Abstract
Purpose
There are few effective biomarkers for neuroendocrine tumors. Precision oncology strategies have provided liquid biopsies for real-time and tailored decision-making. This has led to the development of the first neuroendocrine tumor liquid biopsy (the NETest). The NETest represents a transcriptomic signature of neuroendocrine tumor (NETs) that captures tumor biology and disease activity. The data have direct clinical application in terms of identifying residual disease, disease progress and the efficacy of treatment. In this overview we assess the available published information on the metrics and clinical efficacy of the NETest.
Material and methods
Published data on the NETest have been collated and analyzed to understand the clinical application of this multianalyte biomarker in NETs.
Results
NETest assay has been validated as a standardized and reproducible clinical laboratory measurement. It is not affected by demographic characteristics, or acid suppressive medication. Clinical utility of the NETest has been documented in gastroenteropancreatic, bronchopulmonary NETs, in paragangliomas and pheochromocytomas. The test facilitates accurate diagnosis of a NET disease, and real-time monitoring of the disease status (stable/progressive disease). It predicts aggressive tumor behavior, identifies operative tumor resection, and efficacy of the medical treatment (e.g. somatostatin analogues), or peptide receptor radionuclide therapy (PRRT). NETest metrics and clinical applications out-perform standard biomarkers like chromogranin A.
Conclusions
The NETest exhibits clinically competent metrics as an effective biomarker for neuroendocrine tumors. Measurement of NET transcripts in blood is a significant advance in neuroendocrine tumor management and demonstrates that blood provides a viable source to identify and monitor tumor status.
Keywords: NETest, Carcinoid, CgA, Liquid biopsy, Neuroendocrine
1. Introduction
The management of neuroendocrine tumors (NETs) is a complex problem. Despite advances that include adjustments in nomenclature, new imaging strategies and novel therapies, progress has been slow and limited by lack of knowledge of the molecular biology of the disease [1]. A key set of limitations that have adversely affected advances in management has been the heterogeneous nature of the disease, empiric management strategies and the relative lack of efficacy of many treatments due to the inability to precisely stratify patient cohorts. Although guidelines are widely popularized (e.g., WHO/ENETS classification of 2016 and 2017) [2–4] and repetitively regurgitated, their endemic weakness remains the lack of large databases and registries to analyze, and the intrinsic limitation of pathological categorization based on a single/limited number of lesions compared to tumor heterogeneity. The ability to promulgate rational intervention strategies is hampered by the paucity of information regarding cells of origin of the tumors, and the nature of their proliferative and metabolic regulation [5,6]. Given these limitations, outcome data range from five-year survival rates of 15–95%, contingent upon location of tumor primary site, level of metastatic spread at diagnosis, available treatments and the geographical site of care [7–9].
A wide spectrum of treatments have been proposed which range from surgical resection to drug therapy and peptide receptor radionuclide therapy (PRRT) [3,4,10]. Strategies for management vary from watch-and-wait to aggressive therapy. If identified serendipitously, cure may be achieved in rectal, appendiceal, or gastric NETs [11]. For the most part, however, the disease is disseminated at diagnosis and cure is rare. Overall, therefore, NET management reflects a diversity of interventions with the object of deferring disease advance [12]. Since most NETs exhibit an indolent course, many therapeutic strategies are employed during the evolution of an individual tumor; continual monitoring using morphological and molecular imaging or blood biomarker levels is standard of care. Indeed, monitoring the response of a tumor whether in an individual or as part of a clinical trial, is necessary to ensure appropriate usage of agents that are costly and often have severe adverse events associated with their usage [13]. Alterations in disease status are generally described using a combination of anatomical and functional imaging interfaced with clinical changes and biomarker measurements [14]. The use of Response Evaluation Criteria in Solid Tumors (RECIST) has well-described shortcomings in indolent NETs [15,16] which are reflective of the fact that the criteria were initially developed from data derived from cytotoxic drug studies [17]. Thus, RECIST and imaging assessment in general have limitations in differentiating disease stabilization from ‘pseudo-progression’ (due to therapy effects), are inaccurate in measuring metastatic disease, imprecise in evaluation of lesion size (and changes) and of a low reproducibility. A critical shortcoming of RECIST is its lack of sensitivity in the determination of progressive disease. This issue is of particular relevance in the assessment of NET disease which tends to exhibit slow growth. Thus, low volume disease (e.g., < 5 mm) is especially difficult to accurately monitor. RECIST 1.1 only considers a maximum of five lesions with two per organ. The common clinical situation of extensive metastatic disease is therefore not adequately captured using RECIST 1.1. Of equal concern is that bone disease is difficult to define accurately using CT or MRI. While RECIST 1.1 does include 18FDG-PET for solid tumors as a complement to CT, this functional imaging modality is not effectual in the majority of NETs given the generally indolent nature of the disease. Since data from 68Ga-DOTATATE PET/CT or information derived from a combination of 18FDG- and 68Ga-DOTA-TATE PET/CT is not incorporated in RECIST, the criteria lack fundamentally significant information necessary to accurately delineate NET disease. These are, however, considered in everyday practice, albeit in an empirical, non-standardized and institutional-based fashion. Such modalities, e.g., somatostatin receptor PET techniques, however, have a low sensitivity for capturing progression of disease.
Given the limitations described in the preceding paragraph, NET disease burden (either macroscopic or microscopic) is often under-staged by imaging [18–20]. Pathological examination identifies tumor deposits in > 50% of resected specimens that imaging failed to detect [19,20]. It is likely that these unidentified micrometastases are responsible for the frequent tumor recurrence noted in the aftermath of hepatic resection for liver metastases. In a GEP-NET series, 44% (n = 11 of 25) of patients who exhibited intrahepatic recurrence after margin-negative hepatectomy developed hepatic micrometastasis. Conversely seven patients with no identifiable micrometastases remained disease-free after surgical resection [20]. The incidence of occult disease, particularly hepatic, is therefore likely to be significantly higher than generally considered or reported. This issue is of considerable relevance in accurate staging and assessment of prognosis since metastatic disease is a significant negative prognostic factor [20,21]. Similarly, prognosis is positively influenced by early detection of disease (recurrent/residual disease). Thus, failure to identify either low burden disease or early progression culminates in delay in treatment with consequent adverse effects upon outcome. It should be noted that surgical resection of hepatic metastases is rarely “curative”, and the implementation of adjuvant systemic treatment may well become the norm in the future.
In the era of precision medicine, it is probable that therapeutic individualization (stratification into responders and non-responders by a predictive biomarker) will become standard of care. Treatment itself will be identified by genomic characterization to target specific molecular features of an individual tumor. Currently, detection of small liver metastases and micrometastases is limited by the ability of somatostatin-receptor based/focused imaging to identify lesions with low expression levels of SST receptors [15,19,20,22,23]. Such patients however can effectively be treated by PRRT if molecular strategies that define the tumor circulome are utilized (PRRT Predictive QuotientPPQ). Peptide receptor radionuclide therapies that target these receptors therefore may become a promising systemic treatment, with beta and possibly alpha emitter isotopes, for small volume, metastatic disease once a patient has been demonstrated to exhibit the disease.
A critical question that faces clinicians is how to identify the presence of “non-visible” disease. The second issue is to develop strategies that can identify whether a specific tumor that will respond to a specific treatment. The way forward in these two critical areas suggests that either new imaging modalities should be developed, or more sensitive and specific biomarkers identified.
The NETest has demonstrated utility for the detection of image-negative (CT, MRI, 68Ga-SSA PET/CT, and 68Ga-SSA PET/MRI negative) metastatic disease and for monitoring of disease response particularly to PRRT [23]. PRRT is, to date, the single “real” targeted treatment option in NETs. While mTOR may be a target, there is no effective strategy to identify it pre-treatment for the entirety of disease or objectively and accurately predict treatment effect. The only biomarker currently effective in predicting response to PRRT is the PRRT Predictive Quotient [24,25]. This biomarker strategy allows for stratification of patients into responders and non-responders and is 95% accurate [24].
Given the limitations of imaging [26,27], a sensitive and accurate circulating biomarker would provide important management information. There exist some general biomarkers with clinical utility in neuroendocrine disease though their utility has not proved sustainable. These include chromogranin A (CgA) and urinary 5 hydroxy-indoleacetic acid (5-HIAA) [28]. More effective secretory (monoanalyte) biomarkers for unique functional tumors such as insulin, gastrin and vasoactive intestinal polypeptide (VIP), are accurate measurements of tumor secretory activity. However, these are generated mostly from pancreatic tumors that comprise a very small group of NETs (< 5%). Utility is therefore significantly restricted. CgA is a component of neuroendocrine cell secretory granules and is measurable in blood. Some reports consider it to correlate with tumor biology and size and to provide information in respect of outcome [29,30]. More recently, the enthusiasm for the clinical use of CgA is much diminished as rigorous studies have demonstrated low utility [31–35]. Of particular concern has been the inability of the different assays to produce comparable data [36–41]. A major limitation is that CgA sensitivity ranges from 43 to 100% while the metrics for specificity are < 50% [42]. These low metrics result from abnormal CgA elevations related to acid suppressive medications (e.g., proton pump inhibitors [PPIs]), kidney failure, heart disease and other cancers [29,38,43].
Current clinical practice involves the mutual consideration of both imaging and biomarkers. Current monoanalyte measurements, however, often do not correlate with radiological evaluation [44,45]. Investigation of other cancers has concluded that evaluation of monoanalyte secretory products (exocytotic or secreted proteins) alone fails to adequately define the multiplicity of neoplasia [46]. To overcome this shortcoming, sophisticated biomathematical analyses have been devised to determine the multiple regulators of neoplastic cell biology for breast, bronchopulmonary and blood malignancies [47–49]. This type of strategy remains to be developed for NET disease for which there is no clinically applicable, genomic-based multianalyte biomarker. The development of a technique whereby blood-based molecular information could be integrated with functional imaging would be a substantial clinical advance. The ideal goal would be to deliver non-invasive, real-time, multidimensional information that defined the clinical and molecular functionality of a tumor. Thus, a liquid biopsy that is a precise diagnostic, correlates with imaging and reduces exposure to radiation (and/or cost), would be of clinical value.
This overview contextualizes the scientific basis and the clinical utility of a blood multigene NET signature in the diagnosis and management of NET disease.
