Abstract
Objective
There are widespread efforts to increase symptom awareness of ‘pelvic/abdominal pain, increased abdominal size/bloating, difficulty eating/feeling full and urinary frequency/urgency’ in an attempt to diagnose ovarian cancer earlier. Long-term survival of women with these symptoms adjusted for known prognostic factors is yet to be determined. This study explored the association of symptoms, routes and interval to diagnosis and long-term survival in a population-based cohort of postmenopausal women diagnosed with invasive epithelial tubo-ovarian cancer (iEOC) in the ‘no screen’ (control) UKCTOCS arm.
Methods
Of 101,299 women in the control arm, 574 were confirmed on outcome review to have iEOC between randomisation (2001–2005) and 31 December 2014. Data was extracted from medical notes and electronic records. A multivariable model was fitted for individual symptoms, time interval from symptom onset to diagnosis, route to diagnosis, speciality, morphological Type, age at diagnosis, year of diagnosis (period effect), stage, primary treatment, and residual disease.
Results
Women presenting with symptoms listed in the NICE guidelines (HR1.48, 95%CI1.16–1.89, p = 0.001) or the modified Goff Index (HR1·68, 95%CI1·32–2.13, p < 0.0001) had significantly worse survival than those who did not. Each additional presenting symptom decreased survival (HR1·20, 95%CI1·12–1·28, p < 0.0001). In multivariable analysis, in addition to advanced stage, increasing residual disease and inadequate primary treatment, abdominal pain and loss of appetite/feeling full were significantly associated with increased mortality.
Conclusions
The ovarian cancer symptom indices identify postmenopausal women with a poorer prognosis. This study however cannot exclude the possibility of better outcomes in those who are aware and act on their symptoms.
Keywords: Ovarian cancer, Symptoms, Routes to diagnosis, Survival, UKCTOCS, GOFF index, NICE
Highlights
-
•
This study explored the association of symptoms of ovarian cancer, interval and route to diagnosis with survival.
-
•
Focus on ‘high alert’ symptoms: pelvic/abdominal pain, increase abdominal size/bloating and difficulty eating/feeling full
-
•
The ovarian cancer ‘high alert’ symptom complexes identify postmenopausal women with a significantly poorer prognosis.
-
•
The study could not however exclude the possibility of better outcomes in those who are aware and acted on these symptoms.
1. Introduction
Ovarian cancer continues to be diagnosed at advanced stage with high fatality rates. A significant contributing factor is lack of clear alarm symptoms. To address this, substantial work in exploring symptoms has been undertaken. Although symptoms in women diagnosed with early and late stage disease were described as early as 1985 [1], it was Goff et al. [2,3] in 2004 who gave impetus to this effort by describing a symptom triad (pelvic/abdominal pain, increased abdominal size/bloating and difficulty eating/feeling full) frequently associated with ovarian cancer. This Goff Index [3] with some modifications [4,5] has since been widely used across the globe to drive awareness campaigns [6] among the public and primary care physicians.
Testing symptomatic women was studied in the DOvE randomised controlled trial [7]. Although there was no stage shift, there was a trend to higher complete tumour resection rates in women diagnosed with ovarian cancer in the intervention compared to the standard care arm (73% vs 44%; p = 0.075). Clinical implementation of symptom triggered testing in the UK found that while more women with ovarian cancer accessed expedited care pathways (two-week urgent referral), there was no stage shift [8]. This was in keeping with retrospective studies that reported no difference in stage or survival associated with time to diagnosis in a population cohort of ovarian cancer patients diagnosed in Australia [9] or with two-week urgent referral in an English hospital-based cohort [10].
We are not aware of any studies in women with ovarian cancer that explore the association of symptoms and routes to diagnosis with long term survival adjusted for prognostic factors. Data from the population cohort of women presenting clinically with invasive epithelial tubo-ovarian cancer [11] (iEOC) in the ‘no screening’ (control) arm of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) provides an opportunity to explore this issue in more depth. We report on association of symptoms, intervals and routes to diagnosis with survival adjusted for prognostic factors.
2. Materials and methods
UKCTOCS is a randomised controlled trial assessing the impact of population screening on ovarian cancer mortality [12,13]. In brief, following random invitation from population registers between 2001 and 2005, 202,638 postmenopausal women aged ≥50–74 years, were recruited through 13 regional centres in England, Wales and Northern Ireland. They were randomised to no screening (Control group – 101,359) or annual screening using a multimodal (MMS 50,640) or ultrasound (USS, 50,639) strategy. The cohort only included women with at least one ovary, no significant family history of ovarian cancer and no personal history of ovarian cancer or an active non-ovarian malignancy.
2.1. Follow-up and confirmation of diagnosis
Participants were followed up via electronic health record linkage to national cancer and death registrations (England and Wales - NHS digital; Northern Ireland-Health and Social Care Business Services Organisation and Northern Ireland Cancer Registry). Additional sources included two rounds of postal questionnaires (3–5 years after randomisation and in 2014) and direct communication from participants. For women based in England, data was also obtained from the National Cancer Intelligence Network (April 2001–March 2010) and Hospital Episode Statistics administrative records. Censorship date for this analysis was 31st Dec 2014.
