Abstract
Background
Biologic disease modifying agents (bDMARDs) are an integral part of rheumatoid arthritis treatment guidelines but are associated with significant cost in the US. We present the trends in total spending and unit cost of conventional DMARDs (cDMARDs) as compared to bDMARDs in Medicare program.
Methods
We used the Medicare drug spending data for the year 2012–2017 covering all part B (fee-for-service) and part D drugs. Total spending was calculated by summing spending across various drug formulations and unit drug cost by dividing total spending by number of doses dispensed. We present the 6-year trends in total spending, total beneficiary count and unit costs of each of the commonly used cDMARDs and bDMARDs.
Results
Between 2012 and 2017, the total spending on the cDMARDs increased 5-folds from $98 million to $579 million; this was fraction of total spending on bDMARDs which increased from $4.3 to $10.0 billion. This increase was driven largely by unit costs of drug rather than number of beneficiaries. There was a 6-fold increase in the unit cost of generic hydroxychloroquine followed by methotrexate and leflunomide. Amongst bDMARDs, adalimumab and etanercept unit cost increased by 2-folds. The increase was less pronounced for office-administered products.
Conclusions
Despite the availability of several generic cDMARDs over decades, there were steep increases in the unit cost of these agents to “keep pace” with the increases in bDMARDs. As the number of elderly rheumatoid arthritis patients increases, policy interventions might be required to reduce the spending on both biologics and conventional DMARDs.
Keywords: Rheumatoid arthritis, Medicare, Cost of DMARDs
Introduction
Biologic disease modifying agents (DMARD) are now an integral component of treatment guidelines for rheumatoid arthritis (RA) and other inflammatory arthritis, and currently more than one-third of RA patients use biologics [1], [2], [3]. Several clinical trials have demonstrated the efficacy of biologics in reducing disease activity as well as retarding disease progression and with an increased emphasis on early and tight control of the disease, the use of biologic DMARDs is becoming more common [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Adoption of biologic agents for RA treatment, however, comes at a price. Biologic agents are substantially more expensive per dose than conventional DMARDs, adding an additional $20,000 per year to direct medical care costs ($12,509 if not on a biologic versus $36,053 if on a biologic) in the United States. While the cost of drugs in RA patients using conventional agents is $1500–2000 annually, the cost of drugs in RA patients using biologics is as much as $30,000 a year [15]. Biologic agents administered as an intravenous infusion have a facility/administration fee associated with it which further adds to the cost of care of RA.
RA is twice as prevalent in older population as compared to young (2% versus 1%). And given the chronic nature of the disease and our aging population, the number of elderly RA patients is expected to rise [16], [17], [18], [19]. Medicare is largest payer for healthcare of older individuals. A study conducted by McCormick et al noted more than 2-fold increase in Medicare and Medicaid spending on biologic disease modifying agents commonly used for treatment of rheumatic diseases which was largely driven by post-marketing increases in drug price [20]. In the context of increasing spending on biologic DMARDs, we compared the trends in Medicare spending on conventional and biologic DMARDs commonly used to treat RA, to broadly document the impact of new therapeutic agents alongside more traditional products. While the conventional and biologic agents included have approved indications besides RA, their pricing is independent of the indication for use and trends will be applicable to RA care costs.
Methods
Study design
The current study was an observational study assessing national trends in spending on disease modifying agents used for rheumatoid arthritis using aggregated Medicare data.
Setting & participants
Medicare provides health insurance coverage to individuals greater than 65 years of age and those who are younger but disabled in the US. In 2018 (2019 Annual Report of Medicare Trustees), Medicare provided health insurance for over 60 million individuals, more than 52 million of whom were people aged 65 and older. Medicare provides coverage for prescription medications which are self-administered injections or oral drugs, via Part D, and medications administered as infusions or other office administered agents via Part B. Both Parts D and B are optional benefits for Medicare enrollees and require monthly premiums. For the current study, we used data from CMS's annual, publicly available reports (CMS Medicare Part B and Part D Drug Spending Dashboard, available through the www.cms.gov website) of spending on both conventional and biologic disease modifying agents approved for rheumatoid arthritis between 2012 and 2017 which included spending across all ages.
Data sources and variables
We included all the commonly used disease modifying agents used for rheumatoid arthritis captured through Part B (office-administered) and Part D (self-administered drugs) claims. Conventional agents were methotrexate, sulfasalazine, hydroxychloroquine and leflunomide. Biologic DMARDs available during the period included etanercept, abatacept, adalimumab, certolizumab, golimumab, inflixiumab, rituximab, tocilizumab, and tofacitinib. All conventional DMARDs have been available as generic agents and approved several years before 2012. All the biologic DMARDs were approved for use in RA prior to 2012 except for office-administered certolizumab and golimumab, both approved in 2014. Hence, there was at least 4 years of data to assess the trend of spending on each drug.