1.1. The clinical and scientific need to develop molecular biomarkers
Secretory products of a tumor cell into blood cannot alone adequately define the state or progression of the neoplastic process, nor the efficacy of therapy [50,51]. Such products are unable to adequately define the cellular activity of a tumor since they do not encompass the numerous biological processes that define neoplasia and its progression (growth factor signaling, metabolic status and cell cycling) [51]. The conclusion of a number of consensus meetings and authorities in the field is that a multifactorial measurement that is effective in real-time is required to evaluate the molecular topography of a tumor. Thus, the “hallmarks of cancer”, proposed by Hanahan et al. [50,51] should be assessed by a liquid biopsy which describes and measures the molecular genomic mechanisms of the tumor cell in circulating blood. Such tools would provide more detailed information reflecting the biology of the tumor as it evolves and facilitate management.
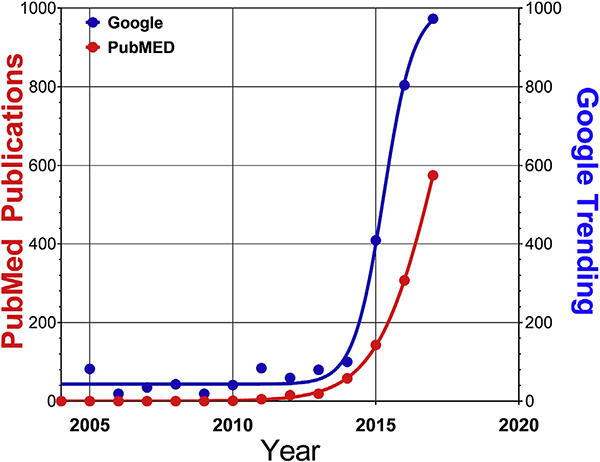
Liquid biopsies, encompass the noninvasive analysis of circulating tumor-derived material (the ‘tumor circulome’), and represent an innovative tool in precision oncology that surmounts many of the current limitations associated with tissue biopsies [52]. This non-invasive strategy exhibits several advantages compared to conventional tissue biopsy (Table 1), in addition to technological advances in sample isolation and detection platforms. It is noteworthy that focus on this biomarker strategy has increased substantially since 2004 and can be predicted to reach a 100-fold increase in relevance by 2050 (Fig. 1).
Table 1.
Comparative assessment of tissue and liquid biopsy.
| Features | Tumor Biopsy | Liquid Biopsy |
|---|---|---|
|
| ||
| Biomaterial | Tissue | Blood |
| Technique | Sharp needle intra-cavity organ penetration | Finger-prick or venous blood sample |
| Interpretation | Standard histopathology | Molecular genomic analysis |
| Timing | One or limited time access | On demand real-time repetition |
| Invasive Level | High | Non-invasive |
| Material | Tissue sample (4–5 mm) | 60 μl-1ml blood |
| Cost | High (US $5000)a | Low-moderatea |
| Data Interpretation | Subjective | Objective mathematical algorithm |
| Adverse Events | High | Negligible |
| Sampling Accuracy | Limited by tumor heterogeneity | Real-time global overview of tumor genomic activity |
| Disease Monitoring | One-time assessment of disease; no insight into tumor evolution | Real-time/continuous overview of tumor evolution |
| Tumor Status | One-time assessment; real-time infeasible; some lesions not accessible | Multiple real-time assessment of molecular biology of tumor |
| Assessment Surgical Resection | Histology of removed tissue; no accurate information on residual disease | Minimal residual disease detectable |
| Monitoring | No information without re-biopsy | Real time assessment accurate and effective |
| Prognostic Information | Based upon one-time subjective assessment | Continuous real time information available |
Costs are defined by insurance and Medicare in the US. The cost of a tissue biopsy, especially if surgery is required is high, ∼US $5000. The costs of current liquid biopsies range in price from US $1000–3500.
Fig. 1. Numbers of publications (PubMed) versus internet interest (Google Trending) related to the search term “liquid biopsy”.
The general public became aware of liquid biopsies as early as 2004 and academic publications followed a similar time course. Interest in liquid biopsies has however significantly escalated since 2012, when technologies such as cell capture and sequencing adequately evolved as clinical tools.
Adapted from Modlin IM, Kidd M, Malczewska A, Drozdov I, Bodei L, Matar S et al. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinology and Metabolism Clinics of North America 2018;47(3):485–504.
While previous focus was on the histology of a tumor there is now substantial interest on the identification and quantification of tumor products in the blood compartment-described broadly as the tumor circulome. This group colliquation represents a variety of tumor derived components, including circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), mRNA, extracellular vesicles, or “tumoreducated” platelets. Each of these components can provide various levels of information [52]. These data can then be integrated using systems biology for better delineation of the molecular biology of a specific tumor and to define, mutations, master regulators and oncogenic check points [5]. Overall, the future must include the introduction of molecular technologies to more accurately demarcate the biological status of a cancer cell. The likely way forward will include identification of mutations in circulating tumor DNA (ctDNA) or transcriptional profiles including mRNA and signal pathway analyses [53,54].
At this time, miRNA measurement in NET is difficult and inadequately standardized for clinical usage [55]. Circulating tumor cell measurement is intriguing given its direct relationship to the tumor, but technological limitations and lack of detailed studies have limited enthusiasm for its widespread application in the clinic [56]. In contradistinction to other neoplasia, activating mutations are infrequent in NETs [5] and most exhibit somatic mutations in tumor suppressor genes e.g., MEN-1, the predominant pancreatic mutation [1]. There is as yet only modest utility for other alterations e.g., in ATRX, DAXX [1] or YY1 [57]. Similarly, chromosomal imbalances and alterations in copy number or chemical-based DNA modifications e.g., methylation, have as yet minimal clinical application [5]. Currently, there exists little information to support the clinical utility of analysis of ctDNA, methylated gene targets or circulating tumor cells in NETs [1]. In more recent times, the approach for NETs has focused on mRNA-based liquid biopsy. This concept has been effective in other diseases. For example, in hepatitis, FibroSure (FibroTest) is a blood-based mRNA test for the assessment of liver damage C [58]. The FibroSure test is a repeatable, blood gene expression test, with high accuracy which avoids liver biopsy. The quantification by QT-PCR (Quantitative Polymerase Chain Reaction) technology of circulating gene expression assays (NETest) has provided real-time characterization of tumor behavior based on tumor genomic information that has clinical applicability) [59].
2. Review
2.1. Materials and methods
Published data on the NETest have been collated (Table 2) and analyzed to describe the clinical applications of this multianalyte biomarker.
Table 2.
Published studies evaluating NETest multigene signature in the Tumor Circulome.
| # | First Author | Year | NET Sites | Compartment | Cohort | Study | Diagnostic Accuracy | Ref |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | Malczewska | 2019 | SI & P | C | 174 | O & IV | 97% | [71] |
| 2 | Al-Toubah | 2019 | GEP & BP | C | 100 | O & IV | 98% | [79] |
| 3 | Malczewska | 2019 | BP | C & T | 352 | O & IV | 92% | [72] |
| 4 | Malczewska | 2019 | SI | C | n/a | CR | N/A | [23] |
| 5 | Malczewska | 2019 | G | C | 109 | O & IV | nd | Sub. |
| 6 | van Treijen | 2018 | GEP | C | 253 | O & IV | 93% | [80] |
| 7 | Barriuso | 2018 | All | C | n/a | Rv | N/A | [97] |
| 8 | Modlin | 2018 | All | C & T | n/a | Rv | N/A | [62] |
| 9 | Liu | 2018 | GEP, BP & U | C | 100 | O | 97% | [64] |
| 10 | Bodei | 2018 | GEP, BP & U | C | 281 | O | N/A | [24] |
| 11 | Genc | 2018 | P | C | 35 | O | 100% | [98] |
| 12 | Chen | 2018 | All | T | 10,224 | O & IV | N/A | [99] |
| 13 | Kyriakopoulos | 2018 | All | T | n/a | Rv | N/A | [100] |
| 14 | Perrier | 2018 | GEP | C | n/a | Rv | N/A | [101] |
| 15 | Filosso | 2018 | BP | C | 328 | O | 95% | [77] |
| 16 | Kidd | 2017 | BP & GEP | T & C | 244 | O | 100% | [76] |
| 17 | Modlin | 2017 | BP & Th | C & T | n/a | Rv | N/A | [102] |
| 18 | Chen | 2017 | GEP | C | n/a | Rv | N/A | [103] |
| 19 | Peczkowska | 2017 | PPGL, GEP, BP | C | 64 | O | nd | [78] |
| 20 | Pavel | 2017 | GEP | C | 34 | O | N/A | [65] |
| 21 | Oberg | 2016 | All | C | n/a | Rv | N/A | [104] |
| 22 | Bodei | 2016 | GEP, BP & U | C | 54 | O | N/A | [25] |
| 23 | Modlin | 2016 | All | C | n/a | Rv | N/A | [105] |
| 24 | Modlin | 2016 | GEP | C | 35 | O | 100% | [73] |
| 25 | Oberg | 2015 | All | C | n/a | Rv | N/A | [42] |
| 26 | Cwikla | 2015 | GEP | C | 63 | O | 100% | [68] |
| 27 | Modlin | 2015 | GEP | C | 179 | O | 93%*, 94%** | [74] |
| 28 | Bodei | 2015 | GEP, BP & U | C | 49 | O | 96% | [106] |
| 29 | Modlin | 2014 | GEP, BP & U | C | 81 | O | 93% | [67] |
| 30 | Modlin | 2013 | GEP | T & C | 365 | O | 90% | [63] |
BP – Bronchopulmonary; C – Circulation; CR – Case Report; G – Gastric; GEP – Gastroenteropancreatic; IV – Independent Validation; O – Original study; nd = no data, N/A = not available; P – Pancreatic; PPGLs – Paragangliomas & Pheochromocytomas; Rv- Review article; Ref- Reference; SI – Small intestine; Sub – Submitted; T – Tissue; Th – Thymic; U – Unknown Primary
In SINETs
In PNETs.
2.2. The scientific platform for the NETest
The basis for the NETest is the objective measurement of multiple NET-related genes in blood. This constitutes the tumor “biological signature” which can be easily measured at regular intervals. This provides a real-time evaluation of the status e.g., stable or progressive [59,60] of the tumor at a tissue level and can be utilized to identify how the biology of the tumor changes with time [61]. As such, this provides a measure of tumor evolution as well as providing an evaluation of treatment impact [59,62].
