Highlights
-
•
PM produced oxidative stress in gastric, liver, and kidney tissue.
-
•
PM-induced toxicity was mediated by ROS and mitochondrial dysfunction.
-
•
PM enhanced the activated caspase-3 expression.
-
•
CoQ10 alleviates PM-induced gastropathy and hepato-renal damage.
-
•
CoQ10 might be effective in COVID-19 treatment regimen.
Keywords: NSAIDs, Nephrotoxicity, Hepatotoxicity, Gastric ulcer, Oxidative stress, COVID-19
Abstract
Piroxicam (PM) is an oxicam-NSAID commonly recommended for various pain and associated inflammatory disorders. However, it is reported to have a gastric and hepato-renal toxic effect. Therefore, the current research was planned to investigate the possible mechanisms behind the mitigating action of the coenzyme (CoQ10), a natural, free radical scavenger, against PM tissue injury. Rats were assigned to five equal groups; Control, CoQ10 (10 mg/kg, orally), PM (7 mg/kg, i.p.), CoQ + PM L, and CoQ + PM H group. After 28 days, PM provoked severe gastric ulceration and marked liver and kidney damage indicated by an elevated gastric ulcer index and considerable alteration in liver and kidney biochemical tests. The toxic effects might be attributed to mitochondrial dysfunction and excess generation of reactive oxygen species (ROS), as indicated by enhanced malondialdehyde (MDA) levels along with decreased reduced-glutathione (GSH) levels and catalase (CAT) activity. Apoptotic cell death also was demonstrated by increased regulation of activated caspase-3 in the stomach, liver, and kidney tissues. Interestingly, external supplementation of CoQ10 attenuated the PM-inflicted deleterious oxidative harm and apoptosis. This ameliorative action was ascribed to the free radical scavenging activity of CoQ10.
1. Introduction
Piroxicam (PM) is a frequently used NSAID and belongs to oxicam class, which is recommended for use in several painful and inflammatory cases, such as rheumatoid arthritis, osteoarthritis, postoperative, post-traumatic inflammation, and other musculoskeletal disorders [1]. Lately, PM also has been used as an effective therapy for cancers. In spite of the beneficial effects of PM, adverse effects limit its use [2]. It primarily works by quelling the prostaglandins, prostacyclins, and thromboxanes genesis via non-selective suppression of cyclooxygenases (COX-I and COX-II). Prostacyclin and prostaglandins E2 have a cytoprotective impact because they reduce gastric mucosal ulceration through the reduction of gastric acid secretions and direct vasodilatation of gastric mucosal vessels. Also, prostanoids induce viscid mucus secretion, which acts as a safeguarding barrier against gastric ulceration [3]. Likewise, prostaglandins are involved in regulating renal blood flow such that acute or chronic intoxication with PM may affect kidney function [4]. Because the liver is the primary organ of drug biotransformation, it is more liable to PM toxicity [5], and in particular, because PM is metabolized in the liver [6]. Consistently, previous studies, including ours, have reported potential gastric mucosal, hepatic, and renal damage in response to PM insult [1,[6], [7], [8]].
Moreover, accumulative evidence has indicated that oxidative stress and disruption of cellular redox homeostasis are involved in PM-induced gastric ulceration and hepato-renal damage. This oxidative cascade can be seen as membrane lipid peroxidation (LPO), protein damage, free radical generation, mitochondrial dysfunction, DNA oxidation, and induction of apoptosis [1,7]. Oxidative damage occurs when the equilibrium of the generation and scavenging of reactive oxygen species (ROS) is disrupted [6]. The chief cellular antioxidant guarding enzymes that play pivotal roles in preserving the redox homeostasis are superoxide dismutase (SOD), glutathione peroxidase, catalase (CAT), and small intrinsic antioxidant molecules like glutathione (GSH), and coenzyme (Co) Q [[9], [10], [11], [12]]. When the endogenous antioxidant system is exhausted, the scavenging of free radicals is insufficient, which results in the initiation of deleterious consequences [6]. Therefore, increasing antioxidant potency may modify PM-inflicted oxidative harm via mitigating oxidative stress and enhancing tissue regeneration.
CoQ10 is a natural antioxidant synthesized in living organisms [13]. It is a 1, 4-benzoquinone; the "Q" indicates the quinone group, and "10" refers to the number of isoprenyl units in its tail end. CoQ10 is present primarily in high energy-requiring organs such as the liver, kidney, and heart, where mitochondria are abundant [14]. CoQ10 acts as a mobile redox agent that plays a substantial role with other molecules in conveying electrons along the mitochondrial electron transport chain, resulting in the synthesis of ATP [15]. In addition, CoQ10 is documented to have a potent free radical scavenging activity that helps to maintain the mitochondrial membrane potential and to prevent LPO, protein oxidation, and DNA damage; thus, it has the capability to conserve cell function as opposed to oxidative stress [13,15,16]. A literature survey has indicated that there is no report on the antioxidant and anti-apoptotic efficacy of CoQ10 during gastric and hepato-renal toxicity caused by PM.
Therefore, in line with this assertion, the present investigation was conducted to assess the protective properties of CoQ10 against PM-generated oxidative stress and apoptosis in the stomach, liver, and kidney and to elucidate the possible mechanisms of action. Liver and kidney biochemical tests, the ulcer index, oxidative status, histological alteration, and apoptotic marker expression (activated caspase-3) were evaluated.
2. Materials and methods
2.1. Drugs
Piroxicam, PM (Feldene®, 20 mg/ ml) was obtained from Pfizer Inc, Cairo, Egypt. Co enzyme-Q 10, CoQ10 (Coenzyme Q 10®, 30 mg) was purchased from MEPACO, Cairo, Egypt.
2.2. Experimental animals
The current research was carried out on 35 Wistar albino male rats weighing 150−180 g, and obtained from the Center of Laboratory Animal, Faculty of Veterinary Medicine, Benha University, Egypt. Prior to the experiment, the rats were acclimated for two weeks (temperature approximately 25 °C) and were given a standard laboratory commercial diet and water ad libitum. Ethical approval from the Ethics Committee of the Faculty of Veterinary Medicine, Benha University, for the study protocol and utilization of rats was obtained (Approval no BUFVTM07012019).
2.3. Experimental design
Rats were assigned into five groups of seven rats each. Group 1 (Vehicle Control), were given saline, i.p. Group 2 (CoQ10) rats received CoQ10 orally at a dose of 10 mg/kg b. wt. [17]. Group 3 (PM) rats were injected with PM intraperitoneally (i.p.) at a dose of 7 mg/kg b. wt. [7]. Group 4 (PM + CoQ10 L) rats were simultaneous administrated PM (7 mg/kg b. wt., i.p.) with CoQ10 (10 mg/kg b. wt., orally). Group 5 (PM + CoQ10 H) rats were given both PM (7 mg/kg b. wt., i.p.) and CoQ10 (20 mg/kg b. wt., orally). All compounds were given as a single dose per day for 28 consecutive days.
2.4. Sampling
At the ending of the experiment, blood samples were taken promptly from the caudal vena cava. After that, the samples intended for biochemical studies were kept at room temperature for serum isolation without anticoagulants. The stomach, liver, and kidneys were harvested and immersed in ice-cold phosphate-buffered saline (PBS).
