10.2196/11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maramba I, et al. Methods of usability testing in the development of eHealth applications: a scoping review. Int. J. Med. Inform. 2019;126:95–104. doi: 10.1016/j.ijmedinf.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Molich, et al. Comparative usability evaluation. Behav. Inf. Technol. 23, 65–74 (2004).
- 28.Zimmerman & Paschal. An exploratory usability evaluation of Colorado State University Libraries’ digital collections and the Western Waters Digital Library Web sites. J. Acad. Librarianship. 35, 227–240 (2009).
- 29.https://userfocus.co.uk/articles/strength-of-evidence.html.
- 30.Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav. Res. Methods. 2003;35:379–383. doi: 10.3758/bf03195514. [DOI] [PubMed] [Google Scholar]
- 31.Jüni P, et al. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, et al. Mobile health technology evaluation: the mHealth evidence workshop. Am. J. Prev. Med. 2013;45:228–236. doi: 10.1016/j.amepre.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker, et al. Digital health: Smartphone-based monitoring of multiple sclerosis using Floodlight. Nature (2019).
- 34.Kohlberger, T. et al. Whole-slide image focus quality: automatic assessment and impact on AI cancer detection. J. Pathol. Inform.10 (2019). [DOI] [PMC free article] [PubMed]
- 35.Chan A-W, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet. 2005;365:1159–1162. doi: 10.1016/S0140-6736(05)71879-1. [DOI] [PubMed] [Google Scholar]
- 36.Basch, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J. Clin. Oncol. 34, 557 (2016). [DOI] [PMC free article] [PubMed]
- 37.Hershman, D. L. et al. Randomized trial of text messaging (TM) to reduce early discontinuation of aromatase inhibitor (AI) therapy in women with breast cancer: SWOG S1105. Oral presentation at: 2019 ASCO Annual Meeting (2019). [DOI] [PMC free article] [PubMed]
- 38.Quinn, et al. WellDoc™ Mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diab. Tech. Therap. 10, 160–168 (2008). [DOI] [PubMed]
- 39.Hammer, R. D. et al. Digital Tumor Board Solutions have significant impact on case preparation. JCO Clinical Cancer Informatics (forthcoming). [DOI] [PMC free article] [PubMed]
- 40.Greaney, et al. Study protocol for Young & Strong: a cluster randomized design to increase attention to unique issues faced by young women with newly diagnosed breast cancer. BMC Public Health. 15, 1–11 (2015). [DOI] [PMC free article] [PubMed]
- 41.Ohl, et al. Impact of availability of telehealth programs on documented HIV viral suppression: A Cluster-Randomized Program Evaluation in the Veterans Health Administration. Open Forum Infect Dis. 6, ofz206 (2019). [DOI] [PMC free article] [PubMed]
- 42.Arnup, et al. The use of the cluster randomized crossover design in clinical trials: protocol for a systematic review. Syst. Rev. 3, 1–6 (2014). [DOI] [PMC free article] [PubMed]
- 43.Eldridge, S. & Kerry, S. APractical Guide to Cluster Randomised Trials in Health Services Research. Vol. 120 (John Wiley & Sons, 2012).
