Key Points
Question
Is neoadjuvant programmed cell death protein 1 (PD-1) or combined PD-1/cytotoxic T-lymphocyte–associated protein 4 inhibition administered prior to surgery tolerated and effective in patients with untreated squamous cell carcinoma of the oral cavity?
Findings
In this phase 2 clinical trial of 29 patients with oral cavity cancer randomized to nivolumab alone or nivolumab and ipilimumab, there were no delays to surgery, and evidence of clinical, radiologic, and pathologic responses in both arms, including 4 patients with major (>90%) or complete pathologic response—3 who were treated with nivolumab and ipilimumab.
Meaning
Both nivolumab and nivolumab plus ipilimumab are feasible in the neoadjuvant setting and result in promising rates of response.
This randomized clinical trial examines the safety and efficacy of neoadjuvant nivolumab or nivolumab plus ipilimumab administered prior to surgery in patients with untreated squamous cell carcinoma of the oral cavity.
Abstract
Importance
Novel approaches are needed to improve outcomes in patients with squamous cell carcinoma of the oral cavity. Neoadjuvant immunotherapy given prior to surgery and combining programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) immune checkpoint inhibitors are 2 strategies to enhance antitumor immune responses that could be of benefit.
Design, Setting, and Participants
In this randomized phase 2 clinical trial conducted at 1 academic center, 29 patients with untreated squamous cell carcinoma of the oral cavity (≥T2, or clinically node positive) were enrolled between 2016 to 2019.
Interventions
Treatment was administered with nivolumab, 3 mg/kg, weeks 1 and 3, or nivolumab and ipilimumab (ipilimumab, 1 mg/kg, given week 1 only). Patients had surgery 3 to 7 days following cycle 2.
Main Outcomes and Measures
Safety and volumetric response determined using bidirectional measurements. Secondary end points included pathologic and objective response, progression-free survival (PFS), and overall survival. Multiplex immunofluorescence was used to evaluate primary tumor immune markers.
Results
Fourteen patients were randomized to nivolumab (N) and 15 patients to nivolumab/ipilimumab (N+I) (mean [SD] age, 62 [12] years; 18 men [62%] and 11 women [38%]). The most common subsite was oral tongue (n = 16). Baseline clinical staging included patients with T2 (n = 20) or greater (n = 9) T stage and 17 patients (59%) with node-positive disease. Median time from cycle 1 to surgery was 19 days (range, 7-21 days); there were no surgical delays. There were toxic effects at least possibly related to study treatment in 21 patients, including grade 3 to 4 events in 2 (N), and 5 (N+I) patients. One patient died of conditions thought unrelated to study treatment (postoperative flap failure, stroke). There was evidence of response in both the N and N+I arms (volumetric response 50%, 53%; pathologic downstaging 53%, 69%; RECIST response 13%, 38%; and pathologic response 54%, 73%, respectively). Four patients had major/complete pathologic response greater than 90% (N, n = 1; N+I, n = 3). With 14.2 months median follow-up, 1-year progression-free survival was 85% and overall survival was 89%.
Conclusions and Relevance
Treatment with N and N+I was feasible prior to surgical resection. We observed promising rates of response in both arms, supporting further neoadjuvant studies with these agents.
Trial Registration
ClinicalTrials.gov Identifier: NCT02919683
Introduction
Neoadjuvant immune checkpoint blockade has demonstrated promise in melanoma,1,2,3 non–small cell lung cancer,4 bladder cancer,5 and glioblastoma.6,7 Preclinical data suggest neoadjuvant immunotherapy may be more effective than adjuvant therapy.8,9 Concurrent blockade of both the programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) immune checkpoints in the neoadjuvant setting was associated with higher objective and pathologic response in melanoma; however, there were also considerable toxic effects with ipilimumab administered at 3 mg/kg for 2 to 3 doses preoperatively.1,2
Locally advanced squamous cell carcinoma (SCC) of the oral cavity (OC) is typically treated with surgical resection followed by adjuvant treatment dictated by pathologic analysis. Evidence supports adjuvant chemoradiation for high-risk patients, although recurrence is common and disease-related death is significant, even at early time points.10,11 Programmed cell death protein 1 inhibitors nivolumab and pembrolizumab are approved for patients with recurrent/metastatic SCC head and neck (HN) cancers including OC cancers, with a response rate of approximately 20% and an overall survival benefit compared with chemotherapy.12,13,14 Use of immunotherapies in the neoadjuvant setting is of particular interest given the potential for an enhanced immune response with potential clinical benefit, and the possibility of tumor debulking to facilitate more limited surgery and deintensified adjuvant therapy.