2.5. Determination of gastric ulcer and preventative indexes
The stomach was removed, washed with 0.1 M cold PBS, and opened along the greater curvature to expose the mucosa then stapled to a corkboard for macroscopic inspection. The ulcer index (UI) was determined corresponding to the formula [18], UI = UN + US + UP × 10−1. UN is the mean value of ulcers per rat as counted using a magnifying glass. US is the mean severity score that corresponds to the following scores; 0 = no lesion; 1 = small-sized ulcers (1–2 mm); 2 = medium-sized ulcers (3–4 mm); 4 = large-sized ulcers (5–6 mm); and 5 = perforated ulcers. UP is the % of ulcerated animals. The preventive index (PI) was assessed using the following equation: UI of PM-ulcerated set - UI of PM + CoQ10 set × 10) /UI of PM-ulcerated set [19]. The incidence of gastric ulcers was assessed independently by a researcher who had no knowledge of the treatment protocol [20].
2.6. Serum biochemical studies
Serum AST, ALT [21], ALP [22], total protein [23], albumin [24], creatinine [25], and urea [26], were assessed using diagnostic kits purchased from Laboratory Biodiagnostics Co, Cairo, Egypt.
2.7. Tissue homogenate preparation for oxidative cascade evaluation
The tissues were dissected and rinsed in a PBS solution that contained 0.16 mg/mL heparin to separate any RBCs and serum clots. Tissues were homogenized using a sonicator homogenizer with 5 mL buffer (i.e., 50 mM potassium phosphate, pH 7.5 1 mM EDTA) added for each gram of tissue. Tissue homogenate aliquots were centrifuged in a refrigerated centrifuge (4000 rpm into 20 min) and kept at −80 °C until used. The oxidative status was determined using the malondialdehyde (MDA) level [27], catalase (CAT) activity [28], and reduced-glutathione (GSH) level [29] with specific diagnostic kits purchased from the Laboratory Biodiagnostic Company.
2.8. Histopathological and immunochemical examinations
The stomach, liver, and kidney were harvested and preserved in a 10 % formalin for 24 h and processed using conventional paraffin-embedding techniques. The paraffin blocks were cut into sections (5 μm thickness), mounted on glass microscope slides, deparaffinized, then stained with H&E for light microscopic inspection [30]. For immunohistochemical evaluation, tissue sections were deparaffinized and dehydrated using a gradated alcohol series. For antigen retrieval, citric acid solution heated to 80 °C, and the sections where placed in the solution for 5 min, cooled, and immersed in H2O2 (3% sol) for 10 min to inhibit endogenous peroxidases. Subsequently, a BSA (5% solution) was added as a blocking agent for 20 min. Then the sections were incubated with anti-activated caspase-3 primary monoclonal antibody overnight. The staining was visualized using an avidin-biotin complex (ABC kit, Vector Laboratories), with a one-hour incubation at approximately 37 °C. The final visualization was produced by reaction with 3,3-diaminobenzidine tetrahydrochloride (DAB), and a counterstain (Mayer's hematoxylin) was used. The immunohistochemistry data were scored as indicated in our previous report [7] using ImageJ software.
2.9. Statistical analysis
Statistical analysis was carried out using SPSS (Version 20; SPSS Inc., Chicago, USA). The differences among the groups were estimated by one-way ANOVA, and the Duncan test was used as the post hoc test. All values were expressed as mean ± SE, with a P-value of < 0.05 considered significantly different.
3. Results
3.1. Gastric ulcer and preventive indexes
Gastric UI and PI were calculated corresponding to the formulas mentioned above and described in Table 1 . Remarkably, PM led to an increase in UI (P ≤ 0.05) compared to the controls. Co-treatment with CoQ10 L and H doses markedly decreased the PM-induced ulceration (P ≤ 0.05) in a dose-dependent manner in relation to PM only exposed rats. This protection also was revealed in the high PI (74.06 %) in the CoQ10 H group and to a lower extent in the CoQ10 L group (66.98 %).
Table 1.
Ulcerative and preventive indexes after administration of PM and/or CoQ10.
| Experimental groups | Ulcer Index | Preventive Index (%) |
|---|---|---|
| Control | 0.00a | -- |
| CoQ10 | 0.00a | -- |
| PM | 24.67 ± 0.79d | -- |
| PM + CoQ10 L | 8.14 ± 0.40c | 66.98 |
| PM + CoQ10 H | 6.39 ± 0.26b | 74.06 |
All values expressed as the mean ± SE, n = 7.
A statistically significant difference (P ≤ 0.05) is indicated by superscript letters in the same row.
3.2. Biochemical study
As shown in Table 2 , the PM-exposed group exhibited liver and kidney damage, as indicated by a significantly elevated level in the serum biomarkers, including ALT, AST, ALP, creatinine, and urea. In addition, a reduction in albumin and total protein levels compared to control rats was observed. On the other hand, there was an improvement in the hepatic and renal parameters when PM was co-administrated with CoQ10. Remarkably, the high dose of CoQ10 significantly improved these parameters compared to the low dose CoQ10 treatment.
Table 2.
Levels of biochemical parameters in serum after administration of PM and/or CoQ10.
| Parameters | Experimental groups |
||||
|---|---|---|---|---|---|
| Control | CoQ10 | PM | PM + CoQ10 L | PM + CoQ10 H | |
| AST (U/L) | 89.75 ± 4.23d | 94.29 ± 3.12d | 182.82 ± 9.48a | 139.68 ± 4.41b | 114.14 ± 3.38c |
| ALT (U/L) | 22.60 ± 0.94c | 23.04 ± 1.39c | 76.74 ± 8.25a | 44.22 ± 0.98b | 32.32 ± 2.39c |
| ALP (U/L) | 206.12 ± 8.05d | 212.18 ± 12.29d | 395.70 ± 23.2a | 327.4 ± 5.94b | 269.14 ± 9.96c |
| Total protein (gm/dl) | 8.73 ± 0.10a | 8.06 ± 0.17a | 6.31 ± 0.23c | 7.33 ± 0.22b | 8.03 ± 0.13a |
| Albumin (gm/dl) | 3.40 ± 0.02a | 3.59 ± 0.08a | 2.21 ± 0.02d | 2.63 ± 0.12c | 3.09 ± 0.04b |
| Creatinine (mg/dl) | 0.73 ± 0.01c | 0.72 ± 0.02c | 0.99 ± 0.04a | 0.87 ± 0.02b | 0.76 ± 0.01c |
| Urea (mg/dl) | 19.72 ± 0.94d | 22.81 ± 1.40d | 74.02 ± 2.97a | 35.87 ± 1.39b | 27.89 ± 0.98c |
All values expressed as the mean ± SE, n = 7.
A statistically significant difference (P ≤ 0.05) is indicated by superscript letters in the same row.
3.3. Oxidative stress markers assay
Data presented in Table 3 show the beneficial effects of CoQ10 against the toxic effects of PM caused by oxidative damage in the hepatic and renal tissues. Significant elevation of MDA values with a substantial decline in GSH values and activities of CAT as a response to PM toxicity confirmed the presence of oxidative stress. PM-caused oxidative stress was significantly improved with concurrent CoQ10 treatment. In particular, the PM + CoQ10 H group displayed significant improvement in contrast to the levels observed for the PM + CoQ10 L group and a dose-dependent improvement of PM-stimulated oxidative damage by the administration of CoQ10.
Table 3.
