Key Points
Question
Are current cochlear implant systems effective when provided to newly implanted Centers for Medicare & Medicaid Services beneficiaries who demonstrate a preoperative sentence recognition score of 41% to 60% in their best-aided condition?
Findings
In this multicenter nonrandomized trial of 31 participants 65 years or older with moderate to profound hearing loss who received cochlear implants, significant improvement in sentence recognition was observed both in the implant ear alone and in the best-aided condition when preoperative and postoperative sentence recognition scores were compared. In addition, significant improvements were noted for some scores on questionnaires dealing with device benefit and health utility.
Meaning
Current cochlear implant systems are appropriate for Centers for Medicare & Medicaid Services beneficiaries who demonstrate preoperative best-aided sentence scores that fall between 41% and 60%.
Abstract
Importance
Current indications for Medicare beneficiaries to receive a cochlear implant are outdated. Multichannel cochlear implant systems may be effective when provided to Medicare beneficiaries using expanded indications.
Objective
To examine the effectiveness of cochlear implants, as measured by improvement on the AzBio Sentence Test, for newly implanted Medicare beneficiaries who meet the expanded indications of an AzBio Sentence Test score of 41% to 60% in their best-aided condition.
Design, Setting, and Participants
A multicenter nonrandomized trial examined preoperative and postoperative speech recognition, telephone communication, hearing device benefit, health utility, and quality of life for 34 participants enrolled at 8 different centers who received a cochlear implant between September 17, 2014, and July 10, 2018. All participants were 65 years or older, had bilateral moderate to profound hearing loss, and had a best-aided preoperative AzBio Sentence Test score in quiet of 41% to 60%. Analysis was performed on an intention-to-treat basis. Statistical analysis of final results took place from July 29 to October 1, 2019.
Intervention
Multichannel cochlear implants.
Main Outcomes and Measures
The study examined the a priori hypothesis that the cochlear implant would improve the AzBio Sentence Test score in the best-aided condition by 25% or more and in the implanted ear–alone condition by 30% or more. The study additionally examined word and telephone recognition and examined device benefit, health utility, and quality of life.
Results
A total of 34 participants received a cochlear implant; 31 (23 men [74%]; median age, 73.6 years [range, 65.7-85.1 years]) completed testing through the 6-month evaluation, and 29 completed testing through the 12-month evaluation. Median preoperative AzBio Sentence Test scores were 53% (range, 26%-60%) for the best-aided condition and 24% (range, 0%-53%) for the cochlear implant–alone condition; median scores 12 months after implantation improved to 89% (range, 36%-100%) for the best-aided condition and 77% (range, 13%-100%) for the cochlear implant–alone condition. This outcome represents a median change of 36% (range, –22% to 75%) for the best-aided condition (lower bound of 1-sided 95% CI, 31%) and a median change of 53% (range, –15% to 93%) for the cochlear implant–alone condition (lower bound of 1-sided 95% CI, 45%).
Conclusions and Relevance
Intervention with a cochlear implant was associated with improved sentence, word, and telephone recognition in adult Medicare beneficiaries whose preoperative AzBio Sentence Test scores were between 41% and 60%. These findings support expansion of the Center for Medicare & Medicaid current indications for cochlear implants.
Trial Registration
ClinicalTrials.gov Identifier: NCT02075229
This multicenter nonrandomized clinical trial examines the effectiveness of cochlear implants, as measured by improvement on the AzBio Sentence Test, for newly implanted Medicare beneficiaries who meet the expanded indications of an AzBio Sentence Test score of 41% to 60% in their best-aided condition.
Introduction
Several clinical trials have clearly demonstrated that cochlear implants are a safe and effective treatment for significant hearing loss in children,1,2 adults,3 and elderly individuals.4,5,6 In addition, many researchers have specifically demonstrated the effectiveness of this treatment in Medicare-eligible recipients, who typically include US citizens older than 65 years.7,8 Such treatment is particularly important for Medicare beneficiaries because hearing loss has been associated with depression, social isolation, and a subjective decrease in well-being in elderly individuals.9,10,11,12,13
Medicare indications for a cochlear implant have been in existence since 2005 and state the following: “The evidence is adequate to conclude that cochlear implantation is reasonable and necessary for treatment of bilateral pre-or postlinguistic, sensorineural, moderate to profound hearing loss in individuals who demonstrate limited benefit from amplification. Limited benefit from amplification is defined by test scores of less than or equal to 40% correct in the best-aided listening condition on tape recorded tests of open-set sentence recognition.”14(p20) Unfortunately, these indications are stricter than the US Food and Drug Administration (FDA)–approved indications for currently available devices (eg, Cochlear Nucleus CI612 cochlear implant indications15), which has resulted in some Medicare beneficiaries being denied a cochlear implant despite experiencing significant difficulties with their hearing and communication.
The Centers for Medicare & Medicaid Services (CMS) decision memo for cochlear implantation also states: “The evidence is sufficient to conclude that a cochlear implant is reasonable and necessary for individuals with hearing test scores of >40% and ≤60% only when the provider is participating in and patients are enrolled in either an FDA-approved category B IDE clinical trial, a trial under the CMS Clinical Trial Policy, or a prospective, controlled comparative trial approved by the CMS as consistent with the evidentiary requirements for National Coverage Analysis and meeting specific quality standards.”14(p20) This study, titled “Evaluation of Revised Indications (ERID) for Cochlear Implant Candidacy for the Adult CMS Population,” is one such study and was approved by the CMS on July 23, 2013, and was registered with ClinicalTrials.gov (NCT02075229).