As previously detailed [13,14], medical notes were retrieved for all women with notifications of a possible ovarian cancer diagnosis. An independent outcomes review committee assigned the final diagnosis [15], date of diagnosis, FIGO 2003 stage, morphological Type [16] (Type I- low-grade serous, low-grade endometrioid, clear cell, mucinous, Type II - mainly high-grade serous carcinoma eTable 1), and cause of death (where applicable).
2.2. Subjects
All women with confirmed diagnosis as per WHO 2014 of primary invasive epithelial tubo-ovarian cancer (includes tubal/peritoneal - ICD-10 C57·0, C48·1, C48·2 in addition to C56.0) on outcomes review were included in the analysis [17]. Women with borderline and non-epithelial ovarian cancer were excluded.
2.3. Symptom ascertainment
Symptom data was retrieved by hand searching of medical records, which included copies of GP and hospital letters, multidisciplinary team (MDT) summaries, hospital notes as well as trial records. All symptoms were captured unless notes review confirmed they were longstanding which was defined as >12 months. Any accompanying diagnoses (e.g. joint pain with diagnosis of osteoarthritis) were noted. No limit was placed on the number of symptoms that could be recorded for each woman.
Women were classified as ‘symptomatic’ if they had presented with any symptoms or ‘asymptomatic’ if this was documented or no symptoms were mentioned despite the availability of comprehensive documentation. Women with ‘insufficient’ documentation were classified as having missing data. The symptoms were grouped as National Institute for Health and Care Excellence (NICE) UK guidance [4] - positive (abdominal or pelvic pain, increased abdominal size or bloating, loss of appetite/feeling full and increased urinary urgency or frequency) or modified Goff Symptom index positive (abdominal or pelvic pain, increased abdominal size or bloating and loss of appetite/feeling full). The Goff symptom index includes duration and frequency of symptoms. The latter was not included in our analysis as frequency was often not captured in the hospital notes. Symptoms were also grouped according to system (gynaecological, abdominal, gastrointestinal, urinary, systemic, other) as described previously [18]. Symptoms not previously described were allocated to the most appropriate system upon agreement of two clinical researchers (JD and UM) (eTable 2).
Symptom interval was calculated from onset to date of diagnosis. Date of onset was derived using GP and secondary care records. Where women had multiple episodes rather than a persistent symptom, the start date of the first episode was recorded as date of onset. When only the month was available, the midpoint (15th) was used. Diagnosis was based on histological confirmation with the date used that of primary surgery or biopsy. In cases with no surgery or biopsy, the date cytology was taken was used. Where none of these were available the date confirmatory imaging was used.
2.4. Route to diagnosis
The date the woman was first seen in hospital, the speciality involved and the route to that appointment was recorded. For those residents in England, this was supplemented where missing, with HES data. The routes were classified as
-
1)
emergency presentation (via accident and emergency department)
-
2)
two-week urgent ‘cancer’ referral to rapid access outpatient diagnostic clinic
-
3)
routine referral to secondary care (e.g. gynaecology or colorectal outpatient clinic). The patients who were seen privately (outside the NHS) were included in this group.
In women who had multiple appointments prior to diagnosis, the first was taken as their route to diagnosis.
2.5. Primary treatment and surgical outcome
The medical notes especially the surgical records and details of chemotherapy received were used to classify women into primary treatment categories (eTable 1). In patients who underwent surgery whether primary or interval debulking, the amount of residual disease as recorded by the surgeon in the operation record was extracted.
2.6. Statistical analysis
Standard survival methods were used to analyse the data, with entry fixed at date of diagnosis, exit at date of death from ovarian cancer, or date of censorship (loss to follow-up or 31st December 2014). Cox models were used to obtain hazard ratio estimates of variables. Formal tests of the proportional hazards assumption were performed using the Schoenfeld residuals.
Survival curves were based on the Kaplan-Meier (KM) estimator. However, a distinction is to be made between analyses considering
-
A)
mutually exclusive variables – (1) women presenting with symptoms listed or not on the modified Goff Index (2) symptoms listed or not in the NICE guidelines (2) (3) route to diagnosis, (4) speciality, (5) cancer type, (6) primary treatment (7) residual disease following surgery, and (8) number of symptoms where KM curves were individually estimated for each variable. KM curves for number of symptoms, were based on the Cox model as this was modelled continuously with fractional polynomials.
-
B)
variables that were not mutually exclusive – (9) individual modified Goff symptoms, (10) symptoms grouped by system where the survival curves based on the Cox model result, resulting in ‘proportional’ curves by design. Here the baseline was implied where all indicator variables were zero.
A multivariable model was fitted for individual symptoms, time interval from symptom onset to diagnosis, route to diagnosis, speciality, Type, age at diagnosis, year of diagnosis (period effect), BMI, stage, primary treatment, and residual disease. To look at period effects, the year of diagnosis was categorised with 2010 as the reference, as this was the year prior to the introduction of NICE guidelines in UK. To address the issue of missing data, we performed a multiple imputation (MI) using chained equations (MICE) to preserve as much information as possible and provide correct inference for the multivariable Cox model. The three modified Goff symptom variables were imputed using a logit model, time from symptom to diagnosis using a linear regression model after first log transforming, stage using an ordered logit model, and route to diagnosis and speciality using a multinomial logit model. 20 fully imputed datasets were created using MICE and following model fitting on all 20 sets, parameter estimates with standard errors were calculated using Rubin's rules [19].