Levels of oxidative stress markers after administration of PM and/or CoQ10.
| Parameters | Experimental groups |
||||||
|---|---|---|---|---|---|---|---|
| Organ | Control | CoQ10 | PM | PM + CoQ10 L | PM + CoQ10 H | ||
| MDA (nmol/g) | Liver | 250.52 ± 15.92d | 259.21 ± 11.99cd | 512.79 ± 26.02a | 314.20 ± 15.21cd | 389.07 ± 28.38b | |
| GSH (mg/g) | 65.85 ± 2.45a | 61.49 ± 2.89ab | 33.83 ± 2.18d | 47.73 ± 3.01c | 57.74 ± 1.53b | ||
| CAT (U/g) | 394.07 ± 13.69a | 393.50 ± 11.46a | 226.71 ± 10.13c | 311.12 ± 26.18b | 385.78 ± 33.54a | ||
| MDA (nmol/g) | Kidney | 144.43 ± 8.11d | 139.21 ± 2.90d | 296.29 ± 9.54a | 228.52 ± 5.71b | 179.96 ± 9.62c | |
| GSH (mg/g) | 73.44 ± 1.23a | 71.71 ± 1.16a | 41.56 ± 2.27d | 53.06 ± 1.51c | 64.68 ± 2.49b | ||
| CAT (U/g) | 440.55 ± 4.48a | 439.91 ± 8.42a | 311.36 ± 17.86c | 363.72 ± 6.43b | 413.74 ± 33.17ab | ||
All values are expressed as the mean ± SE, n = 7.
A statistically significant difference (P ≤ 0.05) is indicated by superscript letters in the same row.
3.4. Histopathological evaluation
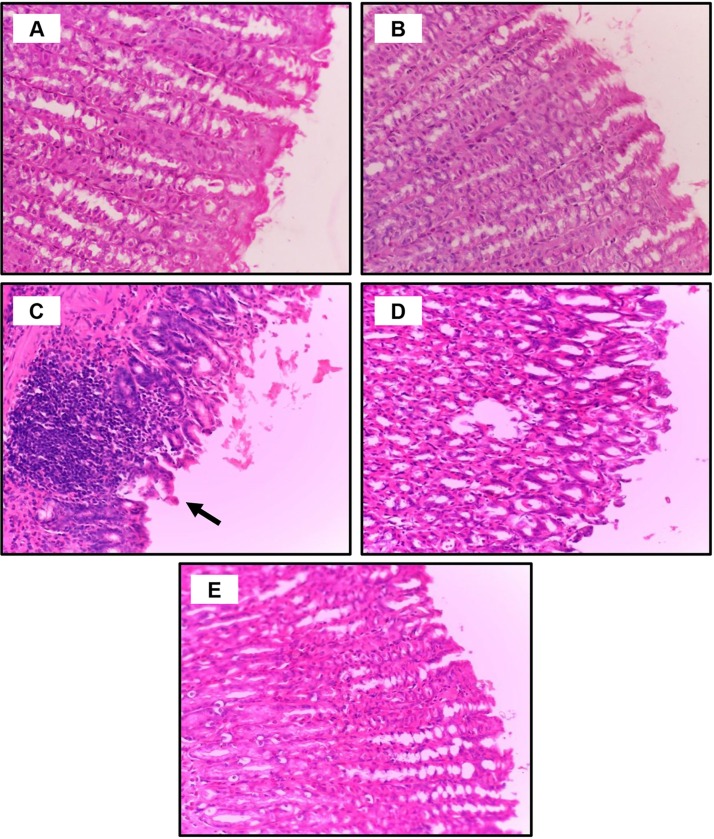
To verify the findings described above, histological alterations were observed in the gastric mucosa, liver, and kidney tissues after PM and CoQ10 treatment. In the gastric sections, as seen in Fig. 1 A and B, the control saline and CoQ10, respectively, exhibited intact gastric histological architecture. In contrast, PM treatment revealed gastric epithelial disintegration, pyknosis of chief cell nucleoli with cytoplasmic vacuolization, and inflammatory cell infiltration (Fig. 1C). In the PM and CoQ10 groups, the gastric mucosa showed minimal loss of epithelial integrity (Fig. 1D; PM CoQ10 L), the PM plus CoQ10 H mucosa displayed remarkably improved gastric histology.
Fig. 1.
Dose-dependent protective effects of CoQ10 on PM intoxication in the stomach. A: Control saline group and B: CoQ10 group, show intact gastric histology. C: PM-exposed rat shows severe disintegration of the gastric epithelial lining, degeneration of chief cells (vacuolation of cytosol with pyknotic nuclei), leucocytic infiltration, and fibrosis in the submucosal layer. D: PM + CoQ10 L group demonstrates minimal loss of gastric epithelial integrity and inflammatory infiltrate. E: PM + CoQ10 H group shows remarkable improvement in gastric architecture; H&E X400).
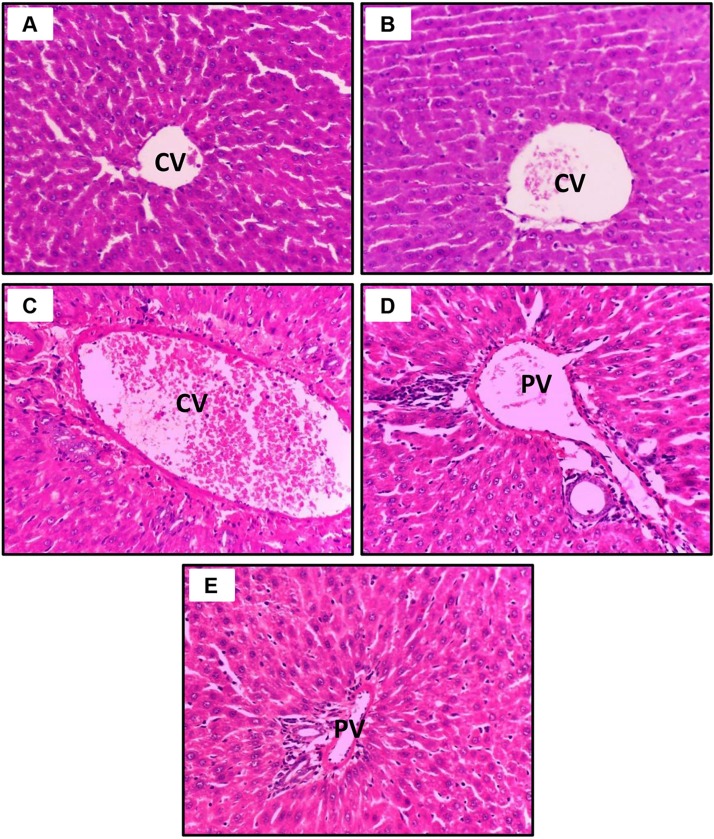
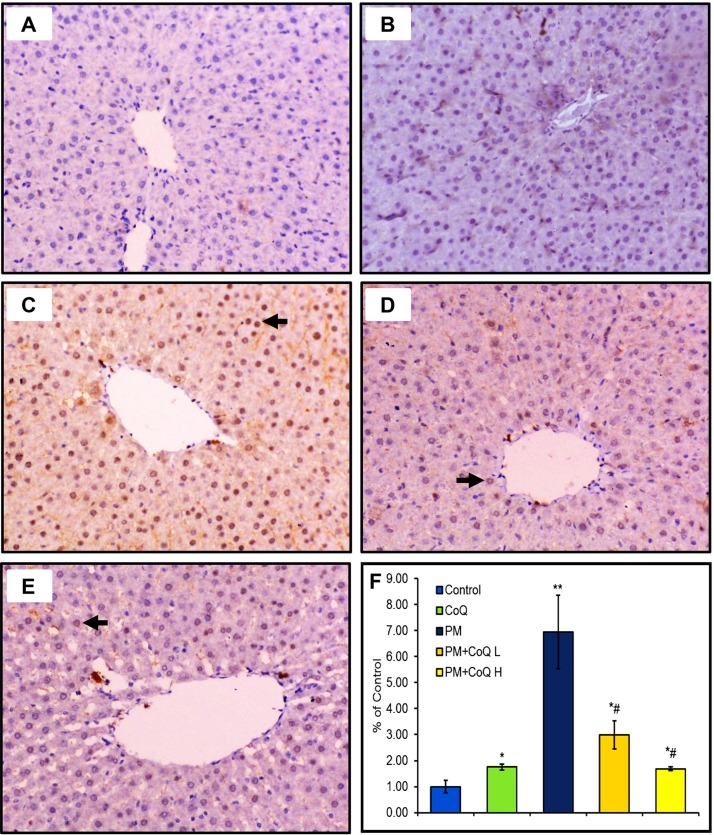
With respect to the liver sections, as seen in Fig. 2 A (control saline) and Fig. 2B (CoQ10), the hepatocytes exhibited uniform polygonal patterns with normal sinusoids and central veins. Conversely, PM treatment resulted in fatty degeneration of the liver cells with pyknotic nuclei, substantial inflammatory cell aggregation, prominent Kupffer cells, and dilation and congestion of the portal veins, pointing to the presence of severe tissue damage (Fig. 2C). In the PM + CoQ10 group, the portal area showed minimal infiltration of inflammatory cells (Fig. 2D; PM + CoQ10 L), and the PM + CoQ10 H group showed notably restored normal hepatic architecture (Fig. 2E; PM + CoQ10 H).