The purpose of this study was to examine the effectiveness of currently available multichannel cochlear implant systems when provided to newly implanted Medicare beneficiaries who meet the expanded indications of a hearing test score of 41% to 60% on recorded tests of open-set sentence recognition. Test measures were selected based on the recommendations provided in the Minimum Speech Test Battery16 and their widespread use in clinical care with recipients of cochlear implants. The study additionally assessed the relationship between measures of speech recognition in candidates for cochlear implants and audiologic and quality-of-life outcomes after implantation. The results of the study will be used to request the opening of a National Coverage Determination under the Social Security Act aimed at expanding current Medicare indications for a cochlear implant.
Methods
The American Cochlear Implant Alliance submitted a proposal to the CMS to investigate expansion of the current National Coverage Determination in July 2013. The study was approved by the CMS and was conducted as a multicenter nonrandomized trial. A total of 10 sites were initially enrolled in the study. Owing to slow enrollment, the CMS approved expansion of the study in 2016 to include 9 additional sites that were geographically spread across the United States to increase access for potential participants. Despite these efforts, only 8 sites enrolled participants. Each site agreed to follow the study protocol approved by the CMS (study protocol in Supplement 1), and each site served as its own study sponsor. All sites obtained approval from their local institutional review boards (Johns Hopkins University, University of Miami, University of Michigan, New York University Langone Medical Center, University of North Carolina, University of Southern California, Vanderbilt University, University of Washington, Washington University in St Louis, University of Texas Southwestern, Loyola University Chicago, Medical College of Wisconsin, University of Pennsylvania, St Luke’s Midwest Ear Institute, Rocky Mountain Ear Center, Massachusetts Eye & Ear Infirmary, Medical University of South Carolina, and The Ohio State University) prior to enrolling participants and prior to collecting data for this study. Informed consent was obtained in writing for each participant from the site that enrolled them in the study. Participating sites did not receive payment for the time devoted to the study but did receive payment from the CMS for the surgery, device, and preoperative and postoperative care provided to participants enrolled in the study. This report includes the final data set submitted to the CMS for this study and follows the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.17
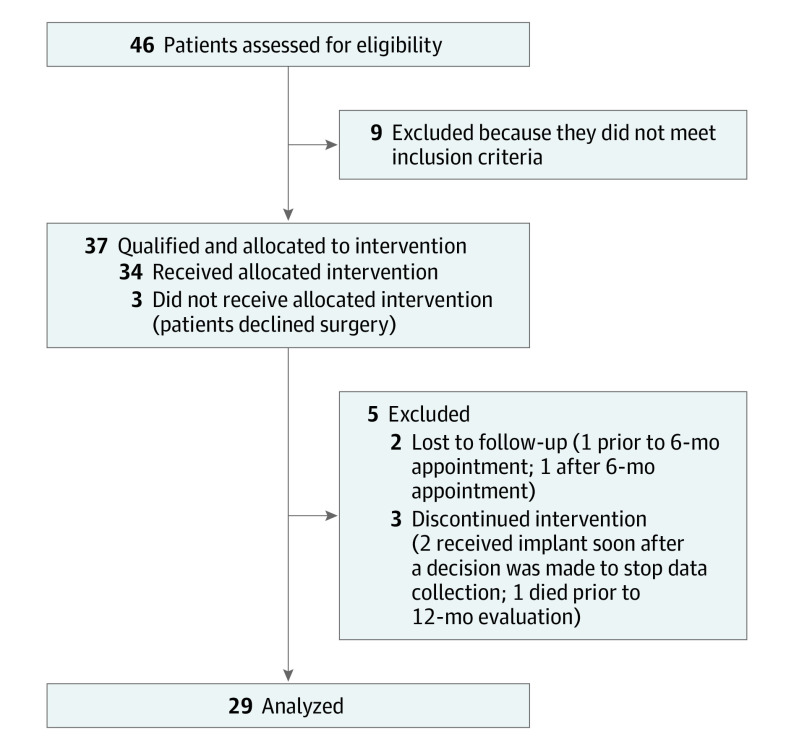
Participant enrollment is summarized in Figure 1. A total of 46 participants were screened for eligibility, and 37 of these met inclusion criteria and were enrolled in the study. Three participants withdrew prior to surgery, resulting in 34 participants who received a cochlear implant between September 17, 2014, and July 10, 2018, at 8 of the 19 participating centers. One participant withdrew from the study and 2 participants were lost to follow-up prior to the 6-month interval visit, resulting in a total of 31 participants. Between the 6-month and 12-month visits, 1 participant was lost to follow-up and 1 participant passed away, resulting in 29 participants tested at the 12-month interval. Twelve participants had a baseline AzBio Sentence Test score of 50% or less (group 1), while 19 participants had a baseline score of 51% or more (group 2). Preoperatively, no significant differences were noted between these 2 groups on the baseline characteristics outlined in Table 1.
Figure 1. Participant Flow Diagram.
