Abstract
Background:
Previous studies show that the use of do-it-yourself artificial pancreas system (DIYAPS) may be associated with better glycemic control characterized by improved estimated hemoglobin A1c (eHbA1c) and time in range among adults with type 1 diabetes (T1D). However, few studies have demonstrated the changes in laboratory-measured HbA1c, which is a more accepted index for glycemic control, after using a DIYAPS.
Methods:
This is a retrospective before-after study approaching patients who reported self-use of AndroidAPS. The main inclusion criteria included: T1D; aged ⩾18 years; having complete record of ⩾3 months of continuous AndroidAPS use; with laboratory-measured HbA1c and quality of life scale data before and after 3 months of AndroidAPS use; and not pregnant. The primary outcome was the change in HbA1c between baseline and 3 months after initiation of AndroidAPS use.
Results:
Overall, 15 patients (10 females) were included; the median age was 32.2 years (range: 19.2–69.4), median diabetes duration was 9.7 years (range: 1.8–23.7) and median baseline HbA1c was 7.3% (range: 6.4–10.1). The 3 months of AndroidAPS use was associated with substantial reductions in HbA1c [6.79% (SD: 1.29) versus 7.63% (SD: 1.06), p = 0.002] and glycemic variability when compared with sensor-augmented pump therapy. A lower level of fear of hypoglycemia [22.13 points (SD: 6.87) versus 26.27 points (SD: 5.82), p = 0.010] was also observed after using AndroidAPS.
Conclusions:
The 3 months of AndroidAPS use was associated with significant improvements in glucose management and quality of life among adults with T1D.
Keywords: do-it-yourself artificial pancreas system, glycemic control, glycemic variability, quality of life, type 1 diabetes
Introduction
A number of artificial pancreas systems (APSs), also known as closed-loop systems, have been developed to automate insulin delivery for patients affected by type 1 diabetes (T1D).1 An APS consists of three components: a continuous glucose monitoring (CGM), an infusion pump and a glucose control algorithm to deliver insulin in a glucose-responsive manner. Studies have indicated that compared with sensor-augmented pump (SAP) therapy, the use of an APS resulted in a decreased hemoglobin A1c (HbA1c) level, an increased time in range (TIR), a lower risk for hypoglycemia and a higher quality of life (QoL) among T1D patients.2–5
Despite the progress of APS development and its promising efficacy, only a few commercial products have just become available in a limited number of countries. Behind the hashtag #WeAreNotWaiting, a community of T1D patients and their families has developed three APSs including OpenAPS,6 AndroidAPS7 and Loop,8 and has openly shared algorithms and instructions online before the launch of any commercial products. These three systems are referred to as do-it-yourself APS (DIYAPS) as they can be self-built by patients with commercially available CGMs and pumps. Thousands of patients are using DIYAPSs and the number is still growing because they are more accessible, economic and flexible than commercial products.9 Notably, none of these three DIYAPSs have yet been approved by any regulatory bodies; their efficacy and safety thus warrant further studies.
Previous studies show that the use of DIYAPS may be associated with better glycemic control characterized by improved estimated HbA1c (eHbA1c) and TIR.10–18 eHbA1c provides a new and convenient way to depict average glucose by using CGM data,19 but it often does not align with laboratory-measured HbA1c, a more accepted index of glycemic control and a predictor for long-term complications,20 in a significant number of individuals.21 Thus, using laboratory-measured HbA1c in the analysis could more reliably reflect the association of AndroidAPS usage with glycemic control. Further, DIYAPS has not yet been evaluated in patients with suboptimally controlled T1D. In addition, in previous studies assessing the impact of commercial APS on QoL, QoL studies are mostly prospective and may be confounded by the frequent contact between patients and healthcare professionals (HCPs), and the scales used were mainly to address the level of fear of hypoglycemia.4 Only two DIYAPS studies assessed the change in QoL, and they used qualitative netnography22 or unvalidated survey-specific questionnaires23 rather than validated QoL scales.
In the present study, we aimed to assess the impact of using AndroidAPS, a type of existing DIYAPSs prevalent due to the compatibility with Bluetooth-enabled insulin pumps,24 on glucose management among adult T1D patients with laboratory-measured HbA1c. The relationship between the use of AndroidAPS and QoL was also analyzed by applying several validated scales with minimum intervention of HCPs.
Methods
Study design and participants
We performed a retrospective cohort study recruiting adults with T1D in the T1D China Registry Study (Supplementary), who self-reported that they had used AndroidAPS. The inclusion criteria were (1) T1D; (2) age ⩾18 years; (3) having a record of ⩾2 weeks of SAP therapy within 3 months prior to the use of AndroidAPS; (4) having a record of ⩾3 months of continuous use of AndroidAPS; and (5) with laboratory-measured HbA1c as well as QoL scale data before and after 3 months of AndroidAPS use. Continuous use of AndroidAPS was defined as being off AndroidAPS for <7 consecutive days. The exclusion criteria included pregnancy, any diseases potentially interfering with the study (Supplementary), and use of oral antidiabetic agents or regular use of acetaminophen. This study was reviewed and approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of University of Science and Technology of China (IRB No. 2019 KY-137). All included participants provided written informed consent.