We performed a randomized study evaluating neoadjuvant nivolumab (N), delivered alone or in combination with ipilimumab (N+I), in patients with OCSCC. In addition to being a high-risk malignant disease, the choice of OC malignant diseases allowed for visual monitoring over the course of therapy. Using a neoadjuvant course of treatment of 2 to 3 weeks (a typical interval between evaluation and surgery with standard-of-care treatment) and administering ipilimumab for a single cycle at a dose of 1 mg/kg, we endeavored to minimize risks associated with this regimen.3
Methods
Study Population and Trial Design
The trial protocol is available in Supplement 1. This protocol was approved by the Dana-Farber Harvard Cancer Center (DF-HCC) institutional review board. Written informed consent was obtained during clinic visits per institutional guidelines. Eligible patients had pathologically confirmed OCSCC, with clinical T2-4b (American Joint Committee on Cancer, 7th Edition [AJCC 7]), or node positive-disease, with no evidence of distant metastases.
Patients were randomized 1:1 to N monotherapy or N+I. All patients received 2 cycles of nivolumab at 3 mg/kg weeks 1 and 3; patients randomized to the N+I arm also received ipilimumab 1 mg/kg week 1 only. All patients were evaluated by a head and neck surgeon prior to enrollment. Surgery was carried out to the initial planned resection margins which were not adjusted in the case of a clinical response. Adjuvant therapy (radiation or cisplatin-radiation therapy) was used as per standard of care based on pathologic analysis unless there were unequivocal indications for adjuvant therapy on baseline clinical staging as determined by treating physicians. For example, patients with radiologic or clinical T3/T4 disease or multiple radiologically suspicious lymph nodes received adjuvant radiation therapy even if pathologic analysis following neoadjuvant therapy did not reveal standard indications for adjuvant treatment.
Coprimary end points were safety (including delays to planned surgical date) and volumetric response. There was a safety run-in for each cohort following a 3 + 3 design to assess dose-limiting toxic effects. Adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Perisurgical events were assessed using the Clavien-Dindo Classification of Surgical Complications.15 Volumetric response was defined as previously described using the product of the longest perpendicular bidirectional tumor measurements16; given the short window period, any tumor volume reduction as determined by the product of the 2 dimensions was counted as a response. Radiologic measurements were performed by the institutional imaging core facility blinded to treatment. Primary lesions were assessed by physical and pathologic measurements when deemed unmeasurable by radiologic review. Responders demonstrated reduction in tumor volume; all other patients were nonresponders. Secondary end points included objective response using RECIST; for this analysis, patients with no radiographically measurable lesions on imaging review were excluded. Positron emission tomography–computed tomography (PET-CT) scans compared the maximum standardized uptake value (SUVmax) in the primary lesion and most avid lymph node. Immunotherapy induced treatment effect was defined as posttreatment lymph nodes that had increased or developed over the course of neoadjuvant therapy using 2 potential cutoffs of SUVmax≥3 or SUVmax≥6. To differentiate immunotherapy induced effect from progression, we only counted patients if they had a lymph node dissection of this nodal region following study treatment and all lymph nodes from this region were pathologically negative. Pathologic response in the primary lesion (percentages of viable tumor vs areas showing nonviable tumor and/or evidence of tissue response to nonviable tumor) was assessed by a head and neck pathologist blinded to treatment arm. Determination of tissue response was based on the presence of inflammatory cells, necrosis, stromal scarring or fibrosis, granulation tissue, and foreign body giant cell reaction in the gross tumor or tumor bed. Clinical to pathologic downstaging was assessed using AJCC 7 staging.
Correlative Analyses
Multiplex immunofluorescence used formalin-fixed, paraffin-embedded slides of the primary tumor stained using BOND RX automated stainers.17 Scoring for programmed cell death ligand 1 (PD-L1) used the percentage of membranous PD-L1–positive (antibody clone 9A11; Cell Signaling Technology/cytokeratin-positive tumor cells using pathologist-assisted image analysis with Inform software; Akoya/PerkinElmer) as described.17
Statistical Analyses
In addition to safety, the primary end point was volumetric response. The goal was to identify a response rate of 15% or more in either arm based on the lower bound of the 1-sided, 90% exact binomial confidence interval. Using these criteria, the study was stopped when there was sufficient evidence that the volumetric response exceeded 15% in both arms.
Analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc) and JMP Pro statistical software (version 15.0; SAS Institute, Inc) based on database as of October 15, 2019. Associations between cell populations determined by multiplex and response were determined using rank sum test, univariate logistic regression, and analysis of variance. Agreement between response metrics was assessed using Cohen κ.
Results
Patient Characteristics and Safety
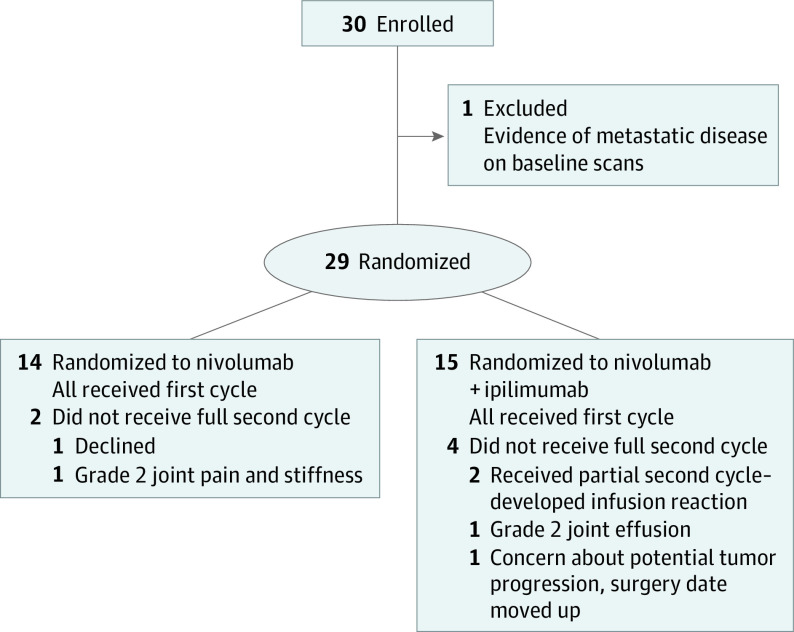
Thirty patients were treated from 2016 to 2019. One patient was excluded from efficacy analyses as she was ineligible because of evidence of distant metastatic disease at baseline (Figure 1). Characteristics of the remaining 29 patients are listed in Table 1. There were 15 (52%) with clinically or radiographically node-positive disease, and 18 (62%) with stage IVA disease (presurgery).
Figure 1. Trial Flow Diagram.
Table 1. Baseline Characteristics of the 29 Study Patients.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Cohort 1: nivolumab (n = 14) | Cohort 2: nivolumab + ipilimumab (n = 15) | Overall (n = 29) | |
| Age, median (range), y | 64.4 (39.1-81.0) | 65.2 (32.5-78.4) | 65.2 (32.5-81.0) |
| Sex | |||
| Male | 10 (71.4) | 8 (53.3) | 18 (62.1) |
| Female | 4 (28.6) | 7 (46.7) | 11 (37.9) |
| Smoker | |||
| No | 11 (78.6) | 8 (53.3) | 19 (65.5) |
| Former, ≤10 pack-year | 1 (7.1) | 6 (40.0) | 7 (24.1) |
| Former, >10 pack-year | 2 (14.3) | 1 (6.7) | 3 (10.3) |
| Primary tumor site | |||
| Oral tongue | 7 (50.0) | 9 (60.0) | 16 (55.2) |
| Gingiva | 1 (7.1) | 1 (6.7) | 2 (6.9) |
| Retromolar trigone | 2 (14.3) | 3 (20.0) | 5 (17.2) |
| Alveolar ridge | 2 (14.3) | 1 (6.7) | 3 (10.3) |
| Buccal mucosa | 1 (7.1) | 1 (6.7) | 2 (6.9) |
| Hard palate | 1 (7.1) | 0 | 1 (3.4) |
| Pretreatment clinical T-stagea | |||
| 2 | 10 (71.4) | 10 (66.7) | 20 (69.0) |
| 3 | 0 | 1 (6.7) | 1 (3.4) |
| 4 | 4 (28.6) | 4 (26.7) | 8 (27.6) |
| Pretreatment clinical N-stagea | |||
| N0 | 5 (35.7) | 6 (40.0) | 11 (37.9) |
| N1 | 4 (28.6) | 0 | 4 (13.8) |
| N2b | 5 (35.7) | 8 (53.3) | 13 (44.8) |
| N2c | 0 | 1 (6.7) | 1 (3.4) |
| AJCC overall clinical disease stagea | |||
| II | 4 (28.6) | 4 (26.7) | 8 (27.6) |
| III | 3 (21.4) | 0 | 3 (10.3) |
| IVA | 7 (50.0) | 11 (73.3) | 18 (62.1) |
American Joint Committee on Cancer, 7th Edition staging.