Table 1. Baseline Characteristics of Study Participants.
| Characteristic | Median (range) | Difference (95% CI) | ||
|---|---|---|---|---|
| All participants (n = 31) | Group 1 AzBio Sentence Test score ≤50% (n = 12) | Group 2 AzBio Sentence Test score >50% (n = 19) | ||
| Age at cochlear implant activation, y | 73.6 (65.7 to 85.1) | 70.2 (66.3 to 84.3) | 73.7 (65.7 to 85.1) | −1.2 (−7.2 to 3.3) |
| Sex, No. (%) | ||||
| Male | 23 (74) | 7 (58) | 16 (84) | −26 (−58 to 6) |
| Female | 8 (26) | 5 (42) | 3 (16) | |
| Duration of HL in cochlear implant ear, y | 23.9 (10.5 to 62.4) | 30.9 (10.5 to 61.3) | 23.6 (10.6 to 62.4) | 1.6 (−7.8 to 21.3) |
| Duration of deafness in cochlear implant ear, y | 10.8 (0.2 to 62.4) | 11.1 (2.6 to 40.3) | 10.8 (0.2 to 62.4) | −1.0 (−8.2 to 7.3) |
| Duration of HL in contralateral ear, y | 9.0 (0.2 to 62.4) | 11.1 (2.6 to 40.3) | 9.0 (0.2 to 62.4) | 0.9 (−4.9 to 8.3) |
Abbreviation: HL, hearing loss.
Inclusion and Exclusion Criteria
Participants were required to be 65 years or older at the time of the study and eligible for the CMS as their primary source of medical insurance coverage, to have bilateral moderate to profound hearing loss in the low frequencies (≤1000 Hz) and profound sensorineural hearing loss in the high frequencies (≥3000 Hz), to use English as their primary spoken language, and to have a preoperative sentence score in quiet that was 41% to 60% correct when recorded sentences were presented in the best-aided condition. In the original study protocol, Hearing in Noise Test (HINT) sentences15 were used to qualify candidates, and 3 participants were enrolled in the study based on their HINT scores. In July 2015, a protocol change was approved by the CMS to use the AzBio Sentence Test18 to qualify candidates for this study because most clinics had ceased using HINT sentences. A total of 34 participants were enrolled in the study based on their AzBio Sentence Test score. Participants were excluded from the study if the onset of their hearing loss occurred prior to the age of 2 years, if there was an indication of cochlear ossification, if they presented with absence of cochlear development or any other cochlear anomaly that might prevent complete insertion of the electrode array, if the hearing loss was suspected to be of neural or central origin, if they had an active middle ear infection, or if the audiologist and/or surgeon thought there were any disabling cognitive limitations that would prevent the patient from providing reliable data for the study.
The initial proposal approved by the CMS in 2013 and the updated proposal approved in 2015 (study protocol in Supplement 1) included retrospective pilot data obtained for 79 implant recipients 65 years or older accrued from 9 different implant centers. These recipients demonstrated scores ranging from 41% to 60% correct on HINT sentences preoperatively and were able to obtain a cochlear implant because of their self-pay status or participation in a CMS-approved, manufacturer-sponsored trial. This group demonstrated a mean gain of 33% on HINT sentences postoperatively in the implant-alone condition. The primary hypothesis for this study was based on this set of pilot data and was formulated to state that intervention with a cochlear implant will significantly improve sentence recognition by 30% in the ear with the cochlear implant and by 25% in the best-aided condition. Although the initial hypothesis of change in sentence score was based on data collected using HINT sentences, ceiling effects previously noted for HINT sentences19 led us to assume that the changes of 30% for the implant ear–alone condition and 25% for the best-aided condition would still apply. In addition, in the initial proposal, it was indicated that a sample size of 27 participants was needed to achieve 80% power based on an accepted current standard of 30% improvement in sentence recognition when preimplant and postimplant scores were compared. We attempted to accrue twice this number of participants (ERID initial submission, 2012), but enrollment was low throughout the study and across many of the participating centers. This reduced enrollment was likely owing to numerous factors, including time constraints involved with participation in a voluntary, nonfunded study; the initial use of HINT sentences, which was not the test measure of choice for many of the participating centers; and the narrow range of 41% to 60% in preoperative sentence scores for study inclusion. An interim analysis of findings was provided to the CMS approximately 4 years after the study was initiated to explore the effect sizes related to cochlear implants. Based on the slow rate of participant accrual combined with the strength of the findings in the interim analysis, the CMS and the investigators agreed to close the study.
At the request of the CMS, participants were divided into 2 groups: those who obtained a preoperative best-aided sentence score that ranged from 41% to 50% (group 1) and those whose scores ranged from 51% to 60% (group 2). The 3 participants who qualified for the study using HINT sentences had preoperative AzBio Sentence Test scores of 26%, 35%, and 35% and were placed in group 1.
Test Protocol
All participants were asked to participate in preoperative and postoperative testing that included unaided audiometric assessment, aided or cochlear implant speech recognition testing, aided or cochlear implant telephone testing, and completion of self-assessment questionnaires. Speech recognition measures included recorded versions of the AzBio Sentence Test and the Consonant Nucleus Consonant (CNC) Monosyllabic Word Test.20 All stimuli were presented in a quiet setting in the sound field at a 60-dB sound pressure level. Stimuli were presented only a single time and feedback was not provided. Preoperatively, both measures were administered when the participant used an appropriately fit hearing aid on their left ear alone, their right ear alone, and on both ears. In addition, City University of New York (CUNY) sentences21 were used to evaluate speech recognition over the telephone. Preoperatively, 2 lists of CUNY sentences were presented via live voice over the telephone by an audiologist while the participant listened using a hearing aid on the ear to receive the implant alone, and while using the settings typical for telephone use by that participant.