Demographic and clinical information as well as QoL scale data were extracted from the dataset of the T1D China Registry Study, and CGM data were donated by participants. Settings on AndroidAPS were collected via an online study-specific survey (Supplementary). Transition from SAP therapy to AndroidAPS was defined as baseline, and the last 2 weeks’ data of SAP therapy before AndroidAPS were used. The first 3 months of AndroidAPS use was defined as the study period.
Devices
AndroidAPS hybrid closed-loop system
AndroidAPS was first developed by Milos Kozak.7 It uses the same heuristic-based algorithm as OpenAPS, which is designed to follow the same math that people with diabetes themselves use to make decisions about insulin adjustments.25 AndroidAPS communicates with an Android-operated phone, a CGM and a pump via a Bluetooth bridge. The algorithm of AndroidAPS provides not only basal rate adjustment but also additional features such as super micro bolus (SMB) and unannounced meal (UAM).26 The SMB feature allows the system to issue an automated correction bolus to front-shift the peak insulin activity; additionally it sets a temporary zero basal rate to prevent overdosing. The UAM feature enables AndroidAPS to effectively detect and handle increases in glucose which are resulted from unannounced carbohydrate intake or hormonal variation.
Basically, real-time CGM data flow into AndroidAPS every 5 min and trigger the embedded algorithm to predict a glucose trajectory in light of the current sensor glucose value, insulin on board, carbohydrates on board, duration of action, insulin sensitivity factor, insulin/carbohydrate ratio and other relevant factors. It then issues a temporary basal rate or an SMB to pump according to the deviation between the future estimated glucose value and predefined glycemic control target, with the purpose of keeping the glucose level within the glycemic control target.
Remote monitoring
After initiating AndroidAPS, an open-source web-based platform (Nightscout)27 could be simultaneously initiated. This platform provides users and their relatives with real-time access to important AndroidAPS data including current glucose value, insulin on board, carbohydrates on board etc. The platform can also automatically sound an audible alarm to users and their relatives. The alarm is activated in the event of hypoglycemia, hyperglycemia or stale data, and these three situations can be customized to the user’s preference.
Outcomes
The primary outcome was the change in HbA1c between baseline and 3 months after initiation of AndroidAPS. Secondary outcomes included changes in percentages of time when sensor glucose concentrations were in clinically relevant ranges (<3.0 mmol/l, <3.9 mmol/l, 3.9–10.0 mmol/l, 3.9–7.8 mmol/l, >10.0 mmol/l, >13.9 mmol/l) between baseline and the study period; change in mean sensor glucose value between baseline and the study period; change in glucose variability (GV) as measured by standard deviation (SD), coefficient of variation (CV) and mean amplitude of glycemic excursion (MAGE) according to sensor glucose values between baseline and the study period; and change in QoL as assessed by the Chinese versions of the Hypoglycemia Fear Survey ii-Worry Scale, Diabetes Distress Scale and EQ-5D-5L questionnaire [utility index (UI) and visual analog scale (VAS)] between baseline and the study period. Well-controlled T1D was defined as baseline HbA1c < 7.5%, whereas suboptimally controlled T1D was defined as baseline HbA1c ⩾ 7.5%. Sensor glucose concentrations <3.0 mmol/l, <3.9 mmol/l, >10.0 mmol/l, > 13.9 mmol/l, 3.9–10.0 mmol/l, and 3.9–7.8 mmol/l were also referred to as time below range (TBR) 3.0, TBR 3.9, time above range (TAR) 10.0, TAR 13.9, TIR and time in target (TIT), respectively. All glycemic outcome measures derived from CGM data were calculated for full 24 h period and for nighttime period (00:00 h to 06:00 h) and daytime period (06:00 h to 24:00 h).28 Little or no distress on the diabetes distress scale was defined as a mean-item score <2.0.29 The UI in the EQ-5D-5L questionnaire was calculated based on the EQ-5D-5L value set for the United Kingdom,30 as no value set for China is available for calculation and the UK version is one of the most commonly used value sets.
Safety outcomes were the frequencies of (1) severe hypoglycemia defined as a severe event characterized by altered mental and/or physical functioning that requires assistance from another person for recovery;31 (2) diabetic ketoacidosis; (3) other serious adverse events including death, life-threatening events, initial or prolonged hospitalization, disability or permanent damage;32 and (4) nonsevere hypoglycemic events during the study phase.
Statistical analyses
CGM data were processed using Glyculator 2.0 software,33 which is designed to calculate every metric of CGM data recommended by the International Consensus.28 Continuous variables in Table 1 are summarized as median (range: minimum–maximum). Continuous variables elsewhere are summarized as mean ± SD if normally distributed or median and interquartile range (IQR: Q1–Q3) if not normally distributed. Categorical variables are summarized as counts and percentages. Variables between baseline and study period were compared using paired-samples Student’s t test for continuous variables if normally distributed, two-related-samples tests for continuous variables if not normally distributed and Chi-square test for categorical variables. Variables between the well-controlled and suboptimally controlled groups were compared using Student’s t test for continuous variables if normally distributed, the Mann–Whitney U-test for continuous variables if not normally distributed and the Chi-square test for categorical variables. In order to investigate whether glycemic metrics change over the time-course of AndroidAPS use, the first 30 days of using AndroidAPS with days 31–60 and 61–90 were compared, respectively. Comparison of consecutive time periods was performed using repeated-measures analysis of variance. Relationships between duration of pump use and glycemic metrics were assessed with Pearson correlation coefficient, or Spearman correlation coefficient if data were not normally distributed. The first 3 months of AndroidAPS use for patient No. 5 were divided into days, and a paired-samples Student’s t test was performed to compare the TBR 3.9% between the first 2 months and the last month of AndroidAPS use. Statistical analyses were performed with SPSS version 25.0 (SPSS Inc, Chicago, IL, USA). Statistical significance was defined as two-tailed p < 0.05.