2.7. Literature review
We searched the Cochrane Library (2010–2017), MEDLINE (1947 to week 372,017), and EMBASE (1947 to week 372,017) using the terms MESH “ovarian cancer” in combination with the terms “symptoms” or “routes to diagnosis”. We selected publications in the past 5 years, but did not exclude commonly referenced and highly regarded older publications (eTable 3).
3. Results
Between randomisation (2001–5) and 31st December 2014, 645 of the 101,299 women were diagnosed with primary tubo-ovarian cancer). This included 574 iEOC, 62 borderline epithelial and 8 non-epithelial cancers. Histology was not available for one woman. This analysis was restricted to the 574 with iEOC. The cohort consists of mainly white (97.9%) women, median age of 62·7 years at recruitment. 8·2% had a personal history of cancer and 2·1% a maternal history of ovarian cancer (eTable 4).
23·9% (137/574) had early (I and II) and 75·9% (436/574) advanced (III and IV) stage disease. The majority (73·9%; 424/574) were Type II, which represents mainly high grade serous cancer. Forty eight per cent (280/547) underwent primary surgery and adjuvant chemotherapy, 19% (110/547) neoadjuvant chemotherapy and interval debulking and 19% only chemotherapy. Complete resection of all disease, leaving no residual disease was recorded as being achieved in 31·5% (181/574) (eTable 1).
90.8% (521/574) reported symptoms (Table 1), most commonly abdominal or pelvic pain (39·5%, 227/574) or increased abdominal size or bloating (39.2%, 225/574). One in five (20%, 115/574) reported change in bowel habit. There were 29 additional symptoms to the ten listed in Table 1, which were reported by 29.8% (171/574) of women. 2.3% (13/574) of women reported no symptoms prior to diagnosis. In 6.9% (40/574) data was insufficient to determine if symptomatic or asymptomatic. In the remaining, number of symptoms ranged from 1 to 9 (median 2).
Table 1.
Presenting symptoms, interval to diagnosis and survival of women diagnosed with invasive epithelial tubo-ovarian cancer (iEOC).
| Presenting symptoms/symptom complexes (only symptoms with ≥10% women) | Number of women who had the symptom n (%) | Histological |
||||
|---|---|---|---|---|---|---|
| Type I (n = 88) | Type II (n = 424) | Type uncertain (n = 62) | p-Value between all Types | p-Value between Type I and II | ||
| Symptoms | ||||||
| Abdominal or pelvic discomfort/pain | 227 (39.5%) | 26(29.5%) | 170(40.1%) | 30(48.4%) | 0.055 | 0.058 |
| Increased abdominal size/bloating | 225 (39.2%) | 22(25%) | 167(39.4%) | 35(56.5%) | 0.001 | 0.023 |
| Change in bowel habit | 115 (20%) | 11(12.5%) | 88(20.8%) | 15(24.2%) | 0.133 | 0.068 |
| Loss of appetite/feeling full | 84 (14.6%) | 7(8%) | 62(14.6%) | 15(24.2%) | 0.022 | 0.096 |
| Weight loss | 67 (11.7%) | 6(6.8%) | 53(12.5%) | 8(12.9%) | 0.304 | 0.129 |
| Lump or mass felt by woman | 45 (7.8%) | 12(13.6%) | 29(6.8%) | 4(6.5%) | 0.089 | 0.033 |
| Respiratory symptoms | 44 (7.7%) | 3(3.4%) | 33(7.8%) | 8(12.9%) | 0.097 | 0.144 |
| Vaginal bleeding | 44 (7.7%) | 11(12.5%) | 30(7.1%) | 3(4.8%) | 0.167 | 0.106 |
| Urinary frequency | 39 (6.8%) | 8(9.1%) | 31(7.3%) | 0(0%) | 0.066 | 0.567 |
| Nausea | 29 (5.1%) | 2(2.3%) | 23(5.4%) | 4(6.5%) | 0.408 | 0.212 |
| Other symptom | 171 (29.8%) | 25(28.4%) | 126(29.7%) | 20(32.3%) | ||
| Asymptomatic | 13 (2.3%) | 2(2.3%) | 16(3.7%) | 0(0%) | ||
| Missing data | 40 (6.9%) | 9(10.2%) | 29(6.8%) | 2(3.2%) | ||
| Abdominal | 375 (65%) | 51(58%) | 277(65.3%) | 47(7.6%) | ||
| Gastrointestinal | 156 (27%) | 18(20.5%) | 118(27.8%) | 20(32.3%) | ||
| Systemic | 133 (23%) | 11(12.5%) | 101(23.8%) | 21(33.9%) | ||
| Urinary | 76 (13%) | 13(14.8%) | 61(14.4%) | 2(3.2%) | ||
| Gynaecological | 60 (10%) | 12(13.6%) | 45(10.6%) | 3(4.8%) | ||
| Other | 65 (11%) | 7(8%) | 49(11.6%) | 9(14.5%) | ||
| Modified Goff | 362 (63%) | 47 (53.4%) | 268 (63.2%) | 47 (75.8%) | ||
| NICE | 381 (66%) | 53 (60.2%) | 281 (66.3%) | 47 (75.8%) | ||
| Overall | Type I | Type II | Type uncertain | |
|---|---|---|---|---|
| Interval from symptom onset to diagnosis in days- median (inter quartile) | ||||
| No. of patients with symptoms | 521 | 77 | 384 | 60 |
| No. of patients with data on interval | 316 | 46 | 232 | 38 |
| Overall | 80 (83) | 93 (122) | 78 (82.5) | 66.5 (86) |
| Early Stage patients | 91 (88.5) | 92 (79) | 88 (71) | 146.5 (175) |
| Late Stage patients | 75 (84) | 98 (144) | 76 (82) | 66 (89) |
| One and five year survival | ||||
| No. of patients | 574 | 88 | 424 | 62 |
| One year survival (IQR) | 76% | 93% | 78% | 42% |
| Five year survival (IQR) | 33% | 76% | 29% | 5% |
| One year survival early stage patients | 94% | 97% | 91% | 50% |
| Five year survival in early stage patients | 79% | 88% | 70% | 50% |
| One year survival in late stage patients | 71% | 78% | 75% | 41% |
| Five year survival in late stage patients | 19% | 28% | 21% | 3% |
Grouping symptoms into systems revealed that 65% (375/574) of women had abdominal symptoms (Table 1). Two thirds of women were positive for NICE symptoms (66%; 381/574) with a slightly lower proportion for modified Goff symptoms (63%; 362/574).