Postoperatively, speech recognition testing was performed 6 and 12 months after activation. At these appointments, participants were asked how often they used a hearing aid in the ear without the implant. If they indicated they used a hearing aid in that ear more than 4 hours each day, all speech recognition tests were additionally administered in the bimodal condition. Thus, the CNC Monosyllabic Word Test and AzBio Sentence Test were performed with all participants when they used the implanted ear alone, and many additionally participated in these tests when using the cochlear implant plus a hearing aid on the contralateral ear. Postoperatively, CUNY sentence testing over the telephone was performed solely in the cochlear implant–only condition.
Self-assessment questionnaires were administered preoperatively and 6 and 12 months postoperatively and included the Abbreviated Profile of Hearing Aid Benefit (APHAB),22 the 36-item Short Form (SF-36),23 and the Health Utility Index Mark 3 (HUI-3).24 Clinicians were provided with study recommendations that included postoperative mapping (recommended parameters for setting the levels of the cochlear implant system for each device), a recommended schedule of follow-up appointments, and guidelines for referral to a speech-language pathologist for formal aural rehabilitation and training if the recipient demonstrated poor postoperative outcomes, demonstrated difficulty adjusting to the sound quality of the cochlear implant, if there was a question regarding the presence of coexisting communication difficulties related to a change in cognitive status, or if the recipient requested additional rehabilitation and training that the audiologist was not able to provide. These recommendations were not regulated because decisions regarding clinical care were left to the individual sites so that results would reflect outcomes received by beneficiaries when treated by their local cochlear implant center.
Statistical Analysis
Statistical analysis of final results took place from July 29 to October 1, 2019. The primary outcome measure was the change in the AzBio Sentence Test score obtained 12 months postoperatively compared with the baseline score obtained prior to receiving the cochlear implant. A 25% improvement in the AzBio Sentence Test score was defined as the minimally clinically important difference in the best-aided condition, and an improvement of 30% was defined as the minimally clinically important difference in the implanted ear. Thus, the study aimed to test the 1-sided hypothesis that the cochlear implant improved the AzBio Sentence Test score in the best-aided condition by 25% or more and in the implanted ear by 30% or more. Shapiro-Wilk test results indicated that data were not normally distributed. Therefore, medians and ranges were used to describe the continuous variables. Median differences and corresponding 1-sided 95% CIs were used as measures of effect size and precision of estimates. The Spearman correlation was used to explore the correlation between speech perception outcome measures and self-assessment questionnaires. At the request of the CMS, participants were additionally divided into 2 groups based on their best-aided preoperative AzBio Sentence Test score in quiet.
A mixed-effects model with an unrestricted variance-covariance matrix was used to examine the change in the outcome measure scores. Estimated marginal means, their differences, and the corresponding 95% CIs were used as measures of effect size. The mixed-effects model allows controlling for potential confounders and for the exploring of group-by-visit interaction. The interaction effect was included in the model to evaluate whether the change in outcome measures through study visits was significantly different for the 2 groups. Mixed-effects modeling follows the intention-to-treat principle and makes maximum use of all available data at each study visit. All statistical analysis was performed in SAS, version 9.4. statistical software (SAS Institute Inc).
Results
Participants
A total of 34 participants received a cochlear implant as part of this study between September 17, 2014, and July 10, 2018, with data available for 31 participants (23 men [74%]; median age, 73.6 years [range, 65.7-85.1 years]) at the 6-month interval and for 29 participants at the 12-month interval. Twelve participants had a baseline AzBio score of 50% or less (group 1), while 19 participants had a baseline score of 51% or more (group 2).
Speech Recognition Scores
Table 2 summarizes the distribution of scores on the AzBio Sentence Test and CNC Monosyllabic Word Test obtained prior to implantation and 6 and 12 months after receiving the cochlear implant for the total cohort and for each of the study groups. Median preoperative AzBio Sentence Test scores for the total cohort were 53% (range, 26%-60%) for the best-aided condition and 24% (range, 0%-53%) for the cochlear implant–alone condition; median scores 12 months after implantation improved to 89% (range, 36%-100%) for the best-aided condition and 77% (range, 13%-100%) for the cochlear implant–alone condition. The median change in the AzBio Sentence Test score in the best-aided condition at 12 months compared with baseline was 36% (range, –22% to 75%). This change is significantly greater than the hypothesized improvement of 25%, with the lower bound of 1-sided 95% CI being 31%. The median change in the AzBio Sentence Test score for the cochlear implant ear alone at 12 months compared with baseline was 53% (range, –15% to 93%). This change is also significantly greater than the hypothesized improvement of 30%, with the lower bound of 1-sided 95% CI being 45%.
Table 2. Distribution of Speech Perception Scores Through All Study Visits.
