Abstract
Fluorescence imaging has made tremendous inroads toward understanding the complexity of biological systems, but in vivo deep-tissue imaging remains a great challenge due to the optical opacity of biological tissue. Recent improvements in laser and detector manufacturing have allowed the expansion of nonlinear and linear fluorescence imaging to the underexplored “tissue-transparent” second near-infrared (NIR-II; 1000–1700 nm) window, opening up new opportunities for optical access deep inside opaque tissue. Molecular fluorophores have historically played a major role in fluorescence bioimaging. It is increasingly important to design new molecular fluorophores to fully unlock the potential of NIR-II imaging techniques. In this outlook, we give an overview of the novel molecular fluorophores developed for deep-tissue bioimaging in the past five years and discuss their pros and cons in applications. Guidelines for designing new molecular fluorophores with the desirable properties are also provided.
Short abstract
Technological advances in imaging setup open up new opportunities for optical access deep inside opaque tissue. This outlook highlights the recent progress on the development of novel molecular fluorophores, which can be operated in the “tissue-transparent” second near-infrared window (NIR-II; 1000−1700 nm) with the help of advanced nonlinear and linear fluorescence imaging techniques.
1. Introduction
Fluorescence imaging has been widely used to investigate the mechanisms underlying various biological phenomena, as this approach can afford much detailed spatiotemporal information in living cells and ex vivo tissue sections than other imaging modalities, such as magnetic resonance imaging, ultrasound, and positron emission tomography. A more complete understanding of biological mechanisms involved in physiology and disease increasingly requires imaging exploration at deeper or intact tissue of living organisms. However, this presents a major challenge for conventional fluorescence imaging that operates in the visible spectrum, because tissues are extremely heterogeneous, and the strong scattering of visible light fundamentally limit the penetration capacity of fluorescence imaging. In recent decades, new windows for deep-tissue biological exploration have been opened in the near-infrared (NIR; 700–1700 nm) spectrum owing to suppressed photon scattering and diminished tissue autofluorescence.1,2 Fluorescence imaging techniques based on NIR nonlinear (multiphoton) excitation and NIR linear (one-photon) emission have enabled unprecedented dynamic and high-contrast exploration of neural activity,3,4 cerebral blood flow,5,6 and tumor microenvironment7−9 in living mice. Very recently, NIR fluorescence imaging of human patients has emerged as a cheap, safe, and real-time navigation modality for surgeons to localize lesions, ensure clear resection margins, and find small metastases during cancer surgery.10 Yet where there are opportunities there are challenges. The emerging deep-tissue fluorescence imaging scenarios call for the development of novel fluorophores with improved photophysical and chemical properties such as longer wavelengths, better brightness, excellent photo-/chem-stability, and diverse chemical function. As once king of fluorescence imaging, molecular fluorophores are undergoing a renaissance to meet the needs of modern biological and biomedical researches.11 In this Outlook, we discuss the recent progress and challenges in the development of novel molecular fluorophores, especially focusing on those operate in the underexplored second near-infrared window (NIR-II; 1000–1700 nm). We have grouped them into two categories, one for NIR-II-excited multiphoton microscopy, and another for NIR-II-emitted one-photon imaging.
2. Pushing the Penetration Limit with NIR-II Light
When considering a photon traveling through tissue, the penetration limit for fluorescence imaging is governed by a physical parameter called the transport mean free path (TMFP) of a photon (Figure 1a).12 TMFP describes the mean propagation distance before the photon’s direction becomes randomized. Imaging beyond TMFP with fluorescence methods results in image blur. In general, TMFP represents an upper limit for the penetration of microscopic imaging. Alternatively, when sacrificing resolution is acceptable, the penetration limit could be equivalent to several times the TMFP, such as for in vivo small animal imaging. For most mammalian tissues, TMFP can be simply defined as 1/μs(1 – g) (μs is the tissue scattering coefficient, g is the anisotropy function defining the degree of forward scattering, g = 0.8–1), when photon scattering is dominant over photon absorption. Reducing the tissue scattering coefficient for fluorescence excitation and/or emission would improve the penetration limit, which requires the exploration of an optimum spectral window.
Figure 1.
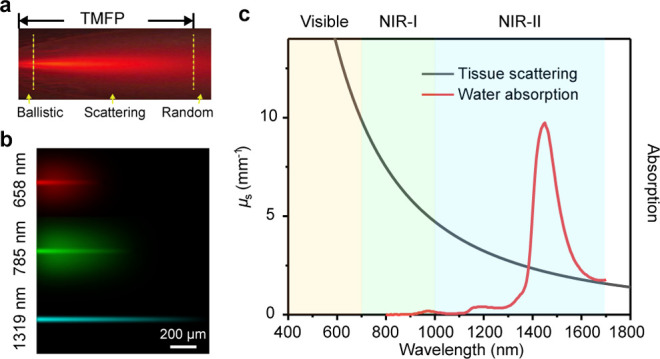
(a) Schematic depiction of TMFP by a light propagation model in tissue.12 (b) Penetration of light of different wavelengths in a tissue phantom (2.5% intralipid).7 (c) Scattering spectrum of a mouse brain model and water absorption spectrum.1
Previous studies on light-tissue interactions revealed that photon scattering nearly scales with λ–α, where λ denotes the wavelength, and the exponent α = 0.2–4.0 depends on tissue types (Figure 1c).1 Longer-wavelength light possesses lower scattering coefficient, and in most cases NIR light penetrates deeper into the scattering medium than visible light (Figure 1b). Nevertheless, in the NIR spectral region, water has a nontrivial contribution to the absorption spectrum of biological tissue (Figure 1c). The trade-off between tissue scattering and absorption sets the boundaries of suitable spectral windows that exclude a narrow water absorption band centered at 1450 nm. In the past decade, fluorescence imaging has focused on the subwindows of 1000–1400 nm and 1500–1700 nm in the NIR-II spectral region, benefiting from the available cost-effective lasers2 and affordable InGaAs detectors that are tunable or respond in these windows.
3. NIR-II Nonlinear Excitation for Deep-Tissue Bioimaging
3.1. NIR-II Multiphoton Fluorescence Imaging Techniques
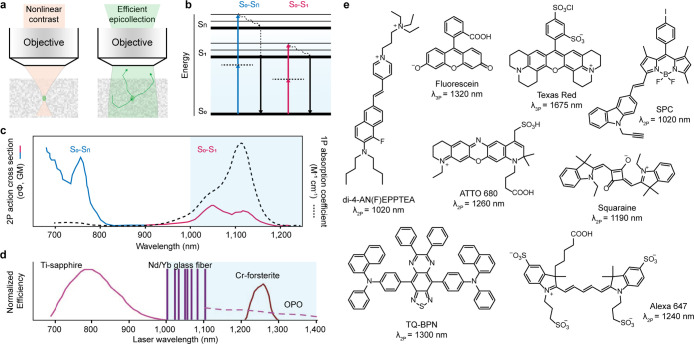
Most widely used nonlinear excitation fluorescence imaging techniques are two-/three-photon (2P/3P) microscopies.6,13 2P excitation accesses a given excited state of fluorophore by using photons of half the energy of the corresponding one-photon (1P) transition, and 3P excitation uses one-third. This nonlinear dependence on excitation intensity confines fluorescence generation to the focal volume, which reduces out of focus background and provides intrinsic three-dimensional resolution (Figure 2a). Through a “whole-area” epifluorescence collection, 2P/3P microscopy collects both scattered and ballistic signal photons to form the final images, resulting in surprisingly little signal loss (Figure 2a). Therefore, the limitation on penetration depth mostly arises from scattering and absorption of excitation light in tissues.14,15 Traditional 2P microscopy relies on Ti:sapphire lasers, which have a wavelength maximum near 1000 nm (Figure 2d), and thus restricts imaging depth to superficial cortical layers (<500 μm thick).16 Driven by advances in laser technology in the past decade, 2P microscopy at a spectral excitation window of 1000–1600 nm have pushed the imaging depth further and visualized the entire mouse cortex (about 1 mm thick) and beyond with single-cell resolution.17−19 3P microscopy extends the excitation wavelength up to 1700 nm and is capable of calcium-activity imaging at high spatial (submicrometer) and temporal resolution through the intact skull at >500 μm depth.3 The development of bright, bioavailable molecular fluorophores that excite in the NIR-II range is a crucial aspect to the advancement of in vivo 2P/3P microscopy. To date, a variety of molecular fluorophores (Figures 2e and 3a) have been used and developed. In this section, we mainly discuss molecular fluorophores designed for NIR-II 2P microcopy, in view of their accomplishments in recent years.
Figure 2.
(a) Signal generation and fluorescence collection in 2P/3P microscopy. (b) Jablonski diagram, illustrating the S0-to-S1 and S0-to-Sn 2P absorption transitions. (c) S0-to-S1 and S0-to-Sn 2P absorption transition bands in a typical 2P absorption spectrum. 1P absorption spectrum (black dotted) is presented for comparison. (d) Commercial femtosecond lasers relevant to 2P excitation.23 (e) Structures of several commercial or reported 2P/3P fluorophores with their maximum 2P/3P absorption wavelengths in the NIR-II range.
Figure 3.
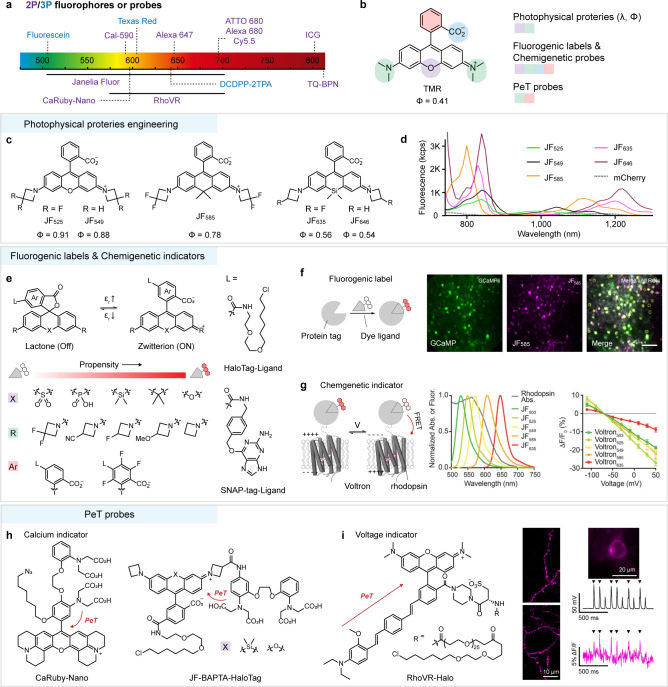
(a) Reported 2P (purple) and 3P (blue) fluorophores and probes. (b) Core structure and modification positions of rhodamine fluorophores. (c) Structure engineering at the bridging heteroatoms and nitrogen substitutes of rhodamine core to increase quantum yield and fine-tuning wavelength; subscripts in e.g. JF525 denote the maximum 1P absorption. (d) 2P peak molecular brightness spectra of JF Fluors, mCherry for comparison.48 (e) Lactone-zwitterion equilibrium in rhodamine fluorophores (εr: solvent dielectric constant) and the influence of substitute structures on the L–Z equilibrium. (f) Rhodamine fluorophore (JF585-HaloTag, magenta) as fluorogenic label for 2P fluorescence imaging of neurons in layer 5 of visual cortex, combined with GCaMP6s (green). Reproduced with permission. Copyright 2017, Springer Nature.48 (g) A hybrid chemigenetic probe (Voltron) composed of rhodamine fluorophores (JF Fluors) and a GEVI (rhodopsin). Reproduced with permission. Copyright 2019, Science Publishing Group.51 (h, i) Calcium probes (h) and voltage indicator (i) based on rhodamine fluorophores. Copyright 2019, American Chemical Society.62
3.2. NIR-II Two-Photon Excitable Molecular Fluorophores
Characterization of molecular brightness at different excitation wavelengths is a crucial first step toward their adoption as fluorophores for 2P microscopy. There are several metrics to evaluate brightness, such as 2P action cross section (σ2′, a product of absorption cross section (σ2) and quantum yield (Φ)),20 figure of merit,21 and peak molecular brightness22 (the maximum molecular brightness with increasing excitation intensity). The latter two consider molecular photostability, a serious constraint in 2P microscopy. In general, donor–acceptor charge transfer dyes have a large 2P absorption cross section at approximately two times the wavelength of their 1P spectra,20,23−25 but their low quantum yield or solvatochromic property may compromise the ability for stable fluorescent labeling. Instead, they may be more suitable for use as aggregation-induced emission (AIE) luminogens (such as TQ-BPN26) or environmental sensitive probes (such as di-4-AN(F)EPPTEA27) (Figure 2e). Traditional dyes including rhodamine, oxazine, BODIPY, cyanine, and squaraine derivatives (Figure 2e) commonly possess two 2P absorption features: one in the NIR-II range corresponds to the S0-to-S1 transition, and one in the NIR-I range corresponds to the S0-to-Sn transition (Figure 2b, c).23,24,28−36 Owing to the resonance enhancement effect, 2P S0-to-Sn transition is often stronger than S0-to-S1 transition.25 However, the benefits of NIR-II excitation, such as increased penetration depth15 and reduced photobleaching,23,30 will outweigh this disadvantage. Although it is a persistent effort goal to improve the absorption cross section, there are other factors—such as quantum yield, photostability, molecular size, functionality, and modification flexibility—that also need to be considered when selecting a fluorophore for 2P applications.