The commonest route to diagnosis was via two-week urgent cancer referral (37%, 214/574) with the majority (85.5%, 183/213) being made to gynaecology. A similar proportion of women were referred by routine referral (32%, 185/574) and a quarter 24% (137/574) presented as an emergency (eTable 5).
The median interval from symptom onset to diagnosis in 316 women for whom data was available was 80 days (IQR 83). There was a trend to shorter median intervals in women diagnosed with Type II (78 days; IQR 82·5) and uncertain Type (66·5 days; IQR 86) (Table 1).
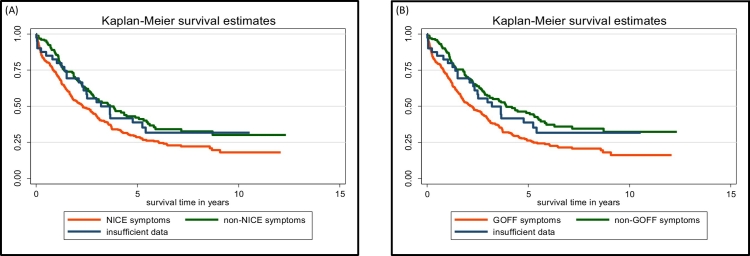
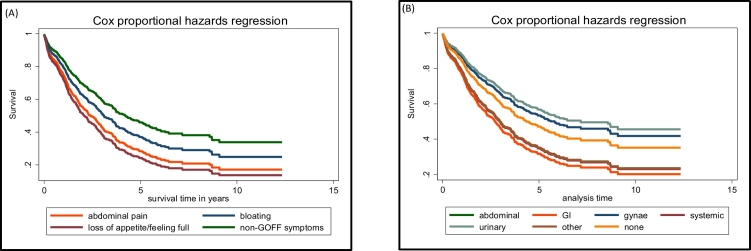
Overall one and five-year survival in this cohort was 76% and 33% respectively, with those with Type I iEOC having significantly better survival than those with Type II or Type uncertain (eFig. 1A, eTable 6). Significantly worse survival was seen in women presenting with NICE (Fig. 1A) or modified Goff (Fig. 1B) symptoms compared to no such symptoms (eTable 6). There was no significant difference in survival between women diagnosed with these symptoms prior to the introduction of NICE guidelines in UK in 2011 compared to 2012 onwards (eFig. 1B, eTable 6). The women's risk of death increased by 20% with each additional symptom beyond the first (eFig. 1C). Of the modified Goff symptoms, abdominal pain and loss of appetite/feeling full were associated with worse survival compared to the absence of those symptoms (Fig. 2A, eTable 6).
Fig. 1.
Survival curves of women with invasive epithelial tubo-ovarian cancer in the ‘no screening’ arm of UKCTOCS, with presenting symptoms included in (A) NICE guidelines vs not included (B) modified Goff symptom index vs not included.
Fig. 2.
Survival curves of women with invasive epithelial tubo-ovarian cancer in the ‘no screening’ arm of UKCTOCS, (A) With the three individual symptoms that constitute the modified Goff index (B) With symptoms affecting different systems*.
*abdominal, GI, gynae, urinary, systemic, other and none.
On univariant analysis when grouped by systems, women with gastrointestinal symptoms had worst survival and those with gynaecological and urinary symptoms the best (Fig. 2B). Compared to those presenting via two-week urgent cancer referral, women presenting as an emergency had significantly worse survival (eFig. 1D, eTable 6). Irrespective of route to diagnosis, compared to women initially managed by a gynaecologist, those initially managed by the emergency physicians or gastrointestinal physicians/surgeons had worse survival (eFig. 1E).
The multivariable analysis involves 573 patients – one women had no treatment data and was excluded. In addition to advanced stage, inadequate primary treatment and increasing residual disease, abdominal pain, loss of appetite/feeling full, were significantly associated with increased mortality (Table 2).