| Measure | Score, median (range) | ||
|---|---|---|---|
| Baseline (before implant) | 6 mo After implant | 12 mo After implant | |
| All participants | |||
| AzBio Sentence Test | |||
| Best-aided condition | 53 (26-60) | 91 (25-100) | 89 (36-100) |
| Cochlear implant ear alone | 24 (0-53) | 79 (0-99) | 77 (13-100) |
| CNC Monosyllabic Word Test | |||
| Best-aided condition | 38 (16-60) | 78 (40-96) | 76 (34-96) |
| Cochlear implant ear alone | 16 (0-42) | 62 (4-92) | 62 (18-93) |
| CUNY sentence test presented via telephone, cochlear implant ear alone | 24 (0-97) | 82 (1-100) | 92 (0-100) |
| Group 1: AzBio Sentence Test baseline score ≤50% | |||
| AzBio Sentence Test | |||
| Best-aided condition | 42 (26-50) | 93 (53-100) | 95 (71-99) |
| Cochlear implant ear alone | 18 (0-38) | 84 (22-99) | 85 (57-98) |
| CNC Monosyllabic Word Test | |||
| Best-aided condition | 37 (16-48) | 78 (52-96) | 77 (60-96) |
| Cochlear implant ear alone | 13 (6-28) | 69 (36-92) | 68 (50-92) |
| CUNY sentence test presented via telephone, cochlear implant ear alone | 28 (0-68) | 89 (32-100) | 97 (29-100) |
| Group 2: AzBio Sentence Test baseline score 51%-60% | |||
| AzBio Sentence Test | |||
| Best-aided condition | 56 (51-60) | 88 (25-100) | 82 (36-100) |
| Cochlear implant ear alone | 27 (0-53) | 71 (0-99) | 72 (13-100) |
| CNC Monosyllabic Word Test | |||
| Best-aided condition | 38 (16-60) | 76 (40-90) | 74 (34-94) |
| Cochlear implant ear alone | 16 (0-42) | 58 (4-86) | 62 (18-93) |
| CUNY sentence test presented via telephone, cochlear implant ear alone | 17 (0-97) | 74 (1-99) | 70 (0-99) |
Abbreviations: CNC, Consonant Nucleus Consonant; CUNY, City University of New York.
Examination of individual scores revealed that 21 of 29 participants (72%) demonstrated an improvement in their AzBio Sentence Test score in the best-aided condition of more than 25% at 12 months. When examined by study group, all 12 of the group 1 participants and 9 of 17 of the group 2 participants (53%) experienced a change of more than 25% in their AzBio Sentence Test score in the best-aided condition at 12 months.
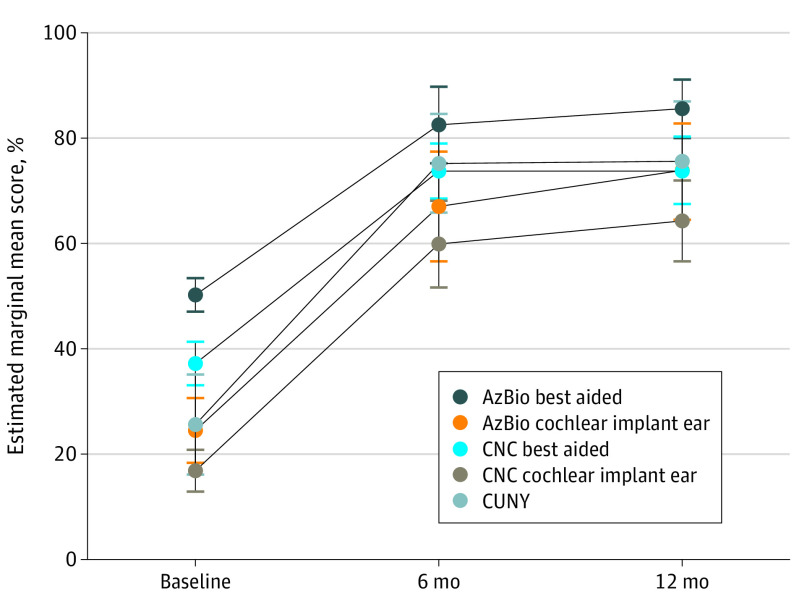
Mixed-model analyses were used to explore the change in estimated marginal mean (EMM) speech recognition scores through study assessment visits and to compare these changes between study groups. Figure 2 shows a statistically significant and clinically important improvement in EMM for the entire group of participants from baseline to the 6-month and 12-month assessments for the AzBio Sentence Test in both the best-aided condition, with EMM differences of 32.3% (95% CI, 23.7%-40.9%) at 6 months and 35.4% (95% CI, 28.2%-42.5%) at 12 months, and in the cochlear implant–alone condition, with EMM differences of 42.4% (95% CI, 30.6%-54.2%) at 6 months and 49.6% (95% CI, 39.2%-60.1%) at 12 months. Important changes were also noted in CNC Monosyllabic Word Test results in both the best-aided condition, with EMM differences from baseline of 36.5% (95% CI, 29.3%-43.8%) at 6 months and 36.7% (95% CI, 28.5%-44.9%) at 12 months, and in the cochlear implant–alone condition, with EMM differences of 43.0% (95% CI, 34.2%-51.8%) at 6 months and 47.4% (95% CI, 38.9%-55.9%) at 12 months. The CUNY sentences telephone test also showed significant and important improvement in the scores at the 6-month (EMM difference, 49.6% [95% CI, 36.1%-63.2%]) and 12-month (50.1% [95% CI, 35.7%-64.6%]) assessments compared with the baseline assessment. Small and insignificant differences were noted when scores obtained at the 6-month and 12-month assessments were compared. When between-group comparisons were examined (eFigure in Supplement 2), EMM differences in AzBio Sentence Test scores between baseline and 12 months were significantly larger for the cochlear implant–alone condition in group 1 (64.9% [95% CI, 49.7%-80.2%]) than in group 2 (39.6% [95% CI, 27.3%-51.9%]). For the best-aided condition, EMM differences in scores between baseline and 12 months were again significantly larger in group 1 (50.1% [95% CI, 40.9%-59.2%]) than in group 2 (26.1% [95% CI, 18.6%-33.5%]) owing to the lower baseline starting point for group 1. One participant in group 2 experienced a decline in score for each of the speech perception measures. This participant was 81 years old and received a diagnosis of dementia after receiving the cochlear implant. Sensitivity analysis revealed that the results did not change significantly with the exclusion of this participant’s data from the analysis. Changes in the other measures were not significantly different when scores for the 2 groups were compared.
