Abstract
SARS-CoV-2 (COVID-19) patients with associated thromboembolic events have demonstrated poor outcomes despite the use of anticoagulation therapy and surgical intervention. We present a COVID-19 patient with acute limb ischemia, secondary to extensive thrombosis of an aortic aneurysm, iliac arteries, and infrainguinal arteries. Initial treatment with systemic thrombolysis, which restored patency of the aortoiliac occlusion, was followed by open thrombectomies of the infrainguinal occlusions.
The current SARS-CoV-2 (COVID-19) pandemic is an unprecedented event that has significantly strained health care systems across the world. In the United States, which has over 4 million cases and 145,000 deaths, the state of New Jersey has the second highest number of deaths, currently at more than 15,000 deaths.1 COVID-19 infection is suspected of inducing a hypercoagulable state, resulting in macro- and micro-vascular thromboses in the arterial and venous systems.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 In particular, surgical interventions to treat arterial thrombosis in these prothrombotic patients have proven to be challenging, with poor outcomes.8, 9, 10
We describe the case of a COVID-19 patient with acute limb ischemia (ALI) secondary to thrombosed aortic aneurysm and bilateral lower extremity arteries, initially treated with systemic thrombolysis, followed by surgical revascularization.
Case Report
A 73-year-old male was transferred to our institution with a one-day history of sudden onset bilateral severe hip and buttock pain, lower extremity paresthesia and paralysis. Initial evaluation was performed by neurology and neurosurgery, with concerns for spinal infarction. His feet were cold to touch, without signs of mottling or gangrene, and his lower extremity muscle compartments were soft. He initially presented with bilateral femoral and popliteal signals, but no pedal doppler signals could be elicited. He had a low-grade fever of 100.8° F and his room air saturation was 96%. His D-dimer level was exceedingly high at 39,875 ng/mL (normal <500 ng/mL), creatinine phosphokinase (CPK) was greater than 30,000 u/L, and SARS-CoV-2 RNA test was positive. Comorbidities included hypertension and an 80 pack-year smoking history. Computed tomography (CT) scan of the chest, abdomen, and pelvis demonstrated a thrombosed 4.5 cm infrarenal abdominal aortic aneurysm, along with thrombosed bilateral common, internal, and proximal external iliac arteries. Flow reconstituted in the distal external iliac arteries (Figs. 1 A and 2A ). Also noted were bilateral lower pole renal infarcts. Peripheral bilateral ground glass opacities consistent with viral pneumonia were present in the lung fields.
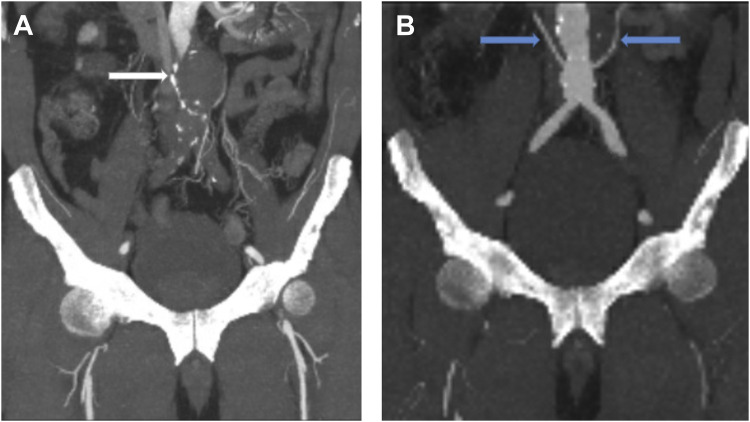
Fig. 1.
CT angiogram pre- and post-thrombolysis, coronal view. CT angiogram (A) before thrombolysis demonstrating thrombosis of the infrarenal abdominal aortic aneurysm (white arrow); (B) after thrombolysis demonstrating patency of the aorta and the aortic bifurcation. Note bilateral accessory renal arteries (blue arrows).