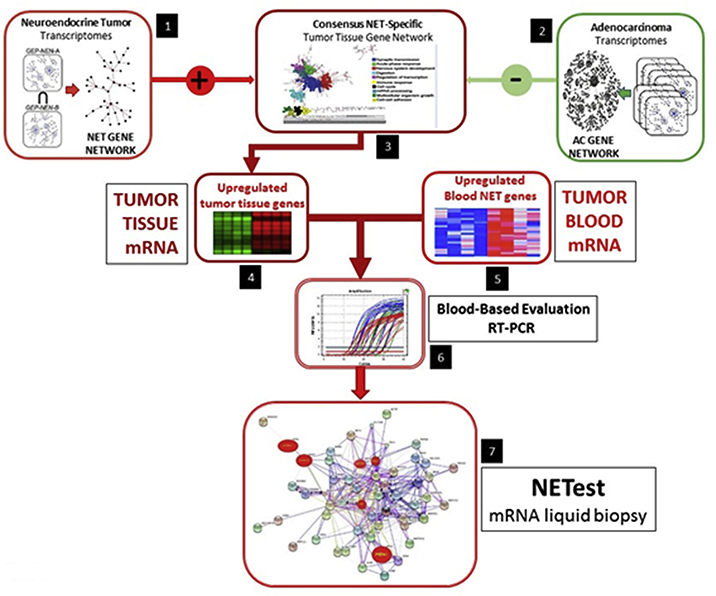
The signature is based on transcriptomic evaluation of NETs per gene discovery followed by evaluation and validation of the assay in training and independent sets (Fig. 2) [63]. The basis of the assay is provided by mRNA isolation from EDTA treated whole blood with subsequent cDNA production measured by PCR (Fig. 3) [63]. Final results are expressed as an activity index (NETest score) from 0 to 100 [24,25]. The normal score cut-off is ≤ 20. NETest values 21–40% are considered representative of “stable” disease and are thus categorized, while values 41–100 reflect “progressive” disease [62,64–66]. Clinical studies confirmed utility of the NETest for the assessment of disease status (stable/progressive); a concordance of 85–95% was identified [62].
Fig. 2. Computational pipeline utilized to derive a set of marker genes, the “NET Marker Panel” that identifies GEP-NEN/NET disease in the blood.
The steps include: the inference of gene co-expression networks and the derivation of a tissue-level GEP-NEN network (Step 1); the derivation of normal and neoplastic networks from other cancers (Step 2); the mathematical derivation of a GEP-NEN “specific network (subtraction of “normal” and “other cancer” networks (Step 3); mapping of upregulated genes to the GEP-NEN network (Step 4); evaluation and expansion of the NETwork to include blood-derived NET genes (Step 5); inclusion of genes from literature and cancer mutation database curation (Step 6) and testing and derivation of the 51 marker gene set (Step 7).
Reprinted from Modlin IM, Kidd M, Malczewska A et al. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinology and Metabolism Clinics of North America 2018;47(3):485–504, with permission from Elsevier, and Modlin IM, Drozdov I, Kidd M (2013) The Identification of Gut Neuroendocrine Tumor Disease by Multiple Synchronous Transcript Analysis in Blood. PLoS ONE 8(5): e63364.
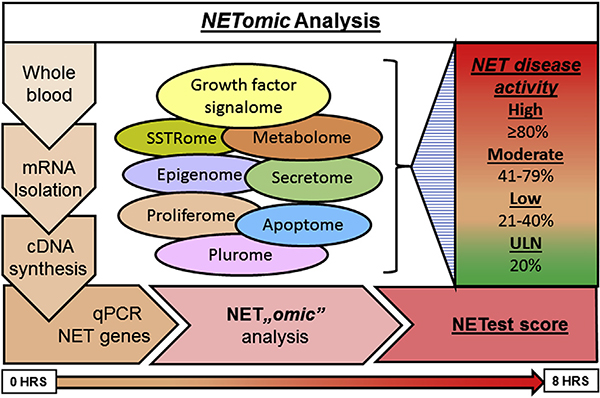
Fig. 3. The multi-step protocol used to provide a multianalyte gene expression assay result for GEP-NETs.
A 2-step protocol (mRNA isolation and cDNA synthesis) is undertaken prior to quantitative PCR gene expression. Normalized 51-marker signature is interrogated using mathematical algorithms to provide a score that is scaled 0–100% (the NETest score). The NETest delineates in a specific patient whether the tumor falls into a category of low (< 40%), moderate (40–79%) and high (≥ 80%) risk for disease activity. HRS, hours; qPCR, quantitative PCR.
The metrics of this signature as a diagnostic exhibit a sensitivity/ specificity of 90–97% [62]. Comparative studies confirmed the utility of the NETest as a diagnostic. Significantly better metrics were determined for the multigene approach (area under curve: 0.95–0.98) versus commonly used biomarkers like CgA (AUC: 0.64), pancreastatin (0.58) or neurokinin A (0.63) [62,67]. Table 3 collates comparative data on the NETest versus chromogranin A as NET biomarkers.
Table 3.
Comparison of the NETest and chromogranin A as NET biomarkers.
| Parameter | Chromogranin A | Reference | NETest | Reference |
|---|---|---|---|---|
|
| ||||
| Assay Invention | 1984 | [107] | 2013 | [63] |
| Assay Technique | Conventional | [36,38,40,105] | QT PCR | [63] |
| Assay Type | ELISA/RIA/IRMA | [36–41] | Multigene PCR based | [63] |
| Biofluid | Serum/Plasma | [36–41] | Whole blood | [62,63] |
| Analyte | Single peptide | [107] | 51 NET genes | [63] |
| Analysis | Scratchard plot analysis of binding ratios | [105] | Multianalyte algorithmic analysis of QT PCR | [62,63] |
| Dimensionality | Monodimensional/Monoanalyte | [105] | Multidimensional/Multianalyte | [67,105] |
| Assay Sensitivity | 43–100% | [42] | 90–100% | [42,62,64,65,67,70–74,76,78–80] |
| Assay Specificity | 10–96% | [42] | 90–100% | [42,62,64,65,67,70–74,76,78–80] |
| False Positive | PPI, renal failure, other tumors, cardiac disease | [29,38,43] | Not known | n/a |
| Clinical Utility | Minimal | [32–35,38,42,108] | Substantial | [62,64–66,68,69,71–74,77–80,98] |
| Diagnostic Characteristics | Assists diagnosis | [33,108] | Documented | [12,62,63,69–72,78–80] |
| GEP NET Clinical Utility | Minimal to modest | [33–35] | Substantial | [12,23,59,62–64,66–71,73,74,79,80,98] |
| BPNET Clinical Utility | None | [31,32] | Substantial | [72,76,77] |
| Assess Completeness of Surgical Resection | Limited efficacy | [31,33–35,108] | Very effective | [73,77,98] |
| Real-Time Disease Status Monitoring | Minimal to moderate effectiveness | [31,33–35,108] | Very effective | [12,25,64,66,68,71–73,98] |
GEP – Gastroenteropancreatic; BPNET – Bronchopulmonary NET; PPI – proton pump inhibitor; QT PCR – Quantitative Polymerase Chain Reaction.
Apart from its use as a diagnostic, the NETest, as noted can be used to determine tumor activity and therefore the clinical status of the patient. In principal, there are three classes of tumor activity: low biological activity ≤ 40%, intermediate biological activity: 41–79% and high (biologically aggressive) activity: 80–100% [59,62,65,68]. Independent clinical assessment of this stratification system was published using a large NIH-Registry study in 2018. This (n = 100) study demonstrated that a low NETest (≤ 40%) had an excellent outcome (PFS not reached) while intermediate and high NETest scores (41–100%) were associated with significantly shorter PFS and treatment failures [64].
The NETest is undertaken in the United Kingdom (Sarah Cannon Molecular Diagnostics Laboratory; London) and in Branford, CT in the USA. The data are identical in both laboratories and the procedure is CLIA-certified [69] (State of Connecticut: 07D2081388). The inter- and intra-assay CV < 2% [69]. There is no test value alteration associated with food, acid suppressive medication, gender, ethnicity or age [69,70]. The summated NETest data assessed in over 5500 samples demonstrate low (< 2%) day-to-day variability and high (sample concordance > 95%) reproducibility [62].
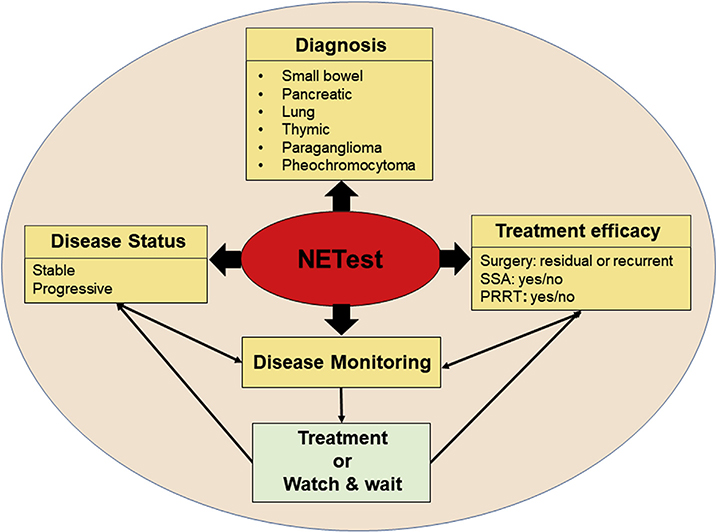
2.3. Diagnostic applications of the NETest (Fig. 4)
Fig. 4. Clinical utility of a multianalyte assay (NETest) for neuroendocrine tumor diagnosis and management.
Diagnosis: The NETest can detect lung, thymic, pancreatic, and gastrointestinal tract NETs as well as paragangliomas and pheochromocytomas (PPGL) with ≥ 90% accuracy.
Management: NETest has clinical utility in three areas: 1) Defining the status of the disease – as either stable or progressive. 2) Monitoring therapy or evaluating patients in watch-and-wait programs. 3) Determining the effectiveness of a treatment modality e.g. determining residual disease or disease “recurrence” after surgery or evaluating responses to somatostatin analogues (SSA) or peptide receptor radionuclide therapy (PRRT).