Fourteen patients were randomized to N and 15 to N+I. Six patients did not receive the full second cycle of therapy, 2 within the N arm and 4 in the N+I arm. Reasons for an incomplete or skipped second cycle summarized in Figure 1 include patient choice (n = 1), infusion reactions (n = 2), and grade 2 arthralgias or joint effusion (n = 2). One N+I patient did not receive the second cycle owing to concern for clinical tumor progression following the first cycle. Restaging scans prior to surgery demonstrated stable disease. The date of surgical resection was moved 1 week earlier than initially planned and complete resection was achieved.
There were no dose-limiting toxic effects in the safety run-in for either cohort. No surgical delays occurred beyond the prespecified surgical date. Adverse events at least possibly related to study treatment occurred in 21 (72%) patients (Table 2). There were no statistically significant differences in the timing of immune-related adverse events relative to surgery between the 2 arms. All grade immune-related toxic effects occurred a median of 7.5 days following surgery in the N arm and 7 days prior to surgery in the N+I arm (P = .33). Grade 3 to 4 immune-related toxic effects occurred a median 26 days after surgery in the N arm and a median of 3 days after surgery in the N+I arm (P = .77). Several of the most serious related events occurred in the perioperative period following surgery or during standard of care adjuvant therapy—these included grade 3 biopsy-proven immune-related colitis and grade 3 pneumonitis in patients in the N+I arm, and a grade 4 event that included new-onset diabetes and associated diabetic ketoacidosis in a patient in the N arm. The grade 3 colitis was responsive to corticosteroid treatment and vedolizumab treatment after 4 months facilitated complete steroid taper and resolution of symptoms. The grade 3 pneumonitis resolved with corticosteroids which were slowly tapered over a period of approximately 1 month. The patient with new-onset diabetes remains insulin dependent. Surgical complications deemed unrelated to study therapy were further assessed using the Clavien-Dindo classification (Table 2).15 Severe events included a pulmonary embolism, evacuation of a postoperative hematoma, and 2 patients who returned to the operating room for revision of an intraoral flap. One of these patients, with a history of hypertension and hyperlipidemia, developed a stroke following flap revision and subsequently died. Median hospital stay following surgery for patients on protocol was 7.5 days (interquartile range [IQR], 6-10 days), and similar between arms (N, median 6 days; IQR, 4-8 days; N+I, median 8 days; IQR, 7-11 days).
Table 2. Treatment-Related Adverse Events (Common Terminology Criteria for Adverse Events Version 4.0), and Surgical-Related Toxic Effects (Clavien-Dindo) in All 30 Originally Enrolled Participants.
| Immune checkpoint blockade-related AE | No. (%) | |||
|---|---|---|---|---|
| Cohort 1: nivolumab (n = 15) | Cohort 2: nivolumab/ipilimumab (n = 15) | |||
| Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | |
| Skina (rash, dryness, dermatitis) | 3 (20) | 0 | 2 (13) | 0 |
| Pain (lymph node and oral) | 1 (7) | 0 | 2 (13) | 0 |
| Fatigue | 3 (20) | 0 | 1 (7) | 0 |
| Hyperthyroidism | 2 (13) | 0 | 1 (7) | 0 |
| Hypothyroidism | 1 (7) | 0 | 0 | 0 |
| Colitis/diarrhea | 0 | 0 | 2 (13) | 1 (7) |
| Oral mucositis/lichenoid reaction/dry mouth/dysphagia | 1 (7) | 1 (7) | 1 (7) | 0 |
| Tongue/face swelling | 0 | 0 | 2 (13) | 0 |
| Infusion/allergic reaction | 0 | 0 | 0 | 2 (13) |
| Joint swelling/effusionb | 1 (7) | 0 | 1 (7) | 1 (7) |
| Autoimmune diabetes and hyperglycemia | 0 | 1 (7) | 0 | 0 |
| Autoimmune disorders | 2 (13) | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 0 | 1 (7) |
| Timing of toxic effect onset (median days from surgery date) | 7 (All grades) | 26 | –7 (All grades)c | 18 |
| Surgical toxic effects–Clavien-Dindo scoring | 4 (31) | 1 (8) | 6 (40) | 3 (20) |
| Hospital stay, median, d (IQR) | 6 (4-8) | 8 (7-11) | ||
Abbreviations: AE, adverse event; IQR, interquartile range.