- 44.Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am. J. Public Health. 2004;94:416–422. doi: 10.2105/ajph.94.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halloran ME, et al. Design and interpretation of vaccine field studies. Epidemiol. Rev. 1999;21:73–88. doi: 10.1093/oxfordjournals.epirev.a017990. [DOI] [PubMed] [Google Scholar]
- 46.Cornfield J. Randomization by group: a formal analysis. Am. J. Epidemiol. 1978;108:100–102. doi: 10.1093/oxfordjournals.aje.a112592. [DOI] [PubMed] [Google Scholar]
- 47.Torgerson DJ. Contamination in trials: is cluster randomisation the answer? BMJ. 2001;322:355–357. doi: 10.1136/bmj.322.7282.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazor, K. et al. Cluster Randomized Trials: opportunities and Barriers Identified by Leaders of Eight Health Plans. Med. Care. S29–S37 (2007). [DOI] [PubMed]
- 49.Raudenbush SW. Statistical analysis and optimal design for cluster randomized trials. Psychol. methods. 1997;2:173. doi: 10.1037/1082-989x.5.2.199. [DOI] [PubMed] [Google Scholar]
- 50.Campbell MK, et al. Analysis of cluster randomized trials in primary care: a practical approach. Family Pract. 2000;17:192–196. doi: 10.1093/fampra/17.2.192. [DOI] [PubMed] [Google Scholar]
- 51.Klasnja P, et al. Micro-Randomized Trials: an experimental design for developing just-in-time adaptive interventions. Health Psychol. 2015;34:1220–1228. doi: 10.1037/hea0000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klasnja P, et al. Efficacy of contextually tailored suggestions for physical activity: a Micro-randomized Optimization Trial of HeartSteps. Ann. Behav. Med. 2018;53:573–582. doi: 10.1093/abm/kay067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bolger & Laurenceau. Intensive Longitudinal Methods: An Introduction to Diary and Experience Sampling Research (Guilford Press, 2013).
- 54.Walls & Schafer. Models for intensive longitudinal data (Oxford University Press, 2006).
- 55.Evans SR. Clinical trial structures. J. Exp. Stroke Transl. Med. 2010;3:8–18. doi: 10.6030/1939-067x-3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowen DJ, et al. How we design feasibility studies. Am. J. Prev. Med. 2009;36:452–457. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimitrov, D. M. & Rumrill, P. D. Pretest–posttest Designs and Measurement of Change. (IOS Press, 2003). [PubMed]
- 58.Beriwal S, et al. How effective are clinical pathways with and without online peer-review? An analysis of bone metastases pathway in a large, integrated National Cancer Institute-Designated Comprehensive Cancer Center Network. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:1246–1251. doi: 10.1016/j.ijrobp.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 59.Bouaud, J. et al. Physicians’ attitudes towards the advice of a guideline-based decision support system: a case study with OncoDoc2 in the Management of Breast Cancer Patients. Stud. Health Technol. Inform. 264–269 (2015). [PubMed]
- 60.Mattsson TO, et al. Non-intercepted dose errors in prescribing anti-neoplastic treatment: a prospective, comparative cohort study. Ann. Oncol. 2015;26:981–986. doi: 10.1093/annonc/mdv032. [DOI] [PubMed] [Google Scholar]
- 61.Berry DL, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J. Clin. Oncol. 2011;29:1029–1035. doi: 10.1200/JCO.2010.30.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caselli E, et al. Influence of sanitizing methods on healthcare-associated infections onset: a multicentre, randomized, controlled pre-post interventional study. J. Clin. Trials. 2016;6:1–6. [Google Scholar]
- 63.Sauerland S, et al. Retrospective clinical studies in surgery: potentials and pitfalls. J. Hand Surg. Br. 2002;27:117–121. doi: 10.1054/jhsb.2001.0703. [DOI] [PubMed] [Google Scholar]
- 64.Kaji AH, et al. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann. Emerg. Med. 2014;64:292–298. doi: 10.1016/j.annemergmed.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 65.Tofthagen C. Threats to validity in retrospective studies. J. Adv. Pract. Oncol. 2012;3:181. [PMC free article] [PubMed] [Google Scholar]
- 66.Geneletti S, et al. Adjusting for selection bias in retrospective, case-control studies. Biostatistics. 2009;10:17–31. doi: 10.1093/biostatistics/kxn010. [DOI] [PubMed] [Google Scholar]
