Abstract
Background
There is increasing evidence supporting coronavirus disease 2019 (COVID-19)-related coagulopathy. In the available literature, only 2 cases of superior mesenteric vein thrombosis have been described.
Methods
We present a peculiar case of high-grade small bowel obstruction in a patient with COVID-19 infection.
Results
Exploratory laparotomy revealed a congenital adhesion band with associated focal bowel ischemia contributed by superior mesenteric vein thrombosis and positive lupus anticoagulant.
Conclusions
It is important to consider the rare differential of mesenteric vein thrombosis and its related sequelae of mesenteric ischemia in a patient with COVID-19 who presents with abdominal pain.
Introduction
Coronavirus disease 2019 (COVID-19) pandemic is the defining global health crisis in recent times. Although this disease is predominantly a respiratory illness with pulmonary manifestations, it has far-reaching consequences in other systems. There is increasing evidence to support COVID-19–related coagulopathy. The commonly observed association is linked to arterial and venous thrombotic events.1, 2, 3
To date, there are only 4 case reports on superior mesenteric artery thrombosis.4, 5, 6, 7 In the available literature, only 2 other cases of superior mesenteric vein thrombosis associated with COVID-19 have been described.7 , 8 We present a peculiar case of high-grade small bowel obstruction secondary to congenital adhesion band with associated focal bowel ischemia contributed by superior mesenteric vein thrombosis and positive lupus anticoagulant in a patient with COVID-19.
Case Report
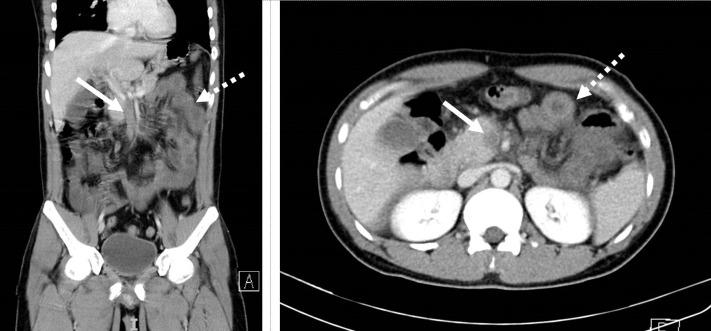
We report a case of a 30-year-old Bangladeshi male with no significant past medical or surgical history. He first presented to a public hospital with a 2-day history of colicky abdominal pain that was associated with vomiting. Chest X-ray showed left lower zone airspace opacities and nasopharyngeal swabs for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction–confirmed COVID-19 infection. A computed tomography scan of the abdomen and pelvis revealed superior mesenteric vein thrombosis with diffuse mural thickening and fat stranding of multiple jejunal loops (Fig. 1 ). Coagulation profile tests showed positive lupus anticoagulant, mildly raised fibrinogen of 4.65 g/L, and a markedly raised D-dimer of more than 20.0 μg/mL, despite normal prothrombin time and activated partial thromboplastin time. These are in keeping with COVID-19–related coagulopathy.9 His clinical condition remained stable, and he was started on twice-daily low-molecular-weight heparin at 1 mg/kg therapeutic dosage. After 17-day length of stay, he was discharged to a community isolation facility with a plan for 3 months of low-molecular-weight heparin as per physician and patient preference.
Fig. 1.
Superior mesenteric vein thrombosis (bold arrow) and diffuse mural thickening (dotted arrow).