Including papulopustular rash and all skin and subcutaneous tissue disorders.
Including all musculoskeletal and connective tissue disorders.
Median interval occurred prior to surgery.
Efficacy Outcomes
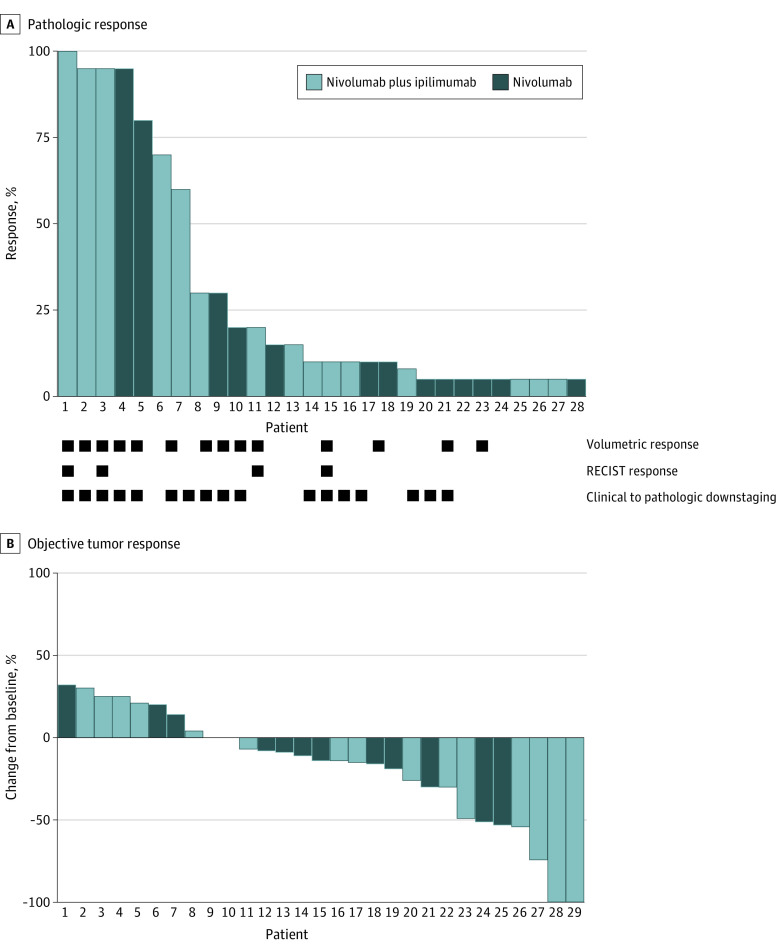
Restaging scans were performed a median of 14 days (range, 6-20 days) and surgery a median of 19 days (range, 7-21 days) after cycle 1 of therapy. Response during the window period is summarized in Figure 2. We observed volumetric response (50% with N; 80% CI, 30.5%-69.5%; 53% with N+I; 80% CI, 34.2%-71.8%), RECIST response (13% with N; 38% with N+I) and clinical to pathologic downstaging (69% with N, 53% with N+I) in both arms. Measures of response were largely concordant between patients (eTable in Supplement 2). Among the 11 patients without clinical to pathologic downstaging, 8 had stable disease. The 3 patients with an increase in stage were: clinical T2N0 (stage II)/pathologic T2N1 (stage III); clinical T2N2b (stage IVA)/pathologic T3N2b (stage IVA); clinical T2N1 (stage III)/pathologic T3N0 (stage III). None of these patients had difficulty with surgical resection or had tumor recurrence.
Figure 2. Summary of Response in Both Treatment Arms.
A, The y-axis represents percent pathologic response. B, The y-axis represents percent unidirectional tumor response.