The dataset contains aggregated national level spending on individual drugs administered through part D and part B (without any administration fee for part B drugs). CMS data summarizes expenditures total spending which includes amounts paid by Medicare, beneficiaries as deductibles and copayments and third parties (for e.g. supplemental insurances). It also includes beneficiary counts, total claims, total dosage units, average cost per unit (calculated as total spending/total dosage units), average spend per claim, average spending per beneficiary, and changes over time at the brand or generic drug name level. Their data combines utilization/cost data across dosage forms to the product brand, if applicable, or generic, if multisource, name.
Analysis
We summed spending for brand and generic versions of drug products for each drug. For the conventional DMARD's, we used the unit price of the most commonly used formulation as reported by CMS. Unit cost was calculated by dividing the total spending on a particular drug formulation by the number of doses dispended and was available in the data. To examine the trends in unit cost of biologic DMARDs and to compare the costs of these drugs with each other, we calculated the annual expenditure of a maintenance regimen of biologic agents for a typical 70-year-old rheumatoid arthritis patient (regimens discussed in supplementary file 1). This was not possible for conventional DMARDs because of lack of information of drug strength. We presented the trends in total spending on individual drugs between 2012 and 2017, along with trends of unit cost of these agents. We also compared the actual drug prices of individual drugs in 2017 to the prices that would be expected because of inflation alone. The latter was calculated using the medical care component inflation provided by Bureau of Labor Statistics and base price of the drug in 2012 [21].
Results
From 2012–2017, Medicare experienced a 2.3-fold increase in their expenditures for DMARDs frequently used for RA: $4.4 billion in 2012 to $10.0 billion in 2017. Biologic DMARDs accounted for ~95% of the total expenditure each year.
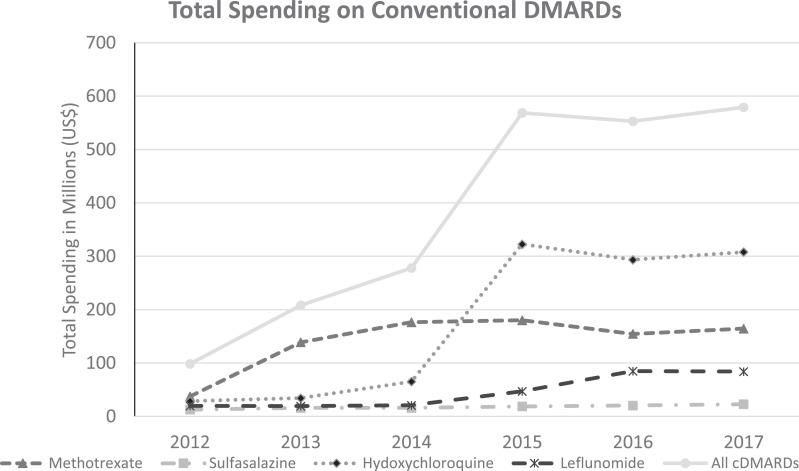
Between 2012 and 2017, the total spending on the conventional DMARDs increased from approximately $98 million to $579 million (Fig. 1 ). The number of beneficiaries using conventional DMARDs also increased (738,195 to 1,134,094 or 1.5-fold) over the same time period. Increases in unit costs drove the rise in total spending, most notably for hydroxychloroquine where cost/unit increased from $0.29 to $1.84. A new brand name hydroxychloroquine product was approved in 2014 with a unit cost of $8.40 in 2017. Unit cost also nearly tripled for methotrexate and leflunomide. Prefilled methotrexate injections cost between $344–$376 per injection, compared to the generic product at $1.63 per dose (Table 1 ). The cost of each in 2017 surpassed the expected cost based on inflation alone except for branded oral and injectable methotrexate.
Fig. 1.
Trends in Total Medicare Spending on conventional DMARDs used for RA between 2012–2017.
Table 1.