Table 2.
Hazard ratios for mortality in the multivariable imputation model.
| Variable | HR | 95% CI | p value | se | t |
|---|---|---|---|---|---|
| Symptoms at presentation | |||||
| Asymptomatic (compared to symptomatic) | 0.78 | 0.28–2.21 | 0.643 | 0.415 | −0.46 |
| Modified Goff Symptoms (compared to absence of symptom) | |||||
| Loss of appetite/Feeling full | 1.43 | 1.04–1.96 | 0.026 | 0.229 | 2.23 |
| Abdominal/Pelvic pain | 1.34 | 1.05–1.72 | 0.018 | 0.168 | 2.37 |
| Increase abdominal size/Bloating | 1.12 | 0.85–1.48 | 0.411 | 0.158 | 0.82 |
| NICE additional symptom in women who did not have the Modified Goff symptom (compared to absence of that symptom) | |||||
| Urinary urgency/frequency | 1.18 | 0.59–2.38 | 0.637 | 0.421 | 0.47 |
| Age | 1.01 | 0.99–1.03 | 0.241 | 0.010 | 1.17 |
| BMI | 1.00 | 0.97–1.02 | 0.888 | 0.012 | −0.14 |
| Initial specialist seen in secondary care (compared to Emergency department) | |||||
| Overall p value for variable | 0.648 | ||||
| Emergency department | 1.00 | ||||
| Gynaecology | 0.80 | 0.48–1.33 | 0.386 | 0.208 | −0.87 |
| Gastrointestinal | 0.87 | 0.55–1.40 | 0.568 | 0.209 | −0.57 |
| Other | 0.73 | 0.42–1.29 | 0.279 | 0.211 | −1.08 |
| Route to diagnosis (compared to two-week urgent cancer) | |||||
| Overall p value for variable | 0.780 | ||||
| Two-week urgent cancer | 1.00 | ||||
| Routine | 1.02 | 0.76–1.37 | 0.892 | 0.152 | 0.14 |
| Emergency | 1.12 | 0.79–1.59 | 0.506 | 0.199 | 0.66 |
| Time symptoms to diagnosis | 0.95 | 0.79–1.14 | 0.589 | 0.087 | −0.54 |
| Stage (compared to Stage 1) | |||||
| Overall p value for variable | <0.001 | ||||
| 1 | 1.00 | ||||
| 2 | 1.36 | 0.63–2.92 | 0.432 | 0.531 | 0.79 |
| 3 | 3.27 | 1.75–6.11 | <0.001 | 1.043 | 3.71 |
| 4 | 2.66 | 1.37–5.15 | 0.004 | 0.897 | 2.89 |
| Type (compared to Type I) | |||||
| Overall p value for variable | 0.326 | ||||
| I | 1.00 | ||||
| II | 1.18 | 0.69–2.03 | 0.55 | 0.327 | 0.6 |
| Uncertain | 1.50 | 0.80–2.81 | 0.209 | 0.482 | 1.26 |
| Year (compared to 2010a) | |||||
| Overall p value for variable | 0.063 | ||||
| 2010 | 1.00 | ||||
| 2002 | 1.52 | 0.51–4.51 | 0.453 | 0.843 | 0.75 |
| 2003 | 1.57 | 0.66–3.75 | 0.312 | 0.697 | 1.01 |
| 2004 | 1.68 | 0.93–3.03 | 0.087 | 0.505 | 1.71 |
| 2005 | 1.49 | 0.87–2.57 | 0.149 | 0.414 | 1.44 |
| 2006 | 1.51 | 0.92–2.49 | 0.105 | 0.384 | 1.62 |
| 2007 | 0.71 | 0.41–1.22 | 0.209 | 0.196 | −1.26 |
| 2008 | 1.02 | 0.63–1.63 | 0.948 | 0.244 | 0.07 |
| 2009 | 1.00 | 0.61–1.63 | 0.997 | 0.250 | 0 |
| 2011 | 1.05 | 0.62–1.78 | 0.846 | 0.281 | 0.19 |
| 2012 | 1.26 | 0.75–2.13 | 0.383 | 0.336 | 0.87 |
| 2013 | 1.27 | 0.72–2.25 | 0.413 | 0.370 | 0.82 |
| 2014 | 2.39 | 1.12–5.10 | 0.024 | 0.924 | 2.26 |
| Surgery- residual disease (compared to 0 mm) | |||||
| Overall p value for variable | <0.001 | ||||
| Zero | 1.00 | ||||
| >0 mm and ≤10 mm | 1.58 | 1.05–2.37 | 0.027 | 0.328 | 2.21 |
| >10 mm and ≤20 mm | 2.67 | 1.71–4.16 | <0.001 | 0.604 | 4.34 |
| >20 mm or no surgery | 2.03 | 0.91–4.50 | 0.083 | 0.825 | 1.73 |
| Primary treatment (compared to Neo adjuvant chemotherapy and interval debulking surgery) | |||||
| Overall p value for variable | <0.001 | ||||
| Neo adjuvant chemotherapy and interval debulking surgery | 1.00 | ||||
| Primary surgery plus adjuvant chemotherapy where appropriate | 0.66 | 0.47–0.91 | 0.012 | 0.110 | −2.5 |
| Primary chemotherapy | 1.91 | 0.89–4.12 | 0.097 | 0.749 | 1.66 |
| No treatment with curative intent | 5.99 | 2.69–13.35 | <0.001 | 2.448 | 4.38 |
| Primary surgery and no adjuvant chemotherapy although recommended | 12.41 | 6.33–24.34 | <0.001 | 4.265 | 7.33 |
Includes Stage I patients where chemotherapy was not recommended.