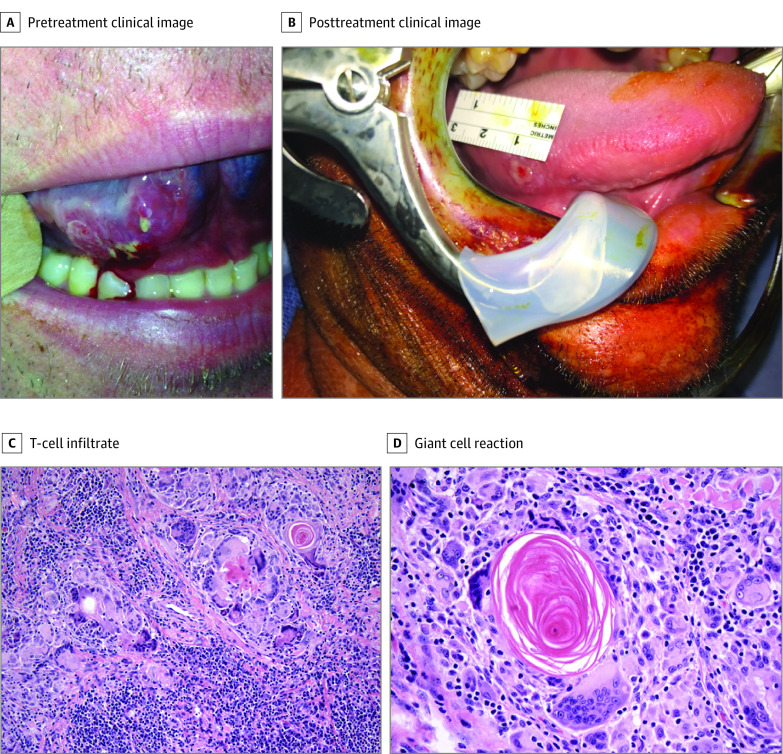
Pathologic response in the primary tumor was assessed using a quantitative grading scheme (pathologic tumor response [nonviable tumor], PTR0 = no or <10% response, PTR1 = ≥10% and <50%, and PTR2 = ≥50%), as has been recently described.18 Pathologic features observed in patients with significant tumor response included visible regressed tumor, inflammation, giant cell reaction, and acellular keratin (Figure 3, C and D). We observed considerable rates of PTR1 and PTR2 in both arms (PTR1: 38% with N, 40% with N+I; PTR2: 15% with N, 33% with N+I). Importantly, we also observed several cases of pathologic near complete (>90%) or complete responses—1 in the N cohort (8%), and 3 (20%) with N+I. Figure 3 demonstrates an example of striking clinical regression in a patient who presented with clinical T2N0 disease involving the right lateral tongue who had dramatic reduction in tumor bulk visible on examination by the time of surgery, 15 days after cycle 1 treatment. Pathologic analysis revealed no evidence of residual invasive disease.
Figure 3. Clinical and Pathologic Features of Response.
A, Pretreatment image shows a cT2 squamous cell carcinoma of the right oral tongue. B, Fifteen days following cycle 1 of preoperative immunotherapy, image at the time of surgery demonstrates near-complete clinical regression of primary lesion (no residual squamous cell carcinoma identified on pathologic analysis). C, Original magnification ×200, and D, original magnification ×400 histopathologic images at the time of surgery following neoadjuvant immunotherapy in another patient with a robust response demonstrate evidence of robust T-cell infiltrate with evidence of regressed tumor, giant cell reaction, and acellular keratin. C and D, Hematoxylin-eosin.
Following study treatment, adjuvant radiation therapy was administered in 10 patients (34%), and chemoradiation therapy with concurrent cisplatin in 9 (31%). One year progression-free and overall survival rates were 85% (95% CI, 72.4%-99.7%) and 89% (95% CI, 78.3%-100%), respectively (eFigure 1 in Supplement 2). One patient had a T1 recurrence in the OC and another patient developed a second primary tumor elsewhere in the OC. These lesions were subsequently resected and both patients have had no further evidence of disease. Thus, at the time of data cutoff, 25 patients (86%) remained alive, without evidence of disease. Note that 1 patient with clinical T4aN2b disease was offered nonsurgical therapy owing to need for a total glossectomy and potential total laryngectomy. This patient had evidence of 15% radiologic tumor shrinkage that did not meet RECIST criteria for response. Treatment was shifted to definitive cisplatin-radiation and he demonstrated an early-on treatment response followed by complete response on first restaging scan findings (eFigure 2 in Supplement 2). He remained disease free more than 34 months after presentation. In addition, the 1 patient who went to surgery early 7 days following cycle 1 owing to concerns regarding clinical progression had evidence of lymphocyte infiltration and a 70% pathologic response at the primary tumor site. He developed distant metastatic disease to the lung, mediastinum, and bone and was subsequently treated with nivolumab monotherapy with evidence of a dramatic near-complete response (eFigure 3 in Supplement 2) and has remained in treatment for more than 14 months.