Unit Cost paid by Medicare of conventional DMARD used for RA between 2012–17.
| Drug Name | 2012 (Actual Cost) | 2017 (Actual Cost) | 2017 (2012 cost in 2017 dollars) | Percentage Increase in Adjusted costs from 2012 to 2017 |
|---|---|---|---|---|
| Leflunomide | ||||
| - Generic | $1.68 | $4.31 | $1.92 | 124% |
| - Branded | $22.49 | $38.49 | $25.65 | 50% |
| Methotrexate | ||||
| - Generic (oral) | $0.64 | $1.89 | $0.73 | 159% |
| - Branded (oral)* | $9.60 | $10.01 | $10.95 | -9% |
| - Branded (injectable)⁎⁎ | $334.90 | $375.90 | $381.91 | -2% |
| Hydroxychloroquine | ||||
| - Generic | $0.29 | $1.84 | $0.33 | 458% |
| - Branded | $4.18 | $8.40 | $4.77 | 76% |
| Sulfasalazine | $0.16 | $0.19 | $0.18 | 6% |
indicates drugs that ceased to be covered in 2016;
indicates drugs that were approved in 2014
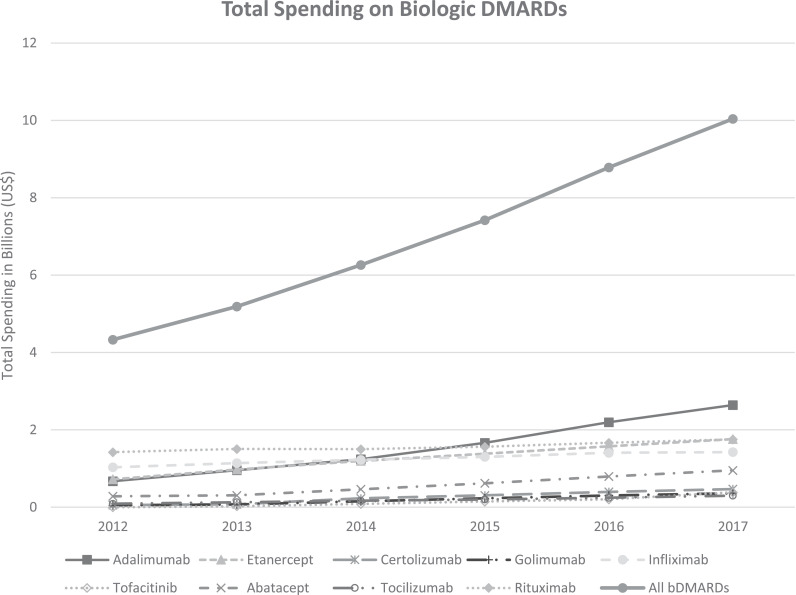
Medicare spending on biologic DMARDs more than doubled from $4.3 billion (2012) to $10.04 billion (2017), also outpacing the gain in the number of beneficiaries using these products (252,225 to 335,960, 1.3-fold increase) (Fig. 2 ). There was nearly a doubling in the price per unit of most self-administered biologic DMARDs, and the increase in unit cost of office administered DMARDs was less pronounced, except for abatacept. Table 2 represents spending for maintenance regimen of biologics for an average 70 kg patient with RA, a function of unit price of the drug. The cost of each drug in 2017 surpassed the expected cost based on inflation alone except for injectable golimumab.
Fig. 2.
Trends in Total Medicare Spending on biologic DMARDs used for RA between 2012–2017.
Table 2.
Average cost of Maintenance Regimen of each of the biologic DMARDs between 2012–2017.
| Drug Name | 2012 (Actual Cost) | 2017 (Actual Cost) | 2017 (2012 cost in 2017 dollars) | Percentage Increase in Adjusted costs from 2012 to 2017 |
|---|---|---|---|---|
| SELF ADMINISTERED DRUGS | ||||
| Golimumab | $52,506 | $93,961 | $59,877 | 57% |
| Adalimumab | $26,491 | $58,135 | $30,210 | 92% |
| Etanercept | $25,875 | $56,897 | $29,507 | 93% |
| Abatacept | $27,087 | $50,314 | $30,889 | 63% |
| Tocilizumab⁎⁎ | $27,056 | $47,155 | $30,854 | 53% |
| Tofacitinib | $25,616 | $46,377 | $29,212 | 59% |
| Certolizumab | $26,036 | $45,830 | $29,691 | 54% |
| OFFICE ADMINISTERED DRUGS | ||||
| Abatacept | $19,494 | $41,652 | $22,231 | 87% |
| Certolizumab* | $30,992 | $38,116 | $35,343 | 8% |
| Rituximab | $24,588 | $32,549 | $28,040 | 16% |
| Tocilizumab | $23,520 | $28,358 | $26,822 | 6% |
| Infliximab | $17,769 | $23,400 | $20,263 | 15% |
| Golimumab* | $23,138 | $23,804 | $26,836 | -8% |
indicates drugs that were approved in 2014;
Tocilizumab was approved in October 2013 and hence figures of 2014 provided.