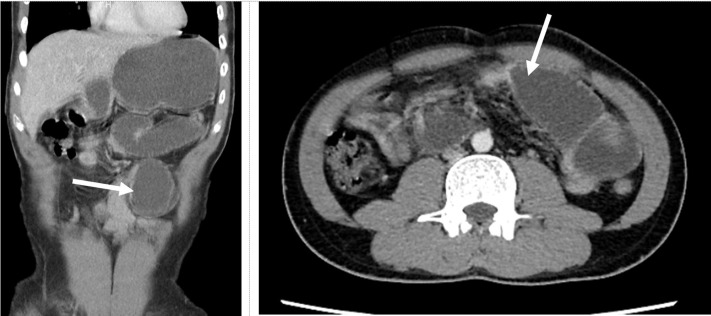
This patient represented to our tertiary hospital a month later for 2-day duration of generalized abdominal pain associated with multiple episodes of bilious vomiting. He reported strict compliance to the twice-daily enoxaparin sodium injections. His vital signs were normal, and examination findings revealed a distended abdomen with no sign of peritonism noted. A computed tomography scan of the abdomen and pelvis revealed proximal small bowel with a transition point seen in mid jejunum (Fig. 2 ). There was no intraabdominal mass or radiological features of ischemia detected. There was partial recanalization of the superior mesenteric vein thrombosis with normal opacification proximal and distal to the thrombosis. He was resuscitated with intravenous fluids, and a nasogastric tube was inserted for prompt decompression of the stomach.
Fig. 2.
High-grade small bowel obstruction with a transition point (bold arrow) seen in mid jejunum.
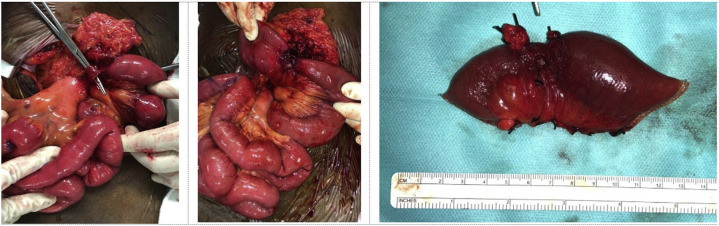
In view of the high-grade small bowel obstruction (Fig. 2), the patient underwent urgent exploratory laparotomy. Intraoperative findings revealed distended proximal small bowel with a single congenital adhesion band at the root of the mesentery 40 cm distal to duodenojejunal flexure (Fig. 3 ), causing a tight stenosis of mid jejunum. The congenital adhesion band was excised, and this revealed a short segment of severely strictured small bowel. The rest of the small bowel was noted to be healthy and viable. The segment of small bowel stricture was resected with primary anastomosis performed. Postoperative recovery was uncomplicated, and he was recommenced on his enoxaparin sodium on postoperative day 2. He made a good recovery and was discharged back to community isolation facility on postoperative day 7. Histology of the small bowel specimen resected revealed ischemic bowel with the presence of organizing thrombosis within large mesenteric venous vessels.
Fig. 3.
Congenital adhesion band (left). Strictured small bowel in mid jejunum (middle). Resected small bowel specimen (right).
Discussion
A congenital adhesion is an uncommon surgical condition causing intestinal obstruction. It is described as an intraperitoneal adhesion that is considered congenital with no relation to previous surgery or inflammatory process. We hypothesized the acute presentation of intestinal obstruction in this patient to be due to a congenital adhesion band exacerbated by small bowel stricture that was contributed by superior mesenteric vein thrombosis–related to COVID-19. The focal short segment transition point in mid jejunum was not demonstrated in this patient's earlier computed tomography scan a month ago when he was admitted for superior mesenteric thrombosis–related to COVID-19. The described fat stranding and diffuse mural thickening seen in jejunal loops in the earlier scan may be related to acute venous congestion. Superior mesenteric vein thrombosis causing venous ischemia and small bowel stricture has been described in scattered case reports in nonCOVID patients.10 , 11 Acute thrombosis of superior mesenteric vein does not allow sufficient time for collateral circulation to develop. Despite therapeutic anticoagulation, secondary arterial spasms may occur, leading to bowel ischemia, transmural infarction, and development of bowel stricture. In this patient, the choice of anticoagulation was enoxaparin sodium. This reduces the frequency of monitoring required to maintain an adequate therapeutic level of anticoagulation in this climate of COVID-19.