Fig. 2.
Dose-dependent ameliorative effects of CoQ10 on PM intoxication in the liver. A: Control saline group and B: CoQ10 group, show normal liver histology. C: PM-exposed rat shows fatty degeneration (signet cells) and vacuolation of hepatocytes, prominent Kupffer cells, and dilatation of central veins and the portal vein with severe lymphocytic infiltration. D: PM + CoQ10 L group demonstrates mild fatty degeneration and inflammatory cell infiltration. E: PM + CoQ10 H group shows remarkable improvement in liver histology (PV, portal vein; CV, central vein; H&E X400).
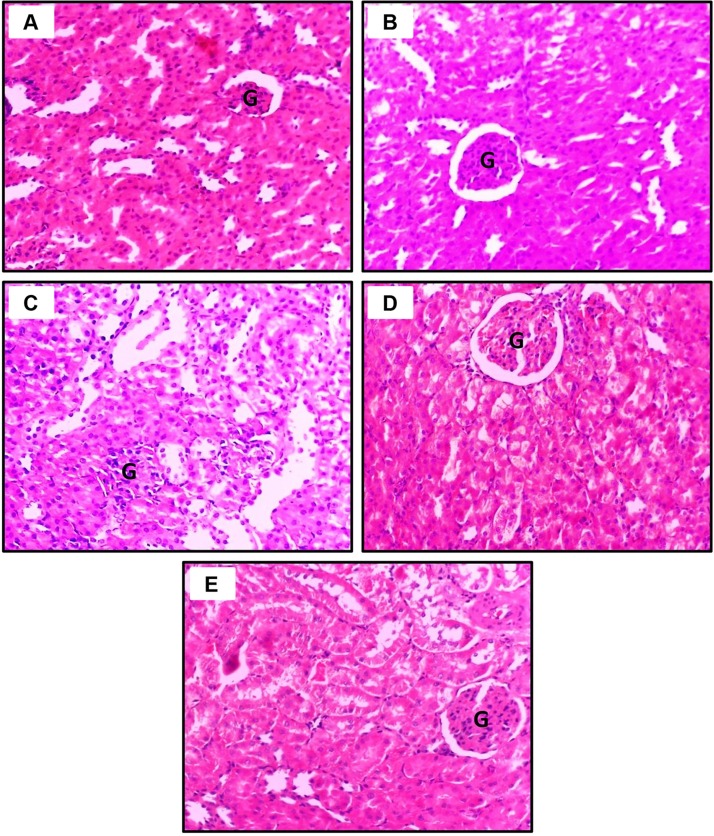
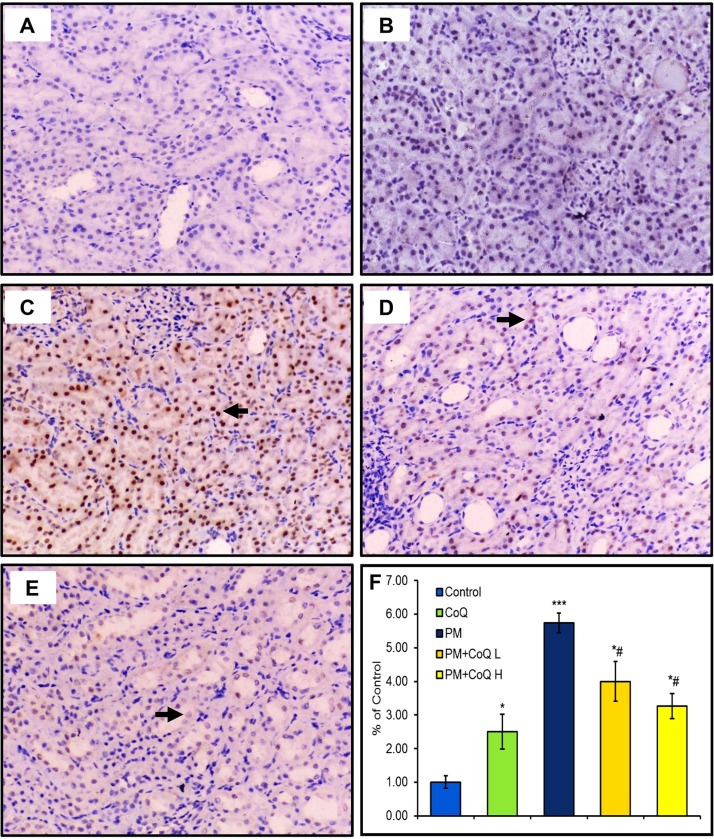
In kidney tissue sections, the control and CoQ10 groups displayed no alterations. The glomeruli and renal tubular epithelia appeared normal (Fig. 3 A and B, respectively). In contrast, in the PM-intoxicated rats, we noticed severe degradation of the proximal convoluted tubules (PCT), as indicated by the presence of tubular dilatation, epithelial degeneration, and severe loss of the brush border due to retraction or destruction of microvilli, intertubular inflammatory cellular leakage, and shrinkage and atrophy of the glomeruli. The various sections of the loop of Henle were minimally affected (Fig. 3C). Nevertheless, the concurrent treatment of PM and CoQ10 demonstrated moderate improvement of histological findings with the low dose of CoQ10 (Fig. 3D) and near-complete recovery at high CoQ10 dose (Fig. 3E).
Fig. 3.