2.3.1. Detection of macroscopic disease and correlation with imaging
While imaging exhibits limitations in NET management, it remains the critical arbiter in NET disease diagnostic work-up and monitoring. A liquid biopsy which would correlate, or ideally out-perform it, would have substantial clinical utility given the opportunity to decrease radiation exposure and costs. A recent independent validation study [71] assessed the concordance of the NETest liquid biopsy with anatomical and functional imaging [71]. The NETest accurately correlates with imaging and the concordances are: CT/MRI 92%, functional imaging 94% and when all modalities are used 96% [64,65,71,72]. In image-positive disease (macroscopically detectable), the NETest was 100% concordant with anatomical imaging, and 98% concordant with 68Ga-SSA PET/CT [71].
2.3.2. Detection of microscopic (occult metastatic) disease
There is an increasing awareness of the underestimation of disease burden [18–20]. This reflects a significant clinical issue in terms of treatment delay. Since metastatic disease (especially in the liver) is a negative prognostic factor, the failure to initiate timely therapy can be predicted to negatively impact outcome. Based upon the limitations in spatial resolution < 2–4 mm (CT/MRI) or ~5 mm (68Ga-SSA PET/CT or 18F-fludeoxyglucose PET/CT), it can be logically inferred that low volume disease will not be detected. Circulating NET genes in contrast can identify as little as one tumor cell/ml [62]. Thus, it is predictable that the presence of micrometastatic disease is more likely to be identified by sophisticated molecular amplification (gene expression–PCR) techniques. In support of this assertion it has been demonstrated that the NETest could detect occult liver metastatic disease that was undetectable by imaging or monoanalyte biomarkers, but confirmed by histology [23]. The assay also enabled monitoring disease progress and response to given therapies (PRRT, SSA) [23]. Furthermore, the NETest has been shown to precede the alterations on imaging by 6–24 months which enables early implementation of effective treatment [23,25,62,64,65,68,73]. This paradigm shift in levels of disease detection opens a novel clinical discussion as to how one may treat disease that is undetectable by imaging but identified by more sensitive molecular technology. What will be the criteria for the treatment of invisible disease and would it be monitored by molecular tools as opposed to the previous norm, represented by imaging? More specifically, will NET experts decide to move from palliation to cure, if disease can be captured earlier?
2.3.3. Diagnostic for gastroenteropancreatic neuroendocrine tumors
2.3.3.1. Small intestine NEN (SINEN)
The NETest has a high accuracy level (~95%) in the diagnosis of neuroendocrine tumors of the small intestine. Furthermore, it can accurately (> 99%) distinguish a neuroendocrine bowel tumor from adenocarcinoma of the small and large intestine. A prospective analysis demonstrated an accuracy of 100% in the identification of small intestinal NENs, with 3 false positives being colorectal cancers [74]. CgA levels were elevated in 80%, but 29% (n = 7) of colorectal tumors had increased CgA levels. Metastatic small intestinal NENs exhibited increased NETest levels compared to localized disease. Overall NETest levels in SINENs were more accurate (76–80%) than CgA levels (20–32%) for detecting disease [74]. In a recent independent validation study, in a GEP-NET cohort (pancreatic NEN, n = 67, SINEN, n = 44), NETest diagnostic accuracy, sensitivity and specificity, were 97, 99 and 95%, respectively. The overall accuracy of the NETest (a cut-off of 40) for differentiating progressive from stable disease based on RECIST 1.1 criteria was 95% [71].
2.3.3.2. Pancreatic NEN (PNEN)
Pancreatic disease is sometimes difficult to accurately diagnose since fibrosis and cystic disease confound clinical and imaging interpretations. The NETest can accurately differentiate (94%) these conditions by identification of PNENs [74]. The NETest identified that 6% (2/31) of intra pancreatic mucinous neoplasia as positive. This is consistent with the co-existence of PNEN and mucinous pancreatic disease [75]. Elevations of blood CgA were identifiable in 29% of PNENs providing an overall diagnostic accuracy of 56% [74] (Fig. 5).
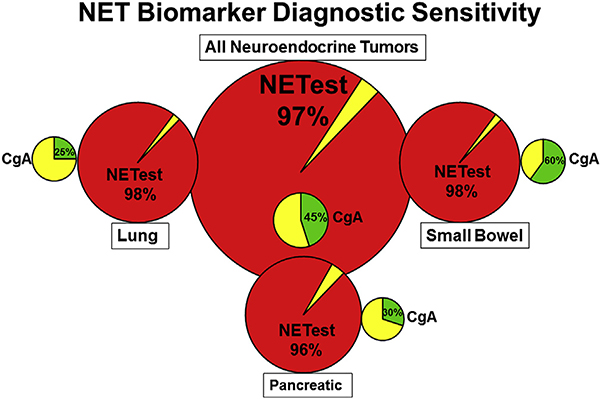
Fig. 5. Comparison of the sensitivity of the blood biomarkers NETest and chromogranin A.
NETest: Positive = red. Negative = yellow. CgA Positive = green.. Negative = yellow. The NETest is overall positive in 96–98% of all NETs (bronchopulmonary, pancreatic and small bowel). CgA is significantly less accurate in small bowel - 60% positive and only ~25% in BPNETs and PNETs. The low diagnostic sensitivity indicates very limited clinical utility for CgA as a biomarker. BPNET – Bronchopulmonary NET; PNET – Pancreatic NET.
2.3.4. Diagnostic for bronchopulmonary neuroendocrine tumors (BPNEN)
The NETest can identify neuroendocrine lung lesions in ~90% and differentiate neuroendocrine lung tumors from controls with ~95% accuracy and an ROC analysis AUC of 0.96–0.99 [72,76]. In contradiction, the monoanalyte biomarker CgA was increased in 19–33%. The sensitivity and specificity range from 93 to 95% and 82–93% respectively [77]. A clinical analysis indicated that stable disease levels (33 ± 17%) were significantly lower than progressive disease (72 ± 23%) irrespective of histology. Surgical resection considered curative decreased levels to or (10 ± 5%) whereas localized disease had levels of (45 ± 21%) compared to disseminated tumors (63 ± 26%). Quantification of the clinical value of NETest vs. CgA using Decision curve analysis indicated a > 75% standardized clinical net benefit up to a risk threshold of 90% for gene expression analysis as compared to CgA. Overall, CgA as a neuroendocrine tumor marker in lung NETs displayed a net clinical benefit in < 30% of patients.
In a recent, multicenter and multinational independent validation study, elevated NETest levels were diagnostic of bronchopulmonary NETs and tumor tissue gene expression correlated with blood levels [72]. Levels were consistent with imaging data and accurately defined disease progression [71]. The NETest was significantly increased in lung carcinoids (n = 99, 45 ± 25) compared to control subjects (9 ± 8, p < 0.0001), the AUROC was 0.96 ± 0.01. The metrics of the test were overall accuracy (92%), sensitivity (84%) and specificity 100%. Levels in stable disease were 35.5 ± 18 as compared to (61 ± 26) in progressive disease (p < 0.0001). Disseminated tumor exhibited NETest elevation regardless of histological subtype (AC: p < 0.02; TC: p = 0.0006). The NETest was also elevated in small cell lung cancer (42 ± 32) and large cell neuroendocrine carcinoma (28 ± 7). The normal cut-off is 20. In non-endocrine lung cancers, adenocarcinoma (18 ± 21) and squamous cell carcinoma (12 ± 11) levels were significantly lower than in carcinoids (BPC) (p < 0.001). Benign lung disease, such as idiopathic pulmonary fibrosis exhibited increased levels (18 ± 25). Paired comparability of tumor to blood levels of gene expression indicate significant correlation for BPC (R: 0.83, p < 0.0001) and SCLC (R: 0.68) but not for SCC and ACC (R: 0.25–0.31). This is consistent with the interpretation that the gene signature originates in the tumor.
2.3.5. Diagnostic for paragangliomas and pheochromocytomas (PPGLs)
The NETest accuracy for identification was 100% in one series [78]. Analysis of the ROC area under the curve demonstrated a 0.98 in distinguishing these neural crest derived lesions from controls. There was no relationship between mutation status and NETest levels. Local disease had lower NETest levels (43 ± 7%) than multicentric (64 ± 9%) or metastatic (80 ± 9%) or disease (p < 0.04). Gene expression levels distinguished stable disease (41 ± 2%) from progressive disease (86 ± 2%) p < 0.0001) [78]. The diagnostic accuracy of the NETest for PPGLs was ~95%.
2.4. Accuracy verification by the independent validation studies
Independent validation studies are important in biomarker implementation. The NETest has been independently validated in clinical GEP [71,79,80] and lung [72] cohorts, providing further evidence for > 90% sensitivity, specificity and accuracy [62]. In 2018 the biological basis of the signature was validated in an independent genomic based multicenter NIH-funded study (10,224 tumor specimens from 32 different tumor types) that demonstrated that NETest gene panel s was significantly and specifically related to neuroendocrine tumors [81].
2.5. Applications of the NETest for therapy efficacy monitoring and longitudinal follow-up (Fig. 4)
2.5.1. Completeness of surgical resection/detection of residual disease
2.5.1.1. BPNEN
Alteration in NETest levels pre and post lung tumor resection provides objective evidence of complete tumor resection or identifies residual disease. In a prospective study of 21 NEN lung resections, all had elevated levels pre-surgery (71 ± 11%). NETest levels at 6 months had returned to normal in 12 patients [77]. Nine patients, however, had elevated levels post-surgery (66 ± 8%) and image evidence of disease recurrence. The 12 patients assessed as “disease free” exhibited a significant decrease in NETest levels to 23 ± 3% (p = 0.0005). Measurements of NETest levels in BPNETs provide objective evidence of tumor removal and identify residual disease or recurrence accurately.
2.5.1.2. GEPNEN
Alteration in NETest levels pre- and post-tumor resection provides objective evidence of complete tumor resection or identifies residual disease. A prospective analysis of 35 pancreatic and small bowel NENs reported that all exhibited elevated NETest levels pre surgery while only 14 had increased CgA levels [73]. Successful removal of the tumor reduced transcript levels from 80 ± 5%–29% ± 5, (p < 0.0001). Alterations in CgA did not correlate with tumor resection. Of the 11 patients that had R0 resections, four still had increased NETest levels at 1 month. At 6 months post-surgery, all 4 had image positive evidence of tumor recurrence.