Table 1.
Baseline characteristics of participants enrolled.
| All participants | Well controlled | Suboptimally controlled | p value‡ | |
|---|---|---|---|---|
| N | 15 | 8 | 7 | - |
| Sex, female (%)† | 10 (66.7) | 5 (62.5) | 5 (71.4) | 1.000 |
| Age (years) | 32.2 (19.2–69.4) | 36.4 (22.6–69.4) | 21.8 (19.2–33.8) | 0.011 |
| Duration of T1D (years) | 9.7 (1.8–23.7) | 10.2 (1.8–23.7) | 9.3 (2.6–23.3) | 0.939 |
| Baseline HbA1c (%) | 7.3 (6.4–10.1) | 6.8 (6.4–7.3) | 8.4 (7.8–10.1) | <0.001 |
| BMI (kg/m²) | 21.9 (15.0–26.3) | 22.4 (17.6–26.3) | 21.9 (15.0–26.0) | 0.606 |
| Duration of pump use (years) | 6.7 (0.1–14.4) | 5.4 (1.0–11.4) | 6.9 (0.1–14.4) | 0.903 |
| Total daily insulin dosage (U/kg/day) | 0.57 (0.35–1.19) | 0.57 (0.47–1.19) | 0.64 (0.35–0.72) | 0.355 |
Variable is presented as number (percentage), other variables are presented as median (min–max) ‡Well controlled versus suboptimally controlled.
BMI, body mass index; HbA1c, hemoglobin A1c; T1D, type 1 diabetes.
Results
Subjects and system use
From 1 May 2018 to 1 September 2019, a total of 49 participants who self-reported AndroidAPS use were screened (the identification of eligibility is shown in Supplementary Figure S1). Among them, 15 patients (10 female individuals) were included in the analysis; the median age was 32.2 years (range: 19.2–69.4), the median diabetes duration was 9.7 years (range: 1.8–23.7) and the median baseline HbA1c was 7.3% (range: 6.4–10.1) (Table 1). The median body mass index (BMI) of these patients was 21.9 kg/m2 (range: 15.0–26.3), the median pump-therapy duration was 6.7 years (range: 0.1–14.4) and the median daily insulin dose was 0.57 U/kg/d (range: 0.35–1.19) (Table 1). All patients used rapid-acting insulin analog (eight used lispro and seven used aspart). Overall, eight patients were well controlled, while the other seven were suboptimally controlled at baseline (Table 1).
During the study period, all participants used Dexcom G5® sensors, Dana R pumps and AndroidAPS (version 2.0, enabling SMB/UAM and remote monitoring features) to close the loop (Supplementary Table S1). A total of 10 patients used 5.6 mmol/l as a prespecified glycemic control target in AndroidAPS, and the other 5 used more than one target at different time points. All patients used SMB/UAM, with only one patient (No. 5) choosing to disable the SMB feature approximately 2 months later considering an increased risk for hypoglycemia. The frequency of reminders patients received from their relatives when Nightscout alarms were activated was shown in Supplementary Table S2. A total of 9 out of 15 patients had received reminders from their relatives after the activation of Nightscout alarms.
Glucose management
Overall, the use of AndroidAPS was associated with reductions in both mean HbA1c [6.79% (SD: 1.29) versus 7.63% (SD: 1.06), p = 0.002] and mean sensor glucose value [7.43 mmol/l (SD: 0.56) versus 8.03 mmol/l (SD: 0.58), p < 0.001], together with a decrease in TBR 3.9% [1.72% (SD: 0.98) versus 2.83% (SD: 1.97), p = 0.011] (Table 2 and Figure 1). After switching from SAP to AndroidAPS, higher TIR% [84.28% (SD: 6.92) versus 75.01% (SD: 10.13), p < 0.001] and TIT% [62.26% (SD: 9.49) versus 48.93% (SD: 8.86), p < 0.001] were also observed, which were mainly attributable to a decrease in hyperglycemia [TAR 10.0%: 14.00% (SD: 6.74) versus 22.16% (SD: 9.60), p < 0.001]. The use of AndroidAPS was also correlated with a decrease in median SD [2.29 mmol/l (IQR: 2.09–2.45) versus 2.63 mmol/l (IQR: 2.30–3.15), p = 0.001] and MAGE (5.60 mmol/l (IQR: 5.27–6.51) versus 6.47 mmol/l (IQR: 5.95–8.37), p = 0.001].
Table 2.