- 67.Somashekhar SP, et al. Watson for Oncology and breast cancer treatment recommendations: agreement with an expert multidisciplinary tumor board. Ann. Oncol. 2018;29:418–423. doi: 10.1093/annonc/mdx781. [DOI] [PubMed] [Google Scholar]
- 68.Graham, R. et al. Clinical Practice Guidelines We Can Trust: Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. (National Academies Press, 2011). [PubMed]
- 69.Goff, et al. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology. American Heart Association Task Force on Practice Guidelines. Circulation63, 2935–2959 (2014). [DOI] [PMC free article] [PubMed]
- 70.Rabar, S. et al. Guideline Development Group Risk assessment of fragility fractures: summary of NICE guidance. BMJ345, p.e3698 (2012). [DOI] [PubMed]
- 71.Bouwmeester, W. et al. Reporting and methods in clinical prediction research: a systematic review. PLoS Med. 9, e1001221 (2012). [DOI] [PMC free article] [PubMed]
- 72.Collins GS, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann. Intern. Med. 2015;131:211–219. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 73.Debray TP, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ. 2017;356:i6460. doi: 10.1136/bmj.i6460. [DOI] [PubMed] [Google Scholar]
- 74.Shaw J, et al. Beyond “implementation”: digital health innovation and service design. NPJ Digit. Med. 2018;1:1–5. doi: 10.1038/s41746-018-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moxey, et al. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J. Am. Med. Inform. Associat. 17, 25–33 (2010). [DOI] [PMC free article] [PubMed]
- 76.O’Sullivan, et al. Decision time for clinical decision support systems. Clin. Med. 14, 338 (2014). [DOI] [PMC free article] [PubMed]
- 77.Mentz RJ, et al. Good Clinical Practice Guidance and Pragmatic Clinical Trials: balancing the best of both worlds. Circulation. 2016;133:872–880. doi: 10.1161/CIRCULATIONAHA.115.019902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ford I, et al. Pragmatic trials. N. Engl. J. Med. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 79.Cunanan KM, et al. An efficient basket trial design. Stat. Med. 2017;36:1568–1579. doi: 10.1002/sim.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dahabreh, I. J. et al. Modeling and Simulation in the Context of Health Technology Assessment: Review of Existing Guidance, Future Research Needs, and Validity Assessment (2017). [PubMed]
- 81.Anderson JG, et al. Evaluation in health informatics: computer simulation. Comput. Biol. Med. 2002;32:151–164. doi: 10.1016/s0010-4825(02)00012-4. [DOI] [PubMed] [Google Scholar]
- 82.Dong Y, et al. Systems modeling and simulation applications for critical care medicine. Ann. Intensive Care. 2012;2:1–10. doi: 10.1186/2110-5820-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts, S. D. Tutorial on the simulation of healthcare systems. Proceedings of the 2011 winter simulation conference (wsc), 1403–1414 (2011).
- 84.Kushniruk A. et. al. From usability testing to clinical simulations: bringing context into the design and evaluation of usable and safe health information technologies. Contribution of the IMIA human factors engineering for healthcare informatics working group. Yearb. Med. Inform. 22, 78–85 (2013). [PubMed]
- 85.Kushniruk, A. W. et al. Low-cost rapid usability engineering: designing and customizing usable healthcare information systems. Healthc, Q. (2006). [PubMed]
- 86.Baylis, T. B. et al. Low-Cost Rapid Usability Testing for health information systems: is it worth the effort? Stud. Health Technol. Inform. (2012). [PubMed]
- 87.Yao, H. et al. Research and design on distributed remote simulation based on Web. In IEEE International Conference on Information Management and Engineering. pp. 522–525 (2010).
- 88.https://imperialbrc.nihr.ac.uk/patients-public/ppi-e-strategy/.
- 89.Jensen S, et al. Clinical simulation: a method for development and evaluation of clinical information systems. J. Biomed. Inform. 2015;54:65–76. doi: 10.1016/j.jbi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 90.Li, et al. Integrating usability testing and think-aloud protocol analysis with “near-live” clinical simulations in evaluating clinical decision support. Int. J. Med. Inform. 81, 761–772 (2012). [DOI] [PubMed]
- 91.Mazur, et al. Association of the usability of electronic health records with cognitive workload and performance levels among physicians. JAMA Netw Open. 2, e191709–e191709 (2019). [DOI] [PMC free article] [PubMed]
- 92.https://www.imperial.ac.uk/global-health-innovation/.