Dose-dependent ameliorative effects of CoQ10 on PM intoxication in the kidney. A: Control Saline group and B: CoQ10 group, demonstrate normal glomerulus and renal tubules. C: PM-exposed rat exhibits proximal tubular dilatation with severe loss of the brush border, intertubular inflammatory cellular leakage with glomerular atrophy. D: PM + CoQ10 L group reveals mild leakage of inflammatory cells with damage to the tubular brush border. E: PM + CoQ10 H group shows prominent improvement in the histology of the kidney (G, glomerulus; H&E X400).
The histopathological results affirmed the biochemical data (Table 2), suggesting that CoQ10 supplementation had substantial protective influence against PM-inflicted hepatorenal injury.
3.5. Expression of activated caspase-3
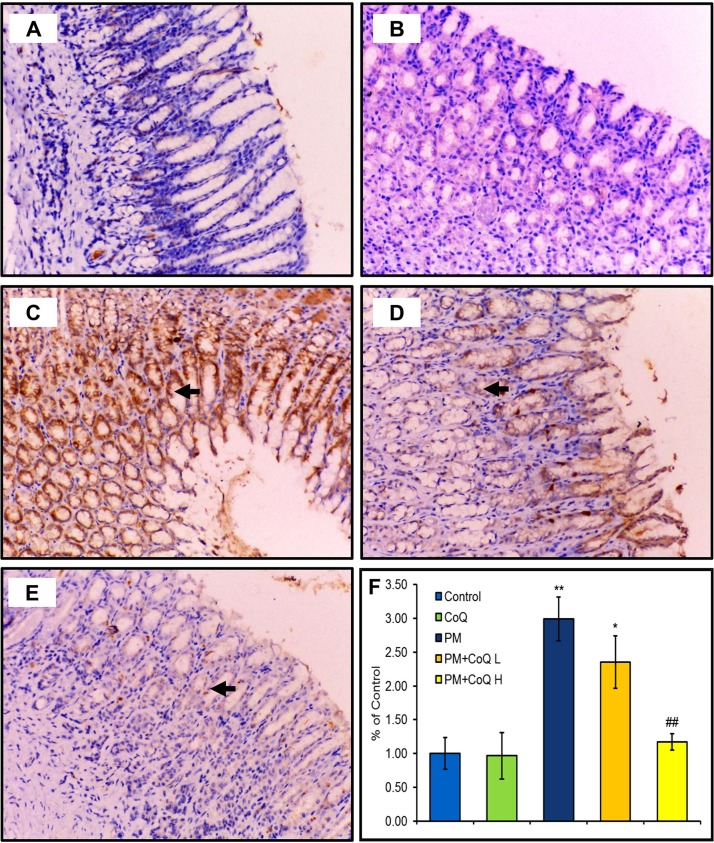
Expression of activated caspase-3 in the stomach, liver, and kidney tissues after treatment by PM and/or CoQ10 are seen in Fig. 4, Fig. 5, Fig. 6 , respectively. PM distinctly up-regulated activated caspase-3 expression in the stomach (Fig. 4C), liver (Fig. 5C), and kidney (Fig. 6C). Moreover, compared to the PM-treated rats, we observed moderate (Fig. 4, Fig. 5, Fig. 6D) and mild (Fig. 4, Fig. 5, Fig. 6E) expressions of activated caspase-3 when rats were given CoQ10 L and H doses, respectively. These findings support the hypothesis that a high dose of CoQ10 could significantly reduce PM-mediated apoptosis and enhanced expression of activated caspase-3 in gastric, renal, and hepatic tissues in a dose-dependent pattern as illustrated in Fig. 4, Fig. 5, Fig. 6F.
Fig. 4.
Effects of CoQ10 on activated caspases-3 expression after PM intoxication in the stomach. A: Control saline and B: CoQ10 groups show no positive expression of activated caspases-3. C: PM-exposed rat reveals a strong expression of caspase-3. D: PM + CoQ10 L and E: PM + CoQ10 H rats show moderate and mild expression, respectively. The positive activated caspase-3 expression is indicated by the brown color of gastric nuclei. F: Immunohistochemical scorning of the activated caspase-3 positive area. Data are expressed in means ± SE (n = 3). * and # significantly different vs control and PM group, respectively. *P ≤ 0.05, **P ≤ 0.01, and ## P ≤ 0.01.
Fig. 5.
Effects of CoQ10 on activated caspases-3 expression after PM intoxication in the liver. A: Control saline and B: CoQ10 groups show no positive expression of activated caspases-3. C: PM-exposed rat reveals a strong expression of caspase-3. D: PM + CoQ10 L and E: PM + CoQ10 H show moderate and mild expression, respectively. The positive activated caspase-3 expression is indicated by the brown color of the hepatic nuclei. F: Immunohistochemical scorning of the activated caspase-3 positive area. Data are expressed in means ± SE (n = 3). * and # significantly different vs control and PM group, respectively. *P ≤ 0.05, **P ≤ 0.01, and # P ≤ 0.05.
Fig. 6.
Effects of CoQ10 on activated caspases-3 expression after PM intoxication in the kidney. A: Control saline and B: CoQ10 groups show no positive expression of activated caspases-3. C: PM-exposed rat reveals a strong expression of caspase-3. D: PM + CoQ10 L and E: PM + CoQ10 H show moderate and mild expression, respectively. The positive activated caspase-3 expression is indicated by the brown color of renal nuclei. F: Immunohistochemical scorning of the activated caspase-3 positive area. Data are expressed in means ± SE (n = 3). * and # significantly different vs control and PM group, respectively. *P ≤ 0.05, ***P ≤ 0.001, and # P ≤ 0.05.
4. Discussion
PM is a classical NSAID in the oxicam category. It is extensively used and possesses anti-inflammatory, analgesic, antipyretic, and antirheumatic properties. Lately, PM also has been investigated as an effective therapy for cancer [1]. Despite its long clinical success, growing evidence has shown that PM can produce adverse effects on the stomach, liver, and kidney. Besides its nonselective-inhibitory effect on COX enzymes, a considerable amount of literature has suggested that the toxicity of PM works through oxidative stress to produce its damaging effects on the stomach, liver, and kidney [1,5,7,[31], [32], [33], [34], [35]]. Oxidative stress is a process that is initiated through well-known ROS (superoxide anion, O2• −; hydrogen peroxide, H2O2; hydroxyl radical, OH•) and reactive nitrogen species, RNS (nitric oxide, NO and peroxynitrite, ONOO−), which are up-regulated due to exhaustion of endogenous antioxidant molecules. Subsequently, tissue damage is elicited by several mechanisms, including the promotion of lipid peroxidation (LPO), mitochondrial perturbation, DNA damage, protein nitration, and apoptotic cell death [[36], [37], [38], [39], [40]].