Changes in glycemic control and variability before and after 3 months of AndroidAPS use among all participants.
| Before use of AndroidAPS | After use of AndroidAPS | Difference | p value | |
|---|---|---|---|---|
| HbA1c (%) | 7.63 ± 1.06 | 6.79 ± 1.29 | −0.85 ± 0.88 | 0.002 |
| Mean glucose value (mmol/l) | 8.03 ± 0.58 | 7.43 ± 0.56 | −0.60 ± 0.52 | <0.001 |
| TIT (3.9–7.8 mmol/l) (%) | 48.93 ± 8.86 | 62.26 ± 9.49 | 13.33 ± 8.99 | <0.001 |
| TIR (3.9–10.0 mmol/l) (%) | 75.01 ± 10.13 | 84.28 ± 6.92 | 9.27 ± 6.71 | <0.001 |
| % CGM time <3.0 mmol/l† | 0.33 (0.17, 1.31) | 0.16 (0.09, 0.44) | −0.18 (−0.49, 0.07) | 0.017 |
| % CGM time <3.9 mmol/l | 2.83 ± 1.97 | 1.72 ± 0.98 | −1.11 ± 1.49 | 0.011 |
| % CGM time >10.0 mmol/l | 22.16 ± 9.60 | 14.00 ± 6.74 | −8.16 ± 6.66 | <0.001 |
| % CGM time >13.9 mmol/l† | 3.15 (0.96, 5.55) | 1.55 (0.88, 2.12) | −0.75 (−1.84, −0.08) | 0.020 |
| SD (mmol/l)† | 2.63 (2.30, 3.15) | 2.29 (2.09, 2.45) | −0.24 (−0.37, −0.03) | 0.001 |
| CV (%)† | 32.63 (31.65, 37.40) | 31.22 (29.51, 33.14) | −1.23 (−4.24, 1.95) | 0.211 |
| MAGE (mmol/l)† | 6.47 (5.95, 8.37) | 5.60 (5.27, 6.51) | −0.68 (−1.20, −0.37) | 0.001 |
Variables are presented as median (interquartile), other variables are presented as mean ± SD.
CGM, continuous glucose monitoring; CV, coefficient of variation; HbA1c, hemoglobin A1c; MAGE, mean amplitude of glycemic excursion; SD, standard deviation; TIR, time in range; TIT, time in target.
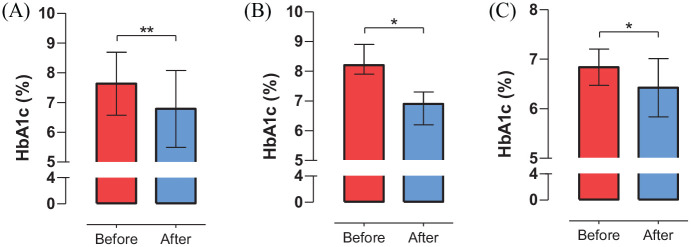
Figure 1.
Changes in HbA1c level among participants before and after 3 months of AndroidAPS use.
Panel A, B and C show the changes in HbA1c level before and after 3 months of AndroidAPS use among all participants, participants with suboptimally controlled T1D and participants with well-controlled T1D, respectively. *: p < 0.05; **: p < 0.01.
HbA1c, hemoglobin A1c; T1D, type 1 diabetes.
The improvements in glycemic control and fluctuation were present when stratified by baseline HbA1c. As shown in Figure 1 and Supplementary Table S3, the use of AndroidAPS was associated with a substantial reduction in median HbA1c [6.90% (IQR: 6.20, 7.30) versus 8.20% (IQR: 7.90, 8.90), p = 0.027] among suboptimally controlled T1D patients. Higher TIR% and TIT% values were also observed during the 3 months of use of AndroidAPS than at baseline due to a combination of decreases in hypoglycemia and hyperglycemia. The use of AndroidAPS was also related to less glycemic fluctuation, which was characterized by decreases in mean SD and MAGE among individuals with suboptimally controlled T1D. For participants with well-controlled T1D, the use of AndroidAPS was associated with a reduction in mean HbA1c [6.42% (SD: 0.59) versus 6.84% (SD: 0.37), p = 0.025], with a nonsignificant decrease in TBR 3.9% (p = 0.265) (Figure 1 and Supplementary Table S4). Significant improvements in other secondary outcomes including mean sensor glucose value, TIR%, TIT%, hyperglycemia, SD and MAGE were also observed after the transition from SAP to AndroidAPS.
The majority of reductions in HbA1c [−1.50% (IQR: −1.90, −1.30) versus −0.48% (IQR: −0.67, −0.05), p = 0.020] were presented in patients with suboptimally controlled T1D compared to those with well-controlled T1D, while the changes in other glycemic metrics were comparable between the two groups (Supplementary Table S5).
The benefits of AndroidAPS on glucose management were observed during both nighttime and daytime periods but were prominent during nighttime among all participants (Supplementary Table S6). The improvement in TIR% was 15.95% for the nighttime versus 7.06% for the daytime period.
No significant improvement in glucose management over time within the first 90 days of AndroidAPS usage was observed among all participants: mean glucose value was 7.46 mmol/l (SD: 0.54), 7.40 mmol/l (SD: 0.58), 7.51 mmol/l (SD: 0.92), p = 0.230; TIR% was 84.55% (SD: 6.66), 85.85% (SD: 7.11), 84.75% (SD: 9.15), p = 0.939; TBR 3.9% was 1.66% (SD: 1.12), 1.82% (SD: 1.20), 1.60% (SD: 0.86), p = 0.663; SD: 2.33 mmol/l (SD: 0.39), 2.35 mmol/l (SD: 0.42), 2.29 mmol/l (SD: 0.52), p = 0.246 for days 1–30, 31–60 and 61–90, respectively.