3.2.1. Rhodamine Derivatives for NIR-II Deep-Brain Two-Photon Imaging
A promising class of fluorophores is the rhodamines, which have received considerable attention due to the superb brightness and excellent photostability of the xanthene scaffold. The photophysical properties and functionalities of rhodamines can be modified at various positions (Figure 3b). In particular, the wavelengths of rhodamines vary widely as the function of bridging heteroatoms such as boron, oxygen, carbon, silicon, sulfur, and phosphorus (namely X-rhodamines, X denotes heteroatom),37−40 allowing the creation of fluorescent labels and probes in different colors. Classic rhodamines such as tetramethylrhodamine (TMR) have a low quantum yield and poor photostability due to the twisted intramolecular charge transfer (TICT) characteristic of dialkylamino donors.41 To suppress TICT, chemists would rigidify donor moieties by forming ring structures, or reduce steric hindrance42−44 and electron-donating strength45 of donor moieties by replacing substitution patterns. All these strategies gained positive returns, while a more general strategy with minimal structural change was to use four-membered azetidine rings as donors (Figure 3c),43 which preserved the requisite small size and high membrane permeability of fluorophores for use in live cells. Proposed and perfected by Lavis and co-workers,46−48 this general method created a palette of X-rhodamines (Janelia Fluor) with improved quantum efficiencies that span the visible (500–700 nm) range by fine-tuning the substitution patterns on azetidine rings (Figure 3c). The improved brightness of these rhodamines under 1P excitation also extended to 2P excitation at a wavelength range of 1000–1300 nm (Figure 3d).48 While the tendency to red shift continues in 2P absorption spectra, much larger 2P absorption cross sections occur at the blue-shifted wavelengths than at twice the 1P absorption wavelengths. This is a common phenomenon for many symmetrical dyes,20 which may allow efficient excitation of spectrally separable dyes with a single wavelength.
A unique property of rhodamines is the equilibrium between the “closed”, nonfluorescent lactone form and the “open”, fluorescent zwitterion form (Figure 3e). Discovered by Johnsson and co-workers,49 Si-rhodamines show a high propensity to form nonfluorescent but cell permeable lactone in solvents of low dielectric constant (εr). The equilibrium shifts to the open form upon an environmental change such as binding to a polar protein surface. This property of rhodamines renders them as effective fluorogenic ligands for live-cell labeling,50 including self-labeling tags, such as the SNAP-tag and HaloTag proteins. The equilibrium exists in almost all X-rhodamines, but it appears that the equilibrium propensity (lactone to zwitterion) is inversely correlated to absorption wavelength; that is, for example, near-infrared rhodamines containing sulfone (−SO2−) and phosphorus (−PO(OH)−) groups are easier to be in lactone form.47 On the basis of azetidine-substituted X-rhodamine structures, Lavis and co-workers have established principles for fine-tuning the fluorogenic properties of various X-rhodamines by exploring substitution patterns on the azetidine rings48 or the pendant phenyl ring47 (Figure 3e). In general, weakening nucleophilicity of the carboxylate anion or improving electron deficiency of the xanthene scaffold will shift the equilibrium to the open form. These improved fluorogenic X-rhodamines integrate a variety of properties, such as high brightness and photostability, compatibility with self-labeling protein tags, and the ability to traverse the blood–brain barrier for in vivo delivery, making them favorable competitors for fluorescent proteins in modern biological experiments. For example, JF585-HaloTag ligands binding to self-labeling HaloTags show superior 2P fluorescence at 1100 nm excitation, which is sufficiently separated from GFP-based indicators such as GCaMP6 (λex = 940 nm) to allow multicolor imaging of neurons in layer 5 of the visual cortex (Figure 3f).48 Recently, a “chemigenetic” indicator (Voltron) was created by fusing a genetically encoded voltage indicator (rhodopsin) with self-labeling HaloTag that irreversibly binds a variety of fluorogenic X-rhodamine ligands (Figure 3g).51 This indicator operates on a transmembrane voltage-dependent modulation of Förster resonance energy transfer (FRET) between the rhodamine dyes and the rhodopsin. Such a “chemigenetic” indicator that combines the genetic targetability and exquisite molecular recognition of proteins with the superb photophysical and sensing properties of synthetic dyes is emerging as a powerful platform for deep-tissue functional imaging.52
Molecular probes based on rhodamines have been studied for a long time,53,54 but few have been explored for 2P imaging,55 especially under NIR-II excitation. Nevertheless, 1P and 2P probes share a common mechanism for fluorescence response, that is, a photoinduced electron transfer (PeT) mechanism between fluorophore and electronic donor.56,57 The recently developed and constantly growing toolbox of PeT probes for neural activity, including calcium and voltage sensors, allows precise measurement of these signaling events with high spatial and temporal resolution under 2P microscopy. For example, a family of red-fluorescent calcium indicators, CaRuby58 and CaRuby-Nano,59 was created by conjugating a calcium chelator BAPTA [bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] to an extended rhodamine dye (Figure 3h). Calcium chelation alters the electronics of the BAPTA, resulting in reduced PeT and increased fluorescence. CaRuby-Nano was used for in vivo 2P calcium imaging from neocortical layer 2/3 (about 200 μm) pyramidal neurons at around 900 nm excitation.59 Extending excitation wavelength to ∼1050 nm by using a commercial calcium probe Cal-590 is capable to record calcium transients at depths of up to ∼900 μm below the pial surface.60 More recently, genetically targetable calcium probes JF-BAPTA-HaloTag based on X-rhodamines were developed by optimizing the relative position of the BAPTA chelator and the fluorophore (Figure 3h).61 These probes may be useful for 2P calcium imaging experiments with cell-type specificity. The Miller group has developed a family of voltage sensitive indicators (RhoVR) by coupling with a molecular wire to the pendant phenyl ring of X-rhodamine dyes (Figure 3i).62−64 These indicators operated in a membrane potential-dependent PeT process, leading to large voltage sensitivities (up to 47% ΔF/F per 100 mV). Coupling with emerging high-speed 2P microscopy, modified RhoVR can provide single-trial resolution of action potentials in neurons under 2P (λex = 1040 nm) illumination.4
4. NIR-II Linear Emission for Deep-Tissue Bioimaging
4.1. NIR-II One-Photon Fluorescence Imaging Techniques
Owing to the low cost and wide-field imaging capability, linear 1P fluorescence imaging techniques remain competitive for a variety of imaging scenarios, such as in vivo cell tracking, small-animal whole-body imaging, and imaging-guided surgery.7,10,65 However, the visible spectrum extensively employed for linear fluorescence imaging has restricted high-resolution optical imaging to thin sections or to superficial layers. In past decades, much effort had been devoted to developing far-red and near-infrared-I (NIR-I; 650–1000 nm) fluorophores to improve the penetration limit by employing the reduced tissue scattering.66,67 But the situation did not change substantially until pioneering work published in 2009, which pushed linear fluorescence imaging to the NIR-II regime by utilizing surface-modified carbon nanotubes as fluorophores.68 Imaging experiments with these materials revealed dramatic benefits in resolution and penetration depth compared with conventional fluorescence imaging at NIR-I wavelengths, for example, achieving sub-10-μm resolution of through-skull imaging of the brain vasculature at a depth of >2 mm in mouse brain.5,69−71 This pioneering work further classified the broadly defined NIR-II spectrum into three optical subwindows such as NIR-IIa′ (1000–1300 nm), NIR-IIa (1300–1400 nm), and NIR-IIb (1500–1700 nm), which yield improved fluorescence-imaging clarity with increased wavelength. Based on this criterion, a large number of novel NIR-II fluorophores, including organic dyes,72−85 quantum dots,86−88 lanthanide nanoparticles,9,89−93 and metal clusters,94 emerged and enriched the NIR-II fluorescent toolbox. Very recently, new NIR-II microscopic imaging setups have been developed such as 3D confocal microscopy and light-sheet microscopy.7,95 The use of 1319 nm illumination and fluorescence detection at 1500–1700 nm in noninvasive NIR-II light-sheet microscopic imaging afforded a penetration depth of approximately 774 μm along the tilted light-sheet direction into the head with sub-10-μm resolution.7 These achievements pave the foundation for deep-tissue biological and biomedical imaging by utilizing NIR-II linear fluorescence imaging techniques.
4.2. NIR-II One-Photon Emitted Molecular Fluorophores
Organic dyes historically play a major role in fluorescence imaging as fluorescent labels, stains, and reporters, mainly due to their advantages of low molecular mass, good biocompatibility, short organism retention time, and ease of modification through chemical design, compared with inorganic contrast agents. In particular, the achievements of two clinical approved NIR-I dyes, indocyanine green (ICG) and methylene blue (MB), greatly motivated the development of NIR-II organic dyes to facilitate clinical translation of the NIR-II fluorescence imaging technique.10,96 However, making bright NIR-II dyes with stable photophysical and chemical properties in a biological environment is a challenging problem for organic chemists. An efficient and convenient approach to obtain NIR-II dyes is to perform structure modification on the available dye chromophores. To date, the reported NIR-II fluorophores mainly include donor–acceptor–donor (D–A–D) chromophores and polymethine cyanine dyes. To improve the biomedical applications of NIR-II dyes, key factors including absorption and emission wavelength, quantum yields, bioavailability, and bioconjugation capacity is discussed in this section.
4.2.1. D–A–D Chromophores
D–A–D chromophore is a typical NIR-II organic molecular structure which contains three parts: one acceptor, two donors, and π bridging groups.103 The spectral properties of D–A–D dyes depend on selecting an electron donor and acceptor as well as π bridging groups. Introducing large π bridging moieties as well as stronger electron acceptors and donors is the most commonly used method to obtain new D–A–D dyes with a longer absorption and emission wavelength.104 To date, various symmetrical NIR-II D–A–D dyes have been reported with strong electron-withdrawing unit benzobisthiadiazole (BBTD) derivatives as the central acceptor and variable groups including thiophene, benzene, triphenylamine, and fluorene as donors, e.g., CH1055, IR-FE, Q4, H1, CH-4T, IR-FP8P, and IR-E1.72,81,83,84,97,99,105 Recently, replacing a sulfur atom by a selenium atom in the BBTD moieties could lead to bathochromically shifted emission for D–A–D dyes, such as SY1100 (Figure 4a).98 The twisted intramolecular charge transfer (TICT) characteristic in D–A–D chromophores significantly lowers the energy gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), yielding an emission peak beyond 1000 nm and a large Stokes shift (usually >200 nm).
Figure 4.