2.5.2. Monitoring peptide receptor radionuclide therapy (PRRT)
This modality of therapy has now achieved FDA approval and is effective in certain groups of patients [82]. The standard technique for stratifying such patients for PRRT is based upon image based subjective assessment of somatostatin receptor uptake [83,84]. Nevertheless, only 66% of individuals with the most promising characteristics for PRRT at somatostatin receptor imaging based on this criteria, respond to the therapy [82]. NETest levels in PRRT patients demonstrate that the NETest correlated accurately (94%) with PRRT responders (97%) vs. non-responders (91%) [25]. Of note was that alterations in NETest scores during PRRT exhibited (89%, p < 10−6) concordance with clinical assessment of response (RECIST). Alterations in CgA levels were concordant in 24% [25].
Since somatostatin receptor (SSTR) expression is a subjective assessment and not very accurate in predicting PRRT efficacy, a gene based Positive Predictive Quotient in blood was developed to accurately stratify patients. This is based upon the integration of Ki67 levels and blood levels of genes from the NET metabolome and growth-factor signalome [25].
The growth factor and the metabolomic genes used in the signature are related to oxidative stress, metabolism and hypoxic signaling [85–87]. The elevated expression of these genes in blood probably therefore identifies tumors that are more radiosensitive given the role of hypoxia, oxidative stress and loss of DNA repair associated with radiation responsivity [88]. The specificity of PRRT efficacy prediction therefore reflects the identification of molecular mechanisms related to radiation response-associated genes which modulate tumor response to PRRT [89].
The PPQ was prospectively evaluated in three independent PRRT studies (n = 158) [24]. The PPQ is a binary predictive tool which identifies a patient as positive (PRRT responsive)) or negative (PRRT non-responsive). Analysis of the data from 158 PRRT-treated patients using Decision curve analysis (DCA) identified a > 90% predictive benefit up to a risk threshold of 80% for the PPQ test. A comparison of CgA or grade as predictors demonstrated that neither were of any benefit (< 10% across comparable risk thresholds). PPQ was in aggregate of the 3 studies 94–97% accurate in the prediction of PRRT efficacy. Clinical correlation of the accuracy of prediction demonstrated that predicted non-responders had an mPFS of 8–14 months while the mPFS in responders was not reached at 31 months after treatment. Hazard ratio calculation demonstrated a value of 47 for the PPQ. NETest levels and PPQ assessment of PRRT demonstrated a > 90% efficacy for prediction and treatment monitoring [25].
2.5.3. Assessment of somatostatin analogue (SSA) efficacy
The NETest is effective in assessing the efficacy of SSA therapy irrespective of whether Sandostatin or Lanreotide are used. In a prospective blinded investigation (n = 28) it was compared to CgA to assess treatment failure [68]. Therapy response could be predicted using univariate analysis of tumor grade and NETest. Using multiple regression analysis only the NETest predicted disease progression during SSA usage (p = 0.0002). Of note was the observation that blood levels of transcripts preceded imaging changes [64]. Analysis of CgA data indicated that it had no predictive value. In a separate study, progression could be identified in 100% of patients [64].
There has been concern that investigative studies do not accurately capture “real world” conditions in which most MDs function [90,91]. However a USA Registry study (NCT02270567) designed to assess the utility of the NETest under such conditions demonstrated considerable efficacy [64]. A USA prospective observational investigation of SSA-treated patients (n = 51) demonstrated with a low score (≤40%) that all (n = 37) patients could be managed without any treatment modification (type or dose). Conversely those (n = 24) with an elevated score (≥80%) experienced a treatment modification (86%). This second high NETest level group all (n = 24) were all identified to have disease progression indicative of failed SSA therapy. In this group 21/ 24) underwent treatment change (dose escalation of SSA, different brand of SSA or addition of other therapy such as liver embolization or PRRT). All (n = 21) were then reported to have image based stable disease after 6months. In the low score group, the median PFS was not demonstrable whereas in the high score group, an mPFS of 5 months was identified (Chi2 = 27.7, HR 60.2 (18–201), p < 0.0001) (Fig. 6).
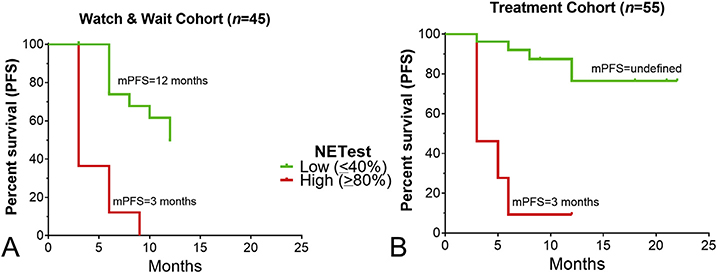
Fig. 6. Relationship between NETest and progression free survival in a prospective observational Registry Cohort (N = 100).
6A. Watch-and-wait cohort: a low NETest score was associated with mPFS of 12 months. A high score was associated with an mPFS of 3 months (HR 30.4, p < 0.0001).
6B. Treatment cohort: a low score was associated with an mPFS that was not reached at 12-months. A high score was associated with an mPFS of 5 months (HR 60.2, p < 0.0001). HR = hazard ratio.
Reprinted from Modlin IM, Kidd M, Malczewska A et al. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinology and Metabolism Clinics of North America 2018;47(3):485–504, with permission from Elsevier.
2.5.4. Monitoring long-term management
2.5.4.1. Retrospective cohort analysis
Assessment of a group (n = 34) studied for 5 years demonstrated that the NETest had both prognostic and predictive utility in GEP-NENs. Image-based progression was only identified ~ one year after elevated NETest levels were detectable [65]. In the assessment of PFS, Cox modeling defined that the only variable that determined identified PFS was the NETest level. Disease progression was definable by NETest level. Thus, median PFS was 0.68 years with levels of > 80% compared to 2.78 years with < 40% levels. Conversely, if a NETest level was > 40% in individuals classified clinically as stable, the value was 100% prognostic for progression. If the baseline NETest level was < 40%, it accurately predicted stability (100%) over 5-years. CgA values in comparison had no value. Thus, a Chi-square analysis evaluating NETest levels to CgA assessed the NETest to be 96% more instructive than CgA p < 0.001) in foretelling alteration in disease status [65].
2.5.4.2. Prospective observational study
This was undertaken to assess whether a NETest level could be used as an adjunct to management decisions in a prospective observational study. Analysis of 100 patients (Registry study (NCT02270567) [64] provided prospective information in regard to management strategy. In a watch-and-wait program (n = 28) a low NETest score defined conservative management. All 28 patients remained stable at 12 months follow up. In the high score group (NETest ≥80%; n = 12), all underwent treatment intervention. At 12 months, all had disease stabilization (imaging interpretation or symptom diminution). The high score group had an mPFS of 3 months which was significantly less than the low score group (Chi2 = 27.7, HR 30.4 [95%CI: 8.5–108], p < 0.0001). CgA in comparison was of no clinical value by McNemar’s test (comparison of two biomarkers in paired sample sets) in decision-making. Of interest in the final assessment was the observation that a low NETest score decreased the utilization of imaging by ~40% (Fig. 7).
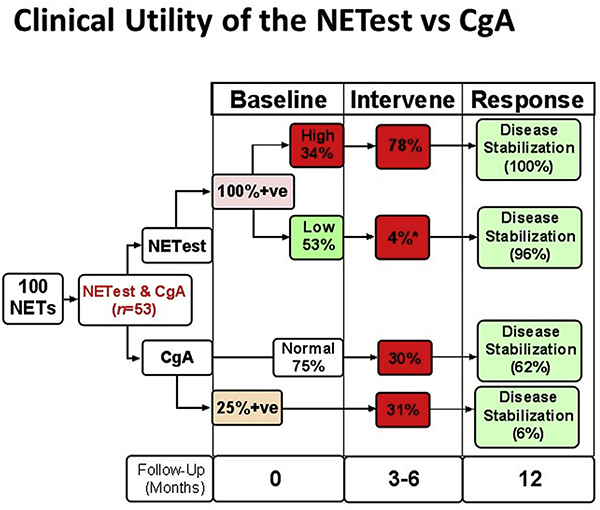
Fig. 7. Comparative clinical utility for CgA and NETest.
Of the one hundred patients enrolled (all of whom had a NETest), fifty-three had both a NETest and CgA. NETest was positive in all 53 samples while CgA was elevated in 13 (25%) and were normal in 40 (75%). High NETest scores were noted in 18 (34%) of the 53 patients. Alterations in clinical management (intervene) were made in 78%. All demonstrated disease stabilization at subsequent follow-up (12 months). Low scores were associated with a management change in 1 patient (4%). This patient, progressed on Affinitor. All other patients (96%) with low scores exhibited disease stabilization. CgA was associated with alterations in clinical management in ~30% of patients, irrespective of whether the CgA level was elevated or not. Disease stabilization ranged from 6 to 62% based on intervention and score. CgA levels therefore are unable to effectively guide disease management. *p < 0.0001 vs. high score. F/ Up = Follow-up; Mo = months; +ve, positive.
Reprinted from Modlin IM, Kidd M, Malczewska A et al. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinology and Metabolism Clinics of North America 2018;47(3):485–504, with permission from Elsevier.
3. Conclusions
3.1. The future
There is a clear need for accurate and sophisticated novel biomarkers. These tools should have three abilities which should embrace diagnosis, prediction and the assessment of disease prognosis. In clinical terms, this would allow a physician that would facilitate early identification of disease, forecast with some certitude the likelihood of therapeutic efficacy and be able to accurately monitor disease status.
The strategy that is most likely to fulfil such criteria is represented by liquid biopsy as opposed to tissue biopsy. The former technique is noninvasive, can be repeated as information is desired and provides real-time information without the potential adverse events associated with tissue biopsy. Such a strategy would provide information adjunctive to imaging and probably decrease the need for an intensive use of the latter. Of particular interest, would be the opportunity to incorporate in to liquid biopsies, information that can identify an appropriate therapy for a particular tumor. This would obviate the use of drugs that fail, provide adverse events and unnecessary burden to the health economic system. A good example is provided by circulating RNA based PRRT-predictive signature (Fig. 8).