- 93.Gardner, et al. A mixed methods study for the evaluation of a digital health solution for cancer multidisciplinary team meetings using simulation-based research methods. ASCO 2020 Annual Conference. American Society of Clinical Oncology. pp. e14063 (2020)
- 94.Khozin, et al. Real-world data for clinical evidence generation in oncology. J. Natl. Cancer Inst.109, djx187 (2017). [DOI] [PubMed]
- 95.Calabria, et al. Open triple therapy for chronic obstructive pulmonary disease: Patterns of prescription, exacerbations and healthcare costs from a large Italian claims database. Pulmon. Pharmacol. Therap. 61, 101904 (2020). [DOI] [PubMed]
- 96.Pal, et al. Real-world treatment patterns and adverse events in metastatic renal cell carcinoma from a large US claims database. BMC Cancer19, 548 (2019). [DOI] [PMC free article] [PubMed]
- 97.http://www.pchalliance.org/resources.
- 98.https://ec.europa.eu/docsroom/documents/17921/attachments/1/translations.
- 99.http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-170921-samd-n41-clinical-evaluation_1.pdf.
- 100.https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device.
- 101.Hayden, et al. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 144, 427–437 (2006). [DOI] [PubMed]
- 102.Higgins, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ343, d5928 (2011). [DOI] [PMC free article] [PubMed]
- 103.Sterne, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355 (2016). [DOI] [PMC free article] [PubMed]
- 104.Wolff, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann. Intern. Med. 170, 51–58 (2019). [DOI] [PubMed]
- 105.https://webstore.iec.ch/preview/info_iec62304%7bed1.0%7den_d.pdf. (2006).
- 106.ISO. https://www.iso.org/standard/52823.html.
- 107.ISO. https://www.iso.org/standard/51344.html.
- 108.ISO. https://www.iso.org/standard/57757.html.
- 109.FDA. https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act.
- 110.https://www.eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0746&from=DE.
- 111.ISO. https://www.iso.org/standard/63553.html.
- 112.ISO. https://www.iso.org/standard/74438.html.
- 113.Denis, et al. Randomized Trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. J. Natl. Cancer Inst. 109 (2017). [DOI] [PubMed]
- 114.Connell, et al. Evaluation of a digitally-enabled care pathway for acute kidney injury management in hospital emergency admissions. Nature Digit. Med. 2, 1–9 (2019). [DOI] [PMC free article] [PubMed]
- 115.Palermo, et al. Mobile health intervention for self-management of adolescent chronic pain (WebMAP mobile): Protocol for a hybrid effectiveness-implementation cluster randomized controlled trial. Contemp. Clin. Trials. 74, 55–60 (2018). [DOI] [PMC free article] [PubMed]
- 116.Frias, et al. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial. J. Med. Internet Res. 19, e246 (2017). [DOI] [PMC free article] [PubMed]
- 117.Aziz MT, et al. Reduction in chemotherapy order errors with computerised physician order entry and clinical decision support systems. Health Inf. Manag. 2015;44:13–22. doi: 10.1177/183335831504400303. [DOI] [PubMed] [Google Scholar]
- 118.Chen AR, et al. Computerized provider order entry in pediatric oncology: design, implementation, and outcomes. J Oncol Pract. 2011;7:218–222. doi: 10.1200/JOP.2011.000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu J, et al. Master clinical medical knowledge at certificated doctor-level with deep learning model. Nat. Commun. 2018;9:4352. doi: 10.1038/s41467-018-06799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ammenwerth, et al. Simulation studies for the evaluation of health information technologies: experiences and results. Health Inf. Manag. J. 41, 14–21 (2012). [DOI] [PubMed]
- 121.Cheng, et al. Utility of a clinical decision support system in weight loss prediction after head and neck cancer radiotherapy. JCO Clin. Cancer Inform. 3, 1–11 (2018). [DOI] [PubMed]
- 122.Palanica, et al. Do you understand the words that are comin outta my mouth? Voice assistant comprehension of medication names. Nat. Digit. Med. 2, 1–6 (2019). [DOI] [PMC free article] [PubMed]
- 123.Seow H, et al. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. J. Oncol. Pract. 2012;8:e142–e148. doi: 10.1200/JOP.2011.000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the paper.