4. Discussion
In this population cohort study of postmenopausal women with invasive epithelial ovarian/tubal/peritoneal cancer, those with symptoms listed on the NICE guidelines or modified Goff Index had significantly worse survival compared to those who did not. Abdominal pain and loss of appetite/feeling full was significantly associated with increased mortality on multivariate analysis alongside advanced stage, increasing residual disease and inadequate primary treatment. It needs to be noted however, that this study cannot exclude the possibility that within the group of symptomatic women who collectively have a poor outcome, those who act early on their symptoms may have a better outcome than those who do not.
4.1. Strength and weakness
The focus is on invasive epithelial cancer, which is the major contributor to mortality in this disease. The key strength is that selection bias is minimised as significant efforts were made via linkage to multiple national registries and postal follow-up to ensure all those with the disease were identified. Site, stage and Type were confirmed by independent outcome review. Similar incidence and survival of ovarian cancer in this group compared to the UK age matched population [14] ensures that the cohort is representative and the findings therefore generalisable. Symptom data were collected using both primary and secondary care records. To allow comparison, similar rules as reported in the literature [18], were used to group symptoms by system. Although symptom data was collected prior to any diagnosis of ovarian cancer, the retrospective review of records is a weakness. Consequently the collection of symptom data is different from the Goff index that uses prospective questionnaire to screen women for symptoms of ovarian cancer. However, previous work [20] has suggested questionnaires and medical records have comparable specificity. The NICE symptom index relies on women presenting to healthcare professionals and volunteering symptoms, a method, which is more closely aligned to that used in this study. The Goff symptoms index uses both duration and frequency in the evaluation process. Though data was collected for duration of symptoms, it was missing in 45% (258/574). Multiple imputation was undertaken using MICE to preserve as much information as possible and provide correct inference. It was not possible to determine the frequency nor severity of symptoms. A further potential weakness is that the model does not include performance status or medical comorbidity, both of which could be confounders when interpreting certain symptoms and in survival.
4.2. Findings in the context of literature
Since the publication of the Goff symptom index [3], symptom awareness has been promoted as an approach that would enable earlier diagnosis of ovarian cancer and has been incorporated into national guidelines such as NICE [4]. Our findings of worse survival in women with NICE or modified Goff symptoms are in keeping with reports that these symptoms are more likely in women with advanced disease [21].
The incidence of common symptoms reported in our study (abdominal/pelvic discomfort or pain, increased abdominal size or bloating and change in bowel habit) closely matches that in similar studies [[22], [23], [24]]. As previously reported [22], increasing number of symptoms led to a worsening of survival. This is a reflection of increasing disease burden, which increases the clinical complexity, adding to the diagnostic challenge.
Our findings that emergency presentation confers worse survival compared with two-week urgent ‘cancer’ referral are in line with Barclay et al. [25] and Altman et al. [26]. It highlights the need to ensure for fast tracking of referrals to gynaecological services. However, the time interval between initial onset of symptoms and diagnosis did not independently influence survival once other factors such as age, stage and Type were included in the analysis. This aligns with Nagle et al. [9] who found that in symptomatic ovarian cancer reducing time to diagnosis does not alter stage or survival. Although reducing the time to diagnosis is associated with more favourable outcomes in breast, colorectal, head and neck, melanoma and testicular cancers [27], the benefit varies between cancers. The lack of benefit in iEOC is probably a reflection of disease biology. Across all stages, larger volumes of residual disease led to a significantly worse survival, which is in keeping with current surgical philosophy of maximal surgical effort to removal all disease [26,28,29].
The introduction of the NICE guidelines did not improve survival in our study. A recent UK based study [8] found that while the introduction of the NICE guidelines did increase in number of case of ovarian cancer being diagnosed, it did not result in a shift in stage at diagnosis.
4.3. Implications
There are two important implications of the findings of this population-based study. The first that the symptom complexes identify women with a poorer prognosis. While it is necessary to continue measures to ensure prompt referral and reduce emergency presentations, it is difficult to assess the impact of these measures on overall survival. To decrease deaths from ovarian cancer, it is critical we remain focussed on understanding disease biology, exploring preventative strategies, refining the current screening strategies by incorporating novel tests and optimising surgical and adjuvant treatment.
The second that the evidence cannot exclude the possibility of better outcomes in those who are aware and act on these symptoms compared to those who do not. A worse survival in women with more symptoms would support this. Definitive proof of this requires further randomised controlled trials like DOvE. Meanwhile, women should be encouraged to seek help as longer intervals to diagnosis and treatment are associated with reduced overall quality of life and decreased patient satisfaction [30].