Consequently, the current study revealed the existence of considerable oxidative damage as indicated by prominent reductions in GSH concentrations and CAT activities in PM-treated rats. It is well known that considerable quantities of OH• are produced from H2O2 (by Fenton's reaction) when CAT is depleted [41]. OH• is the most harmful radical among ROS because it causes membrane damage by direct breaking down the membrane lipid content and initiating LPO and producing MDA [11,36,42]. This process was confirmed in the present study by the dramatic increase in MDA in the liver and kidney, which confirmed the occurrence of membrane damage. That loss of membrane integrity possibly contributed to seeping out of AST, ALT, and ALP enzymes that caused the elevated levels that were detected in the serum (Table 2). These findings are in the same vein with the previous reports [1,6,16]. In other ways, the histopathological examination affirms the existence of LPO in the renal cell membrane, as revealed by the disintegrated brush border (Fig. 3C), which participated in tubular impairment and indicated by a significant rise in serum urea and creatinine levels. It has been confirmed that the enhanced generation of ROS damages mitochondria, the endoplasmic reticulum, and DNA, resulting in altered protein synthesis and gene expression. These events lead to massive necrosis of the liver and disturbances of liver function, as indicated in our study by a considerable decline in serum albumin and, consequently, total protein content. Impaired tubular reabsorption was another possible reason for the increased protein loss [43,44]. Likewise, this could be due to gastric ulceration and bleeding that resulted in malabsorption and decreased nourishment intake [5]. On the other side, direct ROS-induced protein damage was implicated in the reduced activity and synthesis of antioxidant enzymes [45,46].
Make matters worse, MDA is a hazardous molecule that can influence other distant cellular molecules and cause DNA and protein damage. Added to that, regrettably, renal PCT is dissimilar to the other segments of the nephron and depends on circulating GSH rather than synthesizing their own GSH [38]. Intriguingly, PM is a potent chelator of selenium, iron, zinc, copper, and manganese that function as cofactors for different cellular antioxidant components, and for that reason, PM could have a significant negative impact on their scavenging activity [7,46,47].
PM is a preferential COX-I inhibitor that plays a principal role in regulating the glomerular filtration rate and renal hemodynamics. The synthesis of the vasodilator prostaglandins is suppressed, leading to a reduction of the renal blood flow and increased risk of renal ischemia [2,3,48,49]. As a consequence of a renal ischemic insult, mitochondrial bioenergetic disruption takes place, and pathological levels of ROS are generated [16,39,50]. Besides, the current histopathological examination, which revealed gastric ulceration, severe hepatic and renal damage also were observed in response to PM insult. Based on the above-mentioned reports, it is postulated that progressive PM-induced oxidative damage occurred as a result of a cascade of such events.
In the current research, PM exposure resulted in severe gastric ulceration, as revealed by the high UI. The histopathological investigation confirmed marked erosions in the gastric wall lining epithelia with exudes consisting of large polymorphonuclear cells. Thus, our findings suggested that the ulcerogenic gastropathy attributed to the destruction of the gastric mucosal lining was the main target of PM as it caused suppression of prostaglandins, which play a pivotal role in the protection of the gastric mucosa [2]. The destruction of the gastric mucosa was accompanied by induction of lipid peroxidation, DNA oxidation, and damage to stomach cytoskeletal proteins, as demonstrated by enhanced expression of activated caspase-3 in the PM-exposed group. These results are consistent with those obtained from prior studies [7,35,51,52].
In the current investigation, the immunostaining data using the apoptotic marker (activated caspase-3) emphasized that the PM-induced oxidative stress disrupted mitochondrial functions as well as their transmembrane potential, leading to cytochrome-c translocation and induction of programmed cell death. These data are consistent with previous reports from our and other labs [7,38,[53], [54], [55]].
Since mitochondria are abundant in the proximal segment of the nephron and required for the tremendous production of ATP consumed during the massive active transport via Na+/K+ ATPase, the cortical region of the kidney is proposed to be the most vulnerable area for oxidative harm in the kidney [38,39]. In the present research, the histopathological and immunostaining data indicated pronounced injury in the cortical area of the kidney (Fig. 3, Fig. 6) compared to other kidney regions. These findings support our previous work, in which the cortex showed more damage due to oxidative stress in animal models of PM toxicity [7,35], acetaminophen [56], puromycin [57], gentamicin [58], cadmium [44], and mercury [59]. Taken all together, we strongly suggest that the overwhelming toxic effect of PM was mainly associated with mitochondrial dysfunction.
CoQ10 naturally exists as a component of the electron transport chain, where it conveys electrons of complexes I and II to complex III, a process required for energy production [38,60,61]. CoQ10 is considered to be a unique mitochondrial antioxidant, since it suppresses the NADPH oxidase expression, a great source of O2 • − [38], inhibits excess NO production [62], and protects against LPO, protein oxidation, and DNA damage [60]. On the basis of this regard, pretreatment with CoQ10 conferred marked protection against PM-induced oxidative injury, as demonstrated by considerable, dose-dependent improvement in the histopathology and biochemical parameters. These changes might be due to marked enhancement of CAT activities and levels of GSH as well as decreased LPO as a result of the ROS-quenching activity of CoQ10. Our findings concur with those obtained by Khalifa et al. [63] who studied the prophylactic effect of CoQ10 against cisplatin-induced kidney injury. In that study, CoQ10 reduced the cisplatin-induced LPO and restored the decreased level of GSH and activities of CAT and SOD in renal tissue. Also showing the beneficial antioxidant power of CoQ10, another related study was conducted by Eftekhari et al. [64]. This study demonstrated the potential role of CoQ10 in attenuating dichlorvos-induced oxidative damage and mitochondrial dysfunction in hepatic tissue. That protective action was consistent with ours. Thus, CoQ10 suppressed the generated ROS and MDA, along with restoring the GSH levels.
Notably, co-administration of CoQ10 down-regulated the activated caspase-3, which demonstrated its anti-apoptotic activity. The anti-apoptotic activity was exhibited by CoQ10 as it inhibits the opening of the mitochondrial permeability transition pore, which regulates the perturbation of the electrochemical gradient across the mitochondrial membrane [53]. Our results agree with previous investigations that demonstrated CoQ10 protects against chronic kidney disease [65] and optic nerve degeneration [16]. Fig. 7 illustrates the proposed mechanisms of the protective effect of CoQ10 against PM-mediated gastropathy and hepato-renal toxicity.
Fig. 7.
Diagrammatic scheme summarizes the proposed mechanisms of the protective effect of CoQ10 against PM-mediated gastric and hepato-renal toxicity.
5. Conclusions
PM caused gastric, liver, and kidney damage as indicated by alterations in serum biochemistry and changes in histopathological features. This study suggested that mitochondrial dysfunction was the primary mode of action due to PM-induced ROS overproduction, LPO, DNA damage, and apoptosis. It is important to note that treatment with CoQ10 abrogated the PM-inflicted gastric and hepato-renal toxicity in a dose-dependent pattern. We suggest that CoQ10 could improve the therapeutic potential of PM through mitigating its side effects as well as improving the antioxidant state in comorbid conditions. Currently, the COVID-19 pandemic would a good example where CoQ10 supplementation might have a promising therapeutic potential in lowering the deleterious action of COVID-19 on gastric, liver, and kidney tissue and the resulting complex treatment regimen.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors appreciate the Support & Development of Scientific Research Center, Benha University, Egypt, that helped with the implementation of this work. Authors would like to extend their sincere appreciation to King Saud University, Riyadh, Saudi Arabia for financially supporting this work through the Researchers Supporting Projectnumber (RSP-2020/19). The authors are also gratefully thanking Prof. Louise Abbott (Texas A&M University, USA) for techincal and English language editing support.