Medicare spending on office/infusion center administered drugs declined as a percentage of total spending on biologic DMARDs from 64.3% ($2.79 billion of 4.3 billion in 2012) to 45.2% ($4.5 billion of 10.04 billion in 2017). There was a decline in the percentage of total beneficiaries on office/infusion center administered biologic DMARDs. Finally, adalimumab was the most utilized drug amongst beneficiaries receiving biologic DMARDs (73,022 in 2017; 21% share) and had the highest share of total spending ($2.64 billion in 2017; 26% share).
Discussion
Between 2012 and 2017, there was a tremendous increase in spending on both biologic and conventional DMARDs used for the treatment of RA without a similar increase in the number of beneficiaries receiving these treatments. And as expected, most of the spending was on biologic DMARDs. The increase in spending was largely driven by increases in cost/unit of individual drugs; for each conventional and biologic DMARD, the actual cost of drug in 2017 was higher than the estimated cost of drug based on medical care component inflation provided by the consumer price index [22]. The study provides important insights into the spending pattern on individual DMARDs.
We noted that, over the study period, there was a tremendous relative increase in total spending on conventional synthetic DMARDs, almost entirely driven by cost/unit of these drugs and outpacing inflation. The cost/unit of hydroxychloroquine, methotrexate and leflunomide increased almost 3-fold, most notably for hydroxychloroquine which increased 6-fold even though these medications have been available for several decades as generics. Branded oral and injectable methotrexate were the only agents whose actual cost in 2017 was lower than what would be expected by inflation alone. A study by Joyce et al noted an overall decline in the prices of generic medications between 2006 and 2015 covered under the Medicare part D program, however, there were a subset of drugs which saw a tremendous increase during the same time frame [23]. Lack of or limited availability of competitors seemed to be the primary reason for the rise of such costs [24]. In the RA treatment algorithms, there are a limited number of conventional DMARDs to choose from. Biologic DMARDs, on the other hand, are considerably more expensive than conventional DMARDs, hence providing an opportunity to manufacturers of conventional DMARDs to increase the unit cost of these agents.
During the same period, there was also a considerable increase in the total spending on biologic DMARDs; the proportion of spending and beneficiaries on self-administered biologics compared to office-administered biologics increased during the study period. Across all biologic agents, the unit cost of office administered agent increased less than the unit cost of self-administered agents. As such, when drug administration costs are factored in, office administered agents are considerably more expensive than self-administered agents. Across all biologic agents, the price of drug in 2017 was much higher than what would be anticipated with inflation using the 2012 base cost except for office administered golimumab. Biosimilar agents were in their early approval phases during the year 2012–-17 and hence their effects could not be examined. It could be anticipated that these costs would continue to remain high considering the lengthy process of development, regulatory restrictions, patent laws, and clinical trials required to demonstrate safety and efficacy of biosimilar agents [25,26].
Hydroxychloroquine is being extensively investigated as a potential therapeutic option for the treatment of COVID-19 caused by the novel coronavirus (SARS-CoV2) [27], [28], [29], [30]. The global pandemic affecting almost 10 million individuals globally and 2.4 million in the US created an excessive demand for hydroxychloroquine. Tocilizumab and some of the other biologic agents are also being investigated for treatment of COVID-19 [31], [32], [33]. The added demand on these agents may further drive up the unit costs of these medications.
While this study provides useful insights into the spending trends on conventional and biologic DMARDs by Medicare, there are certain limitations that merit discussion. First, we were unable to identify the indications for use of biologic agents. There have been increasing indications of for use of several of the agents for other autoimmune conditions like other inflammatory arthritis, malignancy (for rituximab), uveitis, inflammatory bowel disease and hidradenitis. However, rheumatoid arthritis is the most common inflammatory arthritis amongst senior and hence, most of the spending is likely to be on rheumatoid arthritis. Second, we are unable to assess rebates provided by the pharmaceutical industry to pharmacy benefits manager and Part D plans. Finally, given the categorization of several conventional DMARDs like methotrexate tablets and methotrexate injections under “methotrexate sodium” OR leflunomide 10 mg and 20 mg under “leflunomide”, precluded our ability to calculate annual costs different drug regimens like that for biologics.