Biomarker and Correlative FDG-PET/CT Analyses
Twenty-five patients had pretreatment specimens evaluable for immune marker analysis (N, n = 12; N+I, n = 13). The PD-L1 expression in tumor cells ranged from 0% to 91%. Five patients (20%) had PD-L1 expression lower than 1%; 3 who were treated in the N arm and 2 who received treatment with N+I. There was no significant difference in percent of PD-L1 expressing tumor cells between arms (median 35% for N, 12% for N+I; P = .98). Overall, PD-L1 expression was not correlated with volumetric or pathologic response among the entire cohort (P = .29 and P = .79), nor in the patients treated with nivolumab only (P = .42 and P = .44). We used multiplex immunofluorescence (MIF) to identify specific cell populations that stained positive or negative for cytokeratin, CD8, CD4, PD-1, PD-L1, FOXP3, CTLA-4 and GZMB as potential immune predictors of response to therapy (eFigure 4 in Supplement 2). CD4-positive T-cells prior to treatment was associated with degree of pathologic response in the total population (P = .02) (eFigure 4 in Supplement 2). This association was significant in the cohort of patients treated with N+I (P = .008), but not N alone (P = .83).
Fluorodeoxyglucose PET/CT scans were performed after neoadjuvant immunotherapy and prior to surgery, a median of 14 days after therapy initiation (range, 6-20 days). Decreased fluorodeoxyglucose-avidity in the primary lesion was observed in 15 patients (52%), and 5 patients (83%) with PTR2 (P = .16). We also observed 14 of 15 patients with an increase in fluorodeoxyglucose-avidity with SUVmax of 3 or higher and 7 of 15 with SUVmax of 6 or higher in the cervical lymph nodes where neck dissection did not subsequently reveal pathologic lymph node involvement (eFigure 5 in Supplement 2) suggestive of immunotherapy-induced effect.
Discussion
Combined CTLA-4/PD-1 immune checkpoint inhibition has demonstrated activity across multiple disease types including melanoma, non–small cell lung cancer, renal cell carcinoma, and patients with colorectal cancer with mismatch repair-deficient tumors.19,20,21,22 In melanoma, combined treatment with N+I was notably associated with particularly rapid and deep tumor regression in some patients.1,2,23 A rapid and deep tumor response may be of particular benefit in the neoadjuvant setting, given a more limited window prior to surgery. This benefit does come at the cost of an increased severe immune toxic effects risk, a factor that was limiting in prior studies.1,2
In contrast, we observed that both N and N+I were generally well tolerated. Patients did not experience any delays to surgery, and severe immune-related toxic effects were limited. The difference between these findings and the prior N+I studies likely relates to the decreased dose of ipilimumab and the limited cycles of neoadjuvant therapy administered, consistent with more recent data in other histologic analyses.24 Not unexpectedly, we did observe several cases of severe toxic effects in both arms that manifested after surgery, highlighting the importance of continued multidisciplinary follow-up. We did not observe a concerning effect on surgical outcomes, although larger studies are needed to more definitively indicate that neoadjuvant immune checkpoint blockade does not adversely affect surgical healing.
As was observed with preliminary results presented for neoadjuvant pembrolizumab in patients with head and neck squamous cell carcinoma (HNSCC),18 2 cycles of neoadjuvant nivolumab administered over a few weeks was associated with significant rates of volumetric response, and clinical-to-pathologic downstaging. In addition, we observed multiple patients with pathologic responses with N, including 1 near complete tumor response. Additional cycles of therapy or more prolonged time to resection likely would have led to more significant tumor regression. We also conservatively limited our pathologic response analysis to the primary tumor lesion, which may underestimate the rate of response in involved lymph nodes. Consistent with prior melanoma data, we observed multiple responses including several pathologic near-complete or complete responses with N+I—even though ipilimumab was administered at a lower dose for just a single cycle. This study was not powered to demonstrate superiority for N+I compared with N monotherapy, and we believe caution and vigilant safety monitoring is warranted in future studies investigating N+I in the neoadjuvant setting given the potential for severe toxic effects and lack of proven benefit in the metastatic setting, which could lead to an unfavorable risk-to-benefit ratio with these agents.