Figure 2. Change in Each Speech Perception Outcome Measure Through Study Visits for the Group.
The vertical lines indicate 95% CIs. AzBio indicates AzBio Sentence Test; CNC, Consonant Nucleus Consonant Monosyllabic Word Test; and CUNY, City University of New York sentence test.
Self-assessment Questionnaires
Mixed-model analyses were used to compare EMMs obtained by participants on the APHAB, HUI-3, and SF-36. Summaries of these analyses are provided in eTable in Supplement 2. The analyses indicated statistically significant and clinically important changes in APHAB subscale scores across time. Similar to changes in the speech recognition measures, the largest increase was noted between baseline and the 6-month and 12-month visits. Comparison of EMMs for HUI-3 scores, which includes assessment of 8 different aspects of health utility along with a Multi score (combined scores of all subsections), revealed statistically significant and clinically important changes in the domains of hearing and dexterity as well as a significant change in the HUI-3 Multi score through the different assessments (eTable in Supplement 2); the 95% CIs for the changes were compatible with clinically important changes in all domains except the vision domain. Analysis of the 8 categories of SF-36 scores indicated no significant or important changes in scores at the 3 study visits.
Correlation Between Changes in Each of the Test Scores
The Spearman correlation was used to examine the relationship between the changes in each of the test scores at 12 months compared with baseline. The results of this analysis are summarized in Table 3 and indicate strong correlations between the various speech recognition measures. Results of interest include the significant relationship between the change in AzBio Sentence Test score at 12 months in the cochlear implant–only condition and HUI-3 Hearing (r = 0.405), HUI-3 Multi (r = 0.5559), and APHAB Global (r = 0.4769). A significant relationship was also observed between AzBio Sentence Test score in the best-aided condition and SF-36 fatigue (r = 0.5744) and between CUNY telephone score and HUI-3 Cognition (r = 0.4131) and HUI-3 Multi (r = 0.4781).
Table 3. Correlation Between the Change (12 Months After Baseline) in Speech Perception Scores and Scores Obtained on the Self-assessment Questionnaires.
| Test | AzBio Sentence Test score in cochlear implant ear alone | AzBio Sentence Test score in best-aided condition | CNC Monosyllabic Word Test score in cochlear implant ear alone | CNC Monosyllabic Word Test score in best-aided condition | CUNY sentence test score |
|---|---|---|---|---|---|
| AzBio Sentence Test | NA | NA | NA | NA | NA |
| Cochlear implant ear alone | 1 | NA | NA | NA | NA |
| Best-aided condition | 0.7013 | 1 | NA | NA | NA |
| CNC | |||||
| Cochlear implant ear alone | 0.6377 | 0.6172 | 1 | NA | NA |
| Best-aided condition | 0.5972 | 0.6095 | 0.7084 | 1 | NA |
| CUNY sentence test | 0.6992 | 0.5944 | 0.7077 | 0.4536 | 1 |
| HUI-3 | |||||
| Vision | −0.2857 | −0.0098 | −0.1202 | −0.1153 | −0.0542 |
| Hearing | 0.405 | 0.0911 | −0.0227 | −0.0918 | 0.1035 |
| Speech | 0.2467 | 0.1569 | 0.1755 | 0.1293 | 0.1706 |
| Ambulation | 0.3346 | 0.2522 | 0.3364 | 0.3329 | 0.3259 |
| Dexterity | −0.1382 | 0.0939 | 0.0623 | 0.2495 | −0.0683 |
| Emotion | 0.2238 | −0.0352 | 0.3199 | 0.3499 | 0.2144 |
| Cognition | 0.3399 | 0.3871 | 0.4023 | 0.2982 | 0.4131 |
| Pain | −0.4224 | −0.3314 | −0.2303 | −0.2404 | −0.0284 |
| Multi | 0.5559 | 0.2711 | 0.317 | 0.3396 | 0.4781 |
| APHAB | |||||
| Global | 0.4769 | 0.3777 | 0.1253 | 0.288 | 0.2979 |
| Ease | 0.364 | 0.3028 | −0.0335 | 0.1767 | 0.1755 |
| Reverb | 0.3914 | 0.2946 | 0.1503 | 0.3205 | 0.275 |
| BG noise | 0.3898 | 0.2621 | 0.177 | 0.2208 | 0.2344 |
| Averse | 0.1789 | −0.0007 | 0.3389 | 0.2257 | 0.2483 |
| SF-36 | |||||
| Physical function | 0.254 | 0.1782 | 0.3362 | 0.3592 | 0.306 |
| Limit physically | −0.0219 | −0.0219 | −0.0571 | 0.0385 | −0.03 |
| Limit emotion | 0.1712 | 0.1857 | 0.0191 | −0.0669 | 0.1169 |
| Fatigue | 0.1795 | 0.5744 | 0.3462 | 0.1031 | 0.3138 |
| Emotional | 0.2503 | 0.2576 | 0.4106 | 0.09 | 0.3212 |
| Social | 0.2325 | 0.366 | 0.3107 | 0.2278 | 0.2557 |
| Pain | 0.0668 | 0.199 | 0.2114 | 0.1348 | 0.3843 |
| General health | 0.0381 | 0.1752 | −0.0411 | 0.0016 | −0.0715 |
Abbreviations: APHAB, Abbreviated Profile of Hearing Aid Benefit; BG, background; CNC, Consonant Nucleus Consonant; CUNY, City University of New York; HUI-3, Health Utility Index Mark 3; NA, not applicable; SF-36, 36-item Short Form.