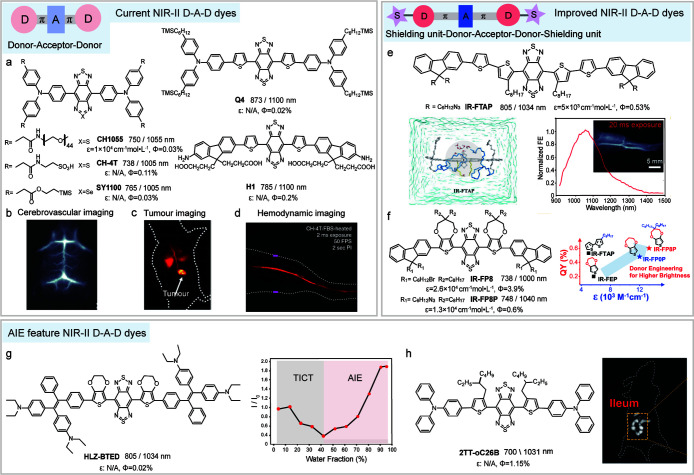
Current D–A–D chromophores for NIR-II bioapplication. (a) Chemical structures of typical NIR-II D–A–D fluorophores CH1055,72 CH-4T,97 SY1100,98 Q4,83 and H1.84 (b–d) NIR-II fluorescence imaging of mouse brain vasculature, tumor, and hemodynamic process with D–A–D fluorophores. Reproduced with permission. Copyright 2016, Wiley-VCH.99 Copyright 2019, Springer Nature.100 Copyright 2017, Springer Nature.97 (e) Chemical structure and the emission spectra of IR-FTAP. Schematic showing the hydrophobic shielding units in IR-FTAP protect the molecule from water polarization. NIR-II imaging of hindlimb vasculature with IR-FTAP administration. Reproduced with permission. Copyright 2018, American Chemical Society.101 (f) Chemical structures of IR-FP8 and IR-FP8P. Schematic showing the quantum yields and molar absorption coefficients of IR-FP8P, IR-FEP, IR-FP0P, and IR-FTAP. Reproduced with permission. Copyright 2020, American Chemical Society.81 (g) Chemical structure of HLZ-BTED and fluorescence intensity ratio (I/I0) of the HLZ-BTED as a function of THF/water ratio, where I0 stands for the maximal intensity. Reproduced with permission. Copyright 2019, The Royal Society of Chemistry.82 (h) Chemical structure of 2TT-oC26B and NIR-II imaging of intestinal peristalsis with 2TT-oC26B administration. Reproduced with permission. Copyright 2020, Springer Nature.102
In 2015, a small-molecule NIR-II fluorophore CH1055 was synthesized by introducing four carboxy groups into the triphenylamine donors of the D–A–D skeleton (Figure 4a).72 This modification not only facilitates PEGylation to impart the molecule with high aqueous solubility and rapid renal clearance but also allows for bioconjugation to targeting ligands, such as antibody, to ultimately produce a molecular imaging agent for tumor diagnostics. The results highlight the potential of D–A–D chromophores for NIR-II fluorescence imaging, in particular, in wide-field through-skull high-resolution brain vasculature imaging (Figure 4b) and high-contrast tumors diagnostic (Figure 4c). However, the quantum yield loss is massive from toluene (7%) to water (0.03%, IR-26 = 0.05% as reference, similarly hereinafter), leaving ample room for further improvement. The fluorescence quenching of D–A–D chromophores in water is associated with the formation of the dark TICT state, in which intramolecular rotation results in nonradiative deactivation of the excited state.102 TICT is governed by a driving force, which depends on solvent polarity, steric hindrance, and the electron-donating strength of the donor groups. Restricting molecular rotation or encapsulating the molecule into a nanomicelle to prevent water contact can straightforwardly produce a brilliant increase in fluorescent brightness. For example, a sulfonate-substituted derivate of CH1055, CH-4T, which forms supramolecular assembly with plasma protein, results in an increased quantum yield of 1.8%. The higher brightness enables fast video-rate imaging at a 50 frames-per-second (FPS) capable of resolving mouse cardiac cycles and blood flow of brain vessels (Figure 4d).97 However, the assembly also brings an undesired blue shift of ∼50 nm and may lose the advantage of small molecules, such as rapid excretion and flexible labeling capability.
Another strategy to improve quantum yield at a single molecule level is to choose a weak, rigid donor and introduce a hydrophobic shielding unit to protect the molecule from solvent polarization, making the TICT state energetically unfavorable.106−108 In recent years, substantial efforts have been made to reveal how changes to donor structures and components influence absorbance, quantum yield, and emission wavelength. Dai and Liang and co-workers introduced shielding units (S) into the D–A–D skeleton to form S–D–A–D–S organic dye.101,105 The dialkoxy modified fluorene was hired as shielding units to construct new NIR-II dye IR-FTAP, which afforded reduced molecule–molecule interaction, leading to a high signal-to-noise ratio (SBR) of 4.9 ± 0.3 for hindlimb vessels (Figure 4e).101 Moreover, these efforts ultimately yield a highest quantum yield record of 0.6% in water for fluorophore IR-FP8P with emission around 1040 nm. The chromophore of IR-FP8P employs a clickable azide-functionalized fluorene as a weak, rigid donor, 3,4-propylenedioxy thiophene as the π-bridge, and the side alkyl chains as protection for the conjugated backbone. The higher quantum yield and absorption coefficient provided an SBR around 2.5 for ovary NIR-II imaging (Figure 4f).81 The improvements in functionalization and brightness at a single molecule level make D–A–D fluorophores a good choice for use in molecular fluorescent labeling in various applications such as targeting imaging of organ/tumor, immune checkpoint, and gut microbiota.
Attempts to extend the emission wavelengths of D–A–D fluorophores remain ongoing, but there exists a trade-off between gently red-shifted emission and a steeply descending quantum yield due to the TICT process. To address this issue, recent efforts have focused on the aggregation induced emission (AIE) effect of D–A–D chromophores such as HLZ-BTED and 2TT-oC26B (Figure 4g and h).82,102 While the intramolecular donor rotation in TICT contributes to red-shifted emission, AIE requires restricting intramolecular motion to enhance fluorescence. Very recently, the two seemingly contradicting individuals were simultaneously realized in the aggregates of the rationally designed fluorophore 2TT-oC26B, thus obtaining a broad emission spectrum with a tail extended to the NIR-IIb window and a high quantum yield of 1.15%. Key elements of the molecular design focus on the branched alkyl chain substituents in thiophene groups, which cause a large distortion of the thiophene-BBTD-thiophene backbone. With 2TT-oC26B administration, a tiny vessel of 10 mm width could be distinguished with an SBR around 2. Furthermore, the small bowel diverticula (∼1 mm) could be observed under 5 mm tissue penetration with an SBR of 2.7 (Figure 4h).102 Such distortion not only prevents intermolecular interactions but also provides certain spatial isolation of the molecules in aggregates to promote the intramolecular rotation of triphenylamine units, which were conducive to the formation of the TICT state.
4.2.2. Polymethine Cyanine Dyes
Polymethine cyanine dyes are other typical small organic NIR-II-emitted chromophores. The dyes generally consist of two heterocyclic terminal groups connected by one polymethine linker with varying length, and have one positive or negative charge in the conjugated system.113−115 The terminal group structures could be heterocyclic ring containing heteroatoms, like sulfur, oxygen, or nitrogen. The maximum absorption and emission wavelength of polymethine cyanine dyes could be tuned by the terminal groups and conjugated chain length. Lengthening the conjugate chain can increase the extent of delocalized π electrons, which is a classic method to afford red-shifted dyes. Extending the π-system in terminal groups, such as adding π-donor substituents or benzene ring groups, could lead to a bathochromic shift of absorption and emission. Replacing strong electronegative heteroatoms by weak electronegative heteroatoms in a similar scaffold results in bathochromic shifted absorption.
Typical cyanine dyes, including IR-1048, IR-1061, Flav7, and IR-26, show intense absorption and emission, both over 1000 nm in nonpolar solvent such as dichloromethane, but are easy to quench when transferring to polar solvents such as dimethyl sulfoxide, methanol, and water.75 Previous studies suggested three major reasons for explaining this phenomenon: (1) Large, hydrophobic conjugated structures favor these fluorophores to form random oligomers or parallel-oriented H-aggregates in water, leading to the fluorescence quenching.78,116 (2) Increased solvent polarity leads to the symmetry breaking effect of electronic structure in ground/excited state, resulting in solvatochromic-induced quenching. This effect connects with the length of polymethine chain and the donor strength of terminal groups, and is generally intensified with the increase of these two factors.75,117,118 (3) Fluorophores like IR-1048, IR-26, and Flav7 appear to be more electron deficient than common cyanine fluorophores and, thereby, are prone to be attacked by nucleophiles. This could even be done by some weak nucleophiles such as methanol, water, and other protic solvents, leading to the break of the π-conjugated system.76,77 A study on IR-1048 suggested that the connected C-position between the heterocycles and the methine chain would be more likely to be the target of nucleophilic attack.119
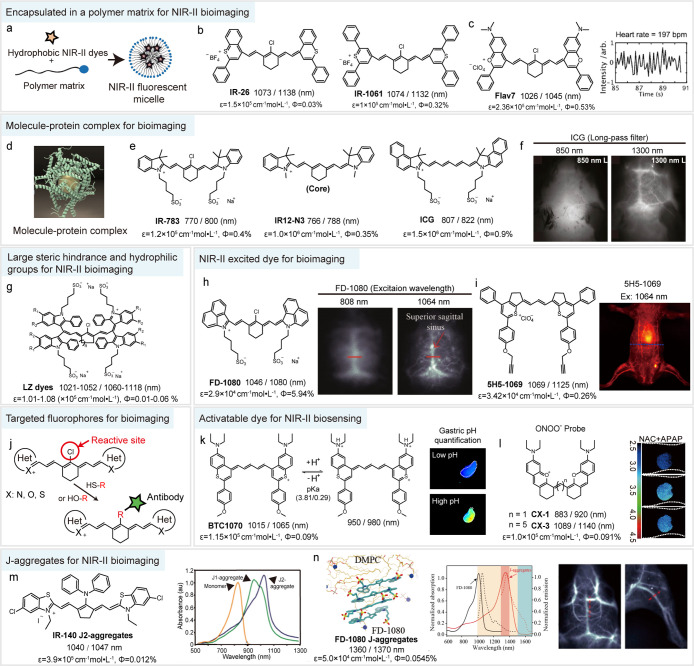
To address these problems, several methods have been developed. First, encapsulating hydrophobic NIR-II dyes into micelles would protect them from water to some extent, thereby be available for in vivo bioimaging (Figure 5a). Dai and co-workers performed abdominal vessels imaging using a phospholipid nanoparticle (IR-PEG) containing the small molecular dye IR-1061 for the first time, from which the smallest discernible vessel width was measured as 150 μm under >1 mm skin penetration depth (Figure 5b).85 Sletten and co-workers have constructed a new panel of polymethine cyanine dyes with emission from 680 to 1045 nm, utilizing a 7-dimethylamino flavylium heterocycle. NIR-II imaging with a micelle formulation of the longest-wavelength dye Flav7 was capable to penetrate through mouse rib and quantify the heart rate of the anesthetized mouse (Figure 5c).76 Second, the assembly of the molecule–protein complex can prevent molecular aggregation and greatly enhance fluorescence by constraining molecular rotation (Figure 5d). For example, previous studies have demonstrated the conventional NIR-I dyes ICG, IR-783, and IR12-N3 show tail fluorescence into the NIR-II window due to the enhanced quantum yield with the molecule–protein complex (4-fold, 6-fold, and 6-fold quantum yield enhancement for ICG, IR-783, and IR12-N3, respectively) (Figure 5e).73,109,120,121 NIR-II bioimaging using them provides many advantages over the NIR-I imaging, such as higher imaging contrast (from 0.20 to 0.29) and superior resolution (210 μm) of brain vasculature (Figure 5f). In particular, the first-in-human liver-tumor surgery guided by NIR-II fluorescence imaging was also realized by ICG.10 Third, molecular engineering by shortening the polymethine chain and further introducing electron-donating moieties at terminal heterocycles is an effective way to overcome solvatochromic-induced quenching. Zhang and co-workers have leveraged this strategy to construct a series of antiquenching benzothiopyrylium pentamethine cyanines (BTC fluorophores), which showed a maximum of 44-fold enhanced brightness as compared with their heptamethine analog IR-26. These fluorophores are capable of resolving lymph vessels with a minimum feature size of 84 μm in in vivo NIR-II lymphatic imaging.75 Fourthly, large steric hindrance groups and small hydrophilic functional groups, such as sulfonate and carboxyl, are introduced into NIR-II dyes to enhance water solubility and prevent nucleophilic attack by solvent. For example, new NIR-II LZ dyes have large steric hindrance groups (diindole) and four water-soluble groups (sulfonate), leading to the superior monodispersity and chemstability in aqueous solution (Figure 5g).110 These fluorophores show promise for in vivo molecular labeling with less influence by fluorophore–biomolecule nonspecific interaction.