Correlative studies confirmed both treatment arms were balanced for baseline features such as tumor PD-L1 expression. We identified a significant correlation between CD4-positive T-cells and pathologic response in patients in the N+I arm. This exploratory finding should be further investigated in future studies to help better identify patients most likely to derive benefits from a neoadjuvant immunotherapy approach. Patients with oral squamous cell carcinoma historically have a poor prognosis; therefore, these patients may derive more benefit from a neoadjuvant immunotherapy strategy that could lead to a durable antitumor immune response. We had 18 patients (62%) with stage IV disease, and at a median follow-up of over 14 months, 25 patients (86%) were alive and disease free. However, larger confirmatory studies are needed to prove meaningful clinical benefit, and whether patients with earlier-stage disease, such as those who were also eligible for the current study, would benefit. There remains uncertainty regarding meaningful surrogate end points in patients with HNSCC undergoing neoadjuvant immunotherapy. Our results anecdotally suggest the potential significance of pathologic response. For example, it is important that there was 1 patient for whom there was discordance prior to surgery between concerns regarding clinical progression following a single cycle of treatment and a 70% response seen during pathologic review. This patient demonstrated a radiologic complete response to nivolumab administered in the setting of metastatic disease, suggesting the original clinical observations were indicative of pseudoprogression and the degree of pathologic response observed was a true indicator of sensitivity.
This study is the first neoadjuvant HNSCC study to report sequential fluorodeoxyglucose-PET/CT examinations before and after neoadjuvant immunotherapy. We observed a high rate of increased fluorodeoxyglucose avidity within the cervical lymph node regions that proved to be pathologically negative. These findings are consistent with the potential generation of an immune response and have ramifications for studies testing neoadjuvant immunotherapy—arguing against adjusting the planned surgical approach or laterality of the neck dissected based on postimmunotherapy scans.
Limitations
Limitations of our study include the absence of a validated end point to establish a clinically meaningful response following neoadjuvant therapy, and limited follow-up. Assessment of major pathologic response in the neoadjuvant setting for oral squamous cell carcinoma is not well defined. We conservatively did not assess pathologic responses in the lymph nodes—given that pretreatment biopsies of lymph node metastases were not performed and thus would be unavailable for comparison. However, we report the first neoadjuvant data for both N and N+I in patients with oral squamous cell carcinoma. Both regimens were tolerated with promising rates of response measured using clinical, radiologic, pathologic, and biomarker end points, suggesting an effective course of neoadjuvant immunotherapy may engender immune responses that could be of benefit.
Trial Protocol
eTable 1. Agreement between the different response metrics used in the trial
eFigure 1. Progression-free (A) and overall-survival (B)
eFigure 2. Images from patient treated with chemoradiation
eFigure 3. Complete metabolic response to nivolumab therapy
eFigure 4. Multiplex immunofluorescent staining
eFigure 5. False positive increase in FDG-avid lymph node
Data Sharing Statement
References
- 1.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649-1654. doi: 10.1038/s41591-018-0197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655-1661. doi: 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 3.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948-960. doi: 10.1016/S1470-2045(19)30151-2 [DOI] [PubMed] [Google Scholar]
- 4.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;379(22):2185. doi: 10.1056/NEJMoa1716078 [DOI] [PubMed] [Google Scholar]
- 5.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol. 2018;36(34):3353-3360. doi: 10.1200/JCO.18.01148 [DOI] [PubMed] [Google Scholar]
- 6.Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477-486. doi: 10.1038/s41591-018-0337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470-476. doi: 10.1038/s41591-018-0339-5 [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382-1399. doi: 10.1158/2159-8290.CD-16-0577 [DOI] [PubMed] [Google Scholar]
- 9.Friedman J, Moore EC, Zolkind P, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernier J, Domenge C, Ozsahin M, et al. ; European Organization for Research and Treatment of Cancer Trial 22931 . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-1952. doi: 10.1056/NEJMoa032641 [DOI] [PubMed] [Google Scholar]
- 11.Cooper JS, Pajak TF, Forastiere AA, et al. ; Radiation Therapy Oncology Group 9501/Intergroup . Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-1944. doi: 10.1056/NEJMoa032646 [DOI] [PubMed] [Google Scholar]
- 12.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi: 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 14.Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer. 2015;3:54. doi: 10.1186/s40425-015-0097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. 2017;130(22):2420-2430. doi: 10.1182/blood-2017-03-770719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uppaluri R, Zolkind P, Lin T, et al. Neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV negative head and neck squamous cell carcinoma (HNSCC). Journal of Clinical Oncology. 2017;35(15_suppl):6012-6012. [Google Scholar]
- 19.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi: 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535-1546. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators . Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 23.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122-133. doi: 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. doi: 10.1186/s13046-019-1259-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Agreement between the different response metrics used in the trial
eFigure 1. Progression-free (A) and overall-survival (B)
eFigure 2. Images from patient treated with chemoradiation
eFigure 3. Complete metabolic response to nivolumab therapy
eFigure 4. Multiplex immunofluorescent staining
eFigure 5. False positive increase in FDG-avid lymph node
Data Sharing Statement