Discussion
The results of this study demonstrate that currently available multichannel cochlear implant systems provide improvements in communication for adult Medicare beneficiaries 65 years or older with qualifying sentence recognition scores between 41% and 60%. Specifically, adults who demonstrate best-aided sentence scores between 41% and 60% correct on the AzBio Sentence Test demonstrated significant improvements in sentence, word, and telephone recognition scores after receiving a cochlear implant. In addition, improvements in speech recognition appear to be related to positive changes on self-reported assessments, such as those noted on the HUI-3 Hearing subtest, the HUI-3 Multi score, and various domains of the APHAB. These findings support the need for change to the current CMS guidelines to allow greater speech recognition for Medicare beneficiaries who present with considerable difficulty communicating and functioning in daily life.
As a group, changes in the primary outcome measures of this study (AzBio Sentence Test scores in the best-aided condition and AzBio Sentence Test scores in the cochlear implant–alone condition) were significantly larger at both the 6-month and 12-month intervals than the predefined minimally clinically important changes of 25% and 30%. When examined on an individual basis, the scores for 8 participants improved less than 25%. However, 4 of these participants (50%) showed a clinically important improvement (≥0.12) in the HUI-3 Multi, indicating that they received important benefit from the device despite not reaching the a priori improvement of 25%. The change in AzBio Sentence Test scores and CNC Monosyllabic Word Test scores at each assessment was correlated with a positive change in patient-reported quality of life for both the HUI-3 Multi and APHAB Global score. This finding is in agreement with those of Crowson et al,25 who found that older adults’ self-reported improvement in quality of life after cochlear implant was often independent of audiologic performance and is also in agreement with numerous previous reports that demonstrate improvements in patient-reported quality of life after intervention with a cochlear implant.6,24,25,26,27,28,29,30
Our findings are particularly important when considering that hearing loss is the most common sensory deficit in elderly individuals.31 In fact, age-related hearing loss is projected to be within the top 15 leading causes of burden of disease by 2030.32 This outcome is likely owing to its association with numerous health issues, including accelerated cognitive and physical aging, depression, poor balance, increased hospitalizations, and early mortality.33,34 In addition, Dowell35 reported that recipients’ chances of a good outcome with a cochlear implant are significantly better if implantation occurs relatively soon after the onset of severe hearing loss and before the loss of all functional auditory skills. The current study participants had a mean duration of deafness of 10 years in the ear with the cochlear implant and had not lost all functional auditory skills (preoperative sentence range of 41%-60%). Waiting until recipients have a decrease in hearing preoperatively and substantially poor speech recognition could be detrimental to their outcome. Adoption of the proposed changes to the CMS indications are needed if we are to treat patients with significant hearing loss expeditiously, as doing so may reduce the effect that the hearing loss will have on their health and quality of life.
The results of this study represent data that will be used to support a formal request to expand the CMS current indications to include patients who obtain a best-aided sentence recognition score that is less than or equal to 60% correct. More important, 31 participants received cochlear implants and demonstrated improved communication skills or improvements in quality of life. Because they did not meet the CMS traditional indications, without this study, they would not have received a cochlear implant. We are hopeful that the results of this study will foster expansion of the CMS indications for a cochlear implant, leading to improved access to this technology for even more Medicare beneficiaries.
Limitations
This study has some limitations. The number of participants was less than projected, and it took longer to enroll participants than anticipated when the study was initially proposed. These events were likely owing to a variety of reasons that were previously described. It could also be possible that few patients exist who meet the study inclusion criteria. In addition, most participants were enrolled at 3 centers, which may limit the ability to generalize our results to the larger population of implant recipients.
Conclusions
Despite these limitations, the outcomes obtained by participants were strong and are consistent with those reported by others for patients older than 65 years,6,28 resulting in significant improvements in speech and telephone recognition as well as self-reported quality of life for the participants who received a cochlear implant in this study.
Study Protocol
eFigure. Comparison of Change in Each of the Speech Perception Outcome Measures at Study Visits Between the Two Study Groups
eTable. Estimated Marginal Means at Each Study Visit and the Comparison With Baseline
References
- 1.Niparko JK, Tobey EA, Thal DJ, et al. ; CDaCI Investigative Team . Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498-1506. doi: 10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osberger MJ, Zimmerman-Phillips S, Koch DB. Cochlear implant candidacy and performance trends in children. Ann Otol Rhinol Laryngol Suppl. 2002;189:62-65. doi: 10.1177/00034894021110S513 [DOI] [PubMed] [Google Scholar]
- 3.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342-360. doi: 10.1097/AUD.0b013e3182741aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, Cosetti M. Safety and outcomes of cochlear implantation in the elderly: a review of recent literature. J Otol. 2016;11(1):1-6. doi: 10.1016/j.joto.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon MT, Buss E, Adunka MC, et al. Long-term speech perception in elderly cochlear implant users. JAMA Otolaryngol Head Neck Surg. 2013;139(3):279-283. doi: 10.1001/jamaoto.2013.1814 [DOI] [PubMed] [Google Scholar]
- 6.Zwolan TA, Henion K, Segel P, Runge C. The role of age on cochlear implant performance, use, and health utility: a multicenter clinical trial. Otol Neurotol. 2014;35(9):1560-1568. doi: 10.1097/MAO.0000000000000583 [DOI] [PubMed] [Google Scholar]
- 7.Mudery JA, Francis R, McCrary H, Jacob A. Older individuals meeting Medicare cochlear implant candidacy criteria in noise but not in quiet: are these patients improved by surgery? Otol Neurotol. 2017;38(2):187-191. doi: 10.1097/MAO.0000000000001271 [DOI] [PubMed] [Google Scholar]
- 8.Lin FR, Chien WW, Li L, Clarrett DM, Niparko JK, Francis HW. Cochlear implantation in older adults. Medicine (Baltimore). 2012;91(5):229-241. doi: 10.1097/MD.0b013e31826b145a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nucleus CI612. Package insert. Cochlear Ltd; 2018.