Figure 5.
Current polymethine cyanine dyes for NIR-II bioapplication. (a) Enhancing bioavailability of cyanine dyes with micelle protection. (b, c) Chemical structure of IR-26, IR-1061, and Flav7. Quantification of the heart rate of the anesthetized mouse by NIR-II dynamic imaging with Flav7 micelle administration. Reproduced with permission. Copyright 2017, Wiley-VCH.76 (d) Schematic diagram of molecule–protein complex. Reproduced with permission. Copyright 2019, Science Publishing Group.109 (e) Chemical structures of IR-783, IR12-N3, and ICG. (f) Fluorescence imaging of mouse brain vasculature with ICG administration using different long-pass filters. Reproduced with permission. Copyright 2018, National Academy of Sciences.73 (g) Chemical structures of monodisperse polymethine LZ dyes.110 (h) Chemical structure of FD-1080 and NIR-II imaging of brain vasculature with FD-1080 administration under 808 and 1064 nm excitation. Reproduced with permission. Copy-right 2018, Wiley-VCH.78 (i) Chemical structure of 5H5-1069 and NIR-II imaging of abdominal vessels under 1064 nm excitation. Reproduced with permission. Copyright 2019, American Chemical Society.74 (j) Schematic illustration of modified method for reported NIR-II dyes with functional groups. (k) NIR-II low-pH ratiometric sensor BTC1070 for noninvasive gastric pH quantification. Reproduced with permission. Copyright 2019, Springer Nature.75 (l) A FRET sensor composed of CX-1 (donor) and CX-3 (acceptor) for ONOO– detection in mouse liver. Reproduced with permission. Copyright 2019, Wiley-VCH.77 (m) Chemical structure of IR-140 and the absorption spectra of IR-140 monomer and J-aggregates in hollow mesoporous silica nanoparticles. Reproduced with permission. Copyright 2019, American Chemical Society.111 (n) J-aggregates of self-assembly between FD-1080 and DMPC and the spectra properties of FD-1080 monomer and J-aggregates. NIR-II imaging of brain and hindlimb vessels with J-aggregates administration. Reproduced with permission. Copyright 2019, American Chemical Society.112
Except for the emission wavelength, the excitation wavelength is also very important to improve the NIR-II 1P fluorescence imaging depth and quality. Zhang and co-workers synthesized a fluorophore FD-1080, which not only resembles ICG but also shows much longer excitation and emission wavelengths at 1064 and 1080 nm, respectively, due to the larger π-system of the benzo[c,d]indolium heterocycle (Figure 5h).78 They performed the first NIR-II excited 1P fluorescence imaging, which demonstrated that imaging under 1064 nm excitation through the scalp and skull can provide higher resolution on brain vessels (0.65 mm) than that under 808 nm excitation (1.43 mm). The same group also used BTC fluorophores and confirmed that excitation at 1064 nm improves the penetration depth, image SBR, and resolution by reducing the signal attenuation coefficient in tissue.75 Recently, high-SBR (7.21) abdominal vessels imaging can also be achieved using 5H5-1069 under 1064 nm excitation (Figure 5i).74
Most of the existing NIR-II polymethine cyanine dyes serve only as nontargeted fluorophores, such as IR-1061, FD-1080, and Flav7. For correct prognosis and therapeutics for disease, effective targeting NIR-II molecular dyes are in urgent demand. Some of the previously reported NIR-II dyes are modified with functional groups, including alkyne, NHS ester, maleimide, succinimidyl ester, isothiocyanate, azide, and carboxyl. Hong and co-workers reported a clickable NIR-II dye 5H5-1069 which bound to integrin αVβ3 targeting peptide c(RGD)fk. Under 1064 nm excitation, the tumor-to-normal tissue ratio (T/NT) of U87 glioma tumor increased 1.7-fold compared to that under 808 nm excitation (from 4.0 to 6.8). Actually, for heptamethine dyes, like IR-1061, FD-1080, and Flav7, a cyclohexene group is introduced to the middle of the conjugated chain to offer a reactive site (Figure 5j). Yan and co-workers have reported a macromolecular NIR-II probe PF by designing Flav7 with covalent linking sulfhydryl groups to realize NIR-II image-guided photothermal therapy.122 To design covalent linkable fluorophores, a simple method is developed by a nucleophilic reaction at the central chloro group with a thionic or oxygenic substituent. These results provide a basis for bioapplication of NIR-II dyes with conjugation capacity.
Near-infrared polymethine cyanine dyes have always been of great interest in in vivo fluorescent biosensing application.114 Modifying substituents on polymethine chain or exploiting intermolecular photophysical interaction (such as FRET, aggregation-induced quenching) are the two common routes to design cyanine-based fluorescent probes. These strategies have been confirmed in NIR-II cyanine dyes; for example, Fan and co-workers designed dual activatable NIR-II nanoparticle HISSNPs to achieve MCF-7 xenografts cancer imaging through changing the IR-1061 aggregation state.116 In addition, manipulating the electronic effects of substituents on terminal heterocycles is another promising route to construct novel NIR-II probes. This is because the extended π-conjugated system of NIR-II cyanine dyes greatly increases the contribution of the substituents of terminal heterocycles on the photophysical properties. Zhang and co-workers investigated the position-dependent effect of diethylamino moieties on the spectra and quantum yield of BTC fluorophores, which allowed the creation of an NIR-II fluorescent low-pH sensor BTC1070 (Figure 5k).75 Protonation of diethylamino moieties changed the electron-donating effect and in turn resulted in ratiometric fluorescence signal output (1065/980 nm). The low scattering and superior sensing accuracy of NIR-II ratiometric signals allowed for noninvasive gastric pH quantification under 4 mm biotissue penetration (Figure 5k). Furthermore, on the basis of increased understanding of the photophysical and chemical properties of NIR-II polymethine cyanine dyes, various activatable or ratiometric NIR-II fluorescent probes have been created for monitoring physiological events or biomarkers in vivo, such as reactive oxygen/nitrogen species (ROS/RNS, Figure 5l)77,123 and enzyme.124
It should be noted that polymethine cyanine dyes hold the property to form head-to-tail dipole arrangement J-aggregates in certain conditions.125 Compared with monomers, J-aggregates exhibit unique photophysical properties of bathochromically shifted absorption and emission bands, enhanced absorption coefficients (ε), and shortened fluorescence lifetimes.126,127 J-aggregates have many advantages for in vivo imaging. For instance, red-shifted absorption and emission wavelength are capable of increasing penetration depth, and in addition, the increased ε will enhance brightness. However, it is difficult to obtain stable and highly purified J-aggregates in solutions. At present, nanocarriers are a promising approach to promote and stabilize J-aggregates. Sletten and co-workers reported IR-140 J-aggregates through encapsulating IR-140 into hollow mesoporous silica nanoparticles, which showed high stability in aqueous solution for several weeks. After aggregation, IR-140 J-aggregates showed bathochromically shifted absorption and emission from 862 and 875 nm to 1040 and 1047 nm, respectively (Figure 5m).111 Holding the longer emission wavelength caused deep tissue penetration; intense signals were observed at lungs, liver, and spleen. Fan and co-workers also reported NIR-II J-aggregate nanoparticles SQP-NPs by encapsulating bispyrrole-sq-bispyrrole (SQP) into PEG-b-PPG-b-PEG (F-127).128 In addition, NIR-II molecular dye also holds promise in forming J-aggregates. Zhang and co-workers developed FD-1080 J-aggregates formed by self-assembly between FD-1080 and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) (Figure 5n). FD-1080 J-aggregates showed a high SBR (5.56 and 4.76 for brain and hindlimb vessels, respectively) for in vivo dynamic vascular imaging beyond 1500 nm.112 Thus, stabilizing by nanocarriers is an efficient way to form J-aggregates as NIR-II imaging agents.
5. Summary and Outlook
The “tissue-transparent” NIR-II spectral window has received considerable interest in the past decade in view of the lower scattering, moderate tissue absorption, and few tissue autofluorescence. By taking advantage of these features, emerging fluorescence methods based NIR-II nonlinear excitation and NIR-II linear emission have revolutionized in vivo bioimaging with unprecedented penetration depth and image resolution. Typical nonlinear methods, 2P/3P microscopies, permit diffraction-limited three-dimensional imaging of tissues in vivo with single-cell or subcellular resolution, but usually need invasive procedures such as implantable chronic windows. Compared with traditional 1P microscopy, the advantages of 2P/3P microscopies are mainly the three-dimensional deep-tissue high-contrast imaging capability due to the nonlinear contrast generation mechanism. Therefore, these methods are preferred for basic biology studies in which interrogating cellular structure and function in thick tissue and living animals is required, for instance, to visualize cell–cell interactions, neurovascular coupling, and neuronal activity in brain research,129,130 and to study the dynamics of immune-cell interactions in intact tissue.131 However, as 2P/3P microscopy operates with small fields of view (hundreds of micrometers) and superficial depth (∼1 mm), it does not yield a complete picture of the underlying activity across varying spatial scales, for example, in mice whole body and even larger human organs. For this purpose, the NIR-II linear fluorescence method with mesoscopic and macroscopic imaging capability may become highly complementary. With an acceptable loss of resolution, this method provides a penetration limit several or a dozen times deeper than 2P/3P microscopy and, thus, is well suited for noninvasive imaging of entire organs and animals in vivo and is more typically part of mouse-to-human clinical translation imaging applications. Its applications include, for example, molecular imaging of entire tumors,8 imaging-guided surgery,1,10 visualizing anatomic structure and hemodynamic information,70 brain imaging,5 and the study of the movement of stem cells or immune cells at deep locations.7,132 With the development of technology, both nonlinear and linear fluorescence methods are constantly overcoming their limitations,4,7 allowing investigation of biological systems across a wide range of spatial and temporal scales. Pushing the frontier of deep-tissue fluorescence bioimaging also requires the design of functional fluorophores that adapted to each method. The complementarity of nonlinear and linear fluorescence methods provides a wide spectral range (500–1700 nm) for chemists to develop novel fluorophores.
In this Outlook, we outline the molecular fluorophores that have evolved over the past five years, driven by the technological advances of nonlinear and linear fluorescence bioimaging in the “tissue-transparent” NIR-II window. The impressive performance of these fluorophores in structural and functional imaging of the mouse brain, tumor and whole body, and in clinical diagnosis provide a huge motivation for people to go forward. Nevertheless, current efforts are still in their infancy, and there remains a gap regarding people’s high expectations for deep-tissue high-resolution fluorescence bioimaging. Regardless of nonlinear or linear fluorescence, the ideal molecular fluorophores would have exquisite brightness and photostability, small size, improved in vivo bioavailability, and ease of use and functionalization, and their nonlinear excitation or linear emission wavelengths better fall in the outstanding spectral area of NIR-II window, for example, around 1300 nm or 1700 nm. Unfortunately, a molecular fluorophore that fulfills all of these criteria has not yet been developed. Some challenges may be objectively difficult to overcome. For example, a trade-off between wavelength and quantum yield is inevitable, because nonradiative relaxation is enhanced at longer wavelengths of the NIR spectrum, partly induced by the vibrational overtone of surrounding chemical bonds such as C–H, O–H, and N–H.75,133 Fortunately, the application of modern synthetic techniques allows further structural refinements to yield dyes with better photophysics,43,134 making us believe that the upper limits of quantum yields of most fluorophores have not yet been reached.