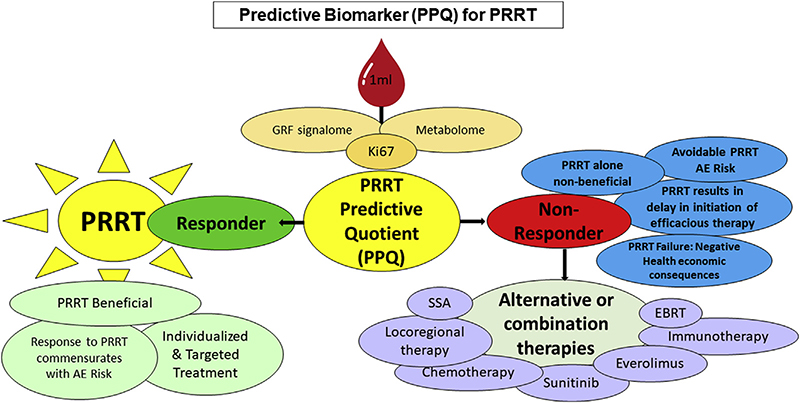
Fig. 8. Strategy for utilizing the PRRT Predictive Quotient (PPQ) to predict an individual patient response to PRRT (177Lu-Octreotate therapy).
The PPQ is derived from algorithmic analysis of growth factor signaling and metabolic pathways. Individuals are stratified into responders (green) and non-responders (red) to PRRT. Responders exhibit intact regulated growth factor signaling pathways and low-level metabolic pathways which indicate the tumor sensitivity/susceptibility to radiation and predict significant tumor DNA damage cell/ apoptosis. The non-responder group are defined by an autonomous growth factor signalome and a high-level metabolome and will not respond to PRRT. The majority (89%) of the predicted non-responders develop disease progression after PRRT. Alternative or combination therapies e.g., chemotherapy, immunotherapy or external beam radiation therapy (EBRT) may improve the likelihood or a response to PRRT.
A predictor strategy is of importance not only for forecasting drug efficacy but also for identifying potential drug toxicity. While PRRT is in general well-tolerated, kidney toxicity is a small but significant issue while rare bone marrow toxicity can be lethal. Both adverse events are not predictable, their pathogenesis undefined and there is a need to understand who may be at risk. The identification of transcript profiles for the blood or kidney that can monitor such emerging risks or predict them are an important developmental goal.
NET diagnosis and management in the USA is associated with significant and consistent resource use irrespective of tumor site or grade [92–94]. In the year prior to a formal NET diagnosis, ~USD$14,000 will be spent on identifying the disease, the majority of which is expended on biomarkers and imaging [94]. A newly diagnosed NET patient pays on average $40,100 in the first year of the disease, excluding somatostatin analogue costs ($60–70,000/year) [93]. Typically, the first 3 months of the disease is associated with the highest costs (up to $30,000) which reflect diagnostic imaging [93]. Thereafter, costs stabilize to a value of between $24,000–30,000 per year, excluding somatostatin analogues or other therapy expenses. The identification and assessment of disease progression is invariably associated with significant additional resource use comprising both diagnostic and therapeutic strategies. At any one time, however, the majority of resources (in 72–78% of patients) are focused on somatostatin analogue use [92]. While effective for symptom control, the clinical utility of this agent as an anti-proliferative drug is extremely modest as can be noted from the recent head to head comparison with PRRT [82].
A real-world assessment identified the NETest functioned as an accurate diagnostic (98–100%), could define whether a patient was responding to somatostatin analogue therapy (100% accurate) and helped reduce the use of imaging by ~50% [64]. These and other published studies have demonstrated the efficacy of the NETest in daily clinical practice [59–61]. The test is available both in the US and in Europe. In addition to the added value from the perspective of clinical utility, the test has obvious applications in terms of economic health benefit.
An accurate biomarker could decrease the costs of making a diagnosis by 50%. In the USA with a NET incidence ~7/100,000 or ~21,000 new cases per year (2012) [95], a decrease of ~$7000/patient per year would result in a cost savings in the range of ~$150 million/ year. A test that could identify a lack of somatostatin analogue treatment efficacy would be expected to result in significant cost reduction. About 50–60% of patients are treated with an analogue at any one time [92]. In the USA, with a NET prevalence of ~170,000 (in 2014) [92], about 90,000–120,000 patients are treated with SSA. In one real-world study, the NETest identified that 40% of patients were not responsive to the analogue [64] which was then discontinued. An extrapolation of this information suggests that using a molecular biomarker would result in cost savings in the range of ~$1–2 billion for non-efficacious use of a SSA. Similarly, if a blood test reduced the requirement for imaging by even one event (current costs ~$6000–24,000/year), a further reduction in annual costs ($200–500 million) would be predicted.
From a separate perspective, it is worth noting the health care cost savings related to CgA. This test is widely considered by experts and national guidelines to have minimal clinical utility [42]. Nevertheless, CgA is prescribed in the majority of NET patients between 1 and 4 times per year [92]. Thus, approximately $30–40 million (~272,000 to 540,000 tests at ~$125/test) is spent on a biomarker that has (almost) no clinical utility [96].
Since the NETest is accurate, easy to use and available, its proven clinical utility [59–61,64] provides a significant health economic cost argument. Inclusion of the NETest in the NET disease management algorithm has obvious implications both as a guide for physicians and patients but also as a strategy to contain medical costs.
3.2. Coda
The previous management strategy of a tissue biopsy followed by CgA measurement in blood requires reconsideration in the 21st century. Tissue information is obtained by random sampling of a heterogeneous tumor. It is invasive and with the evolution of disease over time and with treatment, the original information is unlikely to be as relevant. Repetitive tumor biopsy is unpleasant, has adverse events and has the same drawbacks. Ideally, real-time information from an easily accessible compartment (blood) that captures the complexity of the tumor biology would provide the best opportunity to define tumor evolution and facilitate appropriate management.
An evaluation of a blood based multigene biomarker the NETest in over seven thousand neuroendocrine tumor patients has demonstrated that it is far superior to CgA and has numerous clinical applications. It can assess successful surgical removal of a tumor, identify if recurrent disease is stable or progressing and assess if SSA therapy is effective. If PRRT treatment is considered, the use of predictive genes (PPQ) can accurately identify which patients will benefit from therapy and the NETest can thereafter be used to assess treatment efficacy. Alterations in disease status can be detected in blood by the NETest up to a year before alterations in imaging are evident.
It is probable that future strategies for disease diagnosis and real-time management will reflect the incorporation of functional imaging modalities with blood-based molecular information provided by tumor transcriptome analysis.
Acknowledgments
Financial disclosure
The authors have no funding to disclose.
Footnotes
Declaration of competing interest
Mark Kidd is an employee of Wren Laboratories. Somer Matar formerly worked at Wren Laboratories. Irvin M. Modlin and Ignat Drozdov are consultants to Wren Laboratories.
References
- [1].Kidd M, Modlin I, Oberg K. Towards a new classification of gastroenteropancreatic neuroendocrine neoplasms. Nat Rev Clin Oncol 2016;13(11):691–705. [DOI] [PubMed] [Google Scholar]
- [2].WHO classification of tumours of endocrine organs. In: Lloyd RVOR, Klöppel G, Rosai J, editors. WHO classification of tumours. fourth ed.Lyon: lARC; 2017. [Google Scholar]
- [3].O’Toole D, Kianmanesh R, Caplin M. ENETS 2016 consensus guidelines for the management of patients with digestive neuroendocrine tumors: an update. Neuroendocrinology 2016;103(2):117–8. [DOI] [PubMed] [Google Scholar]
- [4].Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J Natl Compr Cancer Netw 2018;16(6):693–702. [DOI] [PubMed] [Google Scholar]
- [5].Kidd M, Modlin I, Bodei L, Drozdov I. Decoding the molecular and mutational ambiguities of gastroenteropancreatic neuroendocrine neoplasm pathobiology..Cell Mol Gastroenterol Hepatol 2015;1:131–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lewis MA, Yao JC. Molecular pathology and genetics of gastrointestinal neuroendocrine tumours. Curr Opin Endocrinol Diabetes Obes 2013;4:4. [DOI] [PubMed] [Google Scholar]
- [7].Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113(1):5–21. [DOI] [PubMed] [Google Scholar]
- [8].Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26(18):3063–72. [DOI] [PubMed] [Google Scholar]
- [9].Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Diaz-Perez JA, Martinez Del Prado MP, Alonso Orduna V, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol 2010;21(9):1794–803. [DOI] [PubMed] [Google Scholar]
- [10].Kulke MH, Shah MH, Benson AB 3rd, Bergsland E, Berlin JD, Blaszkowsky LS, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Cancer Netw 2015;13(1):78–108. [DOI] [PubMed] [Google Scholar]
- [11].Alexandraki KI, Kaltsas GA, Grozinsky-Glasberg S, Chatzellis E, Grossman AB. Appendiceal neuroendocrine neoplasms: diagnosis and management. Endocr Relat Cancer 2016;23(1):R27–41. [DOI] [PubMed] [Google Scholar]
- [12].Pavel M Translation of molecular pathways into clinical trials of neuroendocrine tumors. Neuroendocrinology 2013;97(1):99–112. [DOI] [PubMed] [Google Scholar]
- [13].Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013;42(4):557–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Mestier L, Dromain C, d’Assignies G, Scoazec JY, Lassau N, Lebtahi R, et al. Evaluating neuroendocrine tumors progression and therapeutic response: state of the art. Endocr Relat Cancer 2013;18:18. [DOI] [PubMed] [Google Scholar]
- [15].Bodei L, Sundin A, Kidd M, Prasad V, Modlin IM. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology 2014;10:10. [DOI] [PubMed] [Google Scholar]
- [16].Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25(13):1753–9. [DOI] [PubMed] [Google Scholar]
- [17].Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer 2016;62:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clift AK, Faiz O, Al-Nahhas A, Bockisch A, Liedke MO, Schloericke E, et al. Role of staging in patients with small intestinal neuroendocrine tumours. J Gastrointest Surg 2016;20(1):180–8. discussion 8. [DOI] [PubMed] [Google Scholar]