5. Conclusions
Symptoms awareness using the NICE Guidance 2011 and modified Goff Index and other similar [5,31] guidance has been widely adopted as a method to identify ovarian cancer earlier. Women with these symptom complexes are likely to have advanced disease and poorer survival. The lack of significant impact on survival of healthcare interventions such as route or interval to diagnosis or secondary care team involved in initial management suggests that in invasive epithelial tubo-ovarian cancer, tumour biology is the overriding driver of survival.
The following are the supplementary data related to this article.
Survival curves for women with invasive epithelial tubo-ovarian cancer in the ‘no screening’ arm of UKCTOCS based on
A – Type
B - NICE symptoms pre 2012 and post 2012
C - Number of presenting symptoms
D - Route of presentation to secondary care
E - Specialty initially managed by in secondary care.
Supplementary tables
CRediT authorship contribution statement
James Dilley: Conceptualization, Investigation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Matthew Burnell: Methodology, Investigation, Writing - original draft, Writing - review & editing. Aleksandra Gentry-Maharaj: Methodology, Investigation, Writing - original draft, Writing - review & editing. Andy Ryan: Methodology, Formal analysis, Writing - review & editing. Christina Neophytou: Formal analysis, Writing - review & editing. Sophia Apostolidou:Writing - review & editing. Chloe Karpinskyj: Formal analysis, Writing - review & editing. Jatinderpal Kalsi: Writing - review & editing. Tim Mould: Writing - review & editing. Robert Woolas: Writing - review & editing. Naveena Singh: Writing - review & editing. Martin Widschwendter: Writing - review & editing. Lesley Fallowfield: Writing - review & editing. Stuart Campbell: Writing - review & editing. Steven J. Skates: Writing - review & editing. Alistair McGuire: Writing - review & editing. Mahesh Parmar: Writing - review & editing. Ian Jacobs: Writing - review & editing. Usha Menon: Conceptualization, Investigation, Methodology, Writing - original draft, Writing - review & editing.
Declaration of competing interest
UM has stocks in Abcodia awarded to her by UCL. IJ and SJS are co-inventors of the Risk of Ovarian Cancer Algorithm (ROCA) which has been licensed to Abcodia Ltd. by Massachusetts General Hospital (MGH) and Queen Mary University of London (QMUL). IJ has a financial interest in Abcodia. Ltd. as a shareholder and director and is entitled to a royalty payments via MGH and QMUL from any commercial use of the ROCA. SJS co-developed the ROCA with all rights assigned to MGH and QMUL. SJS receives personal fees from the LUNGevity Foundation and SISCAPA Assay Technologies as member of their Scientific Advisory Boards and from Abcodia as a consultant. All other authors declare no competing interests.
Acknowledgments
Acknowledgement
We thank the volunteers without whom this trial would not have been possible and everyone involved in conduct and oversight of UKCTOCS. We are very grateful to the current members of the UKCTOCS Trial Steering Committee - Prof Henry Kitchener (chair), Prof Julietta Patnick, Prof Jack Cuzick and Ms. Annwen Jones.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Funding
The current analysis is supported by National Institute for Health Research (NIHR) HTA grant (16/46/01) and The Eve Appeal. UKCTOCS was funded by Medical Research Council (G9901012 and G0801228), Cancer Research UK (C1479/A2884), and the Department of Health, Australia, with additional support from The Eve Appeal. Researchers at UCL were supported by the NIHR University College London Hospitals BRC and MRC core funding (MR_UU_12023).
Views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Paper presentation
An abstract has been presented as a poster at the British Gynaecological Cancer Society Annual meeting in July 2018.
References
- 1.Smith E.M., Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56(11):2727–2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Goff B.A., Mandel L.S., Melancon C.H., Muntz H.G. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. J. Am. Med. Assoc. 2004;291(22):2705–2712. doi: 10.1001/jama.291.22.2705. [DOI] [PubMed] [Google Scholar]
- 3.Goff B.A., Mandel L.S., Drescher C.W. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109(2):221–227. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 4.NICE . Vol. 32. 2011;(April). Ovarian Cancer: Recognition and Initial Management (CG122) [Google Scholar]
- 5.Assesment of symptoms that may be ovarian cancer: a guide for GPs. https://canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/assessment-symptoms-may-be-ovarian-cancer-guide-gps
- 6.Ironmonger L., Ohuma E., Ormiston-Smith N., Gildea C., Thomson C.S., Peake M.D. An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. Br. J. Cancer. 2015;112(1):207–216. doi: 10.1038/bjc.2014.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert L., Basso O., Sampalis J. Assessment of symptomatic women for early diagnosis of ovarian cancer: results from the prospective DOvE pilot project. Lancet Oncol. 2012;13(3):285–291. doi: 10.1016/S1470-2045(11)70333-3. [DOI] [PubMed] [Google Scholar]
- 8.Rai N., Nevin J., Downey G. Outcomes following implementation of symptom triggered diagnostic testing for ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;187:64–69. doi: 10.1016/j.ejogrb.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Nagle C.M., Francis J.E., Nelson A.E. Reducing time to diagnosis does not improve outcomes for women with symptomatic ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2011;29(16):2253–2258. doi: 10.1200/JCO.2010.32.2164. [DOI] [PubMed] [Google Scholar]