Chemists also should not be discouraged in the hurdles that will have to be overcome to create a perfect fluorophore. Indeed, some intriguing and unanticipated phenomena in the application of fluorophores may have a significant impact on biology and biomedicine. The fluorogenic properties of X-rhodamine after binding to self-labeling tags are good examples: In-depth studies of this discovery have created a genetically targetable fluorogenic toolbox and chemigenetic sensor platform,51,52,135 making synthetic dyes progressively attractive and accessible to neurobiologists. Likewise, the phenomena that spectra change and fluorescence enhancement caused by the interaction of NIR-II emitted fluorophores (such as CH-4T and FD-1080) and biomolecules (such as albumin and DMPC lipids) may also imply the emergence of a new imaging and sensing platform based on hybrid structures.
Finally, the overview that is provided in this Outlook aims to call on the research community to strengthen cooperation with scientists among diverse research fields, such as biology, biomedicine, and microscopy. We look forward to the next era of fluorescence bioimaging in which the frontiers of NIR-II nonlinear and linear techniques expand with the help of new molecular fluorophores.
Acknowledgments
The work was supported by the National Key R&D Program of China (2017YFA0207303), National Natural Science Foundation of China (NSFC, 21725502, 51961145403), Key Basic Research Program of Science and Technology Commission of Shang-hai Municipality (17JC1400100), and China Postdoctoral Science Foundation (KLH1615200).
Author Contributions
† S.W. and B.L. contributed equally.
The authors declare no competing financial interest.
References
- Hong G.; Antaris A. L.; Dai H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1 (1), 0010. 10.1038/s41551-016-0010. [DOI] [Google Scholar]
- Xu C.; Wise F. W. Recent advances in fibre lasers for nonlinear microscopy. Nat. Photonics 2013, 7 (11), 875–882. 10.1038/nphoton.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Ouzounov D. G.; Wu C.; Horton N. G.; Zhang B.; Wu C.-H.; Zhang Y.; Schnitzer M. J.; Xu C. Three-photon imaging of mouse brain structure and function through the intact skull. Nat. Methods 2018, 15 (10), 789–792. 10.1038/s41592-018-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemipour A.; Novak O.; Flickinger D.; Marvin J. S.; Abdelfattah A. S.; King J.; Borden P. M.; Kim J. J.; Al-Abdullatif S. H.; Deal P. E.; Miller E. W.; Schreiter E. R.; Druckmann S.; Svoboda K.; Looger L. L.; Podgorski K. Kilohertz frame-rate two-photon tomography. Nat. Methods 2019, 16 (8), 778–786. 10.1038/s41592-019-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G.; Diao S.; Chang J.; Antaris A. L.; Chen C.; Zhang B.; Zhao S.; Atochin D. N.; Huang P. L.; Andreasson K. I.; Kuo C. J.; Dai H. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics 2014, 8 (9), 723–730. 10.1038/nphoton.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton N. G.; Wang K.; Kobat D.; Clark C. G.; Wise F. W.; Schaffer C. B.; Xu C. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 2013, 7, 205. 10.1038/nphoton.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Wan H.; Ma Z.; Zhong Y.; Sun Q.; Tian Y.; Qu L.; Du H.; Zhang M.; Li L.; Ma H.; Luo J.; Liang Y.; Li W. J.; Hong G.; Liu L.; Dai H. Light-sheet microscopy in the near-infrared II window. Nat. Methods 2019, 16 (6), 545–552. 10.1038/s41592-019-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y.; Ma Z.; Wang F.; Wang X.; Yang Y.; Liu Y.; Zhao X.; Li J.; Du H.; Zhang M.; Cui Q.; Zhu S.; Sun Q.; Wan H.; Tian Y.; Liu Q.; Wang W.; Garcia K. C.; Dai H. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 2019, 37 (11), 1322–1331. 10.1038/s41587-019-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.; Wang P.; Lu Y.; Wang R.; Zhou L.; Zheng X.; Li X.; Piper J. A.; Zhang F. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 2018, 13 (10), 941–946. 10.1038/s41565-018-0221-0. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Fang C.; Li B.; Zhang Z.; Cao C.; Cai M.; Su S.; Sun X.; Shi X.; Li C.; Zhou T.; Zhang Y.; Chi C.; He P.; Xia X.; Chen Y.; Gambhir S. S.; Cheng Z.; Tian J. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4 (3), 259–271. 10.1038/s41551-019-0494-0. [DOI] [PubMed] [Google Scholar]
- Lavis L. D. Chemistry Is Dead. Long Live Chemistry!. Biochemistry 2017, 56 (39), 5165–5170. 10.1021/acs.biochem.7b00529. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 2010, 7 (8), 603–614. 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- Helmchen F.; Denk W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2 (12), 932–940. 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Wang M.; Kim M.; Xia F.; Xu C. Impact of the emission wavelengths on in vivo multiphoton imaging of mouse brains. Biomed. Opt. Express 2019, 10 (4), 1905–1918. 10.1364/BOE.10.001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobat D.; Durst M. E.; Nishimura N.; Wong A. W.; Schaffer C. B.; Xu C. Deep tissue multiphoton microscopy using longer wavelength excitation. Opt. Express 2009, 17 (16), 13354–13364. 10.1364/OE.17.013354. [DOI] [PubMed] [Google Scholar]
- Miller D. R.; Jarrett J. W.; Hassan A. M.; Dunn A. K. Deep tissue imaging with multiphoton fluorescence microscopy. Curr. Opin. Biotechnol. 2017, 4, 32–39. 10.1016/j.cobme.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami R.; Sawada K.; Kusama Y.; Fang Y. C.; Kanazawa S.; Kozawa Y.; Sato S.; Yokoyama H.; Nemoto T. In vivo two-photon imaging of mouse hippocampal neurons in dentate gyrus using a light source based on a high-peak power gain-switched laser diode. Biomed. Opt. Express 2015, 6 (3), 891–901. 10.1364/BOE.6.000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. R.; Hassan A. M.; Jarrett J. W.; Medina F. A.; Perillo E. P.; Hagan K.; Shams Kazmi S. M.; Clark T. A.; Sullender C. T.; Jones T. A.; Zemelman B. V.; Dunn A. K. In vivo multiphoton imaging of a diverse array of fluorophores to investigate deep neurovascular structure. Biomed. Opt. Express 2017, 8 (7), 3470–3481. 10.1364/BOE.8.003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M.; Takeuchi A.; Manita S.; Horigane S.-i.; Sakamoto M.; Kawakami R.; Yamaguchi K.; Otomo K.; Yokoyama H.; Kim R.; Yokoyama T.; Takemoto-Kimura S.; Abe M.; Okamura M.; Kondo Y.; Quirin S.; Ramakrishnan C.; Imamura T.; Sakimura K.; Nemoto T.; Kano M.; Fujii H.; Deisseroth K.; Kitamura K.; Bito H. Rational Engineering of XCaMPs, a Multicolor GECI Suite for In Vivo Imaging of Complex Brain Circuit Dynamics. Cell 2019, 177 (5), 1346–1360.e24. 10.1016/j.cell.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Xu C.; Webb W. W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 1996, 13 (3), 481–491. 10.1364/JOSAB.13.000481. [DOI] [Google Scholar]
- Wang X.; Nguyen D. M.; Yanez C. O.; Rodriguez L.; Ahn H.-Y.; Bondar M. V.; Belfield K. D. High-Fidelity Hydrophilic Probe for Two-Photon Fluorescence Lysosomal Imaging. J. Am. Chem. Soc. 2010, 132 (35), 12237–12239. 10.1021/ja1057423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mütze J.; Iyer V.; Macklin J. J.; Colonell J.; Karsh B.; Petrášek Z.; Schwille P.; Looger L. L.; Lavis L. D.; Harris T. D. Excitation Spectra and Brightness Optimization of Two-Photon Excited Probes. Biophys. J. 2012, 102 (4), 934–944. 10.1016/j.bpj.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobizhev M.; Makarov N. S.; Tillo S. E.; Hughes T. E.; Rebane A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 2011, 8, 393. 10.1038/nmeth.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov N. S.; Drobizhev M.; Rebane A. Two-photon absorption standards in the 550–1600 nm excitation wavelength range. Opt. Express 2008, 16 (6), 4029–4047. 10.1364/OE.16.004029. [DOI] [PubMed] [Google Scholar]
- Pawlicki M.; Collins H. A.; Denning R. G.; Anderson H. L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem., Int. Ed. 2009, 48 (18), 3244–3266. 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]
- Qi J.; Sun C.; Li D.; Zhang H.; Yu W.; Zebibula A.; Lam J. W. Y.; Xi W.; Zhu L.; Cai F.; Wei P.; Zhu C.; Kwok R. T. K.; Streich L. L.; Prevedel R.; Qian J.; Tang B. Z. Aggregation-Induced Emission Luminogen with Near-Infrared-II Excitation and Near-Infrared-I Emission for Ultradeep Intravital Two-Photon Microscopy. ACS Nano 2018, 12 (8), 7936–7945. 10.1021/acsnano.8b02452. [DOI] [PubMed] [Google Scholar]
- Yan P.; Acker C. D.; Zhou W.-L.; Lee P.; Bollensdorff C.; Negrean A.; Lotti J.; Sacconi L.; Antic S. D.; Kohl P.; Mansvelder H. D.; Pavone F. S.; Loew L. M. Palette of fluorinated voltage-sensitive hemicyanine dyes. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (50), 20443–20448. 10.1073/pnas.1214850109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanfar S.; Joo C.; Zhan C.; Berezin M.; Akers W.; Achilefu S. Multiphoton microscopy with near infrared contrast agents. J. Biomed. Opt. 2010, 15 (3), 030505 10.1117/1.3420209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J.; Padilha L. A.; Hagan D. J.; Van Stryland E. W.; Przhonska O. V.; Bondar M. V.; Slominsky Y. L.; Kachkovski A. D. Molecular structure—two-photon absorption property relations in polymethine dyes. J. Opt. Soc. Am. B 2007, 24 (1), 56–66. 10.1364/JOSAB.24.000056. [DOI] [Google Scholar]
- Yi R.; Das P.; Lin F.; Shen B.; Yang Z.; Zhao Y.; Hong L.; He Y.; Hu R.; Song J.; Qu J.; Liu L. Fluorescence enhancement of small squaraine dye and its two-photon excited fluorescence in long-term near-infrared I&II bioimaging. Opt. Express 2019, 27 (9), 12360–12372. 10.1364/OE.27.012360. [DOI] [PubMed] [Google Scholar]