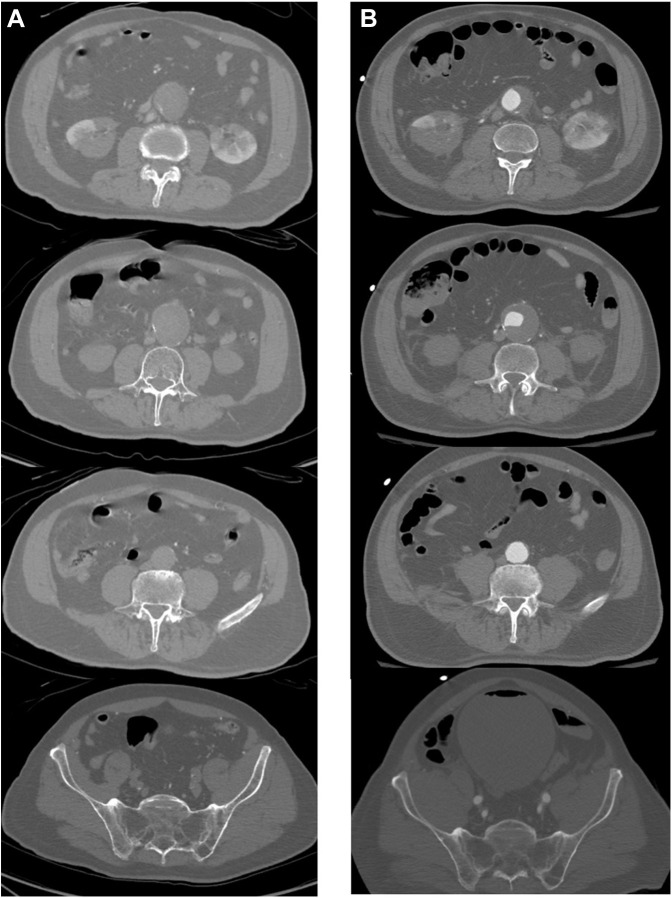
Fig. 2.
CT angiogram pre- and post-thrombolysis, axial view. CT angiogram (A) before thrombolysis demonstrating thrombosed aortic aneurysm, bilateral external and internal iliac arteries; (B) after thrombolysis demonstrating patent infrarenal aorta and bilateral iliac artery bifurcations.
Vascular surgery was consulted at this point. We performed a duplex ultrasound to assess lower extremity outflow and noted occlusions of the common, deep, and superficial femoral arteries, which were not present on the initial CT scan and suggestive of rapid progression of arterial thrombosis. Bilateral popliteal and tibial arteries were occluded without any reconstitution of flow.
In the setting of rapidly progressive macro- and micro-vascular thromboses at multiple levels in this symptomatic COVID-19 patient, we initially administered systemic tissue plasminogen activator (tPA). A 25 mg bolus of tPA was given intravenously over 2 hr, followed by a continuous infusion of 25 mg over the following 22 hr.12 Seventeen hours into his tPA infusion, his hip and buttock pain had completely resolved and he regained motor function in the right lower extremity. A palpable right femoral pulse and a posterior tibial artery doppler signal were restored, while his calf compartments remained soft. However, his left lower extremity was persistently ischemic with no return of motor function or sensation.
Repeat CT imaging demonstrated restoration of the lumen of the aortic aneurysm; along with return of patency of the bilateral common, internal, and external iliac arteries. Of note, patent bilateral accessory renal arteries were now also visualized. (Figs. 1B and 2B). However, the left common, superficial, and deep femoral arteries were thrombosed. Bilateral popliteal and tibial arteries also remained occluded.
Given the resolution of the proximal aortoiliac thrombosis, the patient subsequently underwent open thrombectomy of the left femoral and popliteal arteries. Bilateral tibial thrombectomies and bilateral four compartment fasciotomies were also performed. Completion angiogram confirmed patency of these vessels. There were no bleeding complications from thrombolysis and he was maintained on therapeutic heparin postoperatively. The patient was extubated 12 hr later and his pulmonary status remained stable. His CPK peaked at 72,000 after 2 days but rapidly normalized, and his D-dimer decreased to 2,000. The patient did develop acute renal failure requiring hemodialysis. Although both legs remained perfused, his right lower extremity motor function and sensation returned to baseline, but his left lower extremity did not. Over a span of 2 weeks, he continued to improve and underwent multiple debridements of the left calf muscles for reperfusion injury. Unfortunately, on postoperative day 17, the patient was found in asystole without having had any prior complaints or laboratory derangements, and was presumed to have expired from an acute cardiopulmonary event.
Discussion
As the COVID-19 pandemic continues, the prothrombotic effects of this novel coronavirus are apparent. In addition to venous thromboembolic events, reports have documented multiple arterial thrombotic manifestations of this disease, from lower extremity arterial occlusions to strokes.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 In one recent study of 3,334 consecutive hospitalized COVID-19 patients in a New York City health system,16.0% of patients developed thrombotic events. All-cause mortality was 43.2% in patients with thrombotic events, compared with 21.0% in those without. Thrombosis was independently and significantly associated with mortality in both venous (hazard ratio [HR], 1.37) and arterial (HR, 1.99) thromboses.5
We describe a COVID-19 patient with ALI secondary to macro- and micro-vascular thromboses at multiple levels, including a thrombosed aortic aneurysm, bilateral iliac systems, and bilateral infrainguinal arterial segments. After weighing treatment options, initial therapy with systemic tPA was chosen based on the rapidly progressive and multilevel nature of his arterial thrombosis due to COVID-19 hypercoagulability. Other initial interventions of the aortoiliac segments considered included open thrombectomy and extra-anatomic bypass. With open thrombectomy, there were concerns for disrupting laminar plaque and thrombus in the aortic aneurysm. Extra-anatomic bypass of the thrombosed aortic aneurysm would not have been effective by itself because of compromised outflow. Neither of these procedures would have addressed the microvascular thromboses at the hypogastric and renal artery levels. With his initial presentation of advanced ALI, primary amputation of the lower extremities was considered. However, amputation alone would not have been sufficient in the face of extensive aortoiliac thrombosis and an acutely hypercoagulable state. The patient also did not present with signs of gangrene and his lower extremity muscles were soft without obvious signs of compartment syndrome.