No significant relationship between duration of pump use and change in any glycemic metrics was observed among all participants (Supplementary Table S7).
All glycemic metrics were slightly different between the first 2 months and the last month of AndroidAPS use for patient No. 5 (Supplementary Table S8). No difference on TBR 3.9% between the first 2 months and last month of AndroidAPS use was revealed [1.89% (SD: 1.75) versus 2.19% (SD: 3.27), p = 0.673].
Quality of life
A lower level of fear of hypoglycemia [22.13 points (SD: 6.87) versus 26.27 points (SD: 5.82), p = 0.010] was observed after three months of AndroidAPS use, together with seemingly lower diabetes distress (p = 0.143) and a higher EQ-5D-5L (VAS) score (p = 0.130) among all participants (Table 3). No change in EQ-5D-5L (UI) score was found before and after 3 months of AndroidAPS use.
Table 3.
Changes in quality of life before and after 3 months of AndroidAPS use among all participants.
| Before use of AndroidAPS | After use of AndroidAPS | Difference | p value | |
|---|---|---|---|---|
| Chinese version Hypoglycemia Fear Survey II-Worry Scale (points) | 26.27 ± 5.82 | 22.13 ± 6.87 | −4.13 ± 5.40 | 0.010 |
| Diabetes Distress Scale, little or no distress (%)† | 6 (40.0) | 9 (66.7) | / | 0.143 |
| EQ-5D-5L, UI (points)‡ | 0.88 (0.77, 1.00) | 0.88 (0.84, 1.00) | 0 (0, 0.07) | 0.441 |
| EQ-5D-5L, VAS (points)‡ | 77.0 (70.0, 90.0) | 82.0 (72.0, 90.0) | 5.00 (0, 10.0) | 0.130 |
Variables are presented as median (interquartile) and ‡variable is presented as number (percentage), other variables are presented as mean ± SD.
EQ-5D-5L, EuroQol Five-Dimension 5-Level Health Questionnaire; UI, utility index; VAS, visual analog scale.
Safety outcomes
No severe hypoglycemia, diabetic ketoacidosis or other serious adverse events occurred during the use of AndroidAPS among all participants. The TBR 3.9% and TBR 3.0% [0.16% (IQR: 0.09–0.44) versus 0.33% (IQR: 0.17–1.31), p = 0.017] were both lower during the 3 months of use of AndroidAPS than at baseline among all participants (Table 2).
Discussion
In this 3-month retrospective study, we used laboratory-measured HbA1c values and validated QoL scales and found that compared with SAP therapy, the use of AndroidAPS was associated with better glucose management and higher QoL among adults with T1D.
We observed a profound reduction in HbA1c (−0.85%) after 3 months of AndroidAPS use, which was greater than that reported (−0.5%)34 in the pivotal study using the first commercial APS. One explanation is the lower glycemic control target (mostly 5.6 mmol/l) used in our study than that (6.7 mmol/l35) in the abovementioned study. A lower target may lead to a more aggressive manner of insulin administration and thus a lower HbA1c level. Future head-to-head comparison between AndroidAPS and commercial products is needed to further evaluate the superiority. The multiplicity of other beneficial outcomes, including improvement in TIR% (9.27%, which amounted to 2.2 h per day), 8.16% (2.0 h per day) less time in hyperglycemia and 1.11% (16 min per day) less time in hypoglycemia as well as reduced glycemic variability, is consistent with previous studies using either rigorously developed APSs36–38 or DIYAPSs.9
Many studies have suggested the efficacy of DIYAPSs among patients with averagely good glycemic control at baseline, but these individuals may not be representative of the wider population with T1D. Our study indicated that self-use of AndroidAPS, a type of DIYAPS, may be associated with improvements in both glycemic control and fluctuation among patients with suboptimally controlled T1D, who are considered to be less engaged in diabetes care.39 This suggested that DIYAPSs are easy to use and can reduce the burden of diabetes management, so that patients with less involvement could also benefit.22 Moreover, well-controlled participants achieved further benefits in HbA1c, TIR% and GV, without compromising on undue hypoglycemia. This is in line with previous studies evaluating DIYAPS.10–12,14–18 Our findings collectively underpin the robustness of DIYAPS as a seemingly effective approach among T1D patients irrespective of baseline HbA1c.
Previous prospective studies revealed an improvement in QoL among T1D patients by using commercial APSs.4 The frequent contacts between participants and HCPs in these studies may confound the results of QoL. The relationship between QoL and DIYAPS usage has previously been untested. Here, we evaluated the association between AndroidAPS usage and QoL with minimum intervention of HCPs and revealed similar results as in commercial APS studies. Moreover, we applied validated QoL scales, rather than qualitative netnography22 or unvalidated survey-specific questionnaires23 as in other DIYAPS studies, to generate more objective, reliable, reproducible and generalizable evidence. In addition to the Hypoglycemia Fear Survey, which is commonly used in APS studies, the Diabetes Distress Scale and EQ-5D-5L questionnaire were applied to assess the changes in diabetes-related distress as well as generic health status. Though statistical significance was not reached, improvements in both aspects were observed; a larger sample size is required to confirm the findings.