The pathophysiology of COVID-19–related coagulopathy can be principally attributed to Virchow's triad of thrombosis.12 First, endothelial injury may be caused by SARS-CoV-2 that binds via angiotensin-converting enzyme 2 receptors on endothelial cells. Second, a hypercoagulable state can result from a dysregulated hyperinflammatory response to the viral infection. Third, stasis of blood can be a consequence of immobilization in critically ill patients.12 Antiphospholipid syndrome is an autoimmune disease with hypercoagulable state caused by the presence of antiphospholipid antibodies, which predisposes the patient to having vascular thrombosis. The presence of a lupus anticoagulant in patients with COVID-19 is not uncommon, and in our case, the patient did have a concurrent lupus anticoagulant together with a hypercoagulable state. These antibodies can also arise transiently in patients with critical illness and viral infections. In a case–control study by Louise Bowles et al., among patients with prolonged activated partial thromboplastin time, lupus anticoagulant was significantly higher in COVID-19 patients (91%) compared with control group (26%).13 In another study performed in Mulhouse, France, 25 of 56 patients (45%) with COVID-19 were tested positive for lupus anticoagulant.14 Antiphospholipid syndrome will need to be excluded in this patient with repeat testing for lupus anticoagulant at least 12 weeks after initial testing.
Conclusion
This peculiar case report underscores a few important learning points. One, COVID-19 is a systemic disease not limited to pulmonary manifestations and complications. Two, treating clinicians must be aware of the hypercoagulable state of COVID-19 patients and judicious use of prophylactic anticoagulation in hospitalized patients should be considered. And finally, one must consider the rare differential of mesenteric vein thrombosis and its related sequelae of mesenteric ischemia in a patient with COVID-19 who presents with abdominal pain.
CRediT authorship contribution statement
Jolyn Hui Qing Pang: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Jun Han Tang: Conceptualization, Data curation, Formal analysis, Writing - original draft. Bingwen Eugene-Fan: Data curation, Resources, Writing - review & editing. Chin Li Lee: Resources. Jee Keem Low: Conceptualization, Formal analysis, Resources, Formal analysis, Writing - review & editing.
Footnotes
Declarations of interest: None.
References
- 1.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui S., Chen S., Li X. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azouz E, Yang S, Monnier-Cholley L, et al. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med 46, 1464–1465 [DOI] [PMC free article] [PubMed]
- 5.Beccara L A., Pacioni C., Ponton S. Arterial mesenteric thrombosis as a complication of SARS-CoV-2 infection. Eur J Case Rep Intern Med. 2020;7:001690. doi: 10.12890/2020_001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vulliamy P., Jacob S., Davenport R.A. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol. 2020;189:1053–1054. doi: 10.1111/bjh.16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Barry O., Mekki A., Diffre C. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep. 2020;15:1054–1057. doi: 10.1016/j.radcr.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norsa L., Pietro B., Indriolo A. Poor outcome of intestinal ischemic manifestations of COVID 19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.06.041. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 10.Chou Y.L., Huang T.Y. Delayed bowel stricture complicating superior mesenteric vein thrombosis. J Med Sci. 2018;38:135–136. [Google Scholar]
- 11.Paraskeva P., Akoh J.A. Small bowel stricture as a late sequela of superior mesenteric vein thrombosis. Int J Surg Case Rep. 2015;6C:118–121. doi: 10.1016/j.ijscr.2014.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan B.E., Chia Y.W., Sum C.L.L. Global haemostatic tests in rapid diagnosis and management of COVID-19 associated coagulopathy in acute limb ischaemia. J Thromb Thrombolysis. 2020;50:292–297. doi: 10.1007/s11239-020-02165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowles L., Platton S., Yartey N. Lupus anticoagulant and abnormal coagulation tests in patients with COVID-19. N Engl J Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with COVID-19. J Thromb Haemost. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]