- [19].Elias D, Lefevre JH, Duvillard P, Goere D, Dromain C, Dumont F, et al. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann Surg 2010;251(2):307–10. [DOI] [PubMed] [Google Scholar]
- [20].Gibson WE, Gonzalez RS, Cates JMM, Liu E, Shi C. Hepatic micrometastases are associated with poor prognosis in patients with liver metastases from neuroendocrine tumors of the digestive tract. Hum Pathol 2018;79:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15(1):e8–21. [DOI] [PubMed] [Google Scholar]
- [22].Charoenpitakchai M, Liu E, Zhao Z, Koyama T, Huh WJ, Berlin J, et al. Liver metastases from small intestinal neuroendocrine tumors, SSTR2A expression is heterogeneous. Virchows Arch 2017;470(5):545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Malczewska A, Bodei L, Kidd M, Modlin IM. Blood mRNA measurement (NETest) for neuroendocrine tumor diagnosis of image-negative liver metastatic disease. J Clin Endocrinol Metab 2019;104(3):867–72. [DOI] [PubMed] [Google Scholar]
- [24].Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, et al. PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. EJNMMI 2018;45(7):1155–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bodei L, Kidd M, Modlin IM, Severi S, Drozdov I, Nicolini S, et al. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2016;43(5):839–51. [DOI] [PubMed] [Google Scholar]
- [26].Castano JP, Sundin A, Maecke HR, Villabona C, Vazquez-Albertino R, Navarro E, et al. Gastrointestinal neuroendocrine tumors (NETs): new diagnostic and therapeutic challenges. Cancer Metastasis Rev 2014;5:5. [DOI] [PubMed] [Google Scholar]
- [27].Faivre S, Ronot M, Dreyer C, Serrate C, Hentic O, Bouattour M, et al. Imaging response in neuroendocrine tumors treated with targeted therapies: the experience of sunitinib. Target Oncol 2012;7(2):127–33. [DOI] [PubMed] [Google Scholar]
- [28].Modlin I, Kidd M, Taylor A, Drozdov I, Bodei L. Neuroendocrine tumor biomarkers: current status and perspectives. Neuroendocrinology 2014;100(4):265–77. [DOI] [PubMed] [Google Scholar]
- [29].Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A–biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol 2010;17(9):2427–43. [DOI] [PubMed] [Google Scholar]
- [30].Yao JC, Pavel M, Phan AT, Kulke MH, Hoosen S, St Peter J, et al. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab 2011;96(12):3741–9. [DOI] [PubMed] [Google Scholar]
- [31].Malczewska A, Kidd M, Matar S, Kos-Kudła B, Bodei L, Oberg K, et al. An assessment of circulating chromogranin A as a biomarker of bronchopulmonary neuroendocrine neoplasia: a systematic Review and meta-analysis. Neuroendocrinology 2019. 10.1159/000500525. [DOI] [PubMed] [Google Scholar]
- [32].Matar S, Malczewska A, Oberg K, Bodei L, Aslanian H, Lewczuk A, et al. Blood chromogranin A is not effective as a biomarker for diagnosis or management of bronchopulmonary carcinoids. Neuroendocrinology 2019. 10.1159/000500202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marotta V, Zatelli MC, Sciammarella C, Ambrosio MR, Bondanelli M, Colao A, et al. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer 2018;25(1):R11–29. [DOI] [PubMed] [Google Scholar]
- [34].Pulvirenti A, Rao D, McIntyre CA, Gonen M, Tang LH, Klimstra DS, et al. Limited role of Chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB 2019;21(5):612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rossi RE, Ciafardini C, Sciola V, Conte D, Massironi S. Chromogranin A in the follow-up of gastroenteropancreatic neuroendocrine neoplasms: is it really game over? A systematic Review and meta-analysis. Pancreas 2018;47(10):1249–55. [DOI] [PubMed] [Google Scholar]
- [36].An Italian program of external quality control for chromogranin A (CgA) assay: state of the art of CgA measurement. Int J Biol Mark 2005;20(4):264–8. [DOI] [PubMed] [Google Scholar]
- [37].Leon A, Torta M, Dittadi R, degli Uberti E, Ambrosio MR, Delle Fave G, et al. Comparison between two methods in the determination of circulating chromogranin A in neuroendocrine tumors (NETs): results of a prospective multicenter observational study. Int J Biol Mark 2005;20(3):156–68. [DOI] [PubMed] [Google Scholar]
- [38].Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: biochemical markers. Neuroendocrinology 2017;105(3):201–11. [DOI] [PubMed] [Google Scholar]
- [39].Stridsberg M, Eriksson B, Oberg K, Janson ET. A comparison between three commercial kits for chromogranin A measurements. J Endocrinol 2003;177(2):337–41. [DOI] [PubMed] [Google Scholar]
- [40].Verderio P, Dittadi R, Marubini E, Pizzamiglio S, Gion M, De Apollonia L, et al. An Italian program of External Quality Control for chromogranin A (CgA) assay: performance evaluation of CgA determination. Clin Chem Lab Med 2007;45(9):1244–50. [DOI] [PubMed] [Google Scholar]
- [41].Zatelli MC, Torta M, Leon A, Ambrosio MR, Gion M, Tomassetti P, et al. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian Multicenter Study. Endocr Relat Cancer 2007;14(2):473–82. [DOI] [PubMed] [Google Scholar]
- [42].Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol 2015;16(9):e435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gut P, Czarnywojtek A, Fischbach J, Baczyk M, Ziemnicka K, Wrotkowska E, et al. Chromogranin A - unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci 2016;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kidd M, Bodei L, Modlin IM. Chromogranin A: any relevance in neuroendocrine tumors? Curr Opin Endocrinol Diabetes Obes 2015;30:30. [DOI] [PubMed] [Google Scholar]
- [45].Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 2011;29(7):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Engels CC, Ruberta F, de Kruijf EM, van Pelt GW, Smit VT, Liefers GJ, et al. The prognostic value of apoptotic and proliferative markers in breast cancer. Breast Canc Res Treat 2013;142(2):323–39. [DOI] [PubMed] [Google Scholar]
- [47].Urgard E, Vooder T, Vosa U, Valk K, Liu M, Luo C, et al. Metagenes associated with survival in non-small cell lung cancer. Cancer Inf 2011;10:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Miller WR, Larionov A, Renshaw L, Anderson TJ, Walker JR, Krause A, et al. Gene expression profiles differentiating between breast cancers clinically responsive or resistant to letrozole. J Clin Oncol 2009;27(9):1382–7. [DOI] [PubMed] [Google Scholar]
- [49].Jaeger U, Kainz B. Monitoring minimal residual disease in AML: the right time for real time. Ann Hematol 2003;82(3):139–47. [DOI] [PubMed] [Google Scholar]
- [50].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- [51].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- [52].De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci 2019;40(3):172–86. [DOI] [PubMed] [Google Scholar]
- [53].Walenkamp A, Crespo G, Fierro Maya F, Fossmark R, Igaz P, Rinke A, et al. Hallmarks of gastrointestinal neuroendocrine tumours: implications for treatment. Endocr Relat Cancer 2014;21(6):R445–60. [DOI] [PubMed] [Google Scholar]
- [54].Wang E, Zaman N, McGee S, Milanese JS, Masoudi-Nejad A, O’Connor-McCourt M. Predictive genomics: a cancer hallmark network framework for predicting tumor clinical phenotypes using genome sequencing data. Semin Cancer Biol 2014;18(14):00050–9. [DOI] [PubMed] [Google Scholar]
- [55].Malczewska A, Kidd M, Matar S, Kos-Kudla B, Modlin IM. A comprehensive assessment of the role of miRNAs as biomarkers in gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology 2018;107(1):73–90. [DOI] [PubMed] [Google Scholar]
- [56].Oberg K, Modlin I, DeHerder W, Pavel M, Klimstra D, Frilling A, et al. Biomarkers for neuroendocrine tumor disease: a delphic consensus assessment of multianalytes, genomics, circulating cells and monoanalytes. Lancet Oncol 2015;16. e435046. [Google Scholar]
- [57].Shay JW, Reddel RR, Wright WE. Cancer. Cancer and telomeres–an ALTernative to telomerase. Science 2012;336(6087):1388–90. [DOI] [PubMed] [Google Scholar]
- [58].Patel K, Friedrich-Rust M, Lurie Y, Grigorescu M, Stanciu C, Lee CM, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol 2011;17(41):4581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer 2015;22(4):561–75. [DOI] [PubMed] [Google Scholar]
- [60].Kidd M, Modlin IM, Drozdov I. Gene network-based analysis identifies two potential subtypes of small intestinal neuroendocrine tumors. BMC Genomics 2014;15:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Drozdov I, Kidd M, Nadler B, Camp RL, Mane SM, Hauso O, et al. Predicting neuroendocrine tumor (carcinoid) neoplasia using gene expression profiling and supervised machine learning. Cancer 2009;115(8):1638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Modlin IM, Kidd M, Malczewska A, Drozdov I, Bodei L, Matar S, et al. The NETest: the clinical utility of multigene blood analysis in the diagnosis and management of neuroendocrine tumors. Endocrinol Metab Clin N Am 2018;47(3):485–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Modlin IM, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One 2013;8(5). e63364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liu E, Paulson S, Gulati A, Freudman J, Grosh W, Kafer S, et al. Assessment of NETest clinical utility in a U.S. Registry-based study. The Oncologist 2019. June;24(6):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pavel M, Jann H, Prasad V, Drozdov I, Modlin IM, Kidd M. NET blood transcript analysis defines the crossing of the clinical rubicon: when stable disease becomes progressive. Neuroendocrinology 2017;104(2):170–82. [DOI] [PubMed] [Google Scholar]
- [66].Modlin I, Drozdov I, Kidd M. A multitranscript blood neuroendocrine tumor molecular signature to identify treatment efficacy and disease progress. J Clin Oncol 2013;31(Suppl):A4137. [Google Scholar]
- [67].Modlin IM, Drozdov I, Alaimo D, Callahan S, Teixiera N, Bodei L, et al. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr Relat Cancer 2014;21(4):615–28. [DOI] [PubMed] [Google Scholar]