AndroidAPS also represents a safe method of glucose management, with no serious adverse events occurring during 3 months of use. It may be even safer than SAP therapy given the reduced percentage of time in hypoglycemia. The glycemic control target in the first commercial APS is set to be as high as 6.7 mmol/l and fixed35 to minimize hypoglycemia. In our study, all participants used a target below 6.7 mmol/l. The TBR 3.9%, however, was smaller in our study (1.72%) than that in a pivotal commercial APS study (3.30%).34 This may attribute to AndroidAPS’s remote monitoring feature. Remote monitoring has separately been studied and recommended in other systems to reduce risks for prolonged nocturnal hypoglycemia.40 This feature was used by all patients in our study and most patients self-reported that they had received reminders from their relatives due to the audible alarms on Nightscout platform, which may enable them to promptly deal with hypoglycemia. Despite its benefits, the privacy and security of patient data on remote monitoring platform should be addressed.41 Furthermore, an unfixed glycemic target is also an advantage of AndroidAPS. It enables AndroidAPS to be adapted to a wider range of patients, including pregnant women complicated by pre-existing diabetes who require a lower glycemic goal and older adults with diabetes whose glycemic control is less strict.31
In terms of HbA1c reduction, it appeared that participants with suboptimally controlled T1D in our study benefited more from AndroidAPS use than their counterparts (p = 0.020). Apart from a higher baseline HbA1c level,42 a younger age in our study may also explain this observation. Studies have shown that younger people are more familiar with digital devices and technologies and have less difficulty adopting new technologies than their older counterparts.43 In our study, suboptimally controlled patients were younger (21.8 versus 36.4 years, p = 0.011) and were also more likely to have a higher proportion of time in closed-loop mode due to fewer device pitfalls. A 6-month real-life study suggested that more time spent in closed-loop mode may be associated with lower HbA1c among T1D patients.44 The number of device pitfalls and percentage of time in closed-loop mode were not available due to the retrospective design in our study, and further analysis is yet to be performed.
The benefits of APS were greater overnight in many studies including ours, indicating that carbohydrate intake and physical activity during daytime remain challenges for current APS systems.45 AndroidAPS is progressing towards a full closed-loop system by introducing advanced features such as SMB and UAM, and some patients were reportedly to achieve ideal outcomes without meal announcements.9 This may not be achieved in our study population, however, as the high-carbohydrate diet pattern in Asia46 could be a huge challenge; and patients in our study used rapid-acting insulin rather than ultra-rapid-acting insulin,47 which is still not available in many countries. The application of ultra-rapid-acting insulin in AndroidAPS, in combination with the integration of devices such as a heart rate monitor to detect exercise,48 may help realize fully closed-loop insulin delivery in patients with lower carbohydrate intake.
Here, we did not observe a significant difference in TBR 3.9% for patient No. 5 between the first 2 months and the last month, while the patient self-reported that he disabled SMB due to fear of hypoglycemia. The possible explanations may be: (1) the TBR 3.9% during SAP period for this patient was lower (SAP period versus the first 2 months: 1.00% versus 1.90%); (2) the mean glucose value decreased after switching to AndroidAPS (SAP period versus the first 2 months: 7.79 mmol/l versus 7.02 mmol/l). A lower mean glucose value may increase the level of fear of hypoglycemia in some certain patients.
The strength of this study is that we used laboratory-measured HbA1c rather than eHbA1c and evaluated a DIYAPS among patients with suboptimally controlled T1D. Further, we used validated scales to assess QoL with minimum involvement of HCPs. Several limitations need to be addressed. First, it is not feasible to distinguish between the effect of AndroidAPS use and other factors due to the single-arm, nonrandomized study design; a planned randomized controlled trial will further investigate the efficacy of AndroidAPS.49 Second, the sample size is small and selection bias exists. All participants self-selected to use AndroidAPS. The use of AndroidAPS requires DIY integration of a CGM, a pump and a smartphone; continuous use of a CGM and a pump is costly. Therefore, patients with higher motivation and better economic status were more likely to be involved in this study.
Conclusion
In conclusion, we demonstrated that the 3 months of use of AndroidAPS was associated with significant improvements in glycemic control, GV and QoL among adults with T1D, irrespective of baseline HbA1c. Our results also suggested that AndroidAPS might be comparable with commercial APS products.
Supplemental Material
Supplemental material, sj-pdf-1-tae-10.1177_2042018820950146 for Use of a do-it-yourself artificial pancreas system is associated with better glucose management and higher quality of life among adults with type 1 diabetes by Zekai Wu, Sihui Luo, Xueying Zheng, Yan Bi, Wen Xu, Jinhua Yan, Daizhi Yang and Jianping Weng in Therapeutic Advances in Endocrinology and Metabolism
Acknowledgments
We thank all the participants for kindly donating the CGM data. We also thank the DIYAPS community, especially Ms. Dana Lewis and Mr. Milos Kozak for sharing the open-source closed-loop technology.
Footnotes
Author contributions: Zekai Wu: Conceptualization; Data curation; Formal analysis; Methodology; Software; Writing-original draft; Writing-review & editing.