Although not standard therapy for aortoiliac occlusion, immediate initiation of thrombolysis was found to be effective in restoring the lumen of the aortic aneurysm, iliac arteries, as well as perfusion to the pelvis and accessory renal arteries. Catheter-directed thrombolysis was considered, but we decided systemic tPA would be the fastest and most effective method of treating the acute systemic progression of thrombosis. With aortoiliac patency restored, the infrainguinal thromboses were then able to be treated in a more standard fashion for limb salvage. Although thrombolysis may be contraindicated after aneurysm repairs or in aortic dissection, there is no clear contraindication in patients with untreated aortic aneurysms. We also did not treat the 4.5 cm aneurysm at that time because the patient was in a severely hypercoagulable state from COVID-19 with a primary issue of ALI. Of note, our patient did not develop progression of pulmonary symptoms related to COVID-19 despite his pulmonary findings. The thrombolytic regimen used was similar to recent off-label use of systemic tPA for acute respiratory distress syndrome, in critically ill COVID-19 patients to target microvascular thrombosis in the pulmonary circulation.12
The nascent literature on COVID-19 has started to describe patients with thrombotic complications and the challenges treating this patient population. Klok et al.6 , 7 reported on 184 ICU patients with COVID-19 at a single institution with a 57% crude cumulative incidence of arterial or venous complications. In this group, they noted 65 pulmonary emboli, 3 deep venous thromboses, 5 ischemic strokes, and 2 systemic arterial thromboembolisms. Patients with thrombotic complications had a higher risk of all-cause death (HR, 5.4; 95% confidence interval, 2.4–12).
Perini et al.9 described four COVID-19 patients with ALI. Two patients had no prior risk factors. One patient was a 53-year-old male without risk factors who developed acute aortoiliac occlusion and signs of ALI. Open thrombectomy was performed, but he rethrombosed 2 hr later and expired 2 days later.
Bellosta et al.8 described 20 patients with COVID-19 pneumonia who presented with ALI. Over 3 months, they observed an incidence rate of ALI of 16.3% vs. 1.8% (P < 0.001) during the same period in 2019. Thrombectomy was performed for infrainguinal occlusions in 11 patients, bilateral aortoiliac occlusions in 3 patients, and upper extremity occlusion in 1 patient. Two patients underwent lower extremity bypasses. Intraoperative thrombolysis was also used in 4 patients. Other adjunctive procedures included kissing iliac stents (n = 2), femoral endarterectomy (n = 1), and popliteal angioplasty (n = 1). The authors noted a high failure rate of revascularization, with success only achieved in 12 of 17 patients (70.6%). Two patients required reintervention for recurrent thrombosis on postoperative days 1 and 2. Limb salvage was 93%. Forty percent of patients died during the index admission. One patient with combined aortoiliac and infrainguinal occlusions died on day 0 from multisystem organ failure. The authors hypothesized that the high failure rates for revascularization were due to microvascular thrombosis, resulting in poor outflow.
The literature to date demonstrates poor outcomes in COVID-19 patients with thrombotic complications. The underlying process of endothelial injury can precipitate widespread macro- and micro-vascular thromboses in the venous and arterial systems. Given the potential severity of COVID-19 illness and limited treatment options at this time, it is important to evaluate all possible therapies, such as thrombolysis, for efficacy.
Conclusion
The extensive macro- and micro-vascular arterial thromboses recently witnessed in patients with COVID-19 have proven to challenge the current tenets of medical and surgical standards of care. In patients with extensive arterial thrombosis, systemic thrombolysis might be considered as an adjunct to surgical revascularization in this group of patients. As this pandemic continues to evolve, further investigation of this approach is needed.
References
- 1.COVID-19 Dashboard. New Jersey Department of Health. https://covid19.nj.gov/#live-updates
- 2.Levi M., Thachil J., Iba T. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilaloglu S., Aphinyanaphongs Y., Jones S. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellosta R., Luzzani L., Natalini G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020 doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perini P., Nabulsi B., Massoni C.B. Acute limb ischemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395:1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mestres G., Puigmacia R., Blanco C. Risk of peripheral arterial thrombosis in COVID-19. J Vasc Surg. 2020;72:756–757. doi: 10.1016/j.jvs.2020.04.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., Hajizadeh N., Moore E.E. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]