- 10.Sung Y-K, Li L, Blake C, Betz J, Lin FR. Association of hearing loss and loneliness in older adults. J Aging Health. 2016;28(6):979-994. doi: 10.1177/0898264315614570 [DOI] [PubMed] [Google Scholar]
- 11.Cosh S, Carriere I, Daien V, et al. ; Sense-Cog Consortium . The relationship between hearing loss in older adults and depression over 12 years: findings from the Three-City prospective cohort study. Int J Geriatr Psychiatry. 2018;33(12):1654-1661. doi: 10.1002/gps.4968 [DOI] [PubMed] [Google Scholar]
- 12.Rajasekaran V, Gurulakshmi RB. Assessment of the self perceived hearing handicap and its associated factors in elderly people with hearing loss. J Clin Diagn Res. 2018;12(12):MC09-MC12. [Google Scholar]
- 13.Cosh S, Helmer C, Delcourt C, Robins TG, Tully PJ. Depression in elderly patients with hearing loss: current perspectives. Clin Interv Aging. 2019;14:1471-1480. doi: 10.2147/CIA.S195824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services Decision memo for cochlear implantation (CAG-00107N). Published April 4, 2005. Accessed July 26, 2020. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=134
- 15.Nilsson M, Soli SD, Sullivan JA. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95(2):1085-1099. doi: 10.1121/1.408469 [DOI] [PubMed] [Google Scholar]
- 16.Advanced Bionics LLC, Cochlear Americas, and MED-EL Corp Minimum Speech Test Battery (MSTB) for adult cochlear implant users 2011. Published June 2011. Accessed June 29, 2020. http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf
- 17.Des Jarlais DC, Lyles C, Crepaz N; TREND Group . Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361-366. doi: 10.2105/AJPH.94.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33(1):112-117. doi: 10.1097/AUD.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol. 2008;13(3):193-205. doi: 10.1159/000113510 [DOI] [PubMed] [Google Scholar]
- 20.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62-70. doi: 10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- 21.Boothroyd A, Hanin L, Hnath T A sentence test of speech perception: reliability, set equivalence, and short term learning. CUNY Academic Works. Published September 1985. Accessed February 17, 2020. https://academicworks.cuny.edu/gc_pubs/399
- 22.Cox RM, Alexander GC. The Abbreviated Profile of Hearing Aid Benefit. Ear Hear. 1995;16(2):176-186. doi: 10.1097/00003446-199504000-00005 [DOI] [PubMed] [Google Scholar]
- 23.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 24.Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33(5):375-384. doi: 10.3109/07853890109002092 [DOI] [PubMed] [Google Scholar]
- 25.Crowson MG, Semenov YR, Tucci DL, Niparko JK. Quality of life and cost-effectiveness of cochlear implants: a narrative review. Audiol Neurootol. 2017;22(4-5):236-258. doi: 10.1159/000481767 [DOI] [PubMed] [Google Scholar]
- 26.Contrera KJ, Betz J, Li L, et al. Quality of life after intervention with a cochlear implant or hearing aid. Laryngoscope. 2016;126(9):2110-2115. doi: 10.1002/lary.25848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661-668. doi: 10.1093/geront/43.5.661 [DOI] [PubMed] [Google Scholar]
- 28.Sonnet MH, Montaut-Verient B, Niemier JY, Hoen M, Ribeyre L, Parietti-Winkler C. Cognitive abilities and quality of life after cochlear implantation in the elderly. Otol Neurotol. 2017;38(8):e296-e301. doi: 10.1097/MAO.0000000000001503 [DOI] [PubMed] [Google Scholar]
- 29.Lally JW, Adams JK, Wilkerson BJ. The use of cochlear implantation in the elderly. Curr Opin Otolaryngol Head Neck Surg. 2019;27(5):387-391. doi: 10.1097/MOO.0000000000000569 [DOI] [PubMed] [Google Scholar]
- 30.Vermeire K, Brokx JP, Wuyts FL, Cochet E, Hofkens A, Van de Heyning PH. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol. 2005;26(2):188-195. doi: 10.1097/00129492-200503000-00010 [DOI] [PubMed] [Google Scholar]
- 31.Davis A, McMahon CM, Pichora-Fuller KM, et al. Aging and hearing health: the life-course approach. Gerontologist. 2016;56(suppl 2):S256-S267. doi: 10.1093/geront/gnw033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172(4):369-371. doi: 10.1001/archinternmed.2011.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin FR, Yaffe K, Xia J, et al. ; Health ABC Study Group . Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299. doi: 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowell RC. The case for earlier cochlear implantation in postlingually deaf adults. Int J Audiol. 2016;55(suppl 2):S51-S56. doi: 10.3109/14992027.2015.1128125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eFigure. Comparison of Change in Each of the Speech Perception Outcome Measures at Study Visits Between the Two Study Groups
eTable. Estimated Marginal Means at Each Study Visit and the Comparison With Baseline