Sihui Luo: Conceptualization; Methodology; Writing-review & editing.
Xueying Zheng: Conceptualization; Funding acquisition; Methodology; Writing-review & editing.
Yan Bi: Investigation; Writing-review & editing.
Wen Xu: Investigation; Writing-review & editing.
Jinhua Yan: Investigation; Writing-review & editing.
Daizhi Yang: Investigation; Writing-review & editing.
Jianping Weng: Conceptualization; Funding acquisition; Project administration; Resources; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This work was supported by the National Key R&D Program of China (2017YFC1309603), National Natural Science Foundation of China (Key Programme 81530025), National Natural Science Foundation of China (81941022), The Fundamental Research Funds for the Central Universities (WK9110000137) and Strategic Priority Research Program of Chinese Academy of Sciences (XDB38010100).
Disclosure: This work was presented in part at the 13th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2020), Madrid, Spain, 19–22 February 2020.
ORCID iD: Zekai Wu  https://orcid.org/0000-0002-0671-1888
https://orcid.org/0000-0002-0671-1888
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Zekai Wu, Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Diabetology, Guangzhou, China.
Sihui Luo, Department of Endocrinology and Metabolism, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences of Medicine, University of Science and Technology of China, Hefei, China.
Xueying Zheng, Department of Endocrinology and Metabolism, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences of Medicine, University of Science and Technology of China, Hefei, China.
Yan Bi, Department of Endocrinology, Drum Tower Hospital Affiliated to Nanjing University Medical School, Nanjing, China.
Wen Xu, Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Diabetology, Guangzhou, China.
Jinhua Yan, Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Diabetology, Guangzhou, China.
Daizhi Yang, Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Diabetology, Guangzhou, China.
Jianping Weng, Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Diabetology, Guangzhou 510630, China; Department of Endocrinology and Metabolism, The First Affiliated Hospital of USTC, Division of Life Sciences of Medicine, University of Science and Technology of China, 17 Lujiang Road, Hefei 230001, People’s Republic of China.
References
- 1. Boris K. A century of diabetes technology: signals, models, and artificial pancreas control. Trends Endocrinol. Metab 2019; 30: 432–444 [DOI] [PubMed] [Google Scholar]
- 2. Weisman A, Bai J, Cardinez M, et al. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017; 5: 501–512. [DOI] [PubMed] [Google Scholar]
- 3. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018; 361: k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabet Med 2018; 35: 436–449. [DOI] [PubMed] [Google Scholar]
- 5. Tauschmann M, Hovorka R. Technology in the management of type 1 diabetes mellitus - current status and future prospects. Nat Rev Endocrinol 2018; 14: 464–475. [DOI] [PubMed] [Google Scholar]
- 6. OpenAPS. OpenAPS.org –#WeAreNotWaiting to reduce the burden of Type 1 diabetes https://openaps.org/ (accessed 9 October 2019).
- 7. Kozak M. AndroidAPS. https://androidaps.readthedocs.io/en/latest/index.html (accessed 9 October 2019).
- 8. Loop DIY. LoopDocs. https://loopkit.github.io/loopdocs/ (accessed 9 October 2019).
- 9. Lewis D. Do-it-yourself artificial pancreas system and the OpenAPS movement. Endocrinol Metab Clin North Am 2020; 49: 203–213. [DOI] [PubMed] [Google Scholar]
- 10. Lewis D, Leibrand S. Real-world use of open-source artificial pancreas systems. J Diabetes Sci Technol 2016; 10: 1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi S, Hong E, Noh Y. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes 2018; 67: 964-P. [Google Scholar]
- 12. Lewis D, Swain R, Donner T. Improvements in A1C and time-in-range in DIY closed-loop (OpenAPS) users. Diabetes 2018; 67: 352-OR. [Google Scholar]
- 13. Petruzelkova L, Soupal J, Plasova V, et al. Excellent glycemic control maintained by open-source hybrid closed-loop androidaps during and after sustained physical activity. Diabetes Technol Ther 2018; 20: 744–750. [DOI] [PubMed] [Google Scholar]
- 14. Provenzano V, Guastamacchia E, Brancato D, et al. Closing the loop with OpenAPS in people with type 1 diabetes—experience from Italy. Diabetes 2018; 67: 993-P. [Google Scholar]
- 15. Melmer A, Züger T, Lewis D, et al. Glycemic control in individuals with type 1 diabetes using an open-source artificial pancreas system (OpenAPS). Diabetes Obes Metab 2019; 21: 2333–2337. [DOI] [PubMed] [Google Scholar]
- 16. Braune K, O’Donnell S, Cleal B, et al. 117-LB: DIWHY: factors influencing motivation, barriers, and duration of DIY artificial pancreas system use among real-world users. Diabetes 2019; 68: 117-LB. [Google Scholar]
- 17. Koutsovasilis A, Sotiropoulos A, Antoniou A, et al. 1065-P: the effect of a closed-loop insulin delivery system on glycemic control in type 1 diabetes. Diabetes 2019; 68: 1065-P. [Google Scholar]
- 18. Wilmot E, Langeland L, Mclay A, et al. 1067-P: open-source artificial pancreas system (APS) vs. combination insulin pump with flash glucose monitoring in adults with type 1 diabetes: an observational study. Diabetes 2019; 68: 1067-P. [Google Scholar]
- 19. Bergenstal R, Beck R, Close K, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018; 41: 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nathan D. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014; 37: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pluchino K, Wu Y, Silk A, et al. Comment on Bergenstal et al. glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018; 41:2275–2280. 2019; 42: e28. [DOI] [PubMed] [Google Scholar]
- 22. Litchman M, Lewis D, Kelly L, et al. Twitter analysis of #OpenAPS DIY artificial pancreas technology use suggests improved A1C and quality of life. J Diabetes Sci Technol 2018; 1932296818795705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hng T, Burren D. Appearance of Do-It-Yourself closed-loop systems to manage type 1 diabetes. Intern Med J 2018; 48: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 24. Toffanin C, Kozak M, Sumnik Z, et al. In silico trials of an open-source Android-based artificial pancreas: a new paradigm to test safety and efficacy of do-it-yourself systems. Diabetes Technol Ther 2019; 22: 112–120. [DOI] [PubMed] [Google Scholar]
- 25. OpenAPS. Frequently Asked Questions – OpenAPS.org, https://openaps.org/frequently-asked-questions/ (accessed 31 March 2020).