- 10.Neal R.D., Allgar V.L., France B. Stage, survival and delays in lung, colorectal, prostate and ovarian cancer: comparison between diagnostic routes. Br. J. Gen. Pract. 2007:212–219. [PMC free article] [PubMed] [Google Scholar]
- 11.Singh N., Gilks C.B., Hirshowitz L., Wilkinson N., McCluggage W.G. Adopting a uniform approach to site assignment in Tubo-ovarian high-grade serous carcinoma: the time has come. Int. J. Gynecol. Pathol. 2016;35(3):230–237. doi: 10.1097/PGP.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 12.Menon U., Gentry-Maharaj A., Ryan A. Recruitment to multicentre trials--lessons from UKCTOCS: descriptive study. BMJ. 2008;337:a2079. doi: 10.1136/bmj.a2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon U., Gentry-Maharaj A., Hallett R. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10(4):327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs I.J., Menon U., Ryan A. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2015;6736(15):1–12. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurman R.J., Carcangiu M.L., Herrington C.S. 2014. World Health Organisation Classification of Tumours of the Female Reproductive Organs. International Agency for Research on Cancer. [Google Scholar]
- 16.Kurman R.J., Shih I.-M. The origin and pathogenesis of epithelial ovarian cancer- a proposed unifying theory. Am. J. Surg. Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prat J. For the FC on GO. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obs. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Lim A., Mesher D., Gentry-Maharaj A. Time to diagnosis of Type I or II invasive epithelial ovarian cancers: a multicentre observational study using patient questionnaire and primary care records. BJOG. 2015:1–9. doi: 10.1111/1471-0528.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin D. John Wiley & Sons; New York: 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 20.Lim A.W.W., Mesher D., Gentry-Maharaj A. Predictive value of symptoms for ovarian cancer: comparison of symptoms reported by questionnaire, interview, and general practitioner notes. J. Natl. Cancer Inst. 2012;104(2):114–124. doi: 10.1093/jnci/djr486. [DOI] [PubMed] [Google Scholar]
- 21.Rossing M.A., Wicklund K.G., Cushing-Haugen K.L., Weiss N.S. Predictive value of symptoms for early detection of ovarian cancer. J. Natl. Cancer Inst. 2010;102(4):222–229. doi: 10.1093/jnci/djp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K., Ahn E.H., Prather C.P., Eno M.L., Im D.D., Rosenshein N.B. Patient-reported symptoms and survival in ovarian cancer. Int. J. Gynecol. Cancer. 2011;0(0):1–11. doi: 10.1097/IGC.0b013e3182259c7f. [DOI] [PubMed] [Google Scholar]
- 23.Lurie G., Thompson P.J., McDuffie K.E., Carney M.E., Goodman M.T. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol. Oncol. 2009;114(2):231–236. doi: 10.1016/j.ygyno.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett J., Sharp D.J., Stapley S., Stabb C., Hamilton W. Pathways to the diagnosis of ovarian cancer in the UK: a cohort study in primary care. BJOG An. Int. J. Obstet. Gynaecol. 2010;117(5):610–614. doi: 10.1111/j.1471-0528.2010.02499.x. [DOI] [PubMed] [Google Scholar]
- 25.Barclay M., Gildea C., Poole J., Hirschowitz L., Menon U., Nordin A. Factors affecting short-term mortality in women with ovarian, tubal, or primary peritoneal cancer. Int. J. Gynecol. Cancer. 2016;26(1):56–65. doi: 10.1097/IGC.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 26.Altman A.D., Lambert P., Love A.J. Examining the effects of time to diagnosis, income, symptoms, and incidental detection on overall survival in epithelial ovarian cancer: Manitoba Ovarian Cancer Outcomes (MOCO) Study Group. Int. J. Gynecol. Cancer. 2017;27(8):1637–1644. doi: 10.1097/IGC.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 27.Neal R.D., Tharmanathan P., France B. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer. 2015;112:92–107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Bois A., Reuss A., Pujade-Lauraine E., Harter P., Ray-Coquard I., Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the arbeitsgemeinschaft gynaekologische onkologie studiengruppe ovarialkarzin. Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 29.Vergote Ignace, Tropé Claes G., Amant Frédéric, Kristensen Gunnar B., Ehlen Tom, Johnson Nick, Verheijen René H.M., van der Burg Maria E.L., Lacave Angel J., Panici Pierluigi Benedetti, Kenter Gemma G., Casado Antonio, Mendiola Cesar, Coens Corneel, Leen T of CCG, the NCTG— a GCI Neoadjuvant chemotherapy or primary surgery in advanced ovarian cancer. N. Engl. J. Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 30.Robinson K.M., Christensen K.B., Ottesen B., Krasnik A. Diagnostic delay, quality of life and patient satisfaction among women diagnosed with endometrial or ovarian cancer: a nationwide Danish study. Qual. Life Res. 2012;21(9):1519–1525. doi: 10.1007/s11136-011-0077-3. [DOI] [PubMed] [Google Scholar]
- 31.https://www.cancercareontario.ca/sites/ccocancercare/files/assets/CCOOvaryDiagnosisPathway
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival curves for women with invasive epithelial tubo-ovarian cancer in the ‘no screening’ arm of UKCTOCS based on
A – Type
B - NICE symptoms pre 2012 and post 2012
C - Number of presenting symptoms
D - Route of presentation to secondary care
E - Specialty initially managed by in secondary care.
Supplementary tables