- [68].Cwikla JB, Bodei L, Kolasinska-Cwikla A, Sankowski A, Modlin IM, Kidd M. Circulating transcript analysis (NETest) in GEP-NETs treated with somatostatin analogues defines therapy. J Clin Endocrinol Metab 2015;100(11):E1437–45. [DOI] [PubMed] [Google Scholar]
- [69].Modlin I, Drozdov I, Kidd M. Gut neuroendocrine tumor blood qPCR fingerprint assay: characteristics and reproducibility. Clin Chem 2014;52(3):419–29. [DOI] [PubMed] [Google Scholar]
- [70].Modlin IM, Aslanian H, Bodei L, Drozdov I, Kidd M. A PCR blood test outperforms chromogranin A in carcinoid detection and is unaffected by PPIs. Endocr Connect 2014;14:14–0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Malczewska A, Witkowska M, Makulik K, Bocian A, Walter A, Pilch-Kowalczyk J, et al. NETest liquid biopsy is diagnostic of small intestine and pancreatic neuroendocrine tumors and correlates with imaging. Endocr Connect 2019. 10.1530/EC-19-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Malczewska A, Oberg K, Bodei L, Aslanian H, Lewczuk A, Filosso PL, et al. NETest liquid biopsy is diagnostic of lung neuroendocrine tumors and identifies progressive disease. Neuroendocrinology 2019;108(3):219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Modlin IM, Frilling A, Salem RR, Alaimo D, Drymousis P, Wasan HS, et al. Blood measurement of neuroendocrine gene transcripts defines the effectiveness of operative resection and ablation strategies. Surgery 2016;159(1):336–47. [DOI] [PubMed] [Google Scholar]
- [74].Modlin IM, Kidd M, Bodei L, Drozdov I, Aslanian H. The clinical utility of a novel blood-based multi-transcriptome assay for the diagnosis of neuroendocrine tumors of the gastrointestinal tract. Am J Gastroenterol 2015;110(8):1223–32. [DOI] [PubMed] [Google Scholar]
- [75].Ishida M, Shiomi H, Naka S, Tani T, Okabe H. Concomitant intraductal papillary mucinous neoplasm and neuroendocrine tumor of the pancreas. Oncol Lett 2013;5(1):63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kidd M, Modlin IM, Drozdov I, Aslanian H, Bodei L, Matar S, et al. A liquid biopsy for bronchopulmonary/lung carcinoid diagnosis. Oncotarget 2018;9(6):7182–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Filosso PL, Kidd M, Roffinella M, Lewczuk A, Chung KM, Kolasinska-Cwikla A, et al. The utility of blood neuroendocrine gene transcript measurement in the diagnosis of bronchopulmonary neuroendocrine tumours and as a tool to evaluate surgical resection and disease progression. Eur J Cardiothorac Surg 2018;53(3):631–9. [DOI] [PubMed] [Google Scholar]
- [78].Peczkowska M, Cwikla J, Kidd M, Lewczuk A, Kolasinska-Cwikla A, Niec D, et al. The clinical utility of circulating neuroendocrine gene transcript analysis in welldifferentiated paragangliomas and pheochromocytomas. Eur J Endocrinol 2017;176(2):143–57. [DOI] [PubMed] [Google Scholar]
- [79].Al-Toubah TE, Cives M, Valone T, Blue K, Strosberg JR. Sensitivity and specificity of the NETest: a validation study. J Clin Oncol 2019;37(4_suppl):222. [DOI] [PubMed] [Google Scholar]
- [80].van Treijen MJC, Korse CM, van Leeuwaarde RS, Saveur LJ, Vriens MR, Verbeek WHM, et al. Blood transcript profiling for the detection of neuroendocrine tumors: results of a large independent validation study. Front Endocrinol 2018;9:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen F, Zhang Y, Gibbons DL, Deneen B, Kwiatkowski DJ, Ittmann M, et al. Pancancer molecular classes transcending tumor lineage across 32 cancer types, multiple data platforms, and over 10,000 cases. Clin Cancer Res 2018;24(9):2182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376(2):125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bodei L, Kidd M, Paganelli G, Grana C, Drozdov I, Cremonesi M, editors. Clinical features are not reliable in predicting long-term toxicity after PRRT - evidence from > 800 patients to support genetic screen development. Barcelona: ENETS; 2014. [Google Scholar]
- [84].Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, de Herder WW, Feelders RA, et al. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [(177)Lu-DOTA(0),Tyr(3)]octreotate. Endocr Relat Cancer 2017;24(5):243–51. [DOI] [PubMed] [Google Scholar]
- [85].Valli A, Rodriguez M, Moutsianas L, Fischer R, Fedele V, Huang HL, et al. Hypoxia induces a lipogenic cancer cell phenotype via HIF1alpha-dependent and -independent pathways. Oncotarget 2015;6(4):1920–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Olsson AH, Yang BT, Hall E, Taneera J, Salehi A, Nitert MD, et al. Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes. Eur J Endocrinol 2011;165(4):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Day TF, Mewani RR, Starr J, Li X, Chakravarty D, Ressom H, et al. Transcriptome and proteome analyses of TNFAIP8 knockdown cancer cells reveal new insights into molecular determinants of cell survival and tumor progression. Methods Mol Biol 2017;1513:83–100. [DOI] [PubMed] [Google Scholar]
- [88].Hill RP. The changing paradigm of tumour response to irradiation. Br J Radiol 2017;90(1069):20160474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kidd M, Modlin IM. Therapy: the role of liquid biopsies to manage and predict PRRT for NETs. Nat Rev Gastroenterol Hepatol 2017;14(6):331–2. [DOI] [PubMed] [Google Scholar]
- [90].Ginsburg GS, Kuderer NM. Comparative effectiveness research, genomics-enabled personalized medicine, and rapid learning health care: a common bond. J Clin Oncol 2012;30(34):4233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Malik SM, Pazdur R, Abrams JS, Socinski MA, Sause WT, Harpole DH Jr., et al. Consensus report of a joint NCI thoracic malignancies steering committee: FDA workshop on strategies for integrating biomarkers into clinical development of new therapies for lung cancer leading to the inception of “master protocols” in lung cancer. J Thorac Oncol 2014;9(10):1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Strosberg J, Casciano R, Stern L, Parikh R, Chulikavit M, Willet J, et al. United States-based practice patterns and resource utilization in advanced neuroendocrine tumor treatment. World J Gastroenterol 2013;19(15):2348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shen C, Chu Y, Halperin DM, Dasari A, Zhou S, Xu Y, et al. Carcinoid syndrome and costs of care during the first year after diagnosis of neuroendocrine tumors among elderly patients. The Oncologist 2017;22(12):1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shen C, Dasari A, Xu Y, Zhou S, Gu D, Chu Y, et al. Pre-existing symptoms and healthcare utilization prior to diagnosis of neuroendocrine tumors: a SEER-medicare database study. Sci Rep 2018;8(1):16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3(10):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Matthews C, Doshi S. Cost analysis and economic advantages of the NETest. In: Laboratories W, editor. Cost calculator based analysis of economic value of NETest in current medical climate. Boston: Boston Healthcare Associates; 2018. [Google Scholar]
- [97].Barriuso J, Custodio A, Afonso R, Alonso V, Astudillo A, Capdevila J, et al. Prognostic and predictive biomarkers for somatostatin analogues, peptide receptor radionuclide therapy and serotonin pathway targets in neuroendocrine tumours. Cancer Treat Rev 2018;70:209–22. [DOI] [PubMed] [Google Scholar]
- [98].Genc CG, Jilesen APJ, Nieveen van Dijkum EJM, Klumpen HJ, van Eijck CHJ, Drozdov I, et al. Measurement of circulating transcript levels (NETest) to detect disease recurrence and improve follow-up after curative surgical resection of welldifferentiated pancreatic neuroendocrine tumors. J Surg Oncol 2018;118(1):37–48. [DOI] [PubMed] [Google Scholar]
- [99].Chen F, Zhang Y, Gibbons DL, Deneen B, Kwiatkowski DJ, Ittmann M, et al. Pancancer molecular classes transcending tumor lineage across 32 cancer types, multiple data platforms, and over 10,000 cases. Clin Cancer Res 2018;24(9):2182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kyriakopoulos G, Mavroeidi V, Chatzellis E, Kaltsas GA, Alexandraki KI. Histopathological, immunohistochemical, genetic and molecular markers of neuroendocrine neoplasms. Ann Transl Med 2018;6(12):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Perrier ND. From initial description by Wermer to present-day MEN1: what have we learned? World J Surg 2018;42(4):1031–5. [DOI] [PubMed] [Google Scholar]
- [102].Modlin IM, Kidd M, Filosso PL, Roffinella M, Lewczuk A, Cwikla J, et al. Molecular strategies in the management of bronchopulmonary and thymic neuroendocrine neoplasms. J Thorac Dis 2017;9(Suppl 15). S1458–s73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chen L, Chen M, Chen J. Advances of circulating biomarkers in gastroenteropancreatic neuroendocrine neoplasms. 2017;20(3):357–60. Zhonghua Wei Chang Wai Ke Za Zhi. [PubMed] [Google Scholar]
- [104].Oberg K, Krenning E, Sundin A, Bodei L, Kidd M, Tesselaar M, et al. A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect 2016;5(5):174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Modlin IM, Bodei L, Kidd M. Neuroendocrine tumor biomarkers: from monoanalytes to transcripts and algorithms. Best Pract Res Clin Endocrinol Metabol 2016;30(1):59–77. [DOI] [PubMed] [Google Scholar]
- [106].Bodei L, Kidd M, Modlin IM, Prasad V, Severi S, Ambrosini V, et al. Gene transcript analysis blood values correlate with (6)(8)Ga-DOTA-somatostatin analogue (SSA) PET/CT imaging in neuroendocrine tumors and can define disease status. Eur J Nucl Med Mol Imaging 2015;42(9):1341–52. [DOI] [PubMed] [Google Scholar]
- [107].O’Connor DT, Bernstein KN. Radioimmunoassay of chromogranin A in plasma as a measure of exocytotic sympathoadrenal activity in normal subjects and patients with pheochromocytoma. N Engl J Med 1984;311(12):764–70. [DOI] [PubMed] [Google Scholar]
- [108].Marotta V, Nuzzo V, Ferrara T, Zuccoli A, Masone M, Nocerino L, et al. Limitations of Chromogranin A in clinical practice. Biomarkers 2012;17(2):186–91. [DOI] [PubMed] [Google Scholar]