- 26. Kozak M. OpenAPS features, https://androidaps.readthedocs.io/en/latest/EN/Usage/Open-APS-features.html (accessed 8 April 2020).
- 27. Nightscout. The Nightscout Project–We Are Not Waiting, http://www.nightscout.info/ (accessed 9 October 2019).
- 28. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisher L, Hessler D, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012; 35: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Hout B, Janssen M, Feng Y, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012; 15: 708–715. [DOI] [PubMed] [Google Scholar]
- 31. American Diabetes Association. Standards of medical care in diabetes-2020. Diabetes Care 2020; 43: S1–S212. [DOI] [PubMed] [Google Scholar]
- 32. U.S. Food and Drug Administration. What is a Serious Adverse Event?|FDA, https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed Oct 9, 2019).
- 33. Pagacz K, Stawiski K, Szadkowska A, et al. GlyCulator2: an update on a web application for calculation of glycemic variability indices. Acta Diabetol 2018; 55: 1–4. [DOI] [PubMed] [Google Scholar]
- 34. Bergenstal R, Garg S, Weinzimer S, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016; 316: 1407–1408. [DOI] [PubMed] [Google Scholar]
- 35. Stone J, Haviland N, Bailey T. Review of a commercially available hybrid closed-loop insulin-delivery system in the treatment of Type 1 diabetes. Ther Deliv 2018; 9: 77–87. [DOI] [PubMed] [Google Scholar]
- 36. Brown S, Kovatchev B, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019; 381: 1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boughton C, Hovorka R. Automated insulin delivery in adults. Endocrinol Metab Clin North Am 2020; 49: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cengiz E. Automated insulin delivery in children with type 1 diabetes. Endocrinol Metab Clin North Am 2020; 49: 157–166. [DOI] [PubMed] [Google Scholar]
- 39. Liu L, Yang D, Zhang Y, et al. Glycaemic control and its associated factors in Chinese adults with type 1 diabetes mellitus. Diabetes Metab Res Rev 2015; 31: 803–810. [DOI] [PubMed] [Google Scholar]
- 40. DeSalvo D, Keith-Hynes P, Peyser T, et al. Remote glucose monitoring in cAMP setting reduces the risk of prolonged nocturnal hypoglycemia. Diabetes Technol Ther 2014; 16: 1–7. [DOI] [PubMed] [Google Scholar]
- 41. Fatehi F, Menon A, Bird D. Diabetes care in the digital era: a synoptic overview. Curr Diab Rep 2018; 18: 38. [DOI] [PubMed] [Google Scholar]
- 42. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018; 392: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Czaja S, Charness N, Fisk A, et al. Factors predicting the use of technology: findings from the Center for Research and Education on Aging and Technology Enhancement (CREATE). Psychol Aging 2006; 21: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akturk H, Giordano D, Champakanath A, et al. Long-term real-life glycemic outcomes with hybrid closed-loop system when compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab 2020; 583–589. [DOI] [PubMed] [Google Scholar]
- 45. Lal R, Ekhlaspour L, Hood K, et al. Realizing a closed-loop (artificial pancreas) system for the treatment of type 1 diabetes. Endocr Rev 2019; 40: 1521–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dehghan M, Mente A, Zhang X, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017; 390: 2050–2062. [DOI] [PubMed] [Google Scholar]
- 47. Heise T, Pieber T, Danne T, et al. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet 2017; 56: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Breton M, Brown S, Karvetski C, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther 2014; 16: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. University of Otago. The CREATE Trial, https://www.otago.ac.nz/christchurch/departments/paediatrics/research/otago717634.html (accessed 31 May 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tae-10.1177_2042018820950146 for Use of a do-it-yourself artificial pancreas system is associated with better glucose management and higher quality of life among adults with type 1 diabetes by Zekai Wu, Sihui Luo, Xueying Zheng, Yan Bi, Wen Xu, Jinhua Yan, Daizhi Yang and Jianping Weng in Therapeutic Advances in Endocrinology and Metabolism