References
- 1.Sahu C.R. Mechanisms involved in toxicity of liver caused by piroxicam in mice and protective effects of leaf extract of Hibiscus rosa-sinensis L. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016;9:9–13. doi: 10.4137/CMAMD.S29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichstadt L.R., Moore G.E., Childress M.O. Risk factors for treatment-related adverse events in cancer-bearing dogs receiving piroxicam. Vet. Comp. Oncol. 2017;15:1346–1353. doi: 10.1111/vco.12276. [DOI] [PubMed] [Google Scholar]
- 3.Vane J.R., Botting R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998;2:78–87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 4.Flomenbaum N., Goldfrank L., Hoffman R., Howland M.A., Lewin N., Nelson L. 2006. Goldfranks Toxicologic Emergencies 8e. [Google Scholar]
- 5.Orinya O.A., Adenkola A.Y., Ogbe R.J. Haematological and biochemical studies on the effect of diclofenac sodium on Wistar Rattus norvegicus. Int. J. Biol. Chem. Sci. 2016;10:2231–2242. [Google Scholar]
- 6.Badawi M.S. Histological study of the protective role of ginger on piroxicam-induced liver toxicity in mice. J. Chin. Med. Assoc. 2018;82:11–18. doi: 10.1016/j.jcma.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Abdeen A., Aboubakr M., Elgazzar D., Abdo M., Abdelkader A., Ibrahim S., Elkomy A. Rosuvastatin attenuates piroxicam-mediated gastric ulceration and hepato-renal toxicity in rats. Biomed. Pharmacother. 2019;110:895–905. doi: 10.1016/j.biopha.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Ebaid H., Dkhil M., Danfour M., Tohamy A., Gabry M. Piroxicam-induced hepatic and renal histopathological changes in mice. Libyan J. Med. 2007;2:82–89. doi: 10.4176/070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ognjanović B.I., Marković S.D., Dordević N.Z., Trbojević I.S., Štajn A.Š., Saičić Z.S. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: protective role of coenzyme Q10 and Vitamin E. Reprod. Toxicol. 2010;29:191–197. doi: 10.1016/j.reprotox.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Avery S.V. Molecular targets of oxidative stress. Biochem. J. 2011;434:201–210. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Daim M.M., Abdeen A. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem. Toxicol. 2018;114:69–77. doi: 10.1016/j.fct.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Daim M.M., Eissa I.A.M., Abdeen A., Abdel-Latif H.M.R., Ismail M., Dawood M.A.O., Hassan A.M. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2019;69:44–50. doi: 10.1016/j.etap.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Ulla A., Mohamed M.K., Sikder B., Rahman A.T., Sumi F.A., Hossain M., Reza H.M., Rahman G.M.S., Alam M.A. Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol. Toxicol. 2017;18:29. doi: 10.1186/s40360-017-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J., Hong Y.S., Jeong J.H., Yang E.J., Jhun J.Y., Park M.K., Jung Y.O., Min J.K., Kim H.Y., Park S.H., La Cho M. Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines. PLoS One. 2013;8:4–11. doi: 10.1371/journal.pone.0069362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhagavan H.N., Chopra R.K. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 16.Noh Y.H., Kim K., Shim M.S., Choi S., Choi S., Ellisman M.H., Weinreb R.N., Perkins G.A., Ju W. 2013. Inhibition of Oxidative Stress by Coenzyme Q10 Increases Mitochondrial Mass and Improves Bioenergetic Function in Optic Nerve Head Astrocytes; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen P.Y., Hou C.W., Shibu M.A., Day C.H., Pai P., Liu Z.R., Lin T.Y., Viswanadha V.P., Kuo C.H., Huang C.Y. Protective effect of co-enzyme Q10 on doxorubicin-induced cardiomyopathy of rat hearts. Environ. Toxicol. 2017;32:679–689. doi: 10.1002/tox.22270. [DOI] [PubMed] [Google Scholar]
- 18.Hollander D., Wski A.T.A., Krause W.J., Gergely H. 1985. Protective Effect of Sucralfate Against Alcohol-Induced Gastric Mucosal Injury Functional Time Sequence Analysis. [DOI] [PubMed] [Google Scholar]
- 19.Hano J., Bugajski J., Danek L., Wantuch C. The effect of neuroleptics on the development of gastric ulcers in rats exposed to restraint-cold stress. Pol. J. Pharmacol. Pharm. 1976;28:37–47. [PubMed] [Google Scholar]
- 20.Das D., Banerjee R.K. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol. Cell. Biochem. 1993;125:115–125. doi: 10.1007/BF00936440. [DOI] [PubMed] [Google Scholar]
- 21.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 22.Tietz N.W., Burtis C.A., Duncan P., Ervin K., Petitclerc C.J., Rinker A.D., Shuey D., Zygowicz E.R. A reference method for measurement of alkaline phosphatase activity in human serum. Clin. Chem. 1983;29:751–761. [PubMed] [Google Scholar]
- 23.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Doumas B.T., Watson W.A., Biggs H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 25.Larsen K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta. 1972;41:209–217. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- 26.Coulombe J.J., Favreau L. A new simple semimicro method for colorimetric determination of urea. Clin. Chem. 1963;9:102–108. [PubMed] [Google Scholar]
- 27.Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 28.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 29.Beutler E. Improved method for determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 30.Suvarna K.S., Layton C. 2012. Bancroft’s Theory and Practice of Histological Techniques E-Book: Elsevier Health Sciences. [Google Scholar]
- 31.Benazir S., Ghosh D., Chattyopadhyay A. Protective effect of antioxidant rich aqueous curry leaf (Murraya koenigii) extract against gastro-toxic effects of piroxicam in male Wistar rats. Toxicol. Rep. 2014;1:987–1003. doi: 10.1016/j.toxrep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villegas I., De la Lastra C.A., La Casa C., Motilva V., Martín M.J. Effects of food intake and oxidative stress on intestinal lesions caused by meloxicam and piroxicam in rats. Eur. J. Pharmacol. 2001;414:79–86. doi: 10.1016/S0014-2999(00)00883-9. [DOI] [PubMed] [Google Scholar]
- 33.Bandyopadhyay D., Ghosh G., Bandyopadhyay A., Reiter R.J. Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 2004;36:195–203. doi: 10.1111/j.1600-079X.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- 34.Arunachalam K., Balogun S.O., Pavan E., de Almeida G.V.B., de Oliveira R.G., Wagner T., Cechinel Filho V., de Oliveira Martins D.T. Chemical characterization, toxicology and mechanism of gastric antiulcer action of essential oil from Gallesia integrifolia (Spreng.) harms in the in vitro and in vivo experimental models. Biomed. Pharmacother. 2017;94:292–306. doi: 10.1016/j.biopha.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 35.Abdeen A., Abou-Zaid O.A., Abdel-Maksoud H.A., Aboubakr M., Abdelkader A., Abdelnaby A., Abo-Ahmed A.I., El-Mleeh A., Mostafa O., Abdel-Daim M., Aleya L. Cadmium overload modulates piroxicam-regulated oxidative damage and apoptotic pathways. Environ. Sci. Pollut. Res. 2019;26:25167–25177. doi: 10.1007/s11356-019-05783-x. [DOI] [PubMed] [Google Scholar]