- Podgorski K.; Terpetschnig E.; Klochko O. P.; Obukhova O. M.; Haas K. Ultra-Bright and -Stable Red and Near-Infrared Squaraine Fluorophores for In Vivo Two-Photon Imaging. PLoS One 2012, 7 (12), e51980 10.1371/journal.pone.0051980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Xu G.; Prasad P. N. Conformationally Restricted Dipyrromethene Boron Difluoride (BODIPY) Dyes: Highly Fluorescent, Multicolored Probes for Cellular Imaging. Chem. - Eur. J. 2008, 14 (19), 5812–5819. 10.1002/chem.200800309. [DOI] [PubMed] [Google Scholar]
- Collot M.; Ashokkumar P.; Anton H.; Boutant E.; Faklaris O.; Galli T.; Mély Y.; Danglot L.; Klymchenko A. S. MemBright: A Family of Fluorescent Membrane Probes for Advanced Cellular Imaging and Neuroscience. Cell Chem. Biol. 2019, 26 (4), 600–614. e7 10.1016/j.chembiol.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Contractor A. A.; Miller E. W. Imaging Ca2+ with a Fluorescent Rhodol. Biochemistry 2018, 57 (2), 237–240. 10.1021/acs.biochem.7b01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T.; Xu W.; Jin F.; Cheng D.; Zhang L.; Yuan L.; Zhang X. Rational Engineering of Bioinspired Anthocyanidin Fluorophores with Excellent Two-Photon Properties for Sensing and Imaging. Anal. Chem. 2017, 89 (21), 11427–11434. 10.1021/acs.analchem.7b02538. [DOI] [PubMed] [Google Scholar]
- Minoshima M.; Kikuta J.; Omori Y.; Seno S.; Suehara R.; Maeda H.; Matsuda H.; Ishii M.; Kikuchi K. In Vivo Multicolor Imaging with Fluorescent Probes Revealed the Dynamics and Function of Osteoclast Proton Pumps. ACS Cent. Sci. 2019, 5 (6), 1059–1066. 10.1021/acscentsci.9b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Lesiak L.; Lai R.; Beck J. R.; Zhao J.; Elowsky C. G.; Li H.; Stains C. I. Chemoselective Alteration of Fluorophore Scaffolds as a Strategy for the Development of Ratiometric Chemodosimeters. Angew. Chem., Int. Ed. 2017, 56 (15), 4197–4200. 10.1002/anie.201612628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzybowski M.; Taki M.; Senda K.; Sato Y.; Ariyoshi T.; Okada Y.; Kawakami R.; Imamura T.; Yamaguchi S. A Highly Photostable Near-infrared Labeling Agent Based on a Phospha-rhodamine for Long-term and Deep Imaging. Angew. Chem., Int. Ed. 2018, 57 (32), 10137–10141. 10.1002/anie.201804731. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Lai R.; Beck J. R.; Li H.; Stains C. I. Nebraska Red: a phosphinate-based near-infrared fluorophore scaffold for chemical biology applications. Chem. Commun. 2016, 52 (83), 12290–12293. 10.1039/C6CC05717A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y.; Urano Y.; Hanaoka K.; Piao W.; Kusakabe M.; Saito N.; Terai T.; Okabe T.; Nagano T. Development of NIR Fluorescent Dyes Based on Si–rhodamine for in Vivo Imaging. J. Am. Chem. Soc. 2012, 134 (11), 5029–5031. 10.1021/ja210375e. [DOI] [PubMed] [Google Scholar]
- Vogel M.; Rettig W.; Sens R.; Drexhage K. H. Structural relaxation of rhodamine dyes with different N-substitution patterns: A study of fluorescence decay times and quantum yields. Chem. Phys. Lett. 1988, 147 (5), 452–460. 10.1016/0009-2614(88)85007-3. [DOI] [Google Scholar]
- Song X.; Johnson A.; Foley J. 7-Azabicyclo[2.2.1]heptane as a Unique and Effective Dialkylamino Auxochrome Moiety: Demonstration in a Fluorescent Rhodamine Dye. J. Am. Chem. Soc. 2008, 130 (52), 17652–17653. 10.1021/ja8075617. [DOI] [PubMed] [Google Scholar]
- Grimm J. B.; English B. P.; Chen J.; Slaughter J. P.; Zhang Z.; Revyakin A.; Patel R.; Macklin J. J.; Normanno D.; Singer R. H.; Lionnet T.; Lavis L. D. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12 (3), 244–250. 10.1038/nmeth.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Qiao Q.; Tian W.; Liu W.; Chen J.; Lang M. J.; Xu Z. Aziridinyl Fluorophores Demonstrate Bright Fluorescence and Superior Photostability by Effectively Inhibiting Twisted Intramolecular Charge Transfer. J. Am. Chem. Soc. 2016, 138 (22), 6960–6963. 10.1021/jacs.6b03924. [DOI] [PubMed] [Google Scholar]
- Ye Z.; Yang W.; Wang C.; Zheng Y.; Chi W.; Liu X.; Huang Z.; Li X.; Xiao Y. Quaternary Piperazine-Substituted Rhodamines with Enhanced Brightness for Super-Resolution Imaging. J. Am. Chem. Soc. 2019, 141 (37), 14491–14495. 10.1021/jacs.9b04893. [DOI] [PubMed] [Google Scholar]
- Grimm J. B.; Brown T. A.; Tkachuk A. N.; Lavis L. D. General Synthetic Method for Si-Fluoresceins and Si-Rhodamines. ACS Cent. Sci. 2017, 3 (9), 975–985. 10.1021/acscentsci.7b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm J. B.; Tkachuk A. N.; Choi H.; Mohar B.; Falco N.; Patel R.; Lippincott-Schwartz J.; Brown T. A.; Lavis L. D.. Optimization and functionalization of red-shifted rhodamine dyes. bioRxiv 2019, 10.1101/2019.12.20.881227. [DOI] [Google Scholar]
- Grimm J. B.; Muthusamy A. K.; Liang Y.; Brown T. A.; Lemon W. C.; Patel R.; Lu R.; Macklin J. J.; Keller P. J.; Ji N.; Lavis L. D. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 2017, 14 (10), 987–994. 10.1038/nmeth.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukinavičius G.; Umezawa K.; Olivier N.; Honigmann A.; Yang G.; Plass T.; Mueller V.; Reymond L.; Corrêa I. R. Jr; Luo Z.-G.; Schultz C.; Lemke E. A.; Heppenstall P.; Eggeling C.; Manley S.; Johnsson K. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 2013, 5 (2), 132–139. 10.1038/nchem.1546. [DOI] [PubMed] [Google Scholar]
- Lukinavičius G.; Reymond L.; Umezawa K.; Sallin O.; D’Este E.; Göttfert F.; Ta H.; Hell S. W.; Urano Y.; Johnsson K. Fluorogenic Probes for Multicolor Imaging in Living Cells. J. Am. Chem. Soc. 2016, 138 (30), 9365–8. 10.1021/jacs.6b04782. [DOI] [PubMed] [Google Scholar]
- Abdelfattah A. S.; Kawashima T.; Singh A.; Novak O.; Liu H.; Shuai Y.; Huang Y.-C.; Campagnola L.; Seeman S. C.; Yu J.; Zheng J.; Grimm J. B.; Patel R.; Friedrich J.; Mensh B. D.; Paninski L.; Macklin J. J.; Murphy G. J.; Podgorski K.; Lin B.-J.; Chen T.-W.; Turner G. C.; Liu Z.; Koyama M.; Svoboda K.; Ahrens M. B.; Lavis L. D.; Schreiter E. R. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 2019, 365, 699. 10.1126/science.aav6416. [DOI] [PubMed] [Google Scholar]
- Deo C.; Abdelfattah A. S.; Bhargava H. K.; Berro A. J.; Falco N.; Moeyaert B.; Chupanova M.; Lavis L. D.; Schreiter E. R.. Bright and tunable far-red chemigenetic indicators. bioRxiv 2020, 10.1101/2020.01.08.898783. [DOI] [PubMed] [Google Scholar]
- Koide Y.; Urano Y.; Hanaoka K.; Terai T.; Nagano T. Evolution of Group 14 Rhodamines as Platforms for Near-Infrared Fluorescence Probes Utilizing Photoinduced Electron Transfer. ACS Chem. Biol. 2011, 6 (6), 600–608. 10.1021/cb1002416. [DOI] [PubMed] [Google Scholar]
- Chan J.; Dodani S. C.; Chang C. J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973. 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z.; Jiang H.; Song X.; Hu W.; Liu Z. Development of a Silicon-Rhodamine Based Near-Infrared Emissive Two-Photon Fluorescent Probe for Nitric Oxide. Anal. Chem. 2017, 89 (18), 9620–9624. 10.1021/acs.analchem.7b02697. [DOI] [PubMed] [Google Scholar]
- Chi W.; Chen J.; Liu W.; Wang C.; Qi Q.; Qiao Q.; Tan T. M.; Xiong K.; Liu X.; Kang K.; Chang Y.-T.; Xu Z.; Liu X. A General Descriptor ΔE Enables the Quantitative Development of Luminescent Materials based on Photoinduced Electron Transfer. J. Am. Chem. Soc. 2020, 142 (14), 6777–6785. 10.1021/jacs.0c01473. [DOI] [PubMed] [Google Scholar]
- Daly B.; Ling J.; de Silva A. P. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44 (13), 4203–4211. 10.1039/C4CS00334A. [DOI] [PubMed] [Google Scholar]
- Collot M.; Loukou C.; Yakovlev A. V.; Wilms C. D.; Li D.; Evrard A.; Zamaleeva A.; Bourdieu L.; Léger J.-F.; Ropert N.; Eilers J.; Oheim M.; Feltz A.; Mallet J.-M. Calcium Rubies: A Family of Red-Emitting Functionalizable Indicators Suitable for Two-Photon Ca2+ Imaging. J. Am. Chem. Soc. 2012, 134 (36), 14923–14931. 10.1021/ja304018d. [DOI] [PubMed] [Google Scholar]
- Collot M.; Wilms C. D.; Bentkhayet A.; Marcaggi P.; Couchman K.; Charpak S.; Dieudonné S.; Häusser M.; Feltz A.; Mallet J.-M. CaRuby-Nano: a novel high affinity calcium probe for dual color imaging. eLife 2015, 4, e05808 10.7554/eLife.05808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischbirek C.; Birkner A.; Jia H.; Sakmann B.; Konnerth A. Deep two-photon brain imaging with a red-shifted fluorometric Ca2+ indicator. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (36), 11377–11382. 10.1073/pnas.1514209112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo C.; Sheu S.-H.; Seo J.; Clapham D. E.; Lavis L. D. Isomeric Tuning Yields Bright and Targetable Red Ca2+ Indicators. J. Am. Chem. Soc. 2019, 141 (35), 13734–13738. 10.1021/jacs.9b06092. [DOI] [PubMed] [Google Scholar]
- Deal P. E.; Liu P.; Al-Abdullatif S. H.; Muller V. R.; Shamardani K.; Adesnik H.; Miller E. W. Covalently tethered rhodamine voltage reporters for high speed functional imaging in brain tissue. J. Am. Chem. Soc. 2020, 142 (1), 614–622. 10.1021/jacs.9b12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Miller E. W. Electrophysiology, Unplugged: Imaging Membrane Potential with Fluorescent Indicators. Acc. Chem. Res. 2020, 53 (1), 11–19. 10.1021/acs.accounts.9b00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R. U.; Vandenberghe M.; Thunemann M.; James F.; Andreassen O. A.; Djurovic S.; Devor A.; Miller E. W. In Vivo Two-Photon Voltage Imaging with Sulfonated Rhodamine Dyes. ACS Cent. Sci. 2018, 4 (10), 1371–1378. 10.1021/acscentsci.8b00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q. T.; Tsien R. Y. Fluorescence-guided surgery with live molecular navigation [mdash] a new cutting edge. Nat. Rev. Cancer 2013, 13 (9), 653–662. 10.1038/nrc3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka A. P.; Nani R. R.; Schnermann M. J. Harnessing Cyanine Reactivity for Optical Imaging and Drug Delivery. Acc. Chem. Res. 2018, 51 (12), 3226–3235. 10.1021/acs.accounts.8b00384. [DOI] [PubMed] [Google Scholar]