Despite the limitations, the study provides useful national trends of spending on disease modifying agents in the United States. The findings of this study call for a 2-fold action. First, as the population ages, it is expected that the number of beneficiaries covered through Medicare will increase. If the post-marketing unit costs of DMARDs continue to increase at the pace noted in this study, with the increasing number of Medicare beneficiaries, the total spending on these agents increase at an even faster pace. Hence, policy changes targeting the post-marketing drug prices are required. Second, further studies are required to answer the central question, is the increased spending on DMARDs, particularly biological agents, resulting in improved outcomes in older RA patients. There is growing long-term data to suggest increasing safety of these medications, at least in average RA patients. However, outcomes of interest to geriatric population continue to remain an understudied area in RA care.
Declaration of Competing Interest
None.
Funding source
National Institute of General Medical Sciences, NIH, Grant number: U54GM115677.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.semarthrit.2020.08.002.
Appendix. Supplementary materials
References
- 1.Singh JA, Saag KG, Bridges SL, Jr., Akl EA, Bannuru RR, Sullivan MC. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol (Hoboken, NJ) 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 2.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707. doi: 10.1016/j.clinthera.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yazici Y, Shi N, John A. Utilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapy. Bull NYU Hosp Joint Dis. 2008;66(2):77–85. [PubMed] [Google Scholar]
- 4.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 6.Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet (London, England) 2008;372(9636):375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 7.Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68(6):805–811. doi: 10.1136/ard.2008.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 9.Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet (London, England) 2004;363(9410):675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 10.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet (London, England) 1999;354(9194):1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 11.Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130(6):478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 12.Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67(8):1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 14.Weinblatt ME, Schiff M, Valente R, van der Heijde D, Citera G, Zhao C. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65(1):28–38. doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hresko A, Lin TC, Solomon DH. Medical care costs associated with rheumatoid arthritis in the US: a systematic literature review and meta-analysis. Arthritis Care Res (Hoboken) 2018;70(10):1431–1438. doi: 10.1002/acr.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalal D, Duran J, Brar T, Alqadi R, Halladay C, Lakhani A. Efficacy and safety of biologic therapy in elderly rheumatoid arthritis patients compared to young - a systematic review. Ann Rheum Dis. 2016;76(Suppl 2):SAT0151. doi: 10.1016/j.semarthrit.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48(4):917–926. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 18.Soubrier M, Tatar Z, Couderc M, Mathieu S, Dubost JJ. Rheumatoid arthritis in the elderly in the era of tight control. Drugs Aging. 2013;30(11):863–869. doi: 10.1007/s40266-013-0122-8. [DOI] [PubMed] [Google Scholar]
- 19.Yazici Y, Paget SA. Elderly-onset rheumatoid arthritis. Rheum Dis Clin North Am. 2000;26(3):517–526. doi: 10.1016/s0889-857x(05)70154-x. [DOI] [PubMed] [Google Scholar]
- 20.McCormick N, Wallace ZS, Sacks CA, Hsu J, Choi HK. Decomposition analysis of spending and price trends for biologic antirheumatic drugs in medicare and medicaid. Arthritis Rheumatol. 2019 doi: 10.1002/art.41138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistics UBoL. Consumer Price Index Division of Consumer Prices and Price Indexes, Suite 2120, 2 Massachusetts Avenue NE, Washington DC 20212 [Available from:https://www.bls.gov/cpi/.
- 22.Nass SJ MG, Augustine NR. In: Nass SJ MG, Augustine NR, editors. National Academies Press (US); WashingtonDC: 2017. editors. editors. [Google Scholar]
- 23.Joyce G, Henkhaus LE, Gascue L, Zissimopoulos J. Generic drug price hikes and out-of-pocket spending for medicare beneficiaries. Health Aff (Millwood) 2018;37(10):1578–1586. doi: 10.1377/hlthaff.2018.0628. [DOI] [PubMed] [Google Scholar]
- 24.Office) GUSGA . U.S. Government Accountability Office; Washington, DC: December 2009. Brand-name prescription drug pricing: Lack of therapeutically equivalent drugs and limited competition may contribute to extraordinary price increases. [Google Scholar]
- 25.Ingrasciotta Y, Cutroneo PM, Marciano I, Giezen T, Atzeni F, Trifiro G. Safety of biologics, including biosimilars: perspectives on current status and future direction. Drug Saf. 2018;41(11):1013–1022. doi: 10.1007/s40264-018-0684-9. [DOI] [PubMed] [Google Scholar]
- 26.Kesselheim AS, Sinha MS, Avorn J. Determinants of market exclusivity for prescription drugs in the United States. JAMA Intern Med. 2017;177(11):1658–1664. doi: 10.1001/jamainternmed.2017.4329. [DOI] [PubMed] [Google Scholar]
- 27.Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- 28.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 30.Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 2020;172(11):754–755. doi: 10.7326/M20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.