- 36.Ozbek E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012;2012 doi: 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palipoch Sarawoot. 2013. A Review of Oxidative Stress in Acute Kidney Injury_ Protective Role of Medicinal Plants-Derived Antioxidants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxidants Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galvan D.L., Green N.H., Danesh F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–1057. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdel-daim M.M., Sayed A.A., Abdeen A., Aleya L., Ali D., Alkahtane A.A., Alarifi S., Alkahtani S. Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-LR-Induced hepatotoxicity and neurotoxicity in mice. Oxid. Med. Cell. Longev. 2019;2019:1–10. doi: 10.1155/2019/1309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed W., Zaki A., Nabil T. Prevention of methotrexate-induced nephrotoxicity by concomitant administration of garlic aqueous extract in rat. Turk. J. Med. Sci. 2015:507–516. doi: 10.3906/sag-1408-121. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-daim M.M., Sayed A.A., Abdeen A., Aleya L., Ali D., Alkahtane A.A., Alarifi S., Alkahtani S. Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-LR-Induced hepatotoxicity and neurotoxicity in mice. Oxid. Med. Cell. Longev. 2019;2019:1–10. doi: 10.1155/2019/1309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dwivedi V. Efficacy study of livartho against paracetamol induced hepatotoxicity in adult Sprague Dawley rats. J. Drug Metab. Toxicol. 2015;5 doi: 10.4172/2157-7609.1000175. [DOI] [Google Scholar]
- 44.Abdeen A., Ghonim A., El-Shawarby R., Abdel-Aleem N., El-Shewy E., Abdo M., Abdelhiee E. Protective effect of cinnamon against cadmium-induced hepatorenal oxidative damage in rats. Int. J. Toxicol. 2017;5:17. doi: 10.14419/ijpt.v5i1.7119. [DOI] [Google Scholar]
- 45.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morsy G.M., Abou el-Ala K.S., Ali A.A. Studies on fate and toxicity of nanoalumina in male albino rats: oxidative stress in the brain, liver and kidney. Toxicol. Ind. Health. 2016;32:200–214. doi: 10.1177/0748233713498462. [DOI] [PubMed] [Google Scholar]
- 47.El-Gamel N.E.A. The interactions of metal ions with nonsteroidal anti-inflammatory drugs (oxicams) J. Coord. Chem. 2009;62:2239–2260. doi: 10.1080/00958970902822630. [DOI] [Google Scholar]
- 48.Pehlİvan B., Cuvaş Ö., Başar H., Bakir F., Üstün H., Dİkmen B. Comparison of the effects of repeated dose treatments of lornoxicam and meloxicam on renal functions in rats. Turk. J. Med. Sci. 2010;40:371–376. doi: 10.3906/sag-0806-7. [DOI] [Google Scholar]
- 49.Fatima S., Alsheikh Y.A., Noura H. Vol. 10. 2013. Almohaimeed, protective effect of coenzyme Q10 (CoQ10) and Epigallocatechin gallate (Eccg) against cisplatin-induced acute renal failure in rats; pp. 95–98. (2nd Int. Conf. Biosci. Biochem. Pharm. Sci.). [Google Scholar]
- 50.Plotnikov E.Y., Chupyrkina A.A., Jankauskas S.S., Pevzner I.B., Silachev D.N., Skulachev V.P., Zorov D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta - Mol. Basis Dis. 2011;1812:77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Zhen Y.-Y., Libotte T., Munck M., a Noegel A., Korenbaum E. Nuance, a giant protein connecting the nucleus and actin cytoskeleton. J. Cell. Sci. 2002;115:3207–3222. doi: 10.1242/JCS.01642. [DOI] [PubMed] [Google Scholar]
- 52.Bandyopadhyay D., Chattopadhyay A. Reactive oxygen species-induced gastric ulceration: protection by melatonin. Curr. Med. Chem. 2006;13:1187–1202. doi: 10.2174/092986706776360842. [DOI] [PubMed] [Google Scholar]
- 53.Papucci L., Schiavone N., Witort E., Donnini M., Lapucci A., Tempestini A., Formigli L., Zecchi-Orlandini S., Orlandini G., Carella G., Brancato R., Capaccioli S. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J. Biol. Chem. 2003;278:28220–28228. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 54.El-Sheikh A.A.K., Morsy M.A., Mahmoud M.M., Rifaai R.A., Abdelrahman A.M. Effect of coenzyme-Q10 on doxorubicin-induced nephrotoxicity in rats. Adv. Pharmacol. Sci. 2012;2012 doi: 10.1155/2012/981461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmadian E., Eftekhari A., Babaei H., Nayebi A.M., Eghbal M.A. Anti-cancer effects of citalopram on hepatocellular carcinoma cells occur via cytochrome C release and the activation of NF-kB. Anticancer Agents Med. Chem. 2017;17:1570–1577. doi: 10.2174/1871520617666170327155930. [DOI] [PubMed] [Google Scholar]
- 56.Abdeen A., Abdelkader A., Abdo M., Wareth G., Aboubakr M., Aleya L., Abdel-Daim M. Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ. Sci. Pollut. Res. 2019;26:240–249. doi: 10.1007/s11356-018-3553-2. [DOI] [PubMed] [Google Scholar]
- 57.Abdeen A., Sonoda H., Kaito A., Oshikawa-Hori S., Fujimoto N., Ikeda M. Decreased excretion of urinary exosomal aquaporin-2 in a puromycin aminonucleoside-induced nephrotic syndrome model. Int. J. Mol. Sci. 2020;21:1–12. doi: 10.3390/ijms21124288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdeen A., Sonoda H., El-Shawarby R., Takahashi S., Ikeda M. Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am. J. Physiol. Renal Physiol. 2014;307:F1227–1237. doi: 10.1152/ajprenal.00140.2014. [DOI] [PubMed] [Google Scholar]
- 59.Waheed R., El Asely A.M., Bakery H., El-Shawarby R., Abuo-Salem M., Abdel-Aleem N., Malhat F., Khafaga A., Abdeen A. Thermal stress accelerates mercury chloride toxicity in Oreochromis niloticus via up-regulation of mercury bioaccumulation and HSP70 mRNA expression. Sci. Total Environ. 2020;718 doi: 10.1016/j.scitotenv.2020.137326. [DOI] [PubMed] [Google Scholar]
- 60.Pravst I., Žmitek K., Žmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 2010;50:269–280. doi: 10.1080/10408390902773037. [DOI] [PubMed] [Google Scholar]
- 61.Hernández-Camacho J.D., Bernier M., López-Lluch G., Navas P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 2018;9:1–11. doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdel-rahman G.H. Protective effect of coenzyme Q10 on cadmium-induced testicular damage in male rabbits. Biol. Am.-Eur. J. Toxicol. Sci. 2011;3:153–160. [Google Scholar]
- 63.Khalifa E.A., Nabil Ahmed A., Hashem K.S., Allah A.G. Therapeutic effects of the combination of alpha-lipoic acid (ALA) and coenzyme Q10 (CoQ10) on cisplatin-induced nephrotoxicity. Int. J. Inflam. 2020;2020 doi: 10.1155/2020/5369797. [DOI] [Google Scholar]
- 64.Eftekhari A., Ahmadian E., Azami A., Johari-Ahar M., Eghbal M.A. Protective effects of coenzyme Q10 nanoparticles on dichlorvos-induced hepatotoxicity and mitochondrial/lysosomal injury. Environ. Toxicol. 2018;33:167–177. doi: 10.1002/tox.22505. [DOI] [PubMed] [Google Scholar]
- 65.Ishikawa A., Kawarazaki H., Ando K., Fujita M., Fujita T., Homma Y. Renal preservation effect of ubiquinol, the reduced form of coenzyme Q10. Clin. Exp. Nephrol. 2011;15:30–33. doi: 10.1007/s10157-010-0350-8. [DOI] [PubMed] [Google Scholar]