- Lavis L. D. Teaching Old Dyes New Tricks: Biological Probes Built from Fluoresceins and Rhodamines. Annu. Rev. Biochem. 2017, 86 (1), 825–843. 10.1146/annurev-biochem-061516-044839. [DOI] [PubMed] [Google Scholar]
- Welsher K.; Liu Z.; Sherlock S. P.; Robinson J. T.; Chen Z.; Daranciang D.; Dai H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4 (11), 773–780. 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsher K.; Sherlock S. P.; Dai H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (22), 8943–8. 10.1073/pnas.1014501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G.; Lee J. C.; Robinson J. T.; Raaz U.; Xie L.; Huang N. F.; Cooke J. P.; Dai H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 2012, 18 (12), 1841–1846. 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao S.; Blackburn J. L.; Hong G.; Antaris A. L.; Chang J.; Wu J. Z.; Zhang B.; Cheng K.; Kuo C. J.; Dai H. Fluorescence Imaging In Vivo at Wavelengths beyond 1500 nm. Angew. Chem., Int. Ed. 2015, 54 (49), 14758–62. 10.1002/anie.201507473. [DOI] [PubMed] [Google Scholar]
- Antaris A. L.; Chen H.; Cheng K.; Sun Y.; Hong G.; Qu C.; Diao S.; Deng Z.; Hu X.; Zhang B.; Zhang X.; Yaghi O. K.; Alamparambil Z. R.; Hong X.; Cheng Z.; Dai H. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15 (2), 235–42. 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- Carr J. A.; Franke D.; Caram J. R.; Perkinson C. F.; Saif M.; Askoxylakis V.; Datta M.; Fukumura D.; Jain R. K.; Bawendi M. G.; Bruns O. T. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (17), 4465–4470. 10.1073/pnas.1718917115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B.; Xiao Y.; Zhou H.; Zhang X.; Qu C.; Xu F.; Deng Z.; Cheng Z.; Hong X. Polymethine Thiopyrylium Fluorophores with Absorption beyond 1000 nm for Biological Imaging in the Second Near-Infrared Subwindow. J. Med. Chem. 2019, 62 (4), 2049–2059. 10.1021/acs.jmedchem.8b01682. [DOI] [PubMed] [Google Scholar]
- Wang S.; Fan Y.; Li D.; Sun C.; Lei Z.; Lu L.; Wang T.; Zhang F. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019, 10 (1), 1058. 10.1038/s41467-019-09043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosco E. D.; Caram J. R.; Bruns O. T.; Franke D.; Day R. A.; Farr E. P.; Bawendi M. G.; Sletten E. M. Flavylium polymethine fluorophores for imaging in the near- and shortwave infrared. Angew. Chem., Int. Ed. 2017, 56 (42), 13126–13129. 10.1002/anie.201706974. [DOI] [PubMed] [Google Scholar]
- Lei Z.; Sun C.; Pei P.; Wang S.; Li D.; Zhang X.; Zhang F. Stable, Wavelength-Tunable Fluorescent Dyes in the NIR-II Region for in-vivo High-Contrast Bioimaging and Multiplexed Biosensing. Angew. Chem., Int. Ed. 2019, 58 (24), 8166–8171. 10.1002/anie.201904182. [DOI] [PubMed] [Google Scholar]
- Li B.; Lu L.; Zhao M.; Lei Z.; Zhang F. An Efficient 1064 nm NIR-II Excitation Fluorescent Molecular Dye for Deep-Tissue High-Resolution Dynamic Bioimaging. Angew. Chem., Int. Ed. 2018, 57 (25), 7483–7487. 10.1002/anie.201801226. [DOI] [PubMed] [Google Scholar]
- Shou K.; Qu C.; Sun Y.; Chen H.; Chen S.; Zhang L.; Xu H.; Hong X.; Yu A.; Cheng Z. Multifunctional Biomedical Imaging in Physiological and Pathological Conditions Using a NIR-II Probe. Adv. Funct. Mater. 2017, 27 (23), 1700995. 10.1002/adfm.201700995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.; Xiao Y.; Lin J.; Li S.; Zhou H.; Nong J.; Xu G.; Wang H.; Xu F.; Wu J.; Deng Z.; Hong X. Near-Infrared II Dye-Protein Complex for Biomedical Imaging and Imaging-Guided Photothermal Therapy. Adv. Healthcare Mater. 2018, 7 (18), 1800589. 10.1002/adhm.201800589. [DOI] [PubMed] [Google Scholar]
- Ma H.; Liu C.; Hu Z.; Yu P.; Zhu X.; Ma R.; Sun Z.; Zhang C.-H.; Sun H.; Zhu S.; Liang Y. Propylenedioxy Thiophene Donor to Achieve NIR-II Molecular Fluorophores with Enhanced Brightness. Chem. Mater. 2020, 32 (5), 2061–2069. 10.1021/acs.chemmater.9b05159. [DOI] [Google Scholar]
- Lin J.; Zeng X.; Xiao Y.; Tang L.; Nong J.; Liu Y.; Zhou H.; Ding B.; Xu F.; Tong H.; Deng Z.; Hong X. Novel Near-Infrared II Aggregation-Induced Emission Dots for In Vivo Bioimaging. Chem. Sci. 2019, 10 (4), 1219–1226. 10.1039/C8SC04363A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Qu C.; Chen H.; He M.; Tang C.; Shou K.; Hong S.; Yang M.; Jiang Y.; Ding B.; Xiao Y.; Xing L.; Hong X.; Cheng Z. Novel benzo-bis(1,2,5-thiadiazole) fluorophores for in vivo NIR-II imaging of cancer. Chem. Sci. 2016, 7 (9), 6203–6207. 10.1039/C6SC01561A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Ding M.; Zeng X.; Xiao Y.; Wu H.; Zhou H.; Ding B.; Qu C.; Hou W.; Er-bu A.; Zhang Y.; Cheng Z.; Hong X. Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery. Chem. Sci. 2017, 8 (5), 3489–3493. 10.1039/C7SC00251C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z.; Hong G.; Shinji C.; Chen C.; Diao S.; Antaris A. L.; Zhang B.; Zou Y.; Dai H. Biological Imaging Using Nanoparticles of Small Organic Molecules with Fluorescence Emission at Wavelengths Longer than 1000 nm. Angew. Chem., Int. Ed. 2013, 52 (49), 13002–13006. 10.1002/anie.201307346. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Hong G.; Zhang Y.; Chen G.; Li F.; Dai H.; Wang Q. Ag2S Quantum Dot: A Bright and Biocompatible Fluorescent Nanoprobe in the Second Near-Infrared Window. ACS Nano 2012, 6 (5), 3695–3702. 10.1021/nn301218z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns O. T.; Bischof T. S.; Harris D. K.; Franke D.; Shi Y.; Riedemann L.; Bartelt A.; Jaworski F. B.; Carr J. A.; Rowlands C. J.; Wilson M. W. B.; Chen O.; Wei H.; Hwang G. W.; Montana D. M.; Coropceanu I.; Achorn O. B.; Kloepper J.; Heeren J.; So P. T. C.; Fukumura D.; Jensen K. F.; Jain R. K.; Bawendi M. G. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017, 1, 0056. 10.1038/s41551-017-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Yue J.; Cui R.; Ma Z.; Wan H.; Wang F.; Zhu S.; Zhou Y.; Kuang Y.; Zhong Y.; Pang D.-W.; Dai H. Bright quantum dots emitting at ∼ 1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (26), 6590–6595. 10.1073/pnas.1806153115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.; Wang R.; Li B.; Fan Y.; Wu Y.; Zhu X.; Zhang F. Precise In Vivo Inflammation Imaging Using In Situ Responsive Cross-linking of Glutathione-Modified Ultra-Small NIR-II Lanthanide Nanoparticles. Angew. Chem., Int. Ed. 2019, 58 (7), 2050–2054. 10.1002/anie.201812878. [DOI] [PubMed] [Google Scholar]
- Zhao M.; Li B.; Wang P.; Lu L.; Zhang Z.; Liu L.; Wang S.; Li D.; Wang R.; Zhang F. Supramolecularly Engineered NIR-II and Upconversion Nanoparticles In Vivo Assembly and Disassembly to Improve Bioimaging. Adv. Mater. 2018, 30 (52), 1804982. 10.1002/adma.201804982. [DOI] [PubMed] [Google Scholar]
- Wang S.; Liu L.; Fan Y.; El-Toni A. M.; Alhoshan M. S.; Li D.; Zhang F. In Vivo High-resolution Ratiometric Fluorescence Imaging of Inflammation Using NIR-II Nanoprobes with 1550 nm Emission. Nano Lett. 2019, 19 (4), 2418–2427. 10.1021/acs.nanolett.8b05148. [DOI] [PubMed] [Google Scholar]
- Liu L.; Wang S.; Zhao B.; Pei P.; Fan Y.; Li X.; Zhang F. Er3+ Sensitized 1530 to 1180 nm Second Near-Infrared Window Upconversion Nanocrystals for In Vivo Biosensing. Angew. Chem., Int. Ed. 2018, 57 (25), 7518–7522. 10.1002/anie.201802889. [DOI] [PubMed] [Google Scholar]
- Wang R.; Li X.; Zhou L.; Zhang F. Epitaxial Seeded Growth of Rare-Earth Nanocrystals with Efficient 800 nm Near-Infrared to 1525 nm Short-Wavelength Infrared Downconversion Photoluminescence for In Vivo Bioimaging. Angew. Chem., Int. Ed. 2014, 53 (45), 12086–12090. 10.1002/anie.201407420. [DOI] [PubMed] [Google Scholar]
- Liu H.; Hong G.; Luo Z.; Chen J.; Chang J.; Gong M.; He H.; Yang J.; Yuan X.; Li L.; Mu X.; Wang J.; Mi W.; Luo J.; Xie J.; Zhang X.-D. Atomic-Precision Gold Clusters for NIR-II Imaging. Adv. Mater. 2019, 31 (46), 1901015. 10.1002/adma.201901015. [DOI] [PubMed] [Google Scholar]
- Wan H.; Yue J.; Zhu S.; Uno T.; Zhang X.; Yang Q.; Yu K.; Hong G.; Wang J.; Li L.; Ma Z.; Gao H.; Zhong Y.; Su J.; Antaris A. L.; Xia Y.; Luo J.; Liang Y.; Dai H. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 2018, 9 (1), 1171. 10.1038/s41467-018-03505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek F. P. R.; van der Vorst J. R. v. d.; Schaafsma B. E.; Swijnenburg R.-J.; Gaarenstroom K. N.; Elzevier H. W.; van de Velde C. J. H. v. d.; Frangioni J. V.; Vahrmeijer A. L. Intraoperative Near Infrared Fluorescence Guided Identification of the Ureters Using Low Dose Methylene Blue: A First in Human Experience. J. Urol. 2013, 190 (2), 574–579. 10.1016/j.juro.2013.02.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antaris A. L.; Chen H.; Diao S.; Ma Z.; Zhang Z.; Zhu S.; Wang J.; Lozano A. X.; Fan Q.; Chew L.; Zhu M.; Cheng K.; Hong X.; Dai H.; Cheng Z. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun. 2017, 8, 15269. 10.1038/ncomms15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Ding F.; Zhou Z.; Li C.; Pu M.; Xu Y.; Zhan Y.; Lu X.; Li H.; Yang G.; Sun Y.; Stang P. J. Rhomboidal Pt(II) metallacycle-based NIR-II theranostic nanoprobe for tumor diagnosis and image-guided therapy. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (6), 1968–1973. 10.1073/pnas.1817021116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-D.; Wang H.; Antaris A. L.; Li L.; Diao S.; Ma R.; Nguyen A.; Hong G.; Ma Z.; Wang J.; Zhu S.; Castellano J. M.; Wyss-Coray T.; Liang Y.; Luo J.; Dai H. Traumatic Brain Injury Imaging in the Second Near-Infrared Window with a Molecular Fluorophore. Adv. Mater. 2016, 28 (32), 6872–9. 10.1002/adma.201600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.; Wan H.; Wang W.; Zhang X.; Uno T.; Yang Q.; Yue J.; Gao H.; Zhong Y.; Tian Y.; Sun Q.; Liang Y.; Dai H. A theranostic agent for cancer therapy and imaging in the second near-infrared window. Nano Res. 2019, 12 (2), 273–279. 10.1007/s12274-018-2210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.; Hu Z.; Zhu S.; Ma R.; Ma H.; Ma Z.; Wan H.; Zhu T.; Jiang Z.; Liu W.; Jiao L.; Sun H.; Liang Y.; Dai H. Donor Engineering for NIR-II Molecular Fluorophores with Enhanced Fluorescent Performance. J. Am. Chem. Soc. 2018, 140 (5), 1715–1724. 10.1021/jacs.7b10334. [DOI] [PubMed] [Google Scholar]
- Li Y.; Cai Z.; Liu S.; Zhang H.; Wong S. T. H.; Lam J. W. Y.; Kwok R. T. K.; Qian J.; Tang B. Z. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 2020, 11 (1), 1255. 10.1038/s41467-020-15095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian G.; Dai B.; Luo M.; Yu D.; Zhan J.; Zhang Z.; Ma D.; Wang Z. Y. Band Gap Tunable, Donor–Acceptor–Donor Charge-Transfer Heteroquinoid-Based Chromophores: Near Infrared Photoluminescence and Electroluminescence. Chem. Mater. 2008, 20 (19), 6208–6216. 10.1021/cm801911n. [DOI] [Google Scholar]
- Ding F.; Fan Y.; Sun Y.; Zhang F. Beyond 1000 nm Emission Wavelength: Recent Advances in Organic and Inorganic Emitters for Deep-Tissue Molecular Imaging. Adv. Healthcare Mater. 2019, 8 (14), 1900260. 10.1002/adhm.201900260. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Ma Z.; Wang H.; Zhou B.; Zhu S.; Zhong Y.; Wang J.; Wan H.; Antaris A.; Ma R.; Zhang X.; Yang J.; Zhang X.; Sun H.; Liu W.; Liang Y.; Dai H. Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window. Adv. Mater. 2017, 29 (12), 1605497. 10.1002/adma.201605497. [DOI] [PubMed] [Google Scholar]
- Huang S.; Wang K.; Wang S.; Wang Y.; Wang M. Highly Fluorescent Polycaprolactones with Tunable Light Emission Wavelengths across Visible to NIR Spectral Window. Adv. Mater. Interfaces 2016, 3 (17), 1600259. 10.1002/admi.201600259. [DOI] [Google Scholar]
- Yang C.; Wang X.; Huang S.; Wang M. Tunable Förster Resonance Energy Transfer in Colloidal Nanoparticles Composed of Polycaprolactone-Tethered Donors and Acceptors: Enhanced Near-Infrared Emission and Compatibility for In Vitro and In Vivo Bioimaging. Adv. Funct. Mater. 2018, 28 (7), 1705226. 10.1002/adfm.201705226. [DOI] [Google Scholar]
- Yang C.; Liu H.; Zhang Y.; Xu Z.; Wang X.; Cao B.; Wang M. Hydrophobic-Sheath Segregated Macromolecular Fluorophores: Colloidal Nanoparticles of Polycaprolactone-Grafted Conjugated Polymers with Bright Far-Red/Near-Infrared Emission for Biological Imaging. Biomacromolecules 2016, 17 (5), 1673–1683. 10.1021/acs.biomac.6b00092. [DOI] [PubMed] [Google Scholar]
- Tian R.; Zeng Q.; Zhu S.; Lau J.; Chandra S.; Ertsey R.; Hettie K. S.; Teraphongphom T.; Hu Z.; Niu G.; Kiesewetter D. O.; Sun H.; Zhang X.; Antaris A. L.; Brooks B. R.; Chen X. Albumin-chaperoned cyanine dye yields superbright NIR-II fluorophore with enhanced pharmacokinetics. Sci. Adv. 2019, 5 (9), eaaw0672. 10.1126/sciadv.aaw0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Zhao M.; Feng L.; Dou C.; Ding S.; Zhou G.; Lu L.; Zhang H.; Chen F.; Li X.; Li G.; Zhao S.; Jiang C.; Wang Y.; Zhao D.; Cheng Y.; Zhang F.. Organic NIR-II molecule with long blood half-life for in vivo dynamic vascular imaging. Nat. Commun. 2020. 10.1038/s41467-020-16924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Cheng C.-A.; Cosco E. D.; Ramakrishnan S.; Lingg J. G. P.; Bruns O. T.; Zink J. I.; Sletten E. M. Shortwave infrared imaging with J-aggregates stabilized in hollow mesoporous silica nanoparticles. J. Am. Chem. Soc. 2019, 141 (32), 12475–12480. 10.1021/jacs.9b05195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.; Li B.; Zhao M.; Wang S.; Lei Z.; Lu L.; Zhang H.; Feng L.; Dou C.; Yin D.; Xu H.; Cheng Y.; Zhang F. J-aggregates of Cyanine Dye for NIR-II In-vivo Dynamic Vascular Imaging Beyond 1500 nm. J. Am. Chem. Soc. 2019, 141 (49), 19221–19225. 10.1021/jacs.9b10043. [DOI] [PubMed] [Google Scholar]
- Bricks J. L.; Kachkovskii A. D.; Slominskii Y. L.; Gerasov A. O.; Popov S. V. Molecular design of near infrared polymethine dyes: A review. Dyes Pigm. 2015, 121, 238–255. 10.1016/j.dyepig.2015.05.016. [DOI] [Google Scholar]
- Sun W.; Guo S.; Hu C.; Fan J.; Peng X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116 (14), 7768–7817. 10.1021/acs.chemrev.6b00001. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Behera R. K.; Behera P. K.; Mishra B. K.; Behera G. B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100 (6), 1973–2012. 10.1021/cr990402t. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Li Y.; Hu X.; Zhao H.; Ji Y.; Chen L.; Hu W.; Zhang W.; Li X.; Lu X.; Huang W.; Fan Q. Dual Lock-and-Key”-Controlled Nanoprobes for Ultrahigh Specific Fluorescence Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30 (31), 1801140. 10.1002/adma.201801140. [DOI] [PubMed] [Google Scholar]
- Bouit P.-A.; Aronica C.; Toupet L.; Le Guennic B.; Andraud C.; Maury O. Continuous Symmetry Breaking Induced by Ion Pairing Effect in Heptamethine Cyanine Dyes: Beyond the Cyanine Limit. J. Am. Chem. Soc. 2010, 132 (12), 4328–4335. 10.1021/ja9100886. [DOI] [PubMed] [Google Scholar]
- Lepkowicz R. S.; Przhonska O. V.; Hales J. M.; Fu J.; Hagan D. J.; Van Stryland E. W.; Bondar M. V.; Slominsky Y. L.; Kachkovski A. D. Nature of the electronic transitions in thiacarbocyanines with a long polymethine chain. Chem. Phys. 2004, 305 (1), 259–270. 10.1016/j.chemphys.2004.06.063. [DOI] [Google Scholar]
- Strekowski L.; Mason J. C.; Britton J. E.; Lee H.; Van Aken K.; Patonay G. The addition reaction of hydroxide or ethoxide ion with benzindolium heptamethine cyanine dyes. Dyes Pigm. 2000, 46 (3), 163–168. 10.1016/S0143-7208(00)00046-2. [DOI] [Google Scholar]
- Starosolski Z.; Bhavane R.; Ghaghada K. B.; Vasudevan S. A.; Kaay A.; Annapragada A. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLoS One 2017, 12 (11), e0187563 10.1371/journal.pone.0187563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Hu Z.; Tian R.; Yung B. C.; Yang Q.; Zhao S.; Kiesewetter D. O.; Niu G.; Sun H.; Antaris A. L.; Chen X. Repurposing Cyanine NIR-I Dyes Accelerates Clinical Translation of Near-Infrared-II (NIR-II) Bioimaging. Adv. Mater. 2018, 30, 1802546. 10.1002/adma.201802546. [DOI] [PubMed] [Google Scholar]
- Li T.; Li C.; Ruan Z.; Xu P.; Yang X.; Yuan P.; Wang Q.; Yan L. Polypeptide-Conjugated Second Near-Infrared Organic Fluorophore for Image-Guided Photothermal Therapy. ACS Nano 2019, 13 (3), 3691–3702. 10.1021/acsnano.9b00452. [DOI] [PubMed] [Google Scholar]
- Li D.; Wang S.; Lei Z.; Sun C.; El-Toni A. M.; Alhoshan M. S.; Fan Y.; Zhang F. Peroxynitrite Activatable NIR-II Fluorescent Molecular Probe for Drug-Induced Hepatotoxicity Monitoring. Anal. Chem. 2019, 91 (7), 4771–4779. 10.1021/acs.analchem.9b00317. [DOI] [PubMed] [Google Scholar]
- Meng X.; Zhang J.; Sun Z.; Zhou L.; Deng G.; Li S.; Li W.; Gong P.; Cai L. Hypoxia-triggered single molecule probe for high-contrast NIR II/PA tumor imaging and robust photothermal therapy. Theranostics 2018, 8 (21), 6025–6034. 10.7150/thno.26607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würthner F.; Kaiser T. E.; Saha-Möller C. R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem., Int. Ed. 2011, 50 (15), 3376–3410. 10.1002/anie.201002307. [DOI] [PubMed] [Google Scholar]
- Yang C.; Wang X.; Wang M.; Xu K.; Xu C. Robust Colloidal Nanoparticles of Pyrrolopyrrole Cyanine J-Aggregates with Bright Near-Infrared Fluorescence in Aqueous Media: From Spectral Tailoring to Bioimaging Applications. Chem. - Eur. J. 2017, 23 (18), 4310–4319. 10.1002/chem.201604741. [DOI] [PubMed] [Google Scholar]
- Li B.; Li W.; Xu Y.; Li J.; Tu J.; Sun S. A simple approach for the discrimination of surfactants based on the control of squaraine aggregation. Chem. Commun. 2015, 51 (78), 14652–14655. 10.1039/C5CC06086A. [DOI] [PubMed] [Google Scholar]
- Sun P.; Wu Q.; Sun X.; Miao H.; Deng W.; Zhang W.; Fan Q.; Huang W. J-Aggregate squaraine nanoparticles with bright NIR-II fluorescence for imaging guided photothermal therapy. Chem. Commun. 2018, 54 (95), 13395–13398. 10.1039/C8CC08096H. [DOI] [PubMed] [Google Scholar]
- Kim E. H.; Chin G.; Rong G.; Poskanzer K. E.; Clark H. A. Optical Probes for Neurobiological Sensing and Imaging. Acc. Chem. Res. 2018, 51 (5), 1023–1032. 10.1021/acs.accounts.7b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B. W.; Nuñez V.; Kaplan L.; Granger A. J.; Bistrong K.; Zucker H. L.; Kumar P.; Sabatini B. L.; Gu C. Caveolae in CNS arterioles mediate neurovascular coupling. Nature 2020, 579 (7797), 106–110. 10.1038/s41586-020-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D.; Parker I.; Wei S. H.; Miller M. J. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat. Rev. Immunol. 2002, 2 (11), 872–880. 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.; Lin S.; Wang Q.; Zhang Y.; Li C.; Ji R.; Wang M.; Chen G.; Wang Q. An NIR-II Fluorescence/Dual Bioluminescence Multiplexed Imaging for In Vivo Visualizing the Location, Survival, and Differentiation of Transplanted Stem Cells. Adv. Funct. Mater. 2019, 29 (2), 1806546. 10.1002/adfm.201806546. [DOI] [Google Scholar]
- Doffek C.; Alzakhem N.; Bischof C.; Wahsner J.; Güden-Silber T.; Lügger J.; Platas-Iglesias C.; Seitz M. Understanding the Quenching Effects of Aromatic C–H- and C–D-Oscillators in Near-IR Lanthanoid Luminescence. J. Am. Chem. Soc. 2012, 134 (39), 16413–16423. 10.1021/ja307339f. [DOI] [PubMed] [Google Scholar]
- Michie M. S.; Götz R.; Franke C.; Bowler M.; Kumari N.; Magidson V.; Levitus M.; Loncarek J.; Sauer M.; Schnermann M. J. Cyanine Conformational Restraint in the Far-Red Range. J. Am. Chem. Soc. 2017, 139 (26), 12406–12409. 10.1021/jacs.7b07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Tran M.; D’Este E.; Roberti J.; Koch B.; Xue L.; Johnsson K. A general strategy to develop cell permeable and fluorogenic probes for multicolour nanoscopy. Nat. Chem. 2020, 12 (2), 165–172. 10.1038/s41557-019-0371-1. [DOI] [PubMed] [Google Scholar]